Highlights
-
•
86% of laying hen farmers were aware of antimicrobial resistance.
-
•
49% of laying hen farmers were taking measures to reduce antimicrobial use.
-
•
Flock age was linked to two indicators of responsible medicine use.
-
•
Farm size was linked to two indicators of responsible medicine use.
-
•
Veterinary contact was linked to two indicators of responsible medicine use.
Keywords: Antimicrobial resistance, Antibiotic resistance, Laying hens, Poultry, Surveys
Abstract
Antimicrobial resistance (AMR) is a significant global challenge affecting human health and attention has been drawn to practices of all stakeholders involved in antimicrobial prescription and administration, including in the livestock sector. This survey of free-range egg farmers (n = 117) was conducted to investigate knowledge, attitudes and practices surrounding antimicrobial use, and identify farmer-led solutions towards responsible antimicrobial use. Most participants proved knowledgeable of AMR and selected treatments based on principles of responsible medicine use. ‘Worms’ and ‘infectious diseases’ were the most common reasons for medicine use. Farms with a higher number of poultry houses, younger flock ages at depopulation and farms visited by a vet less than once a year or 3–4 times a year (compared to annually or twice a year) were more likely to select ‘ANTIBIOTICS ONLY’, as opposed to ‘BOTH ANTIBIOTICS AND ANTIPARASITICS’ or ‘ANTIPARASITICS ONLY’ as their most frequently used medicines. Participants from farms with a younger flock age at depopulation, from company-owned farms, and participants purchasing medicines from agricultural merchants instead of veterinary practices were less likely to be taking measures to reduce or replace antimicrobial use. Participants from larger farms and those that had less contact with their vet were less likely to think that they could reduce or replace the amount of antibiotics used. Survey results provided evidence for the important role of veterinarians in guiding antimicrobial stewardship through engagement, collaboration and education. Discussion groups in which farmers share best practices could assist the free-range egg industry in further promoting responsible antimicrobial use.
1. Introduction
Antimicrobial resistance (AMR) is the ability of microbes, including bacteria, viruses or parasites, to grow or survive in the presence of an antimicrobial agent (ECDC, 2008). Antibiotic resistance (i.e. bacterial ability to grow or survive in the present of an antibiotic agent) is one of humanity's most pressing challenges (NOAH, 2016), resulting in mortality and growing pressures on global health care systems (O'Neill, 2014; Warren, 2017). The association between antibiotic use in livestock and the development of antibiotic resistance is now widely acknowledged (Landers, Cohen, Wittum, & Larson, 2012). Preventative use of antibiotics in healthy animals is of particular concern, as is the use of antibiotics essential for human medicine. The World Health Organisation (WHO) has categorised antibiotics as: important; highly important; critically important and highest priority critically important antibiotics (HPCIAs) for human health (WHO, 2017a). HPCIAs represent the sole treatment options for some of the most serious infections in people globally. There are calls for HPCIA use in animals to be restricted or banned (O'Neill, 2015; WHO, 2017b).
Antiparasitics and anthelmintics are used in laying hens for the control of helminths (worms) and mites which are prevalent in UK laying hens (Fiddes et al., 2005; Permin et al., 1999). Antiparasitic resistance to commonly used red mite treatments has been reported, including multi-drug resistance (Beugnet, Chauve, Gauthey, & Beert, 1997; Marangi et al., 2009; Nordenfors, Höglund, Tauson, & Chirico, 2001). With the reliance on a small number of drug classes for the treatment of common helminths such as Ascaridia galli in laying hens, the development of anthelminthic resistance is a concern for production and hen welfare (Tarbiat, Jansson, Tydén, & Höglund, 2017). However, unlike antibiotic resistance, antiparasitic resistance does not pose an immediate threat to human health (O'Neill, 2015).
The UK egg industry is a relatively low user of antibiotics. In 2016, the total tonnage of antibiotics used in the egg industry was less than that of the meat poultry industry by a magnitude of around ten (VMD, 2017). However, antibiotics are used to treat disease outbreaks (e.g. Escherichia coli, Brachyspira sp., Enterococcus sp., Pasteurella sp.) in laying hens. In 2016, tetracyclines (classified as ‘highly important’ by the WHO) and pleuromutilins (classified as ‘important’ by the WHO) accounted for 83% of antibiotics used in UK laying hens (VMD, 2017). HPCIAs account for a small percentage of antibiotic use in UK laying hens as the use of fluoroquinolones at day-old and the use of all 3rd and 4th generation cephalosporins is prohibited by the Lion Code, which is an industry code of practice covering around 90% of UK egg production (Lion Code, 2013, 2017).
Laying hens are exposed to relatively low levels of antibiotics compared to poultry grown for meat production (VMD, 2017). Higher levels of resistance to antibiotics have been found in broilers compared to laying hens (Asai et al., 2006; van den Bogaard, Willems, London, Top, & Stobberingh, 2002). However, in Europe, resistant and multi-drug resistant Salmonella sp. and enterococci bacteria have been isolated from laying hens in commercial (Schwaiger, Schmied, & Bauer, 2008, 2010; Snow et al., 2007) and backyard flocks (Bertelloni et al., 2015). There are a number of potential pathways linking antibiotic use in farm animals and antibiotic resistance in disease-causing bacteria in humans, including direct contact with animals, via environmental contamination with farm effluents and consumption of meat (Landers et al., 2012).
Antibiotic usage is a key driver of resistance (WHO, 2001). In addition to analysing suboptimal use of antibiotics in healthcare settings, assessing behaviours of stakeholders in livestock antibiotic prescription and administration is key to identifying high risk practices and promoting best practice (Jones et al., 2015). Antibiotics for use in UK livestock must be prescribed by veterinarians. However, farmers are generally responsible for the collection, storage and administration of antibiotics to animals under their care or ownership. A better understanding of farmers’ understanding and motivations for implementing prudent antibiotic practices is required to help drive ‘responsible’ antibiotic use. Responsible antibiotic use is defined by the Responsible Use of Medicines in Agriculture Alliance (RUMA) as ‘using medicines as little as possible and as much as necessary’, which includes reducing and replacing the use of antibiotics by controlling disease challenges, and only using antibiotics according to veterinary prescription and advice (RUMA, 2015). There are a number of other organisations that define responsible medicine use, and as previously discussed, some that advocate the restriction of the HPCIAs for use in food-producing animals (WHO, 2017b).
Compared to other farmed species, there is a deficit of published peer-reviewed research on antimicrobial use in the poultry sector, especially within the European Union (Hockenhull et al., 2017). It is therefore important that this area is investigated further to build a picture of antimicrobial use. This farmer knowledge, attitudes and practices (KAP) survey was developed following WHO guidelines (WHO, 2008). The survey was designed to: investigate antimicrobial usage on UK laying hen farms; characterise farms practising responsible antimicrobial use; highlight the key drivers of responsible antimicrobial use; and identify farmer preferences for reducing and replacing antimicrobial use on their farms.
2. Materials and methods
2.1. Participants
Farmers from two companies producing eggs from laying hens reared to RSPCA-assured free-range standards (RSPCA, 2017) or Organic Farmers and Growers (OF&G) (OF&G, 2015) were invited to complete an online questionnaire between March and July 2017 (n = 382 farmers). Questionnaires were sent via email from both companies then followed up by area managers requesting completion. Farmers participated on an informed, voluntary and confidential basis, with the option to remain anonymous. No incentives were provided for completion of the questionnaire. Participation was encouraged by suppliers via email prompts and company newsletters.
2.2. Questionnaire
The KAP questionnaire was developed for dairy cow farmers by Higham et al. (2018) and was adapted and compiled by the authors for laying hens and piloted with five free-range laying hen farmers. Alterations were made to improve clarity of terminology and the final questionnaire (Questionnaire; supplementary information) was distributed via SurveyMonkey (SurveyMonkey, San Mateo, California, USA). The questionnaire was structured in six sections exploring: farm characteristics; farming challenges; characteristics of last flock; veterinary services and support; use of veterinary medicines; and practical solutions.
2.3. Data processing and analysis
All personally identifiable data was removed before data analysis. Questionnaire responses were analysed in IBM SPSS version 22.
