Abstract
A 70-days feeding trial was performed to determine the effect of diets with whole plant-origin proteins added with different ratios of taurine:methionine on the growth, macrophage burst activity and antioxidant capacity of rainbow trout (Oncorhynchus mykiss) fingerlings. Triplicated groups of 70 fingerlings of an initial weight of 0.54±0.1 g (mean±±SD) were fed diets with soy protein isolate and Spirulina powder as protein sources (46% crude protein) and added (10 g/kg diet) with different taurine and methionine ratios: 0.0 and 10.0 (diet T0/M100), 2.5 and 7.5 (diet T25/M75), 5.0 and 5.0 (diet T50/M50), 7.5 and 2.5 (diet T75/M25) and 10.0 and 0.0 (diet T100/M0), respectively. At the end of the trial, growth performance, lipid and protein contents in liver and muscle, macrophage burst activity and liver antioxidant activity, were determined. The growth performance, macrophage burst activity and antioxidant activity were improved as the taurine increased in the diets. The ratio of 7.5 and 2.5 g/kg of taurine:methionine in diets with SPI and Spirulina powder as protein sources (diet T75/M25), seems to be the best inclusion for rainbow trout fingerlings.
Keywords: Antioxidant capacity, Methionine, Rainbow trout, Soy protein isolate, Taurine
Highlights
-
•
Several ratios of taurine:methionine for rainbow trour fed while plant-protein diets.
-
•
Taurine improved antioxidant capacity of heptocites and burst activity of macropahges.
-
•
10 g/kg of taurine:methionine inclusion is optimal for rainbow trout fed whole plant protein diets.
1. Introduction
The rapid growth of the aquaculture during the last 20 years has been accompanied by an increasing demand for aquafeeds (Gatlin et al., 2007). Fishmeal is still the main source of protein in commercial diets and with no expectations to increase the production beyond the current levels (Hardy, 1996) the demand for fishmeal will exceed the supply in a few years (Tacon & Metian, 2008). Plant-origin meals are pointed out as an alternative to fishmeal and particularly, the protein concentrates and isolates of oilseeds are good candidates, as they contain low of levels of fiber, starch, non-soluble carbohydrates and anti-nutrients compounds (Brown, Kaushik & Peres, 2008). Still, such protein sources might be deficient in some indispensable amino acids (such as methionine) and lack certain components such as taurine, hydroxiproline and nucleotides, which appear to have some beneficial proprieties on growth and well-being of fish (Bañuelos-Vargas, López, Pérez-Jiménez & Peres, 2014).
Methionine is a sulfur-containing amino acid and it has several metabolic roles, such as being a precursor in the protein synthesis, production of S-adenosylnethionine (SAM), L-cystein, glutathione, taurine, phosphatidylcholine and other phospholipids. On the other hand, taurine is a non-proteic amino acid (2-aminoethanosulfonic acid), which is involved in several physiological processes: bile acid conjugation, osmoregulation and membrane stabilization and antioxidant activity (Divakaran, 2006, Salze and Davis, 2015). The taurine biosynthesis starts with the methionine conversion to cysteine, which is oxidized to cysteine sulfonic acid mediated by cysteine dioxygenase (CDO), then a decarboxylation to hypotaurine mediated by the cysteine sulfonate decarboxylase (CDS) and a final step of oxidation to taurine (Wang et al., 2015, Salze and Davis, 2015). The taurine biosynthesis from methionine, has been detected in the rainbow trout (Oncorhynchus mykiss) (Yokoyama, Takeuchi, Park & Nakazoe, 2001); but, it seems not to occur at adequate levels when plant proteins sources are used to substitute fish meal (Gaylord et al., 2006, Boonyoung et al., 2013) and might be a conditionally indispensable amino acid (Takeuchi, Park, Seikai & Yokoyama, 2001). It has been reported that inclusion of taurine to diets with plant-origin protein sources improved the growth of rainbow trout (Gaylord et al., 2006). Gaylord et al. (2007) found that supplement diets with three levels of taurine and/or three levels of DL-methionine to diets with soy protein concentrate fed to rainbow trout juvenile showed an improved of growth performance and that methionine supplementation above the requirement did not influence spare taurine. Boonyoung et al. (2013) reported that a methionine hydroxy-analog might be converted to taurine and improve the growth of rainbow trout fingerlings when fed a diet with soy protein concentrate. So far, there is no information available about the inclusion of methionine and/or taurine in diets with soy protein isolation. In addition to these, the importance of methionine as a precursor of taurine, the role of the latter in the immune and antioxidant activity, as well the necessity to include them in diets with plant-origin proteins, the aim of the present work is to determine the effect of diet with soy protein isolate and Spirulina powder as protein sources added with different ratios of taurine:methionine on the growth, macrophage burst activity and antioxidant capacity of rainbow trout (Oncorhynchus mykiss) fingerlings.
