Abstract
Mycobacterium bovis BCG, a live attenuated tuberculosis vaccine offers protection against disseminated TB in children. BCG exhibits heterologous protective effects against unrelated infections and reduces infant mortality due to non-mycobacterial infections. Recent reports have suggested that BCG vaccination might have protective effects against COVID-19, however it is highly unlikely that BCG vaccine in its current form can offer complete protection against SARS-CoV-2 infection due to the lack of specific immunity. Nonetheless, recombinant BCG strains expressing antigens of SARS-CoV-2 may offer protection against COVID-19 due to the activation of innate as well as specific adaptive immune response. Further proven safety records of BCG in humans, its adjuvant activity and low cost manufacturing makes it a frontrunner in the vaccine development to stop this pandemic. In this review we discuss about the heterologous effects of BCG, induction of trained immunity and its implication in development of a potential vaccine against COVID-19 pandemic.
Keywords: SARS-CoV-2, COVID-19, BCG, Trained immunity, Vaccine
1. Introduction
Early reports of mysterious pneumonia caused by pathogen of unknown origin were reported in Wuhan city of China during late December 2019. The causative agent was later identified as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and disease caused was named as COVID-19 [1]. The disease outbreak which started in china, has now spread to 210 countries and territories across the world and as on July 13, 2020, a total of 12,768,307 cases and 566,654 deaths have been reported globally (https://covid19.who.int/). Before COVID-19, human coronaviruses (CoVs) have caused two more epidemics in last two decades, SARS in 2002/2003 and Middle east respiratory syndrome (MERS) in 2012. The infectivity of SARS CoV-2 seems to be higher than that of SARS-CoV and MERS-CoV, the causative agents of SARS and MERS respectively as asymptomatic SARS-CoV-2 infected individuals can transmit the virus to healthy population. On the other hand case fatality rate (CFR) of COVID-19 is higher than that of seasonal influenza but lower than SARS and MERS [2]. COVID-19 patients present common symptoms such as fever, dry cough, fatigue, headache, diarrhea and pneumonia whereas acute respiratory distress syndrome (ARDS) is observed in severe cases [3].
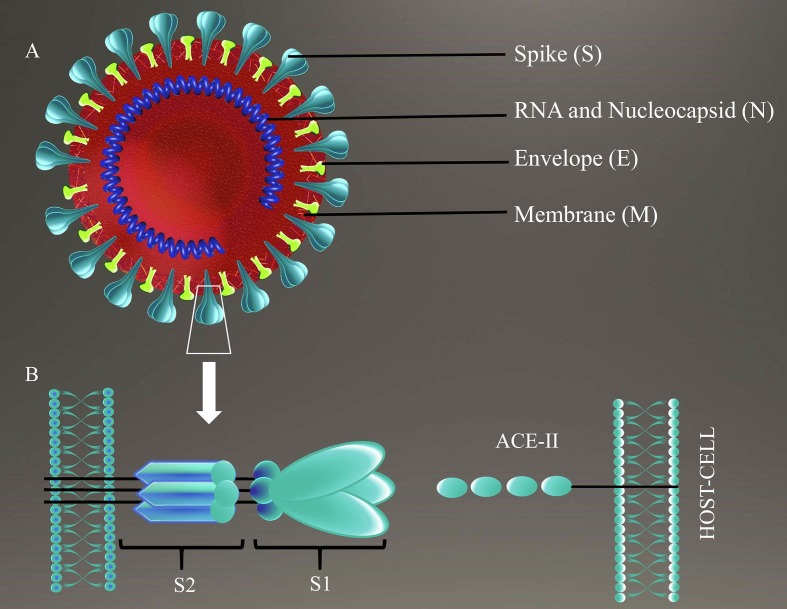
SARS-CoV-2 probably originated from bats and amplified in an intermediate host before reaching to the humans [4]. Like other human CoVs, SARS-CoV-2 is an enveloped beta coronavirus with positive sense, single stranded RNA as its genome [5], [6]. SARSCoV-2, has genome of size 29.9 kb [7] whereas SARS-CoV has 27.9 kb and MERS-CoV has 30.1 kb large genome [8]. SARS-CoV-2 genome contains 5′ capping and 3′ poly(A) tail allowing the expression of viral replicase encoded by nearly two third of genome. The rest of the genome codes for structural and accessory proteins [4], [9]. Total four structural proteins such as spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins are present in SARS-CoV-2 [9]. S protein, a class I fusion protein is expressed on viral the surface and mediates the viral attachment, fusion and entry into host cell by binding to angiotensin-converting enzyme 2 (ACE2) as its receptor [9], [10], [11]. S protein consists of two functional subunits, S1 for host receptor binding and S2 responsible for fusion of viral and host cell membrane [10] (Fig. 1 ). Recently Alba et al predicted the B and T-cell epitopes of SARS-CoV-2 antigens using bioinformatics approach and revealed that S protein is the most immundominant antigen having maximum no of B and T-cell epitopes [12]. Immunogenicity of S protein makes it the most attractive vaccine target against SARS-CoV-2 infection and many groups are working to develop S protein based vaccine.
Fig. 1.
SARS-CoV-2 and its engagement with host cell. SARS-CoV-2 is an enveloped beta coronavirus with positive sense single stranded RNA as its genome. (A) A schematic representation of the virion, that has four main structural proteins: Spike (S) protein, Membrane (M) protein, Envelope (E) protein and Nucleocapsid (N) protein. (B) It depicts the engagement of spike protein of SARS-CoV-2 to angiotensin converting enzyme -2 (ACE-2) of the host cell. S protein has two components: i) S1 that attaches to the ACE-2 of the host cell and ii) S2 that mediates the fusion of host cell membrane and viral membrane leading to the endocytosis of viral particle by host cell.
