Abstract
The objective of this study was to compare the dosages for anesthesia induction in obese dogs using propofol based on lean body weight or total body weight. For this purpose, seven dogs with ideal body condition score (BCS) (BCS 4-5; 17.3 ± 2.5% fat mass) were included in the control group (CG), seven obese dogs (BCS 8-9; 45.7 ± 2.9% fat mass) in the total body weight group (TBWG) and seven obese dogs (BCS 8-9; 42.8 ± 6.3% fat mass) in the lean body weight group (LBWG). Anaesthesia was induced by a constant rate infusion of propofol at 150 mg kg−1 hour−1 through a propofol infusion pump until the loss of consciousness; the animals in CG and TBWG received a propofol infusion based on total body weight; the animals in LBWG received a propofol infusion based on lean body mass (in kg) determined by the deuterium dilution method. The results were compared between the groups using the Tukey test (p < 0.05). The propofol dosage used was 11.4 ± 3.2 mg kg−1, 8.0± 2.0 mg kg−1 and 14.1 ± 4.7 mg kg−1 in groups CG, TBWG and LBWG, respectively, and they were different among all groups (p < 0.001). There was also a statistical difference in the time between the start of propofol infusion and loss of consciousness in which LBWG took longer than CG and TBWG (p = 0.004). This study shows that obese dogs require lower dosages of propofol when inducing anesthesia than ideal BCS dogs anesthetized with dosages based on total body weight, when the propofol dosages are calculated on the basis of muscle mass it should be increased.
Keywords: Canines, Obesity, Overweight, Lean mass, Fat mass
1. Introduction
Canine obesity is a medical condition affecting approximately 30% to 40% of the global canine population (McGreevy et al., 2005; Holmes et al., 2007; Courcier et al., 2010; Mao et al., 2013; Usui et al., 2016); thus, there is a high chance that an obese patient will undergo a surgical procedure and consequently require an anaesthesia since a representative portion of the population is obese (Colliard et al., 2006; Lund et al., 2006).
In addition, the deleterious of being overweight on the health of dogs and cats are widely described in the literature and include effects declining of lifespan (Kealy et al., 2002), orthopedic changes (Brown et al., 1996; Kealy et al., 1997; Kealy et al., 2000), cardiovascular (Edney & Smith, 1986; Pereira-Neto et al., 2010; Pereira-Neto et al., 2014; Piantedosi et al., 2016; Tropf et al., 2017), respiratory (Hendricks, 1992; German, 2006; Bach et al., 2007; Devito et al., 2015; Pereira-Neto et al., 2018) and metabolic disorders (Chikamune et al., 1995; Jeusette et al ., 2005; Brunetto et al., 2011a, Brunetto et al., 2011b). The worsening of all these effects may eventually require surgical intervention, and the rupture of the cranial cruciate ligament is an example in the dog, since obesity a predisposing factor for the excess load on this structure that may be already compromised by the degenerative process and can only be corrected through surgical intervention (Lampman et al., 2003, Bach et al., 2015).
Obesity is characterized by the excess of body fat sufficient to compromise the regular physiology of the organism, with a subsequent decrease of the animal's quality of life (Burkholder & Toll, 2000).
Obese human patients are pre-evaluated and anesthetized considering physiological changes that may influence the pharmacokinetics of the drug used and, consequently, affects anaesthesia (Ingrande & Lemmens, 2010). The physiological changes may include: increased fat percentage, increased lean body mass as a percentage of total blood volume, increased cardiac output, increased kidney filtration, increased volume of distribution of liposoluble drugs, decreased volume of body water and changes in plasmatic protein bonds (Leykin et al., 2011). When considering these changes, drug dosages must be adjusted according to the individual's body composition (Ingrande & Lemmens, 2010; Kirkham & Thomas, 2011).
In a recent review about physiological and pharmacological parameters and their obesity-induced changes in canine and feline patients in perioperative conditions, Love & Cline (2015) described changes similar to those in humans, where small animal obesity may be associated with changes in cardiovascular, respiratory and endocrine function. In addition, body composition changes in obesity may affect pharmacokinetic variables, and alterations in perioperative care may be necessary.
Also, obesity is related to the increased mortality rate during intra e postoperative period (Brodbelt, 2006) and must be considered during anaesthesia of obese dogs. However, unfortunately, studies that evaluated the effects of extreme obesity on the pharmacology of anaesthetics are sparse (Love & Cline, 2015), and although propofol is the induction agent most frequently used in dogs, the recommendation for the appropriate use of this drug remains controversial in obese subjects.
