Abstract
Understanding cellular and molecular damages in oocytes during exposure to extreme conditions is essential to optimize long-term fertility preservation approaches. Using the domestic cat (Felis catus) model, we are developing drying techniques for oocytes’ germinal vesicles (GVs) as a more economical alternative to cryopreservation. The objective of the study was to characterize the influence of desiccation on nuclear envelope conformation, chromatin configuration, and the relative fluorescent intensities of histone H3 trimethylation at lysine 4 (H3K4me3) and at lysine 9 (H3K9me3) compared to vitrification. Results showed that higher proportions of dried/rehydrated GVs maintained normal nuclear envelope conformation and chromatin configuration than vitrified/warmed counterparts. Both preservation methods had a similar influence on epigenetic patterns, lowering H3K4me3 intensity to under 40% while maintaining H3K9me3 levels. Further analysis revealed that the decrease of H3K4me3 intensity mainly occurred during microwave dehydration and subsequent rehydration, whereas sample processing (permeabilization and trehalose exposure) or storage did not significantly affect the epigenetic marker. Moreover, rehydration either directly or stepwise with trehalose solutions did not influence the outcome. This is the first report demonstrating that the incidence of GV damages is lower after desiccation/rehydration than vitrification/warming.
Keywords: desiccation, germinal vesicle, H3K4me3, H3K9me3, nuclear envelope
1 |. INTRODUCTION
Female fertility preservation involves the collection and long-term storage of living germplasms for future use through assisted reproduction. It provides insurance for women against medical- or age-related fertility decline. It is also vital for the maintenance of genetically valuable livestock and the genetic diversity of rare and endangered wildlife. In addition to improving the temporary suspension of cellular and tissue functions, there is a constant need for new fertility preservation strategies to provide safe and cost-effective storage (Comizzoli, Songsasen, & Wildt, 2010; Songsasen & Comizzoli, 2019).
In comparison to sperm freezing, the preservation of oocyte is inherently more challenging. The relatively large size, high intracellular water content as well as complex and delicate cytoskeleton contribute to its low tolerance to nonphysiological conditions encountered during preservation procedures. Comparing to metaphase II (MII) oocytes, the most widely preserved oocyte stage, germinal vesicle (GV) oocytes do not have meiotic spindles that are particularly vulnerable during cryopreservation (Kopeika, Thornhill, & Khalaf, 2015). Additional evidence has suggested that GV alone could be an alternative target for female fertility preservation. Preserved GVs can be used to reconstitute oocytes with the capacity to mature, be fertilized, and develop into blastocysts (Holt, 2013). Moreover, larger numbers of immature oocytes are available in a given ovary; therefore, application of GV preservation could be extended to cases in which MII oocytes are not available due to age or diseases (Comizzoli, Pukazhenthi, & Wildt, 2011; Graves-Herring, Wildt, & Comizzoli, 2013; Holt, 2013).
The higher resilience of GV than the whole oocytes has allowed exploration of alternative preservation methods specifically targeting GV alone (Holt, 2013). A recent article by Dang-Nguyen et al. (2018) reported that oocytes reconstituted with freeze-dried porcine GVs could be matured in vitro. Our laboratory also has previously demonstrated the retention of structure and meiotic competence of GVs after air-dry and rehydration in the domestic cat (Felis catus) model (Graves-Herring et al., 2013). We further improved the dehydration procedure by optimizing a microwave-assisted dehydration technique, which provided more uniform and reproducible drying under thermal control (Cellemme, Van Vorst, Paramore, & Elliott, 2013). With this approach, we have established effective GV drying protocol to reach a moisture content that allowed supra-zero storage temperature. Results also demonstrated the retention of DNA integrity within the GV after up to 8 weeks of storage (Elliott, Lee, Paramore, Van Vorst, & Comizzoli, 2015).
GV contains the maternal genome enclosed in the nuclear envelope. As folliculogenesis progresses, chromatin arranges in particular configurations, which are strongly associated with oocyte competence in multiple species, including the cat model (Comizzoli et al., 2011; Tan et al., 2009). The nuclear envelope consists of two lipid bilayer membranes and serves as the gatekeeper for trafficking of molecules between the nucleus and the cytoplasm. Both lipids and chromatin are vulnerable to freezing or dried conditions. Lipid aggregation, membrane damage, DNA breakage, and chromatin condensation have been reported in cryopreserved and/or dry-preserved cells (Amstislavsky, Mokrousova, Brusentsev, Okotrub, & Comizzoli, 2019; Chen et al., 2001; Golan et al., 2018; Kopeika et al., 2015; Kratochvílová et al., 2018; Shahba et al., 2016). In addition, epigenetic regulation has putative long-lasting or even transgenerational effect in the offspring and therefore is a serious concern for assisted reproduction applications. Among epigenetic modifications, H3 trimethylation at lysine 4 (H3K4me3) and at lysine 9 (H3K9me3) are generally associated with active and repressive gene expression, respectively. These histone modifications are observed in the growing oocytes in the mouse (Kageyama et al., 2007), pig (Endo, Naito, Aoki, Kume, & Tojo, 2005; X. X. Yu et al., 2018), cow (Zhang, Wang et al., 2016), sheep (Russo et al., 2013), cat (Phillips, Wildt, & Comizzoli, 2012), and human (A. Zhang et al., 2012). Disruption of trimethylation of H3K4 in the developing murine oocytes led to failed maturation and impeded maternal-zygote transition (C. Yu et al., 2017). In the domestic cat, increased H3K4me3 coincided with GV competence acquisition, suggesting a potential correlation (Phillips et al., 2012). Deficiency in H3K9 methylation in growing oocytes impaired multiple essential aspects in meiotic maturation in the mouse (Eymery, Liu, Ozonov, Stadler, & Peters, 2016). Previous studies have also reported aberrant changes of H3K9 methylation in vitrified murine and porcine oocytes (Spinaci et al., 2012; Yan, Yan, Qiao, Zhao, & Liu, 2010). As exposure to extreme conditions, such as freezing and desiccation, poses a risk of altering epigenetic regulations and membrane integrity (Jiang et al., 2017; Kopeika et al., 2015), careful evaluation is warranted.
In the cat model, it has been reported that GV chromatin configuration was resistant to osmotic stress but sensitive to freezing (Comizzoli, Wildt, & Pukazhenthi, 2008; Mikołajewska, Müller, Niżański, & Jewgenow, 2012). It is unclear how desiccation affects the nuclear structure, except that nuclear envelope, is still present after drying (Graves-Herring et al., 2013). The influence of these extreme conditions on the epigenetic status of GV chromatin also remains to be investigated.
Understanding the structural and molecular responses of GVs exposed to various stresses (hyperosmotic, freezing, and desiccation) will provide valuable information for optimization of GV preservation strategies. The objectives of the present study were to (a) compare nuclear envelope conformations, chromatin configurations, and key epigenetic patterns of the GVs following newly developed desiccation technique or conventional vitrification, and (b) further examine key epigenetic modifications in GVs during each step of the desiccation procedure, storage, and rehydration.
2 |. RESULTS
2.1 |. Experiment 1: Influence of desiccation versus vitrification on nuclear envelope conformation, chromatin configuration, and epigenetic patterns in GVs
Nuclear envelopes in the majority of control GVs (90%) were spherical (Figure 1A and B). The membrane was not always smooth, as wrinkles were apparent in most of the GVs (Figure 1A–a,b). In 10% of control samples, nuclear envelopes were not perfectly spherical in shape as membrane appeared dented in part of the GVs and therefore were categorized as “irregular” (Figure 1A–c,d and B). Control GV size as marked by lamin-enclosed area was 1,243 ± 63 μm2. Chromatin in all control GVs exhibited a reticular configuration that covered an area of 1,155 ± 58 μm2 (Figure 1A–a’–d’ and C). In each control GV, the area occupied by chromatin was equal or only slightly smaller than the size of nuclear envelope area (Figure 1C), indicating that chromatin spread throughout the whole GV. A clear shift in the proportions of different nuclear envelope conformation and chromatin configuration was found in dried/rehydrated GVs compared to controls (p < .05). The proportion of irregularly shaped nuclear envelope increased to 34%, including 10% that also contained abnormal chromatin (Figure 1A–g,g’,h,h’ and B). Reticular chromatin configuration was preserved in 82% of the dried/rehydrated GVs. Another 8% of the GVs contained abnormal, more condensed chromatin without prominent change in nuclear envelope conformation (Figure 1A–e,e’,f,f’ and B). Both nuclear envelope and chromatin areas (983 ± 41 and 922 ± 41 μm2, respectively) decreased in dried/rehydrated GVs (p < .05), but still maintained a close to 1:1 ratio between the two (Figure 1C). Cryopreservation had the most significant influence (p < .05) on nuclear envelope conformation and chromatin configuration. Over 50% of vitrified GVs had irregular nuclear envelope and condensed chromatin (Figure 1B), many with a more severe level of abnormality compared to desiccated counterparts. Only 27% of cryopreserved GVs maintained normal chromatin and nuclear envelope (Figure 1B). Nuclear envelope and chromatin areas both decreased significantly (p < .05) to 839 ± 67 and 754 ± 64 μm2, respectively, but a roughly 1:1 ratio between the two was still maintained in each GV (Figure 1C).
