Abstract
Due to functionally distinct cell-mediated immunity, newborns and infants are highly susceptible to infection with intracellular pathogens. Indeed, neonatal antigen-presenting dendritic cells (DCs) demonstrate impaired Th1 responses to many candidate adjuvants, including most TLR agonists (TLRAs). Combination adjuvantation systems may provide enhanced immune activation but have typically been developed without regard to the age of the target population. We posited that distinct combinations of TLRAs and C-type lectin receptor agonists (CLRAs) may enhance Th1 responses of newborn DCs. TLRA/CLRA combinations were screened for enhancement of TNF production by human newborn and adult monocyte-derived DCs (MoDCs) cultured in 10% autologous plasma or in newborn cord, infant, adult, and elderly whole blood. MoDC activation was characterized by targeted gene expression analysis, Caspase-1 and NF-κB studies, cytokine multiplex and naïve autologous CD4+ T-cell activation. Dual activation of newborn DCs, via the CLR Mincle (trehalose-6,6-dibehenate) and TLR7/8 (R848) greatly enhanced Caspase-1 and NF-κB activation, Th1-polarizing cytokine production and autologous Th1-polarization. Combined activation via TLR4 (glycopyranosyl lipid A aqueous formulation, GLA-AF) and Dectin-1 (β-glucan peptide) acted synergistically in newborns and adults, but to a lesser extent. Degree of synergy varied dramatically with age, being greatest in newborns and infants with less synergy in adults and elders. Overall, combination adjuvant systems demonstrate markedly different immune activation with age, with combined DC activation via Mincle and TLR7/8 representing a novel approach to enhance the efficacy of early life vaccines.
Introduction
Due to functionally distinct cell-mediated immunity, newborns and young infants are highly susceptible to infection with intracellular pathogens including bacteria such as Listeria spp. and Salmonella spp., and viruses such as herpes simplex virus and respiratory syncytial virus. With age-specific cellular (1) and soluble (2) inhibitors playing a role, the neonatal immune system is distinct from that of infants and adults, with bias towards induction of regulatory T cell (Treg) and T-helper 2 (Th2) type T-cell responses. This distinct ontogeny limits the efficacy of adjuvants to induce a T-helper 1 (Th1) immune response that may be necessary to protect against intracellular pathogens (3–5). Impaired newborn immunity, including reduced function of DCs, key antigen-presenting cells (APCs), puts them at risk for infection and limits vaccine-induced Th1 responses. Most licensed vaccines are not optimally effective at birth or require multiple booster immunizations later in life. As Th1 immunity is needed for protection against multiple early life pathogens (6, 7), there is therefore an unmet need for adjuvantation systems that activate newborn DCs to produce Th1-polarizing cytokines, resulting in T-cell activation (8).
Adjuvants may boost responses to antigens by shaping the type and magnitude of vaccine-induced immune responses (9). While whole-cell vaccines (live or killed) provide intrinsic adjuvant activity, for optimal immunogenicity, subunit vaccines comprised of purified microbial products may require the addition of adjuvants, such as Aluminum salts, MF59 or pattern recognition receptor (PRR) agonists such as TLR agonists (TLRAs), including monophosphoryl lipid A (MPLA; TLR4) currently employed in combination with Aluminum hydroxide in the licensed Cervarix vaccine (10). The synthetic MPLA analog glucopyranosyl lipid adjuvant (GLA) is another TLR4 agonist that has shown promise in vitro and in vivo, including in human clinical trials (11, 12). Importantly, adjuvants can have age-specific immune enhancing effects, and adjuvants that induce a strong Th1 response in adults may induce Th2-biased immune responses early in life (8, 13, 14).
APCs, including monocytes and especially DCs, may mediate adjuvant effects as they express PRRs and are key to optimal vaccine responses (3). Newborn monocytes and DCs demonstrate distinct TLR-mediated responses as compared to adult DCs, producing less T-cell activating and Th1-polarizing cytokines such as Tumor Necrosis Factor (TNF) and IL-12p70, but more IL-6 (15) and anti-inflammatory IL-10, polarizing the immune response to a more regulatory phenotype (16). Skewed TLR-mediated responses in newborn blood monocytes are in part due to cellular factors, such as nucleated CD71+ red blood cells (1) as well as soluble plasma factors, such as adenosine, that enhance intracellular cyclic adenosine monophosphate (cAMP), thereby inhibiting activation of Th1-polarizing signaling pathways (17, 18) upstream of MAP kinase and NF-κB activation (19).
In the process of developing vaccine adjuvant systems that may be optimal in early life, we considered the example of certain live attenuated self-adjuvanted vaccines, which activate the recipients’ immune system through multiple PRRs. For example, Bacille Calmette-Guérin (BCG) activates TLRs, including TLR2 and TLR4, (20), as well as C-type lectin receptors (CLRs) such as Dectin-1 and Mincle (21–23), resulting in strong Th1 biased immune responses. Therefore, combined stimulation of newborn cells through multiple PRRs may potentially overcome the early life bias against Th1 responses. CLRs are a family of carbohydrate-recognizing receptors expressed on a variety of cell types, including leukocytes. DCs have CLRs that mediate endocytosis of pathogens and also induce signaling events (25). In vivo, migratory DCs entering the injection site can be instructed to induce a Th1/17 T-cell response through activation of the CLR Mincle, when trehalose-6,6-dibehenate (TDB), a Mincle agonist, is administered in a liposomal formulation with a vaccinal antigen (26, 27). Some CLR agonists (CLRAs) activate NF-κB via a pathway distinct from that downstream of TLRs, and can act in combination with TLRAs towards human adult leukocytes (21, 25, 28–32). However, little is known about whether TLR/CLR interactions are age-dependent and to what extent their combined action in adults translates to newborns and infants.
In this study, we tested the hypothesis that dual activation with precise combinations of TLRAs and CLRAs can effectively activate neonatal monocyte-derived DCs (MoDCs) and enhance their ability to prime neonatal Th1-polarized immune responses. By screening combinations of TLRAs and CLRAs we identified two TLRA/CLRA combinations that enhanced activation of NF-κB and inflammasome pathways in MoDCs, to reprogram cytokine production and shift the differentiation of human newborn CD4+ T cells to a Th1 phenotype: (a) a combination of TLR4 agonists- either monophosphoryl lipid A (MPLA) or the lipid-based aqueous adjuvant formulation containing glycopyranosyl lipid adjuvant (GLA-AF) (11) with Dectin-1 agonists, Zymosan or β-glucan peptide (BGP) and (b) a highly effective novel combination of TLR7/8 agonists such as R848 and the Mincle agonist Trehalose-6,6-dibehenate (TDB), which synergistically activates newborn, but not adult, MoDCs and enables Th1 polarization in an age-specific fashion. When tested in whole blood assay, these TLRA/CLRA combinations acted in an age-dependent mathematical synergy that varied across four age groups, such that it was greatest in newborns and progressively diminished with age becoming antagonistic in elders over 65 years of age. We have thus discovered unique age-specific synergy between specific TLRA/CLRA combinations suggesting a new paradigm for identification of adjuvantation systems tailored to enhance development of early life vaccines.
Methods
TLR agonists, CLR agonists, and assay reagents
TLRAs included Pam3Cys, and polyinosinic:polycytidylic acid (Poly I:C) (TLR1/2, and TLR3, respectively; InvivoGen, San Diego, CA, USA), ultrapure lipopolysaccharide (LPS) from Salmonella minnesota (TLR4; List Biological Laboratories, Campbell, CA, USA), MPLA (TLR4; InvivoGen, San Diego, CA, USA), lipid-based aqueous formulation GLA-AF (TLR4; Infectious Disease Research Institute, Seattle, WA, USA), R848 (TLR7/8; InvivoGen; San Diego, CA, USA), VTX-294 (TLR8; VentiRx Pharmaceuticals, Inc.; Seattle, WA, USA) and CpC ODN 2006 (TLR9; InvivoGen; San Diego, CA, USA). TLRAs were formulated according to manufacturer’s recommendations. CLR agonists (CLRAs) used were Mannan from Saccharomyces cerevisiae (DC-SIGN/MMR; Sigma-Aldrich Co. LLC. St Louis, MO, USA), Hepatitis C Virus E2 (HCV E2; DCIR/BDCA-2; eENZYME LLC, Gaithersburg, MD, USA), biotin glycoprotein 120 (gp120; BDCA-2/DC-SIGN; Immunodiagnostics, Inc., Woburn, MA, USA), alkaline-treated Zymosan (Dectin-1; InvivoGen; San Diego, CA, USA), Curdlan (Dectin-1; InvivoGen, San Diego, CA, USA), Trehalose-6,6-dibehenate (TDB, Mincle; InvivoGen, San Diego, CA, USA), β-glucan peptide (BGP) (Dectin-1; InvivoGen, San Diego, CA, USA), and Whole Glucan Particles (WGP; InvivoGen, San Diego, CA, USA), which were all formulated according to manufacturer’s recommendations. All TLRAs (other than LPS and MPLA) and CLRAs were verified to be free of endotoxin (<1 EU/ml), as measured by Limulus amoebocyte lysate (LAL) assay per the manufacturer’s instructions (Charles River; Wilmington, MA, USA). Sterile Dulbecco’s Phosphate Buffered Saline (DPBS) without Ca2+, Mg2+, and Phenol Red (Gibco – Life Technologies; Carlsbad, CA, USA) or Roswell Park Memorial Institute (RPMI, Gibco – Life Technologies; Carlsbad, CA, USA) media were included in assays as a negative control when indicated.