Till date, there is no approved treatment or vaccine available to control COVID-19 and researchers across the globe are racing against time to develop new therapeutic agents as well as vaccine against SARS-CoV-2 infection. Several vaccine candidates including RNA, DNA, subunit vaccine targeting spike protein “S”, attenuated virus strains and inactivated viral particles; are in the developmental pipeline [5].
2. Heterologous effects of BCG vaccination: implications in COVID-19 associated mortality
The century old TB vaccine BCG, a live attenuated strain of Mycobacterium bovis is the most widely used vaccine across the world, which protects against disseminated TB in children including miliary TB and TB meningitis, however, in adults, the efficacy of BCG against pulmonary TB is limited [13]. Additionally, BCG offers off target protective effects against several non-mycobacterial infections [14] and many studies have reported BCG mediated reduction in infant mortality due to infection unrelated to tuberculosis [15]. In 1927, Swedish physician Carl Näslund reported three fold lower mortality in BCG vaccinated infants compared to the unvaccinated babies during first year of their lives [16]. Besides, BCG vaccination was associated with lower child mortality due to malaria in Guinea-Bissau [17]. The beneficial effects of BCG in infants have been confirmed in many randomized control trials (RCT) and may be attributed to the protection offered against respiratory infections and neonatal sepsis [18], [19]. Another case control study in Guinea-Bissau reported the reduction in the incidence of acute lower respiratory tract infection (ALRI) caused by respiratory syncytial virus (RSV) in BCG vaccinated infants as compared to infants without BCG vaccination, suggesting the heterologous effects of BCG [20].
Moreover, intravesical BCG has been used as non-specific immunotherapy for the treatment of bladder cancer and immunomodulatory effects exhibited by BCG treatment are attributed to the slowdown of tumor progression in the patients [21]. In a recent randomized controlled trial (RCT) Arts et al reported that BCG vaccination offered protection against vaccine strain of yellow fever virus in adults through epigenetic reprograming in circulating monocytes. This study further asserted that BCG vaccination led to the induction of trained immunity as indicated by upregulation of IL-1β mediated responses which correlated with decrease in the viral load and consequent protection in vaccinated participants compared to placebo treated groups [14]. Another RCT involving H1N1 influenza vaccine strain reported enhanced induction of functional antibody response against this strain if BCG vaccination was given prior to influenza vaccination [22].
Furthermore, BCG vaccination diminished the risk of pneumonia in tuberculin negative elderly people in Japan [23]. A small study by Wardhana et al reported significant reduction in acute upper respiratory tract infections in elderly people after BCG vaccination which was given once a month for three consecutive months [24]. A clinical study from South Africa investigating the effectiveness of BCG vaccination on MTB infections in adolescents reported a 73% decrease in respiratory tract infections compared to non-vaccinated population [25], [26].
The ongoing COVID-19 pandemic has spread over 210 countries and territories till date. Significant differences in COVID-19 associated morbidity and mortality, are visible in different countries, which probably vary according to their population size, geography, socioeconomic status, and healthcare infrastructure of the respective country. Interestingly, in a recent epidemiological study Aron et al attributed the country wise variation in COVID-19 related mortality and morbidity to BCG vaccination program in various countries. This study revealed that countries with proper BCG vaccination program have reported lesser COVID-19 associated mortality as compared to the countries where BCG vaccination has been removed from their vaccination program, suggesting probable protection offered by BCG vaccine against COVID-19 [27]. The findings of this study might be error prone and limiting due to differences in the various factors prevalent in the respective country such as testing capabilities/rates, adequate reporting of the cases and mortality, medical care facilities, disease burden and stage of the disease transmission, hence randomized clinical trials are needed to determine the BCG mediated protection against COVID-19. Till date, 11 clinical trials using BCG vaccine and 3 trials using recombinant BCG vaccine VPM1002 have been initiated with aim to study the BCG mediated protective effects in health care workers handling COVID-19 patients and elderly population.
3. Mechanism underlying heterologous effects of BCG
3.1. Trained immunity: innate memory response
The mechanism underlying the heterologous protective effects of BCG is not yet fully understood however, evidences suggest that induction of memory in innate immune cells such as monocytes, natural killer cells and macrophages, independent of T and B cell response, which is also termed as ‘trained immunity’; plays a critical role in non-specific protection exhibited by BCG vaccination [13]. In severe combined immunodeficiency (SCID) mice which lacks adaptive immunity, BCG vaccination protects from lethal systemic candidiasis by activation of NK cells, further confirming the role of innate immune cells in heterologous benefits of BCG [28]. In a recent study, peripheral blood mononuclear cells (PBMCs) isolated from healthy human volunteers three months post BCG vaccination, produced increased levels of pro-inflammatory cytokines IL-1β and TNF-α, when stimulated with unrelated pathogens in vitro. Further, upregulation in the expression of CD14, CD11b and TLR4 was observed along with increased epigenetic modifications at the promoter sequences of the genes of pro-inflammatory cytokines in human PBMCs, which suggests that increased cytokine production may be responsible for the better protection during secondary infection with non-mycobacterial pathogens [29].
Trained innate immune cells exhibit rapid, stronger and qualitatively different transcriptional responses when exposed to pathogens as compared to untrained cells. The underlying molecular mechanism is poorly understood however; evidences suggest the involvement of several regulatory mechanisms including epigenetic reprogramming and chromatin reorganization in immune cells during the generation of trained immunity by primary stimulus [30], [31].
