Abstract
Plasmodium parasites experience significant bottlenecks as they transit through the mosquito and are transmitted to their mammalian host. Oocyst prevalence on mosquito midguts and sporozoite prevalence in salivary glands are nevertheless commonly used to confirm successful malaria transmission, assuming these are reliable indicators of the mosquito’s capacity to give rise to secondary infections. Here we discuss recent insights in sporogonic development and transmission bottlenecks for Plasmodium. We highlight critical gaps in our knowledge and frame their importance in understanding the human and mosquito reservoirs of infection. A better understanding of the events that lead to successful inoculation of infectious sporozoites by mosquitoes is critical to designing effective interventions to shrink the malaria map.
Keywords: Sporozoite, gametocyte, oocyst, Anopheles, mosquitoes, salivary glands
The rise of sporozoites
Malaria, the deadliest human vector borne disease, is caused by parasites of the Plasmodium genus and transmitted by Anopheles mosquitoes. Transmission of Plasmodium parasites between their mosquito and mammalian hosts is a bottleneck for the parasite and constitutes vulnerabilities that could be leveraged in malaria elimination efforts (Figure 1). Transmission from humans to mosquitoes starts with sexual commitment following activation of Apatella2-g (PfAP2-G) [1, 2], that is under epigenetic control of heterochromatin protein 1 (PfHP1) [3] and gametocyte development 1 (GDV1) [4], leading to the development of male and female gametocytes within the human host [5–7]. Gametocytes sequester away from the circulation during their 8–12 day maturation [8, 9], and circulate once released for an average of 2.7 to 6.4 days in the bloodstream [9–11] to be taken up by blood-feeding mosquitoes. Upon ingestion by mosquitoes, a single activated female gametocyte becomes one macrogamete whilst a single male gametocyte gives rise to 8 motile microgametes [12, 13] that locate and fertilize female macrogametes to form diploid zygotes (see Glossary) [14–16]. Zygotes transform into ookinetes that penetrate the mosquito midgut to form oocysts on the basal side of the midgut [12]. Multiple rounds of genomic DNA replication results in a multinucleated cell, a syncytium, where thousands of daughter cells known as sporozoites are formed after synchronized budding from the sporoblast bodies of the cell [15]. Mature sporozoites exit the oocyst into the open circulatory system of the mosquito with a proportion successfully invading the mosquito salivary glands [17, 18]. Sporozoites remain in the salivary glands and may render adult mosquitoes infectious for the remainder of their lifespan [16]. The rate at which sporozoites are inoculated into the next host, how this is related to the density of gametocytes in the human host, oocyst burden and salivary gland sporozoite burden are matters of current debate [19–21]. It is, however, evident that sporogonic development in mosquitoes involves several bottlenecks where Plasmodium parasites are present in vulnerably low numbers (Figure 1). Here, we review the available literature on developmental bottlenecks in mosquitoes with a focus on recent manuscripts on the transition from salivary gland sporozoites to skin sporozoites. We argue that this understudied area of malaria transmission is of key importance to better appreciate the dynamics of malaria transmission in natural settings, quantify the contribution of different populations of infected individuals (e.g. high- and low-density gametocyte carriers) to onward transmission and predict the impact of malaria interventions.
Figure 1: Transmission bottlenecks in the Plasmodium life cycle.
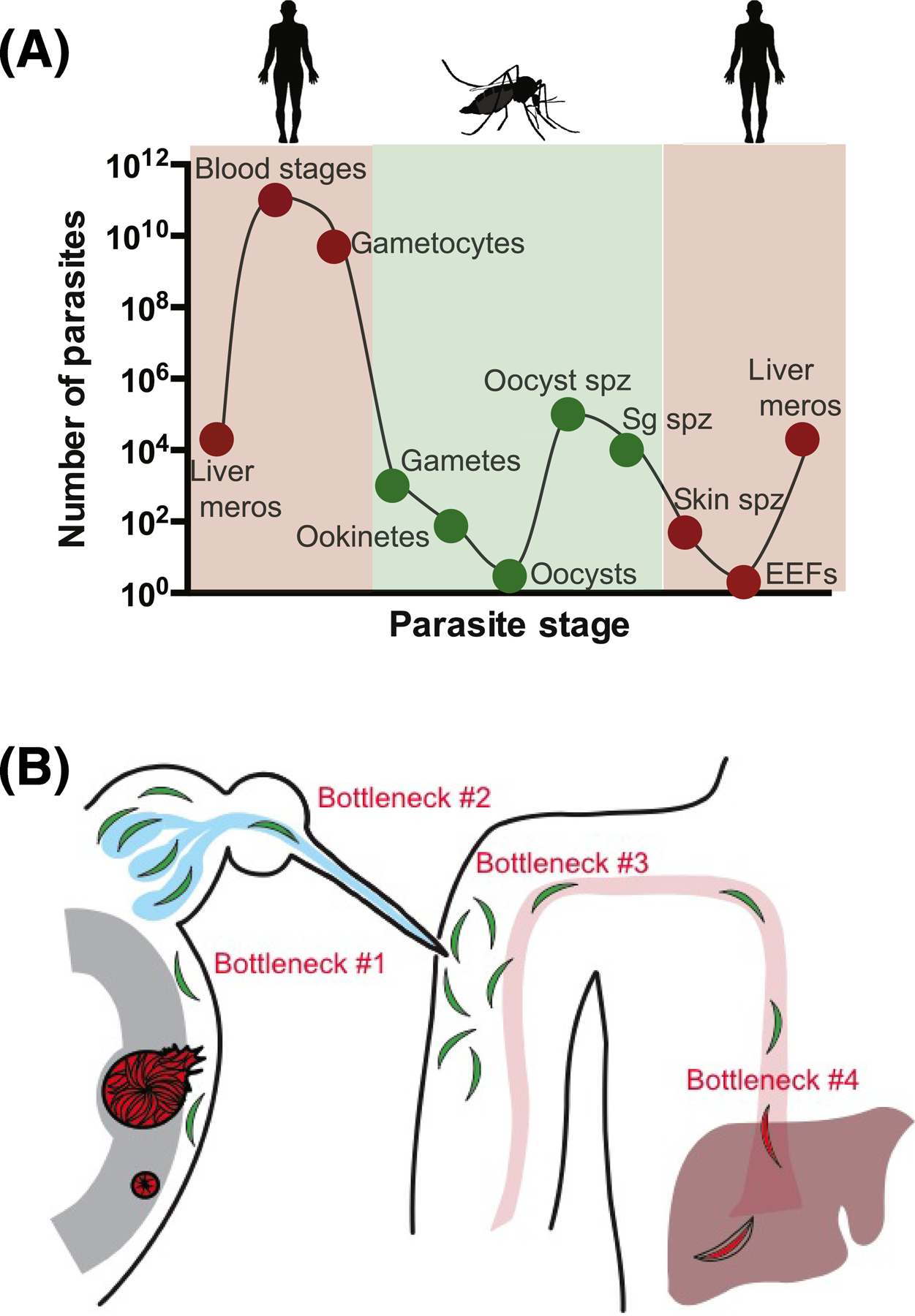
(A) Estimated parasite numbers during the different life cycle stages reveals that transmission to and from the mosquito is associated with significant decreases in parasite numbers (meros, merozoites; spz, sporozoites; sg salivary gland; EEFs, exoerythrocytic forms). Adapted from Povelones et al [111]. (B) Specific bottlenecks faced by Plasmodium sporozoites: Cartoon of the sporozoites journey from mosquito to mammalian host, highlighting the following bottlenecks: #1 Estimates suggest ~20% of oocyst sporozoites reach the salivary glands; #2 Less than 1% of salivary gland sporozoites are expelled during probing; #3 ~20% of inoculated sporozoites enter the bloodstream; #4 represents several unmeasured bottlenecks, namely the efficiency with which sporozoite arrest in the liver, enter hepatocytes, and develop into liver stages. Artwork by Brandy Lee Bennett.
