Highlights
-
•
Sample collection via myringotomy is associated with a high contamination rate.
-
•
The suitability of this method for detection of otitis media is questionable.
-
•
Iatrogenic spread of pathogenic microorganisms into the middle ear is possible.
Keywords: Dog, Myringotomy, Otitis media, Video otoscopy, Microbiota
Abstract
Myringotomy for sample collection from the middle ear cavity for cytology and bacterial culture is considered a routine method to diagnose otitis media in dogs. The objective of this study was to determine the rate of contamination of middle ear aspirates with material from the external ear canal obtained by video-otoscopic guided myringotomy. In canine cadavers (n = 17) free from otitis externa the external ear canals were flushed under video-otoscopic control and a fluorescent dye was instilled. After removal of residual fluid a myringotomy was performed. If air was aspirated, 1 mL of saline was instilled through the same myringotomy needle into the middle ear cavity and re-aspirated. Contamination from the external ear canal was demonstrated by positive fluorescence of the aspirate. Bacterial cultures and cytological examinations of the external ear canals and middle ear cavities were performed.
Data from 28 ears under investigation were included. In 19 of 28 middle ear aspirates (67.9%), clear yellow fluorescent fluid was obtained, indicating a contamination from the external ear canal. Microorganisms were detected in 4 of 26 middle ear samples (15.4%) and in 15 of 26 external ear canals (57.7%). Sample collection by myringotomy in this study was associated with a high contamination rate, implying that the suitability of this method for detection of otitis media in patients with concurrent otitis externa is questionable. Furthermore, the potential for iatrogenic spread of pathogenic microorganisms into the middle ear cavity needs to be considered.
1. Introduction
Ear diseases in dogs are amongst the most common conditions presented to small animal veterinary practice; studies report 12 - 20% of dogs suffering from otitis externa (OE) (Fraser, Gregor, Mackenzie, Spreull & Withers, 1969; Hill et al., 2006; Lund, Armstrong, Kirk, Kolar & Klausner, 1999). Many dogs with chronic OE have concurrent otitis media (OM) that is thought to be a direct extension of OE through a perforated tympanic membrane (TM) (Bruyette & Lorenz, 1993; Fraser et al., 1969; Little, Lane & Pearson, 1991; Schunk & Averill, 1983; Spreull, 1964). OM represents an important perpetuating factor of OE (Logas, 1994). Published prevalence rates range from 32 to 89% of dogs with chronic OE that have concurrent OM (Belmudes et al., 2018; Classen, Bruehschwein, Meyer-Lindenberg & Mueller, 2016; Cole, Kwochka, Kowalski & Hillier, 1998, 2002; Doyle, Skelly & Bellenger, 2004; Herrmann, 2014; Moltzen, 1961; Saridomichelakis, Farmaki, Leontides & Koutinas, 2007; Spreull, 1964; Trower, Gregory, Renfrew & Lamb, 1998). Proper diagnostic evaluation will thus lead to a correct diagnosis of OM and will enable an appropriate treatment (Cole et al., 2002; Rose, 1976).
However, diagnosis of OM in dogs may be challenging and may require several diagnostic procedures. Modalities described for middle ear cavity (MEC) disease include otoscopy, radiology, sonography, canalography, computed tomography (CT) and magnetic resonance imaging (MRI) as well as tympanometry and pneumotoscopy (Cole et al., 2002; Dickie et al., 2003; Garosi, Dennis & Schwarz, 2003; Remedios, Fowler & Pharr, 1991; Spreull, 1974; Trower et al., 1998). Cytology and bacterial culture from the MEC are considered the gold standard for diagnosing or excluding OM. In cases with a ruptured TM, OM can be confirmed by direct sampling of the MEC exudate via a swab or soft feeding tube (Cole et al., 1998; Griffin, 2006; Shell, 1988). Many dogs with suspected OM (based on diagnostic imaging, neurological signs or an abnormal TM appearance) have an intact TM. In such cases, myringotomy by surgical incision or puncture of the TM followed by sampling from the MEC is recommended (Angus & Campbell, 2001; Bruyette & Lorenz, 1993; Gotthelf, 2004; Griffin, 2006; Rose, 1977; Shell, 1988). However, specimen collection from the MEC through the external ear canal (EEC), even if using video-otoscopic guided sampling, may lead to a microbial contamination.
We are not aware of any published data addressing concerns regarding contamination of MEC aspirates. The first aim of this study was therefore to assess the contamination rate of MEC aspirates with material from the EEC obtained by myringotomy, leading to sample mixture from the middle and the external ear. In addition, since little attention has been paid to the microbiota of healthy canine MECs (Defalque, Rosser & Petersen, 2005; Fraser et al., 1969; Matsuda, Tojo, Fukui, Imori & Baba, 1984), the second aim was to determine possible microbial inhabitants of the MEC.
2. Material and methods
2.1. Cadaver selection
Canine cadavers of various breeds, ages and both sexes were used. The dogs had been euthanized for reasons unrelated to this study. Breed, age, sex, weight, as well as reasons for euthanasia and current medications, if known, were documented. Inclusion criteria were normal EECs without clinical signs of OE. Exclusion criteria were pathologic changes of the TM (rupture, abnormal colour, transparency or thickness) or abnormal findings on CT scans of the middle ear (if available). In addition, one ear was excluded due to technical problems (broken myringotomy needle, see results section).
2.2. Procedures
The procedures described below were completed within 4 h after euthanasia. An otoscopic examination was performed using a video otoscope (Karl Storz, GmbH and Co. KG, Tuttlingen, Germany).
In the majority of canine cadavers, CT scans of the MECs were performed with the cadavers placed in sternal recumbency. CT images were acquired using a multislice CT unit (SOMATOM Emotion 16-slice configuration; Siemens AG Medical Solutions, Erlangen, Germany). The detailed technical settings are provided in Appendix: Supplementary File S1.