Blood donors
Non-identifiable cord blood samples were collected with approval from the Ethics Committee of the Beth Israel Deaconess Medical Center, Boston, MA, USA (protocol number 2011P-000118). All de-identified blood samples from adult (age 18–40 years) and elders (age over 65 years) subjects included in the experiments were collected with approval from the Ethics Committee of Boston Children’s Hospital, Boston, MA, USA (protocol number X07- 05-0223), after written informed consent was provided. For collection of de-identified blood samples from infant subjects (age 6-months) written informed consent was obtained from the parents with approval from the Ethics Committee of Boston Children’s Hospital, Boston, MA, USA (protocol numbers P00010750 and P00013867). Blood samples were processed within 4 hours (typically ~1–2 hours), and anti-coagulated with 15 U/ml pyrogen-free heparin sodium (American Pharmaceutical Partners, Inc.; Schaumberg, IL, USA). The number of study subjects (n) used for each experimental approach is presented in the figure legends.
Isolation of mononuclear cells and monocytes
Heparinized blood from newborns and adults was centrifuged for 10 minutes at 500 × g, after which the upper layer of clear yellow plasma was removed. This platelet-rich plasma (PRP) was then centrifuged for 15 minutes at 3000 × g, and platelet-poor plasma (PPP) was collected from the top and stored at −20°C. The remaining blood was reconstituted to its original volume by resuspending in DPBS (Gibco – Life Technologies, Carlsbad, CA, USA). Then, 25 ml of reconstituted blood was layered onto 15 ml of Ficoll-Hypaque gradients (Ficoll-Paque PREMIUM, GE Healthcare; Waukesha, WI, USA) and centrifuged for 30 minutes at 500 × g. After Ficoll separation, the mononuclear cell fraction was collected. Monocytes were then isolated from mononuclear cell fractions by positive selection with magnetic CD14 MicroBeads, performed according to manufacturer’s instructions (Miltenyi Biotec; Auburn, CA, USA). Purity was checked by flow cytometry and was always more than 98%
Blood assay
For assessment of TLRA activity in whole blood, we used an adaptation of a previously described method (5). Neonatal cord blood or peripheral blood from infants, adults or elders was mixed 1:5 with sterile pre-warmed (37°C) RPMI 1640 medium (Invitrogen; Carlsbad, CA, USA) and 135 μL of the 1:5 suspension was added to each well of a 96 well U-bottom plate (Becton Dickinson; Franklin Lakes, NJ, USA) containing 15 μl freshly prepared agonists at 10× the final concentration. Suspensions containing 150 μl/well were gently mixed by pipetting and incubated for 24 hours at 37ºC in a humidified incubator at 5% CO2. After culture, plates were centrifuged at 500xg and supernatant was carefully removed by pipetting without disturbing the cell pellet. Supernatants derived from human leukocyte stimulations were assayed by ELISA for TNF (BD Biosciences; San Jose, CA, USA). The minimum threshold for each analyte was set at the minimum detectable concentration for that particular assay (defined as three standard deviations above the mean background).
Generation and maturation of MoDCs with GM-CSF and IL-4
Isolated monocytes were seeded in 75cm2 tissue culture dishes for 5 days at 37°C in a humidified incubator at 5% CO2 with 106 cells/ml medium. Medium consisted of RPMI 1640 with L-glutamine (Gibco – Life Technologies; Carlsbad, CA, USA) supplemented with 1% Penicillin-Streptomycin-Glutamine (PSG) (Invitrogen – Life Technologies; Carlsbad, CA, USA) and 10% autologous plasma. This was supplemented with 50 ng/ml recombinant human (rh) IL-4 and 100 ng/ml rhGM-CSF (R&D Systems; Minneapolis, MN, USA). Additional fresh media and cytokines were provided on day 3 of incubation. After 5 days, immature MoDCs were harvested by gently pipetting only the loosely adherent fraction and re-plated (105 cells/well) in 96-well U-bottom plates in the presence or absence of TLRAs, and/or CLRAs, and/or sterile DPBS. MoDC arrays were then incubated for 18–24 hours at 37°C in a humidified incubator at 5% CO2. After this stimulation cells and supernatants were harvested and processed for further functional assays.
Gene expression analysis by quantitative RT-PCR array
Total ribonucleic acid (RNA) of newborn and adult MoDCs was isolated with the miRNeasy kit, according to manufacturer’s instructions (Qiagen, Inc.; Valencia, CA, USA). Total RNA from MoDCs was isolated and analyzed for quality and quantity using a NanoDrop 1000 spectrophotometer (Thermo Scientific; Wilmington, DE, USA). Complementary deoxyribonucleic acid (cDNA) was prepared from total RNA from each sample with a miScript II RT Kit, according to manufacturer’s instructions (Qiagen, Inc.). cDNA was quantified on a PAHS-052Z plate (Qiagen, Inc.). Quantitative real time-polymerase chain reaction (RT-PCR) was run on a 7300 real time PCR system (Applied Biosystems – Life Technologies, Life Technologies; Carlsbad, CA, USA). mRNA levels were normalized to housekeeping genes and quantified using the DD comparative threshold (Ct) method using the analysis tools provided by Qiagen (http://www.sabiosciences.com/pcr/arrayanalysis.php). ΔCt values were calculated using normalization to a panel of three housekeeping genes: B2M, GAPDH and RPLP0. For each gene, fold change was subsequently calculated by division of the dct in the treatment condition over that in the control condition.
ELISA
Supernatants from MoDCs stimulated for 6, 18, 24 or 72 hours in a humidified incubator (37°C, 5% CO2) with TLRAs, and/or CLRAs were analyzed for TNF, IL-18 or IL-23 production. For this, human enzyme-linked immunosorbent assay (ELISA) kits were used as per the manufacturer’s instruction. TNF: BD Opteia™ ELISA set (BD Biosciences; San Jose, CA, USA). IL-23: Human IL-23 Quanktikine ® ELISA kit (R&D systems; Minneapolis, MN, USA). IL-18: Human IL-18 ELISA kit (MBL International: Woburn, MA, USA). ELISA plates were read on a Versamax microplate reader with SoftMax Pro Version 5 (both from Molecular Devices; Sunnyville, CA, USA).
Mathematical assessment of TLRA/CLRA interactions
An adaptation of the Loewe method of additivity (33) was used to assess whether cytokine production after stimulation with agonist combinations was synergistic, additive or antagonistic. Concentration-response curves were subjected to regression analysis to determine the slope and Y-intercept of each curve in the exponential phase. The formula D = [Ac]/[Ae] + [Bc]/[Be] was used, where: [Ac] = the concentration of agonist A (TLR agonist) used in the combination of agonists that results in half the maximal TNF production measured with the combination of agonists, [Ae] = the concentration of agonist A (TLR agonist) used alone that results in half the maximal TNF production measured with the combination of agonists, [Bc] = the concentration of agonist B (CLR agonist) used in the combination of agonists that results in half the maximal TNF production measured with the combination of agonists and [Be] = the concentration of agonist B (CLR agonist) used alone that results in half the maximal TNF production measured with the combination of agonists. If D=1: Agonists A and B act additively, if D>1: Agonists A and B act antagonistically, and if D<1: Agonists A and B act synergistically.
Cytokine measurement by multi-analyte fluorescent bead-based array
The cytokine profile of both un-stimulated MoDCs and MoDCs stimulated for 18 hours with TLRAs and/or CLRAs was analyzed using multi-analyte bead array (Milliplex). Cytokines were quantified from culture supernatants with a custom Cytokine Human Magnetic 9-Plex Panel (Invitrogen – Life Technologies), including IFN-α2, IFN-γ, IL-10, IL-12p40, IL-12p70, IL-1α, IL-1β, IL-6, and TNF. Results were obtained with a MAGPIX system with xPONENT software (both from Luminex Corp.; Austin, TX, USA).