Sporozoite development in and egress from oocysts
Oocysts are typically microscopically detected on the Anopheles mosquito midgut wall 7–9 days after an infectious blood meal, although some markers allow much earlier oocyst detection [22]. The density of oocysts is strongly determined by gametocyte density in the peripheral blood of the infectious human host that formed the source of the mosquito infection [23, 24]. Following the formation of oocysts on the mosquito midgut basal wall, a massive expansion of parasite numbers occurs through a replication process known as schizogony. This is a syncytial mode of replication in which genomic DNA replication, after multiple rounds of mitotic nuclear divisions, precedes cytoplasmic compartmentalization into individual sporozoites by the formation of cytoplasmic islands [15], called sporoblasts, from which sporozoites bud, each containing one nucleus and the appropriate number of individual organelles, ultimately filling the oocyst with thousands of crescent-shaped sporozoites (10–15μm by 1μm in diameter). One successful oocysts produces between 1500 to 5000 individual sporozoites [25, 26]. Sporozoite egress is required for sporozoite release in the mosquito’s haemocoel and actively established by parasite-dependent proteolysis. In this process, reviewed by Kojin et al [27], a parasite-derived cysteine protease plays a central role in rupture of the capsule [28] together with the circumsporozoite protein (CSP), that can be found on the oocyst plasma membrane and the inner surface of the capsule [29]. Recently two essential proteins were identified, oocyst rupture protein (ORP) 1 and 2, that promoted heterodimer formation in the oocyst after maturation, possibly leading directly or indirectly to destabilization and the activation of the cysteine protease [30, 31]. Since rupture occurs for the majority of oocysts in low-infected mosquitoes [21], it is generally assumed that oocyst positivity is a reliable indicator of later infectivity of mosquitoes. This, however, depends on the migration of sporozoites into salivary glands.
Sporozoite migration into salivary glands
Upon exit from the oocyst, sporozoites enter the open circulatory system of the mosquito, which consists of a dorsal vessel spanning the length of the mosquito that contracts to generate waves of directional flow. Released sporozoites can enter the abdominal portion of the dorsal vessel through openings called ostia and be passively carried anteriorly with the flow, exiting the vessel in the thoracic cavity near the salivary glands [32–34]. Sporozoites that do not enter the dorsal vessel are carried with the flow of hemolymph throughout the mosquito body and while a proportion likely enter salivary glands, many can be found trapped in the mosquito’s appendages, the alary muscle and other locations [33, 35]. The hemolymph contains immune factors and phagocytic cells called hemocytes and one study found evidence of sporozoite degradation in the hemolymph [33]. Nonetheless, the degree to which this occurs and the mechanism(s) by which this occurs remain understudied. There is a paucity of studies that have looked at the efficiency with which oocyst sporozoites colonize salivary glands [26, 33]. Rosenberg et al., counted the sporozoites in single oocysts of P. falciparum and P. vivax infected mosquitoes and compared these numbers to a previous study in which salivary gland sporozoites were enumerated and estimated that 20% of oocyst sporozoites successfully enter salivary glands [17]. In another study using mosquitoes heavily infected with the rodent malaria parasite P. berghei, a 10-fold lower efficiency of salivary gland entry by sporozoites was estimated [18].
Upon arrival to the glands, entry is dependent on recognition events between sporozoites and salivary gland proteins, a process reviewed in detail by Gosh et al [36], Mueller et al [17], and Kojin et al [27]. On the sporozoite side, CSP, thrombospondin-related anonymous protein (TRAP), TRAP-related protein (TREP), and apical membrane antigen/erythrocyte binding-like protein (MAEBL) have been shown to be involved in this process [37, 38]. CSP is known to bind to heparan sulfate proteoglycans and this may constitute the basis for the initial recognition event as these glycans are found on salivary glands [39, 40]. On the host side, several salivary gland proteins involved in sporozoite invasion have been identified. CSP binding protein (CSPBP), salivary gland surface protein 1 (SGS1), and Saglin being the best characterized [41, 42]. CSPBP and Saglin were identified in screens using CSP or TRAP, respectively. CSPBP or SGS1 specific antibodies and peptides inhibiting the TRAP-Saglin interaction decrease sporozoite salivary glands invasion. However, the specific role of Saglin as an essential salivary gland receptor for sporozoite invasion was recently doubted when no protein expression was observed in the distal lateral lobes of the salivary gland, a primary sporozoite invasion site [43]. Together these studies suggest that entry into salivary glands is a complex process involving several sporozoite proteins, and mosquito glycans and proteins. Indeed, visualization of this process by detailed, sequential electron micrographs suggests that there is an initial recognition event between the sporozoite surface coat and the salivary gland basal lamina, followed by tighter adhesion and entry into the cells [44]. Additional binding studies as well as in vivo knock-down studies in the mosquito using RNAi, are needed to further elucidate the molecular events involved in this process [45, 46]. Experiments that directly examined the number of ruptured oocysts in relation to salivary gland sporozoite load suggest that on average 1250 sporozoites reach the salivary gland per ruptured oocyst. [21]; other studies estimate higher sporozoite numbers per oocyst but do not directly relate this to oocyst rupture [25, 26]. Whilst these numbers are deemed sufficient to render a mosquito infectious for the remainder of her life, there are data suggesting that infectiousness decreases as sporozoites age, further complicating assessments of a mosquito’s infectious potential in the field [47, 48].
Sporozoite residence in salivary glands
The salivary glands of female mosquitoes are paired organs, one on each side of the esophagus, with each gland consisting of three lobes, two lateral lobes and a shorter median lobe. Each lobe is organized as a single layer of cup-shaped epithelial cells surrounding a large secretory cavity and a central salivary canal. In the distal portion of the glands, the salivary duct is continuous with the secretory cavity, however, as one moves anteriorly towards the proboscis, the ducts narrow, about 1μm in diameter which is slightly wider than a single sporozoite, and become chitinized, eventually joining with the duct from the opposite gland to form the common salivary duct [49]. Several studies have found that sporozoites preferentially enter the distal portions of the lateral and median lobes where the ducts are continuous with the secretory cavity [45, 50]. After their entry sporozoites move into the secretory cavity and a few can be found in the salivary duct, awaiting their inoculation into the mammalian host [44]. Sporozoites that enter the more proximal portions of the gland appear to be “landlocked” and may not be able to enter the salivary canal [51]. Thus, the process of localization to the salivary glands and entry into the secretory cavities of the glands presents many barriers to transmission [52].