For the purpose of analysis, the questions from the six sections of the questionnaire were allocated into three categories: characteristics of farm and respondent; services and advice; and, indicators of responsible medicine use (Table S1; supplementary information). Variables within the categories of ‘characteristics of farm and respondent’ and ‘services and advice’ were allocated as explanatory variables, and variables within the category ‘indicators of responsible medicine use’ were analysed further as dependent variables. These variables are described in Table 1. Questions indicative of ‘responsible medicine use’ were devised to reflect the RUMA definition of responsible medicine use (RUMA, 2015), and the WHO recommendation to restrict the use of HPCIAs in food producing animals (WHO, 2017b). Data were analysed using χ2 tests, generalized linear models (GLMs) or ordinal, nominal or binomial logistic regression where appropriate for the dependent variable. Examination of adjusted standardized residuals (>2) was used to identify significant individual responses to questions. Significance of variables was determined at a p < 0.05 level. General questions exploring medicine use on farm were explored using descriptive statistics.
Table 1.
‘Indicators of responsible medicine use’ relating to best practice regarding antimicrobial use.
| Indicator of responsible medicine use | Question | Multiple choice options/example answers | Categorised responses |
|---|---|---|---|
| Antimicrobial use | “What are the three veterinary medicines that you have used most frequently in the past 12 months?” | Open ended response |
“BOTH” Both antibiotics and antiparasitics reported |
| “ANTIBIOTIC ONLY” reported | |||
| “ANTIPARASITIC ONLY” reported | |||
| Factors considered when selecting a treatment | “Please select and rank the three most important factors you consider, when selecting a treatment for an animal on your farm” | 1. Whether you have the medicine available on farm 2. Cost of the product 3. Whether you are familiar with the product and have used it in the past 4. The length of the withdrawal period 5. Whether the product is suitable and licensed for the animal's condition 6. Whether your vet has prescribed the product for the condition 7. The relative importance of the antibiotic in human medicine 8. Othera |
“NOT RESPONSIBLE” Options 1–4: Response inconsistent with responsible medicine use |
|
“RESPONSIBLE” Options 5–7: Response consistent with responsible medicine use | |||
| Knowledge of antimicrobial resistance | “In your own words, what is antimicrobial resistance and what are the threats it presents?” | Responses were categorised as accurate, partly accurate or inaccurate, according to the World Health Organisation (WHO 2017) and the O'Neill Commission's (O'Neill, 2014) definitions: 'When a microorganism changes when exposed to antimicrobial drugs, resulting in the medicines becoming ineffective and infections persist in the body, increasing the risk of spread to others' (WHO, 2017); 'The ability of a microbe to resist the effects of medication previously used to treat them' (O'Neill, 2014). |
“ACCURATE” E.g. reduced effectiveness of agents due to diversity of genetics in target organisms |
|
“PARTLY ACCURATE” e.g. resistance to antibiotics | |||
|
“INACCURATE” e.g. when birds get used to antibiotics | |||
| Undertaking measures to reduce/replace antimicrobials | “Are you currently taking any measures to reduce the amount of antimicrobial medicines (including antibiotics and coccidiostats) used on your farm?” | Yes/No |
YES NO |
| Could reduce or replace antibiotics | “Do you think you could reduce the use of antibiotics on your farm, or replace the use of these medicines with alternatives?” | Yes/No |
YES NO |
| Record keeping | “How do you currently record the use of veterinary products on your farm? (Tick all that apply)” | 1. Paper medicine or record 2. Spreadsheet on a computer 3. Farm management software 4. Our vet records out medicine use 5. We do not record medicine use 6. Other (please specify) |
“RECORDING” Options 1–4 and 6 (if specified) “NOT RECORDING” Option 5 |
One participant answered ‘other’ to this question noting, ‘none-we are organic’. Because the question was regarding general medicine use, not antimicrobials, it was believed he/she had misunderstood the question, so this response was omitted (marked NA).
3. Results
3.1. Data exploration
3.1.1. Questionnaire responses and response rate
382 farmers received access to the questionnaire. 117 responses were received and used in the final analysis, resulting in a 30.6% response rate overall.
3.1.2. Characteristics of farms and respondents
The majority of farms were in the North-West of England (31.6%), followed by the West Midlands (17.9%) and the East of England (10.3%). Scotland was least represented (1.7%). The majority of participants were free-range egg farmers (96.6%; n = 113) whilst 3.4% farmed free-range and organically (n = 4). Most (89.7%) participants’ roles were ‘farmer (owners) with an active role on the farm’ (n = 105) whilst 4.3% (n = 5) of participants were farm managers. Eighty-nine percent of farms were independent-contracted i.e. they are privately owned by the farmer but contracted to supply eggs to the company (n = 104), whilst the other 11% (n = 13) were company-owned i.e. they are owned directly by the company and run by company-employed farm managers. The mean number of birds in lay for all participants was 18,806 (SD ± 14,147.48, range = 3900–76,000, n = 116, one respondent answered zero for this question so was not included); the mean number of poultry houses on each participant's farm was 1.97 (SD ± 1.43, range = 1–10, n = 117).
3.1.3. Characteristics of last flock
Characteristics of participants’ last depopulated flock are summarised in Table 2. Mortality rates were highly variable (range = 0.2–50.0%). The participant whose last depopulated flock had a mortality rate of 50% also stated that these 50% were lost due to disease. One data point was removed from ‘age at last flock peak production’, as the participant stated 72 weeks, which was the same as their stated age at depopulation, so it was assumed to be an error.
Table 2.
Characteristics of participants’ last depopulated flock.
| Characteristic | Mean | Standard deviation | Range | Number of responses |
|---|---|---|---|---|
| Age of last flock at depopulation (weeks) | 72.9 | 3.3 | 64.0–87.0 | 117 |
| Age of last flock at peak production (weeks) | 28.5 | 5.8 | 21.0–51.0 | 116 |
| Percentage floor eggs in last depopulated flock (%) | 2.5 | 2.5 | 0.0–15.0 | 115 |
| Mortality rate of last depopulated flock (%) | 9.1 | 8.0 | 0.2–50.0 | 117 |
| Mortality rate of last depopulated flock due to disease (%) | 3.8 | 7.3 | 0.0–50.0 | 104 |
| Cull rate of last depopulated flock (%) | 0.9 | 1.2 | 0.0–7.0 | 111 |
| End-of-lay feather cover of last depopulated flock (score 1–5) | 2.5 | 1.0 | 1.0–5.0 | 116 |
3.1.4. Challenges on farm
Participants were asked to rank six key challenges in terms of how they affected their egg business (Table 3) and six health and welfare challenges in terms of their effect on their birds in the past 12 months in order from one to six (Table 4). Both questions included a box marked ‘other’ in which respondents could list additional important challenges to their businesses and birds. ‘Other’ important business challenges included: “reliability and provision of quality pullets” and “red tape and paper work”. ‘Other’ important health and welfare challenges included: “distance of transportation between rearer and farm”, “heat stress” and “perch provision and beak tipping”.
Table 3.
Frequency and percentage of participants ranking each challenge as the most important challenge to their business (n = 117).
| Challenge | Number of responses | % of responses |
|---|---|---|
| Input cost volatility | 58 | 49.6 |
| National disease outbreaks | 20 | 17.1 |
| Supply-demand market volatility | 19 | 16.2 |
| On-site disease problems | 14 | 12.0 |
| Labour/employment issues | 4 | 3.4 |
| Weather problems | 2 | 1.7 |
| Total | 117 | 100 |
Table 4.
Frequency and percentage of participants ranking each health and welfare challenge as the most important challenge to their birds (n = 111, 6 participants did not respond).
| Challenge | Number of responses | % of responses |
|---|---|---|
| Red mite | 28 | 25.2 |
| Primary disease infection | 23 | 20.7 |
| Feather pecking | 22 | 19.8 |
| Smothering | 18 | 16.2 |
| Predation | 12 | 10.8 |
| Secondary disease infection | 8 | 7.2 |
| Total | 111 | 100 |
3.1.5. Veterinary services and support
The most common reason for participants to call their vet in the last 12 months was advice on health and flock management (54.5% of responses, n = 61). The second most common reason for participants to call their vet was for ordering and purchasing of veterinary medicines (36.6% of participants, n = 41); whilst the third most common reason for participants to call their vet was for control of infectious diseases (8.9% of participants, n = 10). 4.3% of participants (n = 5) had not called their vet in the last 12 months.
