Highlights
-
•
Pooled oral fluid (OF) sampling from piglets is achievable pre-weaning using a cotton rope.
-
•
OF collection was successful for 62–100% of the litters depending on age and pre-exposure.
-
•
84.7 and 95% of naïve pigs interacted spontaneously with a rope at 21 and 28 days age, respectively.
-
•
Latency to interact with the rope highly varied between piglets within litters.
Keywords: Pig, Diagnostic, Suckling piglets, Oral fluid, Disease surveillance
Abstract
Collection of pooled oral fluid (OF) by allowing pigs to chew on a cotton rope is an alternative to blood sampling. However, little is known about the applicability of this method to suckling piglets. The objectives of the present study were to describe the spontaneous interaction of suckling piglets with a rope and to investigate the influence of a rope pre-exposure on the success rate of sampling. We studied the interaction dynamics of 21 and 28 days-old suckling piglets with a cotton rope presented for 30 min. Ropes were manually wrung out inside plastic bags to release the oral fluid. A total of 49 litters were included. Percentages of success of pooled OF collection for 28-day-old, 21-day-old and 21-day-old pre-exposed litters were 82%, 62% and 100%, respectively. The mean volume collected did not differ between groups. Without pre-exposure, 84.7% and 95% of piglets interacted spontaneously with the rope at 21 and 28 days of age, respectively. The latency between rope presentation and interaction was highly variable between piglets within litters: from < 10 s to 30 min. Among piglets having interacted with the rope, the interaction lasted for at least 60 s for 90% and 91.4% of 21 and 28-day-old piglets, respectively. Pooled OF collection is achievable prior to weaning in piglets of at least 21 days of age. Pooled OF sampling is representative at litter level if collection is successful. In order to improve the success rate of collection, pre-exposing the piglets with a rope one day prior to sampling is effective.
Background
Swine veterinarians and health authorities have to control the spread of infectious diseases at national, regional and herd level. This requires a rapid and efficient collection of infectious disease information. Collection of oral fluid (OF), defined as the fluid obtained by insertion of absorptive collectors in the mouth (Atkinson et al., 1993), seems to be an interesting alternative to blood sampling for some markers. OF is a mixture of salivary gland and oral mucosal secretions (Dawson & Edwards, 2015) with a composition similar to that of serum (Rai, 2006). Although OF collection was introduced to swine health surveillance relatively recently, it has been widely studied as the potential application of OF-based diagnosis is substantial (Prickett & Zimmerman, 2010). Detection of nucleic acids or antibodies in OF have been reported for most swine pathogens (Bjustrom-Kraft, 2018; Rotolo, Main, & Zimmerman, 2018). OF samples are easy to obtain with non-invasive procedures, which utilize the natural behavior of pigs (Kittawornrat & Zimmerman, 2011). Moreover, OF specimens can be frequently collected by a single person and this approach is a welfare-friendly method to monitor pig populations (Rotolo et al., 2017). OF samples are collected from individual pigs by introducing an absorptive device in the mouth (Fablet et al., 2017) or from a group of pigs by allowing them to chew on a cotton rope suspended in the pen for 20–30 min (Prickett, Simer et al., 2008; Prickett, Kim, Simer, Yoon, & Zimmerman, 2008). Pigs are naturally attracted to the rope and deposit OF when interacting with it (Prickett, Kim et al., 2008). However, as pigs may exhibit heterogeneous health status, the sample must be representative of the collected group (Seddon, Guy, & Edwards, 2012).
Diagnosis based on sampling piglets prior to weaning is frequently used to understand the pathogen dynamics within the herd for porcine reproductive and respiratory syndrome virus (PRRSv) (Holtkamp et al., 2011; Lebret et al., 2019), porcine circovirus type 2 (PCV2) (Shen, Wang, Madson, & Opriessnig, 2010) and other pathogens. OF before weaning is therefore of interest. However, most reports on OF sampling for pathogen surveillance are carried out in weaned or adult pigs; only a limited number of publications report on suckling piglets (Kittawornrat et al., 2014; Panyasing et al., 2014; Almeida et al., 2020). Despite these recent reports, the development of a robust sampling protocol under field conditions requires more data on the characteristics of the interaction between piglets and absorptive rope prior to weaning. Principal reasons for OF sampling failure seem to be the lack of interaction with the rope (Hernandez-Garcia et al., 2017) and the lack of sufficient saliva volume collected, especially in recently weaned piglets.
