Highlights
-
•
Infectious disease, including tuberculosis, was the most common cause of death.
-
•
Anthropogenic trauma caused 26% of deaths in mainland sea lions.
-
•
Trauma inflicted by adult male conspecifics caused 24% of deaths.
Keywords: Pinniped, Sea lion, Anthropogenic, Infectious disease, Mortality, Tuberculosis
Abstract
The New Zealand sea lion is an endangered species endemic to New Zealand. While causes of death are well described for pups of this species, mortality in adults is poorly characterised. This study investigated causes of death in 136 New Zealand sea lions in two different populations: a major breeding site on remote, uninhabited Enderby Island in the sub-Antarctic, and a slowly increasing recolonising population on the inhabited mainland. For animals with at least a partial diagnostic investigation (n = 112), the most frequently diagnosed causes of mortality were infectious disease (41/112; 37%), particularly tuberculosis due to M. pinnipedii (20/112; 18%), and conspecific trauma (27/112; 24%). Anthropogenic trauma was an important cause of death in mainland sea lions (9/33; 26%). Deliberate anthropogenic mortality has previously been identified as the greatest potential threat to population recovery for mainland sea lions, and as human and pinniped populations increase, managing interactions between these species will become increasingly important.
Introduction
The New Zealand sea lion (NZSL; Phocarctos hookeri) is endemic to New Zealand and is classified as endangered by the International Union for Conservation of Nature (Chilvers, 2015). The majority of breeding occurs in the sub-Antarctic islands, including Enderby Island, where approximately 20% of total pup production occurs (Chilvers, Wilkinson, & Childerhouse, 2007). Historically, NZSLs were also found throughout mainland New Zealand, but by the mid-19th century had been eliminated from this region due mostly to hunting pressure and habitat change (Childerhouse & Gales, 1998). In the past 20 years, however, recolonisation of the mainland has begun in the form of three small but growing populations: two in the southeast of the South Island and one on Stewart Island (McConkey et al., 2002, Roberts and Doonan, 2016).
Despite these new populations, the NZSL species as a whole has undergone significant decline in the past two decades, with the most recent population estimate being approximately 9400 individuals (Roberts & Doonan, 2016). The exact causes of the decline in numbers are not yet well understood but it is likely that multiple factors play a role (Baker et al., 2010, Chilvers, 2008, Robertson and Chilvers, 2011). Causes of mortality have been thoroughly investigated in NZSL pups on Enderby Island (Castinel, Duignan et al., 2007) but similar investigations are lacking for all other age groups and locations. For pups, the most common cause of mortality is septicaemia due to hypermucoviscous Klebsiella pneumoniae (Roe et al., 2015). The origin of infection and the role of adult sea lions in disease transmission are currently unknown (Castinel et al., 2007b, Roe et al., 2015).
The most well-recognised cause of death in adult NZSLs is incidental capture in commercial fisheries (Chilvers, 2008). Other causes of death are less well characterised. A mass mortality event on Enderby Island in 1998 caused the death of at least 74 adults, characterised by neck abscesses and attributed to a pleomorphic gram negative bacillus that could not be cultured (Baker, 1999). There are several reports of mortality investigations in individual adult sea lions from mainland New Zealand, including infections due to toxoplasmosis (Roe et al., 2017) and tuberculosis (Roe, PJ, L, GW, & DV, 2006).
The nature and the implications of NZSL mortality are likely to differ between mainland New Zealand and the remote breeding sites in the sub-Antarctic. While mainland recolonisation can be seen as positive for the species, it also means that sea lions are brought into closer contact with humans, domestic animals and other wildlife species, and consequently there is increased likelihood of disease transmission. In addition, this proximity increases the chance of contact between humans and sea lions. Given these factors, this study aimed to investigate the causes of mortality in non-pup NZSLs; to compare findings between Enderby Island and the New Zealand mainland; and to assess the presence of pathogens or disease syndromes previously associated with epizootics in NZSLs.