2. Materials and methods
2.1. Experimental diets
A basal diet was formulated with a minimum content of 45% of crude protein and 10% of lipids to cover the requirements previously reported for the species (NRC, 2011). Fishmeal was substituted at 100% with a mixture of soy protein isolate (SPI, crude protein 90±1%, Agridient México S. de R.L. de C.V., Mexico) and Spirulina powder (SP, crude protein 55±2%; Pronaquim, S.A. de C.V., México), according with Table 1. Taurine (catalog number T0625, purity ≥99%, Sigma Aldrich Co., USA) and DL-methionine (Evonik Mexico S.A de C.V., Mexico) were added at a level of 10 g/kg diet, considering the following ratios: 0.0 and 10.0 (diet T0/M100), 2.5 and 7.5 (diet T25/M75), 5.0 and 5.0 (diet T50/M50), 7.5 and 2.5 (diet T75/M25) and 10.0 and 0.0 (diet T100/M0), respectively (Table 2). A diet without addition of taurine or methionine (diet T0/M0) was used as well. Finally, and as control, a commercial diet (Comm, Api-trucha-1 pellet, 45% protein, Malta-Cleyton de Mexico) was also use. Cod liver oil (Drotasa S.A. de C.V., Mexico) and soybean lecithin (Abastecedora de Productos Naturales, S.A. de C.V., México) were used as lipid sources and dextrin (Sigma Aldrich Co., USA) as carbohydrate source. A mixture of vitamins and minerals (Micro Rovimix for carnivorous fish, DSM Nutritional Products of Mexico, Mexico) and wheat gluten (Sigma Aldrich Co., USA) were used. Diets were prepared according to Cruz, Hernández, Fernández, Ramírez and Angeles (2011) by mixing all the powered ingredients for a period of 20 min and then, the oils and purified water (40%) were added and mixed again for other 20 min. The resulting wet dough was passed through a meat chopper to obtain 5-mm diameter pellets, which were dried at 60 °C in a constant temperature oven for 60 min and then, store at −24 °C until used.
Table 1.
Basal formulation of the test diets fed to fingerlings of rainbow trout.
| Ingredients (g/kg diet) | |
|---|---|
| Soy protein isolate | 392 |
| Spirulina powder | 22 |
| Cod liver oil | 50 |
| Soybean lecithin | 50 |
| Dextrin | 186 |
| Vitamin and mineral mixa | 40 |
| Wheat gluten | 150 |
| α-celluloseb | 110 |
| Proximate composition (% on dry weight basis) | |
| Crude protein | 46±3 |
| Crude lipid | 12±1 |
| Ash | 9±2 |
Amino acid profile (% of the diet, dry weight basis): arginine, 2.85±0.05; histidine, 1.02±0.01; isoleucine, 2.00±0.01; lysine, 2.11±0.02; methionine, 0.61±0.01; phenylalanine, 2.34±0.02; threonine, 1.49±0.05; valine, 2.04±0.01.
Vitamin and mineral mixture (g/kg): ρ-aminobenzoic acid, 1.45; biotin, 0.02; myo-inositol, 14.5; nicotinic acid, 2.9; Ca-pantothenate, 1.0; pyridoxine-HCl, 0.17; riboflavin, 0.73; thiamine-HCl, 0.22; menadione, 0.17; α-tocopherol, 1.45; cyanocobalamine, 0.0003; calciferol, 0.03, L-ascorbyl-2-phosphate-Mg, 0.25; folic acid, 0.05; choline chloride, 29.65, NaCl, 1.838; MgSO4·7H2O, 6.85; NaH2PO4·2H2O, 4.36; KH2PO4, 11.99; Ca(H2PO4)2·2H2O, 6.79; Fe-citrate, 1.48; Ca-lactate, 16.35; AlCl3·6H2O, 0.009; ZnSO4·7H2O, 0.17; CuCl2, 0.0005; MnSO4·4H2O, 0.04; KI, 0.008 and CoCl2, 0.05.