3.1.6. Use of veterinary medicines
All answer-providing participants reported using an antimicrobial (including antibiotics and antiparasitics) amongst the top three most frequently used veterinary medicines in the last 12 months (Table 5). However, none of the antibiotics listed by participants were HPCIAs (WHO, 2017a).
Table 5.
Frequency and percentage of participants reporting using antimicrobials as one of the top three most frequently used medicines in the last 12 months (n = 105, 12 participants did not respond).
| Category | Number of responses | % of responses |
|---|---|---|
| ANTIPARASITIC ONLY | 58 | 55.2 |
| BOTH antiparasitics and antibiotics | 34 | 32.4 |
| ANTIBIOTIC ONLY | 13 | 12.4 |
| Total | 105 | 100 |
The most common reasons for treatment with the three most commonly used antimicrobial products were worms followed by infectious diseases (Table 6).
Table 6.
Reasons for treatment with the three most commonly used antimicrobial products (n = 105 participants).
| Reasons for treatment with most commonly used antimicrobial products (1st, 2nd and 3rd most commonly used medicines included) | Number of responses | Percentage of responses (%) |
|---|---|---|
| Worms | 102 | 56.7 |
| Infectious disease | 33 | 18.3 |
| Gastrointestinal issues | 11 | 6.1 |
| Production drop | 10 | 5.6 |
| Red mite | 9 | 5.0 |
| Peritonitis | 7 | 3.9 |
| Histomoniasis (Blackhead disease) | 4 | 2.2 |
| Bird health | 2 | 1.1 |
| Cleaning and disinfection | 1 | 0.6 |
| Fly control | 1 | 0.6 |
| Total | 180a | 100 |
This represents the total number of responses for the three top medicines used so the sum is greater than the number of participants (n = 105 participants). Not all participants provided answers for all three medicines.
Participants were asked to rank their top three most important sources of information regarding the selection and use of veterinary medicines out of ten options (Table 7). The majority (90.6%, n = 106) of respondents ranked their vet as their most important source of advice.
Table 7.
Frequency and percentage of participants ranking each source of information as their first, second and third most important source.
| Source of information | Most important source of advice |
Second most important source of advice |
Third most important source of advice |
|||
|---|---|---|---|---|---|---|
| Number of responses | % of responses | Number of responses | % of responses | Number of responses | % of responses | |
| Your vet | 106 | 90.6 | 6 | 5.4 | 0 | 0.0 |
| Agricultural merchants | 2 | 1.7 | 11 | 9.9 | 2 | 1.9 |
| Industry bodies e.g. BEIC | 2 | 1.7 | 22 | 19.8 | 20 | 19.0 |
| Farming press e.g. poultry magazines, newspapers | 1 | 0.9 | 6 | 5.4 | 18 | 17.1 |
| Training courses and materials | 1 | 0.9 | 25 | 22.5 | 21 | 20.0 |
| Neighbours, friends and other farmers | 0 | 0.0 | 13 | 11.7 | 19 | 18.1 |
| Certification scheme bodies e.g. organic certifiers etc. | 0 | 0.0 | 8 | 7.2 | 3 | 2.9 |
| Websites | 0 | 0.0 | 5 | 4.5 | 6 | 5.7 |
| Drug companies, reps or product advertisements | 0 | 0.0 | 4 | 3.6 | 5 | 4.8 |
| Books | 0 | 0.0 | 1 | 0.9 | 2 | 1.9 |
| Othera | 5 | 4.3 | 10 | 9.0 | 9 | 8.6 |
| Total | 117 | 100 | 111 | 100 | 105 | 100 |
Other responses mainly indicated advice from the egg packer/company.
3.1.7. Practical solutions
Thirty-seven participants (31.6%) said they used farm management software or databases whilst 80 (68.4%) did not. Twenty-nine participants (24.8%) expressed interest in using a mobile device to record future medicine use. One respondent (0.9%) already used a mobile device for this purpose whilst 87 participants (74.4%) said they would not be interested in using a mobile device.
Participants were asked “Are you currently taking any measures to reduce the amount of antimicrobial medicines (including antibiotics and de-wormers) used on your farm? If yes, please describe what actions you are taking”. Fifty-seven of 117 participants (48.7%) said they were taking measures to reduce antimicrobial use. Of those that were taking measures to reduce antimicrobial use, 57 described the measures being taken (Table 8).
Table 8.
Categorised answers describing the measures currently taken to reduce or replace antimicrobial use on their farms (n = 57 participants, 100% of those taking measures).
| Measures taken | Number of responses | % of Responses |
|---|---|---|
| Management practices (e.g. reducing stress/ biosecurity) | 17 | 29.8 |
| Last resort/ only when necessary (e.g. if hens present clear symptoms) | 14 | 24.6 |
| Probiotics | 11 | 19.3 |
| Do not use them at all | 6 | 10.5 |
| Minimise use in general | 3 | 5.3 |
| Vaccination | 3 | 5.3 |
| On advice from vet | 3 | 5.3 |
| Total Responses | 57 | 100 |
Participants answered what would help them and their staff manage animal health issues and promote effective use of medicines, selecting from a list of options. The most common response was attending training days or courses (38.5%) (Table 9). ‘Other’ responses included experience, field officer visits, good bird management, and one-to-one talks.
Table 9.
Responses to “what would help you and your staff manage animal health issues and promote the effective use of medicines on your farm?” (n = 117, all participants responded).
| Farmer led solutions to manage flock health | Number of responses | % of Responses |
|---|---|---|
| Attendance of training courses or training days | 45 | 38.5 |
| Further veterinary visits and support | 19 | 16.2 |
| Training courses–online webinars | 17 | 14.5 |
| Information posters and reading resources | 10 | 8.5 |
| An online farmer network/forum to share knowledge | 9 | 7.7 |
| Other | 7 | 6.0 |
| Training courses–podcasts | 5 | 4.3 |
| A farmer conference | 5 | 4.3 |
| Total Responses | 117 | 100 |
3.2. Indicators of responsible medicine use
3.2.1. Most frequently used veterinary medicines
Categories of products (Table 5) were explored against ‘characteristics’ and ‘services and advice’ variables.
Number of poultry houses on farm was associated with category of antimicrobial reported. As the number of poultry houses increased, the three most commonly used products were more likely to be classified as ‘ANTIBIOTIC ONLY’ compared to ‘ANTIPARASITIC ONLY’ (χ2 = 9.3, df = 2, p = 0.009).
Frequency of vet visits to the farm was a significant predictor of category of antimicrobial reported (χ2 = 15.2, df = 6, p = 0.019). The three most commonly used products were more likely to be classified as ‘ANTIBIOTIC ONLY’ on farms where the vet visited less than once a year or 3–4 times a year, compared to farms where the vet visited twice a year or annually (Fig. 1).
Fig. 1.
Percentage of participants whose three most commonly used products were classified as ‘ANTIPARASITIC ONLY’, ‘ANTIBIOTIC ONLY’ or ‘BOTH’ according to the frequency of vet visits to farm (n = 105 participants, 12 participants did not respond).
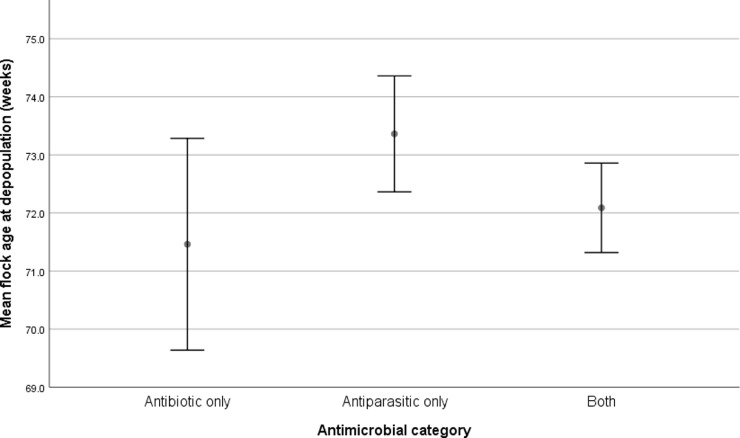
Flock age at depopulation was significantly associated with category of antimicrobial reported (χ2 = 6.1, df = 2, p = 0.047). Where flock age at depopulation was lower, the three most commonly used products were more likely to be classified as ‘ANTIBIOTIC ONLY’ (Fig. 2).
