Abstract
Four major genotypes of Hepatitis E virus (HEV) have been documented worldwide (1–4) with genotypes 1 and 2 found in human in Sub-Saharan Africa. Human Hepatitis cases due to HEV genotype 3 and 4 are zoonotic with various animal identified as possible reservoirs. Recently, HEV genotype 3 was found in pigs and human beings in West Africa, which may change the epidemic in human. Here, we assessed the prevalence of HEV antibodies in various domestic and wild mammalians in Burkina Faso. Random sampling was performed between 2015 and 2017 to collect serum from 100 rabbits (Oryctolagus cuniculus), 19 hares (Lepus africana), 72 cattle (Bos taurus), 75 sheep (Ovis aries) and 81 goats (Capra aegagrus) in three provinces in Burkina Faso. A multi-species ELISA was performed on serum samples from 328 domestic animals and 19 hunting hares. HEV total antibodies were identified in 121 out of 347 specimens (34.9% CI95% [29.9–39.9]). Sera from rabbits (60% CI95% [50.4–69.6]), hares (52.6% CI95% [30.2–75.1]), cattle (26.4% CI95% [16.2–36.6]), sheep (12.0% CI95% [4.6–19.4]), and goats (28.4% CI95% [18.6–38.2]) tested positive for antibodies anti-HEV. In this study we evidence presence of HEV antibodies in various mammalians and highlight the importance of these species in the epidemiology of HEV infection in Burkina Faso.
Keywords: Hepatitis E virus, Seroprevalence, Zoonosis, Lagomorphs, Ungulata, Burkina Faso
1. Introduction
Hepatitis E virus (HEV) is one of the causative agent of acute hepatitis epidemic in human worldwide (Rein, Stevens, Theaker, Wittenborn, & Wiersma, 2012). HEV has a single-stranded, positive-sense RNA genome of approximately from 6.4 to 7.2 Kb (Purdy et al., 2017). Following the new classification, HEV belongs to the family of Hepeviridae, which consists of two genera, Orthohepevirus (with species A, B, C and D) and Piscihepevirus containing one known isolate derived from a trout (ICTV 2014, Smith et al., 2015). Orthohepevirus A contains eight genotypes, the human-pathogenic genotypes HEV-1 to HEV-4 together with additional genotypes from wild boars (HEV-5 and HEV-6) and camels (HEV-7 and HEV-8) (Sridhar, Teng, Chiu, Lau, & Woo, 2017). In developing countries, HEV-1 or 2 appears to have an endemic pattern punctuated with epidemic outbreaks (Liu et al., 2013). HEV-3 and 4 widely found in animal reservoirs are thought to be zoonotic (Pavio, Meng, & Doceul, 2015; Y. Zhang et al., 2017). Indeed, HEV genotype 3 and 4 infections were reported in developed countries in individuals who had consumed raw meat or meat products from deer, wild boars, or pigs. Among the mammals, domestic pigs and wild boars are the main animal's reservoir of these genotypes (Schielke et al., 2009), but serological evidence of HEV infection was also documented in other animal species including chickens, rats, dogs, cats, cows, sheep, goats, buffalo, horses, cattle, camel and rhesus monkeys (Geng et al., 2011a, Geng et al., 2011b, Izopet et al., 2012, Liang et al., 2014, Pavio et al., 2015, Wu et al., 2017, Zhang et al., 2017). Infection with one strain of HEV could confer cross-protection to other strains, even across genotypes 1 to 4, owing to the existence of a single serotype (Krain et al., 2014, Zhang et al., 2017).
Initially HEV was described as the agent of severe forms of acute hepatitis with mortality level ranging from 1 to 4% in the general population to nearly 20% in pregnant women during epidemics, and especially in the third trimester (Worm, van der Poel, & Brandstatter, 2002). However, these epidemic pictures seem to be specific to HEV genotype 1 and 2 and it is also recognized that during inter-epidemic periods a large number of cases were not detected as they were self-limited infections with rapid viral clearance (Mirazo, Ramos, Mainardi, Gerona, & Arbiza, 2014). Most of these self-limited infections were due to genotype 3 and 4 even if severe hepatitis and chronic infection are now identified in at-risk population (Kmush et al., 2015, Pas et al., 2012).
In Burkina Faso the prevalence of Anti-HEV antibodies were 76% in pork butchers, and 47.8% in the general population in 2013 (Traore et al., 2015). Traore et al. also documented HEV antibodies (80%) in swine (Traore et al., 2015). The aim of this study was to assess the presence of anti-HEV antibodies in various wild and domestic mammalians breed find in human food chain in Burkina Faso.
2. Material and method
2.1. Study area
We conducted an epidemiological survey in Kadiogo, Gourma and Houet, three (3) administrative provinces of the semi-arid country of Burkina Faso, West Africa. These provinces are located in the Sudanese agro-ecological area with rainfall ranging from 300 to 1200 mm3/year. The vegetation is represented by the wooded and shrubby savannah. The livestock systems are transhumant and sedentary livestock from the north to the south of the country. It is an agro-pastoral area with maize, sorghum, millet and cowpea as the main crops. The main culinary habits for meat is cooking at high temperatures (Drabo et al., 2009). Ouagadougou (province of Kadiogo) represents the most important city located in the center of the country (Fig. 1). The province of Kadiogo covered about 2805 square Km, with an estimated population of 2854,356 and an estimated livestock population of 22,779 rabbits, 224,056 sheep, 337,912 goats, 155,847 cattle, and other variable species of mammals and poultry (INSD, 2017). Evolution of controlled slaughter by species in province of Kadiogo is 102,384 for cattle, 84,040 for sheep, and 68,898 for goat (INSD, 2017).