Different ratios of the taurine and methinine was added at the expense of 10 g of the α-cellulose
Table 2.
Ratios of taurine and methionine included in the diets for rainbow trout fingerlings.
| Amino acid inclusion (g/kg) | Treatments |
|||||
|---|---|---|---|---|---|---|
| T0/M100 | T25/M75 | T50/M50 | T75/M25 | T100/M0 | T0/M0 | |
| Taurinea | 0.0 | 2.5 | 5.0 | 7.5 | 10.0 | 0.0 |
| DL-methonineb | 10.0 | 7.5 | 5.0 | 2.5 | 0.0 | 0.0 |
Sigma Aldrich Co., USA.
Evonik Mexico S.A de C.V., Mexico.
2.2. Experimental fish
Eyed eggs of rainbow trout (Oncorhynchus mykiss) were obtained from the “Centro Acuícola de Calimaya” (municipality of Calimaya, State of Mexico, Mexico) and translated to the farm “Los Alevines” (municipality of Amanalco de Becerra, State of Mexico, Mexico) and incubated there. After reabsorbing the egg yolk, fingerlings were fed on a commercial diet (Api-trucha 1, crumble, 50% protein and 15% lipid, Malta-Cleyton de Mexico) until the beginning of the feeding trial.
2.3. Feeding trial
The feeding experiment was conducted in 24 glass-fiber tanks of 1000-L. Seventy fingerlings of an initial weight of 0.54±0.1 g (mean±±standard deviation) were randomly stocked in each tank. Each test diet was fed to triplicate groups of fingerlings. Fish were fed the respective diet at 7% of their body weight and the daily ration size was divided into two equal feedings, offered in the morning and evening. The fingerlings were weighed every 10 days and ration size was adjusted accordingly. Water parameters (mean±±SD) were: dissolved oxygen 4.5±1.0 mg/L, pH 7.2±0.5 and water temperature of 13±1 °C. Water flow in each tank was of 5 L/min during the entire experiment. All tanks were maintained under natural photoperiod, which was of 11 h light, 13 h dark during March and April. The feeding trail using the diets was conducted for a period of 70 days. At the end of the feeding experiment, fish were maintained un-fed for 24 h. To obtain the growth performance, they were weighted and measured for total length. Five organisms form each were sacrificed with an overdose (300 mg/L, Popovic et al., 2012) of MS-222 (ethyl 3-aminobenzoate, methanosulfonic acid, Sigma Aldrich Comp., USA) and were dissected to obtain the samples of liver and muscle for the lipid and protein contents analysis. As well, another 5 fingerlings for each tank were sacrificed as previously described and the kidneys were dissected aseptically and put into sterile test tubes containing 5 ml Leibovitz L-15 medium (Sigma Aldrich Co., USA) supplemented with 0.1% fetal bovine serum (FBS, Biowest SAS, France) (Burrells, Williams, Southgate & Crampton, 1999). Then, the liver was also dissected and put into sterile plastic tubes and frozen on liquid N2. All samples were transported to the Laboratorio de Producción Acuícola, Facultad de Estudios Superiores Iztacala, UNAM, where the analysis were performed.
2.4. Chemical analysis
The contents of protein and lipid in the diets, muscle and liver were determined with the technique reported by AOAC (1990) and the Blight and Dyer (1959) of chloroform and methanol extraction, respectively. The amino acid profile of the basal diet was performed in triplicate by Evonik Mexico S.A de C.V, by using a HPLC, after a protein digestion.
2.5. Macrophage burst activity
The isolation and culture of the kidney macrophages was after Chung and Secombes (1988) and Secombes (1990). The macrophage superoxide anion activity (respiratory burst) of the macrophages was determined by the reduction of the redox dye nitro-blue tetrazolium (NBT) by using a Sunrise microplate reader (Tecan Group Ltd., Switzerland) at a wavelength of 620 nm (OD620). Later, the macrophage number in each well was determined. Results are expressed as OD620 per 105 cells.
2.6. Hepatocyte antioxidant capacity
Livers were homogenized in cold (4 °C) phosphate-buffered saline solution (Sigma Aldrich Comp., USA). Samples were centrifuged for 10 min at 14,000 rpm and 20 μl of the supernatant were used. The antioxidant capacity was measured after Cao, Alessio, and Cutler (1993) by using a Quantichrom™ antioxidant assay kit (BioAssay Systems, USA) and optical density was determined with a Sunrise microplate reader (Tecan Group Ltd., Switzerland) at a wavelength of 570 nm. Results are expressed as μM Trolox equivalents per mg per ml.