Fig. 2.
Mean flock age at depopulation (± 95% confidence intervals) where the three most commonly used products were classified as ‘ANTIPARASITIC ONLY’, ‘ANTIBIOTIC ONLY’ or ‘BOTH’ (n = 105 participants, 12 participants did not respond).
3.2.2. Factors considered in the selection of veterinary medicines for treating animals
When asked to determine the most important factors when choosing treatments for birds on their farm, the majority (94.8%, n = 110) of participants provided a “RESPONSIBLE” response as the most important factor. The most common response was whether the participants’ vet had prescribed the product for the condition (65.0% participants, n = 76). Of those providing a “NOT RESPONSIBLE” response, four participants’ most important factor was the length of withdrawal period whilst two participants cited the cost of the product. Due to the small number of “NOT RESPONSIBLE” responses (5.2% of responses, n = 6), associations with this variable were not further explored. Participants’ second most important factors for choosing a treatment were also generally “RESPONSIBLE” (70.0% of responses n = 76). The most common second most important factor considered when selecting a treatment was whether the product is suitable and licensed for the animal's condition (47.0% of responses, n = 55).
3.2.3. Knowledge of antimicrobial resistance
When asked to “Describe antimicrobial resistance and the threats it presents”, 27 participants (23.1%) gave an ‘INACCURATE’ response, 50 gave a ‘PARTIALLY ACCURATE’ response (42.7%) and 40 gave an ‘ACCURATE’ response (34.2%). None of the variables examined had a significant association with the answer to this question.
3.2.4. Measures taken to reduce the amount of antimicrobials used on farm
Participants were asked “are you currently taking any measures to reduce the amount of antimicrobial medicines (including antibiotics and de-wormers) used on your farm? If yes, please describe what actions you are taking”. Fifty-seven of 117 participants (48.7%) said they were taking measures to reduce antimicrobial use.
Whether farms were company-owned or independent-contracted was associated (not significant) with whether participants were taking measures to reduce/replace antimicrobials (χ2 = 3.8, df = 1, p = 0.050). Company-owned farms were more likely to answer ‘no’.
Where participants purchased veterinary medicines from was significantly associated with whether participants were taking measures to reduce/replace antimicrobials (χ2 = 4.2, df = 1, p = 0.041). Participants were more likely to state that they were not taking any measures to reduce antimicrobial use if they purchased veterinary products from an agricultural store/merchant with a prescription from a vet compared to purchasing from a veterinary source and/or online with a prescription from a vet.
Flock age at depopulation was significantly associated with whether participants were taking measures to reduce/replace antimicrobials (GLM with binomial probability distribution and logit link function χ2 = 4.5, df = 1, p = 0.035). Participants with a younger flock age at depopulation were less likely to be taking measures to reduce antimicrobial use.
3.2.5. Ability to reduce or replace the use of antibiotics on farm
Participants were asked, “Do you think you could reduce the use of antibiotics on your farm, or replace the use of these medicines with alternatives?”, 99 of 117 participants (84.6%) answered ‘no’. Participants were significantly more likely to answer no to this question when they had a larger number of birds in lay (GLM with binomial probability distribution and logit link function: χ2 = 6.1, df = 1, p = 0.014), a higher number of poultry houses (GLM with binomial probability distribution and logit link function: χ2 = 4.2, df = 1, p = 0.041) and when they had less contact with their vet (GLM with binomial probability distribution and logit link function: χ2 = 6.6, df = 1, p = 0.010; Fig. 3).
Fig. 3.
Percentage of participants answering ‘yes’ and ‘no’ to the question “do you think you could reduce the use of antibiotics on your farm or replace the use of these medicines with alternatives?” according to the frequency of veterinary contact (either in person or by phone) (n = 117 participants).
3.2.6. Records of use of veterinary medicines
Participants were asked, “How do you currently record the use of veterinary products on your farm?” All participants were recording medicine use. 115 out of 117 participants (98.29%) were using a paper medicine book or record, with 17 of these (14.5%) also acknowledging that their vet records medicine use. The other two participants were recording medicine use via a computer spreadsheet/farm management software. Because all participants were recording medicine use, the results from this question were not further analysed.
4. Discussion
4.1. Challenges on farm
The majority (49.6%) of participants ranked input cost volatility as the most important challenge to their business. This is supported by data showing that, for free-range eggs, the egg price: feed input ratio has been decreasing steadily from 0.54 to 0.34 in the UK since December 2015 (Poultry World, 2018). National disease outbreaks were ranked as the most important challenge to business by 17.1% of participants. During the first half of 2017, when this questionnaire was being completed, highly pathogenic avian influenza (AI) had been identified in the UK, with free-range poultry farmers being ordered to keep their birds inside to reduce the risk of transmission from wild birds. AI is an acute, highly contagious, fatal disease that causes severe disease in chickens and up to 100% mortality (Alexander, 2000). Therefore, it is not surprising that national disease outbreaks were ranked as being the most important challenge for these respondents.
Red mite was most commonly ranked as the most important health and welfare challenge (25.2% participants). Red mite affects around 30% of laying hen flocks (Zloch, Kuchling, Hess, & Hess, 2018). These ectoparasites cause skin irritation, anaemia, and behavioural changes such as increased feather pecking and self-grooming and can result in decreased egg production, leading to a negative economic impact on producers (O’Kilpinen et al., 2005). Severe infestations can cause death due to anaemia (Fossum, Jansson, Etterlin, & Vågsholm, 2009; Zloch et al., 2018). Red mites are also increasingly suspected of being a disease vector (Sparagano, George, Harrington, & Giangaspero, 2014). Antiparasitics were reported as being one of the top three most commonly used medicines in the last 12 months by 87.6% of participants. Multi-drug resistance has been reported to commonly used red mite treatments (Beugnet et al., 1997; Marangi et al., 2009; Nordenfors et al., 2001) demonstrating the importance of responsible use of antiparasitics for bird welfare.
Primary disease infection was rated the second most important health and welfare challenge. This links to infectious diseases being the second most common for treatment in this group of producers, showing that participants’ views on the most significant health and welfare challenges are aligned with the reasons for treating their flock (see: Disease burden and antimicrobial use).
The third most important challenge, as ranked by participants, was feather pecking. Feather pecking is an abnormal behaviour consisting of pulling, plucking and damaging the feathers of conspecifics which can result in poor quality plumage, skin damage and feather loss (Savory, 1995). The average feather score for this group of producers’ last depopulated flock was 2.5, with scores ranging from 1–5 (1 representing no to very little damage and 5 representing severe damage to feathers) (Bright, Brass, Clachan, Drake, & Joret, 2011). Although feather pecking itself is not necessarily linked to antimicrobial use, the occurrence of feather pecking has been linked to increased disease incidence, which may necessitate antimicrobial use (Green et al., 2000; Pötzsch, Lewis, Nicol, & Green, 2001). However, it is unclear as to whether factors stemming from feather pecking, such as feather loss and skin damage, lead to an increased disease susceptibility or whether diseased flocks are more likely to develop feather pecking.
4.2. Veterinary services and support
A high majority (90.6%) of participants ranked their vet as the most important source of advice regarding the selection and use of veterinary medicines on their farm. Therefore, vets have a vital role to play in antimicrobial stewardship within this group of free-range egg producers. More than half (54.5%) of participating farmers stated that the most common reason for calling their vet was for advice on flock health and management. This indicates that these farmers utilise their vets to discuss and improve general flock health rather than just as a means to access treatment. The proportion of farmers that see vets in more of an advisory role could, however, be further improved. Ruston et al. (2016) conducted interviews with 28 farm animal vets in England, revealing that few vets believed themselves to be the main source of information on disease prevention and biosecurity for their clients, whilst some vets felt that their role as an advisor is at risk from non-veterinarian consultants. The majority of vets interviewed by Ruston et al. (2016) stated they were beginning to work in partnership, sharing decision making with their clients rather than acting as ‘experts’ who assume authority over their clients. Such a move towards more collaborative strategies may foster a more positive relationship between farmers and vets based on mutual agreement. This collaborative approach could also be beneficial for motivating farmers to follow veterinary advice (Bard et al., 2017) including the reduction, replacement and refinement of antimicrobials.