Considering the above, the objective of this study was to compare ideal BCS dogs with obese dogs for induction of anaesthesia with propofol using the induction dose based on lean body weight mass or total body weight mass.
2. Material and methods
The present study was conducted with the approval of the Committee of Ethics in Animal Use of the School of Veterinary Medicine and Animal Science of the University of São Paulo, under protocol 2013/15643-4, and all procedures were performed with the consent of the owners.
2.1. Animals and experimental design
Twenty-one adult dogs were used in the study, both male and female, weighing up to 10 kg, of different breeds, healthy or with mild systemic diseases (ASA I or II), which came from the Laboratory of Compared Odontology of the Department of Surgery of the College of Veterinary Medicine and Animal Science of the University of São Paulo. Animals underwent surgical odontological procedures between March 2013 and August 2015.
Dogs were considered obese if they had a body condition score equal to or greater than 8, according to a scale described by Laflamme (1997). Health status was confirmed by general physical examination, complete blood count, biochemical profile, and liver and kidney functions performed before the experiment.
The animals were distributed in three groups: seven dogs with ideal body weight (BCS 4-5; 17.3± 2.5% fat mass) in the control group (CG), seven obese dogs (BCS 8-9; 45.7 ± 2.9% fat mass) in the total body weight group (TBWG) and seven obese dogs (BCS 8-9; 42.8 ± 6.3 fat mass) in the lean body weight group (LBWG), obese animals were randomly assigned to different groups (TBWG or LBWG).
2.2. Determination of body composition
Body composition was determined by the deuterium isotope dilution method. Dogs remained fasted for 8 hours before the beginning of this evaluation and water was withdrawn for two hours. A 0.4 g/kg of deuterium oxide was inoculated subcutaneously. All animals had a prior assessment of the hydration status when starting the protocol, and the analyses were continued only when it was normal (Davis et al., 2013). Blood samples (3mL) were collected from the jugular vein immediately before and after 2 hours at the inoculation of deuterium oxide. These were processed for serum extraction and stored at -20°C. The deuterium enrichment of the samples was determined by isotopic ratio mass spectrometry (IRMS, Calixto System - Sercon Ltda, Gateway, United Kingdom) according to the methodology described by Ferrier et al. (2002) and Brunetto et al. (2011b), at the Laboratory of Mass Spectrometry, Department of Medical Clinic, FMRP / USP, Ribeirão Preto - SP. Isotopic enrichment can be analysed in a serum sample after the injection of a known dose of marked water. The measurement of total body water then allows the calculation of the fat-free mass (FFM), assuming that the hydration of the FFM is constantly by 73.2% (Wang et al., 1999). After quantification of body water, the total lean mass was calculated by difference from the fat mass (expressed as a percentage).
2.3. Anesthetic protocol
All animals were fasted for twelve hours and deprived of water for six hours before. They received pre-anaesthetic medication with tramadol (Tramal: União Química, MG, Brazil) 2 mg kg−1 by intramuscular (IM) injection. After 15 minutes a 20-22 G peripheral catheter was placed aseptically into the saphenous vein and infusion with Ringer's lactate solution (Ringer Lactate, JP Indústria Farmaceutica SA, SP, Brazil) started at a rate of 10 ml kg−1 hour−1 (Mathews, 1998). Another peripheral venous access was placed into the saphenous vein of the contralateral limb for the injection of propofol (Propovan, SP, Brazil) through a specific infusion pump (Digipump Srx8, Digicare Biomedical Technology Inc, FL, USA).
Anaesthesia was induced by a constant rate infusion of propofol at 150 mg kg−1 hour−1 through a propofol infusion pump until the patient lost consciousness. Animals in CG and TBWG received a propofol infusion based on total body weight; the animals in LBWG received a propofol infusion based on lean body mass.
After the propofol induction, the patients were intubated. The animals received isoflurane (1.3 to 1.5 minimum alveolar concentration - MAC) in 100% oxygen through the anesthesia machine (Shogun, K, Takaoka Industry, SP, Brazil). After the odontological procedures, animals received dipyrone (D-500, Fort Dodge, SP, Brazil) 25 mg kg−1 and ketoprofen (Ketofen, Biofarm, SP, Brazil) 1 mg kg−1 intravenously (EMA, 2019).