Mosquito blood feeding physiology and sporozoite inoculation
Plasmodium parasites take advantage of the obligate blood feeding behavior of the mosquito to enter their mammalian host. After the mosquito stylet pierces the skin, it commences to search for blood, the labrum thrusting and bending to survey the entire area within its reach. Release of saliva occurs during probing [53] contributes to the mosquito’s ability to find blood due to saliva proteins that counteract the hemostatic and inflammatory responses of the host. The probing phase ends when the mosquito locates blood, having canulated a vessel or created a hematoma from the rupture of capillaries. There is no evidence of significant salivation during imbibement of blood [49, 53], though if it does occur, the difference in the flow rate of saliva compared to the counter flow of blood into the mosquito, estimated to be 104 to 105 times faster, would result in re-ingestion of the saliva secreted during blood feeding. Indeed, sporozoites have been found in the midguts of blood fed mosquitoes [54, 55]. The physiology of blood feeding suggests that sporozoites are predominately inoculated into the extravascular tissue. Experiments in which the bite site was removed, transplanted to naïve animals, or artificially heated, and in vivo visualization of the process of sporozoite inoculation, all support the notion that the majority of sporozoites are inoculated into the skin [56–61]. This is further supported by a recent study demonstrating that blood meal acquisition is not associated with a higher rate of infection [62]. Due to the length of the mosquito’s stylet the majority of sporozoites are inoculated in the dermis and a small proportion into the epidermis or subcutaneous tissue [63]. To continue their life cycle, sporozoites need to travel great distances compared to other morphological stages. This is achieved by translocating actively, by gliding motility and cell traversal, or passively by entering vessels of the blood stream or lymphatic system. In the dermis they actively glide forward using the actin/myosin based motor [60, 61, 64] to enter vessels of the blood stream. Only those that enter the blood stream, and not those drained in the lymphatic system, can give rise to human infection [61, 64].
Inoculum size and its relation to mosquito salivary gland load
Sporozoite entry into its host is a critical time for both host and parasite, with factors such as inoculum size determining whether the pathogen succeeds in establishing a foothold. Different approaches have been used to estimate the inoculum: initial studies induced infected mosquitoes to salivate [65–69], counting the ejected sporozoites, and more recent studies utilized the rodent malaria model [59, 70], allowing mosquitoes to probe on an anaesthetized mouse, and quantifying inoculated sporozoites by PCR or microscopy. Though these studies differed in their methodologies, some common features emerged: 1) From all cases, mosquitoes inoculated only a small proportion of the sporozoites in their salivary glands, generally less than 1%; 2) The majority of mosquitoes ejected few sporozoites, with median inocula ranging between 8 to 39 sporozoites. A minority of mosquitoes ejected >100 sporozoites; the percentage of these high injectors ranging from 7 to 36% in the different studies. The large range of high injectors may be due to differences in experimental set-up or the distribution of salivary gland sporozoite loads in mosquitoes. Murine models with non-human Plasmodium species typically give higher estimates of expelled sporozoites with 28 to 50% of mosquitoes inoculating >100 sporozoites while such large inocula are less frequently observed in mosquito spitting experiments with human malaria parasites. It is possible that this difference reflects a biological difference between mosquitoes infected with rodent parasites and P. falciparum sporozoites. Alternatively, it could result from differences in protocols with rodent work being performed with live animals that provide more natural biting circumstances, compared to studies that induced infected mosquitoes to salivate, and may thus better stimulate salivation and sporozoite mobilization and ejection. Differences in total sporozoite load in salivary glands also form a plausible factor for a higher inoculum size in murine models: rodent malaria infected mosquitoes frequently achieve high infection burdens. Though these studies did not find a strong correlation between the size of the sporozoite inoculum and sporozoite density in the glands, the number of infected mosquitoes analyzed in each study (n=59) may have been too small to detect differences against the backdrop of high biological variability [59, 71]. Considering the large variation in sporozoite densities observed in mosquito populations, understanding the association between sporozoite load, inoculum size and the likelihood that this inoculum gives rise to secondary infections is of crucial importance to accurately quantify the contribution of different hosts to transmission. The contribution of human hosts to transmission is dependent on mosquito biting behavior. Due to their low reserves, anophelines frequently seek more than one blood meal during a single gonotrophic cycle. Frequent blood meals may not only accelerate sporozoite development [72], but this central aspect of anopheline behavior may also favor parasite transmission with the entomologic inoculation rate (EIR) potentially increasing by a factor equal to the number of bites per gonotrophic cycle [73]. Intriguingly, evidence is accumulating that pathogen-vector manipulation may further enhance transmission (Box 1).
Box 1. Does Plasmodium manipulate its mosquito host to increase transmission likelihood?
There is a growing body of work investigating the influence of Plasmodium infection on mosquito blood feeding behavior. A recent detailed study on mosquito bloodmeal preference observed that, across the transmission season and dry season, ~20% of mosquitoes may take multiple human blood meals during a single night [92]. These repeated, partial bloodmeals may be associated with the mosquito infection status. Behavioral studies using field-caught P. falciparum infected and uninfected An. gambiae and An. funestus found that a higher percentage of sporozoite-infected mosquitoes initiated probing and that they probed longer than uninfected mosquitoes [93]. Additionally, field-caught infected anophelines were more likely to have evidence of ≥ 2 bloodmeals compared to uninfected mosquitoes [94, 95]. This is supported by genotyping of blood-stage parasites from members of the same household in which it was found that a much higher frequency of identical genotypes were found in household members than expected [96], suggestive of inoculations by the same mosquito into multiple human hosts. Furthermore, a laboratory model using rodent Plasmodium yoelii and An. stephensi mosquitoes showed increased biting and number of probes by sporozoite-infected mosquitoes compared to uninfected mosquitoes [97]. One possible mechanism underlying these feeding behavior changes is suggested by data demonstrating that salivary gland sporozoites decrease the level of apyrase in the mosquito host’s saliva, making it more difficult for them to feed to repletion on one host [98, 99]. It is predicted that such changes in feeding behavior would significantly increase the likelihood that a single infected mosquito transmits sporozoites [100, 101].