FIGURE 1.
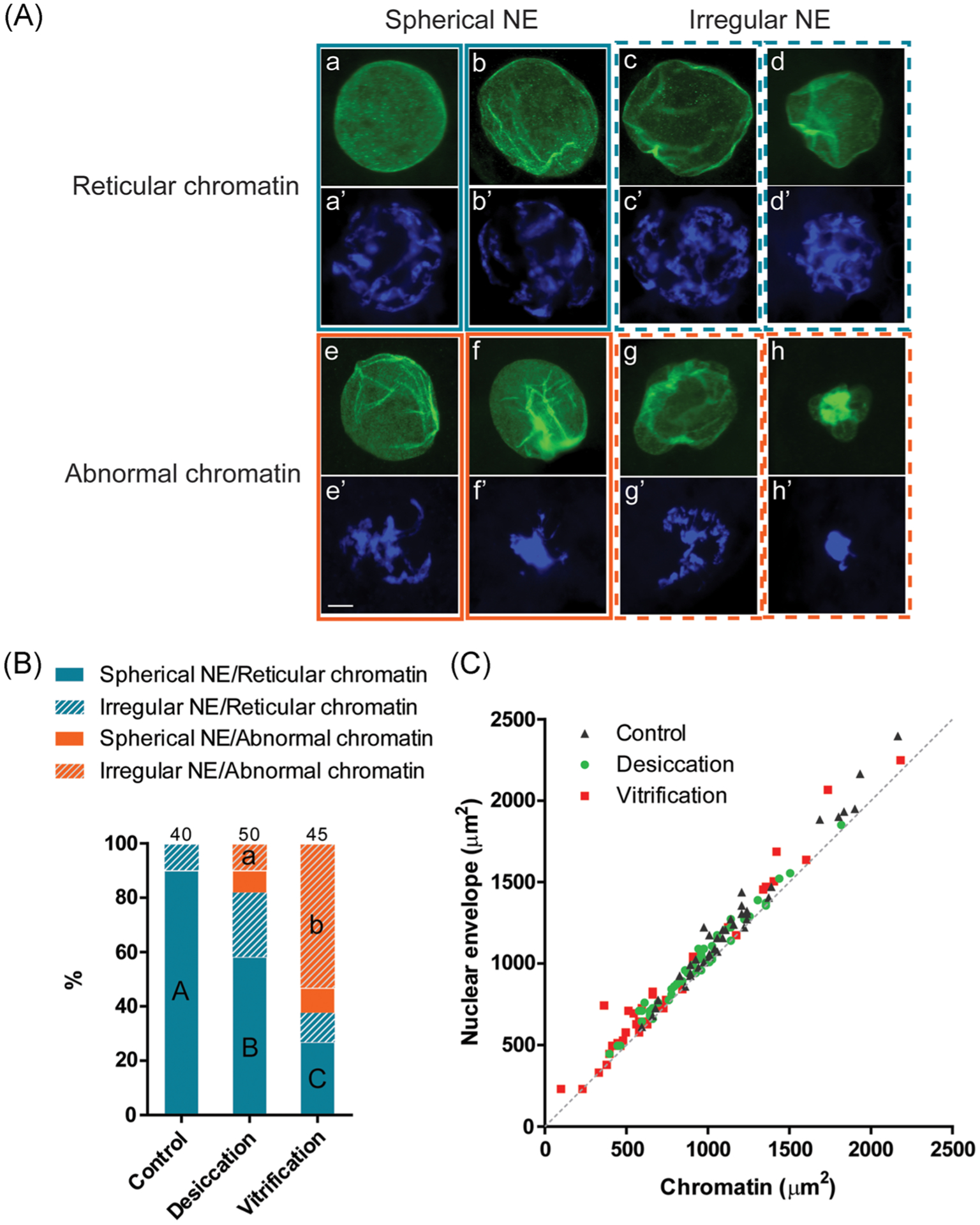
Nuclear envelope conformation and chromatin configuration in cat germinal vesicles after desiccation and vitrification. (A) Representative micrographs of different categories of the nuclear envelope (NE) conformation (immunostaining of lamin A/C; a–h) and chromatin configurations (DAPI staining; a’–h’). Scale bar = 10 μm. (B) Proportion of different categories of nuclear envelope conformation and chromatin configuration in different treatment groups. Proportions with different letters (spherical NE/reticular chromatin category: capital letters; irregular NE/abnormal chromatin category: lowercase letters) differ within the category (p < .05). Numbers on the top of the bars indicate a total number of oocytes in each treatment group. (C) Areas of nuclear envelope relative to the area of the in oocytes after different treatments. The dotted line represents 1:1 ratio in which the size of chromatin equals that of the nuclear envelope
Both H3K4me3 and H3K9me3 immunostaining colocalized with chromatin in all GVs. Staining could be observed in GVs containing either reticular or abnormal chromatin configurations (Figure 2a). The impact of desiccation and vitrification on the levels of epigenetic markers in GVs was similar. Relative fluorescent intensity of H3K4me3 was reduced (p < .05) to a comparable level in both dried/rehydrated and vitrified/warmed GVs (Figure 2b). A 1.2-fold increase of H3K9me3 was observed in desiccated GVs, but it was not significantly different (p > .05) from the control or vitrification groups (Figure 2c).
FIGURE 2.
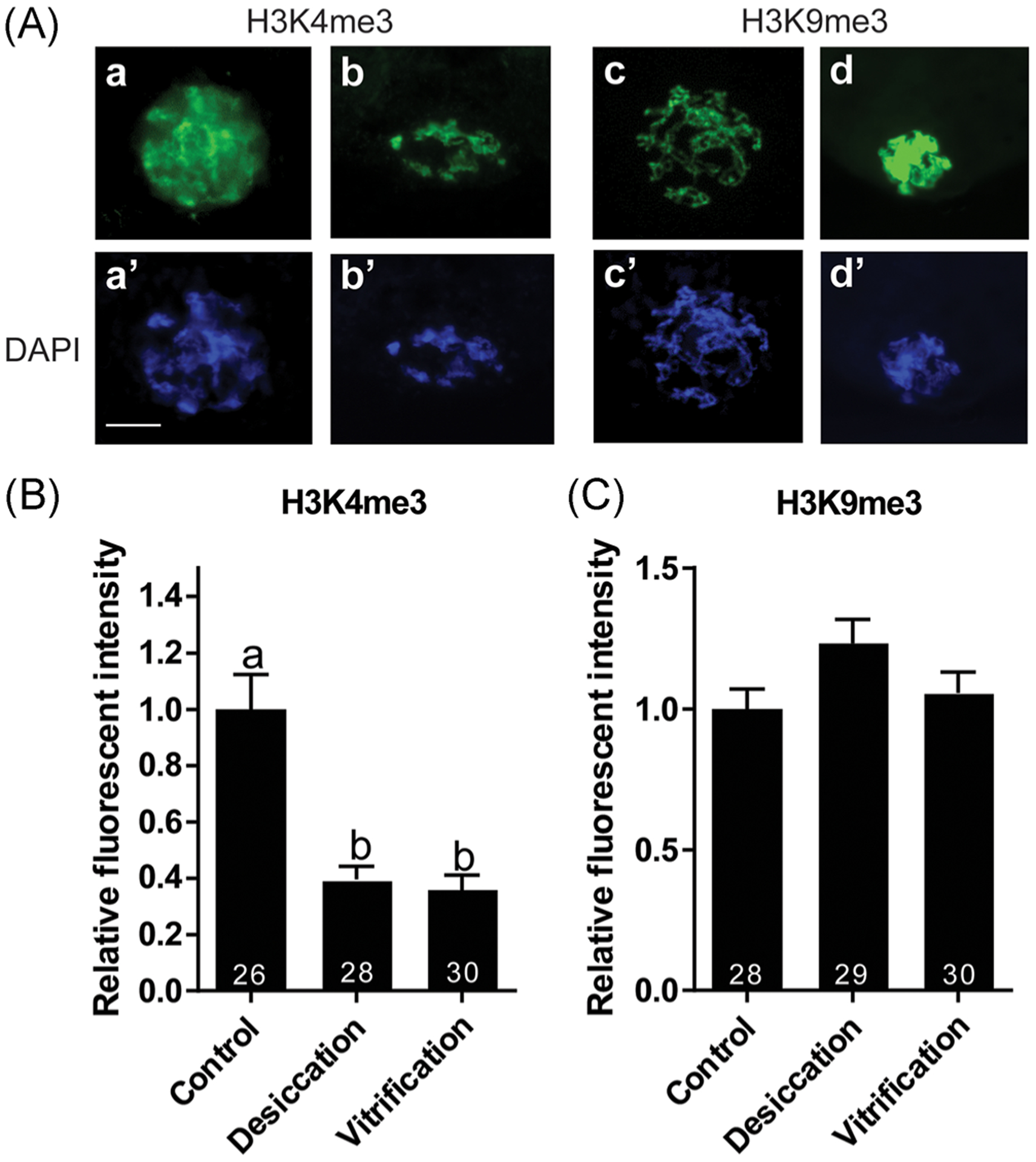
Relative fluorescent intensity of epigenetic markers in cat GVs following desiccation or vitrification. (A) Representatives of GVs immunostained with H3K4me3 (a, b) or H3K9me3 (c, d) antibodies. Corresponding DNA was counterstained with DAPI (a’–d’). Scale bar = 20 μm. (B, C) Influence of desiccation and vitrification on the intensity of (B) H3K4me3 and (C) H3K9me3. Mean fluorescent intensity of control, untreated GVs was set as 1. Values are mean ± SEM. Values with different letters differ (p < .05). Numbers at the bottom of the bars indicate total number of oocytes in each group. GV, germinal vesicle; SEM, standard error of the mean
2.2 |. Experiment 2: Influence of desiccation steps and storage temperatures on epigenetic patterns in GVs
Relative fluorescent intensity of H3K4me3 in the GV showed a modest decrease after hemolysin permeabilization and trehalose exposure compared to the fresh control (p > .05, Figure 3a). When rehydrated immediately after 30 min of drying, H3K4me3 intensity significantly decreased (p < .05) to 25% of the control level. It was partially recovered (40%) after storage at either 4°C or ambient temperature; however, it was still significantly lower than what was detected in the fresh control (p < .05, Figure 3a). Contrarily, H3K9me3 intensity tended to increase mildly 14–36%) throughout the desiccation and storage procedure, although not to a significant degree (p > .05, Figure 3b).