Materials and methods
Enderby Island (50.5°S, 166.3°E) is visited annually by scientists during the NZSL breeding season (December to February), and necropsies are conducted on any NZSLs found dead. Similar investigations are conducted year-round in mainland New Zealand, where colonies are regularly monitored by members of the NZSL Trust as well as staff from the NZ Department of Conservation. All non-pup individuals (i.e. yearlings and older) found dead between 1 January 2000 and 31 December 2017 were included in this study, and are hereafter referred to as “adult” NZSLs, to distinguish them from previous studies focusing on pup mortality. Records were retrieved from an electronic database held at the School of Veterinary Science, Massey University, Palmerston North, New Zealand. For each case the extent of investigation was classified as ‘none’ (dead animal recorded but necropsy not attempted), ‘gross only’ (gross necropsy conducted but no samples collected), ‘partial’ (gross necropsy with limited histology) or ‘full’ (gross necropsy with representative tissues collected for histology). For some animals, archived frozen tissues or swabs were available. These had either been frozen at −20C within 12 h of collection (mainland cases) or frozen in liquid nitrogen before transfer to Massey University and storage at −80C (Enderby Island cases). Formalin-fixed tissues, where available, were processed routinely for histopathology and stained with H&E. Additional stains, including Gram, Ziehl-Neelson, Warthin-Starry silver, Giemsa, Perl's iron, Young's fungal, Congo red, Masson's trichrome, PAS, Von Kossa and Verhoeff's stain were used to further characterise lesions and demonstrate pathogens. Aerobic culture was performed on archived tissues where gross or histological findings suggested an infectious aetiology. These tissues were thawed at room temperature prior to analysis. For cases suspected to have mycobacterial infection on histological examination, the diagnosis was confirmed using culture and PCR as described in Loeffler et al. (2014).
A cause of death (COD) was determined for each animal based on gross and histological lesions. Cases were grouped into the following categories: bacterial infection; conspecific trauma; predation; anthropogenic trauma; miscellaneous; and unknown (no primary COD diagnosed). Clinically significant concurrent diseases were noted for each animal. Fisher's exact test was used to assess differences between study locations and sexes.
Results
A total of 136 adult NZSLs were found dead on Enderby Island (n = 82) and mainland New Zealand (n = 54) between 1 January 2000 and 31 December 2017. Of these, 24 (18%) had no investigation due to decomposition (n = 4), scavenging by birds (n = 1); field restraints such as rising tide or inaccessibility (n = 2); or an unrecorded reason (n = 17). All cases with no recorded reason were from the mainland. Animals with no investigation were excluded from further analysis, but are reported here to indicate the proportion of non-investigated deaths, which was higher on the mainland (21/55 (38%)) than on Enderby Island (3/82 (4%)). The length of the field season on Enderby Island ranged from 33 to 92 days (average 66 days).
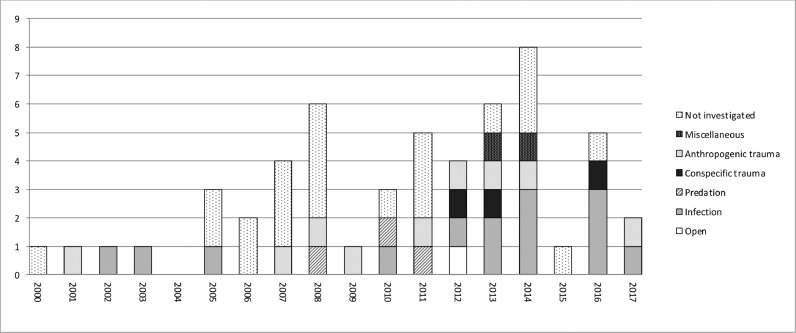
Fig. 1.
Numbers of identified deaths and cause of death, where investigated, for New Zealand sea lions at Enderby Island between 2000 and 2017.