Fig. 1.
Map of sampling sites
Note: The map shows sampling sites (surrounded in green) that correspond to areas of high human population density and hunting areas. It presents three study regions stretching from East to West. The high human density is increasing from yellow to red. The density of the population varies from 3 to 22 inhabitants per hectare.
2.2. Sampling and sample collection
Our study first focused on the most consumed animal species in the city of Ouagadougou (cattle, goats, sheep, and rabbits) except pork, which was studied in our previous survey. Five whole blood sample were collected weekly on red top tube from symptomless animals: 72 cattle (Bos taurus), 81 goats (Capra aegagrus), 75 sheep (Ovis aries), 100 rabbits (Oryctolagus cuniculus) and hares 19 (Lepus africana). Only rabbits are collected in the 3 area (Kadiogo (n = 56), Houet (n = 26) and Gourma (n = 18)) and Hares are collected in the 2 area (Houet (n = 3) and Gourma (n = 16)). Specimens were collected in February and August 2015, October and November 2016 and April and May 2017 at livestock farms, slaughterhouses, animal sale places and hunting areas for the species concerned. The slaughterhouse receives animals from different districts of Burkina Faso and is the most important source of meat supply in the city. Taking into account the literature, we considered that, in addition to pork, the real risks of HEV infection could come from lagomorphs (Liu et al., 2013), both for the high prevalence of the virus in this mammalian order and for genotypes similar to those found in humans (Wang et al., 2013, Zhang et al., 2015). This motivates the extension of the study on rabbits to two other important provinces (Houet and Gourma) and on hares at the same time, since Houet and Gourma include hunting areas.
Serum was obtained by centrifugation at 2500 g for 5 min, aliquoted and stored at −20°C until testing.
2.3. Sample size determination
The sample size (n) was determined using an expected total prevalence within all the various species considered as a whole of 24.1% (pexp) following the data from Junaid et al. acquired in a very close geographic area (Junaid, Agina, & Jaiye, 2014), with a 95% confidence interval level (Z = 1.96) and an error margin of 5% (d) using the formula developed by Fisher, Laing, and Stoeckel (1983). The expected sample size was thus n = 281. As our prospective study involved sampling of various animal species, the estimation of the minimum size was obtained using the lowest prevalence expected for each species reported in the literature (Table 3). Thus, the final target sample size were 50 for rabbits, 33 for hares, 30–86 for cattle, 30–144 for sheep and goats.
Table 3.
Comparison of seroprevalence rates per animal species between seroprevalences recorded in different countries and the results of our study.
| Prevalence from literature | Seroprevalence in our study | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species /Expected genotype | Method ELISA | Method PCR | ||||||||
| Percent | Positive / Total | Method ELISA Used | Percent (%) | Positive / Total | Ref* | Countries / Year of study | Percent | Positive/Total | IC 95% | |
| Rabbit Gen: 3 | 54.6 | 65/119 | Commercial kit (Wantai) | 6.9 | 8/115 | Geng et al. (2011a) | China (North) / 2010 | 60.0 | 60/100 | [50.4–69.6] |
| 37.3 | 47/126 | Commercial kit (Wantai) | 17.1 | 28/164 | Hammerschmidt et al. (2017) | Germany / 2016 | ||||
| 36.0 | 31/85 | In-house test | 16.0 | 14/85 | Cossaboom et al. (2011) | USA (Virginia) / 2011 | ||||
| 57.0 | 191/335 | Commercial kit (Wantai) | 7.6 | 11/144 | Zhao et al. (2009) | China (North) / 2009 | ||||
| 15.4 | 169/1094 | Commercial kit (Wantai) | 2.0 | 22/1094 | Geng et al. (2011a) | China (North; Northeast; North; South East; South) / 2010 | ||||
| 3.4 | 7/206 | In-house test | 0.0 | 0/206 | Di Bartolo et al. (2016) | Italy / 2015 | ||||
| 72.5 and 62.5 | 58/80 and 50/80 | Commercial kit (Wantai) for human and In-house test for swine sera | 46.1 | 24/52 | Liu et al. (2017) | China (Center) / 2017 | ||||
| Hare | 2.2 | 14/624 | Commercial kit (Axiom corresponding to the assay produced by Wantai,) | 0.0 | 0/624 | Hammerschmidt et al. (2017) | Germany / 2016 | 52.6 | 10/19 | [30.2–75.1] |
| 0.0 | Commercial kit (DIA.PRO) | – | – | Mazzei et al. (2015) | Italy / 2015 | |||||
