Abstract
In this report, an overview of the health benefits of omega-3 long-chain (≥C20) polyunsaturated fatty acids (n-3 LC-PUFA) and recent progress in using alpha linolenic acid (ALA) rich sources derived from oilseeds to enhance productive performance, n-3 PUFA profiles and sensory properties of lamb for human consumption is reviewed. Omega-3 LC-PUFA can prevent mental health issues and chronic human disorders including cancer, cardiovascular and inflammatory diseases. The median amount of n-3 LC-PUFA consumption is generally lacking in Western diets. More attention is now being paid to the use of innovative nutritional strategies to improve PUFA content in ruminants, which could subsequently increase the content of health-benefitting n-3 LC-PUFA for human consumption. The richest sources of dietary n-3 LC-PUFA are derived from marine products, while forage and oilseeds such as flaxseed, canola, and their oils are abundant in ALA. Numerous studies have shown that dietary ALA increases n-3 LC-PUFA levels of edible tissues. However, other studies concluded that ALA rich supplementation led to no differences in tissue FA profiles because of extensive biohydrogenation of dietary ALA, limited conversion from ALA to n-3 LC-PUFA and low incorporation of n-3 LC-PUFA into edible tissues. Generally, the inclusion of ALA rich sources in lamb diets potentially increases ALA content in lamb. It is proposed that supplementing ruminants with ALA-rich sources at or below 6% can promote n-3 PUFA profiles in lamb and is unlikely to have negative effects on feed intake, growth, carcass and sensory properties.
Keywords: Alpha linolenic acid, Omega-3 long-chain polyunsaturated fatty acids, Lamb, Canola, Flaxseed, Dietary supplementation
1. Introduction
There is increasing evidence from the current literature to indicate that omega-3 long-chain (≥ C20) polyunsaturated fatty acids (n-3 LC-PUFA) play an important role in the prevention of and decreasing the risk of chronic diseases in humans (Nichols et al., 2014, Walker et al., 2015, Watanabe and Tatsuno, 2017). Consumers have become more aware about the health benefits of dietary n-3 LC-PUFA. Various recommendations for the daily intake of n-3 LC-PUFA also have been proposed. However, Western diets have been reported to be severely lacking in these fatty acids (FA) (Fayet-Moore et al., 2015, Salem and Eggersdorfer, 2015). Although major sources of n-3 LC-PUFA are fish and other marine products, seafood is not a regular part of the traditional diet in many Western countries (Byelashov, Sinclair, & Kaur, 2015). Thus, the ability to increase n-3 LC-PUFA content in other human foods, such as red meat and milk, is required to meet the recommended intakes of these FA.
Edible adipose tissues derived from ruminants are unlikely to be particularly healthy, mainly because of its high levels of saturated fatty acids (SFA) and low content of omega-3 polyunsaturated fatty acids (n-3 PUFA) (Bessa, Alves, & Santos‐Silva, 2015). These features of ruminant fat are directly associated with the biohydrogenation (BH) of dietary unsaturated fatty acids (UFA) in the rumen performed by microorganisms in the rumen (Jenkins, Wallace, Moate, & Mosley, 2008). In the small intestine, the absorption of FA is similar to that in monogastric animals (Woods & Fearon, 2009), with the exception of SFA (described in detail by Bauchart (1993)). In other words, FA leaving the rumen are absorbed unchanged before being incorporated into tissue lipids. Hence, BH is the general metabolic response of ruminal microorganisms that mostly causes an extensive hydrogenation of non-esterified UFA that appears to have a toxic effect on microbiota (Sakurama et al., 2014).
In recent years, numerous studies have agreed that nutrition is the major factor influencing the FA profile of ruminants (Alvarenga et al., 2015, Chikwanha et al., 2017, Shingfield et al., 2013). Great emphasis has been placed in the use of innovative nutritional strategies to improve the content of PUFA in ruminants (Bessa et al., 2015). Much of the research has focused on increasing n-3 PUFA content in ruminant products (meat and milk) using feeds enriched in n-3 PUFA (Díaz et al., 2017, Parvar et al., 2017). The richest sources of n-3 PUFA supplied in ruminant diets are fish, algae, forage and oilseeds (Doreau et al., 2009, Nichols et al., 2010). Flaxseed, canola and their oils, which are rich in alpha linolenic acid (ALA) (Dubois, Breton, Linder, Fanni, & Parmentier, 2007), have been of recent interest in various nutritional studies in order to mainly improve the n-3 LC-PUFA content of lamb meat (Asadollahi et al., 2017, Nguyen et al., 2017).
The main objective of inclusion of n-3 PUFA rich lipid supplements in ruminant diets is to increase the content of health-benefitting PUFA in edible tissues that are then available for human consumption. However, it is necessary to take into account the feed intake, growth responses and meat eating quality of animals to n-3 PUFA supplements, because changes in dietary fat can produce differences in animal health and performance, and sensory properties (Chikwanha et al., 2017). Bessa et al. (2015) stated that maximizing the content of n-3 LC-PUFA in ruminant products would be a highly desirable production target to enhance their nutritional quality, but the nature of lipid digestion and biological limits to that seem to be quite strict. Ideally, increase in n-3 LC-PUFA content should be achieved simultaneously without detrimental impacts on the productive performance of animals and the sensorial attributes of the products. In recent times, it has been shown that although genetics and sex do affect the FA profiles of animal products (Malau-Aduli, Holman, Kashani, & Nichols, 2016), nutritional factors can promote n-3 LC-PUFA deposition in ruminant meat and milk, leading to healthier foods (Chikwanha et al., 2017, Scollan et al., 2014, Shingfield et al., 2013, Vahmani et al., 2015). However, the relationships between the enrichment of n-3 PUFA in lamb diet, and their productive performance, as well as sensory quality are not well documented. The current review will evaluate the recent progress in the field including the contribution of n-3 LC-PUFA consumption to human health promotion, the simultaneous effects of ALA rich supplementation on lamb productivity, the quality of edible tissues including n-3 LC-PUFA content and sensory attributes.
2. Omega-3 polyunsaturated fatty acids: health benefits and sources
Omega-3 PUFA contain their first double bond between the third and fourth carbons, from the methyl end (Swanson, Block, & Mousa, 2012) with the most important ones being alpha-linolenic acid (ALA, 18:3n-3), eicosapentaenoic acid (EPA, 20:5n-3), docosapentaenoic acid (DPA, 22:5n-3) and docosahexaenoic acid (DHA, 22:6n-3).
2.1. Health benefits
Fat consumption is essential for human development, health, and longevity (Gropper & Smith, 2013). Omega-3 LC-PUFA are integral components of all cell membranes in the body as either membrane phospholipids or free molecules (Gorjão et al., 2009, Khan and He, 2017). They contribute considerably to the physical properties of biological membranes, including membrane organization, ion permeability, elasticity and microdomain formation (Gorjão et al., 2009). Ganesan, Brothersen, and McMahon (2014) reported that when released from the cell membranes, the n-3 LC-PUFA become the precursors of eicosanoid hormones such as resolvins (products from EPA) and docosatrienes, protectins, and neuroprotectins (products from DHA) which are important in the defence against, and treatment of various diseases (Calder, 2006, Khanapure et al., 2007).
The broad health benefits of n-3 LC-PUFA in preventing many diseases have been well documented (Nichols et al., 2014, Ruxton et al., 2004, Swanson et al., 2012). DHA has been associated with the function and development of nervous and visual systems because it is present in large amounts in brain and retina membrane phospholipids (Swanson et al., 2012, Walker et al., 2015). Innis (2008) and Gould, Smithers, and Makrides (2013) indicated that n-3 LC-PUFA play a fundamental role in neural development in embryos, as well as in the early postnatal phases. Increase in n-3 LC-PUFA intake has also been associated with brain health benefits in adults, including reduced risk of dementia and delayed decline in cognitive function (Khan and He, 2017, Ruxton et al., 2004, Swanson et al., 2012). Increased intake of n-3 LC-PUFA results in increased amounts of n-3 LC-PUFA and decreased amounts of arachidonic acid in the phospholipids of inflammatory cells (Calder, 2015). This leads to a decrease in the production of inflammatory mediators such as arachidonic acid – derived eicosanoids and cytokines. They act both directly, by replacing arachidonic acid as an eicosanoid substrate and inhibiting arachidonic acid metabolism, and indirectly, by altering the expression of inflammatory genes through effects on transcription factor activation (Calder, 2006). Furthermore, n-3 LC-PUFA are also substrates for anti-inflammatory mediators (Calder, 2013). Thus, many studies in both animals and humans have demonstrated that n-3 LC-PUFA are potential therapeutic agents for suppressing inflammation and thereby have a beneficial role in a variety of inflammatory diseases including diabetes, atherosclerosis, asthma and arthritis (Calder, 2006, Calder, 2013, Calder, 2015, Simopoulos, 2016).
Cardiovascular diseases (CVD) and cancer are the leading causes of death worldwide (Benjamin et al., 2017, Siegel et al., 2016). The cardio-protective effect of PUFA was first postulated in the 1950s (Sinclair, 1956). Scientists observed that Alaskan and Greenlandic Eskimos and Okinawa islanders had a reduced incidence of CVD and other chronic diseases than other groups because of their high consumption of fish and marine mammals, both being rich in n-3 LC-PUFA (Bang et al., 1980, Kagawa et al., 1982). Nowadays, many published studies have comprehensively established that n-3 LC-PUFA play a critical role in the prevention of CVD in humans (Calder, 2017, Nichols et al., 2014, Watanabe and Tatsuno, 2017). The consumption of n-3 LC-PUFA may reduce the risk of CVD by reducing systolic resting heart rate and diastolic blood pressure (Mozaffarian et al., 2005), lowering blood viscosity (Cartwright, Pockley, Galloway, Greaves, & Preston, 1985), inhibiting platelet aggregation (Simopoulos, 2002), improving blood vessel function (Abeywardena & Head, 2001) and reducing plasma fibrinogen (Watanabe & Tatsuno, 2017). Simopoulos (2002) stated that n-3 LC-PUFA, when supplied in high doses, reduce plasma cholesterol and have anti-thrombotic and hypotriglyceridemic properties. The beneficial effects of long-term intake of n-3 LC-PUFA on cancer patients have also received intense attention by both clinicians and epidemiologists (Berquin et al., 2008, Laviano et al., 2013). Based on epidemiological studies, Rose and Connolly (1999) and MacLean et al. (2006) suggested that people whose diets are high in n-3 LC-PUFA, may experience a lower incidence of common cancers such as breast, colon, and prostate. Many mechanisms are involved, including elimination of neoplastic transformation, inhibition of cancer cell growth (Heller et al., 2004), and enhanced apoptosis and anti-angiogenicity, through the prevention of eicosanoid production from arachidonic acid precursors (Rose & Connolly, 1999). Berquin et al. (2008) added that n-3 LC-PUFA can serve as a nutritional source for cancer patients to reduce weight loss, enhance recovery after surgery and modulate the immune system.
As an individual n-3 LC-PUFA, DPA also has positive correlations with lower incidence of CVD and cancers, mental health and inflammatory disorders (Byelashov et al., 2015, Kaur et al., 2011). Moreover, epidemiological studies have demonstrated that DPA consumption has some unique benefits in human health nutrition: (1) DPA prevents platelet aggregation more efficiently than EPA and DHA (Phang, Garg, & Sinclair, 2009); (2) DPA may act as a precursor for production of the DPA-related D-series of resolvins or neuroprotective compounds (Kaur et al., 2011); (3) DPA is a potent stimulator of endothelial cell migration, which is an essential component of embryonic vascular system (Aase et al., 2007) and it acts much more efficiently than EPA (Kaur, Guo, & Sinclair, 2016); (4) DPA is also incorporated into cell phospholipids faster than EPA (Byelashov et al., 2015); and (5) DPA has more potent anti-proliferative and pro-apoptotic effects on cancer cells than both EPA and DHA (Morin, Rousseau, & Fortin, 2013).
Furthermore, DPA can serve as an intermediate reservoir contributing to the biosynthesis of both EPA- and DHA-derived bioactive lipid mediators (Markworth et al., 2016, Miller et al., 2013). Supplementing young female adults with 8 g of pure DPA over a 7-day period, Miller et al. (2013) found that the levels of not only DPA but also both EPA and DHA in plasma triacylglycerol (TAG) fractions were increased. The increases in all three n-3 LC-PUFA indicate that DPA is both being further desaturated to DHA and retro-converted back to EPA. Retro-conversion involves both peroxisomal acyl-CoA oxidase and β-oxidation (Christensen et al., 1993). The retro-conversion has been observed in human fibroblasts (Christensen et al., 1993, Rosenthal et al., 1991). The evidence of retro-conversion of DPA to EPA was found in various animal tissues including rat liver, heart and skeletal muscle (Holub et al., 2011, Kaur et al., 2010), and bovine endothelial cells (Achard, Bénistant, & Lagarde, 1995). The increase in DHA levels in plasma TAG fractions in rat liver following pure DPA supplementation was also reported by Holub et al. (2011) and Kaur et al. (2010). These findings demonstrate that DPA can act as a precursor source for enhancing the production of DHA in the body. However all of aforementioned studies could not clearly describe the desaturation mechanism of DPA to DHA. Markworth et al. (2016) stated that the metabolic fate and novel mechanism that may explain bioactivity of DPA have rarely been investigated. This is in our view, due in part to the lack of sufficient quantities of purified DPA for use in suitable feeding trials. The role of DPA in human health has been largely ignored (Kaur et al., 2011), and knowledge of its beneficial impacts on animal nutrition remains limited (Alvarenga et al., 2015). Hence, more research is required to determine the biological effects and importance of DPA in both animal nutrition and human health, and to systematically investigate its metabolic fate and physiological mechanism.