Subsequently, a sterile swab (Transwab, Medical Wire & Equipment, Wiltshire, England) sample was collected from the horizontal canal of each ear for microbiological examination. Furthermore, a non-sterile cotton swab specimen for cytological examination was obtained from the junction between the vertical and horizontal aspects of each EEC. The sample was rolled on to a glass microscope slide and air-dried.
With the use of video otoscopy each EEC was cleaned and examined with dogs positioned in lateral recumbency. The following procedures were performed using sterile instruments and working materials. Initially the ear canal was flushed with sterile saline (0.9% sodium chloride intravenous infusion, B. Braun Melsungen AG, Melsungen, Germany) and subsequently, 5 mL of fluorescent dye (Fluorescein 1%, Lekaren Unimed Pharma, Bratislava, Slovakia - one drop of fluorescent dye diluted in 5 mL sterile saline) was instilled through one working channel of a double-port adaptor of the endoscopic otoscope. Residual fluid within the ear canal was retrieved by suction via a rigid sterile polyvinyl chloride feeding tube 1 mm in diameter (Rüsch Feeding Tube Eruplast, No. 1, Rüsch Uruguay LTDA, Montevideo, Uruguay) with its end cut to approximately 30 cm in length, introduced through a port of the video otoscope. The tube was connected to a vacuum-pump (Karl Storz Vetpump 2 69,321,620: Karl Storz GmbH and Co. KG, Tuttlingen, Germany). The myringotomy was performed in the ventrocaudal quadrant of the TM using a 1.5 mm diameter myringotomy needle (Myringotomy Needle 67071XS: Karl Storz GmbH and Co. KG, Tuttlingen, Germany) introduced through one operating port of the video otoscope. Immediately after the pars tensa was punctured, attempts were made to aspirate material from the MEC with a 3 mL syringe (B. Braun Injekt, B. Braun Melsungen AG, Melsungen, Germany) attached to the myringotomy needle. If fluid was aspirated, it was collected into a sterile container. In case of aspirated air 1 mL of sterile saline was injected with a 3 mL syringe attached to the myringotomy needle into the MEC and immediately retrieved. The obtained fluid was again collected into a sterile container. Directly after the procedure, the myringotomy needle was removed and the presence of the created hole in the TM was visualized to prove successfully performed myringotomy.
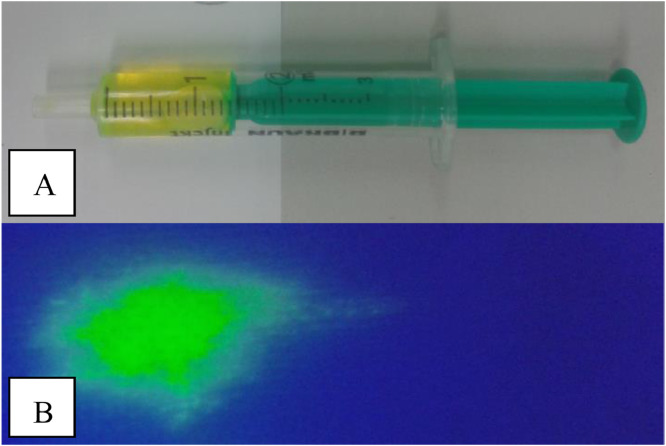
The colour of the fluid obtained from the MEC was judged as either yellow or colourless. A fluid sample was also dropped on a white swab (Medicomp; Paul Hartmann AG, Heidenheim, Germany) and illuminated with an UV-light source (Atlas Fluotest, Karl Schreiner GmbH, Vienna, Austria). In case of positive fluorescence, contamination of the MEC sample with material from the EEC was confirmed (Fig. 1). Subsequently, the specimen was examined cytologically: one drop of the fluid was placed on a slide and smeared with the edge of another slide. The air-dried slides obtained from the EEC and MEC were stained with a modified Wright-Giemsa stain (LT-SYS Diff-Quik, labor + Technik Eberhard Lehmann GmbH, Berlin, Germany). All the samples were analysed by the same investigator (ER, doctoral student of LP, who is a Diplomate of the European College of Veterinary Dermatology). After scanning at low power (x100 magnification), five oil-immersion fields (x1000 magnification) were examined for the presence of leukocytes, bacteria and yeasts. Exact numbers of microorganisms were counted and the mean number per field in a sample was calculated.
Fig. 1.
Yellow coloured fluid obtained from one MEC (A) and positive fluorescence (B) confirming contamination with material from the external ear canal.
For microbiological examination, a sterile swab (Transwab, Medical Wire & Equipment, Wiltshire, England) sample was obtained from the specimen collected from the MEC. Detailed information about cultivation and identification of microorganisms is provided in Appendix: Supplementary file S1.
2.3. Statistic analysis
Descriptive data is presented in the form of cross tabulations. Associations between positive and negative microbial cultures of MEC aspirates and samples from EECs were made by Fisher´s Exact Test. Statistical analysis was performed using IBM SPSS software version 24 (IBM Corp). Statistical significance was set at P<0.05.
3. Results
3.1. Canine cadavers
In total, 17 canine cadavers were included. Nine were males (four intact, five castrated) and eight were females (one intact, seven spayed). Breeds represented included nine mixed-breed dogs and one single representative of the following breeds: dachshund, English pointer, flat coated retriever, golden retriever, Havanese, Lhasa apso, White Swiss shepherd dog and Siberian husky. The age of the dogs ranged from 8 to 17 years (median age 12.3 years). Detailed signalment data, reasons for euthanasia and current medication are provided in Appendix: Supplementary File S2.
3.2. CT
CT imaging of the MECs was completed in 13 of 17 cadavers (CT was not available in the remaining four dogs). The CT report revealed air-filled MECs with no pathologic changes of the osseous bulla in all cases with the exception of one MEC, in which soft tissue densities were detected. This ear was subsequently excluded.