Flow Cytometry
MoDCs were resuspended in PBS/0.5% Human Serum Albumin (HSA, Octapharma USA, Inc., Hoboken, NJ, USA) and stained at 4°C with any of the following fluorescent antibodies: anti-CD14-V450 (clone MϕP9), anti-HLA-DR-PE.Cy7 (clone G46-6), anti-CD80-PE.Cy7 (clone L307.4), anti-CD83-APC (clone HB15e) and anti-CD209-V450 (clone DCN46) were purchased from BD Biosciences (San Jose, CA, USA), anti-TLR4-FITC (clone 76B357.1), anti-TLR7-PE (clone 4G6) and anti-TLR8-PE (clone 44C143) were purchased from Thermo Scientific (Wilmington, DE, USA). Anti-Dectin-1-APC (clone 259931) was purchased from R&D Systems (Minneapolis, MN, USA). Anti-Mincle antibody (clone 15H5) was purchased from InvivoGen (San Diego, CA, USA) and labeled using an AlexaFluor® 488 labeling kit (Life Technologies). Cells were analyzed on an LSRFortessa flow cytometer (Beckton Dickinson). MFI of the entire cell population was determined with Flowjo software version 10 (Tree Star, Inc.; Ashland, OR, USA). For samples that were stained with anti-TLR7 or anti-TLR8, which have intracellular targets, samples were first stained with antibodies against receptors present on the plasma membrane as described above before proceeding to staining for intracellular targets. These samples were fixed for 30 minutes at 4°C with 4% methanol-free paraformaldehyde (Alfa Aesar, Ward Hill, MA, USA) and subsequently permeabilized with BD Perm/Wash™ (BD Biosciences). Samples were then stained at 4°C with anti-TLR7 or anti-TLR8 in BD Perm/Wash™ for 30 minutes and washed with PBS before analysis.
Naïve T-cell stimulation
Naïve (CD45RA+CD45RO−) CD4+ T cells were purified using negative-selection beads (Miltenyi Biotec; Auburn, CA, USA). Isolated naïve T cells (>97% purity) were cultured for 6 days in 96-well plates at a density of 8×104 cells per well in 100% conditioned media from MoDC cultures which were stimulated as indicated, in the presence of CD3/CD28 T Cell Expander Dynabeads (one bead per cell; Life Technologies,). On day 6, beads were removed and replaced with fresh beads. Cells producing IFN-γ, IL-4, IL-10 or IL-17 were analyzed by intracellular cytokine staining after the addition of BD Golgiplug (BD Biosciences) during the final 6 hours of restimulation. Cells were made permeable with Cytofix/Cytoperm reagents (BD Biosciences). Cells were stained with anti-IFN-γ-PE.Cy7 (clone B27, BD Biosciences), anti-IL-17-APC (clone 41802, R&D Systems), anti-IL-4-V450 (clone 8D4-8, BD Biosciences) and anti-IL-10-AlexaFluor® 488 (clone JES3-9D7, Biolegend). Cells were analyzed for production of these four cytokines by flow cytometry on an LSRFortessa flow cytometer (Beckton Dickinson). Flowjo software version 10 (Tree Star, Inc) was used to analyze data and make representative histograms, which are shown in Figure 1, demonstrating gating strategy for IFN-γ- and IL-4-producing cells, based on unstained and single color controls.
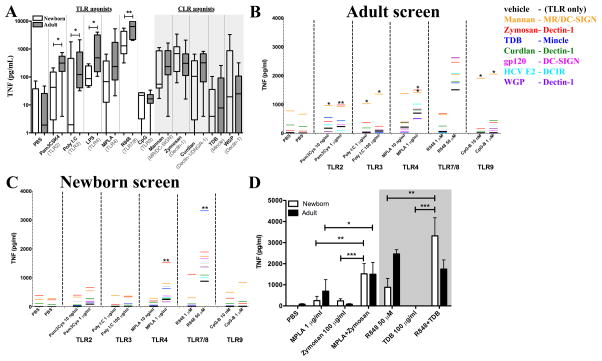
Figure 1. Targeted screening of adult and newborn MoDCs identifies distinct combinations of TLR- and CLR-agonists that markedly enhance TNF production.
A) Production of TNF by newborn and adult MoDCs in response to single agonists was measured by ELISA (n=8). B–C) Black lines indicate the amount of secreted TNF after stimulation with the TLRA alone, as indicated on the horizontal axis. Colored lines indicate TNF secretion after stimulation with the TLRA+CLRA (n=5–7). D) Bar diagram representation of the mean TNF production in response to identified combinations in panels B–C, and each single agonist (n=7). (Mean+SEM, unpaired Student’s t-test, *p<0.05, **p<0.01 and ***p<0.001)
Caspase-1 activity measurement
Newborn MoDCs were generated as described above and stimulated with individual or combined agonists, as indicated in Figure 3, at 5×105 cells per condition for 6 hours at 37°C. During the last hour of the incubation, a fluorescent Caspase-1 inhibitor, FAM-YVAD-FMK (ImmunoChemistry Technologies, LLC; Bloomington, MN, U.S.A.) was added to detect activated Caspase-1. Cells were subsequently fixed in 4% methanol-free paraformaldehyde and fluorescent intensity at 488 nm was measured by flow cytometry on an LSRFortessa flow cytometer (Beckton Dickinson, San Jose, CA, USA). Data was analyzed with Flowjo software version 10 (Tree Star, Inc., Ashland, OR, USA).
Figure 3. Characterization of innate immune pathways associated with synergistic R848+TDB activation of newborn MoDCs.
Newborn MoDCs were stimulated for 18 hours with R848 (50 μM), TDB (100 μg/ml) or the combination. The expression of a panel of 80 innate immune pathway-related genes was measured by qRT-PCR and the mean (n=3) depicted as a volcano plot with the y-axis inversely proportioned to the p-value (Paired Student’s t-test) and the x-axis corresponding to the fold-change in expression relative to unstimulated MoDCs (A). Expression of inflammasome-associated genes NLRP3 and IL1B, and NF-κB-pathway-associated genes IRAK1 and NFKB1 was further analyzed by comparing differences in mean expression between all treatment conditions (Mean+SEM, paired Student’s t-test, *p<0.05, **p<0.01 and ***p<0.001) (B,C). Inflammasome activation was confirmed by incubation of cells that were treated as indicated with FITC-labeled Caspase-1 substrate FAM-YVAD-FMK (representative experiment shown, n=3) (D) Activation of NF-κB was confirmed by detection of IκBa degradation in lysates from cells treated as indicated (representative experiment shown, n=3) (E).
Western blotting
Newborn MoDCs were generated as described above and stimulated with individual or combined agonists as indicated in Figure 3, at 5×105 cells per condition for 30 minutes at 37°C. Cells were lysed in RIPA buffer containing 1% protease inhibitors (Sigma-Aldrich Co. LLC.; St Louis, MO, USA). Protein concentration in cell lysates was determined using a BCA protein determination kit (Life Technologies) and 25 μg of each sample was run on a 10% Bis-Tris protein gel (Life Technologies) and transferred to a nitrocellulose membrane. IκBα and GAPDH were detected using mouse monoclonal antibodies (clone L35A5, Cell Signaling; Danver, MA, USA and clone 6C5, Abcam; Cambridge, MA, USA) and HRP-linked anti-mouse IgG (Cell Signaling, Danvers, MA, USA). After IκBα detection, gels were stripped and re-probed for detection of GAPDH.
Statistical analysis
Statistical analyses employed Prism 4 software (GraphPad Software; La Jolla, CA, USA) using an unpaired or paired Student t test as indicated. P values of less than 0.05 were considered statistically significant and indicated with stars: *p<0.05, **p<0.01 and ***p<0.001.
Results
Screening TLRA/CLRA combinations for synergistic enhancement of TNF production by neonatal MoDCs
As compared to their adult counterparts, neonatal monocytes and DCs demonstrate diminished TLR4-mediated production of TNF (4, 14). To assess whether combinations of PRR-activating agents could overcome this limitation and stimulate robust neonatal cytokine production, we employed an in vitro approach using human MoDCs cultured in 10% (vol/vol) autologous plasma, a rich source of age-specific soluble factors that modulate the immune response (2). The anticipated impairment in TLR-mediated TNF production by newborn MoDCs relative to adult MoDCs was confirmed (Figure 1A), with lesser TNF production in response to Pam3CSK4 (TLR2), Poly I:C (TLR3), MPLA (TLR4), LPS (TLR4), and R848 (TLR7/8). These observations, together with published studies (19, 34) suggest that TLR-mediated NF-κb activation is impaired in newborn innate immune cell populations.