2.4. Monitoring
Sedation and induction scores were classified (Table 1); loss of consciousness was determined by assessing ocular reflexes and eye position at 30-second intervals. Three-time points were defined: time 1 – the presence of palpebral reflex (PR+), presence of corneal reflex (CR+), central eye position (C); time 2 – PR+, CR+, ventromedial rotation of the eye (VMR); and time 3 – PR absent, CR+, VMR. At time point 3, the infusion pump was discontinued, and the time was recorded.
Table 1.
Comparative table of the presence of apnea, and sedation and induction scores among groups.
| CG | LBWG | TBWG | P-value | |
| Sedation (%) | 0.779 | |||
| No effect | 46.2 | 57.1 | 53.3 | |
| Discreet | 53.8 | 42.9 | 40 | |
| Moderate | - | - | 6.7 | |
| Deep | - | - | - | |
| Induction (%) | 0.548 | |||
| No effect | 92.3 | 100.0 | 93.3 | |
| Discreet | 7.7 | - | 6.7 | |
| Moderate | - | - | - | |
| Deep | - | - | - |
CG (control group, seven dogs with ideal body weight, body condition score 4-5; 17.3± 2.5% fat mass, received a propofol infusion based on total body weight); LBWG (lean body weight group, seven obese dogs, with body condition score 8-9; 42.8 ± 6.3 fat mass, received a propofol infusion based on lean body mass); TBWG (total body weight group, seven obese dogs with body condition score 8-9; 45.7 ± 2.9% fat mass, received a propofol infusion based on total body weight).
2.5. Statistical analysis
Data (sedation and induction scores; age, weight, and body composition; time from start of propofol infusion until loss of consciousness, and propofol dosages) were recorded into Excel and then exported to the statistical analysis program SPSS v. 18.0. Parametric data was tested for normality with the chi-square goodness-of-fit test. Quantitative variables (mean and standard deviation) were compared within the groups through ANOVA and post hoc Tukey's tests. One-way repeated-measures ANOVA was also used to compare mean changes related to time. P values < 0.05 were considered statistically significant.
3. Results
There was no statistical difference between sedation and induction scores between the groups (Table 1). There was no statistical difference between mean values of age (p = 0.306) of the groups GC, TBWG, LBWG who were, respectively, 6.0 ± 2.5 years, 5.3 ± 1.0 years and 6.8 ± 2.0 years (Table 2). However, for body composition, there were differences between the related groups. The lean body weight group and total body weight group presented higher fat mass and fat mass percentage compared to the control group. It was also observed a lower percentage of lean mass and body water for these groups, compared to the control group.
Table 2.
Age, weight and body composition among groups.
| CG | LBWG | TBWG | P-value | |
| Age (years) | 6.0 ± 2.5 | 5.3 ± 1.0 | 6.8 ± 2.0 | 0.306 |
| Body weight (kg) | 4.6 ± 2.7 | 7.9 ± 4.6 | 7.8 ± 2.2 | 0.126 |
| Fat mass (kg) | 0.7 ± 0.5 a | 3.5 ± 2.9 b | 3.6 ± 1.0 b | 0.011 |
| Fat mass (%) | 17.3 ± 2.5 a | 42.8 ± 6.3 b | 45.7 ± 2.9 b | <0.001 |
| Total body water (%) | 60.5 ± 1.8 a | 41.9 ± 4.6 b | 39.7 ± 2.1 b | <0.001 |
| Lean mass (kg) | 3.6 ± 2.3 | 4.2 ± 2.0 | 4.3 ± 1.2 | 0.737 |
| Lean mass (%) | 82.7 ± 2.5 a | 57.2 ± 6.3 b | 54.3 ± 2.9 b | <0.001 |
Data reported as means ± standard deviation, compared through ANOVA and Tukey test.
CG (control group, seven dogs with ideal body weight, body condition score 4-5; 17.3± 2.5% fat mass, received a propofol infusion based on total body weight); LBWG (lean body weight group, seven obese dogs, with body condition score 8-9; 42.8 ± 6.3 fat mass, received a propofol infusion based on lean body mass); TBWG (total body weight group, seven obese dogs with body condition score 8-9; 45.7 ± 2.9% fat mass, received a propofol infusion based on total body weight).
a,b Different letters represent statistically different means.
