Abstract
Objective:
Limited data exist on the impact of ascites in pancreatic ductal adenocarcinoma (PDAC). We evaluated the survival outcomes of patients with PDAC and ascites.
Methods:
Retrospective, single-institution, case-control study including patients with newly diagnosed PDAC from 2007 to 2016. One hundred fifty-four patients with ascites at diagnosis (case group) and 154 controls were matched on age, sex, stage, Eastern Cooperative Oncology Group performance, surgical treatment, lymph node, and margin status. Ascites was defined as computed tomography–detected fluid in the pelvic/peritoneal cavity. Overall survival was compared between groups via Cox proportional hazards models with a gamma frailty term to account for the correlation between matched pairs on entire cohort and by disease stages for subgroup analysis.
Results:
The 154 matched cases included 24 resectable, 19 borderline resectable, 51 locally advanced, and 60 metastatic disease. Patients with ascites had higher risk of death compared with those without (conditional hazard ratio, 1.58; 95% confidence interval, 1.23–2.03; P < 0.001). Stratified analysis showed a significant association between ascites and poor prognosis in patients with localized disease (conditional hazard ratio, 1.62; 95% confidence interval, 1.18–2.24; P = 0.003).
Conclusions:
Radiographic ascites is a poor prognostic factor in PDAC. Our findings may aid physicians in considering systemic therapy prior to attempting local treatments.
Keywords: ascites, localized disease, metastatic disease, overall survival, pancreas adenocarcinoma, peritoneal carcinomatosis
Malignant ascites refers to cancer-related accumulation of ascitic fluid in the peritoneal cavity. The prevalence of ascites in patients with gastrointestinal cancers is 21% typically from malignancies such as pancreatic, gastric, colorectal, and gallbladder cancers.1 Several pathophysiological mechanisms described in cancer patients include peritoneal carcinomatosis, lymphatic vessels’ obstruction, portal hypertension, heart failure, or also hypoalbuminemia. The diagnosis is performed in the context of known neoplasia and is typically associated with advanced stage or metastatic disease. Although malignant ascites confers a limited life expectancy regardless of primary malignancy,2 prognosis seems worse in patients with gastrointestinal cancers in which mean survival is 10 weeks from the onset of ascites.3
In patients with pancreatic ductal adenocarcinoma (PDAC), ascites is often diagnosed late with average onset of 11 months from the time of diagnosis. Prognosis is poor after ascites development with an estimated median survival of 1.8 months from time of onset. Positive cytology is present in 58% of patients who undergo diagnostic paracentesis.4 Current treatment options are centered on the palliation of symptoms and treating the underlying malignancy.
Although patients with malignant ascites and advanced meta-static disease have a limited life expectancy, there are limited data on the clinical significance of ascites when it develops early on at presentation or when it occurs in patients with localized nonmetastatic disease. Patients with localized PDAC who have tumor confined within the pancreas are often offered aggressive chemotherapy and/or radiation therapy with the intention of downstaging the tumor to allow potential curative resection. When patients have radiographic ascites in the absence of metastases, clinical decisions are challenging particularly when determining if local therapies such as surgery or radiation are appropriate. In this study, we aimed to determine the prognostic implications of synchronous radiographic ascites in patients with a new diagnosis of PDAC. The findings may help guide future therapeutic options for this specific population of patients.
MATERIALS AND METHODS
Eligibility Criteria
This is a retrospective case-control study, with cases defined as those with evidence of new diagnosis of PDAC and evidence of radiographic ascites on initial computed tomography (CT) scan. Radiographic ascites was defined as the presence of measurable free fluid in pelvis and/or peritoneal cavity on imaging studies at the time of diagnosis. A board-certified radiologist reviewed imaging studies. We included a 1:1 matched control group of patients without evidence of radiographic ascites by age at diagnosis, sex, disease stage, and Eastern Cooperative Oncology Group (ECOG) performance status. If there was a previous surgical resection, patients were matched for lymph node status, margins status, and grade of tumor. If patients underwent surgical resection, cases were matched for lymph node status, margins status, and grade of tumor. Factors that were not matched included race and tumor size. All patients were seen at the Sidney Kimmel Comprehensive Cancer Center of Johns Hopkins Hospital during their initial evaluation at the Pancreas Multidisciplinary Clinics from 2007 to 2016. All studies were carried out with the approval of the Johns Hopkins Hospital Institutional Review Board.