A total of 112 animals (79 from Enderby Island and 33 from mainland New Zealand) had some degree of investigation: either gross post mortem only (n = 30), partial (n = 6) or full investigation (n = 76). A definitive diagnosis of COD was made in 89/112 (79%) of these investigated deaths, including 18/30 (60%) cases that had a gross post-mortem only; 0/6 (0%) cases with partial investigations; and 71/76 (93%) of cases with full investigations. Table 1 shows summarised COD data for each location. The M:F ratio for investigated deaths was 0.4:1 (23M:56F) for Enderby Island and 2.3: 1 (23M:10F) for mainland New Zealand. These ratios are not significantly different from the M:F ratios of live sea lions at either Enderby Island (expected ratio = 1M:2F (Marlow, 1975); P = 0.51) or mainland New Zealand (expected ratio 3.3M:1F (J. Fyfe, pers comm.); P = 0.77). The distribution of CODs for each year are shown for Enderby Island in Fig. 2, and for mainland New Zealand in Fig. 3.
Table 1.
Diagnosed cause of death, with male to female (M:F) ratios, for adult New Zealand sea lions at the two study locations. A superscript denotes categories that are significantly different between locations.
| Cause of death | Enderby Island | Mainland | ||
|---|---|---|---|---|
| No. cases | M:F | No. cases | M:F | |
| Opena | 22 (28%) | 8:15 | 1 (3%) | 0:1 |
| Infection | 27 (34%) | 6:21 | 14 (42%) | 12:2 |
| Conspecific traumab | 24 (30%) | 6:18 | 3 (9%) | 1:2 |
| Anthropogenic traumac | 0 (0%) | 0:0 | 9 (27%) | 6:3 |
| Predation | 4 (5%) | 2:2 | 3 (9%) | 2:1 |
| Miscellaneous | 2 (2%) | 1:0 | 3 (9%) | 2:1 |
| Totals: | 79 | 23:5 | 33 | 23:1 |
P = 0.002;.
P = 0.027;.
P < 0.001.
Fig. 2.
Numbers of identified deaths and cause of death, where investigated, for New Zealand sea lions on mainland New Zealand between 2000 and 2017.
Fig. 3.
Representative examples of gross lesions for different causes of mortality. A. Anthropogenic trauma. This juvenile female from mainland New Zealand was killed by gunshot. Inset shows retrieved bullet fragments. B. Conspecific trauma. A mature adult male attempting to mate a dead female on Enderby Island. C. Infection: cellulitis and abscessation in the neck of a juvenile male sea lion. A beta-haemolytic Streptococcus spp was cultured from the necrotic soft tissue, and M. pinnipedii was cultured from the lymph node (white arrows). D. Predation. Shark bite wounds in the dorsal pelvic region of an Enderby Island adult female.
Infection was the diagnosed COD in 41/112 cases (37%), based on gross necropsy findings, histological lesions, and microbiological testing. In 33 cases death was due to a primary bacterial infection, while in six, all from Enderby Island, death was due to infection of bite wounds from conspecifics (n = 5) or a shark (n = 1). In one further case death was due to intestinal rupture and bacterial peritonitis with concurrent aspiration pneumonia following conspecific trauma (crushing by an adult male). One sea lion had bacterial cellulitis and myositis of the pelvic area with no penetrating wound. The causal agent was identified in 27/33 (82%) cases of fatal primary bacterial infection, with the most frequent diagnosis being tuberculosis caused by Mycobacterium pinnipedii (20/33 (61%)). There was no significant difference in prevalence of this infection between locations (P = 0.18). Non-hypermucoviscous Klebsiella pneumoniae was the COD in two cases, both from the mainland (one case of abscessation and septicaemia, and one of pneumonia and pericarditis). Non-hypermucoviscous K. pneumoniae was also cultured from tissues of two NZSLs that died from conspecific trauma; one had concurrent bacterial septicaemia, with K. pneumoniae isolated from the liver, while in the second K. pneumoniae was cultured from an enlarged tracheobronchial lymph node. β-haemolytic Streptococcus spp. were isolated in pure culture from three animals (one conspecific bite wound infection and two multifocal abscessation with pneumonia), but only one was classified to species level (S. constellatus). E. coli was isolated in pure culture from one animal with suppurative pyelonephritis and cystitis. In six cases mixed bacteria were cultured, with no dominant organism, while no tissues were available for nine animals.