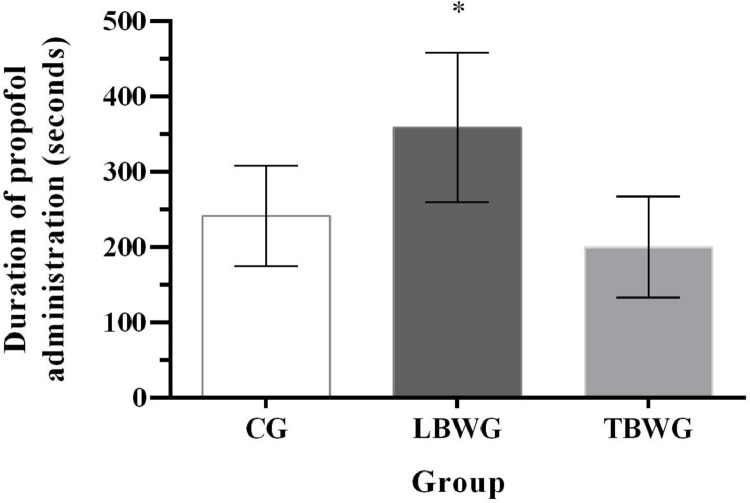
There was a difference in the time between the start of propofol infusion and loss of consciousness (the duration of propofol administration): LBWG took longer (359.1 ± 99.2 seconds) than CG (241.7 ± 66.4 seconds) and TBWG (200.3 ± 66.8 seconds) p = 0.004 (Fig. 1).
Fig. 1.
Times from start of propofol infusion until loss of consciousnes (the duration of propofol administration) CG (control group, seven dogs with ideal body weight, body condition score 4-5; 17.3± 2.5% fat mass, received a propofol infusion based on total body weight), LBWG (lean body weight group, seven obese dogs, with body condition score 8-9; 42.8 ± 6.3 fat mass, received a propofol infusion based on lean body mass), and TBWG (total body weight group, seven obese dogs with body condition score 8-9; 45.7 ± 2.9% fat mass, received a propofol infusion based on total body weight). *Significantly different from LBWG (359.1 ± 99.2 seconds) than CG (241.7 ± 66.4 seconds) and TBWG (200.3 ± 66.8 seconds) (p = 0.004).
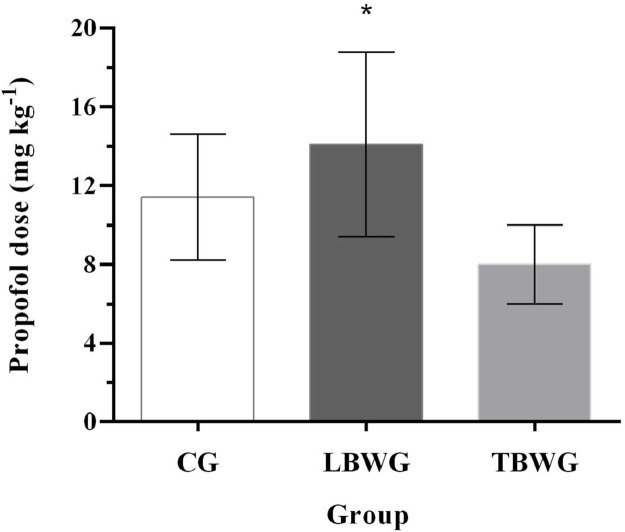
When propofol dosages were compared, there was a difference between LBWG (14.1 ± 4.7 mg kg−1) with CG (11.4 ± 3.2 mg kg−1) or TBWG (8.0 ± 2.0 mg kg−1) (p< 0.001) (Fig. 2).
Fig. 2.
Propofol dosages used in CG (control group, seven dogs with ideal body weight, body condition score 4-5; 17.3± 2.5% fat mass, received a propofol infusion based on total body weight), LBWG (lean body weight group, seven obese dogs, with body condition score 8-9; 42.8 ± 6.3 fat mass, received a propofol infusion based on lean body mass), and TBWG (total body weight group, seven obese dogs with body condition score 8-9; 45.7 ± 2.9% fat mass, received a propofol infusion based on total body weight). *Significantly different from LBWG (14.1 ± 4.7 mg kg-1) than CG (11.4 ± 3.2 mg kg-1) and TBWG (8.0 ± 2.0 mg kg-1) (p = 0.014).
4. Discussion
A pilot study involving four dogs (one TBWG and three CG) was performed to determine suitable and standardized anaesthesia techniques, deuterium dilution application, sampling and storage. It was necessary to adjust propofol dosage to 150 mg kg−1 hour−1, due to excitation observed during anaesthesia induction in two animals with the initially established dosage of 100 mg kg−1 hour−1.