| Cattle Gen: 4d | 15.0 | 52/346 | Commercial kit (Wantai) | – | – | Geng et al. (2010a) | China (North) / 2010 | 26.4 | 19/72 | [16.2–36.6] |
| 28.2 | 257/912 | Commercial kit (Wantai) | 0.8 | 7/912 | Geng et al. (2010a) | China / 2008 (center, North, South, East and West) | ||||
| 6.0 | 6/100 | Commercial kit (Wantai) | 0.0 | 0/100 | Zhang et al. (2008) | China (East) / 2004-2006 | ||||
| 15.0 | 174/1156 | In-house test | – | – | Dong et al. (2011) | USA (Virginia) / 2011 | ||||
| Sheep Gen: 4d | 100.0 And 77.5% | 58/58 And 45/58 | Genelabs Diagnostics (Commercial kit) and In-house test | 0.0 | 0/58 | Shukla et al. (2007) | India / 2006 | 12.0 | 9/75 | [4.6–19.3] |
| 10.5 | 2/19 | Diagnostic Automation (commercial kit for human) | – | – | Junaid et al. (2014) | Nigeria / 2012 | ||||
| 31.8 | 56/176 | ID-vet (commercial kit) | – | – | Shuaibu et al. (2016) | Nigeria / 2016 | ||||
| 35.2 | 176/500 | Commercial kit (Wantai) | 5.3 | 4/75 | Wu et al. (2015) | China (West) / 2014 | ||||
| Goat Gen: 3c | 100.0 | 86/86 | Genelabs Diagnostics (Commercial kit) and In-house test | 0.0 | 0/86 | Shukla et al. (2007) | India / 2006 | 28.4 | 23/81 | [18.6–38.2] |
| 10.4 | 73/700 | Commercial kit (Wantai) | 1.6 | 11/700 | Geng et al., (2010)a | China (center, North, South, East and West) / 2008 | ||||
| 37.2 | 16/43 | Diagnostic Automation (commercial kit for human | – | – | Junaid et al. (2014) | Nigeria / 2012 | ||||
| 16.0 | 13/80 | In-house test | 0.0 | 0/80 | Sanford et al. (2013) | USA (Virginia) / 2002 | ||||
| 24.0 | 12/50 | Commercial kit (Wantai) | 0.0 | 0/50 | Zhang et al. (2008) | China (East) / 2004-2006 | ||||
| 0.0 | 0/5 | In-house test | – | – | Vitral et al. (2005) | Brasil / 2005 | ||||
Note: * references.
2.4. Detection of total HEV antibodies
All 347 Animal sera were tested following manufacturer instructions with the HEV ELISA 4.0v kit (MP Biomedicals, Eschwege, Germany). This commercially available double antigen sandwich ELISA (das-ELISA), developed strictly for veterinary use, is able to detect IgM, IgG and IgA directed against HEV in all animal species. According to the manufacturer, test sensitivity is 99.2% and specificity is 99.2%.
2.5. Ethic statements
Study protocol was approved and published by Burkina Faso Ministry of Environment and Fisheries in its deliberation n°2014 2015 – 001 / MERH/CAB. Blood samples were collected by licensed and certified veterinarians.
2.6. Statistical methods
Data were processed and analyzed using Microsoft Excel 2013 and GraphPad Prism version 7 (GraphPad Software, La Jolla, California USA). Only apparent prevalence were calculated for each species. Binary logistic regression analyses were carried out to determine which variables (Locality, sex and Species) were significantly associated with detection of HEV antibodies. Multivariate logistic regression was performed using R software version 2.13.0 (https://www.r-project.org/). P < 0.05 was considered significant. The lower and upper limits of the 95% confidence interval (CI) for a proportion were also calculated.
3. Results
3.1. Prevalence of anti-HEV antibody in different animal species
We detected anti-HEV total antibodies in 121 (34.9% CI95% [29.9–39.9]) out of the 347 mammalians samples examined by ELISA.
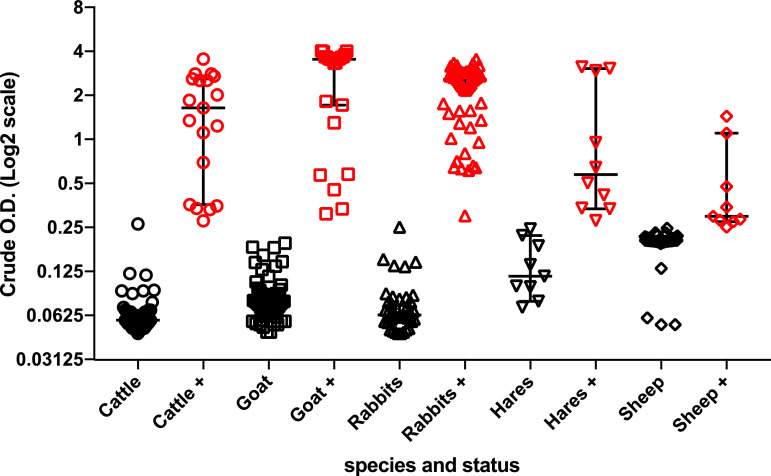
Anti HEV total antibodies were detected in 60/100 rabbits (60.0% CI95% [50.4–69.6]); 10/19 hares (52.6% CI95% [30.2–75.1]); 23/81 goats, (28.4% CI95% [18.6–38.2]); 19/72 cattle (26.4% CI95% [16.2–36.6]) and 9/75 sheep (12.0% CI95% [4.6–19.4]) respectively as shown in Table 1. Fig. 2 presents the distribution of positive and negative cases according to the Optical Density (O.D.) of ELISA test and clearly highlights the indeterminate cases.
Table 1.
Comparison of prevalence of total antibodies anti-hepatitis E 477 virus in the different animal species and the three provinces of Burkina Faso.
| Species | Seroprevalence (%) and 95% CI | Seroprevalence (%) in provinces and 95% CI | ||
|---|---|---|---|---|
| Kadiogo | Houet | Gourma | ||
| Rabbits | 60.0 [50.4–69.6] | 80.4 [70.0–90.8] | 19.2 [4.1–34.4] | 55.6 [32.6–78.5] |
| Hares | 52.6 [30.2–75.1] | NS | 66.67 [13.3–100.0] | 50.00 [25.5–74.5] |
| Cattle | 26.4 [16.2–36.6] | 26.4 [16.2–36.6] | NS | NS |
| Sheep | 12.0 [4.6–19.4] | 12.0 [4.6–19.4] | NS | NS |
| Goats | 28.4 [18.6–38.2] | 28.4 [18.6–38.2] | NS | NS |
Note: CI = confidence interval. NS = No Sample.
