Abstract
Thermal delousing is a new method for removing sea lice from farmed Atlantic salmon (Salmo salar L). We investigated thermally-related tissue injuries in Atlantic salmon in a pilot laboratory trial to describe the acute effect of high water temperatures (34–38 °C). Acute tissue injuries in gills, eyes, brain and possible also nasal cavity and thymus were seen in salmon exposed to water temperatures of 34 - 38 °C in 72 to 140 s. This implies that exposing salmon to such water temperatures is a welfare risk, not only due to the direct tissue injuries that may also be dependent on exposure time, but also due to risk of thermal pain and aversion, including flight reactions.
Key words: Fish, Delousing, Thermal pain, Tissue damage, Welfare
1. Introduction
Thermal delousing is a new method for removing sea lice from farmed Atlantic salmon (Salmo salar L) (Grøntvedt et al., 2015). Although launched as an environmentally and fish welfare friendly technology, it can lead to injuries and acute fish mortality (Hjeltnes, Bang-Jensen, Bornø, Haukaas, & Walde, 2018; Overton et al., 2018). The salmon are crowded and pumped into a chamber with heated seawater for about 30 s, before returning to the sea cage (Noble et al., 2018). The treatment temperature varies between 28 and 34 °C, dependent on sea temperature and delousing effect (Hjeltnes et al., 2018; Roth, 2016). However, extreme temperatures of 36 °C or higher have been reported in anonymous surveys (Hjeltnes et al., 2018).
After commercial thermal delousing, pathologists have observed gill and brain hemorrhages, scale and skin loss and affected thymic and nasal tissues in salmonids in mortality diagnostics (Poppe, Dalum, Røislien, Nordgreen, & Helgesen, 2018). Still, it may be hard to distinguish thermal injuries from other factors like pumping, crowding and the panic reaction in salmon seen during thermal de-licing (Hjeltnes et al., 2018; Noble et al., 2018; Poppe et al., 2018). It has been debated whether it is the heat itself, or panic behavior and collisions in the treatment chambers that causes the injuries. It is known that salmonids have nociceptors (pain receptors) that respond to heat. Ashley, Sneddon, and McCrohan, (2007) reported an average heat threshold temperature of ∼29 °C for polymodal and ∼33 °C for mechanothermal nociceptors in rainbow trout.
High water temperatures are reported as strongly aversive and mortal in salmonids (Beitinger, Bennett, & McCauley, 2000; Elliott, 1991; Frechette, Dugdale, Dodson, & Bergeron, 2018). Lethal water temperature for Atlantic salmon varies according to acclimation temperature, exposure time and life stage (Elliott, 1991; Huntsman, 1942). However, acclimation above ∼28 °C is not considered possible for salmon (Anttila et al., 2014; Elliott & Elliott, 2010). In experiments, parr and smolts die within 10 min at 30 - 33 °C (Elliott & Elliott, 2010). During thermal death of wild salmon, large salmon died first while parr survived (Huntsman, 1942).
Basic knowledge of upper temperatures and holding times that may give acute tissue damage in live salmon is lacking. Here, we investigate thermally-related tissue injuries in Atlantic salmon to describe the acute effect of high water temperatures (34–38 °C).
2. Material and method
The pilot was performed in connection with a behavior trial at the Institute of Marine Research in Matre in May 2018 (reported in Nilsson et al., 2019). The Atlantic salmon smolts were 234 ± 52 g (mean ± SD) and acclimated to 8.5 °C. In the behavior trial the salmon were individually transferred by careful dip-netting to a treatment tank (⌀ = 1.5 m) with different temperatures until they reached the behaviorally defined humane endpoint (laying on the side for two seconds). At this point the salmon were picked up and euthanized by an overdose of tricaine methanesulfonate (Finquel vet.). At treatment temperatures of 36–38 °C, tissue injuries were immediately examined as described below. In addition to individually exposed salmon, the 34 °C group of salmon in the pilot was treated simultaneously. As all individuals in this group could not be netted out in the same netting event, exact exposure times are not known at an individual level but were within 90–140 s for all individuals. These individuals were euthanized and examined in the same manner.
Ten salmon were scored for macroscopic injuries after hot water (36–38 °C) exposure. As a control ten salmon kept at 8.5 °C were individually dip-netted, euthanized and macroscopically scored. Temperatures and exposure time are given in Table 1, also showing the key injuries discovered. The scoring was done on a 0–3 scale; none, mild, moderate, severe (Gismervik, Nielsen, Lind, & Viljugrein, 2017; Grøntvedt et al., 2015; Hjeltnes, Bornø, Jansen, Haukaas & Walde, 2017), for eye damage/bleeding, snout injury, scale loss, skin bleeding, wounds, gill bleeding and paleness and fin injuries. Bleedings in brain, thymus, and palate were scored as present or not. Eye opacity was scored 0–4 (Bass & Wall, Undated; Wall & Bjerkås, 1999), total gill score 0–5 (Grøntvedt et al., 2015; Taylor, Muller, Cook, Kube & Elliott, 2009).
