Abstract
Background
The risk of maternal mortality and morbidity is higher after caesarean section than for vaginal birth. With increasing rates of caesarean section, it is important to minimise risks to the mother as much as possible. This review focused on different skin preparations to prevent infection. This is an update of a review last published in 2018.
Objectives
To compare the effects of different antiseptic agents, different methods of application, or different forms of antiseptic used for preoperative skin preparation for preventing postcaesarean infection.
Search methods
For this update, we searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (9 July 2019), and reference lists of retrieved studies.
Selection criteria
Randomised and quasi‐randomised trials, evaluating any type of preoperative skin preparation (agents, methods or forms). We included studies presented only as abstracts, if there was enough information to assess risk of bias.
Comparisons of interest in this review were between: different antiseptic agents (e.g. alcohol, povidone iodine), different methods of antiseptic application (e.g. scrub, paint, drape), different forms of antiseptic (e.g. powder, liquid), and also between different packages of skin preparation including a mix of agents and methods, such as a plastic incisional drape, which may or may not be impregnated with antiseptic agents. We mainly focused on the comparison between different agents, with and without the use of drapes.
Only studies involving the preparation of the incision area were included. This review did not cover studies of preoperative handwashing by the surgical team or preoperative bathing.
Data collection and analysis
Three review authors independently assessed all potential studies for inclusion, assessed risk of bias, extracted the data and checked data for accuracy. We assessed the certainty of the evidence using the GRADE approach.
Main results
We included 13 individually‐randomised controlled trials (RCTs), with a total of 6938 women who were undergoing caesarean section. Twelve trials (6916 women) contributed data to this review. The trial dates ranged from 1983 to 2016. Six trials were conducted in the USA, and the remainder in India, Egypt, Nigeria, South Africa, France, Denmark, and Indonesia.
The included studies were broadly at low risk of bias for most domains, although high risk of detection bias raised some specific concerns in a number of studies. Length of stay was only reported in one comparison.
Antiseptic agents
Parachlorometaxylenol with iodine versus iodine alone
We are uncertain whether parachlorometaxylenol with iodine made any difference to the incidence of surgical site infection (risk ratio (RR) 0.33, 95% confidence interval (CI) 0.04 to 2.99; 1 trial, 50 women), or endometritis (RR 0.88, 95% CI 0.56 to 1.38; 1 trial, 50 women) when compared with iodine alone, because the certainty of the evidence was very low. Adverse events (maternal or neonatal) were not reported.
Chlorhexidine gluconate versus povidone iodine
Moderate‐certainty evidence suggested that chlorhexidine gluconate, when compared with povidone iodine, probably slightly reduces the incidence of surgical site infection (RR 0.72, 95% CI 0.58 to 0.91; 8 trials, 4323 women). This effect was still present in a sensitivity analysis after removing four trials at high risk of bias for outcome assessment (RR 0.87, 95% CI 0.62 to 1.23; 4 trials, 2037 women).
Low‐certainty evidence indicated that chlorhexidine gluconate, when compared with povidone iodine, may make little or no difference to the incidence of endometritis (RR 0.95, 95% CI 0.49 to 1.86; 3 trials, 2484 women). It is uncertain whether chlorhexidine gluconate reduces maternal skin irritation or allergic skin reaction (RR 0.64, 95% CI 0.28 to 1.46; 3 trials, 1926 women; very low certainty evidence).
One small study (60 women) reported reduced bacterial growth at 18 hours after caesarean section for women who had chlorhexidine gluconate preparation compared with women who had povidone iodine preparation (RR 0.23, 95% CI 0.07 to 0.70).
Methods
Drape versus no drape
This comparison investigated the use of drape versus no drape, following preparation of the skin with antiseptics.
Low‐certainty evidence suggested that using a drape before surgery compared with no drape, may make little or no difference to the incidence of surgical site infection (RR 1.29, 95% confidence interval (CI) 0.97 to 1.71; 3 trials, 1373 women), and probably makes little or no difference to the length of stay in the hospital (mean difference (MD) 0.10 days, 95% CI ‐0.27 to 0.46; 1 trial, 603 women; moderate‐certainty evidence). One trial compared an alcohol scrub and iodophor drape with a five‐minute iodophor scrub only, and reported no surgical site infection in either group (79 women, very‐low certainty evidence). We were uncertain whether the combination of a one‐minute alcohol scrub and a drape reduced the incidence of metritis when compared with a five‐minute scrub, because the certainty of the evidence was very low (RR 1.62, 95% CI 0.29 to 9.16; 1 trial, 79 women). The studies did not report on adverse events (maternal or neonatal).
Authors' conclusions
Moderate‐certainty evidence suggests that preparing the skin with chlorhexidine gluconate before caesarean section is probably slightly more effective at reducing the incidence of surgical site infection in comparison to povidone iodine. For other outcomes examined there was insufficient evidence available from the included RCTs. Most of the evidence in this review was deemed to be very low or low certainty. This means that for most findings, our confidence in any evidence of an intervention effect is limited, and indicates the need for more high‐quality research. Therefore, it is not yet clear what sort of skin preparation may be most effective for preventing postcaesarean surgical site infection, or for reducing other undesirable outcomes for mother and baby.
Well‐designed RCTs, with larger sample sizes are needed. High‐priority questions include comparing types of antiseptic (especially iodine versus chlorhexidine), and application methods (scrubbing, swabbing, or draping). We found two studies that are ongoing; we will incorporate the results of these studies in future updates of this review.
Plain language summary
Skin preparation for preventing infection following caesarean section
This review is an update of a review that was first published in 2012, and updated in 2014 and 2018.
What is the issue? The aim of this Cochrane Review was to find out what methods of skin preparation before caesarean section were most effective in preventing infection after the operation. We collected and analysed all studies that assessed the effectiveness of antiseptics used to prepare the skin before making an incision (or cut) for the caesarean section. We only included analysis of preparations that were used to prepare the surgical site on the abdomen before caesarean section; we did not look at handwashing by the surgical team, or bathing the mother.
Why is this important? Infections of surgical incisions are the third most frequently reported hospital‐acquired infections. Women who give birth by caesarean section are exposed to infection from germs already present on the mother's own skin, or from external sources. The risk of infection following a caesarean section can be 10 times that of vaginal birth. Therefore, preventing infection by properly preparing the skin before the incision is made is an important part of the overall care given to women prior to caesarean birth. An antiseptic is a substance applied to remove bacteria that can cause harm to the mother or baby when they multiply. Antiseptics include iodine or povidone iodine, alcohol, chlorhexidine, and parachlorometaxylenol. They can be applied as liquids or powders, scrubs, paints, swabs, or on impregnated 'drapes' that stick to the skin, which the surgeon then cuts through. Non‐impregnated drapes can also be applied, once the skin has been scrubbed or swabbed, with the aim of reducing the spread of any remaining bacteria during surgery. It is important to know if some of these antiseptics or methods work better than others.