2.7. Statistical analysis
Data on final body weight (FW), weight gain (WG), specific growth rate (SGR), feed intake (FI), total length (TL), condition factor (K), survival, contents of lipid and protein in muscle and liver, macrophage burst activity and hepatocyte antioxidant capacity were tested for normality with the Shapiro and Wilk W tests and the Barletts´s test, respectively (Zar, 1999). Since all data showed to be normal and homoscedastic, a one-way ANOVA test was used (Prism for Mac, Graph Pad Software Inc., USA). Significant differences among the treatments were determined by a Fisher LSD test (Zar, 1999), with a significance level of 5% (p<0.05) for each set of comparisons.
3. Results
Means of final body weight (FW), weight gain (WG), specific growth rate (SGR), total length (TL) and condition factor (K) are show in Table 3. There was a trend in which FW, WG and SGR increased with the increasing level of taurine. These indices increased until the treatment T75/M25, beyond which they decreased significantly. The WG and SGR were the lowest in treatments T100/M0 and T0/M0. No significant differences were observed among the fingerling fed diet T75/M25 and the commercial diet. Regarding TL, a significantly lower value was observed in the fingerlings fed the diet T100/M0. The K values were not significantly different among the treatments. Survival rates were higher than 95% in all the groups and no significant differences were observed among the treatments. Survival rates of all the groups was higher than 95% and no significant differences were observed among the groups (T100/M0, 100%; T75/M25 100%; T50/M50, 95±7%; T25/M75, 98±3%; T0/M100, 97±4%; T0/M0, 100%; Comm, 95±7%).
Table 3.
Growth performance of rainbow trout fingerlings fed diets with soy protein isolate and Spirulina powder as protein sources and the addition of different ratios of taurine:methionine. Each value represents the mean of three replicate groups±±standard error. Columns with different letters differ significantly (P<0.05).
| Diets | FW (g) | WGa (%) | SGRb (%/day) | TL (cm) | Kc |
|---|---|---|---|---|---|
| T0/M100 | 1.8±0.2ac | 177±21a | 1.4±0.1a | 5.4±0.1a | 1.2±0.02 |
| T25/M75 | 1.9±0.2ab | 243±26a | 1.7±0.1b | 5.5±0.1a | 1.1±0.03 |
| T50/M50 | 2.1± 0.1ab | 313±25b | 2.0±0.08bc | 5.6±0.1a | 1.2±0.02 |
| T75/M25 | 2.4±0.1b | 359±27b | 2.1±0.09c | 5.6±0.1a | 1.1±0.01 |
| T100/M0 | 1.4±0.1c | 181±20a | 1.5±0.09a | 4.7±0.1b | 1.2±0.03 |
| T0/M0 | 1.5±0.1c | 232±19a | 1.2±0.06a | 5.1±0.1ab | 1.1±0.05 |
| Comm | 2.0±0.2ac | 302±30b | 1.9±0.1bc | 5.5±0.1a | 1.2±0.03 |
Weight gain =((Final weight – initial weight)/initial weight) *100.
Specific growth rate =((ln final weight – ln initial weight)/70) *100.
Condition factor =(weight/length) *100,000.