Fig. 2.
Distribution of the positive and negative cases according to the Optical Density
Note: Distribution of the positive and negative cases according to the Optical Density (OD) of samples from the various species studied. Positive cases are in red and negative cases are in black. Positive samples were defined according to the Kit CUT-OFF value according to the internal standard of each assay.
3.2. Prevalence of anti-HEV antibodies in study areas
Within a specie, anti-HEV antibodies positivity rates varied according to different locations (Table 2); rabbit in the province of Kadiogo are more likely to be infected than those in province of Gourma and Houet (p ˂ 0.05).
Table 2.
Comparison of prevalence of total antibodies Anti-HEV in domestic and wilds animals in three developed provinces and two rural provinces of Burkina Faso according to different animal species, genders and localities.
| Nr positive/nr tested | Total antibodies anti-HEV% | p-value | Odds ratio | IC95% Odds ratio | ||
|---|---|---|---|---|---|---|
| Rabbits | 60 (/100) | 60.0 ± 9.6 | Ref | Ref | Ref | Ref |
| Cattle | 19 (/72) | 26,4 ± 10.2 | 1.3.10−8 | 11,6 | [5.2–28.2] | <0.001⁎⁎⁎ |
| Goats | 23 (/81) | 28.4 ± 9.8 | 1.9.10−8 | 10.5 | [4.8–24.9] | <0.001⁎⁎⁎ |
| Hares | 10 (/19) | 52.6 ± 22.5 | 0.6 | 0.8 | [0.2–2.6] | >0.05 |
| Sheep | 9 (/75) | 12.0 ± 7.4 | 3.2.10−12 | 31.3 | [12.5–87.5] | <0.001⁎⁎⁎ |
| Female | 61 (/180) | 33.9 ± 6.9 | Ref | Ref | Ref | Ref |
| Males | 60 (/167) | 35.9 ± 7.3 | 0.5 | 0.8 | [0.5–1.4] | >0.05 |
| Houet | 7 (/29) | 24.1 ± 15.6 | Ref | Ref | Ref | Ref |
| Gourma | 18 (/34) | 52.9 ± 16.8 | 0.04 | 3.3 | [1.0–11.2] | <0.05* |
| Kadiogo | 96 (/284) | 33.8 ± 5.5 | 2.4.10−6 | 13.9 | [4.9–44.4] | <0.001⁎⁎⁎ |
Note: OR, odds ratio; CI, confidence interval; *, statistically significant. Statistically significant analyzes in the study were shown in the figure with an asterisk (*); *, statistically significant and P value ˂ 0.05.
Comparing domestic and wild animals within an area where both lagomorph species were tested, prevalence in hares (10/19 (52.6%)) is higher than that in rabbits (15/44 (34.1%)) in both Gourma and Houet provinces (p ˃ 0.05) (Table 1).
Considering the positive cases in a locality without distinction of species (Table 2), the highest seroprevalence was detected in Gourma (52.9%, CI95% [36.2–69.7]) and in Kadiogo area (33.8%, CI95% [28.3–39.3]). The lowest value of 24.1% (CI95% [8.6–39.7]) was recorded for the province of Houet.
Considering all animals species, sex distribution of HEV infection were 62/169 (35.9% CI95% [28.7–43.2]) in males and 61/182 (33.9% CI95% [27.0–40.8]) in females. There was no correlation between gender and HEV seropositivity (p > 0.5).
4. Discussion
Evidence of endemic HEV infections in Burkina Faso was demonstrated through the evaluation of prevalence or incidence of HEV in human population (blood donor and pregnant women) and in asymptomatic domestic swine (Traore et al., 2015, 2016). To the best of our knowledge, this is the first study that investigates anti-HEV antibodies in other mammalians that are important part of local food habits in three provinces of Burkina Faso.
HEV antibodies were detected in 34.9% of tested animals belonging to five (05) mammalian species, which indicate that HEV viruses or HEV infections occur in a wide range of mammalians. These data match findings from a study conducted in Nigeria that found anti-HEV antibodies in pigs, goats, sheep and cattle (Junaid et al., 2014).
Table 3 summarizes previously reported HEV prevalence rate in rabbits, which varies from 3.4% (Di Bartolo et al., 2016) to 72.5% (Liu et al., 2017). Three studies conducted with laboratory developed tests determined prevalence rate of 36.0% (Cossaboom, Cordoba, Dryman, & Meng, 2011), 3.4% (Di Bartolo et al., 2016) and 62.5% (Liu et al., 2017). Five other studies using commercial kit from Wantai Biopharmaceutical reported prevalence rates of 57.0% (Zhao et al., 2009), 15.4% (Geng et al., 2011c), 54.6% (Geng et al., 2010a, Geng et al., 2010b, Geng et al., 2011c, Geng et al., 2011b), 37.3% (Hammerschmidt et al., 2017) and 72.5% (Liu et al., 2017). Our results in Burkina Faso are close to the highest rates previously reported. We strongly feel/believe that lowest prevalence rates can't solely be explained by genotypes variation or immunological characteristics. Other factors such as breeding hygiene conditions, rabbit species, and transmission mode in different regions should also be considered (Liu et al., 2017). During our study we noticed poor hygiene and breeding space management conditions that allow cross-contamination between animals and wide spread of HEV. This can explain the high prevalence rate of HEV found in our study. Further studies are needed in Burkina Faso to explore zoonotic transmission of HEV and the molecular epidemiology of the virus.