The likelihood that a single infected mosquito bite will result in a malaria infection
The entomologic inoculation rate (EIR) is a quantification of malaria exposure that is central to malaria epidemiology. EIR is defined as the number of infected mosquito bites per person per time-unit and estimated based on mosquito density, mosquito biting frequency and the proportion of mosquitoes with sporozoites in their salivary glands [74]. EIR does not explicitly consider how heavily infected mosquitoes are or the likelihood that an infected mosquito bite will result in a blood stage infection [75]. Early malariologists noted a significant discrepancy between human exposure to infected mosquitoes and the incidence of malaria infection [76–79], suggesting that the majority of infected bites may not result in a detectable infection. Attempts to indirectly determine the proportion of infected mosquito bites that result in malaria infection, measuring infant infection rates and mosquito biting rates, estimate that between 1 and 10% of infective bites lead to infection [80]. More recently, a rodent model was utilized to directly quantify infection probability after a single infected mosquito bite and found that 17% of infected bites resulted in a blood stage malaria infection [62]. These data suggest that the majority of infected mosquito bites may not result in a blood stage infection. Whilst human and epidemiological factors also play important roles in determining the relationship between EIR and (clinical) malaria incidence (see Box 2), the discrepancy between both estimates suggests there may be a relevant transmission bottleneck involving sporozoite expelling and downstream barriers in the mammalian host. Does sporozoite density in the salivary glands play a role in infection probability? The few studies addressing this topic, predominantly relying on P. yoelii and P. berghei, reported that bites from mosquitoes with higher sporozoite loads were more likely to initiate infection [19, 77, 79]. Churcher et al. found that mosquitoes with >1000 P. berghei sporozoites initiate infection 78% of the time with considerably lower likelihood of infection at lower sporozoite densities [19]. Using the rodent P. yoelii malaria model, Aleshnick et al. found that a threshold model best describes the data, with a jump in infection likelihood occurring at sporozoite densities of ~10,000. Once this threshold was met, infection probability plateaued at ~ 40% [62]. Whilst both studies found an association between sporozoite salivary gland load and the likelihood of onward infection, the study by Churcher et al. predicted much higher rates of infection by single infected mosquitoes with lower sporozoite burdens. These studies are relevant to controlled human malaria infection (CHMI) trials, a model that is increasingly used to study sporozoite infectivity and evaluate new vaccines or drugs [81]. Naïve volunteers are typically exposed to a minimum number of five bites of blood-fed mosquitoes to ensure homogeneous exposure between volunteers and infection in all control subjects. Analysis of 47 individuals participating in CHMI found that only mosquitoes with >1000 P. falciparum sporozoites remaining in the salivary gland after blood-feeding were capable of inducing an infection in humans [19]. In contrast, review of data from 13 CHMI studies with a total of 75 volunteers found no correlation between the time to parasitemia or height of first parasitemia, a readout indicative of liver load [82], and mean sporozoite load in mosquitoes [20]. This discrepancy may be explained by the fact that all mosquitoes in the studies analyzed by Walk et al. were heavily infected (range 26.500–160.500 P. falciparum sporozoites/salivary gland) [20]. In terms of sporozoite inoculum size and the likelihood of infection, the interpretation of CHMI data is complicated by two factors: 1) In general only highly infected mosquitoes are used and this is not reflective of salivary gland sporozoite densities in naturally infected mosquitoes 2) Blood meal acquisition is used as a readout for a successful encounter, rather than mosquito probing. Whilst this is understandable from a practical point, successful probing being difficult to quantify, it means that CHMI studies do not take into account those mosquitoes that probe but do not take a bloodmeal whilst it is known that these mosquitoes inoculate sporozoites and can initiate infection [56, 58, 59, 62]. This omission of mosquitoes that fail to take a bloodmeal may result in a higher estimated transmission efficiency since some probing mosquitoes (potentially inoculating sporozoites) are excluded. The magnitude and importance of this plausible overestimation of transmission efficiency in CHMI studies are currently unknown.
Box 2. What explains the low transmission efficiency of malaria?
The entomologic inoculation rate (EIR) is the product of the number of mosquito bites experienced by humans per unit time and the proportion of these mosquitoes that is sporozoite positive. Not all infectious bites successful achieve infection in human hosts. Transmission efficiency is defined as the number of infections in humans that is achieved per infectious bite and can be estimated by the number of infections acquired by humans per unit time (the force of infection; FOI) relative to EIR [102]. The observation that malaria incidence is often lower than expected based on EIR may plausibly be related to the fact that not all sporozoite positive mosquitoes are infectious. There are alternative explanators for low transmission efficiency. Transmission efficiency decreases with increasing EIR [102]: in areas of higher malaria transmission intensity where human populations are more heavily exposed, a smaller fraction of all infectious bites results in blood-stage malaria. This suggests that epidemiological characteristics and host factors may be at play. The fact that not all incident infections result in clinical symptoms (or in treatment seeking behavior in case of passively collected data) will result in some infectious bites not contributing to measured clinical incidence. However, also in carefully monitored cohorts with regular active screening for (asymptomatic) infections, the number of incident infections is typically much lower than expected based on EIR estimates [79, 102–104]. Naturally acquired immune responses are unlikely to prevent sporozoite inoculations from achieving blood-stage infection [105, 106] although effective blood-stage immunity may suppresss parasite densities to levels undetectable by microscopy [105, 107] and thus result in incident infections going unnoticed. Another important factor in understanding transmission efficiency is variation in exposure that is experienced by individuals living in endemic areas. This so-called heterogeneity in malaria exposure in space and time is a major determinant of malaria incidence patterns in populations [104, 108]. Whilst heterogenous mosquito exposure can amplify transmission of pathogens if a minority of heavily infected individuals infects many mosquitoes [109], inoculations on the same host may also dampen transmission efficiency [110]. If infectious bites are disproportionally experienced by a subset of the population, especially if this exposure occurs over a short time period of intense exposure, many sporozoite inoculations may result in super-infections but will not be detected as incident infections. These sporozoite inoculations in individuals who are already infected thus contribute to the disconnect between EIR and FOI estimates. A possible impact of inefficient expelling of sporozoites by mosquitoes with low salivary gland sporozoite loads will need to be quantified in the context of these other determinants of transmission efficiency.
The human infectious reservoir: the gap between oocyst prevalence and efficient sporozoite inoculation
Extrapolating these findings to natural malaria transmission is not trivial since very limited data exist from human malarias and the estimated minimum salivary gland sporozoite density required for successful infection differs considerably between studies [19, 79, 83]. Sporozoite numbers in wild-caught mosquitoes are predominantly <10,000 sporozoites per infected mosquito, mirroring the oocyst distribution and the proportion of mosquitoes with 1–2 oocysts (Figure 2) [66, 81, 82]. If the same threshold sporozoite density observed by Aleshnick et al. for P. yoelli applies to natural P. falciparum infections, this would suggests that the majority of naturally infected mosquitoes may be unlikely to transmit their infection. If one successful ookinete produces between 1500–5000 individual sporozoites [25, 26] and a proportion of these sporozoites reach the salivary glands, infections with a natural median of 1–2 oocysts per mosquito would be on the threshold of plausible malaria transmission. Importantly, in xenodiagnostic surveys that aim to quantify the contribution of different populations to the human infectious reservoir for malaria, low oocyst burdens also dominate. High oocyst densities are regularly observed in mosquito feeding experiments on selected high-density gametocyte carriers [84] but since most natural gametocyte carriers harbor very low gametocyte densities [85], low oocyst densities are typically observed in experiments where blood donors were recruited without prior gametocyte screening [86, 87]. In such population-wide assessments of infectivity, it is typically observed that only 1–11% of the general population residing in malaria endemic areas is capable of infecting mosquitoes at the moment of sampling [88]. The majority of these infectious individuals infect only few mosquitoes (34–76% of infectious individuals infect <5% of mosquitoes feeding on their blood sample [87, 89, 90]). This low infection prevalence is accompanied by a low burden of oocysts in infected mosquitoes [23, 84, 91]; 31–60% of infectious individuals in recent xenodiagnostic studies in Burkina Faso infected mosquitoes with 1–2 oocysts only, not achieving higher oocyst burdens [89][87]. If these infected mosquitoes are indeed on the threshold of plausible transmission, this would annul the contribution to transmission of a large proportion of infectious individuals. Better data on sporozoite expelling in relation to gametocyte density, oocyst burden and salivary gland sporozoite burden are thus urgently needed.
Figure 2: Oocyst and sporozoite distributions in wild-caught mosquitoes.