4.3. Disease burden and antimicrobial use
As evidenced by the variable mortality rates of the participants’ last depopulated flocks (Table 2), disease is a major health and welfare challenge for laying hens, and a significant economic challenge for laying hen farmers, with up to 50% of participants’ flocks being lost to disease. All answer-providing participants reported using an antimicrobial amongst their top three most frequently used veterinary medicines in the last 12 months (including antiparasitics and antibiotics; Table 5). None of the participants reported using HPCIAs in line with the restrictions imposed by the Lion Code in the UK (Lion Code, 2013, 2017). The most common reason for medicine use was worms (Table 6). Worms can lead to: direct loss of animals, reduction in growth rate, weight loss and reduced feed conversion ratios (Ramadan et al., 1991). Infection with worms can be a predisposing factor for bacterial infections such as zoonotic Salmonella (Dahl et al., 2002; Eigaard et al., 2006). The second most common reasons for treatment, infectious diseases, are common causes of mortality in laying hens, with free-range/organic hens potentially more at risk (Fossum et al., 2009) (although see Schwaiger et al., 2008, 2010). Resistance to numerous commonly used antibiotics for infectious diseases including Enterococcus sp. Escherichia. coli and Campylobacter sp. isolated from laying hens has been reported (Schwaiger et al., 2008, 2010; Stępień-Pyśniak et al., 2016). The future development and use of vaccines, effective health planning and biosecurity will aid the egg industry in reducing the burden of disease, thus reducing the need for treatment with antibiotics.
4.4. Farm and flock characteristics and antimicrobial use
Respondents were more likely to report the use of ANTIBIOTICS ONLY as their top three most frequently used medicines as the number of poultry houses on farm increased. This suggests that larger farms may be more likely to encounter health problems requiring antibiotic use. In broiler chickens, farms with three or more houses have been found to have an increased risk of contamination with Campylobacter sp. (Refregier-Petton, Rose, Denis, & Salvat, 2001), suggesting that larger farms may have an increased risk of disease transfer across multiple houses. Alternatively, it may be that farms with more houses have more staff and less compliance with set protocols, or fewer staff per bird, so are less able to detect disease outbreaks quickly, resulting in increased antibiotic use. There may also be links between vets’ prescribing habits and the size of the farm; for example, vets may be more risk-averse when dealing with a disease outbreak on a larger farm due to the larger potential economic and welfare costs on larger farms.
Respondents from farms with a larger number of birds in lay and a higher number of poultry houses were more likely to answer ‘no’ to the question “Do you think you could reduce the use of antibiotics on your farm or replace the use of these medicines with alternatives?”. It is interesting that as the number of poultry houses increased, participants were more likely to report the use of ANTIBIOTICS ONLY but less likely to think that they could reduce or replace the use of antibiotics. It may be that respondents that think they could do more to reduce or replace antibiotics are already acting on this awareness, or that larger farms face more disease risk and therefore feel less able to reduce or replace antibiotics.
Lower flock age at depopulation predicted use of ANTIBIOTICS ONLY as the top three most frequently used medicines. This may reflect health problems within a flock resulting in early depopulation. Alternatively, due to the higher prevalence of worms in older hens (Zloch et al., 2018), farmers depopulating at a younger age may be less likely to require antiparasitics due to a decreased worm burden, so more likely to use ANTIBIOTICS ONLY. Alternatively, older flock age may indicate better management in general, with farmers recognising the benefits of a longer flock cycle while managing bird welfare and production (egg quality and number) (Bain, Nys, & Dunn, 2016) with less requirement for using antibiotics. Longer flock cycles reduce resource use through reduced overall number of cleaning (unproductive) periods for the shed; reduce total litter usage; reduce overall number of pullets (and associated hatching and rearing costs) (Bain et al., 2016). If the welfare and production challenges in older flocks can be managed through husbandry, genetic selection and nutrition, longer flock cycles may help to improve the ethical, environmental and economic sustainability of laying hen systems.
4.5. Veterinary contact and antimicrobial use
Farms where the vet visited less than once a year were more likely to use ANTIBIOTICS ONLY, suggesting that farms with less frequent veterinary contact may exhibit less responsible medicine use. Farmers who have regular twice a year or annual visits from their vet may be more informed regarding the judicious use of antimicrobials and may have active, beneficial flock health plans in place including measures to prevent disease and reduce the use of antibiotics. However, interestingly, this pattern was reversed for farms where the vet visited more regularly (3–4 times a year); these farms were more likely to use ANTIBIOTICS ONLY (Fig. 1). On farms where the vet visited 3–4 times per year, there may be ongoing health or management issues requiring the use of antibiotics. Alternatively, this pattern could be linked to vets’ prescribing habits i.e. vets that have more regular contact with their clients may be more risk averse, and liable to over-prescribe antibiotics. However, these are tentative claims and further research is required to investigate the relationship between veterinary contact and antimicrobial use.
4.6. Responsible use of antimicrobials and awareness of AMR
Encouragingly, the majority (94.8%) of participants provided a response indicative of responsible practices when selecting a treatment for their flock. The most commonly selected factor was whether participants’ veterinarians had prescribed the product for the condition, which highlights the reliance on and trust in veterinarians by laying hen farmers. This aligns with a recent survey of medicine use in UK dairy cow farmers (Higham et al., 2018). Similarly, interviews with EU pig and poultry farmers found that the decision to use antibiotics is generally undertaken by veterinarians (EFSA, 2017).
The majority of participants (76.9%) were able to give an accurate or partially accurate description of AMR, which mirrors the high levels of awareness of AMR found from interviews with EU pig and poultry farmers and a survey of Italian rabbit and turkey producers (Di Martino et al., 2018; EFSA, 2017). This finding is encouraging, as farmer awareness and knowledge of AMR has been shown to lead to more judicious use of antibiotics in farm animals (Kramer et al., 2017). However, the online nature of the questionnaire should be considered, as it is possible that some farmers’ definitions of AMR were extracted from an online source rather than their personal knowledge.
4.7. Measures taken to reduce or replace antimicrobial use
Most participants (51.3%) reported that they were not taking measures to reduce antimicrobial use and participants from company-owned farms were more likely to report that they were not taking such measures. If medicine use policies are implemented at company level, rather than by the individual running the farm, participants running company-owned farms may be unaware that the practices they are undertaking are intended to reduce, replace or refine antibiotic use. Both companies have medicine use policies in place and are reviewing antibiotic usage data in recognition of the challenges of AMR. The three company-owned farms that were taking measures to reduce or replace antimicrobials stated ‘probiotics’, ‘bird management’ and ‘not using any’ as the actions they were taking. These results highlight a need for further education regarding AMR and the role of farmers in safeguarding human health by making reductions in antimicrobial use. Equipping farm staff and managers with further knowledge about company policies, and empowering staff to contribute their own ideas to company solutions should foster engagement, and drive improvements in practices at farm level. This could take the form of a company-wide collaborative ‘Farmer Action Group’ where farmers work together towards a shared objective and can gain support and seek advice through peer-to-peer engagement (Bolt et al., 2017).
Participants purchasing veterinary medicines from an agricultural merchant/store (compared to those obtaining medicines from a veterinary source) were significantly less likely to report that they were taking measures to reduce or replace antimicrobial use. This suggests that participants purchasing medicines from a non-veterinary source may not be provided with information required to make an informed choice. In agreement with our findings in the current study, Jones et al. ’s (2015) study of dairy farmers found that veterinary surgeons were the most influential source of information on antibiotic use, therefore contact with veterinarians through medicine sales may provide an important communication channel and opportunity for driving responsible medicine use by farmers.
Participants with a younger flock age at depopulation were significantly less likely to be taking measures to reduce/replace the use of antimicrobials. This aligns with the finding that flock age at depopulation was lower on farms where participants were more likely to report use of ANTIBIOTICS ONLY as the three most frequently used products. As previously discussed, those managing older flocks may be more proactive in flock health management and more likely to implement practices, such as improving genetics, nutrition or housing design (Bain et al., 2016) and this could be linked to being less likely to use ANTIBIOTICS ONLY. However, more research is required to investigate the link between flock cycle length and attitudes towards antimicrobial use.