In resting myeloid cells, genes involved in proinflammatory immune response remain in repressed configuration [32] hampering the access of transcriptional machinery to the important regulatory regions of these genes responsible for their expression [33]. Upon primary stimulation of myeloid cells, chromatin remodeling and histone modifications such as acetylation and methylation enhance the accessibility of the RNA polymerase II to the genes encoding inflammatory factors [34] and thus regulate the activation or repression of the expression of the associated genes. Histones methylation may take place at arginine or lysine residues. Upto three methyl groups can be added to lysine residue and upto two methyl groups can be added to the arginine residues [35].
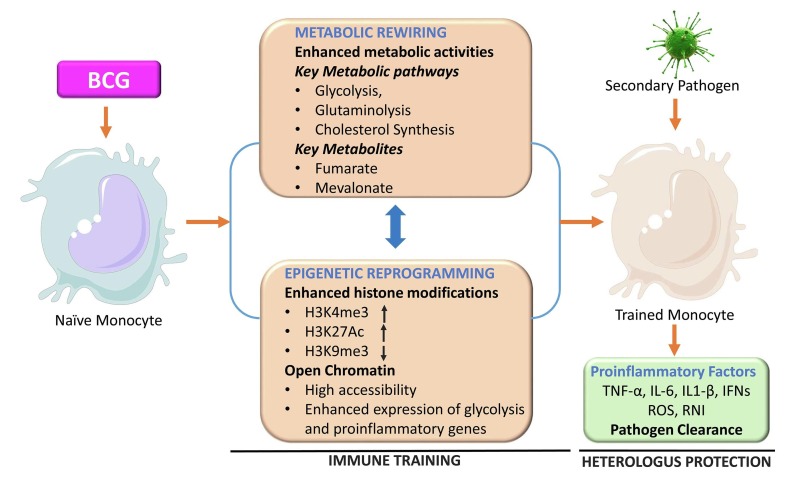
Acquisition of histone 3 methylation of lysine 4, lysine 36 and lysine 79 (H3K4, H3K36 and H3K79) are often associated with the transcriptional activation whereas H3K9, H3K27 and H4K20 methylations are involved in repression of gene expression [35]. BCG vaccination upregulates the expression of IL-6, TNF-α, IL-1β and TLR4 in circulating monocytes in nucleotide-binding oligomerization domain 2 (NOD2) dependent manner through an increase in the H3K4me3 histone modification [29], [36]. Further, due to these epigenetic modifications expression of pattern recognition receptors (PPRs), namely, TLRs, NOD-like receptors and C-type lectin receptors, is upregulated in the innate immune cells [29]. Trained monocytes recognize the pathogen by PRRs during secondary infection and mount a powerful immune response through enhanced cytokine production [37] (Fig. 2 ).
Fig. 2.
Trained immunity and underlying mechanism. Stimulation of naïve monocytes by BCG is accompanied by epigenetic reprogramming and metabolic rewiring in these cells. Histone modifications such as methylation and acetylation leads to chromatin unfolding that facilitates the expression of the genes of proinflammatory factors and metabolic pathways such as glycolysis. Epigenetic changes and metabolic rewiring induced during the initiation of the trained immunity are intricately regulated and work like a positive feedback loop as epigenetic changes enhance the expression of metabolic pathways and metabolites from these pathways cause epigenetic changes in the DNA. These changes vanish only partially after the removal of the stimulus that allows very rapid and enhanced expression of proinflammatory factors following the encounter with unrelated secondary pathogen leading to heterologous protection against this pathogen.
Apart from epigenetic reprogramming, rewiring of cellular metabolic pathways plays a crucial role in development and maintenance of trained immunity in monocytes, macrophages and NK cells [30], [38]. In resting innate immune cells, oxidative phosphorylation is the preferred mode of energy production compared to the glycolysis however a metabolic switch from oxidative phosphorylation to glycolysis takes place in the activated cells. Glycolysis is crucial for epigenetic changes such as histone modifications and other functional modifications underlying trained immunity induced by BCG vaccination [39]. In β-glucan mediated trained immunity, glycolysis genes like pyruvate kinase and hexokinase were upregulated through epigenetic modifications such as H3K4me3 and H3K27Ac at the promoter region of glycolytic genes. The expression of gene encoding mammalian target of rapamycin (mTOR), a target of transcription factor HIF-1α was enhanced by β-glucan. Further, activation of HIF-1α in β-glucan trained monocytes suggested a long-term increase in glycolysis in these cells in mTOR/HIF-1α dependent manner [40]. In line with epigenetic modification induced by β-glucan in monocytes, Arts et al described the comparable epigenetic rearrangements in BCG-induced trained monocytes [39].
Moreover, inhibition of glycolysis in peripheral monocytes impairs the expression of trained immunity phenotypes by hindering the epigenetic modifications. Specifically inhibition of glycolysis reversed the H3K4me3 and H3K9me3 levels in the promoter region of IL-6 and TNF-α in circulating monocytes, suggesting the dependence of these epigenetic modifications on the cellular metabolic pathways [39]. In addition to glycolysis, other metabolic pathways like glutaminolysis, and cholesterol synthesis play a critical role in the induction of trained immunity in innate immune cells. Accumulation of fumarate, a key metabolite of glutamine metabolism and intermediated of TCA cycle helps in induction of trained immune response in myeloid cells by inhibition of KDM5 histone demethylases and thus enhancing the H3K4me3 and H3K27Ac, histone modifications in the promoters of IL-6 and TNF-α, leading to enhanced production of these cytokines after re-stimulation of these cells with LPS [41]. In another study, Bekkering et al demonstrated the induction of trained immunity by mevalonate, a key metabolite of cholesterol metabolism through increased H3K4me3 at the promoter sites of IL-6 and TNF-α [42]. All the studies discussed here support the fact that metabolic rewiring and epigenetic reprogramming are intricately related to each other and play a crucial role in induction of trained immunity after BCG vaccination (Fig. 2).