Given their role in a previous bacterial epizootic in NZSLs, eight cases of neck abscesses were further investigated: seven from Enderby Island and one from the mainland. Two of the Enderby cases were from 2009, while the remaining six cases were spread over six different years. In the mainland case, both M. pinnipedii and a β-haemolytic Streptococcus sp. were isolated from the abscess. Frozen swabs were available from two Enderby sea lions with neck abscesses: one of these produced a heavy growth of an α-haemolytic Streptococcus sp. while the other one grew a pure culture of Staphylococcus sp. A lymph node adjacent to the abscess in one further case from Enderby Island grew M. pinnipedii. No tissues were collected from the remaining three neck abscesses.
The second most frequent COD overall was conspecific trauma (27/112 cases (24%)). For Enderby Island, the M:F ratio of conspecific deaths was not significantly different from that of all investigated deaths at that location (P = 0.80) or for the expected sex ratio of adults present during the breeding season (expected M:F ratio of 1:2) (Marlow, 1975) (P = 0.62). The number of mainland animals with this diagnosis (n = 3) was too small to allow valid statistical comparisons. Lesions present in these animals included bites and bruising; aspiration of sand into deep airways (n = 5); haemoabdomen (n = 2); fractures of the skull (n = 3), ribs (n = 1) or vertebrae (n = 1); intestinal herniation (n = 5); and brain contusions (n = 1). Of these 27 animals, 10 were juveniles (six males and four females), one was a subadult male, and the remaining 16 were adult females. Five Enderby Island conspecific deaths were opportunistically observed, and all involved mating attempts by adult males, including three attacks in shallow water which resulted in drowning/asphyxiation. Five cases had concurrent conditions considered likely to have contributed to death: one with toxoplasmosis (previously described in detail in Roe et al. (2017)), three with severely infected bite wounds, and one with bacterial septicaemia.
Less frequent diagnoses included anthropogenic trauma (n = 9), predation (n = 7), and one case each of starvation, metastatic neoplasia, dystocia, aortic rupture and blunt trauma of unknown origin. Cases of anthropogenic trauma occurred only on the mainland. Seven of these had been shot, one was hit by a train, and one was hit by a car. Seven animals (6%) (six adults and one juvenile) died of predation. One of these deaths was observed to be caused by a great white shark (Carcharodon carcharias). Extensive abdominal wounds with serrated edges characteristic of shark predation were present in all seven animals. Two cases had concurrent tuberculosis, which may have contributed to their mortality. The single case of starvation was a juvenile with a severe shark bite wound as a contributing factor.
No definitive COD could be established in 23 investigated cases. Open diagnoses were significantly more frequent for Enderby Island cases (22/79 (28%) vs 1/33 (3%); P = 0.002). In four of these cases a full set of tissues had been collected, but no significant gross or histological lesions were present. Six cases, all from Enderby Island between 2000 and 2003, had only partial investigations where a very limited selection of tissues had been collected. No significant lesions were detected in these tissues. In the remaining 13 cases (12 from Enderby Island and one from the mainland), only gross examinations were undertaken, with the following reasons given: extensive scavenging (n = 4), decomposition (n = 2), animal too large and in a dangerous location (n = 1) or no recorded reason (n = 6).
Discussion
The NZSL breeding population at Enderby Island has been in decline since the late 1990′s, with a number of possible factors identified, including incidental capture in fishing nets, prey shortage, and disease (Roberts & Doonan, 2016). While small breeding groups are now established on mainland New Zealand, little is known about causes of mortality in these mainland animals. Bacterial infections were the most frequently diagnosed COD in the current study, causing 37% of investigated deaths. In 1998 a bacterial epizootic in the Auckland Islands killed at least 74 adult NZSLs, and was characterised clinically by the presence of subcutaneous abscesses, predominantly affecting the ventral neck (Gales & Childerhouse, 1999). Histologically, these lesions showed acute suppurative dermatitis and vasculitis with fibrinosuppurative lymphadenitis and large colonies of intralesional pleomorphic gram negative bacilli (Duignan, 1999). Grossly similar neck abscesses were present in eight sea lions in the current study, but these cases appear to be unrelated to the 1998 epizootic, as they occurred sporadically, did not contain intralesional pleomorphic gram negative bacteria, and a variety of causal bacteria were isolated from the five cases where tissues were collected.