Our survey shows a HEV seroprevalence of 52.6% (IC 95%; [30.2-75.1]) in hares (Table 1), suggesting that hares could be a reservoir of the virus in the wildlife in West Africa. In contrast, a low seroprevalence rate of 2.2% (Using Axiom test corresponding to the assay produced by Wantai) has been reported in Germany although for different species of hare (Hammerschmidt et al., 2017). This low prevalence, associated with those of two other studies carried out on the same species in Italy (Mazzei et al., 2015, Serracca et al., 2015), could indicate assumed minor importance of European hare in the epidemiology of HEV (Hammerschmidt et al., 2017). This discrepancy needs to be clarified by conducting more studies about HEV infection status in different districts. To that end, different habitats and biological behaviors should be considered.
The anti-HEV seroprevalence rate of 26.4% found in cattle samples was higher than those found in cattle from China or USA (Table 3). In these areas, HEV genotype 4d that is also found in human seems to circulate in the cattle (Yan et al., 2016). If the same strains were found in Burkina's cattle, as infectious HEV can also be excreted into milk, this can cause a new potential zoonotic risk, considering the Burkinabe population's consumption of the unpasteurized milk. In addition, HEV in milk seems not to be inactivated by pasteurization (Huang et al., 2016). However, recent data suggest in cattle a potential antigenic cross-reaction of the antibodies with anti-HEV responsiveness with a related, but as yet unknown agent (Yugo et al., 2019). We thus must be very cautious about the possible role of cattle in HEV transmission until new methods like deep sequencing of transcriptome or proteome of these “HEV positive” cattle may allow to identify the real antigen identity.
In this study, a prevalence of 12.0% (9/75) was recorded in sheep. This was in accordance with prevalence from similar studies in Nigeria and China (Geng et al., 2010a, Geng et al., 2010b, Junaid et al., 2014). Higher prevalence has been recorded with 29.0% to 35.2% in China (Wu et al., 2010, Wu et al., 2015), and 31.8% from Nigeria (Shuaibu et al., 2016). The high prevalence recorded in China could be linked to the sheep breed studied, there specific strains and the geographic area. The low prevalence of 12.0% observed in the sheep of our study can be explained by the presence of indeterminate cases. These indeterminate cases are samples that have an Optical Density value close to or superimposed on those of the cut-off (Fig. 2). In China, phylogenetic analysis based revealed that HEV strains isolated from sheep belong to genotype 4, subtype 4d (Wu et al., 2015), a genotype that can be transmitted from animals to humans. However, the zoonotic potential of HEV in sheep is still under discussion (Junaid et al., 2014).
Our study showed a prevalence of 28.4% (23/81) in goat which was also in accordance with similar studies in Nigeria and China (Table 3). To note, 100% (86/86) of goats in India were tested positive (Shukla, Chauhan, Naik, Anderson, & Aggarwal, 2007), although in the absence of molecular characterization of these putative HEV infection we cannot relate that to the repeated human HEV outbreaks recorded in India. In contrast, other studies demonstrated no anti-HEV antibodies in goats (Vitral et al., 2005). Therefore, HEV infection in goats may be infrequent, or the diagnostic methods used (in-house test) were not totally appropriate for this species (Arankalle et al., 2001).
The main limitation of our study is the usage of the single MP Diagnostics HEV ELISA 4.0v, that is an enzyme-linked immunosorbent assay intended for the detection of antibodies to Hepatitis E Virus in serum or plasma. The second limitation of this study is that was a pre-requisite for complementary study that will focus on molecular detection of the virus RNA. Fig. 2 presents a distribution of the positive and negative cases according to the Optical Density (OD) of each sample. Positive cases are in red and negative cases are in black. Some positive specimens seem stackable to negative; this is explained by the fact that some samples, particularly in sheep, have an OD value close to or equal to the CUT-OFF value. These samples could correspond to indeterminable cases and should be re-examined by another test such as Western Blot, in order to eliminate false positive cases caused by potential agents antigenically-related to HEV (Sridhar et al., 2019, Yugo et al., 2019).
5. Conclusion
Finally, the observed anti-HEV seroprevalence were considerably high in rabbits and local hares an already identified HEV reservoir in other countries, and was significantly lower but still present in cattle, sheep and goats. Our results could suggest that antigenic markers of HEV circulates among several animal species that are in the food chain in Burkina Faso. Further investigations are underway to identify the HEV genome in these animals as well as in sewage samples of animal origin for the dispersion capacity of the virus.
Funding
J.B.O. received funding from the 3rd-cycle university scholarship program of the Embassy of France in Burkina Faso (grant no: 895694D) (http://www.burkina.campusfrance.org).
Declaration of Competing Interest
Corporations that have funded the study played no role in the study design nor in the collection, analysis and interpretation of the data, nor in the decision to submit the manuscript for publication. None of the authors of this paper have a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Acknowledgements
We thank Dr Dahourou Anicet Georges for revising and checking grammatically the manuscript.