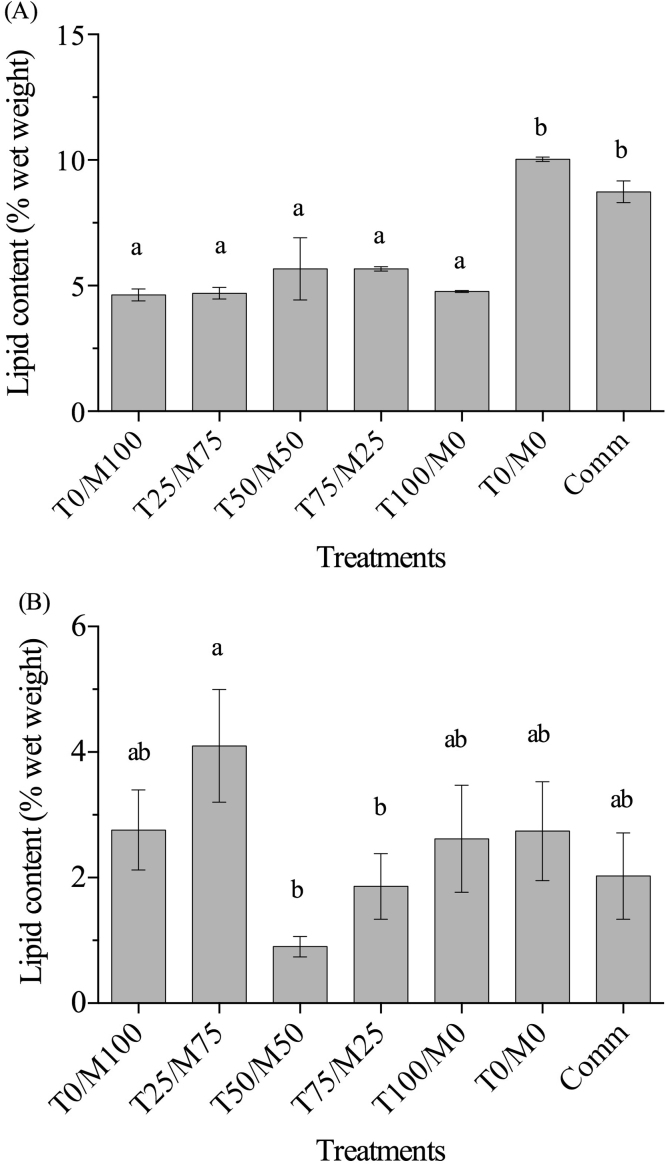
Lipid contents in the liver (Fig. 1A) were significantly lower in the fish fed the diets with the inclusion of taurine and/or methionine than those observed in the fish fed diets T0/M0 and commercial diet. Regarding the lipid contents in muscle (Fig. 1B), a tendency to lower values was observed as the methionine level decreased in the diet, but significant differences were only observed between fish fed the diets T25/M75 and those fed T50/M50 and T75/M25.
Fig. 1.
Lipid content (% wet basis) in liver (A) and muscle (B) of rainbow trout fingerlings fed diets with soy protein isolate and Spirulina powder as protein sources and the addition of different ratios of taurine:methionine. The bars represent the mean of three replicate groups±±standard error. Bars with different letters differ significantly (P<0.05).
The Fig. 2A shows the protein content in liver and a tendency of decreasing values was observed as the methionine concentration lowered in the diets. The lowest value was observed in the fish fed the commercial diet. Muscle protein content (Fig. 2B) did not show significant differences among the treatments.
Fig. 2.
Crude protein contents (% on wet basis) of liver (A) and muscle (B) of rainbow trout fingerlings fed diets with soy protein isolate and Spirulina powder as protein sources and the addition of different ratios of taurine:methionine. The bars represent the mean of three replicate groups±±standard error. Bars with different letters differ significantly (P<0.05).
The antioxidant capacity of hepatocytes is show in Fig. 3 and a trend of increasing values was observed as the taurine concentration increase in the diets. The highest value among the experimental diets was observed in the fish fed the diet T75/M25. Significant higher value was observed in the fish fed the commercial diet when compared with the diets with the other diets, except for that observed in T75/M25.
Fig. 3.
Antioxidant capacity of liver (μeqT/mg/ml) of rainbow trout fingerlings fed diets with soy protein isolate and Spirulina powder as protein sources and the addition of different ratios of taurine:methionine. The bars represent the mean of three replicate groups±±standard error. Bars with different letters differ significantly (P<0.05).
There was a tendency of increased values of the macrophage burst activity with the increment of taurine in the diets (Fig. 4). The burst activity of the fish fed the diet T100/M0 was significantly higher than the other groups, including the fish fed the commercial diet.
Fig. 4.
Kidney macrophage burst activity (OD620/105 cells) of rainbow trout fingerlings fed diets with soy protein isolate and Spirulina powder as protein sources and the addition of different ratios of taurine:methionine. The bars represent the mean of three replicate groups±±standard error. Bars with different letters differ significantly (P<0.05).
4. Discussion
For the first time, here is reported the effect of different ratios of methionine:taurine included in diets with SPI and SP as the main sources of protein in rainbow trout of such small size (0.54 g of initial weight). According to our results, an inclusion of 10 g/kg diet at the ratio of 7.5:2.5 taurine-methinione improved the growth performance and the antioxidant activity in liver, while decreasing the lipid content in the liver. The SPI is considered a good protein source for fish (Glencross et al., 2005), but lacks certain nutrients that are conditionally indispensable when used as a protein sources in formulated diets (Takeuchi et al., 2001).