Table 1.
Overview over the key macroscopic and histological injuries of gills, eyes, brain, snout, nasal cavity and thymus in Atlantic salmon exposed to different water temperatures. Macro; macroscopic bleedings or injuries scored directly after treatment. Opacity scored 0–4 according to Bass and Wall (Undated). Histology: gills; sparse to severe bleedings or epithelial injuries/edema, and/ or epithelial cell necrosis, PGI=proliferative gill infection (non-specific) were seen in all samples including controls, eye; partly to almost totally loss of corneal eye epithelium, brain; sparse to severe focal bleedings in brain and/or meninges, nasal cavity; sparse blood congestion and focal/multifocal bleeding in lamina propria and/or edema in nasal cavity.
| T reatments |
Gills |
Eye |
Brain |
Snout damage | Nasal cavity | Thymus | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | Sec. | Macro | Histology |
Opacity |
Hist. | Macro | Hist. | Macro | Hist. | Hist. | ||
| PGI | Left | Right | Left | |||||||||
| 34 | 90–140 | – | Spa. | D | – | – | ND | – | ND | – | D | ND |
| 34 | 90–140 | – | Spa. | D | – | – | Partly | – | ND | – | ND | ND |
| 34 | 90–140 | – | Spa. | D | – | – | ND | – | ND | – | ND | ND |
| 34 | 90–140 | – | Spa. | D | – | – | ND | – | Sev. | – | ND | Spa. e. c. necrosis |
| 36 | 99 | Sev. | Mod. | D | 1 | 1 | Partly | ND | Spa. | Mod. | D | ND |
| 36 | 100 | Sev. | Mod. | D | 0 | 0 | Partly | D | Mod. | Mod. | – | Sparse bleeding |
| 36 | 105 | Sev. | Mod. | D | 0* | 1 | ND | D | Spa. | Mod. | D | ND |
| 36 | 115 | Sev. | Spa. | D | 1 | 2 | Partly | D | Spa. | Mod. | – | ND |
| 36 | 117 | Sev. | Spa. | D | 0 | 1 | ND | ND | ND | Sev. | × | ND |
| 36 | 130 | Sev. | Mod. | D | 0 | 0 | Partly | ND | ND | Spa. | × | – |
| 37 | 114 | Sev. | Sev. | D | 1 | 0 | Partly | ND | Spa. | Mod. | D | – |
| 37 | 117 | Sev. | Mod. | D | 0 | 2 | Partly | ND | ND | Spa. | D | – |
| 38 | 72 | Sev+ | Sev. | D | 1 | 0 | Partly | ND | ND | Spa. | D | – |
| 38 | 95 | Sev+ | Sev. | D | 3 | 1 | Total | ND | Spa. | Mod | × | × |
| 8.5 | 0 | ND | ND | D | 0 | 0 | ND | ND | ND | ND | – | ND |
| 8.5 | 0 | ND | ND | D | 0 | 0 | ND | ND | ND | Mod. | – | – |
| 8.5 | 0 | ND | ND | D | 0 | 0 | ND | ND | ND | Spa. | – | ND |
| 8.5 | 0 | ND | ND | D | 0 | 0 | ND | ND | ND | ND | – | ND |
| 8.5 | 0 | ND | ND | D | 0 | 0 | ND | ND | ND | ND | – | ND |
| 8.5 | 0 | ND | – | – | 0 | 0 | – | – | – | Spa. | – | – |
| 8.5 | 0 | ND | – | – | 0 | 0 | – | – | – | Spa. | – | – |
| 8.5 | 0 | ND | – | – | 0 | 0 | – | – | – | Spa. | – | – |
| 8.5 | 0 | ND | – | – | 0 | 0 | – | – | – | Spa. | – | – |
| 8.5 | 0 | ND | – | – | 0 | 0 | – | – | – | Spa | – | – |
Spa.= sparse. Mod.= moderate. Sev. = severe. ND = not detected. D = detected. Spa. e. c. necrosis = sparse epithelial cell necrosis. × = sample not suitable for analysis. - = not investigated. *Small bleeding/injury detected.