A previous study reported that the age of piglets influences their ability to interact with enrichment objects (Docking, Van de Weerd, Day, & Edwards, 2008). Furthermore, interaction with a sampling rope is increased if weaned piglets have been in contact with the device previously (White et al., 2014). To our knowledge, this pre-exposure effect has not yet been described in literature for suckling piglets.
The first objective of the present study was to describe the spontaneous interaction of due-to-wean piglets with a rope at both 21 and 28 days of age. The second objective was to investigate the influence of a previous experience of piglets on the success rate of pooled OF sampling.
Materials and methods
Animal selection and experimental design
The selected herd was located at a commercial farm in Brittany (France). Piglets were weaned at 28 days of age. A total of 49 litters of 10–14 piglets ([Large White × Landrace] × [Pietrain]) per farrowing pen (6 m²) were selected from mixed parity sows, according to herd demography. Sows were housed in farrowing crates in a fully slatted pen with one feeder and one drinker in a corner of the pen. Seventeen 28-day-old-litters (n = 177 piglets) and thirty-two 21-day-old-litters (n = 363 piglets) were included from two consecutive batches.
In this study, litters were divided in 3 groups: Group 1 (17 litters with OF collection at 28 days old); Group 2 (18 litters with OF collection at 21 days old); Group 3 (14 litters with OF collection at 21 and 22 days old). Group 3 underwent OF collected in two periods at one day's interval to assess the benefit of a learning process consisting of presenting a rope for four hours one day before sampling. Piglets benefiting from a second rope presentation will be referred to as “pre-exposed” and others as “naïve”.
Rope presentation and oral fluid sampling
In order to collect pooled oral fluid samples from litters, an untreated 100% cotton rope was presented to piglets for 30 min. Samples were taken in the morning. This time was selected as a period of piglet activity and to avoid disturbance of routine farm work. A single oral fluid sample was collected from each farrowing pen. To standardize and simplify oral fluid sampling, a collection kit was used. Each kit contained a 50-cm-long, 0.8-cm-wide pure cotton rope, a single-use disposable plastic bag, a 10 mL tube, gloves, scissors and a permanent marker for labeling tubes. One end of the rope was knotted and fixed with pliers in the middle of the farrowing crate. The opposite (unknotted) end hung at piglet shoulder height (Fig. 1). After a 30-min presentation to piglets, the lower (wet) portion of the rope was inserted into the plastic bag. The rope was manually wrung out inside the plastic bag to release the oral fluid, after which a corner of the bag was cut and the fluid was poured into the 10 mL tube. For each sample, the number of piglets, the sow parity and the volume of collected OF were recorded. We considered sampling as a failure if no OF was yielded by manual wringing of the rope.
Fig. 1.
Collection of Oral Fluid in a farrowing crate.
Dynamic of piglet interaction with the rope
Five naïve litters of 28-day-old piglets (n=57) and five naïve litters of 21-day-old piglets (n=57) were randomly selected. Prior rope presentation, all piglets were individually identified by number marking between the shoulder blades. These litters were video recorded for the full 30 min of rope presentation and behavior of the piglets was analysed. The observation period was divided into 10-s intervals by a single observer and each piglet was scored as either interacting with the rope or not. Rope interaction was defined as chewing the rope or trying to catch it. The obtained data were used to describe (1) the percentage of piglets interacting with the rope over the 30 min observation period, (2) the latency before the first interaction for each piglet, (3) the total duration of piglet contact with the rope for each piglet.
Statistical analysis
Statistical analyses were performed using R Software. The confidence intervals of the percentage of successful OF collection of each groups were assessed using Wilson procedure with a correction for continuity. The difference between groups in the percentage of successful OF collection was assessed using Fisher test.
The difference between two age-groups in the latency between rope presentation and first interaction, and the duration of interaction was assessed using the t-Test for independent samples. Statistical significance was set at p<0.05. OF volumes collected in this trial and individual contact time per piglet on video recorded litters were shown in box-plots using median, quartiles, minimum and maximum.
Results
Oral fluid collection
We compared the percentage of success for 28-day-old naive, 21-day-old naive and 22-day-old pre-exposed litters. OF collection was successful in 82%, 62% and 100% of rope presentation, respectively. Pre-exposure significantly increased the success rate for 3-week-old piglets (Table 1).
Table 1.