2.2. Dietary sources and intakes
The n-3 PUFA are considered essential because they cannot be synthesized by the human body and other vertebrates due to the lack of Δ-12 and Δ-15 desaturase enzymes (Alvarenga et al., 2015) which can form carbon–carbon double bonds beyond the Δ-12 and Δ-15 carbons (Innis, 2008). Therefore, the n-3 PUFA need to be acquired through dietary sources (Rose and Connolly, 1999, Simopoulos, 2016).
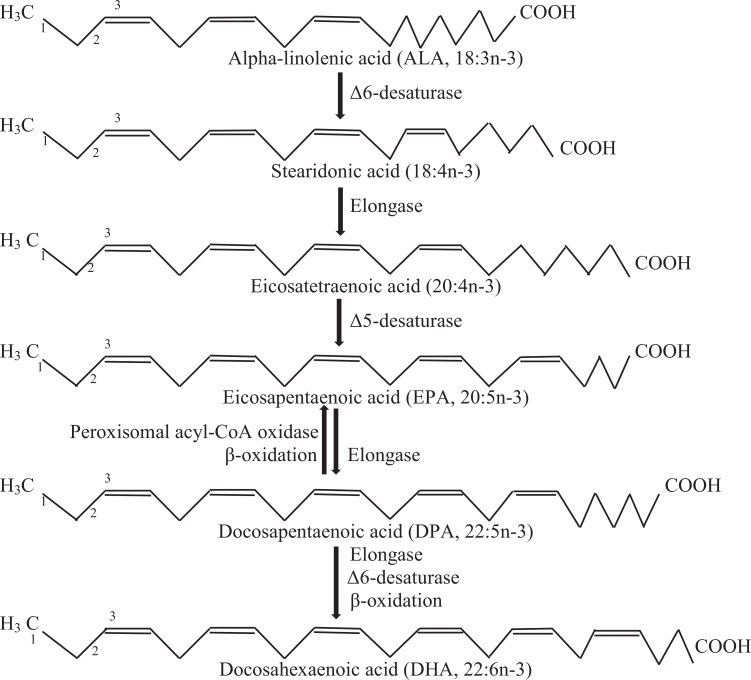
In the human body, the n-3 PUFA can be converted by elongation and desaturation to more unsaturated and long-chain PUFA, which are more bioactive than their precursors. ALA is converted to EPA, which is further elongated to DPA and DHA by the pathway shown in Fig. 1. The conversion of ingested ALA to n-3 LC-PUFA within the body is not usually considered to be a reliable source of n-3 LC-PUFA in the human diet. In healthy adults, conversion rates of dietary ALA to EPA and DHA are almost 6% and 3.8% respectively (Gerster, 1998). Burdge, Jones, and Wootton (2002) showed that, only about 8% of ALA in young male diets is converted to EPA and DPA, and none is converted to DHA. The possible explanations for the inefficient conversions are that most of dietary ALA are utilized as energy sources (Woods & Fearon, 2009) and diets are rich in omega-6 PUFA (Gerster, 1998), with the latter competing against omega-3 PUFA at key pathway steps. Hence, it is more beneficial and practical to directly consume n-3 LC-PUFA from daily food as preformed EPA, DPA and DHA, rather than as ALA.
Fig. 1.
A general pathway for desaturation and chain elongation of omega-3 polyunsaturated fatty acids (adapted from Calder (2017)).
There are many dietary sources of n-3 PUFA including seafood, animal products and land plants. The type and amount of n-3 PUFA varies in the many sources. ALA is mostly found in the chloroplast of green leafy plants, some oilseeds, and in vegetable oils such as flaxseed and canola (Baker et al., 2016, Deckelbaum and Torrejon, 2012). EPA and DHA are found in fairly significant amounts in seafood, especially fish oils (Calder, 2015, Calder, 2017, Nichols et al., 2016).
Although seafood is one the richest sources of DPA, red meat appears to be a main dietary source of DPA and a major source of n-3 LC-PUFA in some countries such as Australia and the United State (Byelashov et al., 2015, Clayton, 2014). In Australia, the largest dietary contributors of DPA in children are red meat, poultry and game products (56%), followed by seafood (23%) (Rahmawaty, Charlton, Lyons-Wall, & Meyer, 2013). The content of DPA in red meat is relatively higher than EPA and DHA. Droulez, Williams, Levy, Stobaus, and Sinclair (2006) reported that DPA content in the lean component of Australian red meat cuts varied from 32 mg/100 g to 54 mg/100 g, whereas the contents of EPA and DHA in the same cuts ranged from 29 mg/100 g to 46 mg/100 g and from 6 mg/100 g to 19 mg/100 g respectively. Another study also showed that DPA in Australian prime lamb muscle was three-fold higher content than DHA and the DPA content was similar to that of EPA (Nguyen, Flakemore et al., 2017).
Due to the potential health benefits, the recommended dietary n-3 LC-PUFA intakes have been published by many organizations. Table 1 summarizes the recommendations for n-3 LC-PUFA consumption from several associations and organizations. In general, consumption of two to three servings per week of oily fish rich in n-3 LC-PUFA is suggested to provide about 500 mg of EPA+DHA daily, for primary prevention of CVD (FFSA 2010, Kris-Etherton and Innis, 2007, NHFA 2015, Nichols et al., 2010). However, it should be noted that different amounts of n-3 LC-PUFA are suggested for males and females (NHMRC 2006). The American Heart Association recommended higher consumption of n-3 LC-PUFA for people with specific diseases. Patients with documented coronary heart disease should consume approximately 1000 mg of n-3 LC-PUFA from oily fish or fish oil capsules. For patients with severe hypertriglyceridemia, the effective doses are higher (2000 to 4000 mg of n-3 LC-PUFA per day) to lower triglyceride levels (Miller et al., 2011).
Table 1.
Recommended weekly fish and/or daily omega-3 polyunsaturated fatty acids (n-3 LC-PUFA) intakes from health organizations.
| Organizations | Focus | Recommendation |
Reference | |
|---|---|---|---|---|
| Weekly fish meal (112 g/serving) | Daily n−3 LC-PUFA (mg/day) | |||
| American Heart Association | Coronary heart disease (CHD) sufferers | 2 servings | 1000 | (Miller et al., 2011) |
| Individuals with hypertriglyceridemia | 2000–4000 | |||
| FAO/WHO Expert Consultation | For secondary prevention of CHD | 250-2000 of EPA+DHA | FAO/WHO (2008) | |
| American Diabetes Association | For primary prevention of CHD | at least 2 servings | Bantle et al. (2008) | |
| Japanese Ministry of Health, Labor and Welfare (JMHLW) | Individuals over the age of 2 | >1000 | JMHLW (2015) | |
| American Dietetic Association and Dietitians of Canada | For primary prevention of CHD | 2 servings | 500 of EPA+DHA | Kris-Etherton and Innis (2007) |
| Academy of Nutrition and Dietetics | All adults | 500 of EPA+DHA | Vannice and Rasmussen (2014) | |
| European Food Safety Authority | All adults | 1-2 servings | 250 of EPA+DHA | Tur, Bibiloni, Sureda, and Pons (2012) |
| French Food Safety Agency (FSSA) | Individuals over the age of 10 | 2 servings | 500 of EPA+DHA | FFSA (2010) |
| National Heart Foundation Australia (NHFA) | CHD sufferers | 2-3 servings | 250-500 of EPA+DHA | NHFA (2015) |
| Australia New Zealand National Health and Medical Research Council (NHMRC) | Healthy female adults | 430 | NHMRC (2006) | |
| Healthy male adults | 610 | |||
FAO/WHO: Food and Agriculture Organization /World Health Organization.
NHMRC: The Australian and New Zealand National Health and Medical Research Council.
In Western countries, various studies have agreed that median levels of n-3 LC-PUFA consumption are insufficient (Byelashov et al., 2015, Walker et al., 2015). Americans currently consume 99 g of seafood per week with considerably lower EPA+DHA than the recommended values (Papanikolaou et al., 2014, Walker et al., 2015). In Australia, Birch and Lawley (2014) showed that the weekly consumption of seafood has slowly increased to approximately 220 g per capita in 2011. However, recent studies on consumption reported that Australians are not consuming recommended quantities of n-3 LC-PUFA (Fayet-Moore et al., 2015, Rahmawaty et al., 2013). Howe, Meyer, Record, and Baghurst (2006) estimated that the average n-3 LC-PUFA intake in Australia was 246 mg/day. Hence, it is necessary to explore more practical options of achieving the n-3 LC-PUFA recommendations.
The low consumption of n-3 LC-PUFA is due to numerous factors. Fishy taste and high cost of seafood are commonly cited reasons for the low consumption of n-3 LC-PUFA (Kennedy, Luo, & Ausman, 2012). Additionally, many people rarely eat fish because of its low availability in many geographical locations (Walker et al., 2015). Birch and Lawley (2014) pointed out that habit, such as regular childhood consumption and seafood familiarity, also influence seafood consumption. The bioaccumulation of toxic contaminants such as mercury, arsenic and lead in fish, is another issue reducing fish consumption (Bosch et al., 2016, Gribble et al., 2016). Other concerns are overfishing and growing global population that could strain the sustainability of the market (Kennedy et al., 2012).
The content of DPA in human milk was higher than that of EPA and similar to DHA content (Koletzko, Mrotzek, & Bremer, 1988), implying that it potentially may play an important role in infant development. The potential health benefits of DPA is currently emerging (Calder, 2017, Kaur et al., 2016). However, many health organizations worldwide presently only offer guidelines for n-3 LC-PUFA intake without DPA (Byelashov et al., 2015). Howe et al. (2006) reported that DPA may contribute almost one-third of total n-3 LC-PUFA in Australian diets. Vahmani et al. (2015) stated that the exclusion of DPA from total n-3 LC-PUFA intake results in reducing the total amount of their actual consumption. Thus, the development of alternative approaches of incorporating n-3 LC-PUFA into the human diet are required. Currently, the enhancement of n-3 LC-PUFA content in sheep meat as an alternative source for human consumption has gained significant research attention (Flakemore et al., 2017, Howes et al., 2015, Nguyen et al., 2017).
3. Metabolism of omega-3 PUFA in ruminants
The nature of lipid digestion by animals has a substantial effect on the transfer of fatty acids from the diet into tissue (Woods & Fearon, 2009). Once dietary lipids enter the rumen, the microbes are thought to be primarily responsible for transforming lipids via two major processes, namely lipolysis and BH (Buccioni et al., 2012, Edwards et al., 2017, Jenkins et al., 2008). The initial stages of lipid digestion are characterised by intense lipolysis. Shortly after esterified dietary lipids are consumed, more than 85% of galactolipids, phospholipids and triacylglycerols are hydrolysed by microbial lipases to release non-esterified fatty acids including UFA (Buccioni et al., 2012, Shingfield et al., 2013). After lipolysis, UFA undergo BH by the rumen microbes (Jenkins, 1993). The process involves the removal of double bonds through microbial enzyme activity and by using hydrogen which is provided from dietary fermentation in the rumen. During BH, PUFA are converted into MUFA and ultimately to SFA with a vast array of trans FA intermediates and isomers being formed simultaneously (Buccioni et al., 2012, Harfoot and Hazelwood, 1998). Thus, many studies have concluded that ruminal BH is one of the main challenges working against attempts to increase n-3 LC-PUFA in ruminant tissues through dietary supplementation (Alves et al., 2017, Bessa et al., 2015, Howes et al., 2015).