Common actions being taken to reduce antimicrobial use primarily focused on changes in management practices (e.g. increased biosecurity, reducing stress; Table 8). The responses in Table 8 align with scientific evidence (McEwen & Fedorka-Cray, 2002) and demonstrate farmers’ knowledge regarding the kind of practices that could help reduce antimicrobial use. Factors such as poor biosecurity have been linked to higher antimicrobial usage in pigs (Laanen et al., 2013), whilst stress is linked to reduced immunocompetence and higher disease prevalence in chickens (Alpigiani et al. 2017), which may increase antibiotic use. A holistic approach to improving health and husbandry practices to prevent disease, as described by the participants, is required to reduce the need for antimicrobial use. Vaarst et al. (2007) showed that participatory farmer discussion groups are a valuable medium for disseminating knowledge. Farmers with knowledge of such measures and their practical implications could share this with peers in discussion groups, widening knowledge of and ability to implement measures to reduce or replace antimicrobial use.
4.8. Benchmarking and recording of medicine use
The Responsible Use of Medicines in Agriculture Alliance (RUMA) identify benchmarking and recording of antibiotic use at farm level as essential to achieve responsible medicine use (RUMA, 2017). In England, under the Welfare of Farmed Animals (England) Regulations (2007), producers are legally required to keep a record of all medicines administered to food producing animals and egg packers are required to report medicine use to Lion Code. As expected, all participants were recording medicine use with 98.3% using a paper medicine book or record. Electronically recording medicine use would ease transfer of data across relevant parties such as researchers, other producers and government bodies. However, the majority of participants (74.4%) stated they would not be interested in using a mobile device to record medicine use. Demonstrable benefits such as saving time, increased flock health and/or enabling producers to compare their usage to anonymised data from similar producers could encourage egg producers to adopt electronic record keeping.
4.9. Farmer training preferences
Participants were asked what would help them and their team manage bird health issues and promote effective medicine use on farm. These results mirror the responses of dairy farmers to the same question (Higham et al., 2018), suggesting that, like dairy farmers, laying hens farmers prefer in-person contact with a trainer or their peers for knowledge exchange. These findings are supported by applied research outcomes in the dairy context, where collaborative development of antimicrobial stewardship policy between farmers and wider stakeholders in a workshop environment led to credible and practical recommendations designed to deliver real and lasting change in antimicrobial use (van Dijk et al., 2016).
4.10. Study limitations
This survey had 117 respondents with a total of 2,181,579 birds in lay, therefore represented approximately 7.6% of the UK free-range/free-range organic laying hen population (DEFRA et al., 2019, Food and Agriculture Organization of the United Nations Database (FAOSTAT) 2017). The response rate for this questionnaire (30.6%) was lower than previous questionnaire-based research within this supply chain (Barrett, Rayner, Gill, Willings, & Bright, 2014). The sample of responding farmers may therefore have been skewed in favour of those with a particular interest in responsible medicine use. The laying hen farms included in this survey were producing to free-range or organic standards therefore the results are not generalisable to other egg production systems. However, as free-range egg production accounts for around 53% of egg production in the UK (and this percentage market-share is increasing year-on-year) (DEFRA, 2019), the results of this study are important in the UK context.
Questionnaires are retrospective, meaning that participants were required to remember or consult records to answer certain questions. In this questionnaire, participants could likely consult medicine books or other records for most of the retrospective information required. In an attempt to avoid misinterpretation, we generally used closed questions with an option for ‘other (please specify)’ where possible to allow expansion where necessary. However, this meant that there were limited options for answers, and some participants may have provided alternative answers if given the chance. The online nature of this questionnaire should also be highlighted, particularly around the definition of AMR, as there is a possibility that participants could have looked this up online. However, most questions pertained to practices undertaken by farmers which are not extractable from an online source. Self-reported behaviours do not always correlate well with actual behaviour, and it possible that some respondents may have answered some questions with what they believed the authors expected them to, rather than their actual practices (Bowling, 2005). Finally, since antimicrobial use in agriculture is an area with close media attention at present, some participants may have been reluctant to disclose certain aspects of their practices in this area. It was clearly stated in the questionnaire that data would remain strictly confidential and analysed by a third party only, to reassure participants that their answers would not be used to identify or penalise them.
5. Conclusions
To our knowledge, the results from this survey represent the first time that the practices and knowledge around antimicrobial use and resistance in UK free-range laying hen farmers have been investigated. The majority of respondents proved to be knowledgeable, providing accurate or partially accurate descriptions of AMR. Most respondents also reportedly chose principles of responsible medicine use as the most important considerations when selecting a treatment.
Larger farms were more likely to use ANTIBIOTICS ONLY as their three most frequently used medicines. Participants from larger farms were also less likely to think they could take measures to reduce or replace the use of antibiotics with alternatives, potentially suggesting a higher disease risk on larger farms, or a link between antibiotic use, farm size and the ability of staff to detect early signs of disease, or vets’ prescribing habits. Respondents from farms with a younger flock age at depopulation were more likely to select ANTIBIOTICS ONLY as their three most frequently used medicines; they were also less likely to be taking measures to reduce or replace the use of antimicrobials. Young flock age at depopulation may reflect health and management problems; however, this link may also be a product of those with older flocks having more parasitic infections and therefore more likely to be using antiparasitics. More research is required to elucidate the link between flock age and antibiotic use; such research could enable targeted monitoring and training for producers that are more likely to be high users of antibiotics.
These results highlighted the important role of veterinarians in antimicrobial stewardship through farmer contact, engagement and education in responsible antimicrobial use. A high majority of participants ranked their vet as the most important source of advice regarding the selection and use of veterinary medicines. Participants whose vet visited once or twice per year were more likely to select both antiparasitics and antibiotics as their three most commonly used medicines, whilst those whose vet visited less than once a year or 3–4 times per year were more likely to select ANTIBIOTICS ONLY. This suggests that there may be some link between regular vet contact and reduced antibiotic usage. Those with more frequent (3–4 times per year) vet contact may have experienced ongoing health issues for which antibiotic use was necessary. Participants purchasing medicines from their vets were also more likely to report that they were taking measures to reduce/replace antibiotic use and participants that had less contact with their vet were less likely to believe they could reduce the use of antibiotics on their farm or replace them with alternatives. However, these links correlate, and further research is required to investigate the relationship between veterinary contact and antibiotic use.
Attendance of training courses or days was ranked by the majority of respondents as the best way to help them and their team manage bird health issues and promote the effective use of medicines, suggesting that laying hen farmers prefer in-person contact with a trainer or their peers for knowledge exchange. Collaborative workshops may represent a useful practical way to engage laying hen farmers on the issue of antimicrobial use and resistance.
Declaration of Competing Interest
McDonald's Restaurants Ltd UK provided financial assistance for this study. McDonald's UK were not involved in the design, data collection or data analysis of this project. J.-P. Michalski and R. Gill both work within the laying hen industry.
Acknowledgements
The authors would like to thank the respondents for their time in completing the questionnaire, Daniel Bray for his statistical expertise and Alice Willet at McDonald's UK for proof reading this manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.vas.2019.100072.