Inclusion of methionine (Mambrini et al., 1999, Espe et al., 2008) to all-plant protein diets has been previously reported to improve the growth performance in rainbow trout. Interestingly, we observed lower values of growth performance in the fingerlings fed the diet T0/M100. Previously, the NRC (2011) reported that methionine is the most toxic of the primary amino acids, and the concentration of 10 g/kg might be toxic at some level for the fingerlings.
The inclusion of taurine has been reported (Gaylord et al., 2006, Gaylord et al., 2007) to improve the growth of fish when fed plant-protein based diets. We could see increasing values of FW, WG and SGR as the taurine increased in the diet. The role of taurine in the growth is still not clear, but according to Salze and Davis (2015), it seems to stimulate some enzymes related to the intermediary metabolism (amino acid catabolism and glucogenesis) and increasing the nutrient utilization (Bañuelos-Vargas et al., 2014). The inclusion of 10 g/kg of taurine (diet T100/M0) decreased significantly the growth performance of the fingerlings, which indicates that even a small quantity of methionine should be added to diets with all plant-origin proteins, which has been previously reported in rainbow trout (Boonyoung et al., 2013) and Atlantic salmon, Salmo salar (Espe et al., 2008).
Contents of lipids in liver were lower than those reported by Enriquez, Ramírez, Hernández, and Fernández (2015) in the rainbow trout fingerlings fed diets with soybean meal or soy protein concentrate. The inclusion of taurine and/or methionine did influence the lipid deposition in the liver, as significantly lower values of lipid content were observed when fish were fed the experimental diets. Previously, the inclusion of taurine has been reported to be related to a decrease in lipid deposition in liver of Atlantic salmon (Espe, Ruohonen & El-Mowafi, 2012) and dentex, Dentex dentex (Chatzifotis, Polemitou, Divanaach & Antonopoulou, 2008). The hypolipidemic effect of taurine seems to be related to an increased formation of bile acid synthesis (Russell, 2003) and a consequent synthesis of bile salt activated lipase (Chatzifotis et al., 2008). Still, more information is necessary to draw a conclusion on this. In the other hand, the inclusion of taurine and/or methionine did not have an effect on the lipid deposition in muscle, as previously reported for Chatzifotis et al. (2008). As for the lipid content, the protein content did not show significant difference in muscle. However, the inclusion of taurine and methionine did influence protein deposition in the liver, as the levels of taurine increased, a tendency toward low levels of protein content were observed. So far, there is no a clear explanation for this, as Bañuelos-Vargas et al. (2014) reported that totoaba (Totoaba macdonaldi) had higher levels of protein in liver when taurine was included in diets with soy protein concentrate.
The antioxidant activity is the capacity of cells to slow or prevent the oxidation from reactive oxygen molecules (Cao et al., 1993) and is one of the most cited benefits of inclusion of taurine in fish diets (Salze & Davis, 2015). We were able to see a significant improvement of antioxidant activity of the hepatocytes, when the rainbow trout received the diets T50/M50 and T75/M25. This has been previously reported for other responses related to antioxidant activity, in several species of fish fed diets with high inclusion of plant proteins (Bañuelos-Vargas et al., 2014). Taurine seems to be related to a modulation in the production of reactive oxygen species, rather than a scavenging activity (Salze & Davis, 2015; Schuller-Levis & Park, 2004) and in fish might be have a similar role (Takagi et al., 2006).
On the other hand, the macrophage burst activity improved as the taurine increased in the diet. The macrophages possess a variety of action mechanisms, but one of the most important is the oxygen-dependent respiratory burst which forms reactive oxygen species such as superoxide anion, hydrogen peroxide and hypochlorus acid (Rubio-Godoy, 2010), particularly with the latter one, which forms the long-lived taurine chloramine (Schulle-Levis & Park, 2004) which might be helping in the responses of the macrophages burst (Salze & Davis, 2015). In mammals, taurine has been pointed out as a potent regulator of immune responses and the present results might indicate immunoregulatory proprieties for rainbow trout, as well (Salze & Davis, 2015).
In conclusion, the ratio of 7.5 and 2.5 g/kg of taurine:methionine in diets with soy protein isolated and Spirulina powder as protein sources (diet T75/M25), did improve the growth and the antioxidant capacity of the liver in rainbow trout fingerlings. As well, the 10 g/kg inclusion of just taurine or methionine affects negatively the growth as much as not inclusion at all in plant protein based-diets. Even if the effects of the inclusion of taurine and methionine are clear, we still need to address research to understand better the modes of action.