Histology examination and scoring was performed in fourteen salmon exposed to hot water (34–38 °C), and five of the control fish. The following organs were sampled and fixed in 10% phosphate-buffered formalin: gills, left eye, thymus, skin and skeletal muscle, brain, nasal cavity, pseudobranch, heart, liver, spleen, kidney, pyloric caeca with pancreas. Skeletal muscle samples were obtained ventral to the dorsal fin, by transverse section in the lateral line area, covering both red and white muscle. Thymus and nasal cavity were not sampled in all fish (see Table 1 for details). After formalin fixation, organs were prepared for histology by standard paraffin wax embedding and stained with haematoxylin and eosin. Pathological lesions were graded from none detected, mild, moderate and severe by light microscopy. All macroscopic and histological scorings were conducted by authorized fish health personnel (Norwegian Veterinary Institute), including full autopsy.
The experiment was approved according to the Norwegian animal welfare act by the Norwegian Food Safety Authority, permit number 15,383.
3. Results and discussion
Key findings were that acute tissue injuries were seen in salmon exposed to water temperatures of 34 - 38 °C in 72 to 140 s (Table 1). Gill injuries were macroscopically seen as gill bleedings and coagulated clots histologically seen as bleedings or congestions, epithelial damage or edema and/or necrosis; the higher the temperature the more severe damage (Table 1 and Fig. 1). That the gill tissue is most affected is not surprising, as gills are extremely delicate, reacting quickly to an unfavorable environment (Strzyzewska, Szarek, & Babinska, 2016).
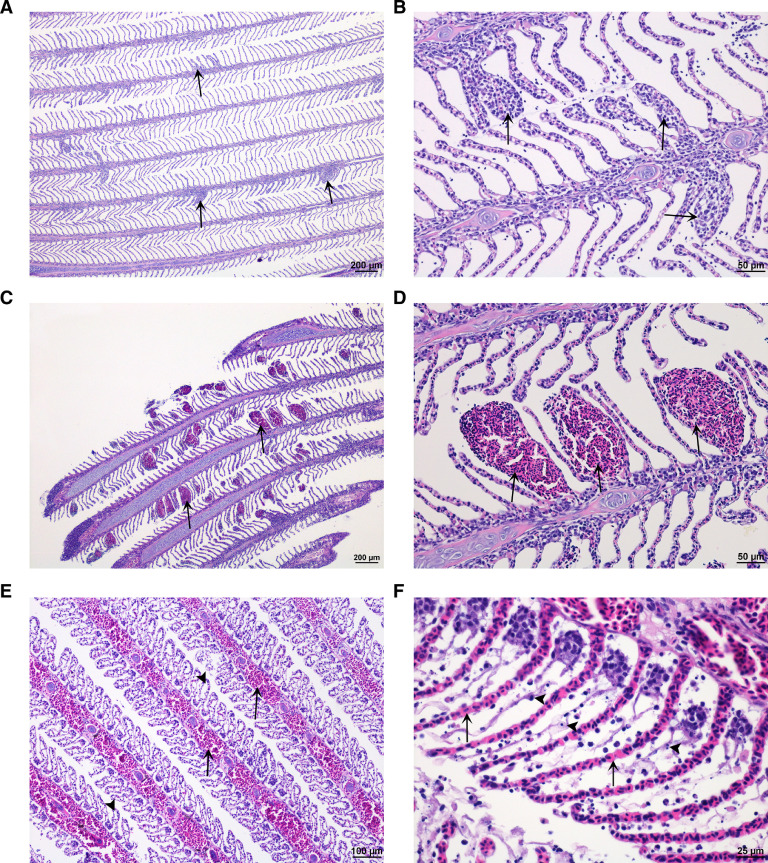
Fig. 1.
Gill tissue. (A and B) Normal gill tissue apart from non-specific multifocal proliferative gill inflammation (arrow) in Atlantic salmon in control group. (C and D) Moderate lamellar bleeding in Atlantic salmon (arrow) exposed to 36 °C water. (E and F) Severe filamental (E) and lamellar congestion (F) (arrow) and severe epithelial lifting/ edema (E, arrow head) and cellular necrosis of epithelial cells (F, arrow head) in Atlantic salmon exposed to 38 °C water. (H&E stain).
Opacity in one or both eyes was seen in eight of the ten salmon exposed to 36–38 °C. One salmon exposed to 38 °C had a score 3 in one eye (Table 1 and Fig. 2). According to the histology, the opacity discovered was not cataract (clouding) in the eye lens, which macroscopically easily can be a mix up (Bass & Wall, Undated), but partly or almost total loss of the corneal eye epithelium assumed painful (Ashley, Sneddon, & McCrohan, 2006). Eyes are vulnerable and can also be damaged by dip-netting of the fish. Still, none of the ten controls showed macroscopic eye damage, five of them also controlled to be negative by histology.
