Abstract
Hereditary nephropathy is a primary progressive glomerular disease in dogs associated with the c.115A>T mutation in the COL4A4 gene in English cocker spaniel (ECS) dogs. The disease is inherited in an autosomal recessive manner. Hereditary nephropathy has been described in this breed since the late 1940s. To date, there are no data on the prevalence of this disease in Brazil, so the aim of this study was to evaluate the allelic frequency of this mutation in ECS dogs in this country. The DNA samples were purified from blood samples or buccal swabs from 221 ECS dogs. Fragments of the DNA containing the mutation were amplified by PCR and submitted to direct gene sequencing. The allele frequency of the mutation was 0.9%. The presence of the mutation in the ECS dog population in Brazil reveals the importance of performing the genotyping tests in these dogs as a method of diagnosing the disease and identifying heterozygous animals, aiming to reduce clinical cases of disease through mating.
Keywords: Diagnosis, Genetic disease, Genotyping, Hereditary nephropathy, COL4A4
Hereditary nephropathy or autosomal recessive familial nephropathy (FN) in the English cocker spaniel (ECS) is caused by a type IV collagen defect in the glomerular basement membranes, i.e., by a nonsense mutation (COL4A4_c.115A>T) (Davidson et al. 2007). FN, described for the first time in ECS dogs by Krook (1957), is a progressive and fatal juvenile disease with clinical signs of chronic renal failure (De Brot et al., 2017). Several studies have described the clinical and laboratory findings of FN in ECS dogs (Steward et al., 1984; Robinson et al., 1985; Koeman et al., 1989); nevertheless, prevalence data concerning the COL4A4_c.115A>T mutation in ECS dogs are scarce worldwide. In Brazil, there is no study that has evaluated this mutation in ECS dogs. Therefore, the aim of this study was to evaluate the allele frequency of COL4A4_c.115A>T in Brazilian ECS dogs.
Our study was performed in accordance with the policies of the Institutional Animal Care and Use Committee (0218/2016-CEUA/UNESP), and samples were collected under a strict confidentiality agreement to ensure the anonymity of establishments, owners and animals. A total of 221 blood or oral swab samples were collected from ECS dogs (149 females and 72 males, with ages ranging from four months to 17 years), of which 131 were registered in the Confederação Brasileira de Cinofilia (CBKC), and 90 were unregistered. The registered dogs were from 18 CBKC kennels.
Genomic DNA was purified from blood or oral swab samples using a commercial kit (GE Healthcare). Specific primers (JPOF_COL4A4_For, 5’-CTGACATAATCATCAGCCCTCTC-3’; and JPOF_COL4A4_Rev, 5’-TCTCTAGGGTTCGTGCATCT-3’) were designed to genotype the COL4A4_c.115A>T mutation (Davidson et al. 2007). Polymerase chain reactions (25 μL) contained 12.5 μL of PCR master mix (Promega), 0.3 μM of each primer, 2.5 μL of template DNA, and 8.5 μL nuclease-free water. The amplification conditions were as follows: initial denaturation at 94°C for 5 minutes; followed by 40 cycles of denaturation at 94°C for 30 seconds, annealing at 59°C for 1 minute, and extension at 72°C for 1 minute; followed by a final extension at 72°C for 5 minutes. Amplicons (432 bp) were analysed by 1.5% agarose gel electrophoresis, purified, and subjected to direct sequencing. The obtained sequences and the electropherograms were analysed using Geneious software (Figure 1). The results of the genotype analysis were descriptively described. In addition, the allele frequencies were compared between males and females and registered and unregistered dogs (Exact Fisher test) using GraphPad Prism software.