What evidence did we find? This updated review included 13 trials with 6938 women. Six trials were conducted in the USA; the remaining trials were in Nigeria, South Africa, France, Denmark, Indonesia, India and Egypt. The review looked at what was best for women and babies when it came to important outcomes including: infection of the site where the surgeon cut the woman to perform the caesarean section; inflammation of the lining of the womb (metritis and endometritis); how long the woman stayed in hospital; and any other adverse effects, such as irritation of the woman's skin, or any reported impact on the baby. Not all of the 13 trials explored all of these outcomes, and the evidence for each outcome was usually based on results from far fewer than 6938 women.
Much of the evidence we found was of relatively poor quality, due to limits in the ways that the studies were conducted. This means that we could not be certain about most of the findings. The evidence suggested that in women who had their skin prepared using the agent chlorhexidine gluconate, there is probably a slight reduction in the incidence of surgical site infection compared to women who had their skin prepared using povidone iodine. For other outcomes there was little or no difference between the various antiseptic agents and methods of application in terms of endometritis, skin irritation, or allergic skin reaction in the mother. In one study, there was a reduction in bacterial growth on the skin at 18 hours after caesarean section for women who received a skin preparation with chlorhexidine gluconate compared with women who received the skin preparation with povidone iodine, but more data are needed to see if this actually reduces infections for women.
What does this mean? The available evidence from the trials that have been conducted was insufficient to tell us the best type of skin preparation for preventing surgical site infection following caesarean section. More high‐quality research is needed. We found two studies that are still ongoing. We will incorporate the results of these studies into this review in future updates.
Summary of findings
Background
Description of the condition
Caesarean section is an increasingly common major surgical procedure performed on women (WHO 2015). For example, in 2015, every third birth (32%) in the USA was a caesarean delivery (Martin 2015). The increasing rate of caesarean birth worldwide in both high‐ and low‐income countries, is well established, and a concern to many (Thomas 2001). Between 2000 and 2015, the global rate of caesarean section almost doubled from 12.1% to 21.1% (Boerma 2018). The risk of maternal morbidity and mortality is higher in caesarean section birth than in vaginal birth; postoperative infection is a common component of morbidity. With the increase in caesarean sections, it is important that the risks to the mother are minimised as far as possible (Thomas 2001). This review focused on different agents, methods and forms of application for preoperative skin preparation to prevent infection; it did not include studies of preoperative handwashing of the surgical team and preoperative bathing.
Women who give birth by caesarean section are exposed to both endogenous (internal) and exogenous (external) sources of infection during birth. Exposure to a hospital environment places these women at risk of developing hospital‐acquired infections. The rate of postcaesarean infection has been estimated to be 10 times greater than that after vaginal birth (Henderson 1995).
The Centers for Disease Control and Prevention (CDC) estimates that 27 million surgical procedures are performed in the USA each year. The CDC's National Nosocomial Infections Surveillance system reports that surgical site infections are the third most frequently reported nosocomial infection, accounting for 14% to 16% of all such infections (CDC 2005). Preventing infection by properly preparing the skin before incision is thus a vital part of the overall care given to women during caesarean birth.
The incidence of abdominal incisional infections following caesarean section ranges from 3% to 15%. A postcaesarean surgical site infection is a bacterial infection in the surgical incision following an abdominal birth. Women who develop a postcaesarean surgical site infection typically experience a temperature of 38.0°C (100.4°F) or higher, and lower abdominal pain (Cunningham 2018). Abdominal incisional abscesses that develop following caesarean birth usually cause fever on about the fourth postoperative day. In many cases, these are preceded by uterine infection, and fever persists from the first or second postoperative day. Wound redness (erythema) and drainage may also be present. Organisms causing these infections are usually the same as those isolated from amniotic fluid at caesarean birth, but hospital‐acquired pathogens may also be the cause (Lewis 2013).
Some women are more likely than others to develop a postcaesarean surgical site infection. Women at increased risk include those who are obese; have diabetes or an immunosuppressive disorder (HIV infection); have chorioamnionitis (infection of the amniotic fluid and fetal membrane) during labour; anaemia; or are taking corticosteroids (by mouth or intravenously (Cunningham 2018)).
In addition to surgical site infections, another common source of morbidity is postcaesarean metritis, including endometritis, an infection that develops within the lining of the uterus after birth. Despite the use of routine antibiotics before or during surgery (perioperative prophylaxis), estimates of metritis following caesarean range form 10% to 20% (Normand 2001).
Description of the intervention
Proper preparation of an incision site involves removing surface dirt and oil with a soap or detergent scrub plus applying a topical antimicrobial agent that will reduce the bacterial population to a minimal level. In surgical patients, the choice of surgical scrub and the duration of scrubbing have not been shown to make any significant difference in the rate of surgical site infection in either clean or clean‐contaminated wounds (such as caesarean skin incision (Dumville 2015; Mangram 1999)).The use of plastic adhesive drape is one of the common method of preventing surgical site infection.
Antiseptics to prevent infection have been in use for over 150 years. Antiseptic handwash solution was first introduced by Semmelweis, in 1847, at the Vienna Maternity Hospital, to reduce maternal mortality due to puerperal sepsis (Loudon 2002). Later, in 1864, Lister introduced carbolic acid spray preparation for the operative site. Since then, many solutions (including alcoholic iodine, mercuric compounds, and ether) have been used to prepare the operative site. However, as another Cochrane Review has shown, there is uncertainty about which antiseptic skin preparation is the most effective for preventing postoperative surgical site infections (Dumville 2015). Iodophore (on its own or as an alcohol‐containing agent) and chlorhexidine gluconate are the primary skin disinfectants used; the CDC's Prevention Guideline for the Prevention of Surgical Site Infection, published in 2017, recommends the use of alcohol‐containing preparations unless there is a contraindication to alcohol (CDC 2017). Iodophore is effective against bacteria, fungi, viruses and spore‐forming bacteria, and its disinfecting effect lasts for a long time. However, it cannot be used on mucous membranes and does not have an immediate antiseptic effect.Chlorhexidine gluconate is characterised by its immediate antiseptic effect, although it cannot kill the spores (Johansson 2007).
There are six types of antiseptics that are designed for topical application: iodine or iodophors, alcohol, chlorhexidine gluconate, hexachlorophene, parachlorometaxylenol, and triclosan (Dumville 2015; Larson 1988). For the purpose of this review, antiseptic agents can be applied in the form of liquids, solutions, or powders, or delivered on impregnated drapes.