The differences observed for fat mass, fat mass percentage, body water percentage and lean mass confirmed the characteristics of the obese group animals, and thus prove that the deuterium dilution method is a safe and precise procedure to determine ideal body condition and body compositions of obese dogs.
Although the control group presents a higher lean mass percentage, as the total body weight (TBW) increases, the lean and fat tissues mass increases, but not in parallel (Ingrande & Lemmens, 2010). The proportion of lean mass decreases when TBW increase (Love & Cline, 2015), which may affect the distribution characteristics of the drug (Hanley et al., 2010).
Boveri et al. (2013) have shown that obese dogs require a lower dose of propofol per kg of total body mass during induction of anaesthesia (1.8 ± 0.4 mg/kg) when compared with CG dogs (2.2 ± 0.5 mg/kg). Our study also confirms that obese dogs (45.7 ± 2.9% body fat; BCS = 8-9) need lower propofol dosages based on total body weight in anaesthesia induction (8.0 ± 2.0 mg/kg) when compared to the dosage (11.4 ± 3.2 mg/kg) needed for ideal BCS dogs (17.3 ± 2.5% body fat; BCS = 4-5).
However, similar propofol doses were expected for CG and LBWG, since studies with human beings (Ingrande & Lemmens, 2010; Ingrande et al., 2011) and animals (Boveri et al., 2013) had shown that by excluding fat weight when calculating the dose for an obese patient, the result would be similar to patients with ideal weight induced using total body weight. However, our study determined significant differences between dosages used in CG and LBWG, and these differences could be associated with the different methods used to determine lean body weight. In the present study, the deuterium dilution method was used whereas Ingrande et al. (2011) adopted bioimpedance analysis in humans, and Boveri et. al., (2013) estimated the ideal body weight according to the size of the dogs. Both the methods used by Ingrande et al. (2011) and by Boveri et. al., (2013) are considered imprecise (Guedes and Rechenchosky, 2008; Toll et al., 2010; Witzel et al., 2014) since some fat weight may not be completely disregarded and end up as part of the propofol dosage calculus. Deuterium isotope dilution methodology is a gold standard technique in human and veterinary medicine because it allows accurate determination of body water volume since water maintains a stable relationship with lean mass, and fat composition (Schoeller et al., 1980; Ferrier et al., 2002; Duarte et al., 2019).
The differences found in the doses used in our study may infer that fat interferes in the pharmacokinetics and pharmacodynamics of propofol and thus raise the question of whether it should be excluded or not when calculating the propofol dosage in induction of anaesthesia in obese dogs.
Propofol is an anesthetic agent commonly used in the induction of anesthesia because it is practical and inexpensive. However, its most prominent undesirable effects are apnea and hypotension, precisely those that should be avoided in the obese patient (Ingrande et al., 2011).
As the blood flow to the adipose tissue is lower than that for the lean mass (Frayn & Karpe, 2014) the propofol administration is an important parameter that requires precision dosing and accurate infusion time, validating the objective of this study. In order for this drug to not accumulate in these tissues and consequently trigger cardiovascular effects such as hypotension, considering that propofol is administered to the effect, remembering that underdosing of anesthetic drugs can lead to insufficiency of loss of consciousness.
The quality of anaesthesia induction was considered satisfactory in all groups, since no animal presented excitation or pedaling movement. Regarding tramadol, this drug is less commonly used in clinical practice, the type of pre-medication protocol is a limitation in the study.
5. Conclusions
The differences observed among fat percentage, water percentage and lean body weight (LBW) confirmed that the deuterium dilution method is a safe and precise procedure to determine ideal body condition and body compositions in obese dogs. In conclusion, the present study shows that obese dogs require smaller effective propofol doses during induction of anaesthesia when compared to ideal weight dogs when the dosage was calculated through total body weight. The induction could not be assessed as being higher or lower quality when calculating propofol doses using either lean body weight or total body weight in obese dogs.
Funding
This study was supported by FAPESP fund (process number: 13 / 15643-4).
Disclosure statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank the staff of the School of Veterinary Medicine and Animal Science for all their support.