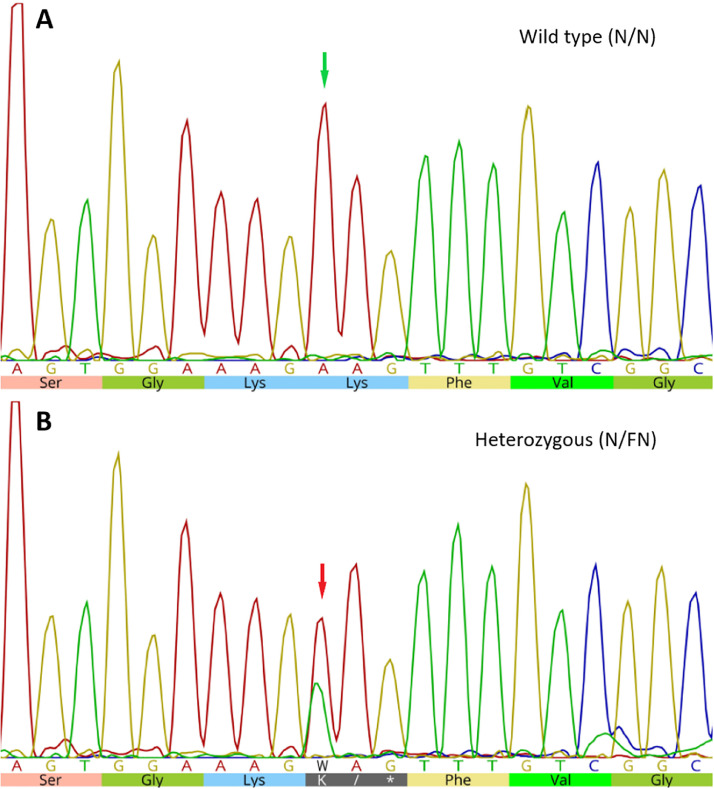
Figure 1.
Partial chromatogram showing capillary sequencing results for wild type (A) and heterozygous (B) allele of the c.115A>T mutation in the COL4A4 gene for hereditary nephropathy in English cocker spaniel dogs. “A” shows wild type allele (adenine) (green arrow) and the respective amino acid Lysine (Lys); in “B”, double peak (adenine/ thymine, W) is observed (red arrow), in addition, note also the amino acid Lysine (K) or the stop codon (*). Image obtained using Geneious® 10.0 software.
Of the 221 dogs tested, 98.2% (217/221) were identified as homozygous for the wild type allele (N/N), and 1.8% (4/221) were heterozygous (N/FN); therefore, the allele frequency was 0.9%. No recessive homozygous individuals were observed in this study. Although there was no significant difference between groups of animals with and without registration (p = 0.3067), we observed a higher allele frequency in the unregistered animals (1.7%, being identified three N/FN and 87 N/N ECS dogs) than that of CBKC registered animals (0.4%, being identified one N/FN and 130 N/N ECS dogs). The only CBKC registered animal identified as heterozygous was a female descended from an Italian male (N/N, clear) and a Brazilian female (N/FN carrier). However, the genealogy of the three heterozygous non-registered animals was unknown, and these three unrelated females were from three different cities. There was no significant difference between males and females (p = 0.3088).
In addition, the allele frequency of this mutation in 3,465 ECS dogs registered in The Kennel Club1 was 0.4% between 2006 and 2018 in the United Kingdom. All the breeders responsible for CBKC registered kennels were aware of the disease. This contributed to the low allele frequencies observed in the CBKC registered group in comparison to the unregistered group. These professional breeders probably use genetic testing to select mating partners, which prevents the birth of carriers or affected animals (FN/FN). As stated before, in relation to this variable, no significant difference was observed between this group and the unregistered group (p = 0.3067). However, when the owners of unregistered animals, which were from non-professional breeders, mate their animals, this is done indiscriminately since they unaware of the disease, and failure to perform animal genotyping does not allow the adoption of measures to control FN. This practice contributes to the maintenance of the mutated allele in this breed, especially in this unregistered group.