How the intervention might work
The removal of transient bacteria and reduction of the number of existing organisms by antiseptic is recommended prior to surgery by several organisations, for example, the Royal College of Surgeons of England (Leaper 2001), the Centers for Disease Control and Prevention (Mangram 1999), and the Association of Operating Room Nurses (AORN 2002). Several antiseptic agents are available for preoperative preparation of skin at the incision site. Leclair 1990 described an antiseptic as 'a chemical agent that reduces the microbial population on the skin'. It is suggested that the ideal agent would kill all bacteria, fungi, viruses, protozoa, tubercle bacilli, and spores; be nontoxic, hypoallergenic, and safe to use in all body regions; not be absorbed; have residual activity, and be safe for repetitive use (Dumville 2015; Hardin 1997).
Antiseptics for preoperative skin preparation should be broad‐spectrum and fast‐acting, and contain an antimicrobial ingredient that results in significant reduction in the number of micro‐organisms on intact skin (Larson 1988). The primary action of antiseptics includes both the mechanical removal and chemical killing, and the inhibition of both contaminating and colonising flora.
Why it is important to do this review
There is a Cochrane Review on the use of preoperative skin antiseptics for preventing infections (Dumville 2015). However, although the scope of the Dumville 2015 review included clean and clean‐contaminated surgical operations, including caesarean section, the focus was solely on preventing surgical site infections. In our review, the focus extended to preventing all types of infection, such as endometritis or metritis. In this review, we did not look at different methods of surgical incision for caesarean, because that was the topic of another Cochrane Review (Mathai 2013).
Objectives
To compare the effects of different antiseptic agents, different methods of application, or different forms of antiseptic used for preoperative skin preparation for preventing postcaesarean infection.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised controlled trials (RCTs) and quasi‐RCTs, including cluster‐RCTs, evaluating any described type of preoperative skin preparation agents, methods and forms of application for caesarean section were eligible for inclusion. No quasi‐ or cluster‐RCTs were identified for inclusion. Cross‐over studies were not eligible for inclusion. We included studies presented only as abstracts, if they provided enough information.
Types of participants
Pregnant women undergoing elective or emergency caesarean section.
Types of interventions
Comparisons between different antiseptic agents used for caesarean section skin preparation (e.g. alcohol, povidone iodine), different methods of antiseptic application (e.g. scrub, paint, drape), or different forms of antiseptic (e.g. powder, liquid).
We only included studies involving the preparation of the incision area. We excluded studies of preoperative handwashing of the surgical team and preoperative bathing. Other Cochrane Reviews cover other methods for preventing infection at caesarean section (e.g. antimicrobial application, skin shaving).
A related Cochrane Review covers vaginal preparation with antiseptics before caesarean section (Haas 2020).
Types of outcome measures
Primary outcomes
Surgical site infection (as defined by trialists)
Metritis or endometritis, or both
Secondary outcomes
Length of stay
Maternal mortality
Repeat surgery
Re‐admission resulting from infection
Reduction of skin bacteria colony count*
Adverse events (maternal or neonatal)*
*Outcome not prespecified in our published protocol (Hadiati 2008); see Differences between protocol and review.
Search methods for identification of studies
The following search methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (9 July 2019)
The Register is a database containing over 25,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned, and ongoing trial reports (9 July 2019) using the methods detailed in Appendix 1.
Searching other resources
We searched the reference lists of all included studies and review articles.
We did not apply any language restrictions.
Data collection and analysis
For methods used in the previous version, please see Hadiati 2012.
For this update, we used the following methods, which are based on a standard template used by the Cochrane Pregnancy and Childbirth.
Selection of studies
Three review authors independently assessed all the potential studies we identified as a result of the search strategy, for inclusion. We resolved any disagreement through discussion, or consulted a fourth person if required.
Data extraction and management
We designed a form to extract data. We also extracted information on the dates of the study, sources of trial funding, and the trial authors' declarations of interest. For eligible studies, at least two review authors extracted the data, using the agreed form. We resolved discrepancies through discussion, or consulted a third review author. if required. We entered data into Review Manager 5 software, and checked for accuracy (RevMan 2014).
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion with the third review author.
(1) Random sequence generation (checking for possible selection bias)
For each included study, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study, we described the method used to conceal the allocation sequence and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study, we described the method used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high, or unclear risk of bias for participants;
low, high, or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study, we described the method used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high, or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
For each included study, and for each outcome or class of outcomes, we described the completeness of data, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups, or were related to outcomes. Where sufficient information was reported, we re‐included missing data in the analyses that we undertook. We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting bias
For each included study, we described how we investigated the possibility of selective outcome reporting bias, and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study’s prespecified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other sources of bias
For each included study, we reported any important concerns we had about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of bias;
high risk of bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the CochraneHandbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias, and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias by undertaking sensitivity analyses (see Sensitivity analysis).
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous outcomes, we used the mean difference with 95% confidence intervals, as outcomes were measured in the same way between trials. In future updates of this review, if there are trials that measure the same outcome, but use different methods, we will use the standardised mean difference.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion in our review. In future updates, if we identify any cluster‐randomised trials, we will include them in the analyses along with individually‐randomised trials. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We will adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions, using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population (Higgins 2011). If we use ICCs from other sources, we will report this, and conduct sensitivity analyses to investigate the effect of variation in the ICC.
We will also acknowledge heterogeneity in the randomisation unit, and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials were not eligible for inclusion in this review.
Studies with more than two intervention arms
If studies included multiple intervention groups (Cordtz 1989; Ngai 2015), we included only the arms relevant to our research question and each arm was included in the analysis only once (Higgins 2011).
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect with a sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we included all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I², and Chi² statistics. We regarded heterogeneity as substantial if the I² was greater than 30%, and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it (Sterne 2017).
Data synthesis
We carried out statistical analysis using Review Manager 5 software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged to be sufficiently similar. Where there was sufficient clinical heterogeneity to expect that the underlying treatment effects differ between trials, or if we detect substantial statistical heterogeneity, we used a random‐effects meta‐analysis to produce an overall summary, where we considered an average treatment effect across trials to be clinically meaningful. We treated the random‐effects summary as the average of the range of possible treatment effects, and discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not considered to be clinically meaningful, we would not have combined the trials.
In future updates, if we use random‐effects analyses, we will present the results as the average treatment effect with its 95% confidence interval, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We planned to investigate substantial heterogeneity by using subgroup analyses and sensitivity analyses, and if an overall summary is meaningful, use random‐effects analysis to produce it. The outcome with substantial heterogeneity in this update, did not include enough trials to make performing these analyses meaningful. Instead, we used a random‐effects analysis for this outcome, and in future updates, we will carry out the following subgroup analyses.
Risk of infection (high versus low risk)
Duration of skin preparation
Dose of preparation
We will restrict subgroup analyses to the primary outcomes.
We will assess differences between subgroups by using interaction tests available in RevMan 2014. We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
To assess the effect of the addition of alcohol to povidone iodine, we performed a post hoc subgroup analysis comparing 'chlorhexidine plus alcohol versus povidone iodine plus alcohol' versus 'chlorhexidine plus alcohol versus povidone iodine alone', see Analysis 2.1.