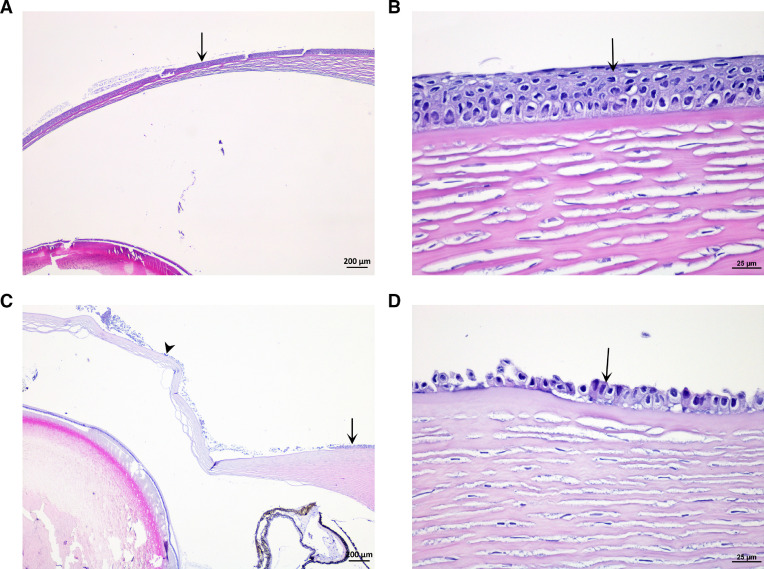
Fig. 2.
Eye. (A and B) Normal cornea of Atlantic salmon (arrow: epithelium) in control group. (C) Partial loss of corneal epithelium in Atlantic salmon exposed to 38 °C water. (arrow: normal epithelium; arrow head: loss of epithelium). (D) Partial loss of epithelial cells (arrow) of cornea. (H&E stain).
Brain bleedings were discovered macroscopically in three of ten salmon and by histology in seven of 13 salmon after hot water exposure (Fig. 3). No brain bleeding was seen in controls. This pilot suggests that hot water itself or the stress may give brain bleedings at the temperatures and holding times examined (Table 1). Going through video recordings from the 36–38 °C groups, collisions with the tank walls cannot be ruled out for all salmon with brain bleedings. Still, one salmon had no clear wall collisions, but a macro- and microscopic bleed, and at least four individuals had no diagnosed brain bleedings despite visible tank wall collisions, suggesting colliding with the walls was not a major contributor to brain injuries in the experimental set up. There was also no macroscopic skin bleedings or wounds detected on the salmon body in either the treated or control group, with the exception of snout and fin damage seen in both groups. The most severe damage appeared in the group exposed to warm water (Table 1).
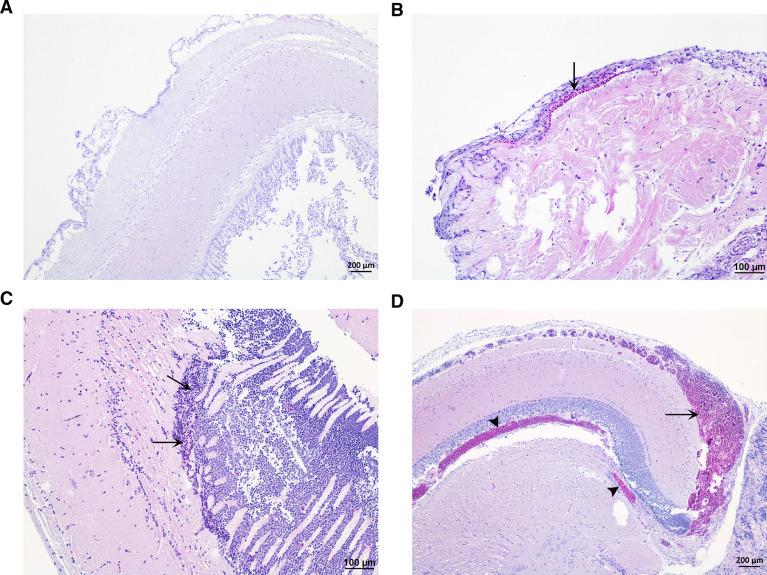
Fig. 3.
Brain. (A) Normal brain of Atlantic salmon in control group. (B) Sparse focal bleeding (arrow) in meninges in Atlantic salmon exposed to 37 °C water. (C) Sparse focal bleeding (arrow) in brain in Atlantic salmon exposed to 38 °C water. (D) Severe focal bleeding in meninges (arrow) and brain (arrow head) in Atlantic salmon exposed to 34 °C water. (H&E stain).