Appendix. Supplementary materials
References
- Alexander D.J. A review of avian influenza in different bird species. Veterinary Microbiology. 2000;74(1–2):3–13. doi: 10.1016/s0378-1135(00)00160-7. [DOI] [PubMed] [Google Scholar]
- Alpigiani I., Abrahantes J.C., Michel V., Huneau-Salaün A., Chemaly M., Keeling L.J., Berthe F. Associations between animal welfare indicators and Campylobacter spp. in broiler chickens under commercial settings: A case study. Preventive veterinary medicine. 2017;147:186–193. doi: 10.1016/j.prevetmed.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Asai T., Esaki H., Kojima A., Ishihara K., Tamura Y., Takahashi T. Antimicrobial resistance in Salmonella isolates from apparently healthy food-producing animal from 2000 to 2003: The first stage of Japanese Veterinary Antimicrobial Resistance Monitoring (JVARM) Journal of Veterinary Medical Science. 2006;68(8):881–884. doi: 10.1292/jvms.68.881. [DOI] [PubMed] [Google Scholar]
- Bain M., Nys Y., Dunn I. Increasing persistency in lay and stabilising egg quality in longer laying cycles. What are the challenges? British Poultry Science. 2016;57:330–338. doi: 10.1080/00071668.2016.1161727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard A.M., Main D.C., Haase A.M., Whay H.R., Roe E.J., Reyher K.K. The future of veterinary communication: Partnership or persuasion? A qualitative investigation of veterinary communication in the pursuit of client behaviour change. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0171380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J., Rayner A.C., Gill R., Willings T.H., Bright A. Smothering in UK free-range flocks. Part 1: Incidence, location, timing and management. Veterinary Record. 2014;175:19. doi: 10.1136/vr.102327. [DOI] [PubMed] [Google Scholar]
- Bertelloni F., Salvadori C., Moni A., Cerri D., Mani P., Ebani V.V. Antimicrobial resistance in Enterococcus spp. isolated from laying hens of backyard poultry flocks. Annals of Agricultural and Environmental Medicine. 2015;22(4):665–669. doi: 10.5604/12321966.1185771. [DOI] [PubMed] [Google Scholar]
- Beugnet F., Chauve C., Gauthey M., Beert L. Resistance of the red poultry mite to pyrethroids in France. Veterinary Record. 1997;140:577–579. doi: 10.1136/vr.140.22.577. [DOI] [PubMed] [Google Scholar]
- Bolt, S.L., Morgans, L., van Dijk, L., Reyher, K,K,. Buller, H., & Main, D.C.J. (2017). Effecting change on farm through Farmer Action Groups: reducing the use of antibiotic medicines on dairy farms. The 23rd European Seminar on Extension (and) Education (ESEE 2017) Transformative learning: new directions in agricultural extension and education, Chania, Greece. July 2017. https://www.researchgate.net/publication/318725825_Effecting_change_on_farm_through_Farmer_Action_Groups_reducing_the_use_of_antibiotic_medicines_on_dairy_farms (accessed 27 December 2018).
- Bowling A. Mode of questionnaire administration can have serious effects on data quality. Journal of public health. 2005;27(3):281–291. doi: 10.1093/pubmed/fdi031. [DOI] [PubMed] [Google Scholar]
- Bright A., Brass D., Clachan J., Drake K.A., Joret A.D. Canopy cover is correlated with reduced injurious feather pecking in commercial flocks of free-range laying hens. Animal Welfare. 2011;20(3):329. [Google Scholar]
- Dahl C., Permin A., Christensen J.P., Bisgaard M., Muhairwa A.P., Petersen K.M. The effect of concurrent infections with Pasteurella multocida and Ascaridia galli on free range chickens. Veterinary Microbiology. 2002;86(4):313–324. doi: 10.1016/s0378-1135(02)00015-9. [DOI] [PubMed] [Google Scholar]
- DEFRA United Kingdom egg statistics – Quarter 1, 2019. 2019 https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/775055/eggs-statsnotice-31jan19.pdf [Google Scholar]
- Di Martino G., Crovato S., Pinto A., Dorotea T., Mascarello G., Brunetta R. Farmers’ attitudes towards antimicrobial use and awareness of antimicrobial resistance: A comparative study among turkey and rabbit farmers. Italian Journal of Animal Science. 2018:1–8. [Google Scholar]
- EFSA . 2017. EU insights – Perceptions on the human health impact of antimicrobial resistance (AMR) and antibiotics use in animals across the EU.http://www.efsa.europa.eu/en/supporting/pub/en-1183 [Google Scholar]
- Eigaard N.M., Schou T.W., Permin A., Christensen J.P., Ekstrøm C.T., Ambrosini F. Infection and excretion of Salmonella enteritidis in two different chicken lines with concurrent Ascaridia galli infection. Avian Pathology. 2006;35(6):487–493. doi: 10.1080/03079450601071696. [DOI] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC) 2008. Factsheet for experts - Antimicrobial resistance.https://ecdc.europa.eu/en/antimicrobial-resistance/facts/factsheets/experts [Google Scholar]
- Fiddes M.D., Le Gresley S., Parsons D.G., Epe C., Coles G.C., Stafford K.A. Prevalence of the poultry red mite (Dermanyssus gallinae) in England. Veterinary Record. 2005;157(8):233. doi: 10.1136/vr.157.8.233. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization of the United Nations Database (FAOSTAT) (2017).FAO, Rome, Italy. http://www.fao.org/faostat/en/#data/QL (accessed 23 July 2019).
- Fossum O., Jansson D.S., Etterlin P.E., Vågsholm I. Causes of mortality in laying hens in different housing systems in 2001 to 2004. Acta Veterinaria Scandinavica. 2009;51(1):3. doi: 10.1186/1751-0147-51-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L.E., Lewis K., Kimpton A., Nicol C.J. Cross-sectional study of the prevalence of feather pecking in laying hens in alternative systems and its associations with management and disease. Veterinary Record. 2000;147(9):233–238. doi: 10.1136/vr.147.9.233. [DOI] [PubMed] [Google Scholar]
- Higham L.E., Deakin A., Tivey E., Porteus V., Ridgway S., Rayner A.C. A survey of dairy cow farmers in the United Kingdom: Knowledge, attitudes and practices surrounding antimicrobial use and resistance. Veterinary Record. 2018;183:746. doi: 10.1136/vr.104986. [DOI] [PubMed] [Google Scholar]
- Hockenhull J., Turner A., Reyher K., Barrett D., Jones L., Hinchliffe S. Antimicrobial use in food-producing animals: A rapid evidence assessment of stakeholder practices and beliefs. Veterinary Record. 2017;181:510. doi: 10.1136/vr.104304. [DOI] [PubMed] [Google Scholar]
- Jones P.J., Marier E.A., Tranter R.B., Wu G., Watson E., Teale C.J. Factors affecting dairy farmers' attitudes towards antimicrobial medicine usage in cattle in England and Wales. Preventive Veterinary Medicine. 2015;121(1–2):30–40. doi: 10.1016/j.prevetmed.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Kilpinen O., Roepstorff A., Permin A., Nørgaard-Nielsen G., Lawson L.G., Simonsen H.B. Influence of Dermanyssus gallinae and Ascaridia galli infections on behaviour and health of laying hens (Gallus gallus domesticus) British Poultry Science. 2005;46(1):26–34. doi: 10.1080/00071660400023839. [DOI] [PubMed] [Google Scholar]
- Kramer T., Jansen L.E., Lipman L.J., Smit L.A., Heederik D.J., Dorado-García A. Farmers’ knowledge and expectations of antimicrobial use and resistance are strongly related to usage in Dutch livestock sectors. Preventive Veterinary Medicine. 2017;147:142–148. doi: 10.1016/j.prevetmed.2017.08.023. [DOI] [PubMed] [Google Scholar]
- Laanen M., Persoons D., Ribbens S., de Jong E., Callens B., Strubbe M. Relationship between biosecurity and production/antimicrobial treatment characteristics in pig herds. The Veterinary Journal. 2013;198(2):508–512. doi: 10.1016/j.tvjl.2013.08.029. [DOI] [PubMed] [Google Scholar]
- Landers T.F., Cohen B., Wittum T.E., Larson E.L. A review of antibiotic use in food animals: Perspective, policy, and potential. Public Health Reports. 2012;127(1):4–22. doi: 10.1177/003335491212700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lion Code . 2013. Code of practice for lion eggs version 7.http://www.britisheggindustrycouncil.co.uk/download/LCoPV7.pdf [Google Scholar]
- Lion Code . 2017. Lion code of practice – Amendments/updates.https://www.nfuonline.com/assets/101582 [Google Scholar]