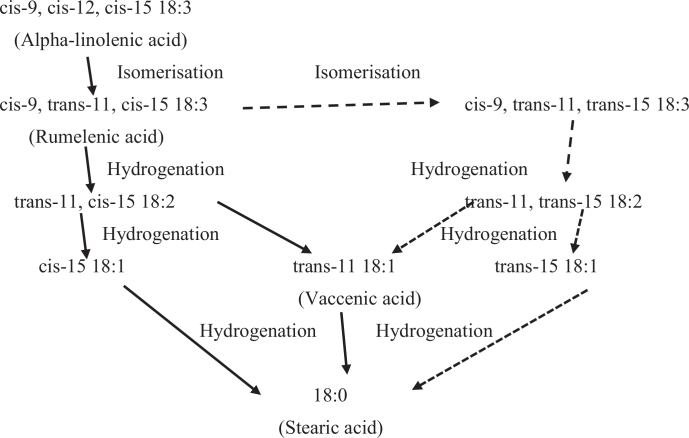
The BH of dietary n-3 PUFA in the rumen is slightly complicated compared to other common UFA. The higher degree of unsaturation of n-3 PUFA requires a greater amount and species of ruminal participated microbes (Lourenço, Ramos-Morales, & Wallace, 2010). These FA also involve numerous steps of isomerization, hydrogenation of double bonds and chain shortening (Jenkins et al., 2008). As a consequence, the BH of n-3 PUFA can produce a wide range of UFA intermediates and final SFA products (Shingfield et al., 2012). The pathways of ALA biohydrogenation are illustrated in Fig. 2. The products of ALA during the ruminal BH are geometric and positional isomers. Doreau and Ferlay (1994) and Glasser, Schmidely, Sauvant, and Doreau (2008) concluded that greater than 85% of dietary ALA is hydrogenated in the rumen. Several studies have reported that EPA and DHA are extensively hydrogenated in the rumen in vivo (Shingfield et al., 2012) and disappear during in vitro incubation with mixed ruminal microorganisms (Kairenius, Toivonen, & Shingfield, 2011). Shingfield et al. (2010) also observed more than 90% of dietary EPA and DHA from fish oil are not recovered in the duodenum, although when supplemented as algae products they might be much less hydrogenated (Sinclair et al., 2005). Chilliard et al. (2007) and Shingfield et al. (2012) stated that the large extent of EPA and DHA biohydrogenation results in a wide array of intermediates including large amount of UFA and a small number of SFA. The presence of these FA inhibits the BH of ALA (Shingfield et al., 2010). The extensive BH of n-3 PUFA occurs because of the toxicity of PUFA to rumen bacteria namely those related to Butyrivibrio (Sakurama et al., 2014). However, the pathways for the BH of EPA, DPA and DHA in the rumen has not been studied in detail (Alvarenga et al., 2015, Sakurama et al., 2014). Thus, further studies are required to clearly characterise the BH pathways of these n-3 LC-PUFA and their products.
Fig. 2.
Ruminal biohydrogenation pathways of alpha-linolenic acid (Adapted from Gómez-Cortés, Tyburczy, Brenna, Juárez, and de la Fuente (2009).
Several factors have been reported to modulate the BH of dietary n-3 PUFA, such as the amount and type of the lipid supplements, the basal diet and duration of feeding (Chikwanha et al., 2017, Realini et al., 2017, Shingfield et al., 2013). The use of secondary plant metabolites such as tannin, saponins and essential oils (Alves et al., 2017, Girard et al., 2016, Willems et al., 2014), and/or the supplementation of concentrate diets with rich sources in n-3 PUFA has been shown to reduce the ruminal BH rate and significantly improve the sensory and nutritional quality of lamb (Nguyen et al., 2017, Ponnampalam et al., 2016, Realini et al., 2017). Other studies minimized the BH rate by using herbage (Buccioni et al., 2012) or changing ruminal pH (Lascano, Alende, Koch, & Jenkins, 2016).
The lipids of post-ruminal digestion includes mainly SFA (stearic acid and palmitic acid), BH intermediates and microbial phospholipids, along with dietary by-pass triglycerides (Alvarenga et al., 2015, Chilliard et al., 2007). The absorption of UFA into the small intestine by ruminants is similar to that in monogastric animals (Woods & Fearon, 2009). The intestinal uptake coefficient of PUFA is up to 92% for conventional low fat diets and higher in ruminants compared to monogastric animals (Doreau & Ferlay, 1994). Doreau and Ferlay (1994) also stated that there is a decrease in FA digestibility in the small intestine when fat intake increases. The apparent digestibility coefficients are on average 70% for n-3 PUFA (Doreau et al., 2016, Glasser et al., 2008). However, small amounts of n-3 LC-PUFA are incorporated into triglycerides in adipocytes. They are mainly incorporated into the membrane phospholipids and are deposited in significant amounts in intramuscular tissue (Alvarenga et al., 2015, De Smet et al., 2004). Thus, lean meat per gram of tissue is richer in n-3 LC-PUFA compared to fattier meat and adipose tissues.
4. Omega-3 PUFA sources for ruminants
Lipid in ruminant diets is very important, not only due to its significant energy contribution, but also because it supplies essential FA and fat-soluble vitamins (Woods & Fearon, 2009). The decision to use fat or oil and the form in which it is included in the feed is influenced by a number of factors. These factors include: (1) the composition of basal diets; (2) lipid form (whole oilseed, processed oilseed or extracted oil) and its digestibility (Gómez-Cortés et al., 2014, Ponnampalam et al., 2015, Shingfield et al., 2013); (3) the price and availability of raw material and (4) feed supply permits and animal welfare regulations (Woods & Fearon, 2009). Since dietary lipids is one of major factors affecting the FA profile of ruminants (De Smet et al., 2004), enrichment of red meat with health benefitting n-3 PUFA can be attained by innovative nutritional approaches (Chikwanha et al., 2017, Doreau et al., 2016, Ponnampalam et al., 2016). The main n-3 PUFA sources supplied in ruminant diets are various including forage sources, fish oils and marine products, oilseed, and terrestrial plant oils (Alvarenga et al., 2015, Scollan et al., 2014, Woods and Fearon, 2009).
4.1. Forage
In general, consumed forage is the main source of n-3 PUFA for ruminants although amounts of n-3 PUFA consumed varies with several factors. For example, fresh grass or pasture has higher n-3 PUFA content than conserved grass (silage and hay) (see recent reviews by Scollan et al. (2014)and Alvarenga et al. (2015)). Fresh grass is not a source of EPA and DHA, but is rich in ALA localised in the chloroplasts, accounting for over 50% of total FA (Chilliard et al., 2007, Dewhurst et al., 2006, Wood et al., 2008). Variations in grass FA profile during ensiling may be due to microbial intervention, which may result in hydrogenation and isomerization similar to that which occurs by microbial action in the rumen (Alves, Cabrita, Jerónimo, Bessa, & Fonseca, 2011). Dewhurst et al. (2006) and Kalač and Samková (2010) observed that field wilting prior to ensiling and drying during hay making can result in major losses of ALA due to oxidation. Glasser, Doreau, Maxin, and Baumont (2013) concluded that ALA content of alfalfa hay is almost half that of fresh alfalfa. The ALA content of grass can also be influenced by variety and maturity (Glasser et al., 2013, Woods and Fearon, 2009). Girard et al. (2016) determined that alfalfa silage had lower an ALA proportion than red clover and sainfoin silages. Furthermore, Koivunen et al. (2015) indicated that within the same variety, advancing maturity reduced ALA content of Timothy – meadow fescue grass and red clover.
A number of studies have demonstrated that n−3 PUFA content in red meat and cow milk are higher in animals fed pasture-based compared to concentrate-based diets (Howes et al., 2015, Jaturasitha et al., 2016, Shingfield et al., 2013, Vazirigohar et al., 2014). Eriksson and Pickova (2007) suggested that fresh grass exerts a greater protection for n-3 PUFA against rumen microorganisms than concentrates, because of the presence of other secondary metabolites that could inhibit ruminal BH (Alves et al., 2017, Girard et al., 2016, Willems et al., 2014). However, forage generally contains a low level of total FA, ranging about 1–3% dry matter (DM) (Chilliard et al., 2007, Jaturasitha et al., 2016). Thus, other lipid sources are commonly supplemented to increase dietary energy density to gain improved animal performance (Parvar et al., 2017, Vazirigohar et al., 2014).
4.2. Marine products
Feeding livestock marine resources provides greater opportunities for increasing a vast range of FA products, from n-3 LC-PUFA and also various BH intermediates although the sustainability (Kitessa et al., 2014, Lenihan-Geels et al., 2013) and cost effectiveness of using marine sources has been questioned (Chikwanha et al., 2017, Shingfield et al., 2012, Vlaeminck et al., 2008). In addition, inclusion of marine sources into ruminant diets may introduce food odors, result in rancidity and abnormal flavor in lamb meat (Scollan et al., 2014, Watkins et al., 2013). The efficiency of supplementing fish oils in ruminant diets is still controversial (Scollan et al., 2014). Several studies demonstrated fish oil supplementation into sheep diets substantially increased the levels of n-3 LC-PUFA in lamb meat and ewe milk (Jaworska et al., 2016, Parvar et al., 2017). In contrast, other studies concluded that the increase in the n-3 LC-PUFA content of edible tissues in ruminants fed fish oils are marginal due to extensive BH in the rumen (Bessa et al., 2015, Shingfield et al., 2012). Thus, new and sustainable sources of n-3 LC-PUFA for supplementation into ruminant diets are required.
Recently, marine algae, an alternative and sustainable source of n-3 LC-PUFA (Kitessa et al., 2014), has been included in sheep diets to increase levels of n-3 LC-PUFA in lamb (Díaz et al., 2017, Ponnampalam et al., 2016). Overall, marine algae are more effective for the incorporation of n-3 LC-PUFA into muscle and adipose tissues because their component oils have lower rate of BH compared to fish oils (Chikwanha et al., 2017, Howes et al., 2015). Sinclair (2007) pointed out that the structure of the algal cell wall might physically protect the algae n-3 LC-PUFA against access by bacteria and the enzymes involved in BH. However, Urrutia et al. (2016) concluded that marine algae have adverse effects on meat quality, with higher lipid oxidation, and reduced odor and flavor rates. Also, high cost, extraction and purification methods are currently limiting the potential of using marine algae on a larger scale in feeding livestock (Lenihan-Geels et al., 2013).
4.3. Oilseed and terrestrial plant oils
Oilseed and vegetable oils generally have a higher ratio of UFA to SFA compared with terrestrial animal fats (Woods & Fearon, 2009). Oilseeds and vegetable oils used in ruminant diets provide a rich source of both energy and protein (Petit, 2010), and their lipid composition is generally more than 20% PUFA (Dubois et al., 2007). As they are derived from terrestrial plants, oilseed and vegetable oils are rich in medium-chain FA, and contain no or very low levels of n-3 LC-PUFA (Dubois et al., 2007, Jaturasitha et al., 2016). A number of vegetable oils are rich in linoleic acid, including those from soybean, sunflower, safflower, and cotton seed (Dubois et al., 2007, Shingfield et al., 2013), while flaxseed and canola oils are abundant in ALA (Baker et al., 2016, Salem and Eggersdorfer, 2015).
Flaxseed, also known as linseed, contains 40–45% oil, 20–25% protein, 20–25% fibre and 1% lignin (Petit, 2010, Ponnampalam et al., 2015). Salem and Eggersdorfer (2015) stated that ALA accounts for approximately 23% of the flaxseed weight. Flaxseed oil contains more than 70% PUFA with ALA generally contributing over 50% of total FA (Baker et al., 2016, Dubois et al., 2007). Kajla, Sharma, and Sood (2015) stated that flaxseed oil is the best terrestrial source of n-3 PUFA.
Canola seed, also known as rapeseed, contains about 40–55% oil, 25–38% protein and 15–20% fibre (Carré et al., 2016, Wroniak et al., 2016). Although oleic acid is the main FA in canola oil, ALA accounts for around 10% of the total FA (Baker et al., 2016, Howes et al., 2015). Ghazani, García‐Llatas, and Marangoni (2014) stated that canola is one of the most economically important food-oil crops. Production of canola oil is the third highest globally, after soybean and palm oils. Thus, canola oil is the most available terrestrial source of n-3 PUFA. In recent years, canola and flaxseed oils have been investigated as sources of n-3 PUFA in prime lamb feeds (Flakemore et al., 2017, Nguyen et al., 2017, Nguyen et al., 2017).
Studies have shown that the extent of ruminal BH and the FA profile in meat products may be affected by the type, form and amount of lipid provided to the animals (Alves et al., 2017, Chikwanha et al., 2017, Ponnampalam et al., 2015). Gómez-Cortés et al. (2014) stated that vegetable oils have a greater effect on depressing ruminal PUFA digestion than that of oilseeds. Furthermore, processed oilseeds (rolled, extruded, roasted, ground) are more effective at increasing the concentrations of ALA and total PUFA in lamb than raw seeds (Petit, Rioux, D'oliveira, & Prado, 1997). In contrast, Paim et al. (2014) observed that whole seeds offer some degree of protection against BH as the seed coat limits access of ruminal microorganisms to oil in the seeds. However, intact oilseeds, without some disruptions of the seed coat, may escape digestion completely (Noci, Monahan, & Moloney, 2011). Other studies also reported that the effects of oilseed and vegetable oils on increasing the n-3 LC-PUFA content in ruminants are minor unless they are protected against BH (Chikwanha et al., 2017, Meignan et al., 2017). Lastly, Kitessa et al. (2009) concluded that the FA profiles were influenced by duration of oil supplementation. Lamb fed ALA rich diets for a longer period contained more ALA and n-3 LC-PUFA in their meat.