Clinical information and patients’ demographics were obtained from the electronic record system including age, sex, clinical staging, time of diagnosis, dates of initial scans, and date of death or last follow-up. For those who underwent surgery, the pathology of the resected cancers was reviewed, including analysis of tumor stage, grade, margin status, perineural invasion, microvascular invasion, and nodal status. Computed tomography scans of the abdomen and pelvis were performed as per standard protocols.5 Clinical staging was determined as resectable disease, borderline resectable (BR) disease, locally advanced unresectable (LAUR), or metastatic disease at the time of patients’ initial evaluation according to the National Comprehensive Cancer Network guidelines.5
Patients with resectable disease were those patients in which tumor was present within the pancreas without arterial or venous involvement. Patients with BR disease were those with superior mesenteric artery involvement of less than 180°, but no extension to celiac axis or hepatic artery bifurcation. Patients with BR disease can have venous encasement (>180° involvement) with superior mesenteric vein or portal vein. Locally advanced unresectable tumors were considered those with arterial encasement (>180° involvement) of the superior mesenteric artery, celiac axis, or unreconstructible superior mesenteric vein/portal vein due to tumor involvement or occlusion. Patients with metastatic disease were those with evidence of any extrapancreatic involvement.
Statistical Analysis
This is a retrospective case-control study with overall survival (OS) as the primary outcome. Overall survival was defined as the time from diagnosis to death from any cause or last follow-up visit if still alive. Overall survival was estimated for subgroups of patients, separately by disease stage, using the Kaplan-Meier method. The proportional hazards assumption was tested, and if met, groups were compared using Cox proportional hazards models. The association between ascites and OS was assessed via Cox proportional hazards models with a gamma frailty term to account for the correlation within the matched pairs. P < 0.05 was considered statistically significant. Statistical software R3.4.2 was used to perform the analyses.6
RESULTS
Demographic, Clinical, and Pathologic Data of Patients
The study included 308 patients with newly diagnosed PDAC evaluated in our clinics from 2007 to 2016. Of these, 154 patients had evidence of ascites at presentation (case group), and 154 patients had no ascites at presentation (control group). Descriptive statistics of clinical characteristics of matched variables and unmatched variables are summarized in Table 1. Flowchart of patients included is shown in Figure 1.
TABLE 1.
Patient Demographics for Matched Variables
| Matched Variables | Entire Cohort (n = 308) |
No Ascites (n = 154) |
Ascites (n = 154) |
|---|---|---|---|
| Age at diagnosis, median (range), y | 67 (31–88) | 67 (31–88) | 67 (35–87) |
| Sex, male, n (%) | 147 (48) | 74 (48) | 73 (47) |
| Disease stage, n (%) | |||
| Resectable | 48 (16) | 24 (16) | 24 (16) |
| BR | 38 (12) | 19 (12) | 19 (12) |
| LAUR | 102 (33) | 51 (33) | 51 (33) |
| Metastasis | 120 (39) | 60 (39) | 60 (39) |
| ECOG, n (%) | |||
| 0 | 88 (29) | 48 (31) | 40 (26) |
| 1 | 137 (44) | 69 (45) | 68 (44) |
| 2 | 24 (8) | 11 (7) | 13 (8) |
| 3 | 2 (1) | 0 (0) | 2 (1) |
| Not evaluable | 57 (19) | 26 (17) | 31 (20) |
| Surgical resection, n (%) | |||
| Yes | 81 (26) | 44 (29) | 37 (24) |
| No | 227 (74) | 110 (71) | 117 (76) |
| Surgical LN,* n (% in SurgR) | |||
| Positive | 61 (75) | 32 (73) | 29 (78) |
| Negative | 17 (21) | 11 (25) | 6 (16) |
| Not evaluable | 3 (4) | 1 (2) | 2 (6) |
| Surgical margin,* n (% in SurgR) | |||
| Positive | 21 (26) | 11 (25) | 10 (27) |
| Negative | 55 (68) | 31 (70) | 24 (65) |
| Not evaluable | 5 (6) | 2 (5) | 3 (8) |
| Grade, n (%) | |||
| 1 | 11 (4) | 2 (1) | 9 (6) |
| 2 | 102 (33) | 55 (36) | 47 (31) |
| 3 | 74 (24) | 38 (25) | 36 (23) |
| Not evaluable | 121 (39) | 59 (38) | 62 (40) |
Percentage within patients with surgical resection (SurgR).