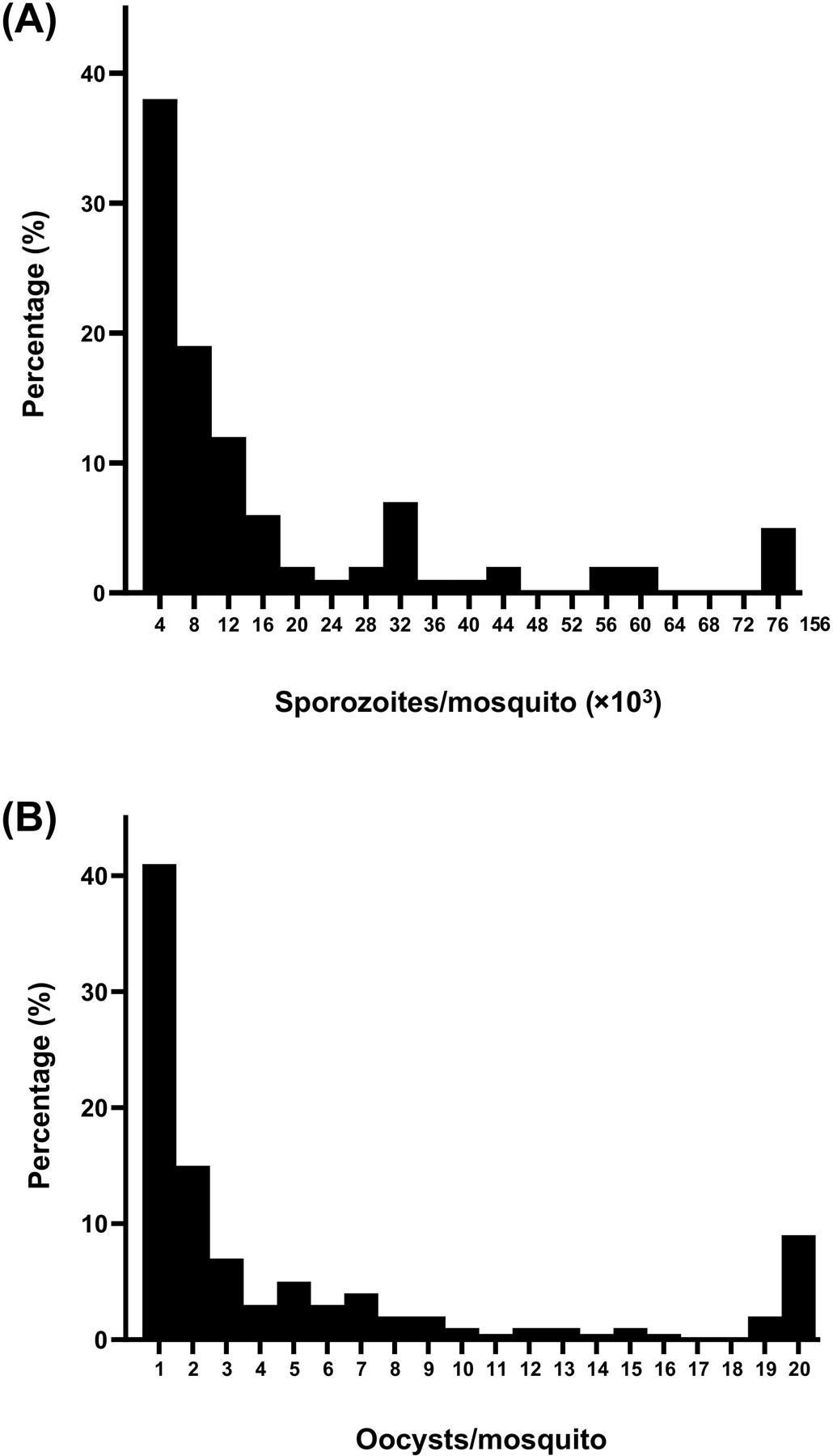
Frequency distribution of (A) oocyst numbers and (B) salivary gland sporozoite numbers in wild-caught P. falciparum infected Anopheles gambiae mosquitoes (n=94). Adapted from Collins et al [71] with permission of the publisher.
Concluding remarks
This review summarizes our current understanding of Plasmodium parasite bottlenecks as they relate to sporozoite development, migration, and transmission. Sporozoite development in oocysts represents the only expansion of parasite numbers in the mosquito host. Following this, the parasite encounters a series of bottlenecks that reduce its numbers considerably by the time the parasite develops into the next life cycle stage, the exoerythrocytic schizont in human hepatocytes. In reviewing what is known of these bottlenecks it is clear that there remain large gaps in our knowledge (see Outstanding Questions). The relationship between oocyst burden and salivary gland sporozoite load remains incompletely understood. Since field work estimates of infection often only involve measuring oocyst burden (and commonly express transmission outcomes in terms of the proportion of oocyst positive mosquitoes), it is of crucial importance to estimate the likelihood of successful sporozoite infection in relation to oocyst density and, if possible, the likelihood of successful hepatocyte infection or subsequent blood-stage infection. Not all of these parameters are easy to obtain for human malarias and some may need to be approximated using non-human malaria models or mathematical models. Current estimates based on P. berghei are likely to underestimate the success rate of oocyst sporozoites because this laboratory model gives rise to high numbers of oocysts, many of which never fully develop [18]. Better measurements on mosquitoes with oocyst numbers in the range of wild-caught mosquitoes (1–5 oocysts/gut) are necessary if we are to close this knowledge gap. If low oocyst densities are unlikely to result in salivary gland sporozoite loads sufficient for efficient onward transmission, transmission blocking interventions might not need to prevent all infected mosquitoes as long as high oocyst burdens are prevented. If, on the contrary, low oocyst densities regularly result in high numbers of expelled sporozoites, transmission blocking intervention would need to completely eliminate mosquito infection. These questions require a better understanding of the quantitative dynamics of the sporozoite’s transition from oocyst to salivary gland. Quantifying differences among different Anopheles - Plasmodium species combinations found in the endemic areas are crucial to the prediction of these dynamics. In addition, a better understanding is required of infection-induced alterations in blood-feeding behavior of mosquitoes. These behavioral studies should be expanded to control for mosquito age and to a range of field-relevant infection levels to be meaningful for our understanding of natural transmission.
Outstanding Questions Box.
How diverse are Anopheles vector species in their transmission potential, due to malaria innate susceptibility and blood feeding physiology?
What is the efficiency with which oocyst sporozoites infect salivary glands? How does oocyst number correlate to salivary gland load?
What is the association between sporozoite load and the number of expelled sporozoites in natural infections?
How are sporozoites expelled during repeated probing events, especially if these occur over a short period of time?
What human populations give rise to the most infectious mosquitoes?
To what extent does mosquito feeding or probing on multiple hosts contribute to transmission dynamics?
Though evidence suggests that the majority of sporozoites are inoculated into the dermis, is there a significant minority that are inoculated directly into the bloodstream and if so, how does this affect transmission efficiency?
In conclusion, successful transmission by Plasmodium-infected mosquitoes depends on several poorly understood factors related to the mosquito vector. These include the efficiency with which sporozoites travel from the midgut to the salivary glands, the impact of mosquito gland load on infection likelihood, differences among mosquito species to transmit sporozoites, and the impact of infection on mosquito behavior. Quantification of these events is critical to understand malaria transmission efficiency and define the minimum efficacy required from transmission blocking interventions. Understanding these factors will allow the identification of human populations that harbor infections capable of rendering mosquitoes not only infected but truly infectious, thus supporting malaria elimination efforts.
Highlights.
The discrepancy between exposure to infected mosquito bites and malaria incidence suggests a transmission bottleneck that is currently understudied
Recent studies from non-human malaria models are indicative of a minimum salivary gland sporozoite density that is required to achieve infection upon mosquito biting
Infection-induced alterations in blood-feeding behavior of mosquitoes may influence natural transmission dynamics
Acknowledgments
This project was supported through grants from the National Institutes of Health (R01AI132359 to PS) and a fellowship from the European Research Council (ERC-2014-StG 639776 to TB). TB and PS are further supported by the Bill and Melinda Gates Foundation (INDIE OPP1173572 to TB and OPP1139130 to PS).