OF collection success at 21 and 28 days of age for naive pigs and at 22 days of age for pre-exposed pigs. Percentages with different superscripts are statistically different (Fisher test, p< 0.05).
| Number of litters | Number of success | Percentage of successful collection (95% CI) |
||
|---|---|---|---|---|
| Group 1 | 28-day-old, naive | 17 | 14 | 82ab (56–95) |
| Groups 2 & 3 | 21-day-old, naive | 32 | 20 | 62 a (44–78) |
| Group 3 | 22-day-old, pre-exposed | 14 | 14 | 100 b (73–100) |
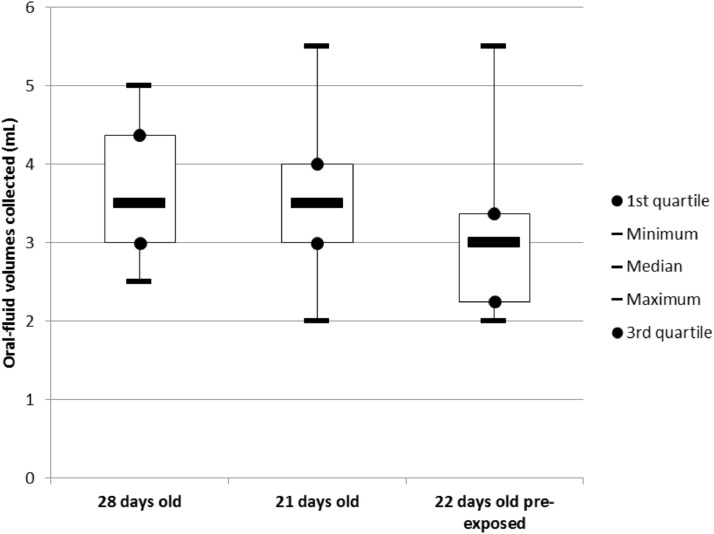
In naive litters, the mean collected volume was 3.6 mL (ranging from 2.5 mL to 5 mL) at 28 days of age and 3.5 mL (2–5.5 mL) at 21 days of age. There was no statistical difference between both groups. In pre-exposed litters, the mean volume collected at 22 days of age was 3.2 mL (2–5.5 mL). There was no statistical difference when compared to naive litters of the same age (Fig. 2).
Fig. 2.
Volumes of OF (mL) collected after a 30-min successful rope presentation from naive 28-day-old, naive 21-day-old and pre-exposed 22-day-old litters.
Rope interaction dynamics
For the 10 naive litters that were video-recorded, the percentage of piglets that had chewed the rope for at least 20 s increased progressively (Fig. 3). We observed no difference between the 21 and 28-day-old group during the first 5 min in the mean percentage of piglets interacting with the rope. But after this first period, the increase was faster in 28-day-old than in 21-day-old piglets. A total of 84.7% (50/59) and 95% (58/61) piglets interacted spontaneously with the rope at 21-day-old and at 28-day-old, respectively (Fig. 3). The greatest increment in the percentage of piglets interacting with the rope occurred between 0 and 15 min, with an average of over 70% (50–83.3%) and over 80% (63.9–91.6%) of piglets chewing the rope at 21 and 28 days of age, respectively. The interaction dynamics were litter-dependent at both ages.
Fig. 3.
Mean cumulative percentage of naïve piglets that interacted with the rope within 30 min of rope presentation at 28 and 21 days of age (5 litters/57 piglets per age group).
Finally, our video recordings revealed heterogeneity in spontaneous behavior of the piglets to the first rope presentation. The latency between rope presentation and interaction was highly heterogeneous and not statistically different (t-Test, p=0.19) between piglets within litters and within the same age: from < 10 s to 1770 s ([29 min 30 s], Standard Deviation (SD)=502 s) and < 10 s to 1550 s ([25 min 50 s], SD=390 s) for 21 and 28-day-old piglets, respectively. Among piglets having interacted with the rope, the interaction lasted for at least 60 s for most: 90% (45/50) and 91.4% (53/58) of 21 and 28-day-old piglets, respectively. The duration of interaction was highly variable and not statistically different (t-Test, p=0.16) between piglets at 21 days of age (514 s on average [8 min 34 s], SD=333) and 28 days of age (612 s on average [10 min 12 s], SD=369) (Fig. 4).
Fig. 4.
Distribution of total contact time (in s) of naive piglets with a rope at 21 and 28 days of age after a 30-minute successful rope presentation (5 litters/57 piglets per age).