Some CLRs activate NF-κB in an IRAK1-independent fashion (29), raising the possibility that they may be able to act together with TLRAs to amplify NF-κB activation. To assess whether combinations of CLRAs and TLRAs may overcome impaired neonatal production of TNF and other Th1-polarizing responses, we screened different combinations of TLRAs and CLRAs for induction of adult-like levels of TNF production from newborn MoDCs (Figure 1B and C).
Adult MoDCs demonstrated increased TNF production after combined stimulation with alkali-treated Zymosan (Dectin-1) and Pam3Cys (TLR2) or MPLA (TLR4), as compared to stimulation with each of the agonists individually. In addition, combined stimulation with Mannan (Mannose Receptor) and CpG (TLR9; Figure 1B) also enhanced TNF production. Stimulation of newborn MoDCs with TLRAs induced lower TNF production compared to adult MoDCs (Figure 1A and C). Two combinations of agonists, however, induced robust TNF secretion in newborns: (1) The combination of R848 (TLR7/8) and TDB (Mincle) induced greater TNF production than any other TLRA/CLRA combination, even in comparison to adult MoDCs; and (2) Zymosan (Dectin-1) and MPLA (TLR4) induced elevated, adult-like TNF secretion. These TLRA/CLRA combinations induced significantly greater TNF production than that induced by each of the individual agonists (Figure 1D). The role of age-specific soluble mediators was explored (Supplementary Figure 1). Newborn MoDCs cultured in fetal bovine serum (FBS) demonstrated less or no impairment of TLR7/8-mediated secretion of TNF or IL-12p70 as compared to adult MoDCs. The impairment in the production of these two Th1-polarizing cytokines, noted in vivo in newborn humans and mice (35, 36), is clearly observed when cells are cultured in autologous plasma (AP). These observations indicate the importance of studying these responses in the presence of age-specific immunomodulatory soluble factors (eg, plasma).
Synergistic stimulation of neonatal MoDCs using R848+TDB enables Th1 polarization of autologous naïve CD4+ T cells
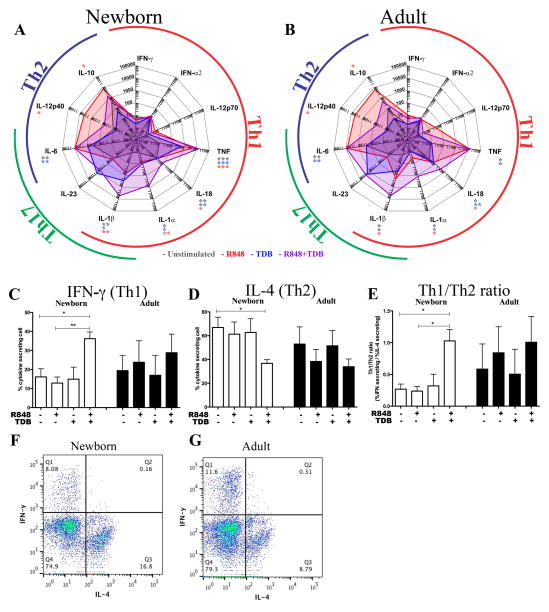
In addition to increasing the production of TNF, we investigated whether the combination R848+TDB could drive newborn MoDCs to secrete additional Th1-inducing cytokines. Interestingly, in comparison to the same concentration of R848 or TDB alone, the combination of R848+TDB induced 5–10 fold lower production of IL-12p40 (Th2) and IL-10 (Treg), coinciding with a significant increase in Th1/17 cytokines IL-1β, IL-1α, IL-18 and TNF (Figure 2A–B). To evaluate the effect of these complex changes of MoDC-derived cytokines on naïve T-cell polarization, we aspecifically stimulated naïve CD4+ T cells with CD3/CD28 beads, in the presence of culture supernatants from R848/TDB-stimulated autologous MoDCs as indicated in Figure 2C–E. A significant increase in IFN-γ-producing T cells (Th1) and corresponding reduction in IL-4-producing Th2 cells was observed after synergistic stimulation with R848+TDB. The percentage of T cells secreting IL-10 or IL-17 did not change significantly under any condition (Supplementary Figure 2). In adult T cells, the ratio between Th1 and Th2 was already more balanced than in newborns in the basal condition, and did not change significantly upon treatment with TLRA/CLRA-treated MoDC supernatants (Figure 2C–G), but the ratio of Th1/Th2 cells in newborns increased significantly after treatment with culture supernatants from synergistically activated autologous MoDCs.
Figure 2. Dual stimulation using R848 and TDB shifts the pattern of cytokine production by human adult and newborn DCs and enables the polarization of newborn naïve CD4+ T cells to Th1 cells.
Adult and newborn DCs were stimulated with R848 (50 μM), TDB (100 μg/ml), or both (A+B). This restored TNF production to adult-like levels in newborn cells and enhanced production IL-1α, IL-1β and IL-18 (n=6, Paired Student’s t-test, *p<0.05, **p<0.01 and ***p<0.001). Naïve (CD4+CD45RA+CD45RO−) T cells were isolated, cultured in 10% (vol/vol) autologous plasma, and activated for 6 days with anti-CD3/CD28 beads, in the presence of culture supernatants of autologous MoDCs activated with agonists as indicated. After 6 days, IFN-γ- and IL-4-producing cells were quantified by flow cytometry following the addition of Brefeldin A. Mean relative percentage of IFN-γ producing cells (C), mean relative percentage of IL-4 producing cells (D), and ratio of IFN-γ producing cells over IL-4 producing cells (E) show an increase in Th1 polarization after treatment with culture supernatants from R848+TDB-activated MoDCs (n=4, Mean+SEM, paired Student’s t-test, *p<0.05, **p<0.01 and ***p<0.001). Representative images of flow cytometric quantification of IFN-γ and IL-4 producing cells are shown. Depicted are control samples of a representative newborn and adult whose T cells were activated with anti-CD3/28 in the presence of supernatant from unstimulated autologous MoDCs. (F–G).
Synergistic activation of neonatal MoDCs through R848+TDB requires restoration of NF-κB activation and inflammasome activation
We next characterized the molecular mechanism of synergistic activation of newborn MoDCs by R848 and TDB by qRT-PCR gene expression analysis of a select panel of 80 innate immune pathway genes. Figure 3A shows that upon stimulation of newborn MoDCs with R848+TDB, 9 genes were upregulated >3-fold (p<0.05). Figures 3B+C demonstrate that treatment of newborn MoDCs with R848+TDB significantly increased the expression of NF-κB- and NLRP3 inflammasome-associated genes, as compared with treatment with either of the agonists alone. As changes in pathway-associated gene expression do not necessarily correspond with pathway activation, we investigated whether synergistic activation of newborn MoDCs coincides with increased inflammasome and NF-κB activation, by measuring Caspase-1 activation and IκBα degradation, respectively. R848 induced moderate Caspase-1 activation (Figure 3D) and moderate IκBα degradation (3E), whereas TDB induced greater Caspase-1 activation but less IκBα degradation. Only when treated with the combination of R848+TDB was robust Caspase-1 activation and near complete IκBα degradation observed.
We used pharmacological inhibitors of signaling adapters IRAK1/4, NF-κB, Syk and Caspase-1 to evaluate whether the NF-κB and inflammasome pathways, noted to be associated with synergistic activation in Figure 3, are required for synergistic enhancement of TNF and IL-1β secretion. A selective IRAK1/4 inhibitor did not inhibit R848-induced TNF production of newborn MoDCs (Figure 4), consistent with minimal IRAK1 expression in these cells (unpublished observations, (34)). Both Syk- and NF-κB-inhibitors reduced synergistically-induced TNF production in a concentration-dependent manner. Both these inhibitors, as well as Caspase-1 inhibitor inhibited synergistic induction of IL-1β production. Absolute values of cytokine secretion are demonstrated in Supplementary Figure 3.
Figure 4. Inhibition of intracellular signaling molecules confirms their role in synergistic induction of TNF and IL-1β by newborn MoDCs.
