Highlights
-
•
Many classes of antibiotic used for humans are also used in food animals, including the highest priority of the critically important antimicrobials for human medicine in the World Health Organisation's list.
-
•
Penicillins and Tetracyclines classes were the most commonly used antibiotics in many countries.
-
•
Improve understanding of the use of antibiotics and factors influencing antibiotic use will help promoting prudent use of antimicrobials in livestock.
Keywords: Antibiotic, Antimicrobial resistance, Antibiotic use, Pig, Systematic review
Abstract
This review assesses the evidence for patterns of antibiotic use in pig on the basis of papers published in peer-reviewed journals in English between 2000 and 2017. Thirty-six articles were identified and reviewed, of which more than 85% of studies were conducted in Europe and North America. Penicillins and Tetracyclines groups were the most commonly used antibiotics in many countries. Oral medication in suckling and post-weaning periods were the most common applications of antibiotic administration in pig production. Antibiotic use is driven by age-specific diseases and the common pathogens causing these conditions where epidemiological profiles varied greatly across countries. In addition, the type and size of farm were associated with antibiotic use with finisher and larger farms using more antibiotics than farrow-to-finish and smaller farms. There is variation in the use of the highest priority critically important antimicrobials in humans across studies. However, this review indicates that they are still commonly used in pig production, for treatment and prevention of infection. This evidence calls for global efforts on the prudent use of antibiotics in response to the emergence of antimicrobial resistance (AMR) in the agricultural sector.
1. Introduction
Antibiotics have been used routinely in farm animal production since the 1950s, in particular during intensive farming, in order to keep animals healthy and to increase productivity. The use of antibiotics in animals has raised concerns that the selective pressure on the bacteria population promotes antibiotic resistance. Despite the difficulties in demonstrating the transmission of resistant bacteria from animals to humans, many studies have shown evidence of human infection from resistant bacteria in animals (Liu et al., 2018, McCrackin et al., 2016, Nhung et al., 2016). The discovery of a plasmid-mediated colistin resistant gene (MCR-1) in commensal Escherichia coli from pigs, pork products and humans in China, triggered global concern (Liu et al., 2016). Colistin is considered a last resort antibiotic as it is one of the only antibiotics active in severe infections caused by hospital acquired multidrug-resistant (MDR) pathogens such as Pseudomonas aeruginosa, Acinetobacter baumannii and Enterobacteriaceae (Catry et al., 2015).
Antibiotics in the same class usually have a similar mode of therapeutic action, with a range of effectiveness. Many classes of antibiotic used for humans are also used in food animals. The World Health Organisation (WHO) produces a list of all antimicrobials grouped into 3 categories based on their importance in treating human infections. (World Health Organization, 2017). The classes of drugs included in the list of critically important antimicrobials (CIA) for human medicine contain the last-resort antibiotics to treat severe infections caused by multidrug-resistant (MDR). The CIA list of Highest Priority Critically Important Antimicrobial includes Quinolones, 3rd and higher generation Cephalosporins, Macrolides and Ketolides, Glycopeptides and Polymixins class which includes colistin (World Health Organization, 2017). This WHO CIA list is referred to in the rest of this report.
In animals, the use of antibiotics is common for not only treatment, but also for controlling the spread of infection (metaphylaxis), preventing infection (prophylaxis) particularly in periods of stress and vulnerability to infections, and improvement of feed efficiency and promotion of animal growth (Aarestrup, 2005). According to American Veterinary Medical Association, the term “therapeutic” includes treatment, control, and prevention of disease (Association American Veterinary Medical, 2019). The use of antibiotics as a growth promoter is considered non-therapeutic. Many countries including USA, Canada and Australia have implemented policies and regulations that medically important antimicrobials are prescription only medicines by licensed veterinarians (Australian Veterinary Association, Guideline for prescribing, authorising and dispensing veterinary medicines, 2005; Government of Canada 2018, US Food and Drug Administration 2011). The use of antibiotics for growth promotion has been banned in the European Union since 2006 (Regulation (EC) No 1831/2003 of the European Parliament and of the councel on additives for use in animal nutrition, 2003). In contrast, other countries – including China and Brazil which are the large livestock producing and exporting countries – do not prohibit the use of antibiotics for growth promotion (Maron, Smith, & Nachman, 2013).
Despite the concerns about the relationship between the use of antibiotics and AMR in food animals and AMR in humans, there are limited studies exploring the use of antibiotic in livestock and the factors that influence how farmers use them. To promote the prudent use of antibiotics in livestock, it is vital to have a better understanding of the current situation. This systematic review aims to analyse and synthesise the available published information on the pattern of antibiotic use in pigs.
2. Method
2.1. Scope of study and research question
This study focuses on antibiotics. Before conducting the systematic review, the terms and explanations to be included were considered as follows. According to World Organisation for Animal Health (OIE) definition, an antimicrobial is considered as a naturally occurring, semi-synthetic or synthetic substance that exhibits antimicrobial activity (to kill or inhibit the growth of micro-organisms) at concentrations attainable in vivo. Anti-helminthic and substances classed as disinfectants or antiseptics are excluded from this definition (World Organisation for Animal Health, 2011). In this study the word ‘pigs’ refers to all stages of swine production including breeding and gestation, farrowing, nursery and feeding and finishing. The word ‘pattern’ explains the use of antibiotics in terms of active ingredient; the route of administration such as injection or medicated feed; the purpose of the use including treatment, metaphylaxis, prophylaxis and growth promotion; and the frequency of the use by different farms and different stages of life cycle of pig production. The research questions in the review is: “What are the patterns of antibiotic use in terms of classes, routes of administration and purpose of the use and its associations with pig production?”.
2.2. Identifying relevant literature
The study applied the “SPIDER” tool, designed specifically to identify relevant quantitative studies (Cooke, Smith, & Booth, 2012). It covers the following: Sample: pig; Phenomenon of Interest: antibiotic use in pigs; Design: Observational studies; Evaluation: pattern of antibiotic use including active ingredient of antibiotic, route of administration, purpose of use including treatment, control, prevention and growth promotion; and Research: Quantitative research.
Literature on the use of antibiotics in pigs was systematically reviewed between July to October 2017. Relevant scientific papers published in English peer-reviewed journals were identified using the keyword combinations (antibiotic OR antimicrobial OR antibacterial) AND (livestock OR swine OR pig* OR farrow OR weaner OR finisher OR sow) AND (use OR utilisation OR consum* OR practice OR administration).
The online electronic database through LSHTM databases: MEDLINE (http://ovidsp.tx.ovid.com; 1946 until present), Scopus (http://www.scopus.com; 1823 until present) and Web of Science (http://apps.webofknowledge.com; 1970 until present) were searched with restriction of the date of publication between 2000 and 2017 to capture up-to-date data. To ensure a wide range of articles from different sources, additional searches were sourced through the reference lists of key articles.
2.3. Eligibility assessment of studies and inclusion criteria
Prior to a study being included within the review, the following criteria were considered: publication in English, and focus on antibiotic usage in pigs with high and moderate ranking of a quality assessment.
Citations of all identified studies were downloaded into a reference management software (EndNote X8.0.2). In the first screening step, the duplicated studies were removed, through consideration of the title and the abstract by comparison with the keywords. Full texts were further considered. Reviews, clinical research, pharmacokinetic, biopharmaceutical and experimental studies were excluded. In addition, studies focusing on antibiotic activity, specific diseases related to drug recommendations, associations of antibiotic use with antimicrobial resistance, relationship between interventions and antibiotic use, and effects of antibiotic treatment to AMR, animal productivity and animal management were excluded.
Studies included in the qualitative synthesis were those that presented the pattern of antimicrobial use, and medium (50–75%) and high-ranking quality assessment (>75%). If a study explored data over many periods of time, then the updated data was selected for the review.
2.4. Quality assessment
The Critical Appraisal Skills Programme (CASP) was applied for quality assessment) (Critical Appraisal Skills Programme, 2014). They were aggregated into a quality score based on four criteria: aim, method, result and application of the literature; Yes, No and Cannot tell are the assessment outcomes. With eleven questions, the score was categorised into three groups: weak means <50% having “yes” answers, moderate means 50–75% having “yes” answers, and high means >75% having “yes” answers (see Table B.1 of annex). If the assessment by the reviewer was ‘no’ or ‘cannot tell’, the score for that question was zero; the score for yes was one. In this review, the studies were ranked by quality criteria. The quality ranking was classified into three groups: High meant >75% of all eleven sub-criteria were met, moderate meant 50–75% were met and weak meant <50% of criteria were met.