- Marangi M., Cafiero M.A., Capelli G., Camarda A., Sparagano O.A., Giangaspero A. Evaluation of the poultry red mite, Dermanyssus gallinae (Acari: dermanyssidae) susceptibility to some acaricides in field populations from Italy. Experimental and Applied Acarology. 2009;48:11–18. doi: 10.1007/s10493-008-9224-0. [DOI] [PubMed] [Google Scholar]
- McEwen S.A., Fedorka-Cray P.J. Antimicrobial use and resistance in animals. Clinical Infectious Diseases. 2002;34(3):S93–S106. doi: 10.1086/340246. [DOI] [PubMed] [Google Scholar]
- NOAH . 2016. Antibiotics for animal health and welfare: An overview.https://www.noah.co.uk/briefingdocument/antibiotics-for-animal-health-and-welfare-an-overview/ [Google Scholar]
- Nordenfors H., Höglund J., Tauson R., Chirico J. Effect of permethrin impregnated plastic strips on Dermanyssus gallinae in loose-housing systems for laying hens. Veterinary Parasitology. 2001;102(1–2):121–131. doi: 10.1016/s0304-4017(01)00528-3. [DOI] [PubMed] [Google Scholar]
- O'Neill J. 2014. Antimicrobial resistance: Tackling a crisis for the health and wealth of nations. review on antimicrobial resistance.https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf [Google Scholar]
- O'Neill J. 2015. Antimicrobials in agriculture and the environment: Reducing unnecessary use and waste, the review on antimicrobial resistance.https://amr-review.org/sites/default/files/Antimicrobials%20in%20agriculture%20and%20the%20environment%20-%20Reducing%20unnecessary%20use%20and%20waste.pdf [Google Scholar]
- Organic Farmers & Growers (OF&G) 2015. Organic standards & certification manual.http://ofgorganic.org/document_type/organic-standards-certification-manual/ Available from: [Google Scholar]
- Permin A., Bisgaard M., Frandsen F., Pearman M., Kold J., Nansen P. Prevalence of gastrointestinal helminths in different poultry production systems. British Poultry Science. 1999;40(4):439–443. doi: 10.1080/00071669987179. [DOI] [PubMed] [Google Scholar]
- Pötzsch C.J., Lewis K., Nicol C.J., Green L.E. A cross-sectional study of the prevalence of vent pecking in laying hens in alternative systems and its associations with feather pecking, management and disease. Applied Animal Behaviour Science. 2001;74(4):259–272. [Google Scholar]
- Poultry World . 2018. Markets: Egg sector feels the pain as prices sink.https://www.poultryworld.net/UK/Articles/2018/8/Markets-Egg-sector-feels-the-pain-as-prices-sink-323937E/ [Google Scholar]
- Ramadan H.H., Znada N.Y.A. Some pathological and biochemical studies on experimental ascaridiasis in chickens. Molecular Nutrition & Food Research. 1991;35(1):71–84. doi: 10.1002/food.19910350120. [DOI] [PubMed] [Google Scholar]
- Refregier-Petton J., Rose N., Denis M., Salvat G. Risk factors for Campylobacter spp. contamination in French broiler-chicken flocks at the end of the rearing period. Preventive Veterinary Medicine. 2001;50(1–2):89–100. doi: 10.1016/s0167-5877(01)00220-3. [DOI] [PubMed] [Google Scholar]
- Responsible Use of Medicines in Agriculture Alliance (RUMA) RUMA; UK: 2015. Responsible use of antimicrobials in cattle production.https://www.ruma.org.uk/wp-content/uploads/2015/07/RUMA_antimicrobial_long_cattle_revised_2015.pdf [Google Scholar]
- Responsible Use of Medicines in Agriculture Alliance (RUMA) RUMA; UK: 2017. Targets task force report 2017.http://www.ruma.org.uk/wp-content/uploads/2017/10/RUMA-Targets-Task-Force-Report-2017-FINAL.pdf [Google Scholar]
- RSPCA . 2017. RSPCA welfare standards for laying hens.https://science.rspca.org.uk/sciencegroup/farmanimals/standards/layinghens [Google Scholar]
- Ruston A., Shortall O., Green M., Brennan M., Wapenaar W., Kaler J. Challenges facing the farm animal veterinary profession in England: A qualitative study of veterinarians’ perceptions and responses. Preventive Veterinary Medicine. 2016;127:84–93. doi: 10.1016/j.prevetmed.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Savory C.J. Feather pecking and cannibalism. World's Poultry Science Journal. 1995;51(2):215–219. [Google Scholar]
- Schwaiger K., Schmied E.M., Bauer J. Comparative analysis of antibiotic resistance characteristics of Gram‐negative bacteria isolated from laying hens and eggs in conventional and organic keeping systems in Bavaria, germany. Zoonoses and Public Health. 2008;55(7):331–341. doi: 10.1111/j.1863-2378.2008.01151.x. [DOI] [PubMed] [Google Scholar]
- Schwaiger K., Schmied E.M., Bauer J. Comparative analysis on antibiotic resistance characteristics of Listeria spp. and Enterococcus spp. isolated from laying hens and eggs in conventional and organic keeping systems in Bavaria, germany. Zoonoses and Public Health. 2010;57(3):171–180. doi: 10.1111/j.1863-2378.2008.01229.x. [DOI] [PubMed] [Google Scholar]
- Snow L.C., Davies R.H., Christiansen K.H., Carrique-Mas J.J., Wales A.D., O'Connor J.L. Survey of the prevalence of Salmonella species on commercial laying farms in the United Kingdom. Veterinary Record. 2007;161:471–476. doi: 10.1136/vr.161.14.471. [DOI] [PubMed] [Google Scholar]
- Sparagano O., George D., Harrington D., Giangaspero A. Significance and control of the Poultry Red Mite, Dermanyssus gallinae. Annual Review of Entomology. 2014;59:447–466. doi: 10.1146/annurev-ento-011613-162101. [DOI] [PubMed] [Google Scholar]
- Stępień-Pyśniak D., Marek A., Banach T., Adaszek Ł., Pyzik E., Wilczyński J. Prevalence and antibiotic resistance of Enterococcus strains isolated from poultry. Acta Veterinaria Hungarica. 2016;64(2):148–163. doi: 10.1556/004.2016.016. [DOI] [PubMed] [Google Scholar]
- Tarbiat B., Jansson D.S., Tydén E., Höglund J. Evaluation of benzimidazole resistance status in Ascaridia galli. Parasitology. 2017;144(10):1338–1345. doi: 10.1017/S0031182017000531. [DOI] [PubMed] [Google Scholar]
- The Welfare of Farmed Animals (England) Regulations (2007). http://www.legislation.gov.uk/uksi/2007/2078/made(accessed 02 July 2019).
- Vaarst M., Nissen T.B., Østergaard S., Klaas I.C., Bennedsgaard T.W., Christensen J. Danish stable schools for experiential common learning in groups of organic dairy farmers. Journal of Dairy Science. 2007;90(5):2543–2554. doi: 10.3168/jds.2006-607. [DOI] [PubMed] [Google Scholar]
- van den Bogaard A.E., Willems R., London N., Top J., Stobberingh E.E. Antibiotic resistance of faecal enterococci in poultry, poultry farmers and poultry slaughterers. Journal of Antimicrobial Chemotherapy. 2002;49(3):497–505. doi: 10.1093/jac/49.3.497. [DOI] [PubMed] [Google Scholar]
- van Dijk L., Hayton A., Main D., Booth A., King A., Barrett D. Participatory policy making by dairy producers to reduce anti-microbial use on farms. Zoonoses and Public Health. 2016;64(6):476–484. doi: 10.1111/zph.12329. [DOI] [PubMed] [Google Scholar]
- Veterinary Medicines Directorate (VMD) 2017. UK – Veterinary antibiotic resistance and sales surveillance report 2016.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/707974/_1274590-v2-VARSS_2016_for_GOV.pdf [Google Scholar]
- Warren H. 2017. How to mitigate antimicrobial resistance in livestock. WATTAgNet.https://www.wattagnet.com/articles/31269-how-to-mitigate-antimicrobial-resistance-in-livestock?v=preview [Google Scholar]
- World Health Organisation (WHO) 2001. WHO global strategy for containment of antimicrobial resistance.https://www.who.int/drugresistance/WHO_Global_Strategy_English.pdf?ua=1 [Google Scholar]
- World Health Organisation (WHO) 2008. A guide to developing knowledge, attitudes and practice surveys.http://apps.who.int/iris/bitstream/10665/43790/1/9789241596176_eng.pdf [Google Scholar]
- World Health Organisation (WHO) 2017. Critically important antimicrobials for human medicine.https://www.who.int/foodsafety/publications/cia2017.pdf?ua=1 5th Revision, 2016. [Google Scholar]
- World Health Organisation WHO . 2017. WHO guidelines on use of medically important antimicrobials in food-producing animals.https://apps.who.int/iris/bitstream/handle/10665/258970/9789241550130-eng.pdf;jsessionid=90A9EB16C641928DCFE958F9F48ACC25?sequence=1 [PubMed] [Google Scholar]
- Zloch A., Kuchling S., Hess M., Hess C. Influence of alternative husbandry systems on postmortem findings and prevalence of important bacteria and parasites in layers monitored from end of rearing until slaughter. Veterinary Record. 2018;182:350. doi: 10.1136/vr.104632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.