Discussion
OF is gaining growing interest in veterinary medicine partly due to its animal welfare friendly aspect and the recent development of diagnostic assays adapted to this sample medium. In agreement with previous studies, around 80% of the pigs deposited pooled OF on the rope and an increase in the percentage of pigs chewing the rope was observed within the first 15 min of rope presentation (Graage, Hennig-Pauka, Arbinger, Ritzmann, & Ladinig, 2019; Seddon et al., 2012). In our study, this high interaction rate could be explained by a higher degree of synchrony of activity for suckling piglets than weaned pigs (Docking et al., 2008). So we observed similar interaction dynamics among unweaned piglets compared with weaned pigs.
In our study, no samples could be obtained from some litters of both age groups after the full 30 min of rope presentation. Failures in pen-based oral fluid sampling were observed for sows with a failure rate of 37.8% in 112 collected sow pens (Pol et al., 2017) and 40.2% in 137 collected sow pens (Fablet et al., 2017). It was already observed for due-to-wean piglets with a failure rate of 77% (Almeida et al., 2020). For weaned pigs, all rope presentation resulted in interaction and OF deposit onto the rope in previous studies for pigs aged of 4/5 weeks (Graage et al., 2019), 8 weeks (Dawson & Edwards, 2015; Graage et al., 2019), 12/14 weeks (Graage et al., 2019), 16 weeks (Dawson & Edwards, 2015; Fablet et al., 2017) and 22 weeks (Fablet et al., 2017). Our results demonstrated that collecting OF at 21-day-old rather than at 28-day-old is less effective (lower success rate). Indeed, contact time between piglets and ropes, and volumes collected did not differ between ages but the percentage of piglets chewing the rope was lower in the younger age group. An explanation could be that before 21 days of age, suckling piglets spend a high proportion of their time suckling the sow's udder (Petersen, Simonsen, & Lawson, 1995) and sleeping, so enrichment objects are largely ignored (Webster, 1998) An older age has been described to positively enhance pig's interaction with enrichment objects (Docking et al., 2008).
To improve OF collection in suckling piglets and to enhance success rate, two strategies are suggested in this study. The first is to sample older piglets. As shown in this study, the OF collection success rate is higher in older piglets. However, weaning at 21 days of age is common practice in most pig farming areas. The other strategy is inspired by the fact that pigs are known to be good learners, whether in trials associated with food delivery (Gieling, Nordquist, & van der Staay, 2011) or through repetition (Nawroth & von Borell, 2015). This strategy would use the pigs’ natural ability by ‘training’ them to interact with the rope. As described in this study, presentation of the rope one day prior to sampling decreases the failure rate of OF sampling in unweaned piglets. This pre-exposure effect has already been demonstrated for pen-based OF collection from growing pigs (White et al., 2014) and sows (Pol et al., 2017). This positive effect has been also demonstrated recently for due-to-wean piglets (17 days for median age) (Almeida et al., 2020). Suspending a rope in farrowing pens the day before sample collection is relatively quick and would not be a time constraint for farmers. Other ways of improving suckling piglets‘ interaction with the rope could be to increase the duration of exposure to the rope and the addition of attractants on the rope, but these protocols had poorly improved pooled OF collection in a recent study (Almeida et al., 2020). A significant improvement in pooled OF sampling is to perform family testing i.e. to place the rope so that both sows and their litters have access (Almeida et al., 2020; Bjustrom-Kraft, 2018). However the latter method does not allow to investigate the health status of piglets alone, which can be of interest when studying sow to piglet transmission of pathogens. Moreover in our study, no difference in volumes collected was demonstrated with or without prior exposure, but the main objective of such protocol is to reduce failure in pooled OF collection.
In groups where the estimated disease prevalence is above 10%, a pooled sample representing 80% of the pen population is sufficient to be 95% sure of finding at least one positive individual (Thrusfield, Ortega, de Blas, Noordhuizen, & Frankena, 2001). In our study (Kittawornrat et al., 2014), a 30-min-rope presentation generated chewing by over 80% and 90% respectively of suckling piglets at 21 and 28 days of age. So in our study, sampling can be considered representative. However, the relative variability in OF deposit on ropes between piglets and between litters is a limitation.
OF quantities extracted from ropes by hand wringing might be dependent on the manipulator. In previous studies, mean volumes collected by hand wringing ranged from 20 mL on average for both weaners and finishers (Dawson & Edwards, 2015) up to 50 mL for finishers (Seddon et al., 2012). A reproducible method could be centrifugation of the absorptive device.