5. Effects of plant-derived ALA sources on lambs
In spite of the extensive BH of UFA by ruminal microbes, dietary lipid supplementation is the most effective way to manipulate the FA profile of ruminant products (Alvarenga et al., 2015, Chikwanha et al., 2017, Jaturasitha et al., 2016). A range of strategies have been employed to improve lamb growth performance, carcass characteristics (Francisco et al., 2015, Nguyen et al., 2017), and meat quality, especially the n-3 LC-PUFA profile in edible tissues (Nguyen et al., 2017, Realini et al., 2017); these studies have all used supplementation of plant-derived ALA sources and have culminated in varied degrees of success. The following section will focus on the effect of plant-derived dietary ALA on lamb growth, tissue FA profile and meat sensory quality.
5.1. Animal performance and carcass traits
It is widely accepted that feeding regime can influence animal growth rate and weight gain. When a basal diet (annual ryegrass hay/clover hay) was supplemented with flaxseed (10.7%, DM basis), Burnett, Jacobs, Norng, and Ponnampalam (2017) and Ponnampalam et al. (2015) observed that lambs fed the flaxseed supplement had similar dry matter intake (DMI), but higher body weight and carcass yield compared to lambs fed the basal diet alone. Burnett, Seymour, Norng, Jacobs, and Ponnampalam (2012) also concluded that lambs receiving flaxseed, either as whole seed or meal (10%, DM basis) while grazing annual pasture, have higher growth performance than lambs grazing annual pasture alone. These differences would be expected because when the metabolisable energy (ME) requirements for a growing lamb are not met, flaxseed served as an energy supplementation source to improve lamb growth response (Burnett et al., 2017).
In iso-energetic and iso-nitrogenous feeding experiments, lamb growth and carcass parameters were not affected by including up to 10% extruded flaxseed (Urrutia et al., 2015, Urrutia et al., 2016) and 10% extruded canola seed (Berthelot, Bas, & Schmidely, 2010) in the diets. Similar trends in DMI, animal performance and carcass measurements amongst dietary treatments were also observed when oil seed was included in the diets of lambs at a rate of between 2% and 5% (Meale et al., 2015, Nguyen et al., 2017, Parvar et al., 2017). In addition, Realini, Bianchi, Bentancur, and Garibotto (2017) observed similarities in cold carcass weight and fat depth when extruded flaxseed (9%, DM basis) was included in lamb basal diet. Several studies agreed that dietary fat levels at or below 60 g/kg DM will not result in any detrimental impact on lamb DMI, growth and carcass traits (Dávila-Ramírez et al., 2017, Jerónimo et al., 2010).
In contrast, high levels of lipid inclusion (> 6%, DM basis) in ruminant diets can result in several negative effects on growth performance. Feeding lipid to ruminants could increase energy density of diets without increasing high-starch concentrate intake or reducing fiber intake, which are both negatively related to rumen function (Meignan et al., 2017, Scollan et al., 2014). Likewise, high-fat diets tend to reduce DMI (Francisco et al., 2015, Parvar et al., 2017) because of the potential decrease in feed palatability (Annett, Carson, Dawson, & Kilpatrick, 2011), fiber digestibility (Bhatt, Soren, Tripathi, & Karim, 2011) and digestive nutrient flows (Ikwuegbu & Sutton, 1982). Doreau et al. (2009) pointed out that dietary supplementation with high levels of fat and oil act as a toxin to ruminal microorganisms by considerably decreasing the population of protozoa which contributes up to 50% of the biomass in the rumen and this subsequently plays a major role in the increased degradation of protein and fiber (Newbold, de la Fuente, Belanche, Ramos-Morales, & McEwan, 2015). Thus, it is suggested that the total fat in the diet should not exceed 6% DM to avoid the impairment of rumen function, digestibility and DMI (Francisco et al., 2015, Meignan et al., 2017).
5.2. Omega-3 PUFA profiles of edible tissues
More recently, great emphasis has been placed on increasing the level of n-3 PUFA in lamb editable tissues by feeding canola seed, flaxseed and their oils as sustainable and cost-effective sources (Kitessa et al., 2014). Supplementation with such sources can increase the concentration of ALA in tissue (Scollan et al., 2014). Furthermore, there is a pronounced tendency for the level of ALA in intramuscular fat (IMF) to be considerably increased due to ALA rich supplementation (Table 2). Nguyen, Flakemore et al. (2017) indicated that ALA dietary inclusion levels directly reflect its content in Longissimus muscle. Additionally, the increase in meat ALA level has been attributed to higher amount of ALA intake (Jerónimo et al., 2010). Consequently, a part of dietary ALA could escape microbial degradation in the rumen and be directly absorbed in the small intestine and then incorporated into meat and other tissues through the circulatory system (Parvar et al., 2017, Urrutia et al., 2015).
Table 2.
Effect of supplementing ALA rich oil sources to lamb on the n-3 PUFA profile of Longissimusmuscle¥.
| ALA rich source | unit | ALA | EPA | DPA | DHA | Reference |
|---|---|---|---|---|---|---|
| Controla | mg/100 g meat | 24.9 | 11.3 | 10.8 | 2.8 | Nguyen, Flakemore et al. (2017) |
| 2.5% canola oil | mg/100 g meat | 39.2 | 13.1 | 13.4 | 4.5 | Nguyen, Flakemore et al. (2017) |
| 5% canola oil | mg/100 g meat | 36.7 | 17.0 | 16.3 | 5.3 | Nguyen, Flakemore et al. (2017) |
| 2.5% flaxseed oil | mg/100 g meat | 40.8 | 14.2 | 13.8 | 4.2 | Nguyen, Flakemore et al. (2017) |
| 5% flaxseed oil | mg/100 g meat | 49.3 | 17.9 | 15.6 | 4.9 | Nguyen, Flakemore et al. (2017) |
| Controlb | g/100 g FA | 0.45 | 0.48 | 0.29 | 0.32 | Parvar et al. (2017) |
| 3% canola oil | g/100 g FA | 1.09 | 0.68 | 0.44 | 0.44 | Parvar et al. (2017) |
| Basal dietc | mg/100 g meat | 18.0 | 2.6 | 5.3 | 1.2 | Realini et al. (2017) |
| 9% extruded flaxseed | mg/100 g meat | 32.0 | 4.0 | 6.3 | 1.4 | Realini et al. (2017) |
| Controld | mg/100 g meat | 5.5 | 2.5 | 7.6 | 1.7 | Asadollahi et al. (2017) |
| 7% roasted canola seed | mg/100 g meat | 14.7 | 5.4 | 15.1 | 4.3 | Asadollahi et al. (2017) |
| Controle | g/100 g FAME | 0.40 | 0.19 | 0.23 | 0.05 | Urrutia et al. (2016) |
| 5% extruded flaxseed and 3.89% marine algae | g/100 g FAME | 0.89 | 1.01 | 0.32 | 0.99 | Urrutia et al. (2016) |
| 10% extruded flaxseed | g/100 g FAME | 1.84 | 0.74 | 0.31 | 0.08 | Urrutia et al. (2016) |
| Controlf | g/100 g FAME | 0.47 | 0.11 | 0.14 | 0.05 | Urrutia et al. (2015) |
| 5% extruded flaxseed | g/100 g FAME | 0.92 | 0.12 | 0.13 | 0.02 | Urrutia et al. (2015) |
| 10% extruded flaxseed | g/100 g FAME | 1.11 | 0.15 | 0.11 | 0.03 | Urrutia et al. (2015) |
| Ryegrass/clover hay | mg/100 g meat | 34.3 | 17.6 | 13.9 | 7.6 | Ponnampalam et al. (2015) |
| 10.7% flaxseed | mg/100 g meat | 59.5 | 18.1 | 11.2 | 6.7 | Ponnampalam et al. (2015) |
| Controlg | mg/100 g meat | 13.7 | 10.6 | 13.9 | 5.7 | Andrés et al. (2014) |
| 8.5% ground flaxseed | mg/100 g meat | 24.6 | 15.8 | 17.6 | 7.2 | Andrés et al. (2014) |
| Controlh | g/100 g FAME | 0.50 | 0.07 | 0.20 | 0.04 | Noci et al. (2011) |
| 6% flaxseed oil | g/100 g FAME | 1.74 | 0.12 | 0.21 | 0.03 | Noci et al. (2011) |
| Basal dieti | g/100 g FA | 0.70 | 0.38 | 0.70 | 0.24 | Jerónimo et al. (2010) |
| 6% flaxseed oil and sunflower oil (2:1, v/v) | g/100 g FA | 2.72 | 0.59 | 0.73 | 0.23 | Jerónimo et al. (2010) |
| 6% sunflower oil | g/100 g FA | 0.93 | 0.19 | 0.46 | 0.14 | Jerónimo, Alves, Prates, Santos-Silva, and Bessa (2009) |
| 6% sunflower oil and flaxseed oil (2:1, v/v) | g/100 g FA | 1.57 | 0.29 | 0.54 | 0.15 | Jerónimo et al. (2009) |
| 6% sunflower oil and flaxseed oil (1:2, v/v) | g/100 g FA | 2.62 | 0.51 | 0.62 | 0.19 | Jerónimo et al. (2009) |
| 6% flaxseed oil | g/100 g FA | 3.05 | 0.50 | 0.54 | 0.20 | Jerónimo et al. (2009) |
ALA: Alpha-linolenic acid; EPA: eicosapentaenoic acid; DPA: docosapentaenoic acid; DHA: docosahexaenoic acid; FA: fatty acid; FAME: fatty acid methyl esters; NA: data not available.
The control diet was concentrate and lucerne hay.
The control diet was a total mixed ration without oil.
The basal diet was based on lucerne hay or corn.
The control diet was mainly composed of milled barley, lucerne hay, soybean meal and canola meal.
The control diet was mainly composed of barley and soybean meal.
The control diet was a total mixed ration with palm oil.
The control diet was based on Megalac (palm-oil based high in 16:0).
The basal diet was composed of lucerne hay and manioc.
As shown in Table 2, various studies have indicated that the inclusion of ALA rich sources in lamb diets generally also increases the level of n-3 LC-PUFA in lamb meat. Nguyen, Flakemore et al. (2017) demonstrated that the inclusion of 5% canola oil or flaxseed oil significantly enhanced n-3 LC-PUFA content in Longissimus muscle. A similar finding was reported by Asadollahi et al. (2017) who supplemented 7% roasted canola seed to Arabian lamb fattening diets. These FA are also absorbed directly in the intestine and then stored in the tissues. However, other studies only observed a substantial increase in EPA in meat when 9% extruded flaxseed (Realini et al., 2017) or a blend of 6% flaxseed oil and sunflower oil (2:1, v/v) were added to lamb finishing diets (Jerónimo et al., 2010), while DPA and DHA were not altered. Furthermore, Urrutia et al. (2015) and Parvar et al. (2017) reported no significant difference in n-3 LC-PUFA levels when feeding lamb with ALA rich sources. These findings show that limited conversion from ALA to n-3 LC-PUFA occurs in some cases for ruminants.
In contrast, several studies have found no differences in n-3 PUFA profiles when lambs were supplemented with an ALA rich oil source in finishing diets. Radunz et al. (2009) supplemented a 3% blend of soybean oil and flaxseed oil (2:1, v/v) into lamb finishing diets and observed only modest changes in ALA and overall n-3 LC-PUFA compositions. Likewise, Berthelot, Bas, Pottier, and Normand (2012) concluded that n-3 PUFA proportions in lamb meat were not affected when 6% extruded flaxseed was included in the animals diet. Some explanations for this limited success in the incorporation of ALA have been proposed: (1) the extensive BH of the dietary ALA, ranging between 85% and 95% (Alves et al., 2017, Glasser et al., 2008); (2) low endogenous conversion of ALA to n-3 LC-PUFA (Scollan et al., 2001); and (3) the capacity of muscle to incorporate and store the n-3 LC-PUFA (Bessa et al., 2015, Wood et al., 2008). However, these studies still confirmed that increasing the dietary n-3 PUFA intake in ruminants is the base of any strategy to enrich red meat in n-3 PUFA level.
In lamb muscle, DPA tends to be present at a higher relative level and absolute content than EPA and DHA (Table 2). The roles of DPA in human health have been largely ignored because it is generally considered as an intermediary between EPA and DHA (Kaur et al., 2016). As previously discussed, the potential health benefits of DPA is now emerging, it is not listed as a beneficial source of n-3 by many health organisations (Byelashov et al., 2015). Vahmani et al. (2015) stated that the exclusion of DPA from total n-3 LC-PUFA intake results in reducing the total amount of their actual consumption. Moreover, Clayton (2014) stated that inclusion of DPA in n-3 LC-PUFA intake would boost the total n-3 LC-PUFA content of lamb to higher values. Thus, several studies have suggested that DPA should be included total in n-3 LC-PUFA intake (Howe et al., 2006, Mozaffarian and Wu, 2012).