Acknowledgements
The authors kindly acknowledge the funding of the Program of Support to Research and Technology Innovation Projects (PAPIIT) number IN213115 of the UNAM. As well thanks are also due to Evonik de Mexico for their kind support to provide the DL-methionine and the amino acid analysis in diets.
References
- AOAC . 15th ed. Official Analytical Chemists; Arlintong, Virginia, USA: 1990. Official methods of analysis. [Google Scholar]
- Bañuelos-Vargas I., López L.M., Pérez-Jiménez A., Peres H. Effect of fishmeal replacement by soy protein concentrate with taurine supplementation on hepatic intermediary metabolism and antioxidant status of totoaba juveniles (Totoaba macdonaldi) Comparative Biochemistry and Physiology B. 2014;170:18–25. doi: 10.1016/j.cbpb.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Blight E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Boonyoung S., Haga Y., Satoh S. Preliminary study on effects of methionine hydroxyl analog and taurine supplementation in a soy protein concentrate-based diet on the biological performance and amino acid composition of rainbow trout [Oncorhynchus mykiss (Walbaum)] Aquaculture Research. 2013;44:1339–1347. [Google Scholar]
- Brown P.B., Kaushik S., Peres H. Protein feedstuffs originating from soybeans. In: Lim C.E., Webster C.D., Lee C.S., editors. Alternative protein sources in aquaculture diets. Haworth Press; New York, USA: 2008. pp. 205–224. [Google Scholar]
- Burrells C., Williams P.D., Southgate P.J., Crampton V.O. Immunological, physiological and pathological response of rainbow trout (Oncorhynchus mykiss) to increasing dietary concentrations of soybean proteins. Veterinary Immunology and Immunopathology. 1999;72:277–288. doi: 10.1016/s0165-2427(99)00143-9. [DOI] [PubMed] [Google Scholar]
- Cao G., Alessio H.M., Cutler R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radical Biology and Medicine. 1993;14:303–311. doi: 10.1016/0891-5849(93)90027-r. [DOI] [PubMed] [Google Scholar]
- Chatzifotis S., Polemitou I., Divanaach P., Antonopoulou E. Effect of the dietary taurine supplementaton on growth performance and bile salt activated lipase activity of common dentex, Dentex dentex, fed a fish meal/soy protein concentrate-based diet. Aquaculture. 2008;275:201–208. [Google Scholar]
- Chung S., Secombes C.J. Analysis of events occurring within teleost macrophages during the respiratory burst. Comparative Biochemistry and Physiology B. 1988;89:539–544. [Google Scholar]
- Cruz C.A.C., Hernández H.L.H., Fernández A.M.A., Ramírez P.T., Angeles L.O. Effects of diets with soybean meal on the growth, digestibility phosphorus and nitrogen excretion of juvenile rainbow trout Oncorhynchus mykiss. Hidrobiológica. 2011;21:118–125. [Google Scholar]
- Divakaran S. Taurine: An amino acid rich in fish meal. In: Suárez L.E.C., Marie D.R., Salazar M.T., López M.G.N., Cavazos D.A.V., Cruz A.C.P., García O.A., editors. Avances en Nutrición Acuícola, VIII Simposium Internacional de Nutrición Acuícola. Universidad Autónoma de Nuevo León, Monterrey; México: 2006. pp. 310–315. [Google Scholar]
- Espe M., Hervrøy E.M., Liaset B., Lemme A., El-Mowafi A. Methionine intake affect hepatic sulphur metabolism in Atlantic salmon Salmo salar. Aquaculture. 2008;274:132–141. [Google Scholar]
- Espe M., Ruohonen K., El-Mowafi A. Effect of taurine supplementation on the metabolism and body lipid-to-protein ratio in juvenile Atlantic salmon (Salmo salar) Aquaculture Research. 2012;43:349–360. [Google Scholar]
- Enriquez R.M.L., Ramírez V.A.E., Hernández H.L.H., Fernández A.M.A. The effect of the substitution of fish oil with a mixture of plant-origin oils in diets of rainbow trout (Oncorhynchus mykiss Walbaum) fingerlings on growth, phosphorus and nitrogen excretion. Israeli Journal of Aquaculture-Bamidgeh. 2015:1–9. [Google Scholar]