Conclusion
Pooled OF collection is achievable prior to weaning in piglets of at least 21 days of age. Our study of the interaction dynamics of suckling piglets with a rope presented for 30 min showed that pooled OF sampling is representative at litter level if collection is successful. In order to improve the success rate of pooled OF collection, we recommend pre-exposure of piglets one day prior to sampling.
Declaration of Competing Interest
None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
Fundings
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Animal welfare
Animals participating in this survey were commercial pigs kept on farms in accordance with French regulations and standards for pig breeding and rearing. Approval of the Ethics committee was not considered necessary as oral fluid collection is not invasive, not painful and pigs were not forced to interact. No other procedures were applied to the animals during the course of this study.
Acknowledgments
The authors would like to thank the herd owner for his cooperation.
References
- Almeida M.N., Rotto H., Schneider P., Robb C., Zimmerman J.J., Holtkamp D.J. Collecting oral fluid samples from due-to-wean litters. Preventive Veterinary Medicine. 2020;174 doi: 10.1016/j.prevetmed.2019.104810. [DOI] [PubMed] [Google Scholar]
- Atkinson J.C., Dawes C., Ericson T., Fox P.C., Gandara B.K., Malamud D. Guidelines for saliva nomenclature and collection. Annals of New York Academy of Science. 1993;694 [Google Scholar]
- Bjustrom-Kraft J. The use of oral fluid diagnostics in swine medicine. JSHAP. 2018;26:262–269. [Google Scholar]
- Dawson L.L., Edwards S.A. The effects of flavored rope additives on commercial pen-based oral fluid yield in pigs. Journal of Veterinary Behavior. 2015;10:267–271. doi: 10.1016/j.jveb.2015.01.003. [DOI] [Google Scholar]
- Docking C.M., Van de Weerd H.A., Day J.E.L., Edwards S.A. The influence of age on the use of potential enrichment objects and synchronisation of behaviour of pigs. Applied Animal Behaviour Science. 2008;110:244–257. doi: 10.1016/j.applanim.2007.05.004. [DOI] [Google Scholar]
- Fablet C., Renson P., Pol F., Dorenlor V., Mahé S., Eono F. Oral fluid versus blood sampling in group-housed sows and finishing pigs: Feasibility and performance of antibody detection for porcine reproductive and respiratory syndrome virus (PRRSV) Veterinary Microbiology. 2017;204:25–34. doi: 10.1016/j.vetmic.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Gieling E.T., Nordquist R.E., van der Staay F.J. Assessing learning and memory in pigs. Animal Cognition. 2011;14:151–173. doi: 10.1007/s10071-010-0364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graage R., Hennig-Pauka I., Arbinger H., Ritzmann M., Ladinig A. Influence of age, group size and the presence of porcine reproductive and respiratory syndrome virus on the collection of oral fluids. The Vet Journal. 2019;244:13–15. doi: 10.1016/j.tvjl.2018.12.005. [DOI] [PubMed] [Google Scholar]
- Hernandez-Garcia J., Robben N., Magnée D., Eley T., Dennis I., Kayes S.M. The use of oral fluids to monitor key pathogens in porcine respiratory disease complex. Porcine Health Management. 2017;3 doi: 10.1186/s40813-017-0055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtkamp D.J., Polson D.D., Torremorell M., Morrison B., Rowland R.R., Snelson H. Terminology for classifying swine herds by porcine reproductive and respiratory syndrome virus status. Journal of Swine Health and Production. 2011;19:13. [PubMed] [Google Scholar]
- Kittawornrat A., Panyasing Y., Goodell C., Wang C., Gauger P., Harmon K. PRRSv surveillance using pre-weaning oral fluid samples detects circulation of wild type PRRSv. Veterinary Microbiology. 2014;168:331–339. doi: 10.1016/j.vetmic.2013.11.035. [DOI] [PubMed] [Google Scholar]
- Kittawornrat A., Zimmerman J.J. Toward a better understanding of pig behavior and pig welfare. Animal Health Research Reviews. 2011;12:25–32. doi: 10.1017/S1466252310000174. [DOI] [PubMed] [Google Scholar]