References
- Arankalle V.A., Joshi M.V., Kulkarni A.M., Gandhe S.S., Chobe L.P., Rautmare S.S. Prevalence of anti-hepatitis E virus antibodies in different Indian animal species. Journal of Viral Hepatitis. 2001;8(3):223–227. doi: 10.1046/j.1365-2893.2001.00290.x. http://www.ncbi.nlm.nih.gov/pubmed/11380801 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Cossaboom C.M., Cordoba L., Dryman B.A., Meng X.J. Hepatitis E virus in rabbits, Virginia, USA. Emerging Infectious Diseases. 2011;17(11):2047–2049. doi: 10.3201/eid1711.110428. http://www.ncbi.nlm.nih.gov/pubmed/22099094 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bartolo I., De Sabato L., Marata A., Martinelli N., Magistrali C.F., Monini M. Serological survey of hepatitis E virus infection in farmed and pet rabbits in Italy. Archives of Virology. 2016;161(5):1343–1346. doi: 10.1007/s00705-016-2778-y. http://www.ncbi.nlm.nih.gov/pubmed/26873813 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Dong C., Meng J., Dai X., Liang J.H., Feagins A.R., Meng X.J. Restricted enzooticity of hepatitis E virus genotypes 1 to 4 in the United States. Journal of Clinical Microbiology. 2011;49(12):4164–4172. doi: 10.1128/JCM.05481-11. http://www.ncbi.nlm.nih.gov/pubmed/21998412 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabo K., Toe L.P., Savadogo L., Zongo I., Tarnagda Z., Rouamba J. Caracteristiques de l'alimentation de rue dans la ville de Bobo-Dioulasso, Burkina Faso. Bulletin de la Societe de Pathologie Exotique. 2009;102(1):36. [PubMed] [Google Scholar]
- Fisher A., Laing J., Stoeckel J. Population Council; 1983. Handbook for family planning operations research design. [Google Scholar]
- Geng J., Wang L., Wang X., Fu H., Bu Q., Liu P. Potential risk of zoonotic transmission from young swine to human: Seroepidemiological and genetic characterization of hepatitis E virus in human and various animals in Beijing, China. Journal of Viral Hepatitis. 2011;18(10):e583–e590. doi: 10.1111/j.1365-2893.2011.01472.x. http://www.ncbi.nlm.nih.gov/pubmed/21914080 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Geng J., Wang L., Wang X., Fu H., Bu Q., Zhu Y. Study on prevalence and genotype of hepatitis E virus isolated from Rex Rabbits in Beijing, China. Journal of Viral Hepatitis. 2011;18(9):661–667. doi: 10.1111/j.1365-2893.2010.01341.x. http://www.ncbi.nlm.nih.gov/pubmed/20609076 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Geng Y., Zhao C., Song A., Wang J., Zhang X., Harrison T.J. The serological prevalence and genetic diversity of hepatitis E virus in farmed rabbits in China. Infection, Genetics and Evolution. 2011;11(2):476–482. doi: 10.1016/j.meegid.2010.12.012. http://www.ncbi.nlm.nih.gov/pubmed/21232633 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Geng J.B., Fu H.W., Wang L., Wang X.J., Guan J.M., Chang Y.B. [Hepatitis E virus (HEV) genotype and the prevalence of anti-HEV in 8 species of animals in the suburbs of Beijing] Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi. 2010;31(1):47–50. http://www.ncbi.nlm.nih.gov/pubmed/20302698 Retrieved from. [PubMed] [Google Scholar]
- Geng Y., Wang C., Zhao C., Yu X., Harrison T.J., Tian K. Serological prevalence of hepatitis E virus in domestic animals and diversity of genotype 4 hepatitis E virus in China. Vector Borne and Zoonotic Diseases (Larchmont, N.Y.) 2010;10(8):765–770. doi: 10.1089/vbz.2009.0168. http://www.ncbi.nlm.nih.gov/pubmed/20021275 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt F., Schwaiger K., Dahnert L., Vina-Rodriguez A., Hoper D., Gareis M. Hepatitis E virus in wild rabbits and European brown hares in Germany. Zoonoses Public Health. 2017 doi: 10.1111/zph.12355. http://www.ncbi.nlm.nih.gov/pubmed/28371421 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Huang F., Li Y., Yu W., Jing S., Wang J., Long F. Excretion of infectious hepatitis E virus into milk in cows imposes high risks of zoonosis. Hepatology. 2016;64(2):350–359. doi: 10.1002/hep.28668. http://www.ncbi.nlm.nih.gov/pubmed/27286751 Retrieved from. [DOI] [PubMed] [Google Scholar]
- ICTV . 2014. International Committee on the Taxonomy of Viruses Hepeviridae Study-Kingston and Montreal, Canada.https://talk.ictvonline.org/taxonomy/ July 2014. Email ratification 2015, (MSL #29) [Google Scholar]
- INSD . 2017. Institut national de la statistique et de la démographie. Annuaire statistique 2016 - Burkina Faso. 370.http://www.insd.bf/n/index.php/publications?id=36 Retrieved from. [Google Scholar]
- Izopet J., Dubois M., Bertagnoli S., Lhomme S., Marchandeau S., Boucher S. Hepatitis E virus strains in rabbits and evidence of a closely related strain in humans, France. Emerging Infectious Diseases. 2012;18(8):1274–1281. doi: 10.3201/eid1808.120057. http://www.ncbi.nlm.nih.gov/pubmed/22840216 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junaid S.A., Agina S.E., Jaiye K. Seroprevalence of Hepatitis E virus among domestic animals in Plateau State–Nigeria. British Microbiology Research Journal. 2014;4(8):11. doi: 10.9734/BMRJ/2014/10203. [DOI] [Google Scholar]