2.1. Analysis.

Comparison 2: Chlorhexidine gluconate versus povidone iodine, Outcome 1: Surgical site infection
Sensitivity analysis
We carried out a sensitivity analysis to explore the effects of blinded outcome assessment on the results of the review. Studies in which blinded outcome assessment (for surgical site infection) was inadequate (i.e. high risk of bias) were excluded from the analysis to assess for any substantive difference to the overall result.
In future updates, we will also carry out sensitivity analysis to explore the effect of allocation concealment on the results of the review, and exclude studies with are high risk for this domain. We will also carry out a sensitivity analysis to explore the effects of fixed‐effect or random‐effects analyses for outcomes with statistical heterogeneity. If, in future updates, we include cluster‐randomised trials along with the individually‐randomised trials, we will carry out sensitivity analysis to investigate the effect of the randomisation unit.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to evaluate the certainty of the evidence, as outlined in the GRADE Handbook (GRADE Handbook; GRADE Working Group 2004). The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence for specific outcomes. The evidence can be downgraded from 'high certainty' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias (GRADE Working Group 2004; Schünemann 2009). In this review, we used the GRADE approach to assess the following outcomes for all comparisons in the review.
Surgical site infection
Metritis or endometritis, or both
Length of stay*
Adverse events (maternal or neonatal)*
*Outcome for GRADE assessment was not prespecified in our previous review (Hadiati 2014); see Differences between protocol and review.
We used GRADEpro GDT to import data from Review Manager 5 to create a 'Summary of findings' table, which presents a summary of the intervention effect and a measure of certainty according to the GRADE approach for each of the outcomes listed above (GRADEpro GDT; RevMan 2014).
Results
Description of studies
Results of the search
See: Figure 1.
1.
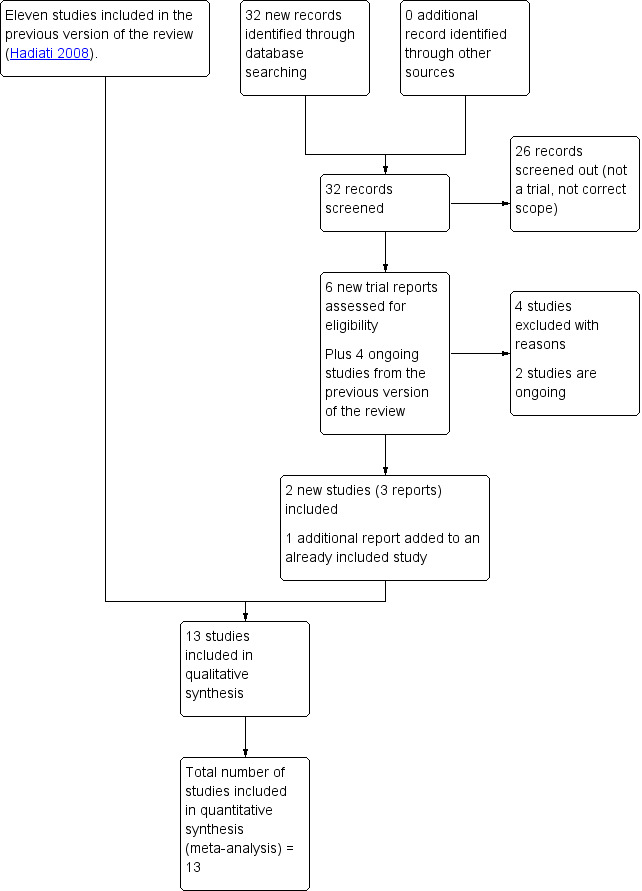
Study flow diagram
For this update, we assessed six new trial reports and reassessed the four studies that were still ongoing in the previous version of the review. We included two new studies (three reports) and added an additional report to an already included study. We also excluded four studies and two are ongoing.
Included studies
We included 13 trials with a total of 6938 women. SeeCharacteristics of included studies.
Method, trial dates, and sample sizes
All of the included studies were randomised controlled trials (RCTs) (Aworinde 2016; Cordtz 1989; Fahmi 2017; Kunkle 2015; Lorenz 1988; Magann 1993; Ngai 2015; Pello 1990; Saha 2019; Salama 2016; Springel 2017; Tuuli 2016; Ward 2001). We did not include any quasi‐ or cluster‐RCTs. The trials were conducted between 1983 and 2016. The trial dates were not provided in three studies (Fahmi 2017; Magann 1993; Pello 1990). Sample sizes ranged from 22 women (Pello 1990), to 1404 women (Ngai 2015).
Settings
Six studies were conducted in the USA (Kunkle 2015; Lorenz 1988; Magann 1993; Ngai 2015; Springel 2017; Tuuli 2016), one study in Denmark (Cordtz 1989), one in France (Pello 1990), one in Nigeria (Aworinde 2016), one in South Africa (Ward 2001), one in Indonesia (Fahmi 2017), one in India (Saha 2019), and one study was conducted in Egypt (Salama 2016). Of the included trials, 10 were single‐centre trials (Aworinde 2016; Kunkle 2015; Lorenz 1988; Magann 1993; Ngai 2015; Saha 2019; Salama 2016; Springel 2017; Tuuli 2016; Ward 2001), two were conducted in multiple centres (Cordtz 1989; Fahmi 2017), and one trial did not provide any details about the facility (Pello 1990).
Participants
Six of the trials recruited women with either scheduled or emergency caesarean section (Fahmi 2017; Magann 1993; Saha 2019; Salama 2016; Springel 2017; Tuuli 2016). Magann 1993 did not include women undergoing caesarean section for fetal distress with inadequate time for skin preparation. Three trials only recruited women with scheduled caesarean section (Aworinde 2016; Kunkle 2015; Ngai 2015). The remaining four trials did not specify type of caesarean section (Cordtz 1989; Lorenz 1988; Pello 1990; Ward 2001). Pello 1990 included women with a male fetus, who were undergoing caesarean section. Kunkle 2015 recruited women aged 18 to 45 years undergoing scheduled caesarean delivery at 36 gestational weeks or greater. Participants in Ngai 2015 were all women in their 37th week of gestation, who were undergoing scheduled or non‐emergency caesarean delivery. Most trials stated that they excluded women with a known sensitivity or allergy to one of the antiseptics used.