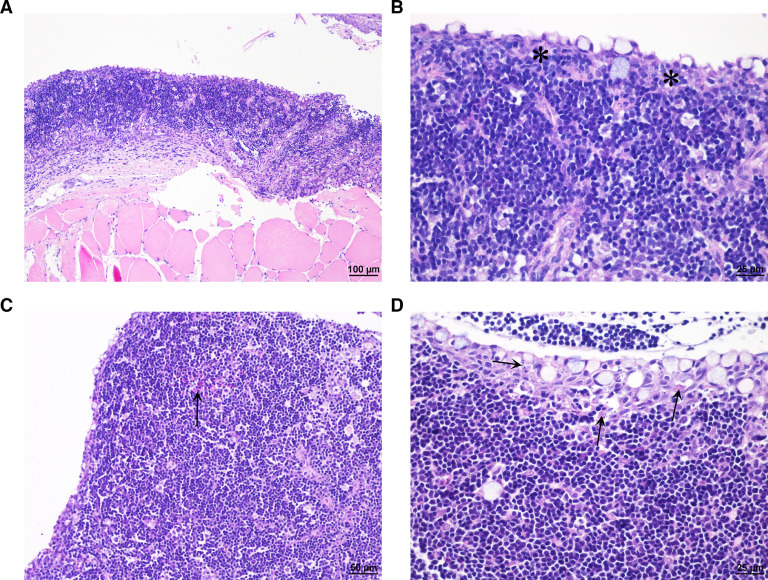
At least one fish exposed to 38 °C water temperature showed macroscopic bleedings in nostrils, but this was not registered systematically on all. Histologically sparse blood congestion and focal or multifocal bleeding were seen in lamina propria and/or edema in the nasal cavity of fish exposed to water temperatures of 36–38 °C, and from one of four salmon exposed to water temperature of 34 °C (Table 1 and Fig. 4). Nasal cavity is an organ normally not sampled, so interpretations of the results must be taken with care as it may be difficult to judge whether the changes are artifacts associated with sampling, also since it was not sampled in the controls. Sparse epithelial cell necrosis was found in the thymus of one salmon, and sparse bleeding in another (Fig. 5). The remaining fish, including four controls, showed no thymus pathology.
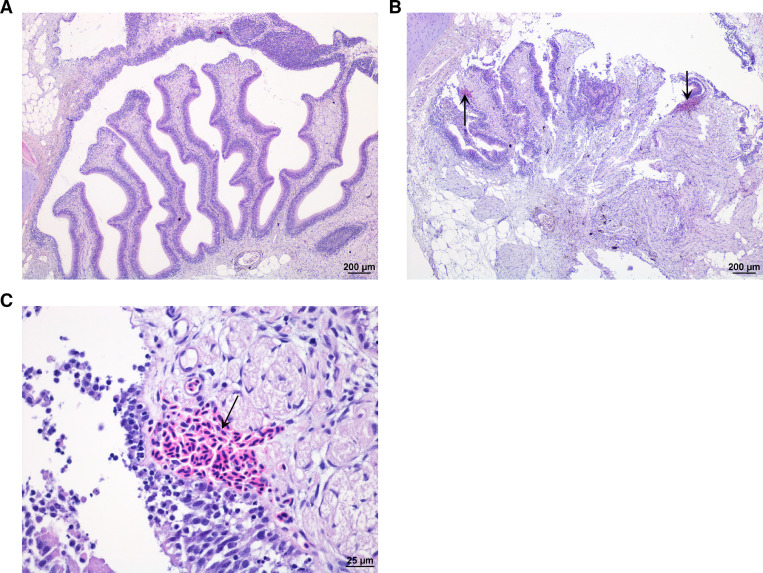
Fig. 4.
Nasal cavity. (A) Normal nasal cavity in Atlantic salmon. (B and C) Focal bleeding (arrow) in lamina propria of mucosa in Atlantic salmon exposed to 37 °C water. (H&E stain).
Fig. 5.
Thymus. (A and B) Normal thymus of Atlantic salmon in control group (asterisk: epithelium). (C) Sparse focal bleeding (arrow) in thymic parenchyma in Atlantic salmon exposed to 36 °C water. (D) Sparse cellular necrosis of epithelial cells (arrow) in Atlantic salmon exposed to 34 °C water. (H&E stain).
No histopathological changes were detected on pseudobranch, heart, liver, spleen, kidney, pyloric caeca, pancreas or muscle from hot water exposed fish or five controls. Concerning skin health, epidermis was partly missing, scales exposed and/or edemas in scale pockets were seen in almost all treated and control fish. This may be due to artifacts during sampling or processing, but it was observed that the salmon generally had “loose scales”.