- Kmush B.L., Nelson K.E., Labrique A.B. Risk factors for hepatitis E virus infection and disease. Expert Review of Anti-Infective Therapy. 2015;13(1):41–53. doi: 10.1586/14787210.2015.981158. http://www.ncbi.nlm.nih.gov/pubmed/25399510 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Krain L.J., Nelson K.E., Labrique A.B. Host immune status and response to hepatitis E virus infection. Clinical Microbiology Reviews. 2014;27(1):139–165. doi: 10.1128/CMR.00062-13. http://www.ncbi.nlm.nih.gov/pubmed/24396140 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., Chen J., Xie J., Sun L., Ji F., He S. Hepatitis E virus serosurvey among pet dogs and cats in several developed cities in China. PLos One. 2014;9(6) doi: 10.1371/journal.pone.0098068. http://www.ncbi.nlm.nih.gov/pubmed/24896257 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Sun Y., Du T., Chen Y., Wang X., Huang B. Rabbit hepatitis E virus is an opportunistic pathogen in specific-pathogen-free rabbits with the capability of cross-species transmission. Veterinary Microbiology. 2017;201:72–77. doi: 10.1016/j.vetmic.2016.10.029. http://www.ncbi.nlm.nih.gov/pubmed/28284626 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Liu P., Bu Q.N., Wang L., Han J., Du R.J., Lei Y.X. Transmission of hepatitis E virus from rabbits to cynomolgus macaques. Emerging Infectious Diseases. 2013;19(4):559–565. doi: 10.3201/eid1904.120827. http://www.ncbi.nlm.nih.gov/pubmed/23628346 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzei M., Forzan M., Pizzurro F., Picciolli F., Bandecchi P., Poli A. Detection of hepatitis E virus antibodies in domestic and wild animal species in central Italy. Clinical Microbiology. 2015;4(06):1–4. [Google Scholar]
- Mirazo S., Ramos N., Mainardi V., Gerona S., Arbiza J. Transmission, diagnosis, and management of hepatitis E: An update. Hepatic Medicine. 2014;6:45–59. doi: 10.2147/HMER.S63417. http://www.ncbi.nlm.nih.gov/pubmed/24966702 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pas S.D., de Man R.A., Mulders C., Balk A.H., van Hal P.T., Weimar W. Hepatitis E virus infection among solid organ transplant recipients, the Netherlands. Emerging Infectious Diseases. 2012;18(5):869–872. doi: 10.3201/eid1805.111712. http://www.ncbi.nlm.nih.gov/pubmed/22516170 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavio N., Meng X.J., Doceul V. Zoonotic origin of hepatitis E. Current Opinion Virology. 2015;10:34–41. doi: 10.1016/j.coviro.2014.12.006. http://www.ncbi.nlm.nih.gov/pubmed/25588602 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Purdy M.A., Harrison T.J., Jameel S., Meng X., Okamoto H., Van der Poel W. ICTV virus taxonomy profile: Hepeviridae. Journal of General Virology. 2017;98(11):2645. doi: 10.1099/jgv.0.000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein D.B., Stevens G.A., Theaker J., Wittenborn J.S., Wiersma S.T. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55(4):988–997. doi: 10.1002/hep.25505. http://www.ncbi.nlm.nih.gov/pubmed/22121109 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Sanford B.J., Emerson S.U., Purcell R.H., Engle R.E., Dryman B.A., Cecere T.E. Serological evidence for a hepatitis e virus-related agent in goats in the United States. Transboundary and Emerging Diseases. 2013;60(6):538–545. doi: 10.1111/tbed.12001. http://www.ncbi.nlm.nih.gov/pubmed/22909079 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schielke A., Sachs K., Lierz M., Appel B., Jansen A., Johne R. Detection of hepatitis E virus in wild boars of rural and urban regions in Germany and whole genome characterization of an endemic strain. Virology Journal. 2009;6:58. doi: 10.1186/1743-422X-6-58. http://www.ncbi.nlm.nih.gov/pubmed/19442307 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serracca L., Battistini R., Rossini I., Mignone W., Peletto S., Boin C. Molecular investigation on the presence of hepatitis E virus (HEV) in wild game in north-western Italy. Food and Environmental Virology. 2015;7(3):206–212. doi: 10.1007/s12560-015-9201-9. [DOI] [PubMed] [Google Scholar]
- Shuaibu A.B., Alkali B.R., Abubakar M.B., Daneji M.B., Shuaibu A.I., Bello S.A. Prevalence of hepatitis E virus (HEV) antibodies in sheep from Sokoto State. Journal of Advances in Microbiology. 2016;1(2):6. doi: 10.9734/JAMB/2016/31378. [DOI] [Google Scholar]