FIGURE 1.

Patient groups based on initial CT scans.
The median age of cases with ascites was 67 years (range, 35–87 years). Of these, 81 (53%) were female, and a total of 24 patients had resectable disease, 19 with BR disease, 51 with LAUR disease, and 60 patients with metastatic disease; 37 patients (24%) underwent surgical resection. The majority of the tumors were located in the head of the pancreas (n = 94 [61%]), and the majority of patients in the case group had performance status in terms of ECOG performance of 0 to 1 (n = 108 [70%]). Patients with ascites had a larger tumor (0.4 cm larger in median of tumor size) when compared with patients without ascites (P = 0.02).
Survival Analysis for Entire Cohort
Median follow-up time was 6.9 years (range, 11 days to 8.7years) based on reverse Kaplan-Meier method. Overall, patients with as-cites had worse OS compared with patients without ascites with median OS of 10.2 versus 15.2 months, respectively (Fig. 2A1), and conditional hazard ratio (cHR) of 1.58 (95% confidence interval [CI], 1.23–2.03; P < 0.001) (Table 2A1).
FIGURE 2.
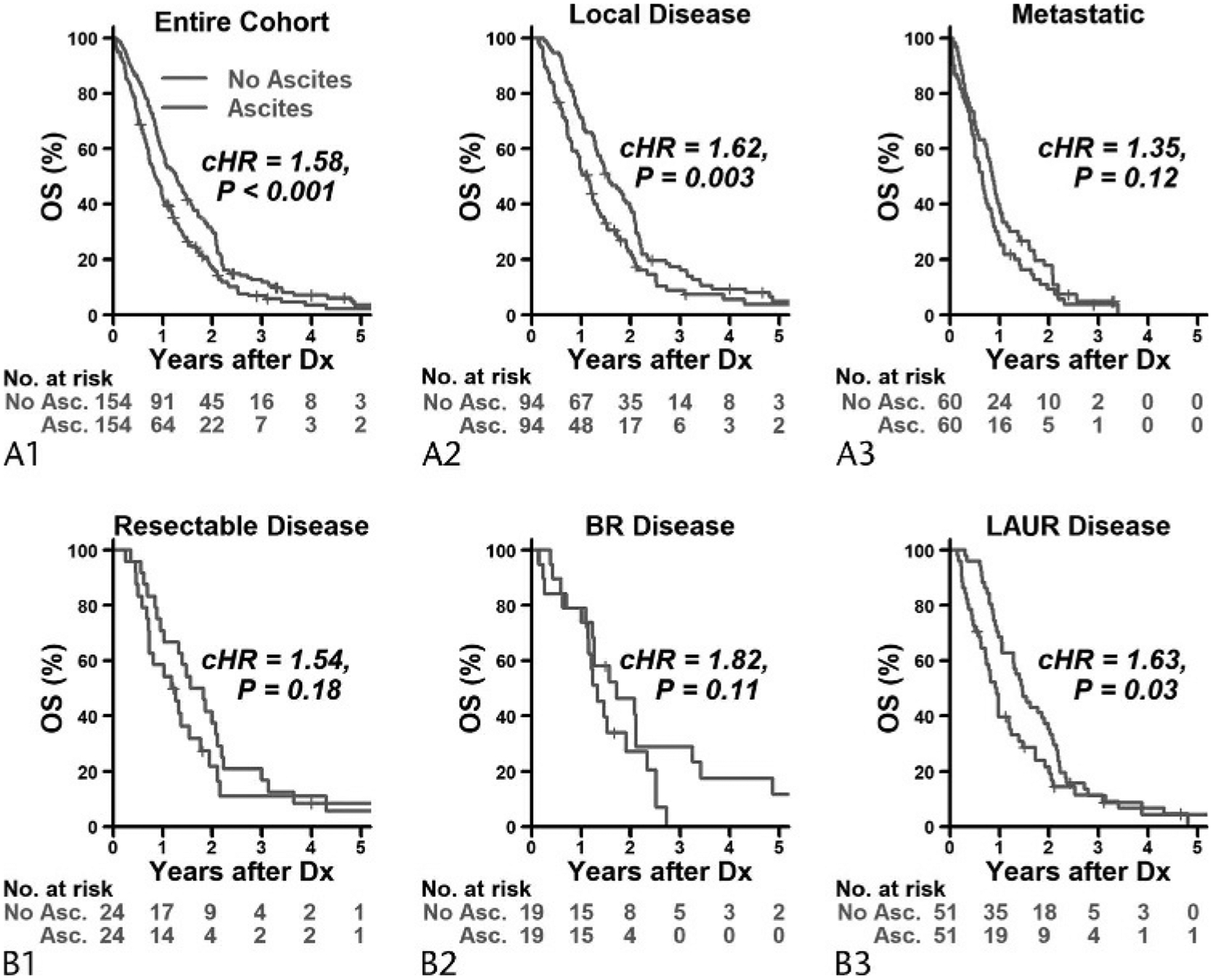
Kaplan-Meier curves for OS by ascites status in the entire cohort (A1) and within patients who had local disease (A2) and metastatic disease (A3); B, within patients who had resectable disease (B1), BR disease (B2), and LAUR disease (B3). Curves were truncated at 5 years because we had a total of only 5 patients who followed up for more than 5 years. Asc indicates ascites; Dx, diagnosis.
TABLE 2.
Summary Results of OS by Ascites Status in Entire Cohort Within Patients Who Had Local Disease and Metastatic Disease and Within Patients Who Had Resectable Disease, BR Disease, and LAUR Disease
| OS Estimates, Probability (95% CI) | |||||||
|---|---|---|---|---|---|---|---|
| Cohort | No. | Median OS, mo | 1 y | 2 y | 3 y | cHR (95% CI) | P |
| A1: Entire cohort | |||||||
| No ascites | 154 | 15.2 | 0.59 (0.52–0.67) | 0.30 (0.24–0.38) | 0.12 (0.08–0.18) | 1 | |
| Ascites | 154 | 10.2 | 0.42 (0.35–0.51) | 0.17 (0.12–0.25) | 0.07 (0.03–0.13) | 1.58 (1.23–2.03) | <0.001 |