3.3. Contamination rate
Data from both ears in 11 dogs and from only one ear in six dogs, with a total of 28 ears (14 left and 14 right ears) were analysed. In the six dogs where only one ear was evaluated, the other ear was excluded due to visible pathologic changes of the TM on video otoscopy (n = 4) or the MEC (n = 1, according to the CT report). The myringotomy needle broke during the myringotomy procedure, leading to the exclusion of the concerned ear. Details are shown in Table 1.
Table 1.
Details of 17 cadavers included in the data.
| Cadaver | CT | Contamination left/right ear | MO EEC | MO MEC |
|---|---|---|---|---|
| 1 | N/A | left: not contaminated | N/A | N/A |
| right: contaminated | ||||
| 2 | N/A | left: contaminated | left: Malassezia pachydermatis | left: Micrococcus sp., |
| right: excluded | Enterococcus gallinarum | |||
| 3 | performed | left: excluded | right: Malassezia pachydermatis | right: Bacillus sp. |
| right: contaminated | ||||
| 4 | performed | left: contaminated | left: Enhydrobacter aerosaccus | left: negative |
| right: not contaminated | right: Acinetobacter johnsonii | right: negative | ||
| 5 | performed | left: excluded | right: Ochrobactrum intermedium | right: Enterococcus gallinarum, |
| right: not contaminated | Microbacterium paraoxidans | |||
| 6 | N/A | left: contaminated | left: negative | left: negative |
| right: contaminated | right: negative | right: negative | ||
| 7 | performed | left: contaminated | left: Malassezia pachydermatis, Candida albicans | left: Malassezia pachydermatis, Candida albicans |
| right: excluded | ||||
| 8 | performed | left: not contaminated | left: negative | left: negative |
| right: contaminated | right: Kocuria sp., Staphylococcus xylosus, | right: negative | ||
| Staphylococcus sciuri ssp. Sciuri | ||||
| 9 | performed | left: contaminated | left: Bacillus sp., Malassezia pachydermatis | left: negative |
| right: contaminated | right: negative | right: negative | ||
| 10 | N/A | left: excluded | right: Staphylococcus equorum | right: negative |
| right: contaminated | ||||
| 11 | performed | left: not contaminated | left: Bacillus sp. | left: negative |
| right: not contaminated | right: negative | right: negative | ||
| 12 | performed | left: not contaminated | left: negative | left: negative |
| right: not contaminated | right: negative | right: negative | ||
| 13 | performed | left: contaminated | left: Staphylococcus warneri | left: negative |
| right: contaminated | right: Bacillus sp. | right: negative | ||
| 14 | performed | left: not contaminated | left: Staphylococcus pseudintermedius | left: negative |
| right: contaminated | right: negative | right: negative | ||
| 15 | performed | left: contaminated | left: Staphylococcus epidermidis | left: negative |
| right: contaminated | right: negative | right: negative | ||
| 16 | performed | left: contaminated | left: Macrococcus | left: negative |
| right: excluded | caseolyticus | |||
| 17 | performed | left: contaminated | left: negative | left: negative |
| right: contaminated | right: negative | right: negative |
MO, microorganisms; EEC external ear canal; MEC middle ear cavity; N/A, not available.
In 19 of 28 MEC aspirates (67.9%) clear yellow and fluorescent fluid was obtained from the MEC, indicating a contamination from the EEC. Each of the yellow stained samples was fluorescent, whereas none of the colourless samples showed fluorescence when illuminated with UV light.
3.4. Microbiological examination
Cultivation of microorganisms was performed with 26 of 28 MEC aspirates (cultivation was not available in 2 samples of one dog). These are tabulated in Table 1. Overall, six different microorganisms were isolated from 4 of 26 MEC samples (15.4%) in total, resulting in 22 negative cultures (84.6%). Three of the positive cultures were associated with contaminated aspirates, but only one of those had the same isolates in the MEC and the EEC detected (case 7, left ear: Malassezia pachydermatis, Candida albicans). From the other two contaminated aspirates Micrococcus sp. and Enterococcus gallinarum in case 2 and Bacillus sp. in case 3 were detected. In these cases, different microorganisms were found in the corresponding ear canals (Malassezia pachydermatis in both). In the not obviously contaminated MEC aspirate (case 5, right ear), the detected bacteria (Enterococcus gallinarum, Microbacterium paraoxidans) also differed from the isolate (Ochrobactrum intermedium) recovered from the EEC. Statistically, there was no significant difference in the frequency of microbial growth between the contaminated and not-contaminated MEC samples (see Table 2). Microbial growth was present in 15 of 26 EECs (57.7%) and negative in 11 samples (42.3%). In cases where both ears of a cadaver were evaluated, isolates from the EECs were never identical for the left and the right side. The different isolates are listed in Table 3. Previous antimicrobial therapy was documented in 3 of 17 dogs (four included ears, with 75% of the EEC samples and 25% of the MEC samples having microbial growth). Ten dogs had no history of recent antibiotic treatment (17 ears, 64.7% of the EEC samples and 11.8% of the MEC samples showed microbial growth) and four dogs had no documentation concerning the current medication (5 ears, 20% of the EEC and the MEC samples showed microbial growth). Detailed data is shown in Table 4.
Table 2.
Association between contamination and positive bacterial cultures from EEC and MEC (n = 26).
| negative bacterial culture MEC | positive bacterial culture MEC | ||
|---|---|---|---|
| Not-contaminated MEC samples (n = 8) | negative bacterial culture EEC | 4 | 0 |
| positive bacterial culture EEC | 3 | 1 | |
| Contaminated MEC samples (n = 18) | negative bacterial culture EEC | 7 | 0 |
| positive bacterial culture EEC | 8 | 3 |
EEC external ear canal, MEC middle ear cavity.
Table 3.