2.5. Data extraction and synthesis
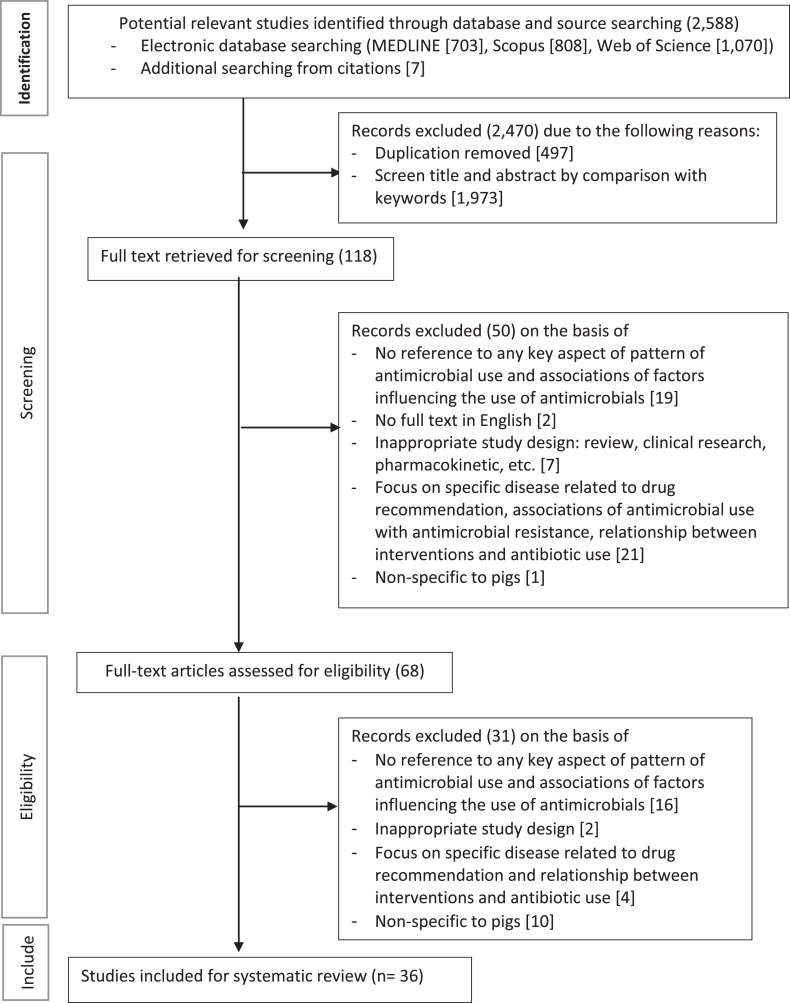
Fig. 1 shows the review process. All relevant articles in full texts were reviewed and summarised using a standardised data extraction table in an Excel spreadsheet.
Fig. 1.
Flow diagram of the screening process of the literature.
3. Results
3.1. Eligible studies
Our search strategies identified a total of 2588 articles (Appendix A). After duplicates were removed and an initial review of titles and abstracts for relevance was conducted and 118 articles were found to be eligible for full-text screening on the basis of the inclusion criteria. Sixty-eight studies were found to be relevant and retained. Further screening excluded 31 papers; of which 16 papers were not related to pattern and factors influencing antibiotic use; two papers had inappropriate study design; four papers focused on specific diseases using recommended antibiotics and relationship between interventions and antibiotic use; and ten papers were not related to pigs. Finally, 36 studies were included in this systematic review. Fig. 1 showed the flow diagram of the process in screening papers.
3.2. Study characteristics
As shown in Table 1, twenty-seven of studies (75%) were conducted between 2010 and 2017; the remaining 9 studies were conducted between 2000 and 2010 (25%). Most studies (72%) were conducted in Europe, with four studies in North America (11%), three in Asia (8%), and one each in Africa (3%) and Australia (3%). Diverse sources of data were used for the study such as farm surveys (39%), national databases (19%), farm-based survey and prescription data (14%), prescription data (8%), antibiotic application records (8%), veterinary survey (6%), pharmaceutical producer survey (3%) and farm-based survey and national data (3%). Among total studies reviewed, 9 studies (25%) were nationally representative. The result of the quality assessment of 36 studies showed that 21 (58%) and 15 (42%) of studies are of high and medium quality respectively (see Table B.1 in Annex).
Table 1.
Characteristics of the reviewed studies.
| Characteristics | N = 36 |
|---|---|
| 2010–2017 | 27 (75%) |
| 2000–2010 | 9 (25%) |
| Geographic area | |
| Europea | 27 (75%) |
| North Americab | 4 (11%) |
| Asiac | 3 (8%) |
| Africad | 1 (3%) |
| Australiae | 1 (3%) |
| Data source of antibiotics | |
| Farm based survey | 14 (39%) |
| National database (consumption/sale/prescription) | 7 (19%) |
| Farm based survey and prescription data | 5 (14%) |
| Prescription data | 3 (8%) |
| Antibiotic application records | 3 (8%) |
| Veterinarian survey | 2(6%) |
| Pharmaceutical producer survey | 1 (3%) |
| Farm based survey and national data | 1 (3%) |
| Quality assessment by authors | |
| High (>75%) | 21 (58%) |
| Moderate (50–74%) | 15 (42%) |
Europe: Denmark (n = 7), Germany (n = 6), Belgium (n = 5), France (n = 3), Netherlands (n = 3), Sweden (n = 3), Switzerland (n = 3), Austria (n = 2), Spain (n = 2), UK (n = 1).
North America: Canada (n = 3), USA (n = 1).
Asia: Vietnam (n = 2), Japan (n = 1).
Africa: Sudan (n = 1).
Australia: Australia (n = 1).
3.3. Patterns of antibiotic use in pigs
3.3.1. Patterns of antibiotic use
3.3.1.1. Classes and active ingredients of antibiotic
Some studies reported antibiotic use by active ingredient and others only by the class. In many studies the most common used antibiotic classes were the Penicillins and Tetracyclines. Benzylpenicillins consisted 61% of total use in a farm study in Sweden (Sjölund et al., 2016). Aminopenicillins were commonly reported accounting for 30–40% of total antibiotic use in studies from Sweden, Germany and Canada (Glass-kaastra et al., 2013, Sjölund et al., 2016, Van Rennings et al., 2015). Twelve studies reported that Tetracyclines class was the most commonly used including studies from Denmark, Japan, Netherlands, Australia, Spain and France (Bondt et al., 2013, Bos et al., 2013, Casal et al., 2007, Chauvin et al., 2002, Dupont et al., 2017, Hosoi et al., 2014, Jordan et al., 2009, Vieira et al., 2011), and was as high as 54.4% in a study from Germany (Merle et al., 2013). Within the Tetracyclines class, doxycycline was used 62.3% of total use in the study in Austria (Moreno, 2012). The share of chlortetracycline use was 23.9% in a farm study in Vietnam (Van Cuong et al., 2016), and formed the majority of antibiotics use in all pig stages in the United States (Apley, Bush, Morrison, Singer, & Snelson, 2012). In the farm study in Switzerland, the most common antibiotic class was the reductase inhibitors and combinations class” of drugs, specifically sulfadimidine, sulfathiazole and trimethoprim, accounting for 62.1% (Arnold, Gassner, Giger, & Zwahlen, 2004) while Bacitracin was the most reported of antibiotic use (24.8%) in the farm study in Vietnam (Van Cuong et al., 2016). Fattening farms in the study from Austria applied Lincosamides in 71.9% of antibiotic use (Trauffler, Griesbacher, Fuchs, & Köfer, 2014).
The use of highest priority Critically Important Antimicrobials in humans was also reported differently across countries. The studies from France and Austria reported the use of Macrolides at 20% and at 7.4% of total use (Chauvin et al., 2002; Trauffler, Obritzhauser, Raith, Fuchs, & Köfer, 2014). Based on the electronic drug application records from 75 pig farms in Austria, Fluoroquinolones were reported at 2.4% of total use, third and fourth generation Cephalosporins were 2.2% of total use (Trauffler et al., 2014), while the use of Fluoroquinolones at 5% and third generation Cephalosporins at 11% were reported from 47 pig farms in the study in Belgium (Sjölund et al., 2016). The study in 60 French pig herds received colistin in 30% of total antibiotic use (Sjölund et al., 2016), 12.2% in the study in Vietnam using the internet-based survey of commercial feed producer (Van Cuong et al., 2016), 33% and 61% in the survey in 45 farrow-to-finish farms and 67 fattening farms in Spain (Moreno, 2012) and 34.4% in fattening farms in 75 pig farms in Austria (Trauffler et al., 2014).