A large number of studies have been undertaken to investigate the impacts of oilseed and/or terrestrial plant oils on lamb n-3 PUFA profiles, with most studies focusing on the effects on Longissimus muscle or intramuscular fat (Table 2). Several studies have determined the changes in FA profiles of subcutaneous, perirenal, and caudal fat (Berthelot et al., 2010, Meale et al., 2015) while other studies have investigated the alterations in the composition of ewe milk (Berthelot et al., 2012, Nudda et al., 2014) and edible non-carcass components (Nguyen, Le et al., 2017). The success rate of increasing n-3 PUFA content in edible tissues is controversial. Thus, further studies are required to better define the optimum level of n-3 PUFA supplementation, and the duration of the supplementation, in order to increase n-3 PUFA content in edible tissues, and limit the risk of adverse effects on animal performance.
5.3. Lamb meat eating quality
In addition to nutritive value, sensory characteristics of meat is another key factor which influences consumers (Pethick, Banks, Hales, & Ross, 2006). Meat palatability can be described by the level of tenderness, juiciness, flavor and overall liking of lamb meat (Pannier, Gardner et al., 2014). The nutritional characteristics of diets significantly influence sensory quality of meat (Girard et al., 2016, Jaworska et al., 2016).
More recently, research has focused on the effects of oilseed and vegetable oils on lamb sensory properties. Francisco et al. (2015) included 4% flaxseed oil and soybean oil (2:1, v/v) in lamb diets but did not find any considerable effect on meat sensory characteristics compared to lambs fed the control diet without oil. A similar result was observed by Urrutia et al. (2016) who included 10% extruded flaxseed in lamb diets. Furthermore, Nguyen, Flakemore et al. (2017) reported that there was no variation in lamb eating quality with up to 5% canola oil or flaxseed oil supplementation. The sensory properties of the Longissimus muscles were not affected by the inclusion of 6% vegetable oil in lamb (Jerónimo et al., 2012) and goat diets (Dávila-Ramírez et al., 2013, Dávila-Ramírez et al., 2017). Overall, these studies in combination reveal that feeding small ruminants with dietary plant lipid at or below 60 g/kg DM will not result in meat eating quality deterioration.
In contrast, Francisco et al. (2015) demonstrated that the supplementation of 8% flaxseed oil and soybean oil (2:1, v/v) in lamb diets had significant effects on juiciness, flavor and overall liking rates. A similar finding was also observed by Abuelfatah, Zuki, Goh, and Sazili (2016) who fed goats with 20% whole flaxseed in their diets. They concluded that supplementing ruminant diets with oilseed and/or vegetable oils can increase meat PUFA, increase susceptibility to oxidative degradation which may hasten a deterioration in meat quality. In general, the oxidation of PUFA during retail display, processing, and cooking is responsible for alterations in sensory properties, especially meat flavor and overall liking (Jaworska et al., 2016, Kouba and Mourot, 2011, Nute et al., 2007). However, individual FA has different impacts on meat eating quality. Increasing the amounts of the n-3 LC-PUFA, such as EPA and DHA, in lamb meat are linked to higher scores of rancid odor and fish flavor, and consequently lower overall liking (Nute et al., 2007, Urrutia et al., 2016). Conversely, increasing ALA content in lamb meat led to increasing flavor and overall liking (Nute et al., 2007, Sanudo et al., 2000). A similar finding was also observed in meat from goats (Abuelfatah et al., 2016).
A number of studies have demonstrated that IMF plays an important role in sensory properties of meat (Sanudo et al., 2000, Watkins et al., 2013). Asadollahi et al. (2017) found that increased IMF content in the Longissimus muscle positively correlated with a significant increase in meat sensory characteristics. High IMF level is directly associated with better juiciness in cooked meat (Wood et al., 2008). Abuelfatah et al. (2016) stressed that juiciness is more influenced by IMF content than individual FA. Pannier, Gardner et al. (2014) also concluded that IMF explained the largest amount of variation in juiciness and flavor. Therefore, finishing lambs would have to obtain high IMF levels to reach optimal eating quality. It has been suggested that lambs need to reach 4% IMF in the meat to achieve consumer satisfaction for palatability (Pannier, Pethick et al., 2014).
6. Conclusions
Omega-3 LC-PUFA can has positive and protective effects on mental health and development, inflammatory diseases, CVD and cancer. Red meat including lamb can be an important source of n-3 LC-PUFA, being especially rich in DPA which currently is often not a health-claimable n-3 LC-PUFA. The processes of lipolysis and BH of dietary lipids in the rumen are the main challenges in modulating n-3 LC-PUFA profiles of ruminant products. However, dietary lipid is the major factor manipulating the FA of ruminant products. Plant-derived sources of ALA such as flaxseed, canola seed and their oils are considered as alternative and sustainable sources of n-3 PUFA for supplementing lamb diets to increase the content of health-benefitting n-3 LC-PUFA.
In iso-energetic and iso-nitrogenous diets, supplementing lipid rich sources at or below 6% (DM basis) seems unlikely to influence animal performance and meat sensory properties. ALA rich supplementation is likely to increase the ALA level in lamb meat. However, increase in n-3 LC-PUFA content due to ALA rich supplementation have been reported in several studies. Further studies are required to focus on (1) the characterization of the desaturations and elongations of the n-3 PUFA pathway; (2) comprehensively determining the pathways for ruminal BH of n-3 LC-PUFA; (3) investigating protection technologies against the ruminal BH, and (4) more deeply understanding the biological role and necessity of DPA in both animal nutrition and human health.
Conflict of interest statement
None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
Acknowledgments
The authors are grateful to the Australian Centre for International Agricultural Research (ACIAR) Grant Number L0019200 — Overcoming technical and market constraints to the emergence of profitable beef enterprises in the North Western Highlands of Vietnam, for the John Allwright Fellowship award and College of Public Health, Medical and Veterinary Sciences of James Cook University, Townsville, Queensland, Australia for PhD scholarship funding to the first-named author.
References
- Aase K., Ernkvist M., Ebarasi L., Jakobsson L., Majumdar A., Yi C. Angiomotin regulates endothelial cell migration during embryonic angiogenesis. Genes & Development. 2007;21:2055–2068. doi: 10.1101/gad.432007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeywardena M.Y., Head R.J. Longchain n-3 polyunsaturated fatty acids and blood vessel function. Cardiovascular Research. 2001;52:361–371. doi: 10.1016/s0008-6363(01)00406-0. [DOI] [PubMed] [Google Scholar]
- Abuelfatah K., Zuki A.B.Z., Goh Y.M., Sazili A.Q. Effects of enriching goat meat with n−3 polyunsaturated fatty acids on meat quality and stability. Small Ruminant Research. 2016;136:36–42. [Google Scholar]
- Achard F., Bénistant C., Lagarde M. Interconversions and distinct metabolic fate of eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in bovine aortic endothelial cells. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1995;1255:260–266. doi: 10.1016/0005-2760(94)00238-t. [DOI] [PubMed] [Google Scholar]
- Alvarenga T.I.R.C., Chen Y., Furusho‐Garcia I.F., Perez J.R.O., Hopkins D.L. Manipulation of omega‐3 PUFAs in lamb: Phenotypic and genotypic views. Comprehensive Reviews in Food Science and Food Safety. 2015;14:189–204. doi: 10.1111/1541-4337.12131. [DOI] [PubMed] [Google Scholar]
- Alves S., Cabrita A., Jerónimo E., Bessa R., Fonseca A. Effect of ensiling and silage additives on fatty acid composition of ryegrass and corn experimental silages. Journal of Animal Science. 2011;89:2537–2545. doi: 10.2527/jas.2010-3128. [DOI] [PubMed] [Google Scholar]
- Alves S.P., Francisco A., Costa M., Santos-Silva J., Bessa R.J.B. Biohydrogenation patterns in digestive contents and plasma of lambs fed increasing levels of a tanniferous bush (Cistus ladanifer L.) and vegetable oils. Animal Feed Science and Technology. 2017;225:157–172. [Google Scholar]
- Andrés S., Morán L., Aldai N., Tejido M.L., Prieto N., Bodas R. Effects of linseed and quercetin added to the diet of fattening lambs on the fatty acid profile and lipid antioxidant status of meat samples. Meat Science. 2014;97:156–163. doi: 10.1016/j.meatsci.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Annett R., Carson A., Dawson L., Kilpatrick D. Effects of dam breed and dietary source of n-3 polyunsaturated fatty acids on the growth and carcass characteristics of lambs sourced from hill sheep flocks. Animal. 2011;5:1023–1035. doi: 10.1017/S1751731110002703. [DOI] [PubMed] [Google Scholar]
- Asadollahi S., Sari M., Erafanimajd N., Kiani A., Ponnampalam E.N. Supplementation of sugar beet pulp and roasted canola seed in a concentrate diet altered carcass traits, muscle (longissimus dorsi) composition and meat sensory properties of Arabian fattening lambs. Small Ruminant Research. 2017;153:95–102. [Google Scholar]
- Baker E.J., Miles E.A., Burdge G.C., Yaqoob P., Calder P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Progress in Lipid Research. 2016;64:30–56. doi: 10.1016/j.plipres.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Bang H., Dyerberg J., Sinclair H.M. The composition of the Eskimo food in North Western Greenland. The American Journal of Clinical Nutrition. 1980;33:2657–2661. doi: 10.1093/ajcn/33.12.2657. [DOI] [PubMed] [Google Scholar]
- Bantle J.P., Wylie-Rosett J., Albright A.L., Apovian C.M., Clark N.G., Franz M.J. Nutrition recommendations and interventions for diabetes: A position statement of the American Diabetes Association. Diabetes care. 2008;31:S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- Bauchart D. Lipid absorption and transport in ruminants. Journal of Dairy Science. 1993;76:3864–3881. doi: 10.3168/jds.S0022-0302(93)77728-0. [DOI] [PubMed] [Google Scholar]
- Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R. Heart disease and stroke statistics—2017 update: A report from the American heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquin I.M., Edwards I.J., Chen Y.Q. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Letters. 2008;269:363–377. doi: 10.1016/j.canlet.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelot V., Bas P., Pottier E., Normand J. The effect of maternal linseed supplementation and/or lamb linseed supplementation on muscle and subcutaneous adipose tissue fatty acid composition of indoor lambs. Meat Science. 2012;90:548–557. doi: 10.1016/j.meatsci.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Berthelot V., Bas P., Schmidely P. Utilization of extruded linseed to modify fatty composition of intensively-reared lamb meat: Effect of associated cereals (wheat vs. corn) and linoleic acid content of the diet. Meat Science. 2010;84:114–124. doi: 10.1016/j.meatsci.2009.08.034. [DOI] [PubMed] [Google Scholar]
- Bessa R.J., Alves S.P., Santos‐Silva J. Constraints and potentials for the nutritional modulation of the fatty acid composition of ruminant meat. European Journal of Lipid Science and Technology. 2015;117:1325–1344. [Google Scholar]
- Bhatt R., Soren N., Tripathi M., Karim S. Effects of different levels of coconut oil supplementation on performance, digestibility, rumen fermentation and carcass traits of Malpura lambs. Animal Feed Science and Technology. 2011;164:29–37. [Google Scholar]
- Birch D., Lawley M. The role of habit, childhood consumption, familiarity, and attitudes across seafood consumption segments in Australia. Journal of Food Products Marketing. 2014;20:98–113. [Google Scholar]
- Bosch A.C., O'Neill B., Sigge G.O., Kerwath S.E., Hoffman L.C. Heavy metals in marine fish meat and consumer health: A review. Journal of the Science of Food and Agriculture. 2016;96:32–48. doi: 10.1002/jsfa.7360. [DOI] [PubMed] [Google Scholar]
- Buccioni A., Decandia M., Minieri S., Molle G., Cabiddu A. Lipid metabolism in the rumen: New insights on lipolysis and biohydrogenation with an emphasis on the role of endogenous plant factors. Animal Feed Science and Technology. 2012;174:1–25. [Google Scholar]
- Burdge G.C., Jones A.E., Wootton S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of α-linolenic acid metabolism in young men. British Journal of Nutrition. 2002;88:355–363. doi: 10.1079/BJN2002662. [DOI] [PubMed] [Google Scholar]
- Burnett V., Seymour G., Norng S., Jacobs J., Ponnampalam E. Lamb growth performance and carcass weight from rotationally grazed perennial pasture systems compared with annual pasture systems with supplements. Animal Production Science. 2012;52:248–254. [Google Scholar]
- Burnett V.F., Jacobs J.L., Norng S., Ponnampalam E.N. Feed intake, liveweight gain and carcass traits of lambs offered pelleted annual pasture hay supplemented with flaxseed (Linum usitatissimum) flakes or algae (Schizochytrium sp.) Animal Production Science. 2017;57:877–883. [Google Scholar]
- Byelashov O.A., Sinclair A.J., Kaur G. Dietary sources, current intakes, and nutritional role of omega‐3 docosapentaenoic acid. Lipid Technology. 2015;27:79–82. doi: 10.1002/lite.201500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. The American Journal of Clinical Nutrition. 2006;83:S1505–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- Calder P.C. n-3 fatty acids, inflammation and immunity: New mechanisms to explain old actions. Proceedings of the Nutrition Society. 2013;72:326–336. doi: 10.1017/S0029665113001031. [DOI] [PubMed] [Google Scholar]
- Calder P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2015;1851:469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Calder P.C. New evidence that omega-3 fatty acids have a role in primary prevention of coronary heart disease. Journal of Public Health and Emergency. 2017;1:35. [Google Scholar]
- Carré P., Citeau M., Robin G., Estorges M. Hull content and chemical composition of whole seeds, hulls and germs in cultivars of rapeseed (Brassica napus) Oilseeds and Fats, Crops and Lipids. 2016;23:A302. [Google Scholar]
- Cartwright I.J., Pockley A.G., Galloway J.H., Greaves M., Preston F.E. The effects of dietary ω-3 polyunsaturated fatty acids on erythrocyte membrane phospholipids, erythrocyte deformability and blood viscosity in healthy volunteers. Atherosclerosis. 1985;55:267–281. doi: 10.1016/0021-9150(85)90106-6. [DOI] [PubMed] [Google Scholar]
- Chikwanha O.C., Vahmani P., Muchenje V., Dugan M.E.R., Mapiye C. Nutritional enhancement of sheep meat fatty acid profile for human health and wellbeing. Food Research International. 2017 doi: 10.1016/j.foodres.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Chilliard Y., Glasser F., Ferlay A., Bernard L., Rouel J., Doreau M. Diet, rumen biohydrogenation and nutritional quality of cow and goat milk fat. European Journal of Lipid Science and Technology. 2007;109:828–855. [Google Scholar]
- Christensen E., Woldseth B., Hagve T.-A., Poll-The B., Wanders R., Sprecher H. Peroxisomal β-oxidation of polyunsaturated long chain fatty acids in human fibroblasts. The polyunsaturated and the saturated long chain fatty acids are retroconverted by the same acyl-CoA oxidase. Scandinavian Journal of Clinical and Laboratory Investigation. 1993;53:61–74. doi: 10.3109/00365519309090698. [DOI] [PubMed] [Google Scholar]
- Clayton E.H. 2014. Graham Centre Monograph No. 4: Long-chain omega-3 polyunsaturated fatty acids in ruminant nutrition: Benefits to animals and humans. NSW Department of Primary Industries, Wagga Wagga, Australia. Available at https://www.csu.edu.au/__data/assets/pdf_file/0007/1155148/Monograph-Clayton-Omega-3-final.pdf. Accessed 16 August 2017.