FIGURE 3.
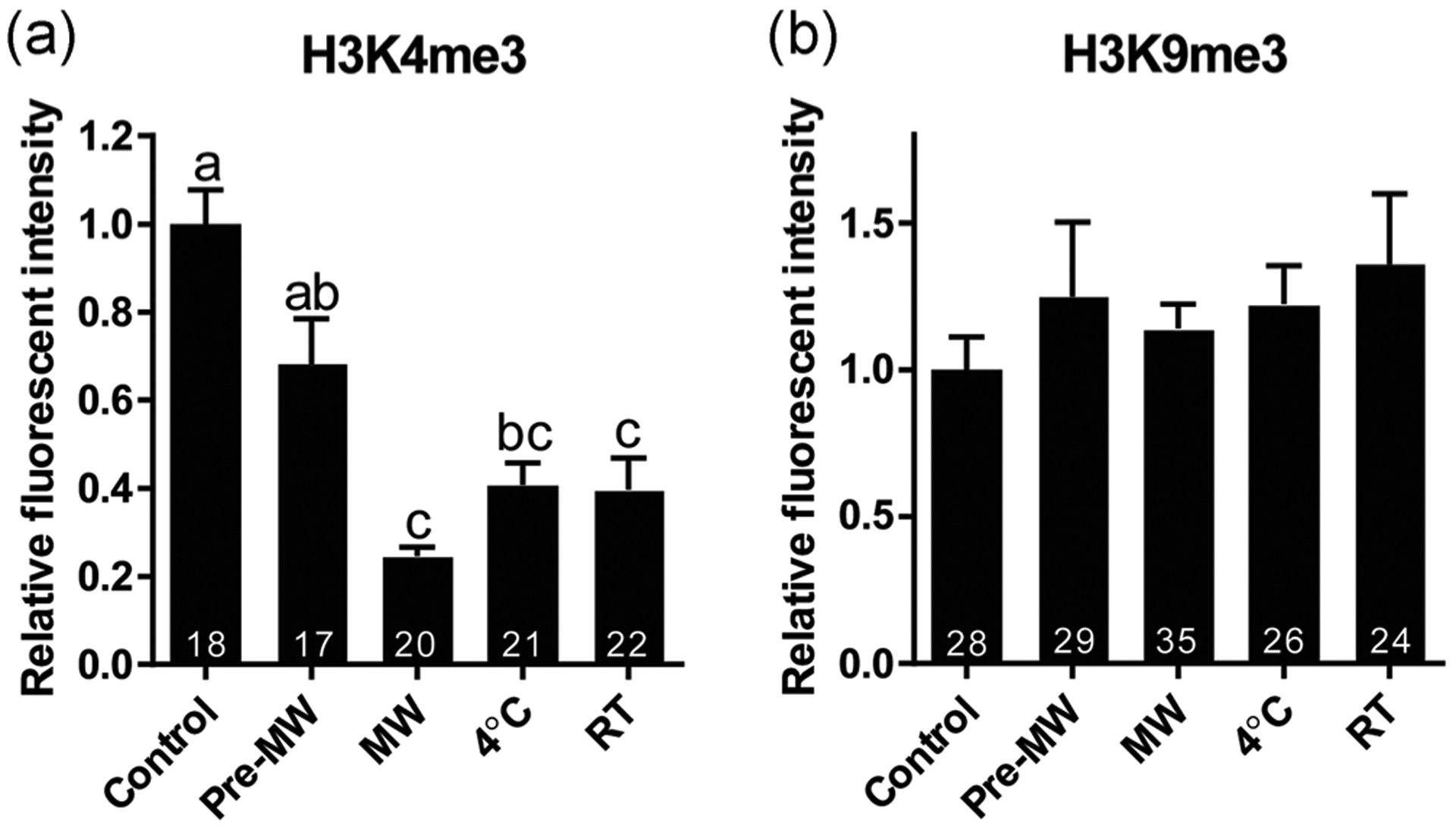
Influence of main steps of the desiccation procedure and storage temperatures on the relative fluorescent intensity of epigenetic markers (a) H3K4me3 and (B) H3K9me3 in cat GVs. Treatment groups were labeled as illustrated in Figure 1. Mean fluorescent intensity of fresh control GVs was set as 1. Values are mean ± SEM. Values with different letters differ (p < .05). Numbers at the bottom of the bars indicate a total number of oocytes in each group. MW, microwave drying; RT, room temperature; GV, germinal vesicle; SEM, standard error of the mean
2.3 |. Experiment 3: Influence of different rehydration methods on epigenetic patterns in GVs
Relative fluorescent intensity of H3K4me3 exhibited a 44–55% decrease (p < .05) in the dried/rehydrated GVs as shown earlier. There was no significant difference (p > .05) between direct and sequential rehydration methods (Figure 4a). Although H3K9me3 intensities were not significantly different from the fresh controls after direct and sequential rehydration, intensities were different between the two rehydration methods (p < .05, Figure 4b).
FIGURE 4.