3.3.1.2. Routes of administration
Generally, oral medication was the most common route of antibiotic administration in pig production. Several studies reported more than 90% of antibiotic substances were administered orally via both feed and water (Chauvin et al., 2002, Merle et al., 2012, Rajić et al., 2006, Van Rennings et al., 2015). About 70–90% of the oral use was reported in many countries, for example 87% in the study from France (Sjölund et al., 2016), 86% in the study from Austria (Trauffler et al., 2014), 73% in the study from Denmark (Dupont et al., 2017), 71% in the study from Germany (Sjölund et al., 2016) and 70% in the study from Belgium (Sjölund et al., 2016). In the UK farm study, 60–75% of the farmers had used medicated feeds for their weaners (Stevens et al., 2007). Another study indicated that oral use of antibiotics was higher than parenteral indication (97.43% VS 2.46%) (Merle et al., 2013).This is in contrast to another study that farmers applied very low levels of oral antibiotics, 13% of all routes (Sjölund et al., 2016).
A wide range of active ingredients was commonly used for oral medication, including: colistin (Filippitzi et al., 2014, Moreno, 2012, Timmerman et al., 2006), amoxicillin(Filippitzi et al., 2014, Timmerman et al., 2006), sulfonamides (Bondt et al., 2013, Timmerman et al., 2006), oxycycline (Bondt et al., 2013), doxycycline (Moreno, 2012, Timmerman et al., 2006), chlortetracycline, lincomycin,tiamulin, tylosin, and penicillin G (in water) (Rosengren et al., 2008). However, ceftiofur (Filippitzi et al., 2014, Timmerman et al., 2006), enrofloxacin (Moreno, 2012), amoxycillin (Moreno, 2012, Timmerman et al., 2006), penicillin (Moreno, 2012, Rosengren et al., 2008) and tulztromycin (Filippitzi et al., 2014) were commonly used for parenteral medication.
The oral administration of antibiotics (either through feed or water) was commonly used for group treatment, while injection was the commonly applied for treatment of individual sick animals (Sjölund et al., 2015, Trauffler et al., 2014). A study showed that 90% of group treatment was administered between birth and ten weeks of age; while only 20% of group treatment was administered during the fattening period (Callens et al., 2012). Group treatments were primarily administered via oral medication in weaners and via parenteral route for individual sucking piglets (Filippitzi et al., 2014), particularly after castration or when diarrhoea occurred (Timmerman et al., 2006). In one study reported ninety-four percent of group treatment at farm for a respiratory infection (prior to a definitive diagnosis) was carried out with tetracycline, beta-lactams and sulphonamides while 90% of group treatment at farm for enteric disease used colistin (Casal et al., 2007).
3.3.1.3. Indications: treatment, metaphylaxis, prophylaxis and growth promotion
Few studies in this review reported the indication for antibiotics use. A vast majority, 93% of total antibiotics administered were for prophylaxis, whereas metaphylaxis or treatments were much smaller at 7% of total antibiotics in Belgium (Callens et al., 2012, Filippitzi et al., 2014). Main therapy indications in farrow-to-finish and fattening farms were metaphylactic/prophylactic measures (Trauffler et al., 2014). Chlrotetracycline and carbadox were the most commonly used antibiotics for growth promotion, prevention and treatment of infectious diseases and tiamulin was commonly used for prevention and treatment of infectious diseases (Apley et al., 2012).
Only few studies reported the use of antibiotic by distinguishing between metaphylaxis and prophylaxis (Callens et al., 2012, Filippitzi et al., 2014), which ‘prophylactic use’ means treatment of healthy pigs to prevent disease from occurring and ‘metaphylactic use’ means treatment of clinically healthy pigs in the same group where some animals had showed clinical symptoms of disease. Based on American Veterinary Medical Association, both ‘prophylaxis’ and ‘metaphylaxis’ means therapeutic (Association American Veterinary Medical, 2019) and commonly described as “preventative use” as a general term. However, both terms are not applied in certain situations such as the use of antibiotics within a group of animals without definite diagnosis.
3.3.2. Association between the use of antibiotics and pig production
3.3.2.4. Phase of pig production
Six studies examined the association of antibiotic use and the phase of pig production. Antibiotics were commonly used during suckling and post-weaning periods. One study reported more than 80% of antibiotics were applied to pigs at less than ten weeks of age (Callens et al., 2012). Four studies reported that weaners received the most antibiotics (Chauvin et al., 2002, Fertner et al., 2015, Jensen et al., 2012, Sjölund et al., 2016). However, another study showed that treatment of suckling piglets was more common than weaners (Sjölund et al., 2016), and similar findings were reported in two studies (Merle et al., 2013, Van Rennings et al., 2015).
Based on the cross-sectional study conducted among 227 farrow-to-finish pig herds in four European countries, there was a significant association between antibiotic use across different age categories. The lowest use of antibiotics among fatteners reported in France and Sweden, while the least use in breeders reported in Belgium and Germany (Sjölund et al., 2016). Similarly, the studies from Denmark and France reported the least application of antibiotics in sows, with about 26% and 17% of total use in all phases respectively (Chauvin et al., 2002, Jensen et al., 2012). Another study using veterinary prescription data reported almost zero use of antibiotic among the finishers in Denmark (Fertner et al., 2015). However, one study from Belgium reported that the use of antibiotics was higher in the farrowing period than fattening period (Callens et al., 2012). Another study showed that eight veterinarians in Saskatchewan and Alberta applied more than 90% of the antibiotics for treatment of disease in sows, compared to less than 20% in other phases (Rosengren et al., 2008). However, the farm study in Vietnam reported there was no significant difference in antibiotics use for prevention across three age groups (piglets, fatteners and sows) (Kim et al., 2013).
The class and active ingredient of antibiotic also varies by different phases of pig production (Table 2). Aminopenicillin, tetracyclines, trimethoprim-sulfonamides, tylosin and colistin were commonly used in all studies (5 studies each). Chlortetracycline (4 and 3 studies), oxytetracycline (3 and 4 studies), tylosin (5 studies) and lincosamide (4 studies) were commonly used in weaners and finishers. Colistin in Polymyxin class was used in weaners in five studies, while aminoglycosides were mostly used in finishers (4 studies).
Table 2.
Number of studies reporting data sources of active by geographical areas.
One study reported the route of administration in relation to the phase of production. Weaners and finishers were more likely to receive oral antibiotics while sows and piglets received parenteral administration (Jensen et al., 2012).
There are variations in the indication for antibiotic use by phases of pig production (Table 3). For example, more than half of pig farms (58%) used oral antibiotics as a routine prophylaxis for fatteners (Casal et al., 2007); medicated feeds are used mostly as growth promoters for weaners (Stevens et al., 2007); and there was less use of antibiotic as a growth promoter and therapeutic use in sows than in piglets; but it was equally used for piglets and fattening pigs (Kim et al., 2013).
Table A.1.
Search terminology to be used in literature review.
| Search term | |
|---|---|
| I | Antimicrobial (Free text) OR antimicrobial (MeSH term) OR antibacterial (Free text) OR antibacterial (MeSH term) OR antibiotic (Free text) OR antibiotic (MeSH term) |
| II | Livestock (Free text) OR swine (Free text) OR pig* (Free text) OR farrow (Free text) OR weaner (Free text) OR finisher (Free text) OR sow (Free text) |
| III | Use (Free text) OR utilisation (Free text) OR consum* (Free text) OR practice (Free text) OR administration (Free text) |
Table 3.
Number of studies reporting use of antibiotic class and active ingredient, by phase of pig production.
3.3.2.5. Diseases in pigs
Five studies reported the use of antibiotics by type of diseases in different geographical areas. A farm study in Denmark, showed herds received more frequent use of antibiotics for gastrointestinal infections (74–83% of total indication in weaners and 56–65% in finishers), 9–24% for respiratory indication, and 15–30% for treatment of locomotor and central nervous systems conditions, skin and urinary tract infections (Jensen et al., 2012). The farm study in Canada showed that 27% of antibiotic treatment reported by ten veterinarians was for multiple systems infection (Glass-kaastra et al., 2013). Base on 303 French pig veterinarians survey, 10% of antibiotics are used for treatment of diseases and conditions such as cough, porcine proliferative enteropathy and post-weaning Escherichia coli (Chauvin et al., 2002).