One aim of this study was to assess the presence of agents previously identified as causing epizootics in NZSLs, including a clonal hypermucoviscous strain of K. pneumoniae, which is the most common cause of mortality in NZSL pups (Roe et al., 2015). K. pneumoniae was cultured from four sea lions in the current study: one isolate from a neck abscess, one from the lung of an animal that died of pneumonia and pericarditis, and two from non-lesional tissues as an incidental finding. None of these isolates were hypermucoviscous, however, hence the syndrome seen in pups does not appear to be a cause of mortality in adult NZSLs. The potential role of adults as reservoirs of hypermucoviscous K. pneumoniae remains to be fully characterised.
The most frequently diagnosed bacterial infection in this study was tuberculosis, which caused the death of 20 animals (18% of total investigated mortalities). Tuberculosis has been described in several wild pinnipeds (Boardman et al., 2014, Cousins et al., 1993, de Amorim et al., 2014, Hunter et al., 1998, Roe et al., 2006, Woods et al., 1995) and the causal organism has now been classified as a distinct species, M. pinnipedii (Cousins et al., 2003). Since these previous reports describe only sporadic cases, although beyond the scope of the current study, the apparently high prevalence of tuberculosis in NZSLs warrants further investigation.
A limitation of this study was the reliance on archived frozen tissues that were collected and processed without using specific techniques designed to increase survival of micro-organisms. Previous studies have demonstrated that bacterial viability decreases with prolonged storage, particularly when tissues are frozen at −20°C (De Paoli, 2005), as was the case for the mainland samples in the current study. This means that pathogens may have been missed here, and future studies should consider modified techniques, including cryopreservative agents, where costs and field constraints allow. Identification of pathogens using molecular techniques could also be considered where appropriate.
Conspecific trauma is a frequent diagnosis in otariids, and can be either a primary cause of death, or an indirect cause following wound infection. Spraker and Lander (2010) found that infected bite wounds were the most common COD for both male and female Northern fur seals (Callorhinus ursinus) at St Paul Island. Similarly, Baker and McCann (1989) found that infected wounds were the main COD in 102 adult male Antarctic fur seals (Arctocephalus gazella) at South Georgia Island. Altukhov et al. (2012), observed Steller sea lion (Eumetopias jubatus) colonies over three years, and attributed almost all observed adult deaths directly to traumatic interactions with mature males, including both mating injuries and fight wounds. In the current study, conspecific trauma was a direct cause of 27 deaths, and a contributing (indirect) cause in six. In contrast to the above studies, none of the affected animals in our study were adult males, which may reflect the more ritualised inter-male territorial behaviour of NZSLs in comparison to the aggressive interactions seen in other otariids (Altukhov et al., 2012, Marlow, 1975). Lesions in NZSLs were consistent with crushing (e.g. ruptured or herniated viscera, fractured ribs and skulls, and aspiration of sand) or biting (puncture wounds with associated subcutaneous bruising), and correlate with the breeding colony behaviour of adult male otariids. In Steller sea lions, adult females and juveniles of both sexes have been observed being crushed during mating attempts by adult males (Altukhov et al., 2012). Marlow (1975) observed adult male NZSLs restraining females by resting the entire weight of their forelimbs and chest across the females’ backs, frequently close to the water-line as females returned from foraging trips. This is consistent with the observed drowning events in the current study. In the 2008/09 season at Enderby Island there were more deaths than in any other year, despite the length of the field season being close to average at 69 days, and conspecific trauma was disproportionately represented. This was accompanied by a marked decrease in numbers of adult females present during that breeding season (unpublished data), the reason for which remains unexplained. It is possible that the increased number of deaths that year was due to an increase in the relative proportion of males present. The significantly lower prevalence of conspecific trauma deaths on mainland New Zealand, in comparison with Enderby Island, likely reflects the non-colonial breeding behaviour that occurs on the mainland (McConkey et al., 2002). Intervention by field scientists when such interactions are observed could be considered as a mitigation action to decrease conspecific deaths.