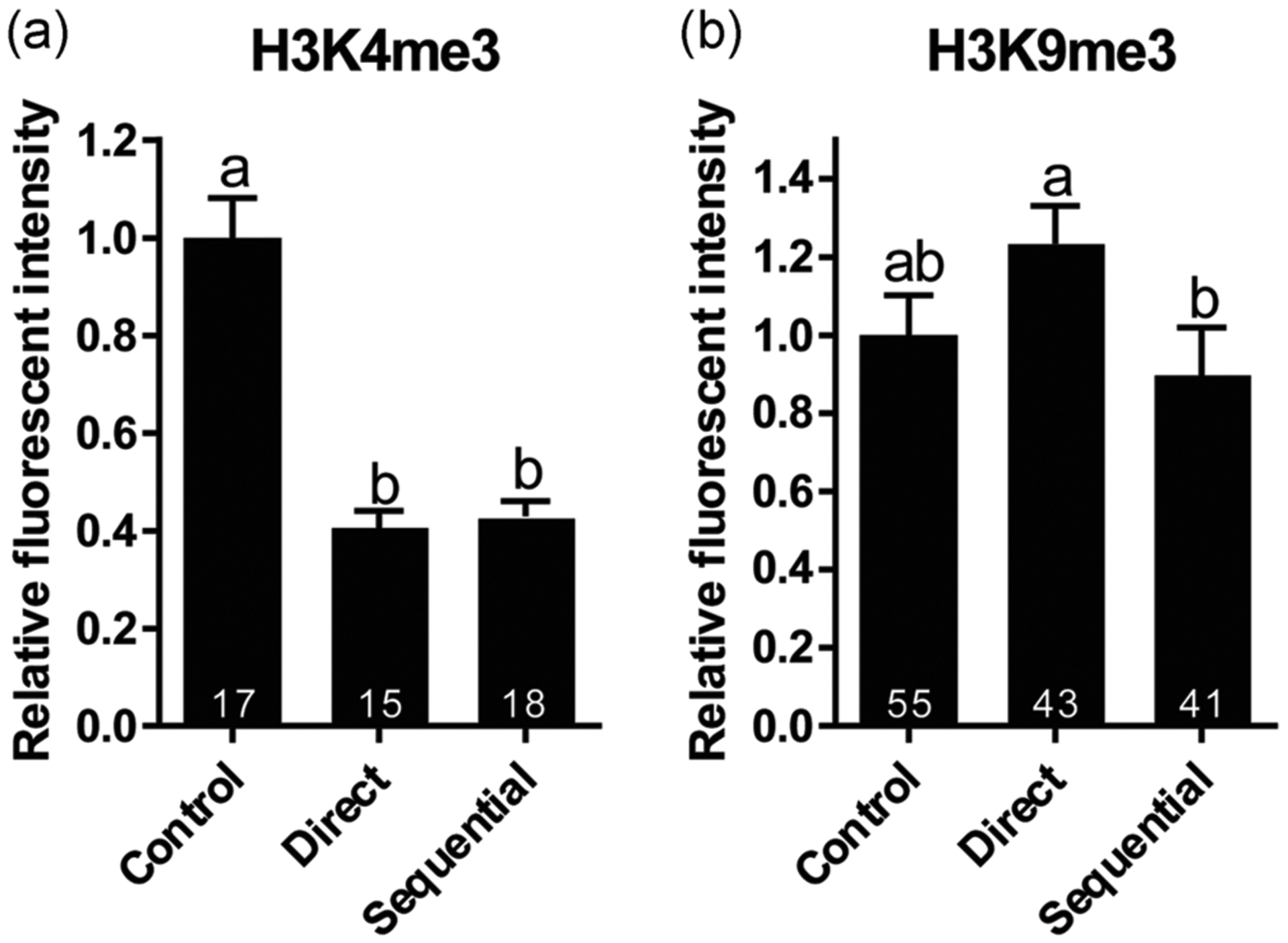
Influence of two rehydration protocols (direct versus sequential) on the relative fluorescent intensity of epigenetic markers (a) H3K4me3 and (b) H3K9me3 in cat GVs. Mean fluorescent intensity of fresh control GVs was set as 1. Values are mean ± SEM. Values with different letters differ (p < .05). Numbers at the bottom of the bars indicate a total number of oocytes in each group. GV, germinal vesicle; SEM, standard error of the mean
3 |. DISCUSSION
When developing gamete preservation methods, understanding cellular response to extreme conditions and maintaining the integrity of cellular components are essential to improve the safety of these techniques. In the present study, we examined the novel microwave-assisted dehydration method and demonstrated that it had a similar impact on the epigenetic status of cat GVs as current cryopreservation approach. Moreover, our data showed that this desiccation technique could better preserve nuclear envelope conformation and chromatin configuration compared to vitrification.
Nuclear envelope not only safeguards the maternal genome but also controls communication between nuclear and cytoplasmic components. Damage of the nuclear envelope may result in aberrant nucleocytoplasmic exchange and alter cellular functions (Robijns, Houthaeve, Braeckmans, & De Vos, 2018). Our results showed that a high proportion of GV oocytes contained irregular nuclear envelope after cryopreservation, ranging from partial shrunken to complete collapse. Similar results were also reported on vitrified bovine oocytes (Chaves et al., 2017). This is not unexpected because lipids are known to be prone to cryoinjuries (Amstislavsky et al., 2019). Although most studies focused on the impact of cryopreservation on cytoplasmic and mitochondrial membranes, nuclear envelope likely endures similar stress during freezing. Lipid membrane is also the prime target for desiccation damage. Lipids tend to merge when surrounding water molecules are removed as dehydration occurs. In our data, the proportion of irregular nuclear envelope was significantly lower in GVs undergone dry preservation than cryopreservation. This could be attributed to the use of trehalose as xeroprotectant, which increases desiccation tolerance and preserves membrane integrity (Chen et al., 2001; Tapia & Koshland, 2014).
In addition to protecting nuclear membrane integrity, desiccation approach also performed better at maintaining GV chromatin conformation than vitrification. Condensed chromatin observed in the cryopreserved samples has been described in previous reports (Falk et al., 2018; Mikołajewska et al., 2012; Royere, Hamamah, Nicolle, & Lansac, 1991). Although ice crystal formation is the usual culprit for cryoinjuries, several other factors during cryopreservation procedure could also impact DNA structure (Kopeika et al., 2015). For example, hyperosmotic stress might cause a change in chromatin compactness and DNA accessibility, and cell shrinkage could alter DNA rigidity and bending (Kültz & Chakravarty, 2001). Some cryoprotectants, such as dimethyl sulfoxide (DMSO) used in this study, have been linked to increased abnormal chromatin despite their function in mitigating freezing damages (Falk et al., 2018; Hu, Marchesi, Qiao, & Feng, 2012). During desiccation, DNA is also susceptible to hyperosmotic and dehydration stress (Palazzese, Gosálvez, Anzalone, Loi, & Saragusty, 2018; Shahba et al., 2016). Results in the present and previous studies indicate that current microwave-assisted dehydration protocol with the use of trehalose can effectively protect DNA integrity and chromatin conformation in the majority of GVs (Elliott et al., 2015).
Interestingly, we observed a strong correlation between the size of the nuclear envelope and chromatin-occupied area in all groups. When chromatin became more condensed, the nuclear envelope also tended to shrink. A similar phenomenon has also been reported in previous studies (Kratochvílová et al., 2018; Royere et al., 1991; Royere, Hamamah, Nicolle, Barthelemy, & Lansac, 1988). Nuclear lamins are known to interact with chromatin and are associated with chromatin organization (Dechat et al., 2008). It is logical to hypothesize that shrinkage of one component will influence the other, although we do not have direct evidence for a causal relation. A recent study analyzed this correlation with different protective agents (Kratochvílová et al., 2018). They demonstrated that DMSO induced higher order of chromatin condensation and nuclear envelope shrinkage in both nonfrozen and frozen/thawed fibroblasts (Kratochvílová et al., 2018), consistent with our observation. Intriguingly, cell viability was positively correlated with DMSO-mediated chromatin structure change yet negatively influenced by trehalose-induced condensation (Kratochvílová et al., 2018). Although the authors suggest that chromatin conformational alteration could be regulated to prevent DNA damage, it could also be a sign of cellular injury if occurred under environmental stress. Analysis at the molecular level may provide more clues to distinguish the difference. It has been reported that many membrane and nuclear proteins were downregulated in cryopreserved oocytes (Turathum et al., 2018). Similarly, mRNA profiling has revealed that oocyte freezing led to decreased expression of genes involved in chromosomal structure maintenance (Monzo et al., 2012). It will be interesting to investigate the effect of different protectants as well as dry preservation on nuclear gene expression.
If chromatin condensation occurred in a controlled manner, it is likely regulated through epigenetic modifications. After examining both active and repressive epigenetic markers, we observed a similar response in desiccated and vitrified oocytes. H3K4me3 decreased to the same extent in both groups and H3K9me3 remained unaffected. It appeared that the chromatin condensation did not correspond to the epigenetic changes assessed in this study. This is not the only case in which H3K4me3 is shown to be sensitive to environmental perturbation incurred in assisted reproductive technologies. Previous studies demonstrated that a lower level of H3K4me3 in embryos produced in vitro compared to in vivo (Wu et al., 2012). Additionally, H3K4 trimethylation is found to be responsive to other external stress factors in different cell types and organisms (W. Ding et al., 2018; Ding, Fromm, & Avramova, 2012; Hunter, McCarthy, Milne, Pfaff, & McEwen, 2009; Ma et al., 2018; Saunderson et al., 2016; Sharma, Kohli, & Brahmachari, 2017; Widiez et al., 2014; Xu, Wang et al., 2017; Zhang, Schroeder, Fong, & Bentley, 2005). Of particular relevance is desiccation stress in fruit flies and plants, in which H3K4me3 was indicated as a memory marker for stress-induced genes in response to droughts (Bedi & Nag Chaudhuri, 2018; Y. Ding et al., 2012; Liu, Fromm, & Avramova, 2014; Sharma et al., 2017). However, this may not be the case for the dried cat oocytes because we observed decreased instead of increased accumulation of H3K4me3 after dehydration.