- Dávila-Ramírez J., Avendaño-Reyes L., Macías-Cruz U., Peña-Ramos E., Islava-Lagarda T., Zamorano-García L. Fatty acid composition and physicochemical and sensory characteristics of meat from ewe lambs supplemented with zilpaterol hydrochloride and soybean oil. Animal Production Science. 2017;57:767–777. [Google Scholar]
- Dávila-Ramírez J.L., Avendaño-Reyes L., Macías-Cruz U., Torrentera-Olivera N.G., Zamorano-García L., Peña-Ramos A. Effects of zilpaterol hydrochloride and soybean oil supplementation on physicochemical and sensory characteristics of meat from hair lambs. Small Ruminant Research. 2013;114:253–257. [Google Scholar]
- De Smet S., Raes K., Demeyer D. Meat fatty acid composition as affected by fatness and genetic factors: A review. Animal Research. 2004;53:81–98. [Google Scholar]
- Deckelbaum R.J., Torrejon C. The omega-3 fatty acid nutritional landscape: Health benefits and sources. The Journal of Nutrition. 2012;142:587S–591S. doi: 10.3945/jn.111.148080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhurst R., Shingfield K., Lee M.a., Scollan N. Increasing the concentrations of beneficial polyunsaturated fatty acids in milk produced by dairy cows in high-forage systems. Animal Feed Science and Technology. 2006;131:168–206. [Google Scholar]
- Díaz M.T., Pérez C., Sánchez C.I., Lauzurica S., Cañeque V., González C., De La Fuente J. Feeding microalgae increases omega 3 fatty acids of fat deposits and muscles in light lambs. Journal of Food Composition and Analysis. 2017;56:115–123. [Google Scholar]
- Doreau M., Aurousseau E., Martin C. Effects of linseed lipids fed as rolled seeds, extruded seeds or oil on organic matter and crude protein digestion in cows. Animal Feed Science and Technology. 2009;150:187–196. [Google Scholar]
- Doreau M., Ferlay A. Digestion and utilisation of fatty acids by ruminants. Animal Feed Science and Technology. 1994;45:379–396. [Google Scholar]
- Doreau M., Meynadier A., Fievez V., Ferlay A. Ruminal metabolism of fatty acids: Modulation of polyunsaturated, conjugated, and trans fatty acids in meat and milk. In: Watson R.R., De Meester F., editors. Handbook of lipids in human function: fatty acids. AOCS Press, Published by Elsevier in cooperation with the American Oil Chemists' Society; 2016. pp. 521–542. [Google Scholar]
- Droulez V., Williams P., Levy G., Stobaus T., Sinclair A. Composition of Australian red meat 2002. 2. Fatty acid profile. Food Australia. 2006;58:335–341. [Google Scholar]
- Dubois V., Breton S., Linder M., Fanni J., Parmentier M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. European Journal of Lipid Science and Technology. 2007;109:710–732. [Google Scholar]
- Edwards H.D., Shelver W.L., Choi S., Nisbet D.J., Krueger N.A., Anderson R.C. Immunogenic inhibition of prominent ruminal bacteria as a means to reduce lipolysis and biohydrogenation activity in vitro. Food Chemistry. 2017;218:372–377. doi: 10.1016/j.foodchem.2016.09.052. [DOI] [PubMed] [Google Scholar]
- Eriksson S.F., Pickova J. Fatty acids and tocopherol levels in M. Longissimus dorsi of beef cattle in Sweden – A comparison between seasonal diets. Meat Science. 2007;76:746–754. doi: 10.1016/j.meatsci.2007.02.021. [DOI] [PubMed] [Google Scholar]
- FAO/WHO 2008. Interim summary of conclusions and dietary recommendations on total fat & fatty acids. From the joint FAO/WHO expert consultation on fats and fatty acids in human nutrition, Geneva, Switzerland. Available at https://www.anses.fr/en/system/files/NUT2006sa0359EN.pdf. Accessed 25 July 2017.
- Fayet-Moore F., Baghurst K., Meyer B.J. Four models including fish, seafood, red meat and enriched foods to achieve Australian dietary recommendations for n-3 lcpufa for all life-stages. Nutrients. 2015;7:8602–8614. doi: 10.3390/nu7105413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FFSA 2010. `Opinion of the French Food Safety Agency on the update of French population reference intakes (ANCs) for fatty acids' Available at https://www.anses.fr/en/system/files/NUT2006sa0359EN.pdf. Accessed 26 July 2017.
- Flakemore A.R., Malau-Aduli B.S., Nichols P.D., Malau-Aduli A.E.O. Omega-3 fatty acids, nutrient retention values, and sensory meat eating quality in cooked and raw Australian lamb. Meat Science. 2017;123:79–87. doi: 10.1016/j.meatsci.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Francisco A., Dentinho M., Alves S., Portugal P., Fernandes F., Sengo S. Growth performance, carcass and meat quality of lambs supplemented with increasing levels of a tanniferous bush (Cistus ladanifer L.) and vegetable oils. Meat Science. 2015;100:275–282. doi: 10.1016/j.meatsci.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Ganesan B., Brothersen C., McMahon D.J. Fortification of foods with omega-3 polyunsaturated fatty acids. Critical Reviews in Food Science and Nutrition. 2014;54:98–114. doi: 10.1080/10408398.2011.578221. [DOI] [PubMed] [Google Scholar]
- Gerster H. Can adults adequately convert a-linolenic acid (18: 3n-3) to eicosapentaenoic acid (20: 5n-3) and docosahexaenoic acid (22: 6n-3) International Journal for Vitamin and Nutrition Research. 1998;68:159–173. [PubMed] [Google Scholar]
- Ghazani S.M., García‐Llatas G., Marangoni A.G. Micronutrient content of cold‐pressed, hot‐pressed, solvent extracted and RBD canola oil: Implications for nutrition and quality. European Journal of Lipid Science and Technology. 2014;116:380–387. [Google Scholar]
- Girard M., Dohme‐Meier F., Silacci P., Ampuero Kragten S., Kreuzer M. Forage legumes rich in condensed tannins may increase n‐3 fatty acid levels and sensory quality of lamb meat. Journal of the Science of Food and Agriculture. 2016;96:1923–1933. doi: 10.1002/jsfa.7298. [DOI] [PubMed] [Google Scholar]
- Glasser F., Doreau M., Maxin G., Baumont R. Fat and fatty acid content and composition of forages: A meta-analysis. Animal Feed Science and Technology. 2013;185:19–34. [Google Scholar]
- Glasser F., Schmidely P., Sauvant D., Doreau M. Digestion of fatty acids in ruminants: A meta-analysis of flows and variation factors: 2. C18 fatty acids. Animal. 2008;2:691–704. doi: 10.1017/S1751731108002036. [DOI] [PubMed] [Google Scholar]
- Gómez-Cortés P., Gallardo B., Mantecón A.R., Juárez M., de la Fuente M.A., Manso T. Effects of different sources of fat (calcium soap of palm oil vs. extruded linseed) in lactating ewes' diet on the fatty acid profile of their suckling lambs. Meat Science. 2014;96:1304–1312. doi: 10.1016/j.meatsci.2013.10.040. [DOI] [PubMed] [Google Scholar]
- Gómez-Cortés P., Tyburczy C., Brenna J.T., Juárez M., de la Fuente M.A. Characterization of cis-9 trans-11 trans-15 C18: 3 in milk fat by GC and covalent adduct chemical ionization tandem MS. Journal of Lipid Research. 2009;50:2412–2420. doi: 10.1194/jlr.M800662-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorjão R., Azevedo-Martins A.K., Rodrigues H.G., Abdulkader F., Arcisio-Miranda M., Procopio J. Comparative effects of DHA and EPA on cell function. Pharmacology & Therapeutics. 2009;122:56–64. doi: 10.1016/j.pharmthera.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Gould J.F., Smithers L.G., Makrides M. The effect of maternal omega-3 (n− 3) LCPUFA supplementation during pregnancy on early childhood cognitive and visual development: A systematic review and meta-analysis of randomized controlled trials. The American Journal of Clinical Nutrition. 2013;97:531–544. doi: 10.3945/ajcn.112.045781. [DOI] [PubMed] [Google Scholar]
- Gribble M.O., Karimi R., Feingold B.J., Nyland J.F., O'Hara T.M., Gladyshev M.I. Mercury, selenium and fish oils in marine food webs and implications for human health. Journal of the Marine Biological Association of the United Kingdom. 2016;96:43–59. doi: 10.1017/S0025315415001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropper S.S., Smith J.L. 6th Edition. Cengage Learning; Belmont, CA: 2013. Advanced nutrition and human metabolism. [Google Scholar]
- Harfoot C.G., Hazelwood G.P. Lipid metabolism in the rumen. In: Hobson P.N., Stewart C.S., editors. The rumen microbial ecosystem. Blackie Academic and Professional; London, UK: 1998. [Google Scholar]
- Heller A.R., Rössel T., Gottschlich B., Tiebel O., Menschikowski M., Litz R.J. Omega‐3 fatty acids improve liver and pancreas function in postoperative cancer patients. International Journal of Cancer. 2004;111:611–616. doi: 10.1002/ijc.20291. [DOI] [PubMed] [Google Scholar]
- Holub B.J., Swidinsky P., Park E. Oral docosapentaenoic acid (22: 5n-3) is differentially incorporated into phospholipid pools and differentially metabolized to eicosapentaenoic acid in tissues from young rats. Lipids. 2011;46:399–407. doi: 10.1007/s11745-011-3535-3. [DOI] [PubMed] [Google Scholar]
- Howe P., Meyer B., Record S., Baghurst K. Dietary intake of long-chain ω-3 polyunsaturated fatty acids: Contribution of meat sources. Nutrition. 2006;22:47–53. doi: 10.1016/j.nut.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Howes N.L., Bekhit A.E.D.A., Burritt D.J., Campbell A.W. Opportunities and implications of pasture‐based lamb fattening to enhance the long‐chain fatty acid composition in meat. Comprehensive Reviews in Food Science and Food Safety. 2015;14:22–36. doi: 10.1111/1541-4337.12118. [DOI] [PubMed] [Google Scholar]
- Ikwuegbu O., Sutton J. The effect of varying the amount of linseed oil supplementation on rumen metabolism in sheep. British Journal of Nutrition. 1982;48:365–375. doi: 10.1079/bjn19820120. [DOI] [PubMed] [Google Scholar]
- Innis S.M. Dietary omega 3 fatty acids and the developing brain. Brain Research. 2008;1237:35–43. doi: 10.1016/j.brainres.2008.08.078. [DOI] [PubMed] [Google Scholar]
- Jaturasitha S., Chaiwang N., Kayan A., Kreuzer M. Nutritional strategies to improve the lipid composition of meat, with emphasis on Thailand and Asia. Meat Science. 2016;120:157–166. doi: 10.1016/j.meatsci.2016.04.014. [DOI] [PubMed] [Google Scholar]
- Jaworska D., Czauderna M., Przybylski W., Rozbicka-Wieczorek A.J. Sensory quality and chemical composition of meat from lambs fed diets enriched with fish and rapeseed oils, carnosic acid and seleno-compounds. Meat Science. 2016;119:185–192. doi: 10.1016/j.meatsci.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Jenkins T., Wallace R., Moate P., Mosley E. Board-invited review: Recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem. Journal of Animal Science. 2008;86:397–412. doi: 10.2527/jas.2007-0588. [DOI] [PubMed] [Google Scholar]
- Jenkins T.C. Lipid Metabolism in the Rumen. Journal of Dairy Science. 1993;76:3851–3863. doi: 10.3168/jds.S0022-0302(93)77727-9. [DOI] [PubMed] [Google Scholar]
- Jerónimo E., Alfaia C.M.M., Alves S.P., Dentinho M.T.P., Prates J.A.M., Vasta V. Effect of dietary grape seed extract and Cistus ladanifer L. in combination with vegetable oil supplementation on lamb meat quality. Meat Science. 2012;92:841–847. doi: 10.1016/j.meatsci.2012.07.011. [DOI] [PubMed] [Google Scholar]
- Jerónimo E., Alves S.P., Martins S.V., Prates J.A., Bessa R.J., Santos-Silva J. Effect of sodium bentonite and vegetable oil blend supplementation on growth, carcass quality and intramuscular fatty acid composition of lambs. Animal Feed Science and Technology. 2010;158:136–145. [Google Scholar]
- Jerónimo E., Alves S.P., Prates J.A., Santos-Silva J., Bessa R.J. Effect of dietary replacement of sunflower oil with linseed oil on intramuscular fatty acids of lamb meat. Meat Science. 2009;83:499–505. doi: 10.1016/j.meatsci.2009.06.033. [DOI] [PubMed] [Google Scholar]
- JMHLW 2015. `Overview of Dietary Reference Intakes for Japanese (2015).' Available at http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/Overview.pdf. Accessed 08 August 2017.