Anthropogenic trauma was a COD only in mainland NZSLs. While fishing-related deaths are widely reported in pinnipeds (Reeves, McClellan, & Werner, 2013), there are few descriptions of other types of anthropogenic deaths. Gunshot wounds are relatively infrequently reported in the literature, although Goldstein et al. (1999) described these in 5% of live-stranded pinnipeds from central California (predominantly California sea lions (Zalophus californianus)), and Gerber, Roletto, Morgan, Smith, and Gage (1993) reported gunshot wounds in 4.2% of live and dead-stranded pinnipeds from the same region. In the study presented here, gunshot wounds caused the deaths of79/33 mainland animals (21%), suggesting that this is an important local cause of mortality. In New Zealand, marine mammals are legally protected under the Marine Mammal Protection Act, hence killing marine mammals is a criminal offence. Amongst known threats to survival, deliberate anthropogenic mortality is suggested to have the greatest potential effect on population size for NZSLs at Otago (Roberts, 2016), further highlighting the importance of these findings. As human and pinniped populations increase, interactions between the species are also likely to increase, and mitigation of human impact on mainland NZSLs becomes increasingly important.
Conclusions
Bacterial infections, particularly M. pinnipedii, were the most common COD in adult NZSLs between 2000 and 2017. There were significant differences in prevalence of trauma-related mortality between locations, with conspecific trauma being more common on Enderby Island and anthropogenic trauma more common on the mainland. Neither the hypermucoviscous phenotype of K. pneumoniae that causes the majority of deaths in NZSL pups, nor the pleomorphic Gram negative bacterial syndrome responsible for a previous mortality event in this species were identified in the current study. The presence of zoonotic mycobacterial infection and high prevalence of anthropogenic deaths in NZSLs highlight the importance of managing interactions between humans and sea lions as populations of this endangered species increase on the mainland.
Conflict of interest statement
None of the authors has any financial or personal relationships that could inappropriately influence or bias the content of the paper.
Acknowledgments
The authors would like to gratefully acknowledge the assistance of Jim Fyfe, Ros Coles, Laura Boren and other New Zealand Department of Conservation staff and scientists who assisted with mainland sea lion post mortems and logistics; New Zealand Sea Lion Trust members, particularly Sean McConkey; Louise Chilvers, plus the many veterinarians and scientists who performed post mortems on Enderby Island; and Komkiew Pinpimai, Lynn Rogers, Mike Hogan, Craig Thomas, Evelyn Lupton and Saritha Gils for technical assistance. This study was part-funded by the Department of Conservation.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.vas.2019.100057.
Appendix. Supplementary materials
References
- Altukhov A.V., Permyakov P.A., Andrews R.D., Burkanov V.N., Calkins D.G., Trukhin A.M. Adult Steller sea lion mortality on rookeries in the Russian Far East, 2002-2010. Russian Journal of Marine Biology. 2012;38:442–447. [Google Scholar]
- Baker A. Department of Conservation; Wellington: 1999. An unusual mortality event of the New Zealand sea lion (Phocarctos hookeri), Auckland Islands, January-February 1998.https://www.doc.govt.nz/documents/science-and-technical/sealion/ [Google Scholar]