Interventions and comparisons
Different antiseptic agents/preparations
Two different antiseptic preparation comparisons were made in the 12 trials. The Magann 1993 trial compared a five‐minute scrub with parachlorometaxylenol followed by a 10% povidone‐iodine scrub and normal saline irrigation of the pelvis and subcutaneous tissue at uterine closure and fascial closure (special preparation) in the experimental group, versus a 7.5% povidone‐iodine surgical scrub followed by 10% povidone iodine and normal saline irrigation of the pelvis and subcutaneous tissue at uterine closure and fascial closure (standard preparation) in the control group. Eight trials compared preoperative application of chlorhexidine gluconate versus povidone iodine, without the use of a drape in either the intervention or control arms (Aworinde 2016; Fahmi 2017; Kunkle 2015; Ngai 2015; Saha 2019; Salama 2016;Springel 2017; Tuuli 2016).Of these eight trials, three trials (Fahmi 2017; Saha 2019; Springel 2017) compared 2% chlorhexidine versus 10% povidone iodine. One trial used antiseptics including 70% isopropyl alcohol in both groups (Fahmi 2017), and the other two trials used antiseptics including 70% isopropyl alcohol in the chlorhexidine group only (Saha 2019; Springel 2017).
Different methods
Four trials compared the use of drape versus no drape, and the drapes used were impregnated with an antiseptic agent (Lorenz 1988; Pello 1990) or without an antiseptic agent (Cordtz 1989; Ward 2001). Cordtz 1989 assessed the effect of incisional plastic drapes versus no drape, combined with standard iodine disinfection with 2.5% iodine in 70% ethanol for all women. For the purpose of this review, we did not include data from this trial relating to additional arms in which women were re‐disinfected, because this secondary disinfection of the skin around the incision took place shortly before skin closure, not before skin incision. Ward 2001 compared plastic incisional drape with no drape. Before surgery in all women, the abdomen was washed with chlorhexidine soap, and then swabbed with 0.5% chlorhexidine in 80% alcohol solution for 30 seconds. In the Lorenz 1988 trial, the comparison of skin preparation for caesarean section was between a one‐minute scrub with 70% isopropyl alcohol followed by application of iodophor‐impregnated adhesive film in the experimental group, versus a five‐minute iodophor scrub followed by application of iodophor solution in the control group. The Pello 1990 trial compared two skin preparations using different agents and either drape or no drape (chlorhexidine 0.5% versus 70% alcohol plus an IOBAN 2 drape).
Outcomes
Surgical site infection (as defined by trialists)
Twelve studies reported on our primary outcome of surgical site infection (Aworinde 2016; Cordtz 1989; Fahmi 2017; Kunkle 2015; Lorenz 1988; Magann 1993; Ngai 2015; Saha 2019; Salama 2016; Springel 2017; Tuuli 2016; Ward 2001). Surgical site infection was assessed from delivery to 30 days postoperative in Aworinde 2016, Springel 2017, Tuuli 2016 and Saha 2019; at three and seven days postoperative in Fahmi 2017; at three days and two weeks in Kunkle 2015; on two separate measurements at least 24 hours postoperative in Lorenz 1988; on two separate occasions six hours apart after the first 24 hours in Magann 1993; at two and six weeks postcaesarean in Ngai 2015; and at seven days and 30 days in Salama 2016. Cordtz 1989 did not describe the time of assessment of surgical site infection. Trials used various definitions for surgical site infections. Cordtz 1989 defined a possible wound infection as localised erythema, serous secretion, or both without the presence of pus, and infected as the presence of pus regardless of the results of bacteriological examination. Kunkle 2015 defined surgical site infection as the presence of purulent drainage, cellulitis, or the need for incision and drainage, or treatment with antibiotics for a clinical diagnosis of infection. In Lorenz 1988, surgical site infection was defined as infectious morbidity with (1) erythema and tenderness of the wound or separation of skin edges, and (2) no uterine tenderness or malodorous, discoloured lochia. Magann 1993 defined this outcome as hyperemic skin incision and a fluctuant mass, which when opened, contained purulent material. In Ward 2001, surgical site infection was diagnosed if two of three features were present: (1) erythematous cellulitis, (2) seropurulent discharge from the wound, and (3) positive swab culture (organisms and leucocytes). In Salama 2016, surgical site infection was diagnosis by pain, tenderness, swelling, redness, heat, purulent discharge from the incision or deliberate reopening of the surgical wound. Fahmi 2017, Ngai 2015, Springel 2017, Tuuli 2016 and Saha 2019 based the diagnosis of surgical site infection on Centers for Disease Control and Prevention (CDC) criteria. One trial did not define surgical site infection in the report (Aworinde 2016).
Metritis or endometritis, or both
Five trials reported on endometritis (Lorenz 1988; Magann 1993; Salama 2016; Springel 2017; Tuuli 2016). In Lorenz 1988, metritis was defined as infectious morbidity with either (1) uterine tenderness on bimanual examination, or (2) no other site of infection identified on physical examination, urinalysis, or urine culture, and if indicated, a chest roentgenogram. In Magann 1993, endometritis was identified through physical examination, urine, and blood cultures. Springel 2017 followed the definition of endometritis according to the CDC, and Tuuli 2016 and Salama 2016 did not provide any definition for this outcome.
Length of stay
Tuuli 2016 and Ward 2001 reported our secondary outcome, length of stay. However, Tuuli 2016 reported only median length of hospital stay, so we only described data in a narrative way.
Re‐admission resulting from infection
Three trials reported on re‐admission resulting from infection (Salama 2016; Springel 2017; Tuuli 2016).
Reduction of skin bacteria colony count
Lorenz 1988 reported on reduction of skin bacteria colony counts, and Kunkle 2015 reported on bacterial growth at 18 hours. Salama 2016 reported on positive post‐sterilisation skin cultures.
Adverse events (maternal or neonatal)
Three trials reported on maternal adverse events involving skin reactions, with two (Aworinde 2016; Tuuli 2016) reporting on erythema and skin irritation, and one (Salama 2016) reporting allergic reaction. Only one trial reported on neonatal adverse events, which was the concentration of iodine in the cord blood (Pello 1990). No other maternal or neonatal adverse events were reported.
The other outcomes investigated by this review, maternal mortality and repeated surgery, were not been reported in any of the included trials.
Sources of trial funding
Seven trials did not mention source of funding (Cordtz 1989; Fahmi 2017; Lorenz 1988; Magann 1993; Ngai 2015; Pello 1990; Ward 2001). The Obafemi Awowolowo University Teaching Hospital Complex, Nigeria supported Aworinde 2016. The University of Southern California supported Kunkle 2015 and Edward Henry Springel, MD supported Springel 2017. Tuuli 2016 received funding from the Women’s Reproductive Health Research Career Development grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health, and the Department of Obstetrics and Gynecology, Washington University School of Medicine in St. Louis. The primary investigator (Dr Luwang) was sponsor in the trial by Saha 2019. The Ain Shams Maternity Hospital in Cairo, Egypt, was sponsor in the trial by Salama 2016.
Trialists' declarations of interest
Nine studies did not report declarations of interest (Aworinde 2016; Cordtz 1989; Fahmi 2017; Lorenz 1988; Magann 1993; Ngai 2015; Pello 1990; Saha 2019; Ward 2001). Three studies stated no conflict of interest (Kunkle 2015; Salama 2016; Springel 2017). In Tuuli 2016, the first author reported having received a grant from the National Institutes of Health during the conduct of the study, but all other authors declared no conflict of interest.