References
- Bach J.F., Rozanski E.A., Bedenice D., Chan D.L., Freeman L.M., Lofgren J.L., Oura T.J., Hoffman A.M. Association of expiratory airway dysfunction with marked obesity in healthy adult dogs. American Journal of Veterinary Research. 2007;68:670–675. doi: 10.2460/ajvr.68.6.670. [DOI] [PubMed] [Google Scholar]
- Bach M.A. A retrospective study of dogs with cranial cruciate ligament rupture: 32 cases (2006-2012) Ciências Agrárias. 2015;36:1409–1418. [Google Scholar]
- Boveri S., Brearley J.C., Dugdale A.H. The effect of body condition on propofol requirement in dogs. Veterinary Anaesthesia and Analgesia. 2013;40:449–454. doi: 10.1111/vaa.12034. [DOI] [PubMed] [Google Scholar]
- Brodbelt D. Royal Veterinary College, Herts; 2006. The confidential enquiry into perioperative small animal fatalities. PhD Thesis. [DOI] [PubMed] [Google Scholar]
- Brown D.C., Conzemius M.G., Shofer F.S. Body weight as a predisposing factor for humeral condylar fractures, cranial cruciate rupture and intervertebral disc disease in cocker spaniels. Veterinary Compedium Orthopedic. 1996;9:75–78. [Google Scholar]
- Brunetto M.A., Nogueira S., SÁ F.C., Peixoto M., Vasconcellos R.S., Ferraudo A.J., Carciofi A.C. Correspondência entre obesidade e hiperlipidemia em cães. Ciência Rural. 2011;41:266–271. [Google Scholar]
- Brunetto M.A., Sá F.C., Nogueira S.P., Gomes, M.de O., Pinarel A.G., Jeremias J.T., de Paula F.J., Carciofi A.C. The intravenous glucose tolerance and postprandial glucose tests may present different responses in the evaluation of obese dogs. British Journal of Nutrition. 2011;106:194–197. doi: 10.1017/S0007114511000870. [DOI] [PubMed] [Google Scholar]
- Burkholder W.J., Toll P.W. Obesity. In: Hand M.S., Tatcher C.D., Remillard R.I., Roudebush P., Lewis L.D., editors. Small animal clinical nutrition. 4th edn. Mark Morris Institute; Topeka: 2000. pp. 401–430. [Google Scholar]
- Chikamune T., Katamoto H., Ohashi F., Shimada Y. Serum lipid and lipoprotein concentrations in obese dogs. Journal of Veterinary Medical Science. 1995;57:595–598. doi: 10.1292/jvms.57.595. [DOI] [PubMed] [Google Scholar]
- Colliard L., Ancel J., Benet J.J., Paragon B.M., Blanchard G. Risk factors for obesity in dogs in France. Journal of Nutrition. 2006;136:1951–1954. doi: 10.1093/jn/136.7.1951S. [DOI] [PubMed] [Google Scholar]
- Courcier E.A., Thomson R.M., Mellor D.J., Yam P.S. An epidemiological study of environmental factors associated with canine obesity. Journal of Small Animal Practice. 2010;51:362–367. doi: 10.1111/j.1748-5827.2010.00933.x. [DOI] [PubMed] [Google Scholar]
- Davis H., Jensen T., Johnson A., Knowles P., Meyer R, Rucinsky R, Shafford H. AAHA/AAFP fluid therapy guidelines for dogs and cats. Journal of the American Animal Hospital Association. 2013;49:149–159. doi: 10.5326/JAAHA-MS-5868. [DOI] [PubMed] [Google Scholar]
- Devito F.C., Patricio G.C.F., Rizzo M.F.C.I., Pacheco P.F., Flor P.B., Brunetto M.A., Cortopassi S.R.G. Anestesia e obesidade canina? revisão de literatura. Clínica Veterinária. 2015;20:42–52. [Google Scholar]
- Duarte M.G.F., Duarte P.O., Pelichek A., Ferriolli E., Moriguti J.C., Pfrimer K., Lima N.K.C. Comparison of body composition analysis methods among centenary women: seeking simpler methods. SAGE Open Medicine. 2019;7 doi: 10.1177/2050312119865126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edney A.T.B., Smith P.M. Study of obesity in dogs visiting veterinary practices in the United Kingdom. Veterinary Record. 1986;118:391–396. doi: 10.1136/vr.118.14.391. [DOI] [PubMed] [Google Scholar]
- EMA - European Medicine Agency (2019). 13 December 2018. EMA/143912/2019. committee for medicinal products for human use (CHMP).