Localisation of isolated organisms in 26 EECs and 26 MECs.
| Isolated microorganism | EEC only | MEC only | Both EEC and MEC |
|---|---|---|---|
| Negative cultures | 11 (42.3%) | 22 (84.6%) | |
| Acinetobacter johnsonii | 1 (3.8%) | ||
| Bacillus sp. | 3 (11.5%) | 1 (3.8%) | |
| Enhydrobacter aerosaccus | 1 (3.8%) | ||
| Enterococcus gallinarum | 2 (7.7%) | ||
| Kocuria sp. | 1 (3.8%) | ||
| Macrococcus caseolyticus | 1 (3.8%) | ||
| Microbacterium paraoxidans | 1 (3.8%) | ||
| Micrococcus sp. | 1 (3.8%) | ||
| Ochrobactrum intermedium | 1 (3.8%) | ||
| Staphylococcus epidermidis | 1 (3.8%) | ||
| Staphylococcus equorum | 1 (3.8%) | ||
| Staphylococcus pseudintermedius | 1 (3.8%) | ||
| Staphylococcus warneri | 1 (3.8%) | ||
| Staphylococcus sciuri ssp sciuri | 1 (3.8%) | ||
| Staphylococcus xylosus | 1 (3.8%) | ||
| Malassezia pachydermatis | 3 (11.5%) | 1 (3.8%) | |
| Candida albicans | 1 (3.8%) |
EEC, external ear canal; MEC, middle ear cavity.
Table 4.
Microbial culture results regarding previous antibiotic treatment.
| EEC positive cultures in total | MEC positive cultures in total | EEC positive cultures of bacteria | MEC positive cultures of bacteria | EEC positive culture of yeast | MEC positive culture of yeast | |
|---|---|---|---|---|---|---|
| total number of samples (n = 26) | 57.7% | 15.4% | 23.1% | 11.5% | 34.6% | 3.9% |
| no history of antibiotic treatment (n = 17) | 64.7% | 11.8% | 29.4% | 11.8% | 35.3% | 0% |
| previous antibiotic treatment (n = 4) | 75% | 25% | 0% | 0% | 75% | 25% |
| not documented (n = 5) | 20% | 20% | 20% | 20% | 0% | 0% |
EEC, external ear canal; MEC, middle ear cavity.
In none of the cytology samples inflammatory cells or rods were detected and a mean number of ≤2 yeast cells and ≤2 cocci per oil-immersion field in all EECs was found. Cytology of all MEC samples was negative, with the exception of case 7, left ear, where yeast organisms (2 per oil-immersion field) were found microscopically and in microbiological culture. Cytological results and microbiological results are compared in Table 5. Microorganisms were found microscopically in 46.7% and 25% of positive cultures of EECs and MECs, respectively.
Table 5.
Comparison of cytological and microbiological culture results.
| Cadaver | Cytology EEC | Culture EEC | Cytology MEC | Culture MEC |
|---|---|---|---|---|
| 1 | left+right: | left+right: | left+right: | left+right: |
| N/A | N/A | N/A | N/A | |
| 2 | left: yeast 2/OIF | left: Malassezia pachydermatis | left: negative | left: Micrococcus sp., Enterococcus gallinarum |
| 3 | right: yeast 2/OIF | right: Malassezia pachydermatis | right: negative | right: Bacillus sp. |
| 4 | left: yeast 2/OIF | left: Enhydrobacter aerosaccus | left: negative | left: negative |
| right: negative | right: Acinetobacter johnsonii | right: negative | right: negative | |
| 5 | right: yeast 1/IOF | right: Ochrobactrum intermedium | right: negative | right: Enterococcus gallinarum, Microbacterium paraoxidans |
| 6 | left+right: negative | left+right: negative | left+right: negative | left+right: negative |
| 7 | left: yeast 2/OIF | left: Malassezia pachydermatis, Candida albicans | left: yeast 2/OIF | left: Malassezia pachydermatis, Candida albicans |
| 8 | left: negative | left: negative | left: negative | left: negative |
| right: negative | right: Kocuria sp., Staphylococcus xylosus, Staphylococcus sciuri ssp. sciuri | right: negative | right: negative | |
| 9 | left: cocci 2/OIF, yeast 1/OIF | left: Bacillus sp., Malassezia pachydermatis | left: negative | left: negative |
| right: cocci 2/OIF, yeast 1/OIF | right: negative | right: negative | right: negative | |
| 10 | right: cocci 2/OIF | right: Staphylococcus equorum | right: negative | right: negative |
| 11 | left: cocci 1/OIF | left: Bacillus sp. | left: negative | left: negative |
| right: cocci 1/OIF | right: negative | right: negative | right: negative | |
| 12 | left+right: negative | left+right: negative | left+right: negative | left+right: negative |
| 13 | left: negative | left: Staphylococcus warneri | left: negative | left: negative |
| right: negative | right: Bacillus sp. | right: negative | right: negative | |
| 14 | left: cocci 1/OIF | left: Staphylococcus pseudintermedius | left: negative | left: negative |
| right: cocci 1/OIF | right: negative | right: negative | right: negative | |
| 15 | left: negative | left: Staphylococcus epidermidis | left: negative | left: negative |
| right: negative | right: negative | right: negative | right: negative | |
| 16 | left: negative | left: Macrococcus caseolyticus | left: negative | left: negative |
| 17 | left+right: negative | left+right: negative | left+right: negative | left+right: negative |
EEC, external ear canal; MEC, middle ear cavity; OIF oil-immersion field; N/A, not available.
4. Discussion
Sample collection from the MEC via myringotomy may lead to microbial contamination not only in infectious OE cases, but also with healthy ear canals. The high contamination rate found in our study may be attributed to several reasons.