Innate immune cells such as monocytes and DCs have relatively shorter life span with mean half-life of 5–7 days and can’t transmit their memory characteristic to their progeny, however, intriguingly, trained immunity can be maintained for months, years and decades. Recent evidences have explained this mystery by demonstrating the induction of trained immunity in bone marrow progenitors cells along with the circulating monocytes and tissue resident macrophages [38]. Evidences suggest that BCG or β-glucan mediated long term trained immunity is maintained by metabolic and epigenetic rewiring in the long lived and self-renewing hematopoietic progenitors cells in the bone marrow [43], [44]. In mice, BCG educated hematopoietic stem cells (HSCs) exhibited enhanced myelopoiesis in IFNγ- dependent manner, leading to the generation of epigenetically modified myeloid cells such as macrophages that provided significantly improved protection against virulent Mycobacterium tuberculosis (MTB) challenge compared to naïve macrophages, which further confirmed the BCG induced protective innate immunity against MTB infection [45]. These findings demonstrate the long standing question that how short lived myeloid cells such as monocytes and macrophages can acquire the memory phenotype.
3.2. Heterologous lymphocyte response
In addition to the mycobacteria specific adaptive response, BCG vaccination induces heterologous lymphocytes that protect from secondary non-mycobacterial infections [46]. BCG immunization of mice protected it from subsequent vaccinia virus infection through enhanced production of IFN-γ by CD4 T cells [47]. BCG vaccination induces nonspecific CD4 and CD8 memory T cells and modulates Th1 and Th17 immune responses to unrelated secondary infections [48]. BCG therapy restored the antiviral T-cell response through the restoration of Th1/Th2/Th17 cytokine balance in the patients suffering from recurrent respiratory papillomatosis caused by caused by human papillomavirus and often associated with imbalance of pro-inflammatory cytokines and Th1/Th2/Th17 cytokines [49]. Above evidences suggest the involvement of non-specific T-cell responses induced by BCG vaccination in infant and adults, which protects them from unrelated infectious disease.
4. BCG: an attractive vector for recombinant vaccines
BCG offers several advantages when it comes to human use such as proven safety records in all age groups i.e. infants, neonates and adults, excellent thermal stability and economic production cost. Besides, it possesses extraordinary adjuvant activity and activates both innate and adaptive immunity. These properties make BCG an attractive bacterial vector to express foreign antigens as vaccine candidates against broad range of pathogens including viral, bacterial and parasite infections [50]. Foreign antigens may be expressed on the surface and in the cytoplasm or secreted out of the BCG, however recombinant BCG (rBCG) strains that express the foreign antigen on the cell surface or secrete the antigen outside the cell are immunogenic whereas strains with cytoplasmic expression of foreign antigens, fail to induce any immune response as they are not accessible to antigen-presenting cells (APCs) [51].
One of the most successful rBCG strains, rBCGΔureC::hly (VPM1002) expressing listeriolysin O (LLO) encoded by Hly gene derived from Listeria monocytogenes and deleted Urease C (ureC) gene, is under phase III clinical trial (NCT03152903) [52]. LLO (Hly) is a pore forming protein which creates pores in the MTB containing phagosomal membrane leading to leakage of MTB antigens to cytoplasm of the host cell that enhances antigen processing and presentation through MHC-I pathway and subsequent augmentation of CD8-T cell activation. Moreover, LLO expression induces apoptosis in infected macrophages which further enhances CD8-T cell response through cross-presentation of antigens. Deletion of ureC encoding urease, which inhibits the acidification of phagosome, helps in the maintenance of optimal pH of phagosome that is critical for the activity of LLO. rBCGΔureC::hly is the most advanced live vaccine candidate that has shown encouraging results in phase I and phase II clinical trials [53].
Several, rBCG vaccines have been successfully developed against wide range of pathogens including viruses, bacteria, parasites and exhibited significant protection against the target pathogens in the animal models. For example, rBCG-pSOV3J1 expressing Env V3 peptide of HIV-1 induced HIV-1 specific T cell response in Guinea pigs following oral immunization [54].
Further, rBCGpan-Gag strain expressing Gag protein of HIV-1 subtype C induced a broad T cell response in baboons [55]. Another rBCG strain rBCG-CtEm which expresses multi-epitope antigen CtEm of HCV induced HCV specific cellular immunity in the transgenic mice [56].
In a recent study, rBCG-N-hRSV which expresses the nucleoprotein (N) of the human respiratory syncytial virus (hRSV), protected mice from hRSV challenge by induction of hRSV specific neutralizing antibodies and reduced the clinical pathology of the lung [57].
Additionally, BCG has been engineered to express antigens of parasites like Plasmodium falciparum. An rBCG-CS strain which expresses circumsporozoite (CS) protein of P. falciparum stimulated dendritic cells, memory T-cells and induced humoral response in BALB/c mice after immunization [58]. Similarly, rBCG-ROP2 that expresses rhoptry protein 2 (ROP2) of Toxoplasma gondii, induced specific humoral and cellular immune responses in mice after the immunization [59]. Above mentioned examples suggest the potential of BCG based vaccine for broad range of diseases including the current pandemic COVID-19.