- Ferrier L., Robert P., Dumon H., Martin L., Nguyen P. Evaluation of body composition in dogs by isotopic dilution using a low-cost technique, Fourier-transform infrared spectroscopy. Journal of Nutrition. 2002;132:1725–1727. doi: 10.1093/jn/132.6.1725S. [DOI] [PubMed] [Google Scholar]
- Frayn K., N., Karpe F. Regulation of human subcutaneous adipose tissue blood flow. International Journal of Obesity. 2014;38:1019–1026. doi: 10.1038/ijo.2013.200. [DOI] [PubMed] [Google Scholar]
- German A.J. The growing problem of obesity in dogs and cats. Journal of Nutrition. 2006;136:1940–1946. doi: 10.1093/jn/136.7.1940S. [DOI] [PubMed] [Google Scholar]
- Guedes D.P., Rechenchosky L. Comparison of predicted body fat from anthropometric methods: body mass index and skinfold-thickness. Revista Brasileira de Cineantropometria & Desempenho Humano. 2008;10:1–7. [Google Scholar]
- Hanley M.J., Abernethy D.R., Greenblatt D.J. Effect of obesity on the pharmacokinetics of drugs in humans. Clinical Pharmacokinetics. 2010;49:71–87. doi: 10.2165/11318100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Hendricks J.C. Brachycephalic airway syndrome. Veterinary Clinics of North America Small Animal Practice. 1992;22:145–1153. doi: 10.1016/s0195-5616(92)50306-0. [DOI] [PubMed] [Google Scholar]
- Holmes K.L., Morris P.J., Abdulla Z., Hackett R., Rawlings J.M. Risk factors associated with excess body weight in dogs in the UK. Journal of Animal Physiology and Animal Nutrition. 2007;91:166–167. [Google Scholar]
- Ingrande J., Brodosky J.B., Lemmens H.J.M. Lean body weight scalar for the anesthetic induction dose of propofol in morbidly obese subjects. Anesthesia & Analgesia. 2011;113:57–62. doi: 10.1213/ANE.0b013e3181f6d9c0. [DOI] [PubMed] [Google Scholar]
- Ingrande J., Lemmens H.J.M. Dose adjustment of anaesthetics in the morbidly obese. British Journal of Anaesthesia. 2010;105:16–23. doi: 10.1093/bja/aeq312. [DOI] [PubMed] [Google Scholar]
- Jeusette J., Detilleux J.D., Shibata H., Saito M., Honjoh T., Delobel A., Istasse L., Diez M. Effects of chronic obesity and weight loss on plasma ghrelin and leptin concentrations in dogs. Research in Veterinary Science. 2005;79:169–175. doi: 10.1016/j.rvsc.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Kealy R.D., Lawler D.F., Ballam J.M., Lust g., Biery D.N., Smith G.K., Mantz S.L. Evaluation of the effect of limited food consumption on radiographic evidence of osteoarthritis in dogs. Journal of the American Veterinary Medical Association. 2000;217:1678–1680. doi: 10.2460/javma.2000.217.1678. [DOI] [PubMed] [Google Scholar]
- Kealy R.D., Lawler D.F., Ballam J.M., Lust G., Smith G.K., Biery D.N., Olsson S.E. Five-year longitudinal study on limited food consumption and development of osteoarthritis in coxofemural joints of dogs. Journal of the American Veterinary Association. 1997;210:222–225. [PubMed] [Google Scholar]
- Kealy R.D., Lawler D.F., Ballam J.M., Mantz S.L., Biery D.N., Greeley E.H., List G., Segre M., Smith G.K., Stowe H.D. Effects of diet restriction on life span and age-related changes in dogs. Journal of the American Veterinary Medical Association. 2002;220:1315–1320. doi: 10.2460/javma.2002.220.1315. [DOI] [PubMed] [Google Scholar]
- Kirkham L., Thomas M. Anaesthesia in obese patients. British Journal of Hospital Medicine. 2011;72:515–520. doi: 10.12968/hmed.2011.72.9.515. [DOI] [PubMed] [Google Scholar]
- Laflamme D. Development and validation of a body condition score system for dogs. A clinical tool. Canine Practice. 1997;22:5–10. [Google Scholar]
- Lampman T.J., Lund E.M., Lipowitz A.J. Cranial cruciate disease: current status of diagnosis, surgery, and risk for disease. Veterinary and Comparative Orthopaedics and Traumatology. 2003;2:122–126. [Google Scholar]