Glossary
- Anteriorly
used to describe anatomy, meaning towards the head, opposite of posterior
- Circumsporozoite protein (CSP)
is the most abundant protein present in the oocyst/sporozoite stage from day 7 post-infection onwards
- Distal
located away from an area, further away from the center, opposite of proximal
- Zygote
a diploid cell formed by fusion of haploid gametes becoming a fertilized ovum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Sinha A, et al. (2014) A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature 507, 253–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kafsack BF, et al. (2014) A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature 507, 248–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brancucci NMB, et al. (2014) Heterochromatin protein 1 secures survival and transmission of malaria parasites. Cell Host Microbe 16, 165–176 [DOI] [PubMed] [Google Scholar]
- 4.Filarsky M, et al. (2018) GDV1 induces sexual commitment of malaria parasites by antagonizing HP1-dependent gene silencing. Science 359, 1259–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inselburg J (1983) Gametocyte formation by the progeny of single Plasmodium falciparum schizonts. The Journal of parasitology 69, 584–591 [PubMed] [Google Scholar]
- 6.Silvestrini F, et al. (2000) Commitment to the production of male and female gametocytes in the human malaria parasite Plasmodium falciparum. Parasitology 121 Pt 5, 465–471 [DOI] [PubMed] [Google Scholar]
- 7.Smith TG, et al. (2000) Commitment to sexual differentiation in the human malaria parasite, Plasmodium falciparum. Parasitology 121 (Pt 2), 127–133 [DOI] [PubMed] [Google Scholar]
- 8.Busula AO, et al. (2017) Mechanisms of Plasmodium-Enhanced Attraction of Mosquito Vectors. Trends Parasitol 33, 961–973 [DOI] [PubMed] [Google Scholar]
- 9.Reuling IJ, et al. (2018) A randomized feasibility trial comparing four antimalarial drug regimens to induce Plasmodium falciparum gametocytemia in the controlled human malaria infection model. Elife 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eichner M, et al. (2001) Genesis, sequestration and survival of Plasmodium falciparum gametocytes: parameter estimates from fitting a model to malariatherapy data. Trans R Soc Trop Med Hyg 95, 497–501 [DOI] [PubMed] [Google Scholar]
- 11.Smalley ME and Sinden RE (1977) Plasmodium-Falciparum Gametocytes - Their Longevity and Infectivity. Parasitology 74, 1–8 [DOI] [PubMed] [Google Scholar]
- 12.Gerald N, et al. (2011) Mitosis in the human malaria parasite Plasmodium falciparum. Eukaryot Cell 10, 474–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinden RE (1991) Mitosis and meiosis in malarial parasites. Acta Leiden 60, 19–27 [PubMed] [Google Scholar]
- 14.Aikawa M, et al. (1984) New observations on gametogenesis, fertilization, and zygote transformation in Plasmodium gallinaceum. J Protozool 31, 403–413 [DOI] [PubMed] [Google Scholar]
- 15.Baton LA and Ranford-Cartwright LC (2005) Spreading the seeds of million-murdering death: metamorphoses of malaria in the mosquito. Trends Parasitol 21, 573–580 [DOI] [PubMed] [Google Scholar]
- 16.Beier JC (1998) Malaria parasite development in mosquitoes. Annu Rev Entomol 43, 519–543 [DOI] [PubMed] [Google Scholar]
- 17.Mueller AK, et al. (2010) Invasion of mosquito salivary glands by malaria parasites: prerequisites and defense strategies. International journal for parasitology 40, 1229–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinden RE, et al. (2007) Progression of Plasmodium berghei through Anopheles stephensi is density-dependent. PLoS pathogens 3, e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Churcher TS, et al. (2017) Probability of Transmission of Malaria from Mosquito to Human Is Regulated by Mosquito Parasite Density in Naive and Vaccinated Hosts. PLoS pathogens 13, e1006108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walk J, et al. (2018) Mosquito Infectivity and Parasitemia after Controlled Human Malaria Infection. Am J Trop Med Hyg 98, 1705–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone WJ, et al. (2013) The relevance and applicability of oocyst prevalence as a read-out for mosquito feeding assays. Scientific reports 3, 3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itsara LS, et al. (2018) PfCap380 as a marker for Plasmodium falciparum oocyst development in vivo and in vitro. Malaria journal 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradley J, et al. (2018) Predicting the likelihood and intensity of mosquito infection from sex specific Plasmodium falciparum gametocyte density. Elife 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiattibutr K, et al. (2017) Infectivity of symptomatic and asymptomatic Plasmodium vivax infections to a Southeast Asian vector, Anopheles dirus. Int J Parasitol 47, 163–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang CYT, et al. (2018) Assessing Plasmodium falciparum transmission in mosquito-feeding assays using quantitative PCR. Malaria journal 17, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg R and Rungsiwongse J (1991) The number of sporozoites produced by individual malaria oocysts. The American journal of tropical medicine and hygiene 45, 574–577 [DOI] [PubMed] [Google Scholar]
- 27.Kojin BB and Adelman ZN (2019) The Sporozoite’s Journey Through the Mosquito: A Critical Examination of Host and Parasite Factors Required for Salivary Gland Invasion. Frontiers in Ecology and Evolution 7 [Google Scholar]
- 28.Aly AS and Matuschewski K (2005) A malarial cysteine protease is necessary for Plasmodium sporozoite egress from oocysts. The Journal of experimental medicine 202, 225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q, et al. (2005) Exit of Plasmodium sporozoites from oocysts is an active process that involves the circumsporozoite protein. PLoS pathogens 1, 72–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curra C, et al. (2016) Release of Plasmodium sporozoites requires proteins with histone-fold dimerization domains. Nat Commun 7, 13846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siden-Kiamos I, et al. (2018) Identification of Plasmodium berghei Oocyst Rupture Protein 2 (ORP2) domains involved in sporozoite egress from the oocyst. International journal for parasitology 48, 1127–1136 [DOI] [PubMed] [Google Scholar]
- 32.Hillyer JF and Pass G (2020) The Insect Circulatory System: Structure, Function, and Evolution. Annu Rev Entomol 65, 121–143 [DOI] [PubMed] [Google Scholar]
- 33.Hillyer JF, et al. (2007) Efficiency of salivary gland invasion by malaria sporozoites is controlled by rapid sporozoite destruction in the mosquito haemocoel. International journal for parasitology 37, 673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glenn JD, et al. (2010) Structural mechanics of the mosquito heart and its function in bidirectional hemolymph transport. J Exp Biol 213, 541–550 [DOI] [PubMed] [Google Scholar]
- 35.Golenda CF, et al. (1990) The distribution of circumsporozoite protein (CS) in Anopheles stephensi mosquitoes infected with Plasmodium falciparum malaria. J Histochem Cytochem 38, 475–481 [DOI] [PubMed] [Google Scholar]
- 36.Ghosh AK and Jacobs-Lorena M (2009) Plasmodium sporozoite invasion of the mosquito salivary gland. Curr Opin Microbiol 12, 394–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kariu T, et al. (2002) MAEBL is essential for malarial sporozoite infection of the mosquito salivary gland. The Journal of experimental medicine 195, 1317–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Combe A, et al. (2009) TREP, a novel protein necessary for gliding motility of the malaria sporozoite. International journal for parasitology 39, 489–496 [DOI] [PubMed] [Google Scholar]