5. Potential of BCG based vaccination strategy against COVID-19
In the current scenario, when no effective vaccine or therapy against COVID-19 is available, BCG vaccine can offer some hope due to its safety records and heterologous effects against range of infectious diseases. BCG in its current form may boost general immunity and offer some protection against SARS-CoV-2 in small group of individuals due to induction of trained immunity, nonetheless lack of specific adaptive immune response against SARS-CoV-2 may be a limiting factor in its large scale usage.
Effective solution of this issue can be provided by the development of recombinant BCG strain expressing proteins of SARS-CoV-2, specifically spike (S) protein and nucleopcapsid (N) protein. S protein is the most immundominant antigen of human CoVs including MERS-CoV, SARS-CoV and SARS-CoV-2. Likewise, N protein is the second most immundominant antigen in SARS-CoV-2 and consists of several B and T cell epitopes [12].
Interestingly, rBCG expressing S or N protein may offer dual benefits by combining the trained immunity induced by BCG and viral specific immunity evoked by S or N protein. Additionally, induction of trained immunity by BCG may induce a wholesome immune response and play a critical role in improving the vaccine efficacy by activation of innate immune cells through the stimulation of pathogen recognition receptors (PRRs). Though, BCG is considered as a good vector to express foreign antigens, its efficacy can be limited by several factors such as the expression level of antigen and choice of vector for antigen expression, hence these factors should be considered during the development of the vaccine. The choice of vector is crucial for the expression of foreign antigen and it can be mediated by both replicative and integrative vectors. Multicopy replicative vectors could be obvious choice as it can increase the expression of foreign antigen, however genes cloned in such vectors exhibit lower stability and may be lost during in vivo experiments due to lack of selective environment. Further, such vectors may raise a safety concern due to the possible horizontal transfer to other bacterial species of the host. On the other hand integrative plasmids demonstrate stable and long lasting expression of the foreign antigen leading to a long term and stable immune response in the recipients [60]. Since viruses use host machinery for the production of their proteins, the difference in the protein translation and their post translation modification such as glycosylation in the prokaryotic system poses another challenge in the development of BCG based vaccines expressing viral proteins. S protein is a glycoprotein and glycosylation pattern of recombinant S protein expressed in BCG may vary from the original pattern leading to the alteration in its immunogenicity and hence this factor should also be considered while vaccine designing. These issues can be avoided by cloning the most immunogenic peptides of the S or N protein in BCG instead of whole protein which in turn may improve the efficacy of rBCG vaccine. In the current scenario, BCG based SARS-CoV-2 vaccine seems a feasible option with immediate translational possibility.
6. Discussion and future challenges
In the absence of any approved therapeutic treatment for COVID-19, development of an effective and safe vaccine against SARS-CoV-2 is urgently needed, that may be a prophylactic, therapeutic and post-exposure in nature. Currently, twenty five COVID-19 vaccine candidates are under clinical trials across the world with aim to minimize the COVID-19 related mortality in the ongoing struggle for survival and rBCG vaccine expressing viral protein might be a potential armor against SARS-CoV-2 infection. Growing evidences suggest that most of the clinical benefits offered by BCG are due to its off target effects through the induction of trained immunity, however it is largely unknown whether recombinant BCGs strains might be able to induce trained immunity following vaccination. Data from ongoing clinical trials to study the efficacy of BCG vaccination in healthcare workers will be available soon and it will play a critical role in the development of rBCG vaccines against COVID-19. Unequivocally, BCG is a suitable vector for the expression of SARS-CoV-2 antigens as it possesses several beneficial properties such as, its inherent adjuvant properties and suitability for neonatal administration [61]. Trained immunity induced by rBCG along with SARS-CoV-2 specific immune response can induce robust protection against COVID-19 and excellent safety profile in humans, low cost production and thermal stability further enhances the possibility of its human use in a limited time frame compared to other candidates where time consuming safety trials and infrastructure are needed before the vaccines reach the end users.
Funding
Intramural funding by Department of Atomic Energy, India.
Conflict of interest disclosure
Author has no conflict of interest to disclose.