Newborn and adult MoDCs were pre-treated with Benzimidazole (IRAK1/4 inhibitor; 5 and 50 μM) (A,B), R406 (Syk inhibitor; 5 and 50 μM) (C,D), Celastrol (NF-κB inhibitor; 1 and 10 μM) (E,F) or VX-765 (Caspase-1 inhibitor; 10 or 100 μM) (G,H) for 1 hour prior to activation with R848, TDB, or R848+TDB. Secretion of TNF and IL-1β was measured in supernatant after an 18-hour stimulation. Cytokine production was normalized to condition with no pharmacological inhibitors to visualize the percentage of inhibition. (n=6, mean+SEM, paired Student’s t-test, *p<0.05, **p<0.01 and ***p<0.001)
Synergistic activation of MoDCs through TLR4 and Dectin-1 is less age-restricted and less robust
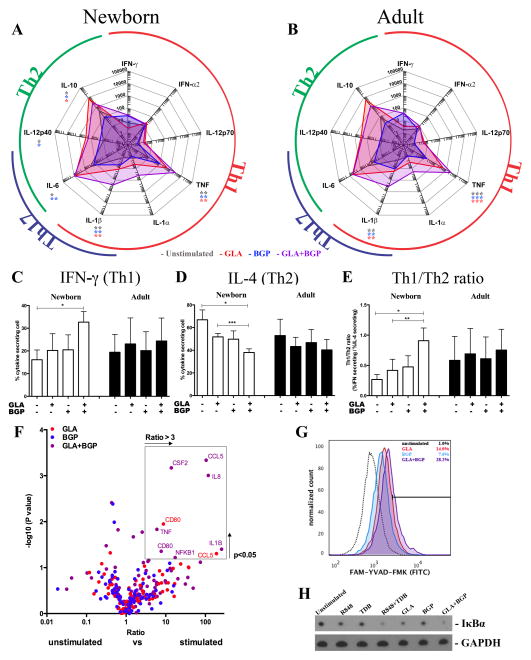
The combination of MPLA+Zymosan (Figure 1) also elevated the production of TNF, in newborns and adults. The observed enhancement in TNF production using MPLA and Zymosan is also present when GLA-AF is used instead of MPLA, and when BGP, another Dectin-1 agonist, is used instead of alkali-treated Zymosan (Figure 5 A). Stimulation of MoDCs through GLA-AF+BGP also induced a shift cytokine secretion, with a statistically significant increase in TNF and IL-1β (Figure 5A/B). In contrast to R848+TDB (Figure 2), this GLA-AF+BGP combination also enhanced both TNF and IL-1β in adult MoDCs, and there was a decrease in IL-10 observed, but no decrease in IL-12p40. Culture supernatants from (GLA-AF+BGP)-treated newborn MoDCs also induced Th1 polarization of newborn CD4+ T cells to a Th1 phenotype (Figure 5C–E). Analysis of NF-κB- and NLRP3 inflammasome pathways revealed that GLA-AF+BGP also induces near complete degradation of IκBα, but Caspase-1 activation was less pronounced than observed with R848+TDB (Figure 5F–G, Supplementary Figure 3).
Figure 5. Characterization of synergistic dual GLA-AF+BGP activation of newborn MoDCs.
Adult and newborn MoDCs were stimulated for 18 hours with GLA-AF (1000 ng/ml), BGP (100 μg/ml) or the combination. Secreted cytokines were measured by multiplexing bead array (A,B) (n=3–6, Paired Student’s t-test, *p<0.05, **p<0.01 and ***p<0.001). IFN-γ- and IL-4-producing cells were quantified by flow cytometry following treatment of autologous naïve CD4+ T cells with MoDC culture supernatant for 6 days and subsequent addition of Brefeldin A. Mean relative percentage of IFN-γ producing cells (C), mean relative percentage of IL-4 producing cells (D), and ratio of IFN-γ producing cells over IL-4 producing cells (E) show an increase in Th1 polarization after treatment with culture supernatants from R848+TDB-activated MoDCs (n=4, mean+SEM, paired Student’s t-test, *p<0.05, **p<0.01 and ***p<0.001). The expression of a panel of 80 innate immune pathway-related genes was measured by qRT-PCR and the mean (n=3) depicted as a volcano plot (F). Inflammasome activation was confirmed by incubation of cells that were treated as indicated with FITC-labeled Caspase-1 substrate FAM-YVAD-FMK (representative experiment shown, n=3) (G) Activation of NF-κB was confirmed by detection of IκBa degradation in lysates from cells treated as indicated (representative experiment shown, n=3) (H).
Synergistic cytokine induction by TLRA/CLRA combinations is quantifyably age-specific
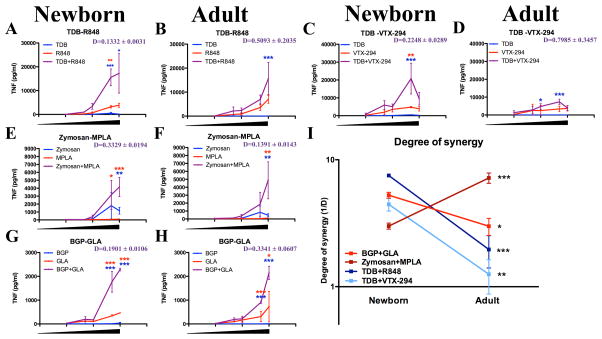
We further characterized age-specific adjuvantation by quantifying the amount of synergy induced by R848+TDB (Figure 6A/B). Concentration-response curves were used to assess whether the observed enhancement by combining agonists is equal to the sum of the effects observed with individual agonists (i.e., was additive) or if these agonists activate the newborn cells synergistically. A modification of the Loewe definition of additivity was applied to determine whether the agonists act synergistically (D<1), additively (D=1) or antagonistically (D>1, see methods section) (33). A similar effect was observed when R848 (TLR7/8) was substituted for VTX-294 (Figure 6 C/D), a TLR8-selective benzazepine derivative (37), indicating that synergy was not a compound-specific effect, but rather a general effect of stimulation through the receptors TLR7/8 and Mincle. The combination of (MPLA+Zymosan) (Figure 6 E/F) was synergistic in both newborns and adults. Similarly, the observed synergy between (MPLA+Zymosan) is also present using (GLA-AF+BGP) (Figure 6 G/H). Plotting the degree of synergy (1/D) confirmed that synergistic enhancement of TNF production induced by (R848+TDB) or (VTX-294+TDB) was most prominent in newborn MoDCs (Figure 6I), as this combination did not significantly act in synergy towards adult MoDCs (Figure 1B). The combinations (MPLA+Zymosan) and (GLA+BGP) acted synergistically to a similar degree in newborn and adult cells. These observations confirm and expand upon the synergistic pathway in newborn MoDCs triggered via dual activation of TLR4 and Dectin-1 (38). To determine whether the synergistic enhancement of cytokine production correlated with increased protein expression of costimulatory receptors CD80 and CD83 or the receptors engaged by the agonists, these receptors were quantified by flow cytometry (Supplementary Figure 4 J–M). A modest increase in TLR8 was detected after dual stimulation with TDB+R848, as compared to stimulation with single agonists, as well as a modest increase in Dectin-1 after stimulation with GLA+BGP. These findings, however, were not statistically significant.
Figure 6. Specific TLR/CLR agonist combinations demonstrate age-specific synergistic induction of TNF production.
MoDCs were stimulated with a concentration range of TLR7/8-agonist (R848 or VTX-294, 1, 5, 10, 25 and 50 μM), Mincle-agonist (TDB, 1, 5, 10, 50 and 100 μg/ml), or both (A–D) or with a TLR4-agonist (MPLA or GLA-AF, 10, 50, 100, 500 and 1000 ng/ml), Dectin-1-agonist (Zymosan or BGP, 1, 5, 10, 50 and 100 μg/ml), or both (E–H) (n=5–7). Blue and red stars indicate statistically significant differences between combination and the CLR agonist alone or TLR agonist alone, respectively. The Loewe definition of additivity was applied to determine whether the agonists act synergistically (D<1), additive (D=1) or antagonistically (D>1). Degree of synergy (1/D) was compared between age groups (I). (Mean+SEM, unpaired Student’s t-test, *p<0.05, **p<0.01 and ***p<0.001).
Innate immune responses vary markedly with age (8, 14). To assess the ability of (R848+TDB) to act in synergy across different age groups, their ability to activate primary leukocytes across four different age groups was determined using a whole-blood assay. Newborn cord blood as well as peripheral blood from infants (~6 months), adults (18–40 years) and elders (>65 years) was diluted (5x) in RPMI and directly stimulated with agonists as indicated in Supplementary Figure 4 A–D. Analysis of TNF secretion in culture supernatants indicated synergistic activation across different concentrations of agonists in newborn, but not infant, adult or elderly donors. Indeed, for elders the combinations appeared to be antagonistic highlighting dramatic differences in adjuvant interaction with age.. The degree of synergy induced by (R848+TDB) in purified monocytes was similar to MoDCs (Supplementary Figure 4 E/F and G–I, respectively).
Discussion
The impaired ability of newborns to mount a Th1 response after exposure to microbes or vaccines represents a major challenge in protecting against intracellular pathogens in early life (35). In this study, we employed human MoDCs cultured in 10% autologous plasma, a rich source of age-specific immunomodulatory factors (2, 16–18), as a model to compare the functionality of the newborn APCs to that of adults and discovered novel age-dependent synergy between the receptors TLR7/8 and Mincle.