We have here shown that a sudden exposure to water at 34–38 °C can be lethal after 72 to 140 s in salmon acclimated to 8.5 °C. We used equilibrium loss and cease of movements for two seconds as a humane endpoint. Such behavior has also been suggested by others as a sign of irreversible thermal shock (Beitinger et al., 2000; Elliot, 1991), and wild salmon would be an easy meal lying on their sides. Still, we did not check reflexes like the eye roll (Kestin, Van de Vis, & Robb, 2002). Acute injuries, especially in gills but also eyes and other tissue, were detected in the salmon. In humans, the cornea is acknowledged as the most richly innervated body structure when it comes to nerves (Al-Aqaba, Dhillon, Mohammed, Said & Dua, 2019), 40 times more sensitive than dental pulp, 400 times more than the skin (Bonini, Rama, Olzi, and Lambiase, (2003). Polymodal nociceptors in corneas of cats are excited by temperatures over 37–38 °C, and are sensitized to repeated heating (Belmonte & Giraldez, 1981; Gallar, Pozo, Tuckett, & Belmonte, 1993). Cats have a normal rectal temperature ranging from 36.7–38.9 °C (Levy, Nutt, & Tucker, 2015). Ashley et al. (2006) identified corneal nociceptors in rainbow trout, polymodal temperature threshold 31.70 ± 2.09, still ranging from 21.4 °C. Ashley et al. (2007) reported an average heat threshold temperature of ∼29 °C for polymodal and ∼33 °C for mechanothermal nociceptors on the head of the rainbow trout, still ranging from 20.1–22.0 °C. Ashley et al. (2007) argues that a lower noxious thermal level than mammals found in rainbow trout, can be explained by the function nociception has, and evolved to match habitat temperatures. Lethal limit for trout is described to be >25 °C, where trout actively avoide such temperatures, even at the risk of hypoxia (Matthews & Berg, 1997). Research on more thermally tolerant fish species like the goldfish supports thermal nociception levels in accordance with thermal limits. Nordgreen et al. (2009) found a mean heat noxious level of 38 °C in goldfish based on behaviour, that correspond to their critical maximal limit of 37,5–38,3 °C (Ford & Beitinger, 2005). Adult wild Atlantic salmon are found to maintain a narrow range of body temperatures (16–20 °C) using behavioral thermoregulation via use of cool refuge in rivers (Frechette et al., 2018). Elliot (2010) states that salmon will soon die with water temperatures exceeding 22–28 °C.
Based on the biological function of nociception and pain keeping an animal alive (Sneddon, 2017), it is likely that water at 34–38 °C, here shown to give acute tissue injuries within minutes, also is potentially painful and strongly aversive to Atlantic salmon. Fish pain in general is reviewed elsewhere (ie. Chatigny, 2018; EFSA, 2009; Pouca & Brown, 2017; Sneddon, 2009). The salmon showed immediate strong aversive behaviors like bursts, colliding in tank walls, headshake and increased swimming speed at these temperatures (reported in details in Nilsson et al., 2019 and at ensured oxygen conditions). Others have reported similar aversive behavioral responses to high water temperatures (Elliot, 1991). Heat stress is also found to give physiological responses, like elevation of glucose and lactate when raising the temperature from 15 to 26 °C over 15 h (Gallant, LeBlanc, MacCormack & Currie, 2017).
4. Conclusion
Exposure to water at 34–38 °C in 72 to 140 s can lead to acute tissue injuries in gills, eyes, brain and possible also nasal cavity and thymus in Atlantic salmon. This implies that exposing salmon to such water temperatures is a welfare risk, not only due to the direct tissue injuries that may also be dependent on exposure time, but also due to risk of thermal pain and aversion, including flight reactions.
Ethical statement
The experiment was conducted in accordance with Norwegian regulations on animal experimentation under permit number 15,383.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgements and funding
This work was supported by the Norwegian Veterinary Institute and Institute of Marine Research in connection with research- based governmental support ordered by the Norwegian Food Safety Authorities. We would like to thank the staff at Matre Research station for technical assistance. Thanks to Claire Peterson for proof reading.
Contributor Information
Kristine Gismervik, Email: stine.gismervik@vetinst.no.
Siri K. Gåsnes, Email: siri.gaasnes@vetinst.no.
Jinni Gu, Email: jinni.gu@vetinst.no.
Lars H. Stien, Email: lars.helge.stien@hi.no.
Angelico Madaro, Email: angelico.madaro@hi.no.
Jonatan Nilsson, Email: jonatan.nilsson@hi.no.