Acknowledgments
I acknowledge Department of Atomic Energy, India.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cellimm.2020.104187.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y.Z., Holmes E.C. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell. 2020;181:223–227. doi: 10.1016/j.cell.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020 doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.W. Tai, L. He, X. Zhang, J. Pu, D. Voronin, S. Jiang, Y. Zhou, L. Du, Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine, Cell. Mol. Immunol., (2020). [DOI] [PMC free article] [PubMed]
- 10.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020 doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qing E., Gallagher T. SARS coronavirus redux. Trends Immunol. 2020 doi: 10.1016/j.it.2020.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grifoni A., Sidney J., Zhang Y., Scheuermann R.H., Peters B., Sette A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moorlag S., Arts R.J.W., van Crevel R., Netea M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019;25:1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Arts R.J.W., Moorlag S., Novakovic B., Li Y., Wang S.Y., Oosting M., Kumar V., Xavier R.J., Wijmenga C., Joosten L.A.B., Reusken C., Benn C.S., Aaby P., Koopmans M.P., Stunnenberg H.G., van Crevel R., Netea M.G. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23:89–100 e105. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Shann F. Nonspecific effects of vaccines and the reduction of mortality in children. Clin. Ther. 2013;35:109–114. doi: 10.1016/j.clinthera.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Covián C., Fernández-Fierro A., Retamal-Díaz A., Díaz F.E., Vasquez A.E., Lay M.K., Riedel C.A., González P.A., Bueno S.M., Kalergis A.M. BCG-induced cross-protection and development of trained immunity: implication for vaccine design. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.02806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth A., Gustafson P., Nhaga A., Djana Q., Poulsen A., Garly M.L., Jensen H., Sodemann M., Rodriques A., Aaby P. BCG vaccination scar associated with better childhood survival in Guinea-Bissau. Int. J. Epidemiol. 2005;34:540–547. doi: 10.1093/ije/dyh392. [DOI] [PubMed] [Google Scholar]
- 18.Aaby P., Roth A., Ravn H., Napirna B.M., Rodrigues A., Lisse I.M., Stensballe L., Diness B.R., Lausch K.R., Lund N., Biering-Sørensen S., Whittle H., Benn C.S. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 2011;204:245–252. doi: 10.1093/infdis/jir240. [DOI] [PubMed] [Google Scholar]
- 19.Biering-Sørensen S., Aaby P., Napirna B.M., Roth A., Ravn H., Rodrigues A., Whittle H., Benn C.S. Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guérin vaccination at first health center contact. Pediatr. Infect. Dis. J. 2012;31:306–308. doi: 10.1097/INF.0b013e3182458289. [DOI] [PubMed] [Google Scholar]
- 20.Stensballe L.G., Nante E., Jensen I.P., Kofoed P.E., Poulsen A., Jensen H., Newport M., Marchant A., Aaby P. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: a beneficial effect of BCG vaccination for girls community based case-control study. Vaccine. 2005;23:1251–1257. doi: 10.1016/j.vaccine.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Han R.F., Pan J.G. Can intravesical bacillus Calmette-Guerin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology. 2006;67:1216–1223. doi: 10.1016/j.urology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Leentjens J., Kox M., Stokman R., Gerretsen J., Diavatopoulos D.A., van Crevel R., Rimmelzwaan G.F., Pickkers P., Netea M.G. BCG vaccination enhances the immunogenicity of subsequent influenza vaccination in healthy volunteers: A randomized, placebo-controlled pilot study. J. Infect. Dis. 2015;212:1930–1938. doi: 10.1093/infdis/jiv332. [DOI] [PubMed] [Google Scholar]
- 23.Ohrui T., Nakayama K., Fukushima T., Chiba H., Sasaki H. [Prevention of elderly pneumonia by pneumococcal, influenza and BCG vaccinations] Nihon Ronen Igakkai zasshi. Jpn. J. Geriatr. 2005;42:34–36. doi: 10.3143/geriatrics.42.34. [DOI] [PubMed] [Google Scholar]
- 24.Wardhana E.A., Datau A., Sultana V.V., Mandang E.J. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med. Indones. 2011;43:185–190. [PubMed] [Google Scholar]
- 25.E. Nemes, H. Geldenhuys, V. Rozot, K.T. Rutkowski, F. Ratangee, N. Bilek, S. Mabwe, L. Makhethe, M. Erasmus, A. Toefy, H. Mulenga, W.A. Hanekom, S.G. Self, L.G. Bekker, R. Ryall, S. Gurunathan, C.A. DiazGranados, P. Andersen, I. Kromann, T. Evans, R.D. Ellis, B. Landry, D.A. Hokey, R. Hopkins, A.M. Ginsberg, T.J. Scriba, M. Hatherill, C.S. Team, Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination, N. Engl. J. Med., 379 (2018) 138–149. [DOI] [PMC free article] [PubMed]
- 26.Netea M.G., Giamarellos-Bourboulis E.J., Dominguez-Andres J., Curtis N., van Crevel R., van de Veerdonk F.L., Bonten M. Trained immunity: a tool for reducing susceptibility to and the severity of SARS-CoV-2 infection. Cell. 2020;181:969–977. doi: 10.1016/j.cell.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.A. Miller, M.J. Reandelar, K. Fasciglione, V. Roumenova, Y. Li, G.H. Otazu, Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study, medRxiv, (2020) 2020.2003.2024.20042937.