Excluded studies
We excluded 10 trials (Bianco 2018; Brown 1984; Jindal 2019; Kosus 2010; Lukabwe 2018; NCT00528008; NCT01700803; NCT02027324; Nili 2015; Robins 2005). Bianco 2018 was excluded as it focused on preoperative washing on the surgical site by the surgical team. The Brown 1984 study was excluded as the trial did not present the results for caesarean section cases separately from other surgical cases. Jindal 2019 was excluded as they compared different antiseptic agents and different surgical closing techniques. The Kosus 2010 trial was excluded as they used an antibiotic compared with or without Rifamycin SV (250 mg) before closure of subcutaneous tissue, in addition to povidone iodine 10% for preoperative antisepsis and after closure of the skin. Lukabwe 2018 was excluded as they focused on preoperative bathing. One trial (NCT00528008) was stopped following interim analysis and results of interim analysis are unclear. Two trials (NCT01700803; NCT02027324) were excluded as they focused on preoperative handwashing of the surgical team. We excluded one study because it was a cohort study and not a randomised controlled trial (Nili 2015). Robins 2005 compared the effectiveness of chlorhexidine spray and single‐use sachets for skin preparation before regional nerve blockade, not for the preparation of the incision site before caesarean section.
For further details of the excluded studies, seeCharacteristics of excluded studies.
Risk of bias in included studies
See 'Risk of bias' tables and figures for the 13 included studies in Characteristics of included studies, Figure 2, and Figure 3.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

'Risk of bias' graph: review authors' judgements about each risk of bias item for each included study
Allocation
Sequence generation
Sequence generation (computer‐generated sequence) was adequate in nine trials (Aworinde 2016; Fahmi 2017; Lorenz 1988; Magann 1993; Ngai 2015; Saha 2019; Salama 2016; Tuuli 2016; Ward 2001). The remaining four trials provided insufficient or no details about sequence generation, and therefore, we judged them to be of unclear risk of bias (Cordtz 1989; Kunkle 2015; Pello 1990; Springel 2017).
Allocation concealment
The majority of included trials provided insufficient or no details regarding the measures taken to ensure that the treatment allocation could not be foreseen, and so we assessed them as unclear risk of bias for this item (Cordtz 1989; Fahmi 2017; Kunkle 2015; Lorenz 1988; Magann 1993; Pello 1990; Springel 2017; Tuuli 2016). In four trials, allocation concealment was performed using sequentially numbered sealed envelopes, so we assessed the trials at low risk of bias (Aworinde 2016; Ngai 2015; Salama 2016; Ward 2001). Saha 2019 used an open list of random numbers and was therefore judged to be of high risk of bias for allocation concealment.
Blinding
Blinding of participants and personnel (performance bias)
Although blinding was not reported in most of the trials, it was unlikely that this lack of blinding could have caused different treatments or behaviour between groups, such that outcomes would be affected, and therefore, we judged performance bias to be low in 11 trials (Aworinde 2016; Cordtz 1989; Fahmi 2017; Kunkle 2015; Lorenz 1988; Magann 1993; Ngai 2015; Salama 2016; Springel 2017; Tuuli 2016; Ward 2001). In Saha 2019 and Pello 1990, the information provided was too limited to exclude any performance bias.
Blinding of outcome assessment (detection bias)
Seven trials were at high risk of detection bias (Aworinde 2016; Cordtz 1989; Kunkle 2015; Lorenz 1988; Magann 1993; Ngai 2015; Springel 2017). In these trials, outcome assessors were not blinded, and some outcomes involved an important element of subjective assessment. It was possible that lack of blinding could have caused differences in the reported outcomes, especially with respect to our primary outcome of surgical site infection, and therefore, this brings into question the certainty of the estimated treatment effect. Outcome assessment was blinded in four trials, so we judged them at low risk of bias (Fahmi 2017; Salama 2016; Tuuli 2016; Ward 2001). In Pello 1990, blinding of outcome assessment was not described, but lack of blinding was unlikely to affect the outcome because it was objectively measured, and therefore was at low risk of bias. Saha 2019 did not provide sufficient information to exclude detection bias and was judged to be unclear.
Incomplete outcome data
In 11 trials, we judged attrition bias to be low, as all women were followed up until the end of the study, or the number of missing participants was balanced between intervention groups, and it was unlikely that the missing data substantially influenced the outcomes (Aworinde 2016; Cordtz 1989; Fahmi 2017; Kunkle 2015; Lorenz 1988; Magann 1993; Ngai 2015; Salama 2016; Springel 2017; Tuuli 2016; Ward 2001). Two studies were judged as unclear risk of bias, because the number of women in each group was not reported (Pello 1990; Saha 2019).
Selective reporting
We found no evidence of selective reporting in four trials, and assessed them at low risk of bias (Aworinde 2016; Kunkle 2015; Ngai 2015; Springel 2017). Protocols were not available for us to assess originally planned outcomes in six trials, and so these we assessed as unclear risk of reporting bias (Cordtz 1989; Fahmi 2017; Lorenz 1988; Magann 1993; Pello 1990; Ward 2001). Saha 2019 was also assessed as unclear because only an abstract of this study was available which may not report all the outcomes assessed. We judged Salama 2016 and Tuuli 2016 as being at a high risk of reporting bias, because some prespecified outcomes were not reported in the trial report.
Other potential sources of bias
Overall, we judged 11 trials to be at low risk of bias for other potential sources of bias. In the Cordtz 1989 trial, antibiotics for prophylaxis therapy were given to about 10% of the women in the study. Even though more women in the drape group (10.7%) received antibiotics than in the group without drapes (8.2%), infections were more common in the drape group. Therefore, the 'real' effect of skin preparation may have been different, but this was likely to be small, because of the overall small difference between groups. In the Lorenz 1988, Magann 1993, and Kunkle 2015 trials, the use of prophylaxis antibiotics was not specified in the study report. Saha 2019 and Pello 1990 did not provide enough information to exclude other bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Parachlorometaxylenol with iodine versus iodine alone for preventing infection following caesarean section.
| Parachlorometaxylenol with iodine versus iodine alone | ||||||
|
Population: women undergoing caesarean section
Settings: a hospital in the USA
Intervention: parachlorometaxylenol with iodine Comparison: iodine alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with iodine alone | Risk with parachlorometaxylenol with iodine | |||||
| Surgical site infection | Study population | RR 0.33 (0.04 to 2.99) | 50 (1 study) | ⊕⊝⊝⊝ VERY LOW a,b | ||
| 120 per 1000 | 40 per 1000 (5 to 359) | |||||
| Endometritis | Study population | RR 0.88 (0.56 to 1.38) | 50 (1 study) | ⊕⊝⊝⊝ VERY LOW a,b | ||
| 640 per 1000 | 563 per 1000 (358 to 883) | |||||
| Length of stay | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the included study. |
| Adverse events (maternal or neonatal) | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in the included study. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate;the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Wide confidence interval crossing the line of no effect, single study with small sample size (imprecision ‐2).
b Blinding of outcome assessor was at high risk of bias (risk of bias ‐1).