To identify the source of stress that induced epigenetic changes observed in desiccated GVs, we examined relative fluorescent intensities of epigenetic markers at different steps of the procedure. Neither H3K4me3 nor H3K9me3 was significantly affected by hemolysin and trehalose exposure, consistent with previous studies that illustrated high osmotic tolerance of GV oocytes in cat (Comizzoli et al., 2008). While H3K9me3 remained mostly unchanged, loss of H3K4me3 predominantly occurred after microwave drying and rehydration. This indicated that H3K4me3 was more sensitive than H3K9me3 to the desiccation stress. Encouragingly, the H3K4me3 level was able to partially recover from the initial stress response and stabilized during storage. There was no difference between GVs stored at 4°C and room temperature, consistent with our previous studies that GVs could reach a low water content compatible for storage at either temperature (Elliott et al., 2015). Results also demonstrated that the low level of H3K4me3 was not mitigated by using a sequential rehydration method. This suggested that the decrease might not have resulted from imbibition damage during rehydration and trehalose removal (Crowe, Crowe, Hoekstra, & Wistrom, 1989; Wolkers, Walker, Tablin, & Crowe, 2001). Interestingly, the difference in relative fluorescent intensity of H3K9me3 was observed between dried GVs rehydrated directly and sequentially, which might be a result of differential local histone methylation events. It is important to point out that even when the observed global histone methylation levels were equal, we could not exclude the possibility that local differences may exist. Higher-resolution examination will be required in the future to analyze the local chromatin occupancy of modified histones.
What influence does lower H3K4me3 have on the oocyte? In murine oocytes, decreased H3K4me3 level has been linked to misexpression of genes essential for meiotic resumption, leading to failure to maturation (Xu, Chen et al., 2017; C. Yu et al., 2017). Consistent with its role in promoting oocyte maturation, increased H3K4me3 was associated with improving meiotic and developmental competence of porcine oocytes (X. X. Yu et al., 2018). In contrast, a steady decrease of H3K4me3 level was observed as human oocytes progress from GV to MII stage (A. Zhang et al., 2012). Close examination on the distribution of H3K4me3 revealed dynamic reprogramming of epigenome in mouse oocyte and zygotes. H3K4me3 occupied broader, noncanonical domains in both GV oocytes and mature oocytes. In the preimplantation embryos, H3K4me3 marks become restricted to transcription start site regions. Such confinement of H3K4me3 is critical for normal zygotic gene activation (Dahl et al., 2016; Stewart et al., 2015; Zhang, Zheng et al., 2016). More studies are needed to understand alteration in H3K4me3 distribution in dry-preserved oocytes and its effect on oocyte function and development.
The ultimate goal of GV preservation is to generate oocytes that can fully support normal early embryogenesis by transferring GVs to fresh ooplasts. It has been demonstrated that oocytes reconstituted with vitrified GVs (using the same vitrification protocol in the present study) could mature, be fertilized, and develop into blastocysts (Comizzoli et al., 2009; Comizzoli et al., 2011), despite the altered nuclear structure and epigenetic modifications. We also have demonstrated that functional oocytes can be reconstructed with desiccated GVs (Graves-Herring et al., 2013). Intriguingly, a recent report finds that removing H3K4 methylation in donor cells improved the development of nuclear transfer embryos (Hörmanseder et al., 2017). This suggests that under certain circumstances, changes in epigenetic modifications could even be beneficial. Ongoing research is focusing on the functionality of dry-preserved GVs and any lasting epigenetic influence on the resulting embryos.
In conclusion, the incidence of GV damages is lower after desiccation/rehydration than vitrification/warming. This essential finding will help us to further explore underlying molecular protection toward cellular or tissue integrity following exposure to extreme conditions.
4 |. MATERIALS AND METHODS
4.1 |. Oocyte collection
All methods were carried out in accordance with relevant guidelines and regulations. The study did not require the approval of the Animal Care and Use Committee of the Smithsonian Conservation Biology Institute because cat ovaries were collected at local veterinary clinics as byproducts from owner-requested routine ovariohysterectomies. Collected adult cat ovaries were then transported at 4°C to the laboratory. Cumulus-oocyte complexes (COCs) were collected from antral follicles by repeatedly slicing the ovaries in HEPES-buffered minimum essential medium (H-MEM; Gibco Laboratories, Grand Island, NY) supplemented with 1 mM pyruvate, 2 mM L-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 4 mg/ml bovine serum albumin (Sigma–Aldrich, St. Louis, MO) within 24 hr of excision. Each COC from antral follicles was classified according to the standard quality criteria (Wood and Wildt, 1997). Only Grade 1 (uniformly dark cytoplasm, ≥5 compact layers of cumulus cells) and Grade 2 (same as Grade 1, but with <5 cell layers) oocytes were collected. The oocytes were then denuded of cumulus cells by exposure to 0.2% hyaluronidase (Sigma–Aldrich) for 5 min at 38°C followed by pipetting. Denuded oocytes were rinsed with H-MEM before undergoing additional treatments. While GVs were the main preservation targets in this study, we kept them enclosed in the zona pellucida in the following procedures for the ease of manipulations.
4.2 |. Microwave-assisted dehydration, storage, and rehydration
Microwave drying was performed following a previously described procedure (Elliott et al., 2015) with slight modification. Denuded oocytes were permeabilized with 10 μg/ml hemolysin (Sigma–Aldrich) for 15 min before exposure to 1.5 M trehalose in Tris-EDTA buffer for 10 min. Up to six oocytes along with 40 ul of trehalose were transferred onto each conjugate-release filter and dehydrated with a SAM 255 microwave system (CEM, Matthews, NC) for 30 min at 20% power with the upper temperature threshold set at 40°C. For storage, filters were individually sealed in Dri-shield moisture barrier bags (3M, St. Paul, MN) and stored at 4°C or room temperature at a relative humidity around 30%. To rehydrate, filters were immersed in rehydration medium (H-MEM medium supplemented with pyruvate, l-glutamine, penicillin, and streptomycin) at room temperature. Oocytes were allowed to recover in rehydration medium for at least 30 min at 38°C before fixation for further assessment.
4.3 |. Oocyte vitrification and warming
Oocyte vitrification was performed as described previously (Comizzoli, Wildt, & Pukazhenthi, 2009). Denuded oocytes were first equilibrated for 30 s in a solution containing 10% ethylene glycol (EG) and 10% DMSO in phosphate-buffered saline (PBS) followed by a 20-s exposure to vitrification solution containing 20% EG, 20% DMSO, and 1 M sucrose at room temperature. After exposure, oocytes were deposited in droplets of minimal volume onto a plastic gutter (up to four oocytes per gutter) made with a 0.25 ml semen straw and then plunged directly into liquid nitrogen. For warming, vitrified oocytes were sequentially exposed to 2, 1, and 0.5 M sucrose in H-MEM for 2 min each at room temperature and rested in H-MEM for 30 min at 38°C before fixation.
4.4 |. Immunofluorescent staining
Oocytes were fixed in 4% paraformaldehyde in PBS (USB Corporation, Cleveland, OH) for 30 min at 38°C or for overnight at 4°C. After rinsing with wash solution (PBS with 2% fetal bovine serum and 0.5% Triton X-100) and blocking with saturation solution (PBS with 20% fetal bovine serum and 0.5% Triton X-100) for 30 min at 38°C, oocytes were incubated with primary antibodies in saturation solution for overnight at 4°C. Primary antibodies used in this study were antihistone H3 tri-methyl K4 (Abcam, Cambridge, MA) at 1:100 dilution, antihistone H3 tri-methyl K9 (Abcam) at 1:400, and Lamin A/C antibody (Santa Cruz Biotechnology, Dallas, TX) at 1:50. A negative control using rabbit or mouse IgG was included in each trial. After three washes with wash solution, oocytes were incubated with secondary antibodies tagged with either fluorescein isothiocyanate or Texas red for 1 hr at 38°C. Excess antibodies were washed away by three additional washes before mounting with Vectashield mounting medium (Vector Laboratories, Burlingame, CA) containing 4′,6-diamidino-2-phenylindole (DAPI) for DNA visualization. Resulting images (×400) were obtained using an Olympus BX41 epifluorescence microscope (Olympus Corporation, Center Valley, PA) with SPOT advanced software 5.0 (Diagnostic Instruments, Inc., Sterling Heights, MI). Images of oocytes from the same cohort under different treatments were taken under the same configuration to allow comparisons. Total fluorescent intensity within a GV as well as chromatin and nuclear envelope enclosed areas were measured using the ImageJ software (National Institutes of Health, Bethesda, MD). Background fluorescent signal from the cytoplasm was subtracted before calculating total intensity. Fluorescent intensity was normalized with DAPI intensity in each GV. Nuclear envelopes with a spherical shape, with or without wrinkles, were considered normal; otherwise, they were classified as irregular. Fresh oocytes from antral follicles contained GV chromatin with reticular conformation as previously described (Comizzoli et al., 2011). All other conformations were considered abnormal.