FN is a rare and fatal juvenile disease (Steward et al., 1984), and its early diagnosis requires specialized methods (e.g., electron microscopy and immunostaining) (Lees, 2013), which are almost always unavailable in clinical practice. In addition, some Brazilian veterinary practitioners may not be aware of it, which may also contribute to the fact that clinical reports of the disease in Brazil have not been found in the literature. In addition, mating of animals known to produce offspring suffering from a genetic disease, such as FN, is considered a practice that affects animal welfare and cannot be practiced by Brazilian veterinarians. Therefore, the genotyping for the c.115A>T mutation is an important method of diagnosis, mainly for dogs used for breeding. Despite the low prevalence of heterozygous animals, this mutation is present in the Brazilian ECS dog population. Our study provides a better understanding of the disease in Brazil and provides prevalence data of FN in ECS dogs that may support additional studies in other countries, since the genealogical basis of Brazil's ECS dogs originates in Europe.
Therefore, genotyping for the c.115A>T mutation is an important method of diagnosis, mainly for dogs used for breeding, because despite the low prevalence of heterozygous animals, this mutation is present in the Brazilian ECS dog population. As described in the literature, heterozygous animals do not present alterations associated with hereditary nephropathy (Lees et al., 1998). The absence of clinical signs in heterozygous dogs makes it difficult to control the disease, and genotyping is necessary for the prevention of clinically affected animals.
Ethical Statement
This study was performed in accordance with the policies of the Institutional Animal Care and Use Committee (0218/2016-CEUA/UNESP).
Declaration of competing interest
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Acknowledgements
The authors acknowledge the support of the National Council for Scientific and Technological Development (CNPq, 423519/2016-6). This study was carried out at the São Paulo State University (UNESP) and in part as fulfilment of the master science thesis of LRA at São Paulo State University (UNESP), Brazil.
Footnotes
See: Spaniel (Cocker) DNA screening. https://www.thekennelclub.org.uk/health/for-breeders/dna-screening-schemes-and-results/dna-screening-for-breeds-s-z/spaniel-cocker-dna-screening/ (accessed 20 February 2019)
References
- Davidson A.G., Bell R.J., Lees G.E., Kashtan C.E., Davidson G.S., Murphy K.E. Genetic Cause of Autosomal Recessive Hereditary Nephropathy in the English Cocker Spaniel. Journal of Veterinary Internal Medicine. 2007;21:394–401. doi: 10.1892/0891-6640(2007)21[394:gcoarh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- De Brot S., Adamany J., Baiker K., Dhumeaux M., Allegrucci C., Polledo I., Grau-Roma L. Pathology in practice. Hereditary nephropathy (familial nephropathy) in an english cocker spaniel. Journal of the American Veterinary Medical Association. 2017;251:661–664. doi: 10.2460/javma.251.6.661. [DOI] [PubMed] [Google Scholar]
- Koeman J.P., Ezilius J.W., Biewenga W.J., Van Den Brom W.E., Gruys E. Familial nephropathy in cocker spaniels. Deutsche Tierärztliche Wochenschrift. 1989;96:174–179. [PubMed] [Google Scholar]
- Krook L. The pathology of renal cortical hypoplasia in the dog. Nordisk veterinaermedicin. 1957;9:161–176. [Google Scholar]
- Lees G.E., Helman R.G., Kashtan C.E., Michael A.F., Homco L.D., Millichamp N.J., Ninomiya Y., Sado Y., Naito I., Kim Y. A model of autosomal recessive Alport syndrome in English cocker spaniel dogs. Kidney International. 1998;54:706–719. doi: 10.1046/j.1523-1755.1998.00062.x. [DOI] [PubMed] [Google Scholar]
- Lees G.E. Kidney diseases caused by glomerular basement membrane type IV collagen defects in dogs. Journal of Veterinary Emergency and Critical Care (San Antonio) 2013;23:184–193. doi: 10.1111/vec.12031. [DOI] [PubMed] [Google Scholar]
- Robinson W.F., Huxtable C.R., Gooding J.P. Familial nephropathy in cocker spaniels. Australian Veterinary Journal. 1985;62:109–112. doi: 10.1111/j.1751-0813.1985.tb07253.x. [DOI] [PubMed] [Google Scholar]
- Steward A.P., Macdougall D.F. Familial nephropathy in the Cocker Spaniel. Journal Small Animal Practice. 1984;25:15–24. [Google Scholar]