| A2: Local disease | |||||||
| No ascites | 94 | 18.6 | 0.71 (0.63–0.81) | 0.38 (0.29–0.49) | 0.16 (0.10–0.26) | 1 | |
| Ascites | 94 | 13.6 | 0.53 (0.43–0.64) | 0.23 (0.15–0.34) | 0.09 (0.04–0.18) | 1.62 (1.18–2.24) | 0.003 |
| A3: Metastatic disease | |||||||
| No ascites | 60 | 10.4 | 0.40 (0.29–0.55) | 0.18 (0.10–0.31) | 0.05 (0.01–0.16) | 1 | |
| Ascites | 60 | 7.8 | 0.27 (0.18–0.41) | 0.09 (0.04–0.21) | 0.04 (0.01–0.14) | 1.35 (0.92–1.98) | 0.12 |
| B1: Resectable disease | |||||||
| No ascites | 24 | 20.3 | 0.71 (0.55–0.92) | 0.38 (0.22–0.63) | 0.17 (0.07–0.41) | 1 | |
| Ascites | 24 | 14.5 | 0.58 (0.42–0.82) | 0.22 (0.10–0.49) | 0.11 (0.03–0.39) | 1.54 (0.82–2.91) | 0.18 |
| B2: BR disease | |||||||
| No ascites | 19 | 20.6 | 0.79 (0.63–1) | 0.46 (0.28–0.76) | 0.29 (0.14–0.60) | 1 | |
| Ascites | 19 | 16.0 | 0.79 (0.63–1) | 0.27 (0.12–0.59) | 0 | 1.82 (0.87–3.77) | 0.11 |
| B3: LAUR disease | |||||||
| No ascites | 51 | 17.6 | 0.69 (0.57–0.83) | 0.35 (0.24–0.51) | 0.11 (0.05–0.25) | 1 | |
| Ascites | 51 | 11.3 | 0.40 (0.28–0.56) | 0.21 (0.12–0.37) | 0.11 (0.05–0.27) | 1.63 (1.06–2.51) | 0.03 |
Survival Analysis by Disease Stage
Although there was no statistically significant interaction (P = 0.48) between disease stages and ascites status on OS, we observed that patients with ascites with local disease had worse OS than those with local disease but without ascites (resectable + BR + LAUR) (median OS, 13.6 versus 18.6 months; cHR, 1.62 [95% CI, 1.18–2.24]; P = 0.003). There was no significant difference in OS between those with and without ascites in the cohort with metastatic disease (median OS, 7.8 vs 10.4 months; cHR, 1.35 [95% CI, 0.92–1.98]; P = 0.12) (Figs. 2A2, A3; Table 2A2, 2A3). When looking at the subgroup of patients with localized disease stage, patients with ascites had a higher risk of death compared with those with no ascites (cHR, 1.63 [95% CI, 1.06–2.51]; P = 0.03). In resectable and BR disease patients, patients with ascites also had relatively worse OS compared with patients with no ascites, but this difference was not statistically significant (Figs. 2B1, B3; Table 2B1–B3).