4.5 |. Experimental design and statistical analysis
Oocytes selected from a pool of ovaries collected on a given day were considered as one replicate. For each of the three described experiments, oocytes were randomly allocated to different treatment groups, including for controls.
Experiment 1 compared the impact of desiccation (microwave-assisted dehydration) versus vitrification (cryopreservation) on nuclear envelope conformations, chromatin configuration, and epigenetic pattern. A portion of untreated oocytes was fixed as controls. The rest of the oocytes was separated into two groups. One group was vitrified and stored in liquid nitrogen, whereas the other group was dehydrated and kept at 4°C for up to 7 days. Oocytes were either warmed or directly rehydrated after storage for evaluation by immunostaining (Figure 5a). The total number of oocytes was 135 in four replicates for Lamin A/C and chromatin analysis. The total numbers were 84 and 87 in three replicates for H3K4me3 and H3K9me3 staining, respectively. Relative humidity during the course of the experiment ranged from 4.0% to 42.0%, and room temperature ranged from 19.3 to 26.3°C.
FIGURE 5.
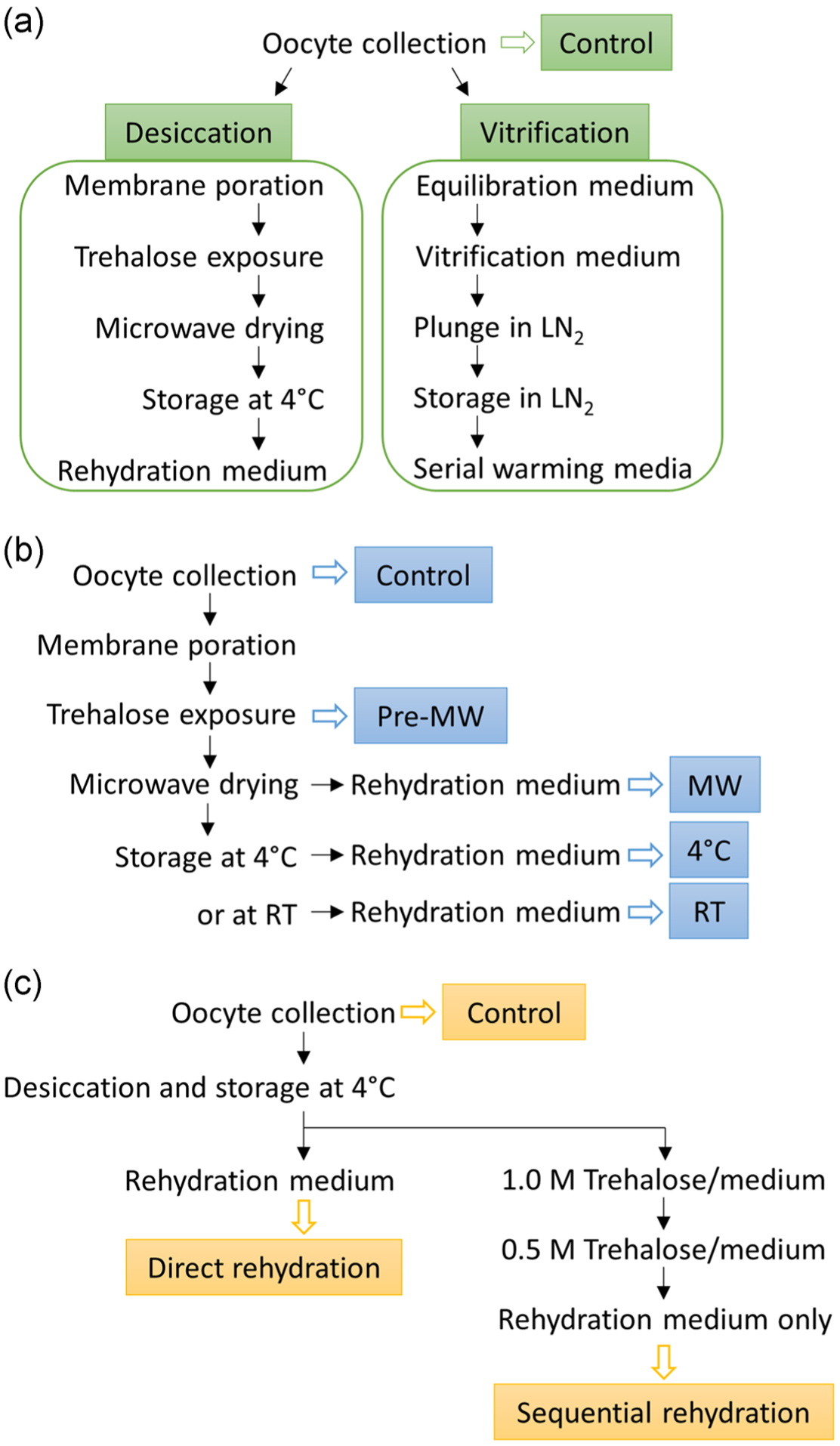
Flow charts for (a) Experiment 1, (b) Experiment 2, and (c) Experiment 3. Open arrows indicate samples collected at the end of the procedure and boxed texts indicate the names of the treatment groups. MW, microwave drying. RT, room temperature
Experiment 2 examined relative fluorescent intensities of H3K4me3 and H3K9me3 after different steps of microwave-assisted dehydration and storage temperatures. Oocytes were randomly allocated to different treatment groups. Oocytes were either fixed after (a) collection and denuding; (b) hemolysin and trehalose treatment; (c) rehydration immediately after 30 min microwave drying; (d) rehydration after storage at 4°C; or (e) rehydration after storage at room temperature for up to 7 days (Figure 5b). A total number of 98 and 142 oocytes in three replicates was stained for H3K4me3 and H3K9me3, respectively. Relative humidity during the course of the experiment ranged from 21.1% to 69%, and room temperature ranged from 20.8 to 28.9°C.
Experiment 3 explored the influence of two different rehydration methods on H3K4me3 and H3K9me3 relative fluorescent intensities. Oocytes were randomly allocated to the different treatment groups. A portion of untreated oocytes was fixed as controls while the rest was dehydrated and stored at 4°C for up to 7 days. Dried oocytes then were separated into two groups. One group was directly rehydrated by adding rehydration medium onto the oocytes and allowing recovery for 30 min. The other underwent sequential rehydration by exposing to 1.0 M trehalose in MEM for 10 min, 0.5 M trehalose in MEM for 10 min, and lastly rehydration medium for 30 min (Figure 5c). A total number of 50 and 118 oocytes in three replicates was stained for H3K4me3 and H3K9me3, respectively. Relative humidity during the course of the experiment ranged from 3.7% to 69%, and room temperature ranged from 20.8 to 25.4°C.
Differences in nuclear envelope conformations and chromatin configurations were analyzed by χ2 testing. Chromatin and nuclear envelope areas, as well as H3K4me3 and H3K9me3 data, did not pass normality test (D’Agostino-Pearson omnibus normality test) and therefore were evaluated by the Kruskal–Wallis test followed by post-hoc Dunn’s analysis. Differences were considered significant at p < .05 (GraphPad Prism 6, San Diego, CA).