As described previously, we found that TLR-mediated responses of newborn MoDCs are distinct from those of adult MoDCs, with an impairment of TNF production but robust production of Th2/Treg-polarizing cytokines IL-6 and IL-10 (Figure 1, 2)(18, 37). In response to most TLRAs, newborn MoDCs produced less TNF (Figure 1). This raises the possibility that impaired NF-κB activation contributes to impaired TLR-mediated cytokine production of newborn DCs, a hypothesis supported by prior observations (34). Besides the canonical TRAF6-IRAK1-mediated pathway of NF-κB activation that is commonly induced by TLR activation, there are alternative pathways leading to NF-κb activation. Some CLRs, such as Dectin-1, can activate NF-κB via signaling through PLC and CARD9 (30, 39, 40). Stimulation of DCs through CLRs induces modest TNF production (Figure 1) and often results in Th2 polarization (41–45). Dual engagement of TLRs and CLRs, however, can enhance Th1 or Th17 polarization (21, 28, 31, 32, 46–48). Conversely, there are also examples of CLRs inhibiting Th1 immunity (49, 50). The rationale for testing TLR-CLR synergy was three-fold: (1) TLR-CLR synergy has been described in adults, (2) TLR-CLR crosstalk can activate NF-κB via alternative pathways raising the possibility that it may overcome impairment of newborn DCs to elicit TLR-mediated NF-κB activation, and (3) the live attenuated BCG vaccine, which is effective at birth, activates both TLRs and CLRs (21, 22, 51). We therefore investigated whether dual stimulation of newborn MoDCs with combinations of TLRAs and CLRAs induced Th1-polarization of newborn MoDCs and CD4+ T cells.
Using a targeted screen approach for synergistic PRR agonist combinations we measured the ability of combinations of TLRAs and CLRAs to induce TNF, a Th1-polarizing cytokine important to innate and adaptive immune responses by newborn and adult MoDCs (52). Distinct combinations of TLRAs and CLRAs activated newborn and adult MoDCs. With respect to adult MoDCs, several combinations, many of which have been previously described (21, 48, 53), induced markedly greater TNF production compared to individual agonists: (a) Zymosan (alkali-treated, Dectin-1 agonist) and Pam3Cys (TLR2), (b) Zymosan and MPLA (TLR4), (c) Mannan (Mannose Receptor agonist) and Pam3Cys (TLR2) and (d) Mannan and CpG (TLR9).
Of note, distinct combinatorial interactions between TLRAs and CLRAs were observed in newborn MoDCs. The combination of TLR4 and Dectin-1 agonists is known to induce robust TNF production by both newborn and adult MoDCs (38, 53). More specifically, the Dectin-1 agonist Curdlan induces Th1 polarization by itself (in vivo) and in combination with certain TLRAs (in vitro) (38). Our results confirm that, indeed, stimulation of newborn MoDCs through Dectin-1 and TLR4, using MPLA and Zymosan can induce Th1 polarization in newborns (Figures 2 and 5). Interestingly, dual stimulation of newborn MoDCs with R848 (TLR7/8) and TDB (Mincle) also enhanced TNF production. Remarkably, the synergy evident with Mincle and TLR7/8 was not only unique to newborn MoDCs, but also induced neonatal TNF production greater than that induced by any combination in adult MoDCs.
Synergistic stimulation of newborn MoDCs with (R848+TDB) not only increased Th1/17 cytokines, but also reduced production of Th2/Treg cytokines (Figure 2). Overall, synergistic activation of newborn MoDCs shifted the balance of Th-polarizing cytokines, favoring a Th1 response over a Th2 response. A study employing MoDCs generated in fetal bovine serum suggested that induction of a Th1 response through Dectin-1 and TLRs in vitro using newborn MoDCs depended on induction of IL12p70 secretion by unlocking transcriptional control over the p35 subunit (38). In our study, to maximize the physiologic relevance of our findings, we cultured MoDCs in autologous plasma as previously described (18), a rich source of age-specific immunomodulatory factors that shape a distinct immune response (2) that may have suppressed IL-12p70 production. Impairment in production of IL-12p70 and TNF by neonatal MoDCs was only observed when cells were cultured in autologous plasma (Supplementary Figure 1). Contrary to the study of Lemoine et al. (38), our study demonstrates a robust Th1 polarization of neonatal MoDCs characterized by synergistic induction of TNF, IL-18 and IL-1β production, coupled with suppression of IL-12p40 and IL-10 production. We speculate that our distinct observations may be due to the use of autologous plasma instead of FBS.
Given the age-specific complexity of the soluble environment, it was important to establish how the soluble fraction, including the distinct cytokine composition, affected polarization of newborn CD4+ T cells. To this end, naïve CD4+ T cells were activated with CD3/CD28 beads in the presence of culture supernatant from activated MoDCs, in an adaptation of the method of Volpe et al. (54). Intracellular cytokine staining demonstrated that culture supernatants from dual-stimulated autologous newborn MoDCs reduced the proportion of cells that are Th2-polarized, and enhanced polarization to Th1 cells (Figure 2). In addition to DC-intrinsic factors and soluble mediators, T-cell-intrinsic factors also contribute to the phenotype of neonatal CD4+ T cell polarization (55, 56). In our study, >65% of newborn naïve CD4 T cells cultured in conditioned media from unstimulated MoDCs differentiated into Th2 cells (Figure 2). The aim of this study was not to identify T-cell associated signaling events required for differentiation of newborn T cells to a Th1 phenotype, but because an agonist combination was identified that has the ability to induce a Th1 phenotype in newborn naïve T cells, future studies could address this phenomenon.
Characterization of the impact of dual TLRA/CLRA stimulation on the innate immune transcriptome (Figure 3) suggested that both R848+TDB and GLA-AF+BGP enhance signaling through NF-κB and the NLRP3 inflammasome. Indeed, enhanced activation of both NF-κB and Caspase-1 was required for synergistic production of TNF and IL-1β by newborn MoDCs (Figure 4). Concentration-response curves indicated that the amount of TNF produced after dual stimulation with either combination was more than the sum of its parts, as confirmed by an adaptation of the Loewe model of additivity (33). Synergy was not compound-specific, but rather appeared to be a common mechanism elicited through activation of certain combinations of TLRs and CLRs (Figure 2). For example, substitution of R848 for VTX-294 (TLR8) resulted in a similar synergy, as did substitution of MPLA for GLA-AF (TLR4) and substitution of Zymosan for BGP (Dectin-1) (Figure 6 and Supplementary Figure 4). Using a whole blood assay platform, the extent of TLRA/CLRA synergy varied with the agonist used for a given receptor and was age-specific with greatest synergy in early life with diminished or even antagonistic interactions in elders (>65 years old). TLRA/CLRA synergy was also observed when stimulating newborn and adult primary monocytes (Supplementary Figure 4 E/F), which also express Mincle (57). The degree of synergistic TNF secretion upon stimulation with (R848+TDB) is similar between MoDC and monocytes, and greater than synergy in the whole blood platform. Of note, age-specific synergy was not due to different kinetics of cell activation, as synergistic activation of newborn and adult MoDCs using R848+TDB did not change notably over time (Supplementary Figure 4 H–I).
Th1-polarizing adjuvantation systems may be particularly useful for vaccines targeting intracellular microbes including multiple intracellular pathogens for which there are unmet vaccine needs such as tuberculosis, malaria, respiratory syncytial virus and human immunodeficiency virus. As vaccines are given to healthy individuals, vaccine adjuvant development must emphasize safety bearing in mind that synergistic adjuvantation systems have potential for reactogenicity and should be used only as necessary for vaccine effectiveness, as may be the case for populations with relatively weak immune responses. In this context, future development of such synergistic adjuvantation systems may employ methods to localize their action such as chemical modification to enhance hydrophobicity and/or nanoparticle encapsulation to target such formulations to APCs (58, 59). Of note, targeting vaccine antigens to the endocytic CLRs by covalent linkage to CLRAs may also enhance internalization by DCs, further enhancing vaccine efficacy. Encapsulation of TLRA/CLRA combinations, as has been demonstrated with TDB and Poly (I:C) (60), may facilitate co-delivery, resulting in Th1-polarization.
Overall, dual stimulation of newborn MoDCs with combinations of: 1) a TLR7/8 agonist (e.g., R848 or VTX-294) and a Mincle agonist (e.g., TDB) or 2) TLR4 agonists (e.g., MPLA or GLA-AF) and Dectin-1 agonists (e.g., Zymosan or BGP) induced adult-like levels of Th1-/Th17- polarizing cytokines (e.g., TNF and IL-1β, respectively). As the newborn immune system is prone to develop a Th2 response after stimulation with either TLRAs or CLRAs individually and as the TLRA/CLRA combinations demonstrated distinct degrees of synergy with the age of the study particpants, our results suggest a new paradigm in which immune ontogeny - i.e., age - is taken into account in assessing the potential of combination adjuvant systems. Indeed, the TLRA/CLRA combinations identified in our study may represent novel adjuvantation systems to enable early life immunization against intracellular pathogens.