DISCUSSION
Although malignant ascites has been associated with a worse prognosis, there are limited data in patients with PDAC.7–11
Hicks et al4 reported the largest series in 180 patients with pancreatic cancer who either presented with or developed ascites during their disease course. In their study, the authors reported a median OS of 12 months (range, 10–14 months [95% CI]) from diagnosis and 1.8 months (range, 1.6–2.3 months [95% CI]) after ascites developed. The time from diagnosis to ascites development for the entire cohort was approximately 8.8 months with a range of 0 to 45 months. Few of their patients had ascites without radio-graphic evidence of metastases (n = 13); thus, the outcomes in this group of patients are still not well known.
In our study, we included patients with any amount of radio-graphic fluid in the peritoneal or pelvic cavity at diagnosis, whereas previous studies were mostly limited to patients with either carcinomatosis or cytology-confirmed malignant ascites. In our study, radiographic ascites emerged as an important negative prognostic factor: patients had higher risk of death if with ascites compared with patients without ascites, cHR of 1.58 (95% CI, 1.23–2.03; P < 0.001), taking into account all disease stages.
An interesting finding in our study is the description of survival data in patients with localized disease and synchronous radio-graphic ascites at presentation. While ascites is a common finding later in the disease course with the appearance of metastases, little is known about its implications at diagnosis when local therapies are being considered. Our study sheds more light on this topic as we included a significant number of patients with nonmetastatic disease (n = 94). We showed that patients with localized disease have worse OS compared with patients without ascites. The estimated hazard ratios suggested worse prognosis in patients with resectable and BR disease, but the difference was too small to detect with our sample size in these 2 subgroups.
Our findings correlate with smaller previous studies. Zervos et al3 investigated the prognostic implications of new-onset ascites in patients with both resectable and unresectable PDAC, showing that the appearance of ascites was associated with a significant shorter life expectancy, with an OS after ascites developed of approximately 2 months, regardless of tumor stage and prior intervention. Takahara et al12 evaluated the prognostic role of cytology-confirmed malignant ascites. In their series of 73 patients, 29% (n = 21) presented with synchronous ascites at diagnosis. In the metachronous group, the median time of ascites onset from the initial diagnosis of PDAC was 224 days. Regardless of the time of onset, patients with malignant ascites had poor survival, with a median survival of only 47 days. A study conducted by Clark and Traverso,13 also demonstrated that positive cytology in locally advanced disease is associated with decreased survival. Confirmation of this in the clinical setting would require diagnostic laparos-copy in patients with ascites, which is an invasive procedure and may delay start of chemotherapy. Similarly, diagnostic cytology may have a low yield in patients with PDAC, with only half of cases of patients with malignant ascites having a positive cytology.4
These findings may have clinical implications. When patients present with radiographic ascites at the time of diagnosis without evidence of metastasis or measurable peritoneal carcinomatosis, it could be challenging to establish the best treatment plan, particularly when considering local therapies such as radiation or surgery. We believe radiographic ascites should be considered a poor prognostic factor or high-risk feature for worse OS even in patients with local disease. Such cases may benefit from initial systemic approach with upfront chemotherapy to determine disease biology prior to attempting local therapies. Using radiographic ascites as a clinical criterion to establish the need of systemic treatment is less invasive than laparoscopic procedures or diagnostic paracentesis in patients with ascites without delay on initiation of treatment. We hypothesize that radiographic ascites may represent a phenomenon of early carcinomatosis prior to the appearance of measurable omental disease or carcinomatosis on imaging at least for patients with LAUR disease. The peritoneal cavity is a frequently encountered metastatic site in patients with PDAC and a negative factor for survival.12 In certain cases, peritoneal nodules could be hard to detect with available imaging techniques owing to the small size of the peritoneal tumor implants.