- Kagawa Y., Nishizawa M., Suzuki M., Miyatake T., Hamaoto T., Goto K. Eicosapolyenoic acids of serum lipids of Japanese islanders with low incidence of cardiovascular diseases. Journal of Nutritional Science and Vitaminology. 1982;28:441–453. doi: 10.3177/jnsv.28.441. [DOI] [PubMed] [Google Scholar]
- Kairenius P., Toivonen V., Shingfield K.J. Identification and ruminal outflow of long-chain fatty acid biohydrogenation intermediates in cows fed diets containing fish oil. Lipids. 2011;46:587–606. doi: 10.1007/s11745-011-3561-1. [DOI] [PubMed] [Google Scholar]
- Kajla P., Sharma A., Sood D.R. Flaxseed—a potential functional food source. Journal of Food Science and Technology. 2015;52:1857–1871. doi: 10.1007/s13197-014-1293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalač P., Samková E. The effects of feeding various forages on fatty acid composition of bovine milk fat: A review. Czech Journal of Animal Science. 2010;55:521–537. [Google Scholar]
- Kaur G., Begg D.P., Barr D., Garg M., Cameron-Smith D., Sinclair A.J. Short-term docosapentaenoic acid (22: 5n-3) supplementation increases tissue docosapentaenoic acid, DHA and EPA concentrations in rats. British Journal of Nutrition. 2010;103:32–37. doi: 10.1017/S0007114509991334. [DOI] [PubMed] [Google Scholar]
- Kaur G., Cameron-Smith D., Garg M., Sinclair A.J. Docosapentaenoic acid (22: 5n-3): A review of its biological effects. Progress in Lipid Research. 2011;50:28–34. doi: 10.1016/j.plipres.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Kaur G., Guo X.-F., Sinclair A.J. Short update on docosapentaenoic acid: A bioactive long-chain n-3 fatty acid. Current Opinion in Clinical Nutrition & Metabolic Care. 2016;19:88–91. doi: 10.1097/MCO.0000000000000252. [DOI] [PubMed] [Google Scholar]
- Kennedy E.T., Luo H., Ausman L.M. Cost implications of alternative sources of (n-3) fatty acid consumption in the United States. The Journal of Nutrition. 2012;142:605S–609S. doi: 10.3945/jn.111.152736. [DOI] [PubMed] [Google Scholar]
- Khan M.Z., He L. The role of polyunsaturated fatty acids and GPR40 receptor in brain. Neuropharmacology. 2017;113:639–651. doi: 10.1016/j.neuropharm.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Khanapure S.P., Garvey D.S., Janero D.R., Gordon Letts L. Eicosanoids in inflammation: Biosynthesis, pharmacology, and therapeutic frontiers. Current Topics in Medicinal Chemistry. 2007;7:311–340. doi: 10.2174/156802607779941314. [DOI] [PubMed] [Google Scholar]
- Kitessa S.M., Abeywardena M., Wijesundera C., Nichols P.D. DHA-containing oilseed: A timely solution for the sustainability issues surrounding fish oil sources of the health-benefitting long-chain omega-3 oils. Nutrients. 2014;6:2035–2058. doi: 10.3390/nu6052035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitessa S.M., Williams A., Gulati S., Boghossian V., Reynolds J., Pearce K.L. Influence of duration of supplementation with ruminally protected linseed oil on the fatty acid composition of feedlot lambs. Animal Feed Science and Technology. 2009;151:228–239. [Google Scholar]
- Koivunen E., Jaakkola S., Heikkilä T., Lampi A.-M., Halmemies-Beauchet-Filleau A., Lee M. Effects of plant species, stage of maturity, and level of formic acid addition on lipolysis, lipid content, and fatty acid composition during ensiling. Journal of Animal Science. 2015;93:4408–4423. doi: 10.2527/jas.2014-8813. [DOI] [PubMed] [Google Scholar]
- Koletzko B., Mrotzek M., Bremer H. Fatty acid composition of mature human milk in Germany. The American Journal of Clinical Nutrition. 1988;47:954–959. doi: 10.1093/ajcn/47.6.954. [DOI] [PubMed] [Google Scholar]
- Kouba M., Mourot J. A review of nutritional effects on fat composition of animal products with special emphasis on n-3 polyunsaturated fatty acids. Biochimie. 2011;93:13–17. doi: 10.1016/j.biochi.2010.02.027. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton P.M., Innis S. Position of the american dietetic association and dietitians of Canada: Dietary fatty acids. Journal of the American Dietetic Association. 2007;107:1599–1611. [PubMed] [Google Scholar]
- Lascano G.J., Alende M., Koch L.E., Jenkins T.C. Changes in fermentation and biohydrogenation intermediates in continuous cultures fed low and high levels of fat with increasing rates of starch degradability. Journal of Dairy Science. 2016;99:6334–6341. doi: 10.3168/jds.2016-11032. [DOI] [PubMed] [Google Scholar]
- Laviano A., Rianda S., Molfino A., Fanelli F.R. Omega-3 fatty acids in cancer. Current Opinion in Clinical Nutrition & Metabolic Care. 2013;16:156–161. doi: 10.1097/MCO.0b013e32835d2d99. [DOI] [PubMed] [Google Scholar]
- Lenihan-Geels G., Bishop K.S., Ferguson L.R. Alternative sources of omega-3 fats: Can we find a sustainable substitute for fish. Nutrients. 2013;5:1301–1315. doi: 10.3390/nu5041301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço M., Ramos-Morales E., Wallace R.J. The role of microbes in rumen lipolysis and biohydrogenation and their manipulation. Animal. 2010;4:1008–1023. doi: 10.1017/S175173111000042X. [DOI] [PubMed] [Google Scholar]
- MacLean C.H., Newberry S.J., Mojica W.A., Khanna P., Issa A.M., Suttorp M.J. Effects of omega-3 fatty acids on cancer risk: A systematic review. JAMA. 2006;295:403–415. doi: 10.1001/jama.295.4.403. [DOI] [PubMed] [Google Scholar]
- Malau-Aduli A.E.O., Holman B.W.B., Kashani A., Nichols P.D. Sire breed and sex effects on the fatty acid composition and content of heart, kidney, liver, adipose and muscle tissues of purebred and first-cross prime lambs. Animal Production Science. 2016;56:2122–2132. [Google Scholar]
- Markworth J.F., Kaur G., Miller E.G., Larsen A.E., Sinclair A.J., Maddipati K.R. Divergent shifts in lipid mediator profile following supplementation with n-3 docosapentaenoic acid and eicosapentaenoic acid. The FASEB Journal. 2016;30:3714–3725. doi: 10.1096/fj.201600360R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meale S.J., Chaves A.V., He M.L., Guan L.L., McAllister T.A. Effects of various dietary lipid additives on lamb performance, carcass characteristics, adipose tissue fatty acid composition, and wool characteristics. Journal of Animal Science. 2015;93:3110–3120. doi: 10.2527/jas.2014-8437. [DOI] [PubMed] [Google Scholar]
- Meignan T., Lechartier C., Chesneau G., Bareille N. Effects of feeding extruded linseed on production performance and milk fatty acid profile in dairy cows: A meta-analysis. Journal of Dairy Science. 2017;100:4394–4408. doi: 10.3168/jds.2016-11850. [DOI] [PubMed] [Google Scholar]
- Miller E., Kaur G., Larsen A., Loh S.P., Linderborg K., Weisinger H.S. A short-term n-3 DPA supplementation study in humans. European Journal of Nutrition. 2013;52:895–904. doi: 10.1007/s00394-012-0396-3. [DOI] [PubMed] [Google Scholar]
- Miller M., Stone N.J., Ballantyne C., Bittner V., Criqui M.H., Ginsberg H.N. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- Morin C., Rousseau É., Fortin S. Anti-proliferative effects of a new docosapentaenoic acid monoacylglyceride in colorectal carcinoma cells. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2013;89:203–213. doi: 10.1016/j.plefa.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D., Geelen A., Brouwer I.A., Geleijnse J.M., Zock P.L., Katan M.B. Effect of fish oil on heart rate in humans. Circulation. 2005;112:1945–1952. doi: 10.1161/CIRCULATIONAHA.105.556886. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D., Wu J.H. (n-3) fatty acids and cardiovascular health: Are effects of EPA and DHA shared or complementary. The Journal of Nutrition. 2012;142:614S–625S. doi: 10.3945/jn.111.149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold C.J., de la Fuente G., Belanche A., Ramos-Morales E., McEwan N.R. The role of ciliate protozoa in the rumen. Frontiers in Microbiology. 2015;6:1313. doi: 10.3389/fmicb.2015.01313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D.V., Flakemore A.R., Otto J.R., Ives S.W., Smith R.W., Nichols P.D. Nutritional value and sensory characteristics of meat eating quality of Australian prime lambs supplemented with pelleted canola and flaxseed oils: Fatty acid profiles of muscle and adipose tissues. Internal Medicine Review. 2017;3:1–21. [Google Scholar]
- Nguyen D.V., Le V.H., Nguyen Q.V., Malau-Aduli B.S., Nichols P.D., Malau-Aduli A.E. Omega–3 long-chain fatty acids in the heart, kidney, liver and plasma metabolite profiles of Australian prime lambs supplemented with pelleted canola and flaxseed oils. Nutrients. 2017;9:893. doi: 10.3390/nu9080893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D.V., Malau-Aduli B., Nichols P., Malau-Aduli A.E.O. Growth performance and carcass characteristics of Australian prime lambs supplemented with pellets containing canola oil or flaxseed oil. Animal Production Science. 2017 doi: 10.1071/AN16812. [DOI] [Google Scholar]
- NHFA 2015. `Fish and seafood position statement.' Available at https://www.heartfoundation.org.au/images/uploads/main/Programs/PRO-169_Fish_and_seafood_position_statement.pdf. Accessed 26 July 2017.