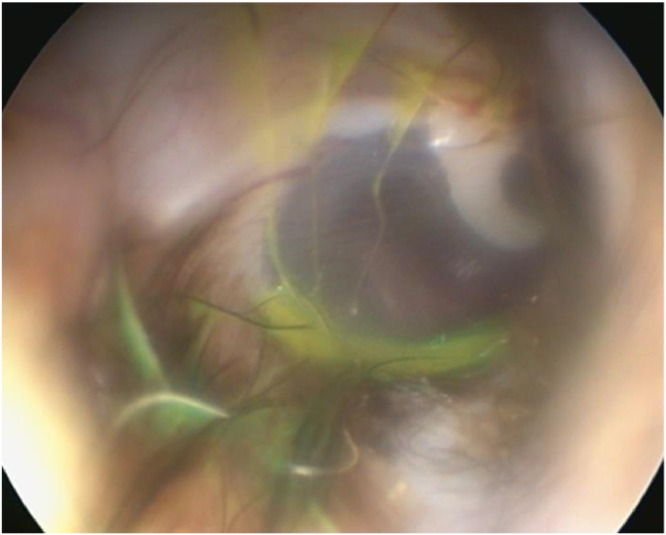
First, the TM is orientated at an approximately 30 to 45° angle with its dorsal portion closer to the viewer than the ventral aspect (Angus & Campbell, 2001; Griffin, 2006; Spreull, 1964). Njaa, Cole and Tabacca (2012) described this angle as helpful, as it allows evacuation of fluid residuals without rupturing the TM. In contrast, we commonly observed a small remnant of accumulated liquid in front of the TM after the drying procedure, especially when the angulation between the TM and ear canal wall appeared very sharp (Fig. 2). This may also be explained by the facilitated fluid visualisation due to its yellow character. The residual fluid accumulates ventrally and - following gravity - may contaminate the MEC after myringotomy has been performed. In our study, the TM was punctured in the caudoventral quadrant of the pars tensa as advised in the literature (Angus & Campbell, 2001; Gotthelf, 2004; Griffin, 2006; Rose, 1977). Perforating the TM in the dorsal half may reduce contamination; however, a myringotomy in the dorsal aspect is not recommended because the corresponding region of the MEC contains several important structures (Rose, 1977). To prevent fluid accumulation at the ventral TM, the ear canal and the TM might be approached from ventrally with the animal positioned above the performer (Mohammaddavoodi et al., 2018).
Fig. 2.
Remnant of yellow coloured fluid in front of the TM after the drying procedure.
Large defects in the TM created by the instrument used for myringotomy may also contribute to a possible contamination. In our study, a needle with 1.5 mm diameter was used. In the literature a variety of instruments for the procedure is described; Griffin (2006) recommended utilisation of a 0.64 mm diameter spinal needle and Cole (2004) suggested a 1.17 mm diameter tomcat catheter for myringotomy, which may cause a smaller defect. On the contrary, other authors used larger instruments such as a 1.7 mm diameter polypropylene catheter (Cole, Rajala-Schultz, Lorch & Daniels, 2019; Gotthelf, 2004) or a myringotomy knife (Guerin, Hampel & Ter Haar, 2015; Rose, 1977; Stern-Bertholtz, Sjöström & Håkanson, 2003), which may create an even bigger defect.
It might be speculated that the use of cadavers in this study could have influenced the outcome. To minimize autolytic processes, the cadavers underwent examination within 4 h post-mortem. Furthermore, the TMs included did not show any signs of inflammation. As healthy TMs require more force to rupture in comparison to diseased membranes (Spreull, 1964), it may be derived that myringotomy in OE cases with more fragile TMs may create larger defects, further augmenting the contamination risk. In addition, the cadavers used in our study with clinically and cytologically healthy EECs (according to guidelines used by Ginel, Lucena, Rodriguez & Ortega, 2002; Lehner, Louis & Mueller, 2010; Nuttall & Bensignor, 2014), made video otoscopy easier compared to stenotic or hyperplastic ear canals. The difficulties of the procedure associated with the latter probably will increase sample contamination.
Our results are in line with recommendations to avoid myringotomy in cases with only weak criteria for OM, such as changes in the appearance of the TM (Rosychuk & Bloom, 2010). As suggested by Colombini, Merchant and Hosgood (2000) in cases with intact TMs, sampling the MEC via bulla osteotomy might be the preferred technique to get a true representation of the MEC content. Additionally, contamination may compromise antimicrobial selection (Colombini et al., 2000), since the microbiota in the MEC and the EEC frequently differ from each other (Cole et al., 1998). However, in our opinion, myringotomy appears to be appropriate in patients with suspected MEC disease based on diagnostic imaging (CT or MRI of the MEC) in the absence of OE. Further, myringotomy may not only be a diagnostic, but also an important therapeutic procedure in OM (Cole, 2004; Gotthelf, 2004; Rose, 1977).
In this study, we have also obtained information on the microorganisms in dogs with normal EECs and MECs. Cytological examination of all obtained samples depicted similar results as reported for healthy canine ears (Ginel et al., 2002; Lehner et al., 2010; Nuttall & Bensignor, 2014). Microorganisms were found microscopically in 46.7% and 25% of positive cultures of EECs and MECs, respectively. According to previous studies, the microscopic evaluation of samples is not a reliable predictor of microbiological culture results (Cole et al., 1998, 2019; Graham-Mize & Rosser, 2004). There are two reasons for the poor correlation between cytological presence of bacteria with bacterial culture results. First, only few organisms for growth are necessary to occur on the media under optimal laboratory conditions, they can easily be missed on a single slide with a small number of bacteria present. Moreover, not the entire slide is evaluated when doing cytological examination (Graham-Mize & Rosser, 2004).
Only three studies have evaluated the microbiota of healthy canine MECs (Defalque et al., 2005; Fraser et al., 1969; Matsuda et al., 1984). In our study, 84.6% of the MEC cultures yielded no microbial growth; in contrast, Matsuda et al. (1984) and Defalque et al. (2005) reported only 52% and 17%, respectively, of healthy MECs without microorganisms. On the other hand, bacterial cultures of primary secretory otitis media (PSOM) cases are also negative in the majority of cases (Cole et al., 2019; Foster, Morandi & May 2015; Paterson, 2018; Stern-Bertholtz et al., 2003;). Pre-treatment with systemic antibiotics might have influenced microbial culture results, since 3 of 17 dogs in our study had received systemic antibiotics. No sample of those four EECs and MECs showed bacterial growth but they were more likely to have positive cultures of yeast compared to the group without antimicrobial treatment. Based on published data and our results, there is a microbiota in the MEC that may have ascended from the nasopharynx via the Eustachian tube (Matsuda et al., 1984). Fraser et al. (1969) cultured healthy MECs, nares and tonsils and found similar microbiota. It is not recommended to establish a diagnosis of OM only based on positive bacterial culture. Additional cytological examination represents a diagnostic procedure for differentiating between resident colonization, overgrowth and infection (Angus, 2004).