Supplementary Material
Acknowledgments
The authors thank Drs. Michael Wessels, Richard Malley and Matthew Pettengill for helpful conversations. We thank Dr. Gary Fleisher for his support for the Precision Vaccines Program. We also thank Dr. Ronald Samuels, Ms. Michaela Banks and the nursing staff at the Children’s Hospital Primary Care Center for their assistance with infant blood donations. The assistance of the labor and delivery staff at Beth Israel Deaconess Hospital is gratefully acknowledged. We thank Dr. Sweta Joshi and members of the Brigham and Women’s Hospital (BWH) Vaccine Clinical Research Site for assistance with elderly blood collections.
OL’s laboratory is supported by a Boston Children’s Hospital Department of Medicine award to the Precision Vaccines Program, Global Health (OPPGH5284) and Grand Challenges Explorations (OPP1035192) awards from the Bill & Melinda Gates Foundation and by NIH grants 1R01AI100135-01 and 3R01AI067353- 05S1 and National Institute of Allergy & Infectious Diseases Adjuvant Discovery Program, Contract No. HHSN272201400052C as well as a UO1 award on Molecular Mechanisms of Combination Adjuvants (U01AI124284-01). SvH was supported by an Early Career Award from the Thrasher Research Fund. The Levy Laboratory has also received sponsored research support from VentiRx Pharmaceuticals, 3M Drug Delivery Systems, MedImmune, Crucell (Johnson & Johnson) and Shire.
Abbreviations
- APC
Antigen-presenting cell
- BCG
Bacille Calmette-Guérin
- BGP
Beta-glucan peptide
- cAMP
cyclic adenosine monophosphate
- CLR
C-type Lectin Receptor
- CLRA
C-type Lectin Receptor agonist
- DC
Dendritic cell
- GLA
Glucopyranosyl Lipid Antigen
- IL
Interleukin
- Mincle
Macrophage-inducible C-type Lectin
- MoDC
Monocyte-derived dendritic cell
- MPLA
Monophosphoryl Lipid A
- PRR
Pattern-recognition Receptor
- TDB
Trehalose-6,6-dibehenate
- TLR
Toll-like Receptor
- TLRA
Toll-like Receptor agonist
- Th
T-helper cell
- TNF
Tumor Necrosis Factor
- Treg
Regulatory T cell
References
- 1.Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher KA, Kalfa TA, Shaaban AF, Way SS. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504:158–162. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pettengill MA, van Haren SD, Levy O. Soluble mediators regulating immunity in early life. Front Immunol. 2014;5:457. doi: 10.3389/fimmu.2014.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willems F, Vollstedt S, Suter M. Phenotype and function of neonatal DC. Eur J Immunol. 2009;39:26–35. doi: 10.1002/eji.200838391. [DOI] [PubMed] [Google Scholar]
- 4.Angelone DF, Wessels MR, Coughlin M, Suter EE, Valentini P, Kalish LA, Levy O. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-alpha production in vitro and in vivo. Pediatr Res. 2006;60:205–209. doi: 10.1203/01.pdr.0000228319.10481.ea. [DOI] [PubMed] [Google Scholar]
- 5.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES, 3rd, Hajjar AM, Hawkins NR, Self SG, Wilson CB. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen AC, Mills KH. Improved pertussis vaccines based on adjuvants that induce cell-mediated immunity. Expert Rev Vaccines. 2014;13:1253–1264. doi: 10.1586/14760584.2014.936391. [DOI] [PubMed] [Google Scholar]
- 7.Kamphuis T, Meijerhof T, Stegmann T, Lederhofer J, Wilschut J, de Haan A. Immunogenicity and protective capacity of a virosomal respiratory syncytial virus vaccine adjuvanted with monophosphoryl lipid A in mice. PLoS One. 2012;7:e36812. doi: 10.1371/journal.pone.0036812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy O, Goriely S, Kollmann TR. Immune response to vaccine adjuvants during the first year of life. Vaccine. 2013;31:2500–2505. doi: 10.1016/j.vaccine.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarz TF. AS04-adjuvanted human papillomavirus-16/18 vaccination: recent advances in cervical cancer prevention. Expert Rev Vaccines. 2008;7:1465–1473. doi: 10.1586/14760584.7.10.1465. [DOI] [PubMed] [Google Scholar]
- 11.Clegg CH, Roque R, Perrone LA, Rininger JA, Bowen R, Reed SG. GLA-AF, an emulsion-free vaccine adjuvant for pandemic influenza. PLoS One. 2014;9:e88979. doi: 10.1371/journal.pone.0088979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coler RN, Bertholet S, Moutaftsi M, Guderian JA, Windish HP, Baldwin SL, Laughlin EM, Duthie MS, Fox CB, Carter D, Friede M, Vedvick TS, Reed SG. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One. 2011;6:e16333. doi: 10.1371/journal.pone.0016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Biggelaar AH, Pomat WS. Immunization of newborns with bacterial conjugate vaccines. Vaccine. 2013;31:2525–2530. doi: 10.1016/j.vaccine.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity. 2012;37:771–783. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burl S, Townend J, Njie-Jobe J, Cox M, Adetifa UJ, Touray E, Philbin VJ, Mancuso C, Kampmann B, Whittle H, Jaye A, Flanagan KL, Levy O. Age-dependent maturation of Toll-like receptor-mediated cytokine responses in Gambian infants. PLoS One. 2011;6:e18185. doi: 10.1371/journal.pone.0018185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belderbos ME, Levy O, Stalpers F, Kimpen JL, Meyaard L, Bont L. Neonatal plasma polarizes TLR4-mediated cytokine responses towards low IL-12p70 and high IL-10 production via distinct factors. PLoS One. 2012;7:e33419. doi: 10.1371/journal.pone.0033419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy O, Coughlin M, Cronstein BN, Roy RM, Desai A, Wessels MR. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J Immunol. 2006;177:1956–1966. doi: 10.4049/jimmunol.177.3.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philbin VJ, Dowling DJ, Gallington LC, Cortes G, Tan Z, Suter EE, Chi KW, Shuckett A, Stoler-Barak L, Tomai M, Miller RL, Mansfield K, Levy O. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J Allergy Clin Immunol. 2012;130:195–204 e199. doi: 10.1016/j.jaci.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood. 2006;108:1284–1290. doi: 10.1182/blood-2005-12-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heldwein KA, Liang MD, Andresen TK, Thomas KE, Marty AM, Cuesta N, Vogel SN, Fenton MJ. TLR2 and TLR4 serve distinct roles in the host immune response against Mycobacterium bovis BCG. J Leukoc Biol. 2003;74:277–286. doi: 10.1189/jlb.0103026. [DOI] [PubMed] [Google Scholar]
- 21.Yadav M, Schorey JS. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108:3168–3175. doi: 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsunaga I, Moody DB. Mincle is a long sought receptor for mycobacterial cord factor. J Exp Med. 2009;206:2865–2868. doi: 10.1084/jem.20092533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, Agger EM, Stenger S, Andersen P, Ruland J, Brown GD, Wells C, Lang R. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol. 2010;184:2756–2760. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen KJ, Larsen N, Biering-Sorensen S, Andersen A, Eriksen HB, Monteiro I, Hougaard D, Aaby P, Netea MG, Flanagan KL, Benn CS. Heterologous immunological effects of early BCG vaccination in low-birth-weight infants in Guinea-Bissau: a randomized-controlled trial. J Infect Dis. 2015;211:956–967. doi: 10.1093/infdis/jiu508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cambi A, Figdor CG. Dual function of C-type lectin-like receptors in the immune system. Curr Opin Cell Biol. 2003;15:539–546. doi: 10.1016/j.ceb.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Kamath AT, Mastelic B, Christensen D, Rochat AF, Agger EM, Pinschewer DD, Andersen P, Lambert PH, Siegrist CA. Synchronization of dendritic cell activation and antigen exposure is required for the induction of Th1/Th17 responses. J Immunol. 2012;188:4828–4837. doi: 10.4049/jimmunol.1103183. [DOI] [PubMed] [Google Scholar]
- 27.Kamath AT, Rochat AF, Christensen D, Agger EM, Andersen P, Lambert PH, Siegrist CA. A liposome-based mycobacterial vaccine induces potent adult and neonatal multifunctional T cells through the exquisite targeting of dendritic cells. PLoS One. 2009;4:e5771. doi: 10.1371/journal.pone.0005771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eberle ME, Dalpke AH. Dectin-1 stimulation induces suppressor of cytokine signaling 1, thereby modulating TLR signaling and T cell responses. J Immunol. 2012;188:5644–5654. doi: 10.4049/jimmunol.1103068. [DOI] [PubMed] [Google Scholar]