In all age-groups, the most commonly-used antibiotic classes for the treatment of gastrointestinal infections were tetracyclines, lincosamides, Pleuromutilins (Jensen et al., 2012) and Macrolides (Jensen et al., 2012, Trauffler et al., 2014), while the most common use for the treatment of respiratory infections were chlortetracycline, tetracycline and amoxicillin (Van Rennings et al., 2015). Considering the phase of pig, the most commonly used was colistin in piglets and weaners, and tylosin in fatteners for gastrointestinal conditions (Van Rennings et al., 2015). Pleuromutilins were commonly used for respiratory tract infections in sow and piglets (Jensen et al., 2012). Use of antibiotics for gastrointestinal infection in breeding farms was also common (Trauffler et al., 2014).
3.3.2.6. Farm characteristic and management
Six studies reported a relationship between antibiotic use and type of farm. Overall, finisher farms were more likely to use antibiotics than farrow-to-finish farms (84–94% versus 43%–92% respectively) (Merle et al., 2012), (90% versus 54.3% respectively) (Moreno, 2012). (van der Fels-Klerx, Puister-Jansen, van Asselt, & Burgers, 2011). Sow farms used fewer antibiotics than farrow-to-finish farms (van der Fels-Klerx et al., 2011). Moreover, finisher farms had the highest use (14.91%) of the highest priority and critically important antimicrobials, while it was 7.83% in breeding farms and 12.54% in farrow-to-finish farms (Trauffler et al., 2014). In finishing farms, fattening units were more likely to use a routine antimicrobial prophylaxis than farrow-to-finish farms (OR = 11.7, 95CI: 4.1 − 33.3) and use antibiotics for growth promotion (OR = 2.8, 11.7, 95CI:1.2 − 6.9) (Casal et al., 2007); about a half (46%) and one third (30%) of antibiotics were applied for metaphylatic and prophylactic purposes in farrow-to-finish and fattening farms (Trauffler et al., 2014).
Pig density had a positive association with the use of antibiotics (Bos et al., 2013, Stevens et al., 2007). The number of sows presented on the farm has a positive correlation with the amount of antibiotic use (van der Fels-Klerx et al., 2011). Different findings in few studies showed that small herd size had significantly higher antibiotic use than moderate and large herd size (Vieira et al., 2011); and the number of pigs in farms had no association with the use of antibiotics (Casal et al., 2007) and the use of growth promoters (Stevens et al., 2007). One study reported that industrial production system had higher antibiotic use than a semi-industrial production system and small farm holders (Kim et al., 2013).
There was only one study that documented the association between vaccination and antibiotic use. The vaccination of suckling piglets and weaners was significantly associated with the greater use of in-feed antibiotics (Stevens et al., 2007). In terms of farm management, one study found that weaner production in indoor pig farming systems had higher use of antibiotics in medicated feed (64–74%) than the outdoor farming (60%); it is noted that UK is the only country that raise commercial sow outdoor(Stevens et al., 2007). One study showed that improved farm sanitation and management contributes to a reduction in antibiotic consumption without productivity losses (Fertner et al., 2015); however, another study reported that the use of antibiotics had no association with farm management (Casal et al., 2007).
3.3.2.7. Other factors
Other factors also contribute to the use of antibiotics. The volume of tetracycline used in the spring was five-fold higher than other seasons (Van Rennings et al., 2015). In the study from the UK, there was a large variations of in-feed antibiotics in weaners and growers, and in individual weaners in different pork quality assurance schemes (Stevens et al., 2007). In term of farm location, farms located in high pig-density areas have a positive correlation with the amount of antibiotic use (van der Fels-Klerx et al., 2011).
Only one study examined the educational status of farmers, and there was a significant association between low education and poor knowledge on antibiotic use (Eltayb, Barakat, Marrone, Shaddad, & Sta, 2012). Farmers perceived that use of antibiotic contributes to their profitability from raising pigs (Stevens et al., 2007).
4. Discussion
Understanding the current pattern of antibiotic use in livestock is important in order to support optimal antibiotic use, which may potentially slow down the emergence of AMR in animal production. Studies on antibiotic use have increased considerably over the last decade, and in this review, the majority of studies were conducted between 2010 and 2017. Most of the studies were conducted in Europe, particularly in high-income countries (HICs) where there are higher research capacities and data availability. Due to the population size, demand for animal-source food is higher among low- and middle-income countries (LMICs) than in HICs (Robinson & Pozzi, 2011). More evidence on consumption of antibiotics in LMICs is required for proper and timely responses to AMR such as optimizing consumptions and uses of antibiotics.
4.1. Pattern of antibiotic use
Common classes of antibiotics used varied across countries. Overall, Penicillins and Tetracyclines class were the most commonly used antibiotic in pigs. These findings were similar to another review which reported Penicillins, Tetracyclines and Macrolides were the most common use in pig production (Cuong, Padungtod, Thwaites, & Carrique-Mas, 2018). This was probably because they are relatively cheap and cost-effective compared to other antibiotics (“OIE LIST OF ANTIMICROBIALS OF VETERINARY IMPORTANCE Criteria used for categorisation List of antimicrobials,” 2007). Penicillins have bactericidal actions against Gram-negative and Gram-positive pathogens (Lobanovska & Pilla, 2017). They were commonly used for prophylaxis and treatment of septicaemia, respiratory and urinary tract infections in a broad range of animal species. Tetracyclines were widely used for the treatment of respiratory diseases caused by Actinobacillus pleuropneumonia and Pasteurella multocida; however, resistance to tetracyclines is common. For example, 22% of Pasteurella multocida, 15% of Actinobacillus pleuropneumoniae and 82% of Streptococcus suis were reported to be resistant to tetracyclines (de Jong et al., 2014).
This review confirms that antibiotics of veterinary importance defined in the highest priority critically important antimicrobials in human in WHO's list (World Health Organization, 2017), were still commonly used in swine production.
The use of antibiotics without definitive diagnosis and proper indications has raised global concern, especially with the emergence of AMR in the agricultural sector. The first attempt to withdraw non-therapeutic antibiotics was in the UK in 1969 when the Swann Joint Committee suggested restricting the use of medicated feed at a sub-therapeutic level in livestock (Swann, Baxter, & Field, 1969). Many countries have banned antibiotic use for growth promotion. However, this review shows that the use of antibiotics for infection prevention is still globally common in pig production, in order to prevent production loss in particular in intensive industrial farming. The standard prophylactic protocol for the whole herd can be more convenient to administer and less labour-intensive to manage than treatment of individual sick animals.
4.2. Antibiotics choice and route of administration associated with specific diseases and age groups
Choices of antibiotics were driven by age-specific diseases and the common pathogens for these conditions. Gastrointestinal and respiratory tract infections are common in pigs at all stages and are easily transmitted within and between herds. However, some specific diseases are more common in weaners such as septicaemia caused by Actinobacillus suis and Mycoplasma infection than others. In finishers, diarrhoea, porcine haemorrhagic enteropathy (Lawsonia intracellulalis) and swine dysentery (Bachyspira hyodysentary) are common pathogens causing gastrointestinal infection, whereas enzootic pneumonia (Mycoplama hyopnuemoniae), mycoplasma induced respiratory disease (Pasturella multocida), pleuropnuemonia (Actinobacillus pleuropnueumoniae) are common pathogens causing respiratory infection (Burch, 2013). Type of bacteria in animal are drivers for type of antibiotic use (Jordan et al., 2009).
Colistin was most commonly used for gastrointestinal conditions in piglets and weaners, tylosin in fatteners and sows (Van Rennings et al., 2015). Farmers used Pleuromutilins for respiratory tract infections in sow and piglets (Jensen et al., 2012) and beta-lactam antibiotics in piglets, weaners and fattening pigs (Van Rennings et al., 2015). However, the choice of antibiotics depends on market availability and cost in different countries. For example, while the most common respiratory pathogens in Danish swine production, Actinobacillus pleuropneumoniae, Pasturella multocida, and Streptococcus suis infections are fully susceptible to penicillin (Aarestrup, Oliver Duran, & Burch, 2008), penicillin only constitutes a minor share of the prescriptions for respiratory disease, whereas Tetracyclines and Pleuromutilins are widely used. Possibly they have relatively lower costs; aminopenicillins are more expensive and parenteral use of benzylpenicillins is less convenient in administration (Jensen et al., 2012).