- 28.Kleinnijenhuis J., Quintin J., Preijers F., Joosten L.A., Jacobs C., Xavier R.J., van der Meer J.W., van Crevel R., Netea M.G. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin. Immunol. 2014;155:213–219. doi: 10.1016/j.clim.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleinnijenhuis J., Quintin J., Preijers F., Joosten L.A.B., Ifrim D.C., Saeed S., Jacobs C., van Loenhout J., de Jong D., Stunnenberg H.G., Xavier R.J., van der Meer J.W.M., van Crevel R., Netea M.G. Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. PNAS. 2012;109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.M.G. Netea, L.A. Joosten, E. Latz, K.H. Mills, G. Natoli, H.G. Stunnenberg, L.A. O'Neill, R.J. Xavier, Trained immunity: A program of innate immune memory in health and disease, Science, 352 (2016) aaf1098. [DOI] [PMC free article] [PubMed]
- 31.Netea M.G., Dominguez-Andres J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., Joosten L.A.B., van der Meer J.W.M., Mhlanga M.M., Mulder W.J.M., Riksen N.P., Schlitzer A., Schultze J.L., Stabell Benn C., Sun J.C., Xavier R.J., Latz E. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smale S.T., Tarakhovsky A., Natoli G. Chromatin contributions to the regulation of innate immunity. Annu. Rev. Immunol. 2014;32:489–511. doi: 10.1146/annurev-immunol-031210-101303. [DOI] [PubMed] [Google Scholar]
- 33.Ghisletti S., Barozzi I., Mietton F., Polletti S., De Santa F., Venturini E., Gregory L., Lonie L., Chew A., Wei C.L., Ragoussis J., Natoli G. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity. 2010;32:317–328. doi: 10.1016/j.immuni.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Liu H., Li P., Wei Z., Zhang C., Xia M., Du Q., Chen Y., Liu N., Li H., Yang X.P. Regulation of T cell differentiation and function by epigenetic modification enzymes. Sem. Immunopathol. 2019;41:315–326. doi: 10.1007/s00281-019-00731-w. [DOI] [PubMed] [Google Scholar]
- 36.Arts R.J., Blok B.A., Aaby P., Joosten L.A., de Jong D., van der Meer J.W., Benn C.S., van Crevel R., Netea M.G. Long-term in vitro and in vivo effects of gamma-irradiated BCG on innate and adaptive immunity. J. Leukoc. Biol. 2015;98:995–1001. doi: 10.1189/jlb.4MA0215-059R. [DOI] [PubMed] [Google Scholar]
- 37.Kleinnijenhuis J., van Crevel R., Netea M.G. Trained immunity: consequences for the heterologous effects of BCG vaccination. Trans. R. Soc. Trop. Med. Hyg. 2015;109:29–35. doi: 10.1093/trstmh/tru168. [DOI] [PubMed] [Google Scholar]
- 38.Netea M.G., Joosten L.A.B. Trained immunity and local innate immune memory in the lung. Cell. 2018;175:1463–1465. doi: 10.1016/j.cell.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Arts R.J.W., Carvalho A., La Rocca C., Palma C., Rodrigues F., Silvestre R., Kleinnijenhuis J., Lachmandas E., Goncalves L.G., Belinha A., Cunha C., Oosting M., Joosten L.A.B., Matarese G., van Crevel R., Netea M.G. Immunometabolic pathways in BCG-induced trained immunity. Cell Rep. 2016;17:2562–2571. doi: 10.1016/j.celrep.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng S.-C., Quintin J., Cramer R.A., Shepardson K.M., Saeed S., Kumar V., Giamarellos-Bourboulis E.J., Martens J.H.A., Rao N.A., Aghajanirefah A., Manjeri G.R., Li Y., Ifrim D.C., Arts R.J.W., van der Veer B.M.J.W., Deen P.M.T., Logie C., O’Neill L.A., Willems P., van de Veerdonk F.L., van der Meer J.W.M., Ng A., Joosten L.A.B., Wijmenga C., Stunnenberg H.G., Xavier R.J., Netea M.G. mTOR- and HIF-1α–mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arts R.J., Novakovic B., Ter Horst R., Carvalho A., Bekkering S., Lachmandas E., Rodrigues F., Silvestre R., Cheng S.C., Wang S.Y., Habibi E., Goncalves L.G., Mesquita I., Cunha C., van Laarhoven A., van de Veerdonk F.L., Williams D.L., van der Meer J.W., Logie C., O'Neill L.A., Dinarello C.A., Riksen N.P., van Crevel R., Clish C., Notebaart R.A., Joosten L.A., Stunnenberg H.G., Xavier R.J., Netea M.G. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 2016;24:807–819. doi: 10.1016/j.cmet.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.S. Bekkering, R.J.W. Arts, B. Novakovic, I. Kourtzelis, C. van der Heijden, Y. Li, C.D. Popa, R. Ter Horst, J. van Tuijl, R.T. Netea-Maier, F.L. van de Veerdonk, T. Chavakis, L.A.B. Joosten, J.W.M. van der Meer, H. Stunnenberg, N.P. Riksen, M.G. Netea, Metabolic induction of trained immunity through the mevalonate pathway, Cell, 172 (2018) 135–146 e139. [DOI] [PubMed]
- 43.I. Mitroulis, K. Ruppova, B. Wang, L.S. Chen, M. Grzybek, T. Grinenko, A. Eugster, M. Troullinaki, A. Palladini, I. Kourtzelis, A. Chatzigeorgiou, A. Schlitzer, M. Beyer, L.A.B. Joosten, B. Isermann, M. Lesche, A. Petzold, K. Simons, I. Henry, A. Dahl, J.L. Schultze, B. Wielockx, N. Zamboni, P. Mirtschink, U. Coskun, G. Hajishengallis, M.G. Netea, T. Chavakis, Modulation of myelopoiesis progenitors is an integral component of trained immunity, Cell, 172 (2018) 147–161 e112. [DOI] [PMC free article] [PubMed]
- 44.Chavakis T., Mitroulis I., Hajishengallis G. Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation. Nat. Immunol. 2019;20:802–811. doi: 10.1038/s41590-019-0402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.E. Kaufmann, J. Sanz, J.L. Dunn, N. Khan, L.E. Mendonca, A. Pacis, F. Tzelepis, E. Pernet, A. Dumaine, J.C. Grenier, F. Mailhot-Leonard, E. Ahmed, J. Belle, R. Besla, B. Mazer, I.L. King, A. Nijnik, C.S. Robbins, L.B. Barreiro, M. Divangahi, BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis, Cell, 172 (2018) 176–190 e119. [DOI] [PubMed]