- Baker C.S., Chilvers B.L., Constantine R., DuFresne S., Mattlin R.H., van Helden A. Conservation status of New Zealand marine mammals (suborders Cetacea and Pinnipedia), 2009. New Zealand Journal of Marine and Freshwater Research. 2010;44:101–115. [Google Scholar]
- Baker J.R., McCann T.S. Pathology and bacteriology of adult male Antarctic fur seals, Arctocephalus gazella, dying at Bird Island, South Georgia. British Veterinary Journal. 1989;145:263–275. doi: 10.1016/0007-1935(89)90079-1. [DOI] [PubMed] [Google Scholar]
- Boardman W.S.J., Shephard L., Bastian I., Globan M., Fyfe J.A.M., Cousins D.V. Mycobacterium pinnipedii tuberculosis in a free-ranging Australian fur seal (Arctocephalus pusillus doriferus) in South Australia. Journal of Zoo and Wildlife Medicine. 2014;45:970–972. doi: 10.1638/2014-0054.1. [DOI] [PubMed] [Google Scholar]
- Castinel A., Duignan P.J., Pomroy W.E., Lopez-Villalobos N., Gibbs N.J., Chilvers B.L. Neonatal mortality in New Zealand sea lions (Phocarctos hookeri) at Sandy Bay, Enderby Island, Auckland Islands from 1998 to 2005. Journal of Wildlife Diseases. 2007;43:461–474. doi: 10.7589/0090-3558-43.3.461. [DOI] [PubMed] [Google Scholar]
- Castinel A., Grinberg A., Pattison R., Duignan P., Pomroy B., Rogers L. Characterization of Klebsiella pneumoniae isolates from New Zealand sea lion (Phocarctos hookeri) pups during and after the epidemics on Enderby Island, Auckland Islands. Veterinary Microbiology. 2007;122:178–184. doi: 10.1016/j.vetmic.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Childerhouse S., Gales N. Historical and modern distribution and abundance of the New Zealand sea lion Phocarctos hookeri. New Zealand Journal of Zoology. 1998;25:1–16. [Google Scholar]
- Chilvers B.L. New Zealand sea lions Phocarctos hookeri and squid trawl fisheries: Bycatch problems and management options. Endangered Species Research. 2008;5:193–204. [Google Scholar]
- Chilvers, B. L. (2015). Phocarctos hookeri. The IUCN Red List of Threatened Species, http://www.iucnredlist.org/details/17026/0 Downloaded 31 March 2018.
- Chilvers B.L., Wilkinson I.S., Childerhouse S. New Zealand sea lion, Phocarctos hookeri, pup production 1995 to 2006. New Zealand Journal of Marine and Freshwater Research. 2007;41:205–213. [Google Scholar]
- Cousins D.V., Bastida R., Cataldi A., Quse V., Redrobe S., Dow S. Tuberculosis in seals caused by a novel member of the Mycobacterium tuberculosis complex: Mycobacterium pinnipedii sp. nov. International Journal of Systematic and Evolutionary Microbiology. 2003;53:1305–1314. doi: 10.1099/ijs.0.02401-0. [DOI] [PubMed] [Google Scholar]
- Cousins D.V., Williams S.N., Reuter R., Forshaw D., Chadwick B., Coughran D. Tuberculosis in wild seals and characterization of the seal bacillus. Australian Veterinary Journal. 1993;70:92–97. doi: 10.1111/j.1751-0813.1993.tb03284.x. [DOI] [PubMed] [Google Scholar]
- de Amorim D.B., Casagrande R.A., Alievi M.M., Wouters F., De Oliveira L.G.S., Driemeier D. Mycobacterium pinnipedii in a stranded South American sea lion (Otaria byronia) in Brazil. Journal of Wildlife Diseases. 2014;50:419–422. doi: 10.7589/2013-05-124. [DOI] [PubMed] [Google Scholar]
- De Paoli P. Biobanking in microbiology: From sample collection to epidemiology, diagnosis and research. FEMS Microbiology Reviews. 2005;29:897–910. doi: 10.1016/j.femsre.2005.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duignan P.J. Gross pathology, histopathology, virology, serology and parasitology. Unusual mortality of the New Zealand sea lion, Phocarctos hookeri, Auckland Islands, January–February 1998. Department of Conservation, Wellington; New Zealand; 1999. pp. 29–33. [Google Scholar]