- Lebret A., Boulbria G., Berton P., Moalic P.-Y., Le Guennec J., Bouchet F. Monitoring PRRSV-1 in suckling piglets in an endemic herd using reverse transcriptase quantitative real time polymerase chain reaction: Comparison of the rate of detection in serum and oral fluid samples and evaluation of pooling. Porcine Health Management. 2019;5:8. doi: 10.1186/s40813-019-0115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth C., von Borell E. Domestic pigs’ (Sus scrofa domestica) use of direct and indirect visual and auditory cues in an object choice task. Animal Cognition. 2015;18:757–766. doi: 10.1007/s10071-015-0842-8. [DOI] [PubMed] [Google Scholar]
- Panyasing Y., Goodell C., Kittawornrat A., Wang C., Levis I., Desfresne L. Influenza A virus surveillance based on pre-weaning piglet oral fluid samples. Transboundary and Emerging Diseases. 2014 doi: 10.1111/tbed.12307. [DOI] [PubMed] [Google Scholar]
- Petersen V., Simonsen H.B., Lawson L.G. The effect of environmental stimulation on the development of behaviour in pigs. Applied Animal Behaviour Science. 1995;45:215–224. doi: 10.1016/0168-1591(95)00631-2. [DOI] [Google Scholar]
- Pol F., Dorenlor V., Eono F., Eudier S., Eveno E., Liégard-Vanhecke D. Individual and pen-based oral fluid sampling: A welfare-friendly sampling method for group-housed gestating sows. Preventive Veterinary Medicine. 2017;147:58–65. doi: 10.1016/j.prevetmed.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Prickett J., Simer R., Christopher-Hennings J., Yoon K.-J., Evans R.B., Zimmerman J.J. Detection of Porcine reproductive and respiratory syndrome virus infection in porcine oral fluid samples: A longitudinal study under experimental conditions. Journal of Veterinary Diagnostic Investigation. 2008;20:156–163. doi: 10.1177/104063870802000203. [DOI] [PubMed] [Google Scholar]
- Prickett J.R., Kim W., Simer R., Yoon K.-J., Zimmerman J. Oral-fluid samples for surveillance of commercial growing pigs for porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 infections. Journal of Swine Health and Production. 2008;16:6. [Google Scholar]
- Prickett J.R., Zimmerman J.J. The development of oral fluid-based diagnostics and applications in veterinary medicine. Animal Health Research Reviews. 2010;11:207–216. doi: 10.1017/S1466252310000010. [DOI] [PubMed] [Google Scholar]
- Rai B. Oral Fluid in Toxicology. The Internet Journal of Toxicology. 2006;3 [Google Scholar]
- Rotolo M.L., Main R.G., Zimmerman J.J. Herd-level infectious disease surveillance of livestock populations using aggregate samples. Animal Health Research Reviews. 2018;19:53–64. doi: 10.1017/S1466252318000038. [DOI] [PubMed] [Google Scholar]
- Rotolo M.L., Sun Y., Wang C., Giménez-Lirola L., Baum D.H., Gauger P.C. Sampling guidelines for oral fluid-based surveys of group-housed animals. Veterinary Microbiology, Alternative Strategies for the Control of Porcine Reproductive and Respiratory Syndrome. 2017;209:20–29. doi: 10.1016/j.vetmic.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Seddon Y.M., Guy J.H., Edwards S.A. Optimising oral fluid collection from groups of pigs: Effect of housing system and provision of ropes. Veterinary Journal. 2012;193:180–184. doi: 10.1016/j.tvjl.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Shen H., Wang C., Madson D.M., Opriessnig T. High prevalence of porcine circovirus viremia in newborn piglets in five clinically normal swine breeding herds in North America. Preventive Veterinary Medicine. 2010;97:228–236. doi: 10.1016/j.prevetmed.2010.09.020. [DOI] [PubMed] [Google Scholar]
- Thrusfield M., Ortega C., de Blas I., Noordhuizen J.P., Frankena K. WIN EPISCOPE 2.0: Improved epidemiological software for veterinary medicine. Veterinary Record. 2001;148:567–572. doi: 10.1136/vr.148.18.567. [DOI] [PubMed] [Google Scholar]
- Webster The use of a rooting substrate by fifteen day old piglets. Proceedings of the British Society of Animal Science. 1998 [Google Scholar]
- White D., Rotolo M., Olsen C., Wang C., Prickett J., Kittawornrat A. Recommendations for pen-based oral-fluid collection in growing pigs. Journal of Swine Health and Production. 2014;22:4. [Google Scholar]