- 46.Goodridge H.S., Ahmed S.S., Curtis N., Kollmann T.R., Levy O., Netea M.G., Pollard A.J., van Crevel R., Wilson C.B. Harnessing the beneficial heterologous effects of vaccination. Nat. Rev. Immunol. 2016;16:392–400. doi: 10.1038/nri.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mathurin K.S., Martens G.W., Kornfeld H., Welsh R.M. CD4 T-cell-mediated heterologous immunity between mycobacteria and poxviruses. J. Virol. 2009;83:3528–3539. doi: 10.1128/JVI.02393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kleinnijenhuis J., Kleinnijenhuis J., Quintin J., Preijers F., Benn C.S., Joosten L.A.B., Jacobs C., van Loenhout J., Xavier R.J., Aaby P., van der Meer J.W.M., van Crevel R., Netea M.G. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2014;6:152–158. doi: 10.1159/000355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vetskova E.K., Muhtarova M.N., Avramov T.I., Stefanova T.R., Chalakov I.J., Nikolova M.H. Immunomodulatory effects of BCG in patients with recurrent respiratory papillomatosis. Folia Med. 2013;55:49–54. doi: 10.2478/folmed-2013-0005. [DOI] [PubMed] [Google Scholar]
- 50.Zheng Y.Q., Naguib Y.W., Dong Y., Shi Y.C., Bou S., Cui Z. Applications of bacillus Calmette-Guerin and recombinant bacillus Calmette-Guerin in vaccine development and tumor immunotherapy. Expert Rev. Vacc. 2015;14:1255–1275. [PMC free article] [PubMed] [Google Scholar]
- 51.Grode L., Kursar M., Fensterle J., Kaufmann S.H.E., Hess J. Cell-mediated immunity induced by recombinant <em>Mycobacterium bovis</em> Bacille Calmette-Guérin strains against an intracellular bacterial pathogen: importance of antigen secretion or membrane-targeted antigen display as lipoprotein for vaccine efficacy. J. Immunol. 2002;168:1869–1876. doi: 10.4049/jimmunol.168.4.1869. [DOI] [PubMed] [Google Scholar]
- 52.Desel C., Dorhoi A., Bandermann S., Grode L., Eisele B., Kaufmann S.H.E. Recombinant BCG ΔureC hly+ induces superior protection over parental BCG by stimulating a balanced combination of type 1 and type 17 cytokine responses. J. Infect. Dis. 2011;204:1573–1584. doi: 10.1093/infdis/jir592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saiga H., Nieuwenhuizen N., Gengenbacher M., Koehler A.-B., Schuerer S., Moura-Alves P., Wagner I., Mollenkopf H.-J., Dorhoi A., Kaufmann S.H.E. The recombinant BCG ΔureC::hly vaccine targets the AIM2 inflammasome to induce autophagy and inflammation. J. Infect. Dis. 2014;211:1831–1841. doi: 10.1093/infdis/jiu675. [DOI] [PubMed] [Google Scholar]
- 54.Kawahara M., Hashimoto A., Toida I., Honda M. Oral recombinant Mycobacterium bovis bacillus Calmette-Guerin expressing HIV-1 antigens as a freeze-dried vaccine induces long-term, HIV-specific mucosal and systemic immunity. Clin. Immunol. 2002;105:326–331. doi: 10.1006/clim.2002.5292. [DOI] [PubMed] [Google Scholar]
- 55.Chege G.K., Burgers W.A., Stutz H., Meyers A.E., Chapman R., Kiravu A., Bunjun R., Shephard E.G., Jacobs W.R., Jr., Rybicki E.P., Williamson A.L. Robust immunity to an auxotrophic Mycobacterium bovis BCG-VLP prime-boost HIV vaccine candidate in a nonhuman primate model. J. Virol. 2013;87:5151–5160. doi: 10.1128/JVI.03178-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei S.H., Yin W., An Q.X., Lei Y.F., Hu X.B., Yang J., Lu X., Zhang H., Xu Z.K. A novel hepatitis C virus vaccine approach using recombinant Bacillus Calmette-Guerin expressing multi-epitope antigen. Arch. Virol. 2008;153:1021–1029. doi: 10.1007/s00705-008-0082-1. [DOI] [PubMed] [Google Scholar]
- 57.Soto J.A., Galvez N.M.S., Rivera C.A., Palavecino C.E., Cespedes P.F., Rey-Jurado E., Bueno S.M., Kalergis A.M. Recombinant BCG vaccines reduce pneumovirus-caused airway pathology by inducing protective humoral immunity. Front. Immunol. 2018;9:2875. doi: 10.3389/fimmu.2018.02875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arama C., Waseem S., Fernandez C., Assefaw-Redda Y., You L., Rodriguez A., Radosevic K., Goudsmit J., Kaufmann S.H., Reece S.T., Troye-Blomberg M. A recombinant Bacille Calmette-Guerin construct expressing the Plasmodium falciparum circumsporozoite protein enhances dendritic cell activation and primes for circumsporozoite-specific memory cells in BALB/c mice. Vaccine. 2012;30:5578–5584. doi: 10.1016/j.vaccine.2011.09.054. [DOI] [PubMed] [Google Scholar]
- 59.Wang H., Liu Q., Liu K., Zhong W., Gao S., Jiang L., An N. Immune response induced by recombinant Mycobacterium bovis BCG expressing ROP2 gene of Toxoplasma gondii. Parasitol. Int. 2007;56:263–268. doi: 10.1016/j.parint.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Méderlé I., Bourguin I., Ensergueix D., Badell E., Moniz-Peireira J., Gicquel B., Winter N. Plasmidic versus insertional cloning of heterologous genes in <em>Mycobacterium bovis</em> BCG: impact on in vivo antigen persistence and immune responses. Infect. Immun. 2002;70:303–314. doi: 10.1128/IAI.70.1.303-314.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kilpeläinen A., Saubi N., Guitart N., Moyo N., Wee E.G., Ravi K., Hanke T., Joseph J. Priming with recombinant BCG expressing novel HIV-1 conserved mosaic immunogens and boosting with recombinant ChAdOx1 is safe, stable, and elicits HIV-1-specific T-cell responses in BALB/c mice. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.