This study has several limitations, given its retrospective nature. It was conducted within a single tertiary referral center; thus, referral bias could not be eliminated. Additionally, some patients were lost to follow-up if they decided to undergo therapies at outside institutions, although every attempt was taken to follow up for their survival. Third, our definition of malignant ascites was in the context of radiographic ascites in a patient with known PDAC but no cytologic confirmation. In practice, usually fewer than 20% of patients will have cytologic confirmation attempt, and similarly the yield is still 36%.4 Thus, radiographic ascites is a reasonable and clinically acceptable way to diagnose malignant ascites. This study is the largest case-control study to investigate the relationship between CT-detected ascites at diagnosis and outcomes in patients with PDAC, with more than half of the patients (n = 94 pairs) having localized disease without evidence of metastases.
In conclusion, radiographic ascites adversely affects OS in patients with newly diagnosed pancreatic cancer and should be considered a high-risk feature. Systemic chemotherapy should be considered upfront in all patients with evidence of radiographic ascites at presentation.
Abbreviations:
- BR
borderline resectable
- cHR
conditional hazard ratio
- CI
confidence interva
- GI
gastrointestinal
- LAUR
locally advanced unresectable
- OS
overall survival
- PDAC
pancreatic ductal adenocarcinoma
- PV
portal vein
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Cavazzoni E, Bugiantella W, Graziosi L, et al. Malignant ascites: pathophysiology and treatment. Int J Clin Oncol. 2013;18:1–9. [DOI] [PubMed] [Google Scholar]
- 2.Saâda E, Follana P, Peyrade F, et al. [Pathogenesis and management of refractory malignant ascites]. [Article in French]. Bull Cancer. 2011;98: 679–687. [DOI] [PubMed] [Google Scholar]
- 3.Zervos EE, Osborne D, Boe BA, et al. Prognostic significance of new onset ascites in patients with pancreatic cancer. World J Surg Oncol. 2006;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hicks AM, Chou J, Capanu M, et al. Pancreas adenocarcinoma: ascites, clinical manifestations, and management implications. Clin Colorectal Cancer. 2016;15:360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:1028–1061. [DOI] [PubMed] [Google Scholar]
- 6.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 7.Ayantunde AA, Parsons SL. Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol. 2007;18:945–949. [DOI] [PubMed] [Google Scholar]
- 8.DeWitt J, Yu M, Al-Haddad MA, et al. Survival in patients with pancreatic cancer after the diagnosis of malignant ascites or liver metastases by EUS-FNA. Gastrointest Endosc. 2010;71:260–265. [DOI] [PubMed] [Google Scholar]
- 9.Fang N, Zhang HQ, He BX, et al. Clinicopathological characteristics and prognosis of gastric cancer with malignant ascites. Tumour Biol. 2014;35: 3261–3268. [DOI] [PubMed] [Google Scholar]
- 10.Viguier J, De Muret A, Bacq Y. [Ascites due to portal hypertension from breast cancer- related metastatic liver infiltration]. [Article in French]. Gastroenterol Clin Biol. 2006;30:903–905. [DOI] [PubMed] [Google Scholar]
- 11.Woopen H, Sehouli J. Current and future options in the treatment of malignant ascites in ovarian cancer. Anticancer Res. 2009;29:3353–3359. [PubMed] [Google Scholar]
- 12.Takahara N, Isayama H, Nakai Y, et al. Pancreatic cancer with malignant ascites: clinical features and outcomes. Pancreas. 2015;44:380–385. [DOI] [PubMed] [Google Scholar]
- 13.Clark CJ, Traverso LW. Positive peritoneal lavage cytology is a predictor of worse survival in locally advanced pancreatic cancer. Am J Surg. 2010; 199:657–662. [DOI] [PubMed] [Google Scholar]