ACKNOWLEDGMENTS
We thank Dr. Brent Whitaker (Animal Rescue Inc.), Dr. Joy Lewis (Spay Now Animal Surgery Clinic), and Dr. Keiko Antoku, and their staff for providing domestic cat ovaries. The research reported in this publication was supported by the Office of The Director, National Institutes of Health under Award Number R01OD023139. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- Amstislavsky S, Mokrousova V, Brusentsev E, Okotrub K, & Comizzoli P (2019). Influence of cellular lipids on cryopreservation of mammalian oocytes and preimplantation embryos: A review. Biopreservation and Biobanking, 17(1), 76–83. 10.1089/bio.2018.0039 [DOI] [PubMed] [Google Scholar]
- Bedi S, & Nag Chaudhuri R (2018). Transcription factor ABI3 auto-activates its own expression during dehydration stress response. FEBS Letters, 592(15), 2594–2611. 10.1002/1873-3468.13194 [DOI] [PubMed] [Google Scholar]
- Cellemme SL, Van Vorst M, Paramore E, & Elliott GD (2013). Advancing microwave technology for dehydration processing of biologics. Biopreservation and Biobanking, 11(5), 278–284. 10.1089/bio.2013.0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves DF, Corbin E, Almiñana C, Locatelli Y, Souza-Fabjan JMG, Bhat MH, … Mermillod P (2017). Vitrification of immature and in vitro matured bovine cumulus-oocyte complexes: Effects on oocyte structure and embryo development. Livestock Science, 199, 50–56. 10.1016/j.livsci.2017.02.022 [DOI] [Google Scholar]
- Chen T, Acker JP, Eroglu A, Cheley S, Bayley H, Fowler A, & Toner M (2001). Beneficial effect of intracellular trehalose on the membrane integrity of dried mammalian cells. Cryobiology, 43(2), 168–181. 10.1006/cryo.2001.2360 [DOI] [PubMed] [Google Scholar]
- Comizzoli P, Pukazhenthi BS, & Wildt DE (2011). The competence of germinal vesicle oocytes is unrelated to nuclear chromatin configuration and strictly depends on cytoplasmic quantity and quality in the cat model. Human Reproduction, 26(8), 2165–2177. 10.1093/humrep/der176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comizzoli P, Songsasen N, & Wildt DE (2010). Protecting and extending fertility for females of wild and endangered mammals In Woodruff TK, Zoloth L, Campo-Engelstein L, & Rodriguez S (Eds.), Oncofertility, Cancer Treatment and Research (156, pp. 87–100). New York, NY: Springer Science and Business Media. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comizzoli P, Wildt D, & Pukazhenthi B (2009). In vitro compaction of germinal vesicle chromatin is beneficial to survival of vitrified cat oocytes. Reproduction in Domestic Animals, 44(Suppl2), 269–274. 10.1111/j.1439-0531.2009.01372.x [DOI] [PubMed] [Google Scholar]
- Comizzoli P, Wildt DE, & Pukazhenthi BS (2008). Impact of anisosmotic conditions on structural and functional integrity of cumulus-oocyte complexes at the germinal vesicle stage in the domestic cat. Molecular Reproduction and Development, 75(2), 345–354. 10.1002/mrd.20769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe JH, Crowe LM, Hoekstra FA, & Wistrom CA (1989). Effects of water on the stability of phospholipid bilayers: The problem of imbibition damage in dry organisms. Crop Science, 14, 1–14. [Google Scholar]
- Dahl JA, Jung I, Aanes H, Greggains GD, Manaf A, Lerdrup M, … Klungland A (2016). Broad histone H3K4me3 domains in mouse oocytes modulate maternal-to-zygotic transition. Nature, 537(7621), 548–552. 10.1038/nature19360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang-Nguyen TQ, Nguyen HT, Nguyen MT, Somfai T, Noguchi J, Kaneko H, & Kikuchi K (2018). Maturation ability after transfer of freeze-dried germinal vesicles from porcine oocytes. Animal Science Journal, 89, 1253–1260. 10.1111/asj.13067 [DOI] [PubMed] [Google Scholar]
- Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, & Goldman RD (2008). Nuclear lamins: Major factors in the structural organization and function of the nucleus and chromatin. Genes & Development, 22(7), 832–853. 10.1101/gad.1652708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Fromm M, & Avramova Z (2012). Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis. Nature Communications, 3, 740 10.1038/ncomms1732 [DOI] [PubMed] [Google Scholar]
- Ding W, Higgins DP, Yadav DK, Godbole AA, Pukkila-Worley R, & Walker AK (2018). Stress-responsive and metabolic gene regulation are altered in low S-adenosylmethionine. PLoS Genetics, 14(11), e1007812 10.1371/journal.pgen.1007812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott GD, Lee PC, Paramore E, Van Vorst M, & Comizzoli P (2015). Resilience of oocyte germinal vesicles to microwave-assisted drying in the domestic cat model. Biopreservation and Biobanking, 13(3), 164–171. 10.1089/bio.2014.0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Naito K, Aoki F, Kume S, & Tojo H (2005). Changes in histone modifications during in vitro maturation of porcine oocytes. Molecular Reproduction and Development, 71(1), 123–128. 10.1002/mrd.20288 [DOI] [PubMed] [Google Scholar]
- Eymery A, Liu Z, Ozonov EA, Stadler MB, & Peters AHFM (2016). The methyltransferase Setdb1 is essential for meiosis and mitosis in mouse oocytes and early embryos. Development, 143(15), 2767–2779. 10.1242/dev.132746 [DOI] [PubMed] [Google Scholar]
- Falk M, Falková I, Kopečná O, Bačíková A, Pagáčová E, Šimek D, … Kratochvílová I (2018). Chromatin architecture changes and DNA replication fork collapse are critical features in cryopreserved cells that are differentially controlled by cryoprotectants. Scientific Reports, 8(1), 14694 10.1038/s41598-018-32939-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan M, Pribyl J, Pesl M, Jelinkova S, Acimovic I, Jaros J, … Kratochvilova I (2018). Cryopreserved cells regeneration monitored by atomic force microscopy and correlated with state of cytoskeleton and nuclear membrane. IEEE Transactions On Nanobioscience, 17(4), 485–497. 10.1109/TNB.2018.2873425 [DOI] [PubMed] [Google Scholar]
- Graves-Herring JE, Wildt DE, & Comizzoli P (2013). Retention of structure and function of the cat germinal vesicle after air-drying and storage at suprazero temperature. Biology of Reproduction, 88(6), 139 10.1095/biolreprod.113.108472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt WV (2013). Who needs cytoplasm? Genomic preservation for the 21st century. Biology of Reproduction, 88(6), 140 10.1095/biolreprod.113.109959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Marchesi D, Qiao J, & Feng HL (2012). Effect of slow freeze versus vitrification on the oocyte: An animal model. Fertility and Sterility, 98(3), 752–760. 10.1016/j.fertnstert.2012.05.037.e753 [DOI] [PubMed] [Google Scholar]
- Hunter RG, McCarthy KJ, Milne TA, Pfaff DW, & McEwen BS (2009). Regulation of hippocampal H3 histone methylation by acute and chronic stress. Proceedings of the National Academy of Sciences, 106(49), 20912–20917. 10.1073/pnas.0911143106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörmanseder E, Simeone A, Allen GE, Bradshaw CR, Figlmüller M, Gurdon J, & Jullien J (2017). H3K4 methylation-dependent memory of somatic cell identity inhibits reprogramming and development of nuclear transfer embryos. Cell Stem Cell, 21(1), 135–143.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Wang Y, Lin J, Xu J, Ding G, & Huang H (2017). Genetic and epigenetic risks of assisted reproduction. Best Practice & Research Clinical Obstetrics & Gynaecology, 44, 90–104. 10.1016/j.bpobgyn.2017.07.004 [DOI] [PubMed] [Google Scholar]
- Kageyama S, Liu H, Kaneko N, Ooga M, Nagata M, & Aoki F (2007). Alterations in epigenetic modifications during oocyte growth in mice. Reproduction, 133(1), 85–94. 10.1530/REP-06-0025 [DOI] [PubMed] [Google Scholar]
- Kopeika J, Thornhill A, & Khalaf Y (2015). The effect of cryopreservation on the genome of gametes and embryos: Principles of cryobiology and critical appraisal of the evidence. Human Reproduction Update, 21(2), 209–227. 10.1093/humupd/dmu063 [DOI] [PubMed] [Google Scholar]
- Kratochvílová I, Kopečná O, Bačíková A, Pagáčová E, Falková I, Follett SE, … Falk M (2018). Changes in cryopreserved cell nuclei serve as indicators of processes during freezing and thawing. Langmuir, 35, 7496–7508. 10.1021/acs.langmuir.8b02742 [DOI] [PubMed] [Google Scholar]
- Kültz D, & Chakravarty D (2001). Maintenance of genomic integrity in mammalian kidney cells exposed to hyperosmotic stress. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 130(3), 421–428. [DOI] [PubMed] [Google Scholar]
- Liu N, Fromm M, & Avramova Z (2014). H3K27me3 and H3K4me3 chromatin environment at super-induced dehydration stress memory genes of Arabidopsis thaliana. Molecular Plant, 7(3), 502–513. 10.1093/mp/ssu001 [DOI] [PubMed] [Google Scholar]