References
- Al-Aqaba M.A., Dhillon V.K., Mohammed I., Said D.G., Dua H.S. Corneal nerves in health and disease. Progress in Retinal and Eye Research. 2019 doi: 10.1016/j.preteyeres.2019.05.003. [DOI] [PubMed] [Google Scholar]
- Anttila K., Couturier C.S., Overli O., Johnsen A., Marthinsen G., Nilsson G.E. Atlantic salmon show capability for cardiac acclimation to warm temperatures. Nature Communications, 5. 2014:4252. doi: 10.1038/ncomms5252. [DOI] [PubMed] [Google Scholar]
- Ashley P.J., Sneddon L.U., McCrohan C.R. Properties of corneal receptors in a teleost fish. Neuroscience Letters. 2006;410(3):165–168. doi: 10.1016/j.neulet.2006.08.047. [DOI] [PubMed] [Google Scholar]
- Ashley P.J., Sneddon L.U., McCrohan C.R. Nociception in fish: Stimulus-response properties of receptors on the head of trout. Oncorhynchus Mykiss. Brain Research, 2007;1166:47–54. doi: 10.1016/j.brainres.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Bass, N., & Wall, T.(Undated). A standard procedure for the field monitoring of cataracts in farmed Atlantic salmon and other species.Dublin, Ireland: BIM, Irish Sea Fisheries Board, Dun Laoghaire, Co., 2p.
- Beitinger T.L., Bennett W.A., McCauley R.W. Temperature tolerances of north american freshwater fishes exposed to dynamic changes in temperature. Environmental Biology of Fishes. 2000;58(3):237–275. doi: 10.1023/A. doi:DOi1007676325825. [DOI] [Google Scholar]
- Belmonte C., Giraldez F. Responses of cat corneal sensory receptors to mechanical and thermal stimulation. J Physiol. 1981;321:355–368. doi: 10.1113/jphysiol.1981.sp013989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini S., Rama P., Olzi D., Lambiase A. Neurotrophic keratitis. Eye. 2003;17(8):989–995. doi: 10.1038/sj.eye.6700616. [DOI] [PubMed] [Google Scholar]
- Chatigny F. The controversy on fish pain: A veterinarian's perspective. Journal of Applied Animal Welfare Science : JAAWS. 2018:1–11. doi: 10.1080/10888705.2018.1530596. [DOI] [PubMed] [Google Scholar]
- EFSA AHAW panel (EFSA panel on animal health and welfare), General approach to fish welfare and to the concept of sentience in fish. The EFSA Journal. 2009;954:1–26. doi: 10.2903/j.efsa.2009.954. [DOI] [Google Scholar]
- Elliott J.M. Tolerance and resistance to thermal-stress in juvenile atlantic salmon. Salmo Salar. Freshwater Biology. 1991;25(1):61–70. doi: 10.1111/j.1365-2427.1991.tb00473.x. [DOI] [Google Scholar]
- Elliott J.M., Elliott J.A. Temperature requirements of atlantic salmon salmo salar, brown trout salmo trutta and arctic charr salvelinus alpinus: Predicting the effects of climate change. Journal of Fish Biology. 2010;77(8):1793–1817. doi: 10.1111/j.1095-8649.2010.02762.x. [DOI] [PubMed] [Google Scholar]
- Ford T., Beitinger T.L. Temperature tolerance in the goldfish, carassius auratus. Journal of Thermal Biology. 2005;30(2):147–152. [Google Scholar]
- Frechette D.M., Dugdale S.J., Dodson J.J., Bergeron N.E. Understanding summertime thermal refuge use by adult atlantic salmon using remote sensing, river temperature monitoring, and acoustic telemetry. Canadian Journal of Fisheries and Aquatic Sciences. 2018;75(11):1999–2010. doi: 10.1139/cjfas-2017-0422. [DOI] [Google Scholar]
- Gallant M.J., LeBlanc S., MacCormack T.J., Currie S. Physiological responses to a short-term, environmentally realistic, acute heat stress in atlantic salmon, salmo salar. Facets. 2017;2(1):330–341. [Google Scholar]
- Gallar J., Pozo M.A., Tuckett R.P., Belmonte C. Response of sensory units with unmyelinated fibres to mechanical, thermal and chemical stimulation of the cat's cornea. J Physiol. 1993;468:609–622. doi: 10.1113/jphysiol.1993.sp019791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gismervik K., Nielsen K.V., Lind M.B., Viljugrein H. Norwegian Veterinary Institute (in Norwegian); Oslo: 2017. Mekanisk avlusing med FLS-avlusersystem- dokumentasjon av fiskevelferd og effekt mot lus; p. 41. Norwegian Veterinary Institute`s Report Series 6-2017. [Google Scholar]
- Grøntvedt R.N., Nervikbø I., Viljugrein H., Lillehaug A., Nilsen H., Gjevre A. Norwegian Veterinary Institute; Oslo: 2015. Thermal de-licing of salmonid fish- documentation of fish welfare and effect; p. 32. Norwegian Veterinary Institute`s Report Series 13-2015. [Google Scholar]