- Shukla P., Chauhan U.K., Naik S., Anderson D., Aggarwal R. Hepatitis E virus infection among animals in northern India: An unlikely source of human disease. Journal of Viral Hepatitis. 2007;14(5):310–317. doi: 10.1111/j.1365-2893.2006.00815.x. http://www.ncbi.nlm.nih.gov/pubmed/17439520 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Smith D.B., Simmonds P., ameel S., Emerson S.U., Harrison T.J., Meng X.J. Consensus proposals for classification of the family Hepeviridae. Journal of General Virology. 2015;96(Pt 5):1191–1192. doi: 10.1099/vir.0.000115. http://www.ncbi.nlm.nih.gov/pubmed/26015322 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar S., Cheng V.C., Wong S.-C., Yip C.C., Wu S., Lo A.W. Donor-derived genotype 4 hepatitis E virus infection, Hong Kong, China, 2018. Emerging Infectious Diseases. 2019;25(3):425. doi: 10.3201/eid2503.181563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar S., Teng J.L.L., Chiu T.H., Lau S.K.P., Woo P.C.Y. Hepatitis E virus genotypes and evolution: emergence of camel hepatitis E variants. International Journal of Molecular Sciences. 2017;18(4) doi: 10.3390/ijms18040869. http://www.ncbi.nlm.nih.gov/pubmed/28425927 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traore K.A., Ouoba J.B., Huot N., Rogee S., Dumarest M., Traore A.S. Hepatitis E virus exposure is increased in pork butchers from Burkina Faso. American Journal of Tropical Medicine and Hygiene. 2015;93(6):1356–1359. doi: 10.4269/ajtmh.15-0321. http://www.ncbi.nlm.nih.gov/pubmed/26438027 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traore K.A., Ouoba J.B., Rouamba H., Nebie Y.K., Dahourou H., Rossetto F. Hepatitis E virus prevalence among blood donors, Ouagadougou, Burkina Faso. Emerging Infectious Diseases. 2016;22(4):755–757. doi: 10.3201/eid2204.151728. http://www.ncbi.nlm.nih.gov/pubmed/26982195 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitral C.L., Pinto M.A., Lewis-Ximenez L.L., Khudyakov Y.E., dos Santos D.R., Gaspar A.M. Serological evidence of hepatitis E virus infection in different animal species from the Southeast of Brazil. Memorias Do Instituto Oswaldo Cruz. 2005;100(2):117–122. doi: 10.1590/s0074-02762005000200003. http://www.ncbi.nlm.nih.gov/pubmed/16021297 Retrieved from. doi:/S0074-02762005000200003. [DOI] [PubMed] [Google Scholar]
- Wang S., Cheng X., Dai X., Dong C., Xu M., Liang J. Rabbit and human hepatitis E virus strains belong to a single serotype. Virus Research. 2013;176(1–2):101–106. doi: 10.1016/j.virusres.2013.05.013. http://www.ncbi.nlm.nih.gov/pubmed/23742853 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Worm H.C., van der Poel W.H., Brandstatter G. Hepatitis E: An overview. Microbes and Infection. 2002;4(6):657–666. doi: 10.1016/s1286-4579(02)01584-8. http://www.ncbi.nlm.nih.gov/pubmed/12048035 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Wu J., Si F., Jiang C., Li T., Jin M. Molecular detection of hepatitis E virus in sheep from southern Xinjiang, China. Virus Genes. 2015;50(3):410–417. doi: 10.1007/s11262-015-1194-9. http://www.ncbi.nlm.nih.gov/pubmed/25833205 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.Y., Kang Q., Bai W.S., Bai Z.H. [Seroepidemiological survey of sheep hepatitis E virus infection in Aksu region of Xinjiang Autonomous] Bing Du Xue Bao. 2010;26(3):234–237. http://www.ncbi.nlm.nih.gov/pubmed/20572346 Retrieved from. [PubMed] [Google Scholar]
- Wu Q., An J., She R., Shi R., Hao W., Soomro M. Detection of genotype 4 swine hepatitis E virus in systemic tissues in cross-species infected rabbits. PLos One. 2017;12(1) doi: 10.1371/journal.pone.0171277. http://www.ncbi.nlm.nih.gov/pubmed/28129390 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B., Zhang L., Gong L., Lv J., Feng Y., Liu J. Hepatitis E virus in yellow cattle, Shandong, Eastern China. Emerging Infectious Diseases. 2016;22(12):2211–2212. doi: 10.3201/eid2212.160641. http://www.ncbi.nlm.nih.gov/pubmed/27869603 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yugo D.M., Cossaboom C.M., Heffron C.L., Huang Y.W., Kenney S.P., Woolums A.R. Evidence for an unknown agent antigenically related to the hepatitis E virus in dairy cows in the United States. Journal of Medical Virology. 2019;91(4):677–686. doi: 10.1002/jmv.25339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Shen Q., Mou J., Gong G., Yang Z., Cui L. Hepatitis E virus infection among domestic animals in eastern China. Zoonoses Public Health. 2008;55(6):291–298. doi: 10.1111/j.1863-2378.2008.01136.x. http://www.ncbi.nlm.nih.gov/pubmed/18638181 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang L., Zeng H., Gong W., Wang L., Zhuang H. Development and validation of a new serum standard for the measurement of anti-HEV antibodies in animals. Journal of Medical Virology. 2017;89(3):497–501. doi: 10.1002/jmv.24651. http://www.ncbi.nlm.nih.gov/pubmed/27487450 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zeng H., Liu P., Liu L., Xia J., Wang L., et al. Hepatitis E vaccine immunization for rabbits to prevent animal HEV infection and zoonotic transmission. Vaccine. 2015;33(38):4922–4928. doi: 10.1016/j.vaccine.2015.07.040. http://www.ncbi.nlm.nih.gov/pubmed/26212003 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Zhao C., Ma Z., Harrison T.J., Feng R., Zhang C., Qiao Z. A novel genotype of hepatitis E virus prevalent among farmed rabbits in China. Journal of Medical Virology. 2009;81(8):1371–1379. doi: 10.1002/jmv.21536. http://www.ncbi.nlm.nih.gov/pubmed/19551838 Retrieved from. [DOI] [PubMed] [Google Scholar]