- NHMRC . Australian Government; Canberra, Australia: 2006. Nutrient reference values for Australia and New Zealand including recommended dietary intakes. [Google Scholar]
- Nichols P.D., Dogan L., Sinclair A. Australian and New Zealand fish oil products in 2016 meet label omega-3 claims and are not oxidized. Nutrients. 2016;8:703. doi: 10.3390/nu8110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols P.D., McManus A., Krail K., Sinclair A.J., Miller M. Recent advances in omega-3: Health benefits, sources, products and bioavailability. Nutrients. 2014;6:3727–3733. doi: 10.3390/nu6093727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols P.D., Petrie J., Singh S. Long-chain omega-3 oils–an update on sustainable sources. Nutrients. 2010;2:572–585. doi: 10.3390/nu2060572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noci F., Monahan F.J., Moloney A.P. The fatty acid profile of muscle and adipose tissue of lambs fed camelina or linseed as oil or seeds. Animal. 2011;5:134–147. doi: 10.1017/S1751731110001485. [DOI] [PubMed] [Google Scholar]
- Nudda A., Battacone G., Boaventura Neto O., Cannas A., Francesconi A.H.D., Atzori A.S. Feeding strategies to design the fatty acid profile of sheep milk and cheese. Revista Brasileira de Zootecnia. 2014;43:445–456. [Google Scholar]
- Nute G.R., Richardson R.I., Wood J.D., Hughes S.I., Wilkinson R.G., Cooper S.L. Effect of dietary oil source on the flavour and the colour and lipid stability of lamb meat. Meat Science. 2007;77:547–555. doi: 10.1016/j.meatsci.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Paim T.d.P., Viana P., Brandão E., Amador S., Barbosa T., Cardoso C. Carcass traits and fatty acid profile of meat from lambs fed different cottonseed by-products. Small Ruminant Research. 2014;116:71–77. [Google Scholar]
- Pannier L., Gardner G.E., Pearce K.L., McDonagh M., Ball A.J., Jacob R.H. Associations of sire estimated breeding values and objective meat quality measurements with sensory scores in Australian lamb. Meat Science. 2014;96:1076–1087. doi: 10.1016/j.meatsci.2013.07.037. [DOI] [PubMed] [Google Scholar]
- Pannier L., Pethick D.W., Geesink G.H., Ball A.J., Jacob R.H., Gardner G.E. Intramuscular fat in the longissimus muscle is reduced in lambs from sires selected for leanness. Meat Science. 2014;96:1068–1075. doi: 10.1016/j.meatsci.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Papanikolaou Y., Brooks J., Reider C., Fulgoni V.L. US adults are not meeting recommended levels for fish and omega-3 fatty acid intake: Results of an analysis using observational data from NHANES 2003–2008. BMC Nutrition Journal. 2014;13:31. doi: 10.1186/1475-2891-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvar R., Ghoorchi T., Shargh M.S. Influence of dietary oils on performance, blood metabolites, purine derivatives, cellulase activity and muscle fatty acid composition in fattening lambs. Small Ruminant Research. 2017;150:22–29. [Google Scholar]
- Pethick D., Banks R., Hales J., Ross I. Australian prime lamb–a vision for 2020. International Journal of Sheep and Wool Science. 2006;54:66–73. [Google Scholar]
- Petit H. Review: Feed intake, milk production and milk composition of dairy cows fed flaxseed. Canadian Journal of Animal Science. 2010;90:115–127. [Google Scholar]
- Petit H.V., Rioux R., D'oliveira P., Prado I.D. Performance of growing lambs fed grass silage with raw or extruded soybean or canola seeds. Canadian Journal of Animal Science. 1997;77:455–463. [Google Scholar]
- Phang M., Garg M.L., Sinclair A.J. Inhibition of platelet aggregation by omega-3 polyunsaturated fatty acids is gender specific - Redefining platelet response to fish oils. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2009;81:35–40. doi: 10.1016/j.plefa.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Ponnampalam E.N., Burnett V.F., Norng S., Hopkins D.L., Plozza T., Jacobs J.L. Muscle antioxidant (vitamin E) and major fatty acid groups, lipid oxidation and retail colour of meat from lambs fed a roughage based diet with flaxseed or algae. Meat Science. 2016;111:154–160. doi: 10.1016/j.meatsci.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Ponnampalam E.N., Lewandowski P.A., Fahri F.T., Burnett V.F., Dunshea F.R., Plozza T. Forms of n-3 (ALA, C18: 3n-3 or DHA, C22: 6n-3) fatty acids affect carcass yield, blood lipids, muscle n-3 fatty acids and liver gene expression in lambs. Lipids. 2015;50:1133–1143. doi: 10.1007/s11745-015-4070-4. [DOI] [PubMed] [Google Scholar]
- Radunz A., Wickersham L., Loerch S., Fluharty F., Reynolds C., Zerby H. Effects of dietary polyunsaturated fatty acid supplementation on fatty acid composition in muscle and subcutaneous adipose tissue of lambs. Journal of Animal Science. 2009;87:4082–4091. doi: 10.2527/jas.2009-2059. [DOI] [PubMed] [Google Scholar]
- Rahmawaty S., Charlton K., Lyons-Wall P., Meyer B.J. Dietary intake and food sources of EPA, DPA and DHA in Australian children. Lipids. 2013;48:869–877. doi: 10.1007/s11745-013-3812-4. [DOI] [PubMed] [Google Scholar]
- Realini C.E., Bianchi G., Bentancur O., Garibotto G. Effect of supplementation with linseed or a blend of aromatic spices and time on feed on fatty acid composition, meat quality and consumer liking of meat from lambs fed dehydrated alfalfa or corn. Meat Science. 2017;127:21–29. doi: 10.1016/j.meatsci.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Rose D.P., Connolly J.M. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacology & Therapeutics. 1999;83:217–244. doi: 10.1016/s0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- Rosenthal M.D., Garcia M.C., Jones M.R., Sprecher H. Retroconversion and δ4 desaturation of docosatetraenoate (22: 4 (n- 6)) and docosapentaenoate (22: 5 (n− 3)) by human cells in culture. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism. 1991;1083:29–36. doi: 10.1016/0005-2760(91)90121-w. [DOI] [PubMed] [Google Scholar]
- Ruxton C., Reed S.C., Simpson M., Millington K. The health benefits of omega‐3 polyunsaturated fatty acids: A review of the evidence. Journal of Human Nutrition and Dietetics. 2004;17:449–459. doi: 10.1111/j.1365-277X.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- Sakurama H., Kishino S., Mihara K., Ando A., Kita K., Takahashi S. Biohydrogenation of C20 polyunsaturated fatty acids by anaerobic bacteria. Journal of Lipid Research. 2014;55:1855–1863. doi: 10.1194/jlr.M045450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem N., Eggersdorfer M. Is the world supply of omega-3 fatty acids adequate for optimal human nutrition. Current Opinion in Clinical Nutrition & Metabolic Care. 2015;18:147–154. doi: 10.1097/MCO.0000000000000145. [DOI] [PubMed] [Google Scholar]
- Sanudo C., Enser M., Campo M., Nute G., Marıa G., Sierra I. Fatty acid composition and sensory characteristics of lamb carcasses from Britain and Spain. Meat Science. 2000;54:339–346. doi: 10.1016/s0309-1740(99)00108-4. [DOI] [PubMed] [Google Scholar]
- Scollan N.D., Choi N.-J., Kurt E., Fisher A.V., Enser M., Wood J.D. Manipulating the fatty acid composition of muscle and adipose tissue in beef cattle. British Journal of Nutrition. 2001;85:115–124. doi: 10.1079/bjn2000223. [DOI] [PubMed] [Google Scholar]
- Scollan N.D., Dannenberger D., Nuernberg K., Richardson I., MacKintosh S., Hocquette J.F. Enhancing the nutritional and health value of beef lipids and their relationship with meat quality. Meat Science. 2014;97:384–394. doi: 10.1016/j.meatsci.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Shingfield K., Bonnet M., Scollan N. Recent developments in altering the fatty acid composition of ruminant-derived foods. Animal. 2013;7:132–162. doi: 10.1017/S1751731112001681. [DOI] [PubMed] [Google Scholar]
- Shingfield K.J., Kairenius P., Ärölä A., Paillard D., Muetzel S., Ahvenjärvi S. Dietary fish oil supplements modify ruminal biohydrogenation, alter the flow of fatty acids at the omasum, and induce changes in the ruminal Butyrivibrio population in lactating cows. The Journal of Nutrition. 2012;142:1437–1448. doi: 10.3945/jn.112.158576. [DOI] [PubMed] [Google Scholar]
- Shingfield K.J., Lee M.R.F., Humphries D.J., Scollan N.D., Toivonen V., Reynolds C.K. Effect of incremental amounts of fish oil in the diet on ruminal lipid metabolism in growing steers. British Journal of Nutrition. 2010;104:56–66. doi: 10.1017/S0007114510000292. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA: A Cancer Journal for Clinicians. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Simopoulos A. An increase in the omega-6/Omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8:128. doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. Journal of the American College of Nutrition. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- Sinclair H.M. Deficiency of essential fatty acids and atherosclerosis, etcetera. Lancet (London, England) 1956;270:381–383. [PubMed] [Google Scholar]
- Sinclair L. Nutritional manipulation of the fatty acid composition of sheep meat: A review. The Journal of Agricultural Science. 2007;145:419–434. [Google Scholar]
- Sinclair L., Cooper S., Chikunya S., Wilkinson R., Hallett K., Enser M., Wood J. Biohydrogenation of n-3 polyunsaturated fatty acids in the rumen and their effects on microbial metabolism and plasma fatty acid concentrations in sheep. Animal Science. 2005;81:239–248. [Google Scholar]
- Swanson D., Block R., Mousa S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Advances in Nutrition: An International Review Journal. 2012;3:1–7. doi: 10.3945/an.111.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tur J., Bibiloni M., Sureda A., Pons A. Dietary sources of omega 3 fatty acids: Public health risks and benefits. British Journal of Nutrition. 2012;107:S23–S52. doi: 10.1017/S0007114512001456. [DOI] [PubMed] [Google Scholar]
- Urrutia O., Mendizabal J., Insausti K., Soret B., Purroy A., Arana A. Effect of linseed dietary supplementation on adipose tissue development, fatty acid composition, and lipogenic gene expression in lambs. Livestock Science. 2015;178:345–356. [Google Scholar]
- Urrutia O., Mendizabal J.A., Insausti K., Soret B., Purroy A., Arana A. Effects of addition of linseed and marine algae to the diet on adipose tissue development, fatty acid profile, lipogenic gene expression, and meat quality in lambs. PloS One. 2016;11 doi: 10.1371/journal.pone.0156765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahmani P., Mapiye C., Prieto N., Rolland D.C., McAllister T.A., Aalhus J.L. The scope for manipulating the polyunsaturated fatty acid content of beef: A review. Journal of Animal Science and Biotechnology. 2015;6:29. doi: 10.1186/s40104-015-0026-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannice G., Rasmussen H. Position of the academy of nutrition and dietetics: Dietary fatty acids for healthy adults. Journal of the Academy of Nutrition and Dietetics. 2014;114:136–153. doi: 10.1016/j.jand.2013.11.001. [DOI] [PubMed] [Google Scholar]
- Vazirigohar M., Dehghan-Banadaky M., Rezayazdi K., Krizsan S.J., Nejati-Javaremi A., Shingfield K.J. Fat source and dietary forage-to-concentrate ratio influences milk fatty-acid composition in lactating cows. Animal. 2014;8:163–174. doi: 10.1017/S175173111300181X. [DOI] [PubMed] [Google Scholar]
- Vlaeminck B., Mengistu G., Fievez V., De Jonge L., Dijkstra J. Effect of in vitro docosahexaenoic acid supplementation to marine algae-adapted and unadapted rumen inoculum on the biohydrogenation of unsaturated fatty acids in freeze-dried grass. Journal of Dairy Science. 2008;91:1122–1132. doi: 10.3168/jds.2007-0537. [DOI] [PubMed] [Google Scholar]
- Walker R., Decker E.A., McClements D.J. Development of food-grade nanoemulsions and emulsions for delivery of omega-3 fatty acids: Opportunities and obstacles in the food industry. Food & Function. 2015;6:41–54. doi: 10.1039/c4fo00723a. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Tatsuno I. Omega-3 polyunsaturated fatty acids for cardiovascular diseases: Present, past and future. Expert Review of Clinical Pharmacology. 2017;10:865–873. doi: 10.1080/17512433.2017.1333902. [DOI] [PubMed] [Google Scholar]
- Watkins P.J., Frank D., Singh T.K., Young O.A., Warner R.D. Sheepmeat flavor and the effect of different feeding systems: A review. Journal of Agricultural and Food Chemistry. 2013;61:3561–3579. doi: 10.1021/jf303768e. [DOI] [PubMed] [Google Scholar]
- Willems H., Kreuzer M., Leiber F. Alpha-linolenic and linoleic acid in meat and adipose tissue of grazing lambs differ among alpine pasture types with contrasting plant species and phenolic compound composition. Small Ruminant Research. 2014;116:153–164. [Google Scholar]
- Wood J., Enser M., Fisher A., Nute G., Sheard P., Richardson R. Fat deposition, fatty acid composition and meat quality: A review. Meat Science. 2008;78:343–358. doi: 10.1016/j.meatsci.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Woods V.B., Fearon A.M. Dietary sources of unsaturated fatty acids for animals and their transfer into meat, milk and eggs: A review. Livestock Science. 2009;126:1–20. [Google Scholar]
- Wroniak M., Rękas A., Siger A., Janowicz M. Microwave pretreatment effects on the changes in seeds microstructure, chemical composition and oxidative stability of rapeseed oil. LWT - Food Science and Technology. 2016;68:634–641. [Google Scholar]