Choices of antibiotic are also guided by route of administration. Oral application is a major route in weaners and fatteners, whereas parenteral is applied more in sows than piglets and fatteners, such as through the use of benzylpenicillin (Merle et al., 2014). In finishers, however, parenteral benzylpenicillins are applied to individual treatment of sick pigs, although other drugs such as tylosin and lincomycin are mainly administered through feed (Rajić et al., 2006, Rosengren et al., 2008).
4.3. The use of antibiotic associated with farm management
Farm management can be associated with antibiotic use, such as the type of farm, size of farm and vaccination status. This review shows that finisher farms used higher volumes of antibiotics than farrow-to-finish farms (24,30), in particular for metaphylactic and prophylactic measures, and growth promotion (Casal et al., 2007, Trauffler et al., 2014).
Large farms were more likely to use medicated feeds compared to smaller-sized farms (Bos et al., 2013, Kim et al., 2013, Stevens et al., 2007, van der Fels-Klerx et al., 2011). One possible explanation of the high use of antibiotics is that larger-sized farms have a greater risk of transmission of pathogens within herds than smaller farms, although this review is inconclusive as contradictory findings were reported (Vieira et al., 2011). However, there are multiple factors which influence the use of antibiotics, such as farm biosecurity practices, density, level of stress in the herd, vaccination status, quality of feed, farmer knowledge and disease prevalence rates. Good farm biosecurity such as all-in all-out production and a single supplier of weaners has been identified as common practice in herds which lead to a reduction in disease transmissions and lower antibiotic use (Fertner et al., 2015) . Vaccination has been recommended as an alternative strategy to prevent disease contributing to optimizing antibiotic usage (Postma et al., 2015). It has shown beneficial return of investment despite the costs for vaccines (Alarcon, Rushton, Nathues, & Wieland, 2013). However, the higher use of in-feed antibiotics was significantly associated with the vaccination of pigs in some age groups including suckling piglets, weaners and sows (Stevens et al., 2007). This finding can be confounded by other factors such as poor bio-security and farm management, low health status of the herd and high disease prevalence.
4.4. The use of antibiotic associated with other factors
The use of antibiotics was highly dependent on the farm management and person in charge of the daily routines. The initiation of treatment depended on the ability of early detection of diseased animals and the level of farmers’ perceptions and responses to the clinical signs in animals (Fertner et al., 2015). Farmers were likely to have a limited understanding of antibiotics, particularly those in low- and middle-income countries. One study in Cambodia indicated that none of the farmers demonstrated an understanding about the action and indication for antibiotics (Om & Mclaws, 2016). A study in Sudan found a significant association between farmers’ low education level and poor knowledge on antibiotic use and AMR awareness (Eltayb et al., 2012). All these challenges can lead to inappropriate use of antibiotics.
Veterinarians play important roles in animal health and antibiotic stewardship; and often farmers rely on veterinarian's advice on pig health, choices and use of antibiotics (Visschers et al., 2015). Despite their critical role, veterinarians’ prescription decisions are based on “expert opinion” or views from “opinion leaders” or from internet sources, rather than scientific and peer-reviewed data (Vandeweerd et al., 2012) or laboratory resistant profiles. Representatives from pharmaceutical companies, when serving as advisors to farmers on disease management, may have conflict of interests to offer their products. In Belgium, on average, 43% of the income among pig veterinarians came from selling pharmaceutical products (Maes et al., 2010) for which prudent use of antibiotics can be at risk due to potential conflict of interests.
The prevalence of pathogens in pigs and levels of resistance and susceptibility to different antibiotics is an important evidence to guide antibiotic selection and support prudent use. Despite critical contributions, nearly half of all veterinarians in a study in 25 European countries (44.3%) seldom collect a sample for bacterial identification and drug sensitivity tests in laboratory (De Briyne, Atkinson, Pokludová, Borriello, & Price, 2013). In addition, law and enforcement and availability of antibiotics influences the use of antibiotics. Sweden and Denmark's law restricts the use of fluoroquinolones and third and fourth generation Cephalosporins in pigs (Guidelines for the prudent use of antimicrobials in veterinary medicine. Practical examples., 2015).
4.5. Limitations of the review
There are a number of limitations which need to be considered in interpreting the findings from this review. This review covers 36 studies published in English; where studies in non-English speaking countries may offer different or similar findings. More than 85% of the studies reviewed were conducted of in Europe and North America, limiting the relevance of the findings to LMIC. Diversity in study design is a major challenge for an in-depth comparative analysis and synthesis; a few studies could be considered nationally representative, while others were small scale studies. These challenges require careful interpretation. Different data collection methods including face-to-face interviews with farmers, internet-based surveys, and mail surveys to pig farmers or veterinarians may affect the validity of the findings. Use of antibiotics based on survey questionnaires cannot detect the misuse and off-label use, where other approaches are needed such as prescription reviews. As noted in our recent review there is also huge variability in how studies have measured the quantity of antibiotic use making it very difficult to make any comparisons. Some studies measure the use of antibiotics as a percentage of total use, while other studies calculated in specific units such as animal daily dose. Some studies reported the use by class of antibiotic, while other studies reported active ingredient of antibiotic (Lekagul, Tangcharoensathien, & Yeung, 2018). The EU is developing a standard unit for antibiotic measurement, called defined daily dose for animal (DDDvets) of active ingredient which take into account differences in dosing, pharmaceutical forms and routes of administration used (European Medicines Agency, 2015).
5. Conflict of interest statement
None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
Acknowledgements
The authors wish to thank Professor Anne Mills and Professor Jonathan Rushton for providing guidance and support. We also thank Alison Dunn for their support in English proof reading.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.vas.2019.100058.
Appendix A
I. Search Strategy
Structured Database Search (Search terms and results)
-
○MEDLINE: N = 703 articles
-
○
-
-
(Antibiotic.mp. or exp Anti-Bacterial Agents) (704,921)
-
-
(Antimicrobial agents.mp. or Anti-Infective Agents) (61,091)
-
-
(livestock or swine or pig* or farrow or weaner or sow).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] (519,570)
-
-(Use* or usage or consume or consumption or practiceor or administration or oral or feed or injection).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] (6635,975)
-
○Scopus: N = 808 articles
-
○
(TITLE-ABS-KEY (antibiotic OR antimicrobial OR antibacterial) AND TITLE-ABS-KEY (livestock OR swine OR pig* OR farrow OR weaner OR finisher OR sow) AND TITLE-ABS-KEY (use OR utilisation OR consum* OR practice OR administration)) AND (LIMIT-TO (PUBYEAR, 2017) OR LIMIT-TO (PUBYEAR, 2016) OR LIMIT-TO (PUBYEAR, 2015) OR LIMIT-TO (PUBYEAR, 2014) OR LIMIT-TO (PUBYEAR, 2013) OR LIMIT-TO (PUBYEAR, 2012) OR LIMIT-TO (PUBYEAR, 2011) OR LIMIT-TO (PUBYEAR, 2010) OR LIMIT-TO (PUBYEAR, 2009) OR LIMIT-TO (PUBYEAR, 2008) OR LIMIT-TO (PUBYEAR, 2007) OR LIMIT-TO (PUBYEAR, 2006) OR LIMIT-TO (PUBYEAR, 2005) OR LIMIT-TO (PUBYEAR, 2004) OR LIMIT-TO (PUBYEAR, 2003) OR LIMIT-TO (PUBYEAR, 2002) OR LIMIT-TO (PUBYEAR, 2001) OR LIMIT-TO (PUBYEAR, 2000)) AND (LIMIT-TO (DOCTYPE, "ar") OR LIMIT-TO (DOCTYPE, "sh")) AND (LIMIT-TO (SUBJAREA, "AGRI") OR LIMIT-TO (SUBJAREA, "VETE")) AND (LIMIT-TO (LANGUAGE, "English")) AND (LIMIT-TO (SRCTYPE, "j"))
-
○Web of Science: N = 1070 articles
-
○
TOPIC: (antibiotic or antimicrobial or antibacterial) AND TOPIC: (livestock or swine or pig or farrow or weaner or finisher or sow) AND TOPIC: (use or utilisation or consum* or practice or administration)
Refined by: PUBLICATION YEARS: (2016 OR 2006 OR 2015 OR 2005 OR 2014 OR 2004 OR 2012 OR 2002 OR 2013 OR 2003 OR 2017 OR 2000 OR 2011 OR 2001 OR 2009 OR 2010 OR 2007 OR 2008) AND WEB OF SCIENCE CATEGORIES: (VETERINARY SCIENCES) AND DOCUMENT TYPES: (ARTICLE)
Indexes=SCI-EXPANDED, SSCI, A&HCI, CPCI-S, CPCI-SSH, ESCI Timespan=1970–2018
Appendix B
.