- 39.Sinnis P, et al. (2007) Mosquito heparan sulfate and its potential role in malaria infection and transmission. The Journal of biological chemistry 282, 25376–25384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frevert U, et al. (1993) Malaria circumsporozoite protein binds to heparan sulfate proteoglycans associated with the surface membrane of hepatocytes. The Journal of experimental medicine 177, 1287–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, et al. (2013) Anopheles gambiae circumsporozoite protein-binding protein facilitates plasmodium infection of mosquito salivary glands. The Journal of infectious diseases 208, 1161–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghosh AK, et al. (2009) Malaria parasite invasion of the mosquito salivary gland requires interaction between the Plasmodium TRAP and the Anopheles saglin proteins. PLoS pathogens 5, e1000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Brochta DA, et al. (2019) Is Saglin a mosquito salivary gland receptor for Plasmodium falciparum? Malaria journal 18, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pimenta PF, et al. (1994) The journey of malaria sporozoites in the mosquito salivary gland. J Eukaryot Microbiol 41, 608–624 [DOI] [PubMed] [Google Scholar]
- 45.Okulate MA, et al. (2007) Identification and molecular characterization of a novel protein Saglin as a target of monoclonal antibodies affecting salivary gland infectivity of Plasmodium sporozoites. Insect molecular biology 16, 711–722 [DOI] [PubMed] [Google Scholar]
- 46.Brennan JDG, et al. (2000) Anopheles gambiae salivary gland proteins as putative targets for blocking transmission of malaria parasites. Proceedings of the National Academy of Sciences of the United States of America 97, 13859–13864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Porter RJ, et al. (1954) Studies on malarial sporozoites. II. Effect of age and dosage of sporozoites on their infectiousness. Experimental parasitology 3, 267–274 [DOI] [PubMed] [Google Scholar]
- 48.Boyd MF, et al. (1936) On the Duration of Infectiousness in Anophelines Harboring Plasmodium Falciparum1. The American journal of tropical medicine and hygiene s1–16, 157–158 [Google Scholar]
- 49.Clements AN (1992) The Biology of Mosquitoes: Development, nutrition, and reproduction. Chapman & Hall [Google Scholar]
- 50.Sinden RE (1984) The biology of Plasmodium in the mosquito. Experientia 40, 1330–1343 [DOI] [PubMed] [Google Scholar]
- 51.Sterling CR, et al. (1973) The passage of Plasmodium berghei sporozoites through the salivary glands of Anopheles stephensi: an electron microscope study. The Journal of parasitology 59, 593–605 [PubMed] [Google Scholar]
- 52.Wells MB and Andrew DJ (2019) Anopheles Salivary Gland Architecture Shapes Plasmodium Sporozoite Availability for Transmission. MBio 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Griffiths RB and Gordon RM (1952) An apparatus which enables the process of feeding by mosquitoes to be observed in the tissues of a live rodent; together with an account of the ejection of saliva and its significance in Malaria. Annals of tropical medicine and parasitology 46, 311–319 [DOI] [PubMed] [Google Scholar]
- 54.Beier MS, et al. (1992) Ingestion of Plasmodium falciparum sporozoites during transmission by anopheline mosquitoes. The American journal of tropical medicine and hygiene 47, 195–200 [DOI] [PubMed] [Google Scholar]
- 55.Kebaier C and Vanderberg JP (2006) Re-ingestion of Plasmodium berghei sporozoites after delivery into the host by mosquitoes. The American journal of tropical medicine and hygiene 75, 1200–1204 [PubMed] [Google Scholar]
- 56.Matsuoka H, et al. (2002) A rodent malaria, Plasmodium berghei, is experimentally transmitted to mice by merely probing of infective mosquito, Anopheles stephensi. Parasitol Int 51, 17–23 [DOI] [PubMed] [Google Scholar]
- 57.Lloyd OC and Sommerville T (1949) The Fate of Sporozoites of Plasmodium-Cynomolgi Injected into the Skin of Rhesus Monkeys. J Pathol Bacteriol 61, 144–146 [Google Scholar]
- 58.Sidjanski S and Vanderberg JP (1997) Delayed migration of Plasmodium sporozoites from the mosquito bite site to the blood. The American journal of tropical medicine and hygiene 57, 426–429 [DOI] [PubMed] [Google Scholar]
- 59.Medica DL and Sinnis P (2005) Quantitative dynamics of Plasmodium yoelii sporozoite transmission by infected anopheline mosquitoes. Infection and immunity 73, 4363–4369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hopp CS, et al. (2015) Longitudinal analysis of Plasmodium sporozoite motility in the dermis reveals component of blood vessel recognition. eLife 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amino R, et al. (2006) Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med 12, 220–224 [DOI] [PubMed] [Google Scholar]
- 62.Aleshnick M, et al. (2019) Experimental Determination of the Force of Malaria Infection Reveals a Non-Linear Relationship to Mosquito Sporozoite Loads. bioRxiv, 830299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sinnis P and Zavala F (2012) The skin: where malaria infection and the host immune response begin. Semin Immunopathol 34, 787–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Douglas RG, et al. (2015) Active migration and passive transport of malaria parasites. Trends Parasitol 31, 357–362 [DOI] [PubMed] [Google Scholar]
- 65.Beier JC, et al. (1992) Sporozoite transmission by Anopheles freeborni and Anopheles gambiae experimentally infected with Plasmodium falciparum. J Am Mosq Control Assoc 8, 404–408 [PubMed] [Google Scholar]
- 66.Beier JC, et al. (1991) Quantitation of Plasmodium falciparum sporozoites transmitted in vitro by experimentally infected Anopheles gambiae and Anopheles stephensi. The American journal of tropical medicine and hygiene 44, 564–570 [DOI] [PubMed] [Google Scholar]
- 67.Frischknecht F, et al. (2004) Imaging movement of malaria parasites during transmission by Anopheles mosquitoes. Cell Microbiol 6, 687–694 [DOI] [PubMed] [Google Scholar]
- 68.Ponnudurai T, et al. (1991) Feeding behaviour and sporozoite ejection by infected Anopheles stephensi. Transactions of the Royal Society of Tropical Medicine and Hygiene 85, 175–180 [DOI] [PubMed] [Google Scholar]
- 69.Rosenberg R, et al. (1990) An estimation of the number of malaria sporozoites ejected by a feeding mosquito. Transactions of the Royal Society of Tropical Medicine and Hygiene 84, 209–212 [DOI] [PubMed] [Google Scholar]
- 70.Jin Y, et al. (2007) Direct microscopic quantification of dynamics of Plasmodium berghei sporozoite transmission from mosquitoes to mice. Infection and immunity 75, 5532–5539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Collins FH, et al. (1984) First field trial of an immunoradiometric assay for the detection of malaria sporozoites in mosquitoes. The American journal of tropical medicine and hygiene 33, 538–543 [DOI] [PubMed] [Google Scholar]
- 72.Ponnudurai T, et al. (1989) Sporozoite load of mosquitoes infected with Plasmodium falciparum. Transactions of the Royal Society of Tropical Medicine and Hygiene 83, 67–70 [DOI] [PubMed] [Google Scholar]
- 73.Tedrow RE, et al. (2019) Multiple Blood Feeding: A Force Multiplier for Transmission. Trends Parasitol 35, 949–952 [DOI] [PubMed] [Google Scholar]
- 74.Tusting LS, et al. (2014) Measuring changes in Plasmodium falciparum transmission: precision, accuracy and costs of metrics. Advances in parasitology 84, 151–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith DL, et al. (2012) Ross, macdonald, and a theory for the dynamics and control of mosquito-transmitted pathogens. PLoS pathogens 8, e1002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Macdonald G (1956) Epidemiological basis of malaria control. Bulletin of the World Health Organization 15, 613–626 [PMC free article] [PubMed] [Google Scholar]