- Ma C, Niu R, Huang T, Shao LW, Peng Y, Ding W, … Liu Y (2018). N6-methyldeoxyadenine is a transgenerational epigenetic signal for mitochondrial stress adaptation. Nature Cell Biology, 21, 319–327. 10.1038/s41556-018-0238-5 [DOI] [PubMed] [Google Scholar]
- Mikołajewska N, Müller K, Niżański W, & Jewgenow K (2012). Vitrification of domestic cat oocytes--effect on viability and integrity of subcellular structures. Reproduction in Domestic Animals, 47(Suppl 6), 295–299. 10.1111/rda.12044 [DOI] [PubMed] [Google Scholar]
- Monzo C, Haouzi D, Roman K, Assou S, Dechaud H, & Hamamah S (2012). Slow freezing and vitrification differentially modify the gene expression profile of human metaphase II oocytes. Human Reproduction, 27(7), 2160–2168. 10.1093/humrep/des153 [DOI] [PubMed] [Google Scholar]
- Palazzese L, Gosálvez J, Anzalone DA, Loi P, & Saragusty J (2018). DNA fragmentation in epididymal freeze-dried ram spermatozoa impairs embryo development. Journal of Reproduction and Development, 64(5), 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips T, Wildt D, & Comizzoli P (2012). Increase in histone methylation in the cat germinal vesicle related to acquisition of meiotic and developmental competence. Reproduction in Domestic Animals, 47(Suppl 6), 210–214. 10.1111/rda.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robijns J, Houthaeve G, Braeckmans K, & De Vos WH (2018). Loss of nuclear envelope integrity in aging and disease. International Review of Cell and Molecular Biology, 336, 205–222. 10.1016/bs.ircmb.2017.07.013 [DOI] [PubMed] [Google Scholar]
- Royere D, Hamamah S, Nicolle JC, Barthelemy C, & Lansac J (1988). Freezing and thawing alter chromatin stability of ejaculated human spermatozoa: Fluorescence acridine orange staining and Feulgen-DNA cytophotometric studies. Gamete Research, 21(1), 51–57. 10.1002/mrd.1120210107 [DOI] [PubMed] [Google Scholar]
- Royere D, Hamamah S, Nicolle JC, & Lansac J (1991). Chromatin alterations induced by freeze-thawing influence the fertilizing ability of human sperm. International Journal of Andrology, 14(5), 328–332. [DOI] [PubMed] [Google Scholar]
- Russo V, Bernabò N, Di Giacinto O, Martelli A, Mauro A, Berardinelli P, … Barboni B (2013). H3K9 trimethylation precedes DNA methylation during sheep oogenesis: HDAC1, SUV39H1, G9a, HP1, and Dnmts are involved in these epigenetic events. Journal of Histochemistry and Cytochemistry, 61(1), 75–89. 10.1369/0022155412463923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunderson EA, Spiers H, Mifsud KR, Gutierrez-Mecinas M, Trollope AF, Shaikh A, … Reul JMHM (2016). Stress-induced gene expression and behavior are controlled by DNA methylation and methyl donor availability in the dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America, 113(17), 4830–4835. 10.1073/pnas.1524857113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahba MI, El-Sheshtawy RI, El-Azab A-SI, Abdel-Ghaffar AE, Ziada MS, & Zaky AA (2016). The effect of freeze-drying media and storage temperature on ultrastructure and DNA of freeze-dried buffalo bull spermatozoa. Asian Pacific Journal of Reproduction, 5(6), 524–535. 10.1016/j.apjr.2016.11.002 [DOI] [Google Scholar]
- Sharma V, Kohli S, & Brahmachari V (2017). Correlation between desiccation stress response and epigenetic modifications of genes in Drosophila melanogaster: An example of environment-epigenome interaction. Biochimica et Biophysica Acta. Gene Regulatory Mechanisms, 1860(10), 1058–1068. 10.1016/j.bbagrm.2017.08.001 [DOI] [PubMed] [Google Scholar]
- Songsasen N, & Comizzoli P (2019). Protecting and extending fertility for females of wild and endangered mammals In Woodruff TK, Shah DK, & Vitek WS (Eds.), Textbook of oncofertility research and practice (1 ed, pp. 401–412). Cham: Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinaci M, Vallorani C, Bucci D, Tamanini C, Porcu E, & Galeati G (2012). Vitrification of pig oocytes induces changes in histone H4 acetylation and histone H3 lysine 9 methylation (H3K9). Veterinary Research Communications, 36(3), 165–171. 10.1007/s11259-012-9527-9 [DOI] [PubMed] [Google Scholar]
- Stewart KR, Veselovska L, Kim J, Huang J, Saadeh H, Tomizawa S, … Kelsey G (2015). Dynamic changes in histone modifications precede de novo DNA methylation in oocytes. Genes and Development, 29(23), 2449–2462. 10.1101/gad.271353.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JH, Wang HL, Sun XS, Liu Y, Sui HS, & Zhang J (2009). Chromatin configurations in the germinal vesicle of mammalian oocytes. Molecular Human Reproduction, 15(1), 1–9. 10.1093/molehr/gan069 [DOI] [PubMed] [Google Scholar]
- Tapia H, & Koshland DE (2014). Trehalose is a versatile and long-lived chaperone for desiccation tolerance. Current Biology, 24(23), 2758–2766. 10.1016/j.cub.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Turathum B, Roytrakul S, Changsangfa C, Sroyraya M, Tanasawet S, Kitiyanant Y, & Saikhun K (2018). Missing and overexpressing proteins in domestic cat oocytes following vitrification and in vitro maturation as revealed by proteomic analysis. Biological Research, 51(1), 27 10.1186/s40659-018-0176-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widiez T, Symeonidi A, Luo C, Lam E, Lawton M, & Rensing SA (2014). The chromatin landscape of the moss Physcomitrella patens and its dynamics during development and drought stress. The Plant Journal, 79(1), 67–81. 10.1111/tpj.12542 [DOI] [PubMed] [Google Scholar]
- Wolkers WF, Walker NJ, Tablin F, & Crowe JH (2001). Human platelets loaded with trehalose survive freeze-drying. Cryobiology, 42(2), 79–87. 10.1006/cryo.2001.2306 [DOI] [PubMed] [Google Scholar]
- Wood TC, & Wildt DE (1997). Effect of the quality of the cumulus- oocyte complex in the domestic cat on the ability of oocytes to mature, fertilize and develop into blastocysts in vitro. Journal of reproduction and fertility, 110, 355–360. [DOI] [PubMed] [Google Scholar]
- Wu FR, Liu Y, Shang MB, Yang XX, Ding B, Gao JG, … Li WY (2012). Differences in H3K4 trimethylation in in vivo and in vitro fertilization mouse preimplantation embryos. Genetics and Molecular Research, 11(2), 1099–1108. 10.4238/2012.April.27.9 [DOI] [PubMed] [Google Scholar]
- Xu K, Chen X, Yang H, Xu Y, He Y, Wang C, … Gao S (2017). Maternal Sall4 is indispensable for epigenetic maturation of mouse oocytes. Journal of Biological Chemistry, 292(5), 1798–1807. 10.1074/jbc.M116.767061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wang Q, Freeling M, Zhang X, Xu Y, Mao Y, … Lu Y (2017). Natural antisense transcripts are significantly involved in regulation of drought stress in maize. Nucleic Acids Research, 45(9), 5126–5141. 10.1093/nar/gkx085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L-Y, Yan J, Qiao J, Zhao P-L, & Liu P (2010). Effects of oocyte vitrification on histone modifications. Reproduction, Fertility, and Development, 22(6), 920–925. [DOI] [PubMed] [Google Scholar]
- Yu C, Fan X, Sha QQ, Wang HH, Li BT, Dai XX, … Fan HY (2017). CFP1 regulates histone H3K4 trimethylation and developmental potential in mouse oocytes. Cell Reports, 20(5), 1161–1172. 10.1016/j.celrep.2017.07.011 [DOI] [PubMed] [Google Scholar]
- Yu XX, Liu YH, Liu XM, Wang PC, Liu S, Miao JK, … Yang CX (2018). Ascorbic acid induces global epigenetic reprogramming to promote meiotic maturation and developmental competence of porcine oocytes. Scientific Reports, 8(1), 6132 10.1038/s41598-018-24395-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Schroeder S, Fong N, & Bentley DL (2005). Altered nucleosome occupancy and histone H3K4 methylation in response to ‘transcriptional stress’. EMBO Journal, 24(13), 2379–2390. 10.1038/sj.emboj.7600711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang F, Fan C, Tang B, Zhang X, & Li Z (2016). Dynamic changes of histone H3 lysine 9 following trimethylation in bovine oocytes and pre-implantation embryos. Biotechnology Letters, 38(3), 395–402. 10.1007/s10529-015-2001-3 [DOI] [PubMed] [Google Scholar]
- Zhang A, Xu B, Sun Y, Lu X, Gu R, Wu L, … Xu C (2012). Dynamic changes of histone H3 trimethylated at positions K4 and K27 in human oocytes and preimplantation embryos. Fertility and Sterility, 98(4), 1009–1016. 10.1016/j.fertnstert.2012.06.034 [DOI] [PubMed] [Google Scholar]
- Zhang B, Zheng H, Huang B, Li W, Xiang Y, Peng X, … Xie W (2016). Allelic reprogramming of the histone modification H3K4me3 in early mammalian development. Nature, 537(7621), 553–557. 10.1038/nature19361 [DOI] [PubMed] [Google Scholar]