- Gales N., Childerhouse S. Field observations and sampling regime. Unusual mortality of the New Zealand sea lion, Phocarctos hookeri, Auckland Islands, January–February 1998. Department of Conservation, Wellington; New Zealand; 1999. pp. 8–14. [Google Scholar]
- Gerber J.A., Roletto J., Morgan L.E., Smith D.M., Gage L.J. Findings in pinnipeds stranded along the central and northern California coast, 1984-1990. Journal of Wildlife Diseases. 1993;29:423–433. doi: 10.7589/0090-3558-29.3.423. [DOI] [PubMed] [Google Scholar]
- Goldstein T., Johnson S.P., Phillips A.V., Hanni K.D., Fauquier D.A., Gulland F.M.D. Human-related injuries observed in live stranded pinnipeds along the central California coast 1986-1998. Aquatic Mammals. 1999;25:43–51. [Google Scholar]
- Hunter J.E.B., Duignan P.J., Dupont C., Fray L., Fenwick S.G., Murray A. First report of potentially zoonotic tuberculosis in fur seals in New Zealand. The New Zealand Medical Journal. 1998;111:130–131. [PubMed] [Google Scholar]
- Loeffler S.H., de Lisle G.W., Neill M.A., Collins D.M., Price-Carter M., Paterson B. The seal tuberculosis agent, Mycobacterium pinnipedii, infects domestic cattle in New Zealand: Epidemiologic factors and DNA strain typing. Journal of Wildlife Diseases. 2014;50:180–187. doi: 10.7589/2013-09-237. [DOI] [PubMed] [Google Scholar]
- Marlow B.J. The comparative behaviour of the Australasian sea lions Neophoca cinerea and Phocarctos hookeri (Pinnipedia: Otariidae) Mammalia. 1975;39:159–230. [Google Scholar]
- McConkey S.D., McConnell H., Lalas C., Heinrich S., Ludmerer A., McNally N. A northward spread in the breeding distribution of the New Zealand sea lion Phocarctos hookeri. Australian Mammalogy. 2002;24:97–106. [Google Scholar]
- Reeves R.R., McClellan K., Werner T.B. Marine mammal bycatch in gillnet and other entangling net fisheries, 1990 to 2011. Endangered Species Research. 2013;20:71–U140. [Google Scholar]
- Roberts, J., & Doonan, I. (2016). Quantitative risk assessment of threats to New Zealand sea lions. Aquatic Environment Biodiversity Report No. 166. Ministry for Primary Industries, Wellington, NZ, p. 111. http://www.mpi.govt.nz/news-and-resources/publications/.
- Robertson B.C., Chilvers B.L. The population decline of the New Zealand sea lion Phocarctos hookeri: A review of possible causes. Mammal Review. 2011;41:253–275. [Google Scholar]
- Roe W.D., Michael S., Fyfe J., Burrows E., Hunter S.A., Howe L. First report of systemic toxoplasmosis in a New Zealand sea lion (Phocarctos hookeri) New Zealand Veterinary Journal. 2017;65:46–50. doi: 10.1080/00480169.2016.1230526. [DOI] [PubMed] [Google Scholar]
- Roe W.D., Duignan P.J., desLisle G.W., Collins D. Tuberculosis in a New Zealand (Hooker's) sea lion. New Zealand Veterinary Journal. 2006;54:51. [Google Scholar]
- Roe W.D., Rogers L., Pinpimai K., Dittmer K., Marshall J., Chilvers B.L. Septicaemia and meningitis caused by infection of New Zealand sea lion pups with a hypermucoviscous strain of Klebsiella pneumoniae. Veterinary Microbiology. 2015;176:301–308. doi: 10.1016/j.vetmic.2015.01.019. [DOI] [PubMed] [Google Scholar]
- Spraker T.R., Lander M.E. Causes of mortality in Northern fur seals (Callorhinus ursinus), St. Paul Island, Pribilof Islands, Alaska, 1986-2006. Journal of Wildlife Diseases. 2010;46:450–473. doi: 10.7589/0090-3558-46.2.450. [DOI] [PubMed] [Google Scholar]
- Woods R., Cousins D.V., Kirkwood R., Obendorf D.L. Tuberculosis in a wild Australian fur seal (Arctocephalus pusillus doriferus) from Tasmania. Journal of Wildlife Diseases. 1995;31:83–86. doi: 10.7589/0090-3558-31.1.83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.