- 77.Davidson G and Draper CC (1953) Field Studies of Some of the Basic Factors Concerned in the Transmission of Malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 47, 522–535 [DOI] [PubMed] [Google Scholar]
- 78.Pull JH and Grab B (1974) A simple epidemiological model for evaluating the malaria inoculation rate and the risk of infection in infants. Bulletin of the World Health Organization 51, 507–516 [PMC free article] [PubMed] [Google Scholar]
- 79.Davey TH and Gordon RM (1933) The Estimation of the Density of Infective Anophelines as a Method of Calculating the Relative Risk of Inoculation with Malaria from Different Species or in Different Localities. Annals of Tropical Medicine & Parasitology 27, 27–52 [Google Scholar]
- 80.Davidson G and Draper CC (1953) Field studies of some of the basic factors concerned in the transmission of malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 47, 522–535 [DOI] [PubMed] [Google Scholar]
- 81.Sauerwein RW, et al. (2011) Experimental human challenge infections can accelerate clinical malaria vaccine development. Nature Reviews Immunology 11, 57–64 [DOI] [PubMed] [Google Scholar]
- 82.Douglas AD, et al. (2013) Comparison of modeling methods to determine liver-to-blood inocula and parasite multiplication rates during controlled human malaria infection. The Journal of infectious diseases 208, 340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pumpuni CB, et al. (1997) Plasmodium yoelii sporozoite infectivity varies as a function of sporozoite loads in Anopheles stephensi mosquitoes. The Journal of parasitology 83, 652–655 [PubMed] [Google Scholar]
- 84.Da DF, et al. (2015) Experimental study of the relationship between Plasmodium gametocyte density and infection success in mosquitoes; implications for the evaluation of malaria transmission-reducing interventions. Experimental parasitology 149, 74–83 [DOI] [PubMed] [Google Scholar]
- 85.Slater HC, et al. (2019) The temporal dynamics and infectiousness of subpatent Plasmodium falciparum infections in relation to parasite density. Nat Commun 10, 1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gonçalves BP, et al. (2017) Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity. Nature Communications 8, 1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tadesse FG, et al. (2018) The Relative Contribution of Symptomatic and Asymptomatic Plasmodium vivax and Plasmodium falciparum Infections to the Infectious Reservoir in a Low-Endemic Setting in Ethiopia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 66, 1883–1891 [DOI] [PubMed] [Google Scholar]
- 88.Stone W, et al. (2015) Assessing the infectious reservoir of falciparum malaria: past and future. Trends Parasitol 31, 287–296 [DOI] [PubMed] [Google Scholar]
- 89.Ouedraogo AL, et al. (2016) Dynamics of the Human Infectious Reservoir for Malaria Determined by Mosquito Feeding Assays and Ultrasensitive Malaria Diagnosis in Burkina Faso. The Journal of infectious diseases 213, 90–99 [DOI] [PubMed] [Google Scholar]
- 90.Goncalves BP, et al. (2017) Examining the human infectious reservoir for Plasmodium falciparum malaria in areas of differing transmission intensity. Nat Commun 8, 1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Churcher TS, et al. (2012) Measuring the blockade of malaria transmission--an analysis of the Standard Membrane Feeding Assay. International journal for parasitology 42, 1037–1044 [DOI] [PubMed] [Google Scholar]
- 92.Guelbeogo WM, et al. (2018) Variation in natural exposure to anopheles mosquitoes and its effects on malaria transmission. eLife 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bovino JA (1979) Scleral spreading forceps for drainage of subretinal fluid. Am J Ophthalmol 87, 721. [DOI] [PubMed] [Google Scholar]
- 94.Koella JC, et al. (1998) The malaria parasite, Plasmodium falciparum, increases the frequency of multiple feeding of its mosquito vector, Anopheles gambiae. Proc Biol Sci 265, 763–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tedrow RE, et al. (2019) Anopheles mosquito surveillance in Madagascar reveals multiple blood feeding behavior and Plasmodium infection. PLoS Negl Trop Dis 13, e0007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Conway DJ and McBride JS (1991) Genetic evidence for the importance of interrupted feeding by mosquitoes in the transmission of malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 85, 454–456 [DOI] [PubMed] [Google Scholar]
- 97.Anderson RA, et al. (1999) The effect of Plasmodium yoelii nigeriensis infection on the feeding persistence of Anopheles stephensi Liston throughout the sporogonic cycle. Proc Biol Sci 266, 1729–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thievent K, et al. (2019) Malaria load affects the activity of mosquito salivary apyrase. J Insect Physiol 116, 10–16 [DOI] [PubMed] [Google Scholar]
- 99.Rossignol PA, et al. (1984) Increased intradermal probing time in sporozoite-infected mosquitoes. The American journal of tropical medicine and hygiene 33, 17–20 [DOI] [PubMed] [Google Scholar]
- 100.Koella JC (1999) An evolutionary view of the interactions between anopheline mosquitoes and malaria parasites. Microbes Infect 1, 303–308 [DOI] [PubMed] [Google Scholar]
- 101.Rossignol PA and Rossignol AM (1988) Simulations of enhanced malaria transmission and host bias induced by modified vector blood location behaviour. Parasitology 97 (Pt 3), 363–372 [DOI] [PubMed] [Google Scholar]
- 102.Smith DL, et al. (2010) A quantitative analysis of transmission efficiency versus intensity for malaria. Nat Commun 1, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beier JC, et al. (1994) Plasmodium-Falciparum Incidence Relative to Entomologic Inoculation Rates at a Site Proposed for Testing Malaria Vaccines in Western Kenya. American Journal of Tropical Medicine and Hygiene 50, 529–536 [DOI] [PubMed] [Google Scholar]
- 104.Churcher TS, et al. (2015) Human-to-mosquito transmission efficiency increases as malaria is controlled. Nature Communications 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tran TM, et al. (2013) An Intensive Longitudinal Cohort Study of Malian Children and Adults Reveals No Evidence of Acquired Immunity to Plasmodium falciparum Infection. Clinical Infectious Diseases 57, 40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barry A, et al. (2018) Functional antibodies against Plasmodium falciparum sporozoites are associated with a longer time to qPCR-detected infection among schoolchildren in Burkina Faso. Wellcome Open Res 3, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pinkevych M, et al. (2012) The dynamics of naturally acquired immunity to Plasmodium falciparum infection. PLoS Comput Biol 8, e1002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Johnston GL, et al. (2013) Malaria’s missing number: calculating the human component of R0 by a within-host mechanistic model of Plasmodium falciparum infection and transmission. PLoS Comput Biol 9, e1003025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Woolhouse MEJ, et al. (1997) Heterogeneities in the transmission of infectious agents: Implications for the design of control programs. Proceedings of the National Academy of Sciences of the United States of America 94, 338–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smith DL, et al. (2014) Recasting the theory of mosquito-borne pathogen transmission dynamics and control. Transactions of the Royal Society of Tropical Medicine and Hygiene 108, 185–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Povelones M, et al. (2016) The Complement System of Malaria Vector Mosquitoes. Adv Insect Physiol 51, 223–242 [Google Scholar]