Summary of findings 2. Chlorhexidine gluconate compared to povidone iodine for preventing infection following caesarean section.
| Chlorhexidine gluconate compared to povidone iodine | ||||||
|
Population: women undergoing caesarean section
Settings: single‐centre or multicentre trials in Nigeria, USA, India and Indonesia
Intervention: chlorhexidine gluconate Comparison: povidone iodine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with povidone iodine | Risk with chlorhexidine gluconate | |||||
| Surgical site infection | Study population | RR 0.72 (0.58 to 0.91) | 4323 (8 RCTs) | ⊕⊕⊕⊝ MODERATE a | ||
| 75 per 1000 | 54 per 1000 (43 to 68) | |||||
| Endometritis | Study population | RR 0.95 (0.49 to 1.86) | 2484 (3 RCTs) | ⊕⊕⊝⊝ LOW a,b | ||
| 14 per 1000 | 13 per 1000 (7 to 25) | |||||
| Length of stay | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in any of the included studies. |
| Adverse events (maternal) ‐ skin irritation or allergic skin reaction | Study population | RR 0.64 (0.28 to 1.46) | 1926 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW a,c | No neonatal adverse events were reported in any of the included studies. | |
| 15 per 1000 | 9 per 1000 (4 to 21) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Selection bias was unclear and blinding of outcome assessor was high risk of bias (risk of bias ‐1).
b Wide confidence interval crossing the line of no effect (imprecision ‐1).
c Wide confidence interval crossing the line of no effect and few events (imprecision ‐2).
Summary of findings 3. Drape compared to no drape for preventing infection following caesarean section.
| Drape compared to no drape | ||||||
|
Population: women undergoing caesarean section
Settings: hospitals in Denmark (8 hospitals), USA (1 hospital) and South Africa (1 hospital)
Intervention: antiseptic application using drape Comparison: no drape | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no drape | Risk with drape | |||||
| Surgical site infection | Study population | RR 1.29 (0.97 to 1.71) | 1373 (3 RCTs) | ⊕⊕⊝⊝ LOW a,b | ||
| 112 per 1000 | 144 per 1000 (109 to 191) | |||||
| Metritis | 49 per 1000 | 79 per 1000 (14 to 447) |
RR 1.62 (0.29 to 9.16) |
79 (1 study) | ⊕⊝⊝⊝ VERY LOW a,c |
|
| Length of stay (days) | The mean length of stay with no drape was 5.7 days | The mean number of days with a drape was 0.10 higher (0.27 days lower to 0.46 days higher) | ‐ | 603 (1 RCT) | ⊕⊕⊕⊝ MODERATE b | |
| Adverse events (maternal or neonatal) | ‐ | ‐ | ‐ | ‐ | ‐ | This outcome was not reported in any of the included studies. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Selection bias was unclear and blinding of outcome assessor was at high risk of bias (risk of bias ‐1).
b Wide 95% CI (imprecision ‐1).
c Single study with small sample size and wide 95% CI (imprecision ‐2).
We included 13 trials, involving 6938 women, in this review. One small study, involving 22 women, only reported neonatal outcomes, and did not contribute any data towards any of the prespecified outcomes of this review (Pello 1990). We were only able to conduct meta‐analyses for Comparisons 2 (8 trials) and 3 (3 trials).
Comparison 1: Parachlorometaxylenol with iodine versus iodine alone
One trial, involving 50 women, contributed data to this comparison (Magann 1993). See Table 1.
Primary outcomes
Surgical site infection
We were uncertain if parachlorometaxylenol with iodine made any clear difference to the reduction of surgical site infection when compared with iodine alone, because the certainty of the evidence was very low (risk ratio (RR) 0.33, 95% confidence interval (CI) 0.04 to 2.99; 1 trial, 50 women; very low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Parachlorometaxylenol with iodine versus iodine alone, Outcome 1: Surgical site infection
Metritis or endometritis, or both
We were uncertain if parachlorometaxylenol with iodine made any difference to the reduction of metritis, endometritis, or both, when compared with iodine alone, because the certainty of the evidence was very low (RR 0.88, 95% CI 0.56 to 1.38; 1 trial, 50 women; very low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Parachlorometaxylenol with iodine versus iodine alone, Outcome 2: Endometritis
Secondary outcomes
Length of stay
No data were reported for length of stay.
Maternal mortality
No data were reported for maternal mortality.
Repeat surgery
No data were reported for repeated surgery.
Re‐admission resulting from infection
No data were reported for re‐admission resulting from infection.
Reduction of skin bacteria colony count
No data were reported for reduction of skin bacteria colony count.
Adverse events (maternal or neonatal)
No adverse events were reported for mother or baby in the included study.
Comparison 2: Chlorhexidine gluconate verus povidone iodine
Eight trials, involving 4807 women, contributed data to this comparison (Aworinde 2016; Fahmi 2017; Kunkle 2015; Ngai 2015; Saha 2019; Salama 2016; Springel 2017; Tuuli 2016). See Table 2.
Primary outcomes
Surgical site infection
Moderate certainty of evidence suggested that chlorhexidine gluconate before caesarean section probably slightly reduces the incidence of surgical site infection compared with povidone iodine (RR 0.72, 95% CI 0.58 to 0.91; 8 trials, 4323 women; moderate‐certainty evidence; Analysis 2.1). The effect of chlorhexidine gluconate compared with povidone iodine was still present in sensitivity analysis (RR 0.87, 95% CI 0.62 to 1.23; P = 0.44; 4 trials, 2037 women) after removing four trials at high risk of bias for outcome assessment (Aworinde 2016; Kunkle 2015; Ngai 2015; Springel 2017). To assess the effect of the addition of alcohol to povidone iodine we performed a post hoc subgroup analysis comparing 'chlorhexidine plus alcohol versus povidone iodine plus alcohol' versus 'chlorhexidine plus alcohol versus povidone iodine alone'. We found no evidence of a subgroup difference according to the test for subgroup differences: Chi² = 0.25, df = 1 (P = 0.61), I² = 0%. However, it should be noted that there were too few trials included to carry out any meaningful subgroup analysis.
Metritis or endometritis, or both
Low certainty of evidence indicated that using chlorhexidine gluconate before caesarean section, when compared with povidone iodine, may make little or no difference to the reduction of endometritis (RR 0.95, 95% CI 0.49 to 1.86; 3 trials, 2484 women; low certainty of evidence; Analysis 2.2).