Staphylococcus spp, Streptococcus spp or Escherichia coli, which are reported to be commonly found in healthy MECs and healthy nasopharyngeal regions (Defalque et al., 2005; Fraser et al., 1969; Matsuda et al., 1984), were not cultured in our study. Instead, we recovered Bacillus sp, Enterococcus gallinarum and Micrococcus sp, which were also found in at least one of the above-mentioned studies. Fraser et al. (1969) also cultured Micrococcus sp and Bacillus sp in healthy nares and tonsils. Moreover, Microbacterium paraoxidans was cultured from one MEC in our study; however, it has not previously been detected in healthy MECs. Yeast organisms were found in 8% of healthy MECs by Matsuda et al. (1984); in our study, Malassezia pachydermatis and Candida albicans were isolated from one MEC. However, this was a contaminated sample and the same organisms were also cultured from the corresponding EEC; if the yeast organisms were part of the MEC microbiota, or rather a contamination from the EEC is under question. There are no other reports that found yeast organisms in healthy MECs or PSOM cases (Cole et al., 2019; Corfield, Burrows, Imani & Bryden, 2008; Defalque et al., 2005; Fraser et al., 1969; Guerin et al., 2015; Stern-Bertholtz et al., 2003).
It should be stressed, that we had a different sampling method, which could have affected the results of bacterial cultures. Fraser et al. (1969) did not report how the samples were taken, Matsuda et al. (1984) and Defalque et al. (2005) sampled canine MECs by bulla osteotomy. Also, we should reflect, that we have collected material by injecting 1 mL of fluid. In case of a very sparse bacterial population, this might have negatively influenced the ability of bacteria to multiply. A sample directly taken from the MEC mucosa with a sterile swab could potentially reveal different results.
As previously shown, isolated microbial populations from the vertical/horizontal ear canal and corresponding MEC may differ in healthy and inflamed ears (Cole et al., 1998; Matsuda et al., 1984). In our study, there was only one ear with the same microorganisms (Candida albicans, Malassezia pachydermatis) in the EEC and MEC, although, the positive culture of the MEC could also represent contamination, since this was a contaminated MEC sample. The contamination rate in our study was high, but only one dog had the same microorganisms in the ear canal and MEC, questioning the high risk of introduction of microorganisms into the MEC. Potentially, because there was no infection in the EEC, the risk of introducing bacteria into the MEC may be higher in cases of OE where a higher number of microorganisms is present. Besides the possibility of contamination, it has also been shown that the reliability of cultures taken from the EEC is questionable; Graham-Mize and Rosser (2004) reported that only 60% of paired samples for bacterial culture and susceptibility testing taken from the same location of the EEC in dogs with OE had identical results. These authors concluded, that a single sample may not reflect the total population of microorganisms present in the EEC. Based on the aforementioned study, bacterial cultures must be interpreted with caution. It is not possible to determine if the microorganisms found in the contaminated MEC samples represented the physiological MEC microbiota or were a result of contamination, although different microorganisms were found in the corresponding EECs. Comparison between bacterial isolates gained by myringotomy and by bulla osteotomy would be an interesting topic for future studies.
The major limitation of this study is the relatively small number of ears included, preventing profound statistical analysis. Another limitation is, that not all of the MECs were scanned by CT in order to exclude MEC disease. We did not exclude the concerned six ears, because even if MEC disease was present, it would not have influenced the outcome of the contamination rate, since the use of fluorescent dye excluded false positive contaminations. However, MEC infection would have influenced the outcome of the microbiological examination; two ears were not sampled for cytology or microbiological analysis, three MECs had negative culture results and one MEC sample showed bacterial growth, but negative cytology, making infection very unlikely. Furthermore, it was not investigated, if there is any antibacterial activity of the fluorescent dye that may have influenced the results of bacterial cultures; this should be excluded before fluorescent dye is used in further studies.
5. Conclusions
With respect to the high contamination rate found in our study, the validity of cytological and microbial examinations of MEC aspirates collected via myringotomy to diagnose OM needs to be challenged. Our findings suggest that in patients with OE, the diagnosis of concurrent OM may be erroneously established if solely based on the examination of possibly contaminated MEC aspirates. In addition, the risk of iatrogenic spread of microorganisms needs to be considered.
Conflict of interest statement
The company Karl Storz, GmbH and Co. KG supported this project with the loan of the video otoscope (Karl Storz Telepack Vet X 69,045,020, Video Camera Head SN MD649776-H, 50 W HiLux Light Source) and the vacuum-pump (Karl Storz Vetpump 2 69,321,620). The company played no role in the study design, in the collection, analysis and interpretation of data, nor in the decision to submit the manuscript for publication. None of the authors of this article has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Ethical statement
None
Declaration of Competing Interest
None
Acknowledgements
The authors thank the company Karl Storz, GmbH and Co. KG for supporting this project with the loan of the video otoscope (Karl Storz Telepack Vet X 69045020, Video Camera Head SN MD649776-H, 50 W HiLux Light Source) and the vacuum-pump (Karl Storz Vetpump 2 69321620). We acknowledge A.Tichy for the statistical analysis and A. Mohammaddavoodi for his help during sample collection. Preliminary results were presented as an oral communication at the 8thWorld Congress of Veterinary Dermatology, Bordeaux, May 31 to June 4, 2016
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.vas.2020.100125.