- Gatlin D.M., Barrows F.T., Brown P., Dabrowski K., Gaylord T.G., Hardy W.R.…Wilson R. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquaculture Research. 2007;38:551–579. [Google Scholar]
- Gaylord T.G., Teague A.M., Barrows F.T. Taurine supplementation of all-plant protein diets for rainbow trout (Oncorhynchus mykiss) Journal of the Aquaculture Society. 2006;37:509–517. [Google Scholar]
- Gaylord T.G., Barrows F.T., Teague A.M., Johansen K.A., Overturf K.E., Sherpherd B. Supplementation of taurine and methionine to all-plant protein diets for rainbow trout (Oncorhynchus mykiss) Aquaculture. 2007;269:514–524. [Google Scholar]
- Glencross B.D., Hawkins W.E., Evans D., McCafferty P., Dods K., Maas R., Sipsas S. Evaluation of the digestible value of lupin and soybean protein concentrates and isolates when fed to rainbow trout, Oncorhynchus mykiss, using either stripping or settlement faecal collection methods. Aquaculture. 2005;245:211–220. [Google Scholar]
- Hardy R.W. Alternate protein sources for salmon and trout diets. Animal Feed Science and Technology. 1996;59:71–80. [Google Scholar]
- Mambrini M., Roem A.J., Cardevi J.P., Lalles J.P., Kaushik S.J. Effects of replacing fish meal with soy protein concentrate and of DL-methionine supplementation in high-energy, extruded diets on the growth and nutrient utilization of rainbow trout, Oncorhynchus mykiss. Journal of Animal Science. 1999;77:2990–2999. doi: 10.2527/1999.77112990x. [DOI] [PubMed] [Google Scholar]
- NRC . The National Academies Press; Washington, D.C: 2011. Nutrient requirements of fish and shrimp. [Google Scholar]
- Popovic N.T., Strunjak-Perovi I., Coz-Rakovac R., Barisic J., Jadan M., Berakovic A.P., Klobucar S.R. Tricanine methane-sulfonate (MS-222) application in fish anathesthesia. Journal of Applied Ichthiology. 2012;28:553–564. [Google Scholar]
- Rubio-Godoy M. Teleost fish immunology, review. Revista Mexicana de Ciencias Pecuarias. 2010;1:47–57. [Google Scholar]
- Russell D.W. The enzymes, regulation, and genetics of bile acid synthesis. Annual Review of Biochemistry. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- Salze G.P., Davis D.A. Taurine: A critical nutrient for future fish feed. Aquaculture. 2015;437:215–229. [Google Scholar]
- Secombes C.J. Isolation of salmonid macrophages and analysis of their killing activity. In: Stolen J.S., Fletcher T.C., Anderson D.P., Robertson B.S., van Muiswinkel W.B., editors. Techniques in fish immunology. SOS Publications; NJ, USA: 1990. pp. 139–154. [Google Scholar]
- Schuller-Levis G.B., Park E. Taurine and its chloramine: Modulators of immunity. Neurochem. Res. 2004;29(1):117–126. doi: 10.1023/b:nere.0000010440.37629.17. [DOI] [PubMed] [Google Scholar]
- Tacon A.G.J., Metian M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture. 2008;285:146–158. [Google Scholar]
- Takagi S., Murata H., Goto T., Hayashi M., Hatate H., Endo M.… Hemolytic suppression roles of taurine in yellowtail Seriola quinqueradiata fed non-fishmeal diet based on soybean protein. Fisheries Science. 2006;72:546–555. [Google Scholar]
- Takeuchi T., Park G.S., Seikai T., Yokoyama M. Taurine content in Japanese flounder Paralichthys olivaceus T. & S. and red sea bream Pagrus major T. & S. during the period of seed production. Aquaculture Research. 2001;32:244–248. [Google Scholar]
- Yokoyama M., Takeuchi T., Park G.S., Nakazoe J. Hepatic cysteinesulphinate decarboxylase activity in fish. Aquaculture Research. 2001;32:216–220. [Google Scholar]
- Wang X., He G., Mai K., Xu W., Zhou H. Ontogenic taurine biosynthesis ability in rainbow trout (Oncorhynchus mykiss) Comparative Biochemistry and Physiology B. 2015;185:10–15. doi: 10.1016/j.cbpb.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Zar J.H. Biostatistical analysis. 4th ed. Prentice Hall; New Jersey, USA: 1999. p. 663. [Google Scholar]