- Hjeltnes B., Bang-Jensen B., Bornø G., Haukaas A., Walde C.S.E. Norwegian Veterinary Institute; Oslo: 2018. The health situation in Norwegian aquaculture 2017; p. 108. Norwegian Veterinary Institute Report No. 1b-2018. [Google Scholar]
- Hjeltnes B., Bornø G., Jansen M.D., Haukaas A., Walde C.S.E. Norwegian Veterinary Institute; Oslo: 2017. The health situation in Norwegian aquaculture 2016; p. 127. Norwegian Veterinary Institute Report No. 4b-2017. [Google Scholar]
- Huntsman A. Death of salmon and trout with high temperature. Journal of the Fisheries Board of Canada. 1942;5(5):485–501. [Google Scholar]
- Kestin S.C., Van de Vis J.W., Robb D.H.F. Protocol for assessing brain function in fish and the effectiveness of methods used to stun and kill them. Veterinary Record. 2002;150(10):302–307. doi: 10.1136/vr.150.10.302. [DOI] [PubMed] [Google Scholar]
- Levy J.K., Nutt K.R., Tucker S.J. Reference interval for rectal temperature in healthy confined adult cats. Journal of Feline Medicine and Surgery. 2015;17(11):950–952. doi: 10.1177/1098612x15582081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K.R., Berg N.H. Rainbow trout responses to water temperature and dissolved oxygen stress in two southern california stream pools. Journal of Fish Biology. 1997;50(1):50–67. doi: 10.1111/j.1095-8649.1997.tb01339.x. [DOI] [Google Scholar]
- Nilsson J., Moltumyr L., Madaro A., Kristiansen T.S., Gåsnes S.K., Mejdell C.K., Gismervik K., Stien L.H. Sudden exposure to warm water causes instant behavioural responses indicative of nociception or pain in Atlantic salmon. Veterinary and Animal Science. 2019 doi: 10.1016/j.vas.2019.100076. [DOI] [PMC free article] [PubMed] [Google Scholar]; In press.
- Noble C., Gismervik K., Iversen M.H., Kolarevic J., Nilsson J., Stien L.H. 2018. Welfare indicators for farmed atlantic salmon: Tools for assessing fish welfare.http://www.nofima.no/fishwell/english (Eds.) Norway. [Google Scholar]
- Nordgreen J., Garner J.P., Janczak A.M., Ranheim B., Muir W.M., Horsberg T.E. Thermonociception in fish: Effects of two different doses of morphine on thermal threshold and post-test behaviour in goldfish (Carassius auratus) Applied Animal Behaviour Science. 2009;119(1–2):101–107. [Google Scholar]
- Overton K., Dempster T., Oppedal F., Kristiansen T., Gismervik K., Stien L. Salmon lice treatments and salmon mortality in norwegian aquaculture: A review. Reviews in Aquaculture. 2018:1–20. doi: 10.1111/raq.12299. [DOI] [Google Scholar]
- Poppe T., Dalum A.S., Røislien E., Nordgreen J., Helgesen K.O. Termisk behandling av laks (In Norwegian, English summary) Norsk veterinærtidsskrift. 2018;(3):130–155. [Google Scholar]
- Pouca C.V., Brown C. Contemporary topics in fish cognition and behaviour. Current Opinion in Behavioral Sciences. 2017;16:46–52. doi: 10.1016/j.cobeha.2017.03.002. [DOI] [Google Scholar]
- Roth B. Nofima; Tromsø: 2016. Avlusing av laksefisk med Optilice: Effekt på avlusing og fiskevelferd (In Norwegian, English summary) p. 41. Nofima report 59/2016. [Google Scholar]
- Sneddon L.U. Pain perception in fish: Indicators and endpoints. Ilar Journal. 2009;50(4):338–342. doi: 10.1093/ilar.50.4.338. [DOI] [PubMed] [Google Scholar]
- Sneddon L.U. Comparative physiology of nociception and pain. Physiology. 2017;33(1):63–73. doi: 10.1152/physiol.00022.2017. [DOI] [PubMed] [Google Scholar]
- Strzyzewska E., Szarek J., Babinska I. Morphologic evaluation of the gills as a tool in the diagnostics of pathological conditions in fish and pollution in the aquatic environment: A review. Veterinarni Medicina. 2016;61(3):123–132. doi: 10.17221/8763-Vetmed. [DOI] [Google Scholar]
- Taylor R.S., Muller W.J., Cook M.T., Kube P.D., Elliott N.G. Gill observations in atlantic salmon (Salmo salar, L.) during repeated amoebic gill disease (AGD) field exposure and survival challenge. Aquaculture (Amsterdam, Netherlands) 2009;290(1–2):1–8. doi: 10.1016/j.aquaculture.2009.01.030. doi:DOi. [DOI] [Google Scholar]
- Wall T., Bjerkås E. A simplified method of scoring cataracts in fish. Bulletin of the European Association of Fish Pathologists. 1999;19(4):162–165. [Google Scholar]