Table B.1.
Quality assessment of included studies.
| Author, year | Q1 | Method | Result | Application | Rank* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | |||
| 2017 | ||||||||||||
| Dupont et al. (2017) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | H |
| Jensen, Jorsal, and Toft (2017) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | H |
| 2016 | ||||||||||||
| Van Cuong et al. (2016) | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | H |
| Sjölund et al. (2016) | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | H |
| 2015 | ||||||||||||
| Fertner et al. (2015) | Y | CT | N | N | Y | N | Y | Y | Y | Y | Y | M |
| Van Rennings et al. (2015) | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | H |
| Visschers et al. (2015) | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | H |
| (Sjölund et al.( 2015) | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | H |
| 2014 | ||||||||||||
| Filippitzi et al. (2014) | Y | Y | CT | N | CT | CT | Y | Y | Y | Y | Y | M |
| Hosoi et al. (2014) | Y | Y | CT | N | N | N | Y | N | Y | Y | Y | M |
| Merle et al. (2014) | Y | Y | N | N | Y | N | Y | Y | Y | Y | Y | M |
| Trauffler et al. (2014) | Y | Y | N | N | Y | N | Y | Y | Y | Y | Y | M |
| Trauffler et al. (2014) | Y | Y | N | N | Y | N | Y | Y | Y | Y | Y | M |
| Visschers et al. (2014) | Y | Y | CT | Y | Y | Y | Y | Y | Y | Y | Y | H |
| 2013 | ||||||||||||
| Bondt et al. (2013) | Y | Y | Y | CT | CT | Y | Y | Y | Y | Y | Y | H |
| Bos et al. (2013) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | H |
| Glass-kaastra et al. (2013) | Y | Y | N | N | Y | CT | Y | Y | Y | Y | Y | M |
| Kim et al. (2013) | Y | Y | N | N | Y | CT | Y | Y | Y | Y | Y | M |
| Merle, et al. (Merle et al., 2013) | Y | Y | N | N | Y | N | Y | Y | Y | Y | Y | M |
| 2012 | ||||||||||||
| Apley et al. (2012) | Y | Y | Y | N | Y | Y | Y | N | Y | Y | Y | H |
| Callens et al. (2012) | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | H |
| Eltayb et al. (2012) | Y | Y | CT | N | Y | CT | Y | N | Y | Y | Y | M |
| Merle et al. (2012) | Y | Y | N | N | Y | N | Y | Y | Y | Y | Y | M |
| Moreno (2012) | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | H |
| 2011 | ||||||||||||
| JJensen et al. (2012) | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | H |
| van der Fels-Klerx et al. (2011) | Y | Y | Y | N | Y | Y | N | Y | N | Y | Y | M |
| Vieira et al. (2011) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | H |
| Aarestrup, Vibeke, Jacobsen, and Wegener (2010) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | H |
| 2009 | ||||||||||||
| Jordan et al. (2009) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | H |
| 2008 | ||||||||||||
| Rosengren et al. (2008) | Y | CT | N | N | Y | N | Y | Y | Y | Y | Y | M |
| 2007 | ||||||||||||
| Casal et al. (2007) | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | M |
| Stevens et al. (2007) | Y | Y | N | N | Y | N | Y | Y | Y | Y | Y | M |
| 2006 | ||||||||||||
| Rajić et al. (2006) | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | M |
| Timmerman et al. (2006) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | H |
| 2004 | ||||||||||||
| Arnold et al. (2004) | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | H |
| 2002 | ||||||||||||
| Chauvin et al. (2002) | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | H |
Note:
Q1 = Did the study address a clearly focused issue?.
Q2 = Did the authors use an appropriate method to answer their question?.
Q3 = Were the subjects recruited in an acceptable way?.
Q4 = Were the measures accurately measured to reduce bias?.
Q5 = Were the data collected in a way that addressed the research issue?.
Q6 = Did the study have enough participants to minimize the play of chance?.
Q7 = How are the results presented and what is the main result?.
Q8 = Was the data analysis sufficiently rigorous?.
Q9 = Is there a clear statement of findings?.
Q10 = Can the results be applied to the local population?.
Q11 = How valuable is the research?.
Y = Yes (clearly described).
N = No (Not described).
CT = Cannot tell (described but with limited detail.
Score >75 = high (H), 50–74 = medium (M) and <50 = low (L) *score >75 = high (H), 50–74 = medium (M) and <50 = low (L).
Appendix C. Supplementary materials
References
- Aarestrup F.M., Jensen V.F., Emborg H.D., Jacobsen E., Wegener H.C. Changes in the use of antimicrobials and the effects on productivity of swine farms in Denmark. American Journal of Veterinary Research. 2010;71(7):726–733. doi: 10.2460/ajvr.71.7.726. [DOI] [PubMed] [Google Scholar]
- Aarestrup F.M. Veterinary drug usage and antimicrobial resistance in bacteria of animal origin. Basic and Clinical Pharmacology and Toxicology. 2005;96(4):271–281. doi: 10.1111/j.1742-7843.2005.pto960401.x. [DOI] [PubMed] [Google Scholar]
- Aarestrup F.M., Oliver Duran C., Burch D.G.S. Antimicrobial resistance in swine production. Animal Health Research Reviews. 2008;9(02):135–148. doi: 10.1017/S1466252308001503. [DOI] [PubMed] [Google Scholar]
- Alarcon P., Rushton J., Nathues H., Wieland B. Economic efficiency analysis of different strategies to control post-weaning multi-systemic wasting syndrome and porcine circovirus type 2 subclinical infection in 3-weekly batch system farms. Preventive Veterinary Medicine. 2013;110(110):103–118. doi: 10.1016/j.prevetmed.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apley M.D., Bush E.J., Morrison R.B., Singer R.S., Snelson H. Use estimates of in-feed antimicrobials in swine production in the United States. Foodborne Pathogens and Disease. 2012;9(3):272–279. doi: 10.1089/fpd.2011.0983. [DOI] [PubMed] [Google Scholar]
- Arnold S., Gassner B., Giger T., Zwahlen R. Banning antimicrobial growth promoters in feedstuffs does not result in increased therapeutic use of antibiotics in medicated feed in pig farming. Pharmacoepidemiology and Drug Safety. 2004;13(5):323–331. doi: 10.1002/pds.874. [DOI] [PubMed] [Google Scholar]
- Association American Veterinary Medical. (2019). American Veterinary Medical Association, therapeutic means treatment, control, and prevention of disease. Retrieved February 1, 2019, from https://www.avma.org/KB/Policies/Pages/AVMA-Definitions-of-Antimicrobial-Use-for-Treatment-Control-and-Prevention.aspx.
- Australian Veterinary Association, Guideline for prescribing, authorising and dispensing veterinary medicines, 2005, Retrieved from https://www.ava.com.au/sites/default/files/documents/Other/Guidelines_for_prescribing_authorising_and_dispensing_veterinary_medicines.pdf.