Appendix. Supplementary materials
References
- Angus J.C. Otic cytology in health and disease. The Veterinary Clinics of North America. Small Animal Practice. 2004;34:411–424. doi: 10.1016/j.cvsm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Angus J.C., Campbell K.L. Uses and indications for video-otoscopy in small animal practice. The Veterinary Clinics of North America. Small Animal Practice. 2001;31:809–828. doi: 10.1016/s0195-5616(01)50072-8. [DOI] [PubMed] [Google Scholar]
- Belmudes A., Pressanti C., Barthez P.Y., Castilla-Castaño E., Fabries L., Cadiergues M.C. Computed tomographic findings in 205 dogs with clinical signs compatible with middle ear disease: A retrospective study. Veterinary Dermatology. 2018;29:45–e20. doi: 10.1111/vde.12503. [DOI] [PubMed] [Google Scholar]
- Bruyette D.S., Lorenz M.D. Otitis externa and otitis media: Diagnostic and medical aspects. Seminars in Veterinary Medicine and Surgery (Small Animal) 1993;8:3–9. [PubMed] [Google Scholar]
- Classen J., Bruehschwein A., Meyer-Lindenberg A., Mueller R.S. Comparison of ultrasound imaging and video otoscopy with cross-sectional imaging for the diagnosis of canine otitis media. The Veterinary Journal. 2016;217:68–71. doi: 10.1016/j.tvjl.2016.09.010. [DOI] [PubMed] [Google Scholar]
- Cole L.K. Otoscopic evaluation of the ear canal. Veterinary Clinics of North America. Small Animal Practice. 2004;34:397–410. doi: 10.1016/j.cvsm.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Cole L.K., Kwochka K.W., Kowalski J.J., Hillier A. Microbial flora and antimicrobial susceptibility patterns of isolated pathogens from the horizontal ear canal and middle ear in dogs with otitis media. Journal of the American Veterinary Medical Association. 1998;212:534–538. [PubMed] [Google Scholar]
- Cole L.K., Kwochka K.W., Podell M., Hillier A., Smeak D.D. Evaluation of radiography, otoscopy, pneumotoscopy, impedance audiometry and endoscopy for the diagnosis of otitis media. In: Thoday K.L., Foil C.S., Bon R., editors. Eds. Volume 4. Blackwell Science; Oxford, UK: 2002. pp. 49–55. (Advances in veterinary dermatology). [Google Scholar]
- Cole L.K., Rajala-Schultz P.J., Lorch G., Daniels J.B. Bacteriology and cytology of otic exudates in 41 cavalier King Charles spaniels with primary secretory otitis media. Veterinary Dermatology. 2019;30:152–e44. doi: 10.1111/vde.12724. [DOI] [PubMed] [Google Scholar]
- Colombini S., Merchant S.R., Hosgood G. Microbial flora and antimicrobial susceptibility patterns from dogs with otitis media. Veterinary Dermatology. 2000;11:235–239. [Google Scholar]
- Corfield G.S., Burrows A.K., Imani P., Bryden S.L. The method of application and short term results of tympanostomy tubes for the treatment of primary secretory otitis media in three Cavalier King Charles Spaniel dogs. Australian Veterinary Journal. 2008;86:88–94. doi: 10.1111/j.1751-0813.2008.00254.x. [DOI] [PubMed] [Google Scholar]
- Defalque V.E., Rosser E.J., Petersen A.D. Aerobic and anaerobic bacterial microflora of the middle ear cavity in normal dogs. Journal Veterinary Dermatology. 2005;16:196. [Google Scholar]
- Dickie A.M., Doust R., Cromarty L., Johnson V.S., Sullivan M., Boyd J.S. Comparison of ultrasonography, radiography and a single computed tomography slice for the identification of fluid within the canine tympanic bulla. Research in Veterinary Science. 2003;75:209–216. doi: 10.1016/s0034-5288(03)00118-8. [DOI] [PubMed] [Google Scholar]
- Doyle R.S., Skelly C., Bellenger C.R. Surgical management of 43 cases of chronic otitis externa in the dog. Irish Veterinary Journal. 2004;57:22–30. doi: 10.1186/2046-0481-57-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster A., Morandi F., May E. Prevalence of ear disease in dogs undergoing multidetector thin-slice computed tomography of the head. Veterinary Radiology and Ultrasound. 2015;56:18–24. doi: 10.1111/vru.12180. [DOI] [PubMed] [Google Scholar]
- Fraser G., Gregor W.W., Mackenzie C.P., Spreull J.S.A., Withers A.R. Canine Ear Disease. Journal of Small Animal Practice. 1969;10:725–754. doi: 10.1111/j.1748-5827.1969.tb03995.x. [DOI] [PubMed] [Google Scholar]
- Garosi L.S., Dennis R., Schwarz T. Review of diagnostic imaging of ear diseases in the dog and cat. Veterinary Radiology and Ultrasound. 2003;44:137–146. doi: 10.1111/j.1740-8261.2003.tb01262.x. [DOI] [PubMed] [Google Scholar]
- Ginel P.J., Lucena R., Rodriguez J.C., Ortega J. A semiquantitative cytological evaluation of normal and pathological samples from the external ear canal of dogs and cats. Veterinary Dermatology. 2002;13:151–156. doi: 10.1046/j.1365-3164.2002.00288.x. [DOI] [PubMed] [Google Scholar]
- Gotthelf L.N. Diagnosis and treatment of otitis media in dogs and cats. The Veterinary Clinics of North America. Small Animal Practice. 2004;34:469–487. doi: 10.1016/j.cvsm.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Graham-Mize C.A., Rosser E.J. Comparison of microbial isolates and susceptibility patterns from the external ear canal of dogs with otitis externa. Journal of the American Animal Hospital Association. 2004;40:102–108. doi: 10.5326/0400102. [DOI] [PubMed] [Google Scholar]