- 29.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol. 2009;9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kingeter LM, Lin X. C-type lectin receptor-induced NF-kappaB activation in innate immune and inflammatory responses. Cell Mol Immunol. 2012;9:105–112. doi: 10.1038/cmi.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feinberg H, Jegouzo SA, Rowntree TJ, Guan Y, Brash MA, Taylor ME, Weis WI, Drickamer K. Mechanism for recognition of an unusual mycobacterial glycolipid by the macrophage receptor mincle. J Biol Chem. 2013;288:28457–28465. doi: 10.1074/jbc.M113.497149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dennehy KM, Ferwerda G, Faro-Trindade I, Pyz E, Willment JA, Taylor PR, Kerrigan A, Tsoni SV, Gordon S, Meyer-Wentrup F, Adema GJ, Kullberg BJ, Schweighoffer E, Tybulewicz V, Mora-Montes HM, Gow NA, Williams DL, Netea MG, Brown GD. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38:500–506. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berenbaum MC. Criteria for analyzing interactions between biologically active agents. Adv Cancer Res. 1981;35:269–335. doi: 10.1016/s0065-230x(08)60912-4. [DOI] [PubMed] [Google Scholar]
- 34.Lederhuber H, Baer K, Altiok I, Sadeghi K, Herkner KR, Kasper DC. MicroRNA-146: tiny player in neonatal innate immunity? Neonatology. 2011;99:51–56. doi: 10.1159/000301938. [DOI] [PubMed] [Google Scholar]
- 35.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adkins B, Bu Y, Guevara P. The generation of Th memory in neonates versus adults: prolonged primary Th2 effector function and impaired development of Th1 memory effector function in murine neonates. J Immunol. 2001;166:918–925. doi: 10.4049/jimmunol.166.2.918. [DOI] [PubMed] [Google Scholar]
- 37.Dowling DJ, Tan Z, Prokopowicz ZM, Palmer CD, Matthews MA, Dietsch GN, Hershberg RM, Levy O. The ultra-potent and selective TLR8 agonist VTX-294 activates human newborn and adult leukocytes. PLoS One. 2013;8:e58164. doi: 10.1371/journal.pone.0058164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lemoine S, Jaron B, Tabka S, Ettreiki C, Deriaud E, Zhivaki D, Le Ray C, Launay O, Majlessi L, Tissieres P, Leclerc C, Lo-Man R. Dectin-1 activation unlocks IL12A expression and reveals the TH1 potency of neonatal dendritic cells. J Allergy Clin Immunol. 2015;136:1355–1368. e1351–1315. doi: 10.1016/j.jaci.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 39.Lang R, Schoenen H, Desel C. Targeting Syk-Card9-activating C-type lectin receptors by vaccine adjuvants: findings, implications and open questions. Immunobiology. 2011;216:1184–1191. doi: 10.1016/j.imbio.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Strasser D, Neumann K, Bergmann H, Marakalala MJ, Guler R, Rojowska A, Hopfner KP, Brombacher F, Urlaub H, Baier G, Brown GD, Leitges M, Ruland J. Syk kinase-coupled C-type lectin receptors engage protein kinase C-sigma to elicit Card9 adaptor-mediated innate immunity. Immunity. 2012;36:32–42. doi: 10.1016/j.immuni.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vijayan D, Radford KJ, Beckhouse AG, Ashman RB, Wells CA. Mincle polarizes human monocyte and neutrophil responses to Candida albicans. Immunol Cell Biol. 2012;90:889–895. doi: 10.1038/icb.2012.24. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi D, Takii T, Fujiwara N, Fujita Y, Yano I, Yamamoto S, Kondo M, Yasuda E, Inagaki E, Kanai K, Fujiwara A, Kawarazaki A, Chiba T, Onozaki K. Comparable studies of immunostimulating activities in vitro among Mycobacterium bovis bacillus Calmette-Guerin (BCG) substrains. FEMS Immunol Med Microbiol. 2009;56:116–128. doi: 10.1111/j.1574-695X.2009.00559.x. [DOI] [PubMed] [Google Scholar]
- 43.Williams JW, Tjota MY, Clay BS, Vander Lugt B, Bandukwala HS, Hrusch CL, Decker DC, Blaine KM, Fixsen BR, Singh H, Sciammas R, Sperling AI. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat Commun. 2013;4:2990. doi: 10.1038/ncomms3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Geijtenbeek TB. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat Immunol. 2009;10:1081–1088. doi: 10.1038/ni.1778. [DOI] [PubMed] [Google Scholar]
- 45.Openshaw PJ. The mouse model of respiratory syncytial virus disease. Curr Top Microbiol Immunol. 2013;372:359–369. doi: 10.1007/978-3-642-38919-1_18. [DOI] [PubMed] [Google Scholar]
- 46.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Netea MG, Marodi L. Innate immune mechanisms for recognition and uptake of Candida species. Trends Immunol. 2010;31:346–353. doi: 10.1016/j.it.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Nakaira-Takahagi E, Golim MA, Bannwart CF, Puccia R, Peracoli MT. Interactions between TLR2, TLR4, and mannose receptors with gp43 from Paracoccidioides brasiliensis induce cytokine production by human monocytes. Med Mycol. 2011;49:694–703. doi: 10.3109/13693786.2011.565485. [DOI] [PubMed] [Google Scholar]
- 49.Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, Van Kooyk Y. Mycobacteria target DC-SIGN to suppress dendritic cell function. The Journal of experimental medicine. 2003;197:7–17. doi: 10.1084/jem.20021229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wevers BA, Kaptein TM, Zijlstra-Willems EM, Theelen B, Boekhout T, Geijtenbeek TB, Gringhuis SI. Fungal engagement of the C-type lectin mincle suppresses dectin-1-induced antifungal immunity. Cell host & microbe. 2014;15:494–505. doi: 10.1016/j.chom.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 51.Mortaz E, I, Adcock M, Tabarsi P, Masjedi MR, Mansouri D, Velayati AA, Casanova JL, Barnes PJ. Interaction of Pattern Recognition Receptors with Mycobacterium Tuberculosis. J Clin Immunol. 2015;35:1–10. doi: 10.1007/s10875-014-0103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Becher B, Blain M, Giacomini PS, Antel JP. Inhibition of Th1 polarization by soluble TNF receptor is dependent on antigen-presenting cell-derived IL-12. J Immunol. 1999;162:684–688. [PubMed] [Google Scholar]
- 53.Ferwerda G, Meyer-Wentrup F, Kullberg BJ, Netea MG, Adema GJ. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008;10:2058–2066. doi: 10.1111/j.1462-5822.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 54.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 55.Hsu H, Boudova S, Mvula G, Divala TH, Mungwira RG, Harman C, Laufer MK, Pauza CD, Cairo C. Prolonged PD1 Expression on Neonatal Vdelta2 Lymphocytes Dampens Proinflammatory Responses: Role of Epigenetic Regulation. J Immunol. 2016 doi: 10.4049/jimmunol.1600284. published ahead of print July 29, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White GP, Watt PM, Holt BJ, Holt PG. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO- T cells. J Immunol. 2002;168:2820–2827. doi: 10.4049/jimmunol.168.6.2820. [DOI] [PubMed] [Google Scholar]
- 57.Ostrop J, Jozefowski K, Zimmermann S, Hofmann K, Strasser E, Lepenies B, Lang R. Contribution of MINCLE-SYK Signaling to Activation of Primary Human APCs by Mycobacterial Cord Factor and the Novel Adjuvant TDB. J Immunol. 2015;195:2417–2428. doi: 10.4049/jimmunol.1500102. [DOI] [PubMed] [Google Scholar]
- 58.Smirnov D, Schmidt JJ, Capecchi JT, Wightman PD. Vaccine adjuvant activity of 3M-052: an imidazoquinoline designed for local activity without systemic cytokine induction. Vaccine. 2011;29:5434–5442. doi: 10.1016/j.vaccine.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 59.Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462:449–460. doi: 10.1038/nature08604. [DOI] [PubMed] [Google Scholar]
- 60.Hansen J, Lindenstrom T, Lindberg-Levin J, Aagaard C, Andersen P, Agger EM. CAF05: cationic liposomes that incorporate synthetic cord factor and poly(I:C) induce CTL immunity and reduce tumor burden in mice. Cancer Immunol Immunother. 2012;61:893–903. doi: 10.1007/s00262-011-1156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.