- Bondt N., Jensen V.F., Puister-Jansen L.F., van Geijlswijk I.M. Comparing antimicrobial exposure based on sales data. Preventive Veterinary Medicine. 2013;108(1):10–20. doi: 10.1016/j.prevetmed.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Bos M.E.H., Taverne F.J., van Geijlswijk I.M., Mouton J.W., Mevius D.J., Heederik D.J.J. Consumption of Antimicrobials in Pigs, Veal Calves, and Broilers in the Netherlands: Quantitative results of nationwide collection of data in 2011. PLoS ONE. 2013;8(10) doi: 10.1371/journal.pone.0077525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch D.G.S. 5th. Wiley Blackwell. Iowa State University Press; Iowa: 2013. Antimicrobial drug use in swine: In antimicrobial therapy in veterinary medicine. [Google Scholar]
- Callens B., Persoons D., Maes D., Laanen M., Postma M., Boyen F. Prophylactic and metaphylactic antimicrobial use in Belgian fattening pig herds. Preventive Veterinary Medicine. 2012;106(1):53–62. doi: 10.1016/j.prevetmed.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Casal J., Mateu E., Mejía W., Martín M. Factors associated with routine mass antimicrobial usage in fattening pig units in a high pig-density area. Veterinary Research. 2007;38(3):481–492. doi: 10.1051/vetres:2007010. [DOI] [PubMed] [Google Scholar]
- Catry B., Cavaleri M., Baptiste K., Grave K., Grein K., Holm A. Use of colistin-containing products within the European Union and European Economic Area (EU/EEA): Development of resistance in animals and possible impact on human and animal health. International Journal of Antimicrobial Agents. 2015;46(3):297–306. doi: 10.1016/j.ijantimicag.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Chauvin C., Beloeil P.A., Orand J.P., Sanders P., Madec F. A survey of group-level antibiotic prescriptions in pig production in France. Preventive Veterinary Medicine. 2002;55(2):109–120. doi: 10.1016/S0167-5877(02)00091-0. [DOI] [PubMed] [Google Scholar]
- Cooke A., Smith D., Booth A. Beyond PICO. Qualitative Health Research. 2012;22(10):1435–1443. doi: 10.1177/1049732312452938. [DOI] [PubMed] [Google Scholar]
- Critical Appraisal Skills Programme. 2014. CASP appraisal checklists. Retrieved March 1, 2019., from https://casp-uk.net/casp-tools-checklists/.
- Cuong N.V., Padungtod P., Thwaites G., Carrique-Mas J.J. Antimicrobial usage in animal production: a review of the literature with a focus on low- and middle-income countries. Antibiotics (Basel, Switzerland) 2018;7(3) doi: 10.3390/antibiotics7030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Briyne, N., Atkinson, J., Pokludová, L., Borriello, S. P., & Price, S. (2013). Factors influencing antibiotic prescribing habits and use of sensitivity testing amongst veterinarians in Europe. 10.1136/vr.101454. [DOI] [PMC free article] [PubMed]
- de Jong A., Thomas V., Simjee S., Moyaert H., El Garch F., Maher K. Antimicrobial susceptibility monitoring of respiratory tract pathogens isolated from diseased cattle and pigs across Europe: The VetPath study. Veterinary Microbiology. 2014;172(1–2):202–215. doi: 10.1016/J.VETMIC.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Dupont N., Diness L.H., Fertner M., Kristensen C.S., Stege H. Antimicrobial reduction measures applied in Danish pig herds following the introduction of the “Yellow Card” antimicrobial scheme. Preventive Veterinary Medicine. 2017;138:9–16. doi: 10.1016/j.prevetmed.2016.12.019. [DOI] [PubMed] [Google Scholar]
- Eltayb A., Barakat S., Marrone G., Shaddad S., Sta C. 2012. Antibiotic use and resistance in animal farming : A quantitative and qualitative study on knowledge and practices among farmers in Khartoum, Sudan; pp. 330–338. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency . 2015. Principles on assignment of defined daily dose for animals (DDDvet) and defined course dose for animals (DCDvet)https://www.ema.europa.eu/documents/scientific-guideline/principles-assignment-defined-daily-dose-animals-dddvet-defined-course-dose-animals-dcdvet_en.pdf London. Retrieved from. [Google Scholar]
- Fertner M., Boklund A., Dupont N., Enøe C., Stege H., Toft N. Weaner production with low antimicrobial usage: A descriptive study. Acta Veterinaria Scandinavica. 2015;57(1):1–8. doi: 10.1186/s13028-015-0130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippitzi M.E., Callens B., Pardon B., Persoons B., Dewulf J. Antimicrobial use in pigs, broilers and veal calves in Belgium. Vlaams Diergeneeskundig Tijdschrift. 2014;83:214–224. [Google Scholar]
- Glass-Kaastra S.K., Pearl D.L., Reid-smith R.J., Mcewen B., Mcewen S.A., Amezcua R. 2013. Describing antimicrobial use and reported treatment efficacy in Ontario swine using the Ontario swine veterinary-based surveillance program; pp. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government of Canada . 2018. Responsible use of medically important antimicrobials in animals.https://www.canada.ca/en/public-health/services/antibiotic-antimicrobial-resistance/animals/actions/responsible-use-antimicrobials.html Retrieved February 1, 2019, from. [Google Scholar]
- Guidelines for the prudent use of antimicrobials in veterinary medicine. Practical examples. (2015). (No. 10.9.2015). Brussels. Retrieved from https://ec.europa.eu/health//sites/health/files/antimicrobial_resistance/docs/2015_prudent_use_guidelines_annex_en.pdf.
- Hosoi Y., Asai T., Koike R., Tsuyuki M., Sugiura K. Sales of veterinary antimicrobial agents for therapeutic use in food-producing animal species in Japan between 2005 and 2010. Revue Scientifique et Technique (International Office of Epizootics) 2014;33(3):1007–1015. doi: 10.20506/rst.33.3.2337. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L603987574%5Cnhttp://sfx.library.uu.nl/utrecht?sid=EMBASE&issn=02531933&id=doi:&Atitle=Sales+of+veterinary+antimicrobial+agents+for+therapeutic+use+in+food-producing+animal+species+i Retrieved from. [DOI] [PubMed] [Google Scholar]
- Jensen V.F., Emborg H.D., Aarestrup F.M. Indications and patterns of therapeutic use of antimicrobial agents in the Danish pig production from 2002 to 2008. Journal of Veterinary Pharmacology and Therapeutics. 2012;35(1):33–46. doi: 10.1111/j.1365-2885.2011.01291.x. [DOI] [PubMed] [Google Scholar]
- Jensen V.F., Jorsal S.L., Toft N. A cross-sectional study of oral antibacterial treatment patterns in relation to specific diarrhoeal pathogens in weaner pigs. 2017;203:18–27. doi: 10.1016/j.vetmic.2017.01.038. [DOI] [PubMed] [Google Scholar]
- Jordan D., Chin J.J.C., Fahy V.A., Barton M.D., Smith M.G., Trott D.J. Antimicrobial use in the Australian pig industry: Results of a national survey. Australian Veterinary Journal. 2009;87(6):222–229. doi: 10.1111/j.1751-0813.2009.00430.x. [DOI] [PubMed] [Google Scholar]
- Kim D.P., Saegerman C., Douny C., Dinh T.V., Xuan B.H., Vu B.D. First survey on the use of antibiotics in pig and poultry production in the Red River Delta Region of Vietnam. Food Public Health. 2013;3(5):247–256. doi: 10.5923/j.fph.20130305.03. [DOI] [Google Scholar]
- Lekagul A., Tangcharoensathien V., Yeung S. The use of antimicrobials in global pig production: A systematic review of methods for quantification. Preventive Veterinary Medicine. 2018;160:85–98. doi: 10.1016/j.prevetmed.2018.09.016. [DOI] [PubMed] [Google Scholar]
- Liu C., Stegger M., Aziz M., Johnson T.J., Waits K., Nordstrom L. Escherichia coli ST131-H22 as a Foodborne Uropathogen. MBio. 2018;9(4) doi: 10.1128/mBio.00470-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.-Y., Wang Y., Walsh T.R., Yi L.-X., Zhang R., Spencer J. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. The Lancet. Infectious Diseases. 2016;16(2):161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- Lobanovska M., Pilla G. Penicillin's discovery and antibiotic resistance: Lessons for the future? Yale Journal of Biology and Medicine. 2017;90:135–145. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5369031/pdf/yjbm_90_1_135.pdf Retrieved from. [PMC free article] [PubMed] [Google Scholar]
- Maes, D., Vander Beken, H., Dewulf, J., De Vliegher, S., Castryck, F., & De Kruif, A. 2010. The functioning of the veterinarian in the Belgian pig sector: A questionnaire survey of pig practitioners. Retrieved from http://vdt.ugent.be/sites/default/files/art79308.pdf.