- Leykin Y., Miotto L., Pellis T. Pharmacokinetic consideration in the obese. Clinical Anaesthesia. 2011;25:27–36. doi: 10.1016/j.bpa.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Love L., Cline M.G. Perioperative physiology and pharmacology in the obese small animal patient. Veterinary Anaesthesia and Analgesia. 2015;42:119–132. doi: 10.1111/vaa.12219. [DOI] [PubMed] [Google Scholar]
- Lund E.M., Armstrong P.J., Kirk C.A., Klausner J.S. Prevalence and risk factors for obesity in adult dogs from private US veterinary practices. International Journal of Applied Research in Veterinary Medicine. 2006;4:177–186. [Google Scholar]
- Mao J., Xia Z., Chen J., Yu J. Prevalence and risk factors for canine obesity surveyed in veterinary practices in Beijing. Chinese Journal of Preventive Veterinary Medicine. 2013;112:438–442. doi: 10.1016/j.prevetmed.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Mathews K.A. The various types of parenteral fluids and their indications. Veterinary Clinic of North American – Small Animals Practice. 1998;28:483–513. doi: 10.1016/s0195-5616(98)50052-6. [DOI] [PubMed] [Google Scholar]
- McGreevy P.D., Thomson P.C., Pride C., Fawcett A., Grassi T., Jones B. Prevalence of obesity in dogs examined by Australian veterinary practices and the risk factors involved. Veterinary Record. 2005;156:695–702. doi: 10.1136/vr.156.22.695. [DOI] [PubMed] [Google Scholar]
- Pereira-Neto G.B., Brunetto M.A., Champion T., Ortiz E., Carciofi A.C., Camacho A.A. Avaliação da pressão arterial sistêmica em cães obesos: comparação entre os métodos oscilométrico e doppler ultrassônico. Pesquisa Veterinária Brasileira. 2014;34:87–91. [Google Scholar]
- Pereira-Neto G.B., Brunetto M.A., Oba P.M., Champion T., Villaverde C., Vendramini T.H.A., Balieiro J.C.C., Carciofi A.C., Camacho A.A. Weight loss improves arterial blood gases and respiratory parameters in obese dogs. Journal of Animal Physiology and Animal Nutrition. 2018;102:1743–1748. doi: 10.1111/jpn.12963. [DOI] [PubMed] [Google Scholar]
- Pereira-Neto G.B., Brunetto M.A., Sousa M.G., Carciofi A.C., Camacho A.A., Effects of weight loss on the cardiac parameters of obese dogs Pesquisa Veterinária Brasileira. 2010;30:167–171. [Google Scholar]
- Piantedosi D., Di Loria A., Guccione J., De Rosa A., Fabbri S., Cortese L., Carta S., Ciaramella P. Serum biochemistry profile, inflammatory cytokines, adipokines and cardiovascular findings in obese dogs. The Veterinary Journal. 2016;216:72–78. doi: 10.1016/j.tvjl.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Schoeller D.A., Santen E.V., Peterson D.W., Dietz W., Jaspan J., Klein P.D. Total body water measurement in humans with 18O- and 2H-labelled water. American Journal of Clinical Nutrition. 1980;33:2286–2293. doi: 10.1093/ajcn/33.12.2686. [DOI] [PubMed] [Google Scholar]
- Toll P.W., Yamkaha R.M., Schoenherr W.D., Hand M.S. Obesity. In: Hand M.S., Thatcher C.D., Remillard R.L., Roudebusch P., Novotny B.J., editors. Small animal clinical nutrition. 5th edn. Mark Morris Institute; Topeka: 2010. pp. 501–542. [Google Scholar]
- Tropf M., Nelson O.L., Lee P.M., Weng H.Y. Cardiac and metabolic variables in obese dogs. Journal of Veterinary Internal Medicine. 2017;31:1000–1007. doi: 10.1111/jvim.14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui S., Yasuda H., Koketsu Y. Characteristics of obese or overweight dogs visiting private Japanese veterinary clinics. Asian Pacific Journal of Tropical Biomedicine. 2016;6:338–343. [Google Scholar]
- Wang Z., Deurenberg P., Wang W., Pietrobelli A., Baumgartner R.N., Heymsfield S.B. Hydration of fat-free body mass: review and critique of a classic body-composition constant. American Journal of Clinical Nutrition. 1999;69:833–841. doi: 10.1093/ajcn/69.5.833. [DOI] [PubMed] [Google Scholar]
- Witzel A.L., Kirk C.A., Henry G.A., Toll P.W., Brejda J.J., Paetau-Robinson I. Use of a novel morphometric method and body fat index system for estimation of body composition in overweight and obese dogs. Journal of the American Veterinary Medical Association. 2014;244:1279–1284. doi: 10.2460/javma.244.11.1279. 2014. [DOI] [PubMed] [Google Scholar]