- Griffin C.E. Otitis techniques to improve practice. Clinical Techniques in Small Animal Practice. 2006;21:96–105. doi: 10.1053/j.ctsap.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Guerin V., Hampel R., Ter Haar G. Video-otoscopy-guided tympanostomy tube placement for treatment of middle ear effusion. Journal of Small Animal Practice. 2015;56:606–612. doi: 10.1111/jsap.12397. [DOI] [PubMed] [Google Scholar]
- Herrmann I. Master of Veterinary Medicine; Vetmeduni Vienna, Austria: 2014. Chronische Otitis beim Hund: Nutzen der Videootoskopie und Spülung. Eine retrospektive Studie. Thesis. [Google Scholar]
- Hill P.B., Lo A., Eden C.A.N., Huntley S., Morey V., Ramsey S., Richardson, C., Smith, D.J., Sutton, C., Taylor, M.D., Thorpe, E., Tidmarsh, R., &. Survey of the prevalence, diagnosis and treatment of dermatological conditions in small animals in general practice. The Veterinary Record. 2006;158:533–539. doi: 10.1136/vr.158.16.533. [DOI] [PubMed] [Google Scholar]
- Lehner G., Louis C.S., Mueller R.S. Reproducibility of ear cytology in dogs with otitis externa. The Veterinary Record. 2010;167:23–26. doi: 10.1136/vr.c3523. [DOI] [PubMed] [Google Scholar]
- Little C.J., Lane J.G., Pearson G.R. Inflammatory middle ear disease of the dog: The pathology of otitis media. The Veterinary Record. 1991;128:293–296. doi: 10.1136/vr.128.13.293. [DOI] [PubMed] [Google Scholar]
- Logas D.B. Diseases of the ear canal. The Veterinary Clinics of North America. Small Animal Practice. 1994;24:905–919. doi: 10.1016/s0195-5616(94)50108-6. [DOI] [PubMed] [Google Scholar]
- Lund E.M., Armstrong P.J., Kirk C.A., Kolar L.M., Klausner J.S. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. Journal of the American Veterinary Medical Association. 1999;214:1336–1341. [PubMed] [Google Scholar]
- Matsuda H., Tojo M., Fukui K., Imori T., Baba E. The aerobic bacterial flora of the middle and external ears in normal dogs. Journal of Small Animal Practice. 1984;25:269–274. [Google Scholar]
- Mohammaddavoodi A., Hirt R., Kneissl S., Aghapour M., Reinbacher E., Spergser J. Videoendoscope-guided myringotomy: Value of a vertical access to the tympanic membrane during myringotomy with respect to avoidance of contamination in dogs – a pilot study. Journal Veterinary Dermatology. 2018;29:367. [Google Scholar]
- Moltzen H. Otitis media in the dog and cat. In: Jone B.V., editor. Advances in small animal practice. Ed. Pergamon Press; Oxford, UK: 1961. pp. 62–65. [Google Scholar]
- Njaa B.L., Cole L.K., Tabacca N. Practical otic anatomy and physiology of the dog and cat. The Veterinary Clinics of North America. Small animal practice. 2012;42:1109–1126. doi: 10.1016/j.cvsm.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Nuttall T., Bensignor E. A pilot study to develop an objective clinical score for canine otitis externa. Veterinary Dermatology. 2014;25:530–537. doi: 10.1111/vde.12163. [DOI] [PubMed] [Google Scholar]
- Paterson S. Otitis media with effusion in the boxer: A report of seven cases. Journal of Small Animal Practice. 2018;59:646–650. doi: 10.1111/jsap.12801. [DOI] [PubMed] [Google Scholar]
- Remedios A.M., Fowler J.D., Pharr J.W. A comparison of radiographic versus surgical diagnosis of otitis media. Journal of the American Animal Hospital Association. 1991;27:183–188. [Google Scholar]
- Rose W.R. Otitis media. Number ten in a series. Veterinary Medicine, Small Animal Clinician. 1976;71:1443–1451. [PubMed] [Google Scholar]
- Rose W.R. Small animal clinical otology. Surgery 1-myringotomy. Number twenty-two in a series. Veterinary Medicine, Small Animal Clinician. 1977;72:1646–1650. [PubMed] [Google Scholar]
- Rosychuk R.A.W., Bloom P. Otitis media: How common and how important. In: DeBoer D.J., Affolter V.K., Hill P.B., editors. Eds. Volume 6. Wiley-Blackwell; Oxford, UK: 2010. pp. 345–352. (Advances in veterinary dermatology). [Google Scholar]
- Saridomichelakis M.N., Farmaki R., Leontides L.S., Koutinas A.F. Aetiology of canine otitis externa: A retrospective study of 100 cases. Veterinary Dermatology. 2007;18:341–347. doi: 10.1111/j.1365-3164.2007.00619.x. [DOI] [PubMed] [Google Scholar]
- Schunk K.L., Averill D.R. Peripheral vestibular syndrome in the dog: A review of 83 cases. Journal of the American Veterinary Medical Association. 1983;182:1354–1357. [PubMed] [Google Scholar]
- Shell L.G. Otitis media and otitis interna. Etiology, diagnosis, and medical management. Veterinary Clinics of North America. Small Animal Practice. 1988;18:885–899. doi: 10.1016/s0195-5616(88)50088-8. [DOI] [PubMed] [Google Scholar]
- Spreull J.S.A. Treatment of Otitis Media in the Dog. Journal of Small Animal Practice. 1964;5:107–122. [Google Scholar]
- Spreull J.S.A. Otitis media of the dog. In: Kirk R.W., editor. Current veterinary therapy; small animal practice. Ed. W.B. Saunders; Philadelphia, USA: 1974. pp. 675–683. [Google Scholar]
- Stern-Bertholtz W., Sjöström L., Håkanson N.W. Primary secretory otitis media in the Cavalier King Charles spaniel: A review of 61 cases. Journal of Small Animal Practice. 2003;44:253–256. doi: 10.1111/j.1748-5827.2003.tb00151.x. [DOI] [PubMed] [Google Scholar]
- Trower N.D., Gregory S.P., Renfrew H., Lamb C.R. Evaluation of the canine tympanic membrane by positive contrast ear canalography. The Veterinary Record. 1998;142:78–81. doi: 10.1136/vr.142.4.78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.