- Maron D.F., Smith T.J., Nachman K.E. Restrictions on antimicrobial use in food animal production: An international regulatory and economic survey. Globalization and Health. 2013;9 doi: 10.1186/1744-8603-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrackin M.A., Helke K.L., Galloway A.M., Poole A.Z., Salgado C.D., Marriott B.P. Effect of antimicrobial use in agricultural animals on drug-resistant foodborne campylobacteriosis in humans: A systematic literature review. Critical Reviews in Food Science and Nutrition. 2016;56(13):2115–2132. doi: 10.1080/10408398.2015.1119798. [DOI] [PubMed] [Google Scholar]
- Merle R., Hajek P., Käsbohrer A., Hegger-Gravenhorst C., Mollenhauer Y., Robanus M. Monitoring of antibiotic consumption in livestock: A German feasibility study. Preventive Veterinary Medicine. 2012;104(1–2):34–43. doi: 10.1016/j.prevetmed.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Merle R., Mollenhauer Y., Hajek P., Robanus M., Hegger-Gravenhorst C., Honscha W. Monitoring of antibiotic consumption in pigs on agricultural farms. Berliner Und Munchener Tierarztliche Wochenschrift. 2013;126(7–8):326–332. doi: 10.2376/0005-9366-126-326. [DOI] [PubMed] [Google Scholar]
- Merle R., Robanus M., Hegger-Gravenhorst C., Mollenhauer Y., Hajek P., Käsbohrer A. Feasibility study of veterinary antibiotic consumption in Germany - comparison of ADDs and UDDs by animal production type, antimicrobial class and indication. BMC Veterinary Research. 2014;10(1):7. doi: 10.1186/1746-6148-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M.A. Survey of quantitative antimicrobial consumption in two different pig finishing systems. Veterinary Record. 2012;171(13):325. doi: 10.1136/vr.100818. [DOI] [PubMed] [Google Scholar]
- Nhung N.T., Cuong N.V., Thwaites G., Carrique-Mas J. Antimicrobial usage and antimicrobial resistance in animal production in southeast Asia: A review. Antibiotics (Basel, Switzerland) 2016;5(4) doi: 10.3390/antibiotics5040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE List of Antimicrobials of Veterinary Importance Criteria used for categorisation list of antimicrobials. 2007. Retrieved from https://www.oie.int/doc/ged/D9840.PDF.
- Om, C., & Mclaws, M.-L. 2016. Antibiotics: Practice and opinions of Cambodian commercial farmers, animal feed retailers and veterinarians. 10.1186/s13756-016-0147-y. [DOI] [PMC free article] [PubMed]
- Postma M., Stärk K.D.C., Sjölund M., Backhans A., Beilage E.G., Lösken S. Alternatives to the use of antimicrobial agents in pig production: A multi-country expert-ranking of perceived effectiveness, feasibility and return on investment. Preventive Veterinary Medicine. 2015;118(4):457–466. doi: 10.1016/J.PREVETMED.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Rajić A., Reid-Smith R., Deckert A., Dewey C., McEwen S. Antibiotic use in swine farms in Alberta - A reply [2] Canadian Veterinary Journal. 2006;47(12):1153. [PMC free article] [PubMed] [Google Scholar]
- Regulation (EC) No 1831/2003 of the European Parliament and of the councel on additives for use in animal nutrition (2003). Retrieved from http://eur-lex.europa.eu/legal-content/En/TXT/PDF/?uri=CELEX:02003R1831-20100901&rid=1.
- Robinson T., Pozzi F. 2011. Mapping supply and demand for animal-source foods to 2030 (No. 2)http://www.fao.org/docrep/014/i2425e/i2425e00.pdf RomeRetrieved from. [Google Scholar]
- Rosengren, L. B., Waldner, C. L., Reid-smith, R. J., Harding, J. C. S., Gow, S. P., & Wilkins, W. L. (2008). Antimicrobial use through feed, water, and injection in 20 swine farms in Alberta and Saskatchewan Résumé Herd selection and data collection, 1(306), 143–150. [PMC free article] [PubMed]
- Sjölund M., Backhans A., Greko C., Emanuelson U., Lindberg A. Antimicrobial usage in 60 Swedish farrow-to-finish pig herds. Preventive Veterinary Medicine. 2015;121(3–4):257–264. doi: 10.1016/j.prevetmed.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Sjölund M., Postma M., Collineau L., Lösken S., Backhans A., Belloc C. Quantitative and qualitative antimicrobial usage patterns in farrow-to-finish pig herds in Belgium, France, Germany and Sweden. Preventive Veterinary Medicine. 2016;130:41–50. doi: 10.1016/j.prevetmed.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Stevens K.B., Gilbert J., Strachan W.D., Robertson J., Johnston A.M., Pfeiffer D.U. 2007. Papers & articles characteristics of commercial pig farms in Great Britain and their use of antimicrobials. [DOI] [PubMed] [Google Scholar]
- Swann M.M., Baxter K., Field H.I. 1969. Report of the Joint Committee on the use of antibiotics in animal husbandry and veterinary medicine. [Google Scholar]
- Timmerman T., Dewulf J., Catry B., Feyen B., Opsomer G., Kruif A.de. Quantification and evaluation of antimicrobial drug use in group treatments for fattening pigs in Belgium. Preventive Veterinary Medicine. 2006;74(4):251–263. doi: 10.1016/j.prevetmed.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Trauffler M., Griesbacher A., Fuchs K., Köfer J. Antimicrobial drug use in Austrian pig farms: Plausibility check of electronic on-farm records and estimation of consumption. The Veterinary Record. 2014;175(16):402. doi: 10.1136/vr.102520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauffler M., Obritzhauser W., Raith J., Fuchs K., Köfer J. The use of the “highest priority critically important antimicrobials” in 75 Austrian pig farms–evaluation of on-farm drug application data. Berl Munch Tierarztl Wochenschr. 2014;127(9–10):375–383. doi: 10.2376/0005-9366-127-375. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration . 2011. Guidance for industry 213: New animal drugs and new animal drug combination products administered in or on medicated feed or drinking water of food- producing animals: Recommendations for drug sponsors for voluntarily aligning product use conditions with.https://www.fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/UCM299624.pdf Rockville, MD. Retrieved from. [Google Scholar]
- Van Cuong N., Nhung N.T., Nghia N.H., Mai Hoa N.T., Trung N.V., Thwaites G. Antimicrobial consumption in medicated feeds in Vietnamese pig and poultry production. EcoHealth. 2016;13(3):490–498. doi: 10.1007/s10393-016-1130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fels-Klerx H.J., Puister-Jansen L.F., van Asselt E.D., Burgers S.L.G.E. Farm factors associated with the use of antibiotics in pig production. Journal of Animal Science. 2011;89(6):1922–1929. doi: 10.2527/jas.2010-3046. [DOI] [PubMed] [Google Scholar]
- Van Rennings L., Von Münchhausen C., Ottilie H., Hartmann M., Merle R., Honscha W. Cross-sectional study on antibiotic usage in pigs in Germany. PLoS ONE. 2015;10(3) doi: 10.1371/journal.pone.0119114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeweerd J.-M., Vandeweerd S., Gustin C., Keesemaecker G., Cambier C., Clegg P. Understanding veterinary practitioners’ decision-making process: Implications for veterinary medical education. Journal of Veterinary Medical Education. 2012;39(2):142–151. doi: 10.3138/jvme.0911.098R1. [DOI] [PubMed] [Google Scholar]
- Vieira A.R., Pires S.M., Houe H., Emborg H.D. Trends in slaughter pig production and antimicrobial consumption in Danish slaughter pig herds, 2002–2008. Epidemiology and Infection. 2011;139(10):1601–1609. doi: 10.1017/S0950268810002724. [DOI] [PubMed] [Google Scholar]
- Visschers V.H.M., Iten D.M., Riklin A., Hartmann S., Sidler X., Siegrist M. Swiss pig farmers' perception and usage of antibiotics during the fattening period. Livestock Science. 2014;162:223–232. doi: 10.1016/j.livsci.2014.02.002. [DOI] [Google Scholar]
- Visschers V.H.M., Backhans A., Collineau L., Iten D., Loesken S., Postma M. Perceptions of antimicrobial usage, antimicrobial resistance and policy measures to reduce antimicrobial usage in convenient samples of Belgian, French, German, Swedish and Swiss pig farmers. Preventive Veterinary Medicine. 2015;119(1–2):10–20. doi: 10.1016/j.prevetmed.2015.01.018. [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2017. Critically important antimicrobials for human medicine.http://apps.who.int/iris/bitstream/handle/10665/255027/9789241512220-eng.pdf;jsessionid=FC65DF119DE54907C6E5D457093EC97E?sequence=1 Geneva. Retrieved from. [Google Scholar]
- World Organisation for Animal Health . 2011. Terrestrial animal health code.www.oie.int Retrieved from. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.