2.2. Analysis.

Comparison 2: Chlorhexidine gluconate versus povidone iodine, Outcome 2: Endometritis
Secondary outcomes
Length of stay
In Tuuli 2016, a median length of hospital stay of four days (interquartile range three to four days) was reported for both the chlorhexidine gluconate group and the povidone iodine group; there was no difference in these reported data between groups.
Maternal mortality
No data were reported for maternal mortality in any of the included studies.
Repeat surgery
No data were reported for repeated surgery in any of the included studies.
Re‐admission resulting from infection
The results from three trials reporting on this outcome suggested that there may be little or no difference in re‐admissions due to infection between the chlorhexidine gluconate and the povidone‐iodine groups (average RR 0.51, 95% CI 0.25 to 1.02; 3 trials, 2484 women; Analysis 2.3).
2.3. Analysis.

Comparison 2: Chlorhexidine gluconate versus povidone iodine, Outcome 3: Re‐admission resulting from infection
Reduction of skin bacteria colony count
Results from one small trial suggested that women who received chlorhexidine gluconate skin preparation may have slightly reduced bacterial growth at 18 hours after caesarean section compared with women who received skin preparation with povidone iodine (RR 0.23, 95% CI 0.07 to 0.70; 1 trial, 60 women; Analysis 2.4).
2.4. Analysis.

Comparison 2: Chlorhexidine gluconate versus povidone iodine, Outcome 4: Bacterial growth 18 hours
Adverse events (maternal or neonatal)
We are unclear about the effects for skin irritation or allergic skin reaction (RR 0.64, 95% CI 0.28 to 1.46; 3 trials, 1926 women; very low certainty of evidence; Analysis 2.5), because the evidence was very‐low certainty. Wide confidence intervals crossing the line of no effect were also present for skin reactions (RR 0.79, 95% CI 0.32 to 1.96; 1 trial, 374 women; Analysis 2.5), and erythema (RR 1.13, 95% CI 0.57 to 2.26; 2 trials, 1521 women; Analysis 2.5). No neonatal adverse events were reported in the included studies.
2.5. Analysis.

Comparison 2: Chlorhexidine gluconate versus povidone iodine, Outcome 5: Adverse events (maternal)
Comparison 3: Drape versus no drape
Four trials involving 2046 women contributed data to this comparison (Cordtz 1989; Ward 2001; Lorenz 1988; Pello 1990). See Table 3
Primary outcomes
Surgical site infection
For women undergoing caesarean section, low certainty of the evidence suggested that using a drape before surgery compared with no drape may make little or no difference to surgical site infection (risk ratio (RR) 1.29, 95% confidence interval (CI) 0.97 to 1.71; 3 trials, 1373 women; low‐certainty evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3: Drape versus no drape, Outcome 1: Surgical site infection
Metritis or endometritis, or both
One trial, involving 79 women, was measured (Lorenz 1988). We were uncertain whether the combination of a one‐minute alcohol scrub with an iodophor drape clearly reduced the occurrence of metritis when compared with a five‐minute iodophor scrub, because the certainty of the evidence was very low (RR 1.62, 95% CI 0.29 to 9.16; 1 trial, 79 women; very low‐certainty evidence; Analysis 3.2).
3.2. Analysis.

Comparison 3: Drape versus no drape, Outcome 2: Metritis
Secondary outcomes
Length of stay
The length of stay was measured in one trial involving 603 women, which compared the use of a drape with no drape (Ward 2001). Moderate‐certainty evidence suggested that the use of a drape probably makes little or no difference to the length of time spent in hospital (mean difference (MD) 0.10 day, 95% CI ‐0.27 to 0.46; Analysis 3.3).
3.3. Analysis.

Comparison 3: Drape versus no drape, Outcome 3: Length of stay
Maternal mortality
No data were reported for maternal mortality in either of the included studies.
Repeat surgery
No data were reported for repeated surgery in either of the included studies.
Re‐admission resulting from infection
No data were reported for re‐admission resulting from infection in either of the included studies.
Reduction of skin bacteria colony count
The single trial (Lorenz 1988) providing data on this comparison suggested that there may be little or no difference between groups for reduced skin bacteria colony counts (MD 0.07 colony forming unit per plate, 95% CI ‐0.34 to 0.48; 1 trial, 79 women; Analysis 3.4).
3.4. Analysis.

Comparison 3: Drape versus no drape, Outcome 4: Reduction of skin bacteria colony counts
Adverse events (maternal or neonatal)
Pello 1990 compared skin preparation with chlorhexidine 0.5% and 70% alcohol with a drape (IOBAN 2), and reported the newborn's exposure to iodine. Cord blood iodine concentration (CBI) was higher in the IOBAN 2 drape group (18.38 ± 20.34 versus 6.44 ± 0.66 μg/100 mL; P < 0.05). There was no clear difference between the two groups in 48‐hour urine iodine excretion or thyroid‐stimulating hormone (TSH) blood concentration on the fifth day. No maternal adverse events were reported in the included study.
Discussion
Summary of main results
This updated review included 13 trials and 6938 women, with 12 trials and 6916 women contributing data to the meta‐analyses. In relation to the primary outcomes of metritis or endometritis, our analyses suggested that there may be little or no difference between different skin preparations for caesarean section. In one comparison, we found that there is probably a slight reduction in surgical site infection with chlorhexidine gluconate compared to povidone iodine, based on seven trials including 4323 women. We assessed the evidence for this result to be of moderate certainty due to limitations in study design. The effect of chlorhexidine gluconate compared with povidone iodine was still present in sensitivity analysis after removing four trials at high risk of bias for outcome assessment (Aworinde 2016; Kunkle 2015; Ngai 2015; Springel 2017). The evidence for the other comparisons between skin preparations in this review all came from only one or two trials, involved small numbers of women, and also yielded effect estimates with wide confidence intervals in most cases. There were also wide‐ranging concerns regarding risk of bias. In some studies, it was not possible to know whether randomisation processes were adequate, and we were particularly concerned about the high number of studies where there was no blinding of subjective assessments relating to infection. Moreover, in assessing surgical site infection, pooled results may have also been only indirectly comparable, due to variability between the included studies in the criteria used to assess surgical site infection.
Regarding the secondary outcomes, we were unclear about the effect between chlorhexidine gluconate and povidone iodine for maternal skin irritation or allergic skin reaction because the certainty of the evidence was found to be very low. However, the included trials provided either very little or no data for almost all of our specified secondary outcomes, most notably, adverse neonatal effects, maternal mortality, or repeat surgery. Therefore, this review was limited in its power to detect meaningful differences between antiseptic agents and methods of skin preparation, with respect to most of the outcomes under consideration.
Overall completeness and applicability of evidence
Of the 13 included trials, seven were reasonably large, while the other six trials each recruited only small numbers of women. Twelve trials contributed to the evaluation of four main comparisons, limiting the ability to pool results (we were only able to carry out meta‐analysis for two comparisons). Twelve trials reported on surgical site infection, while five trials reported on endometritis; only one to two trials reported on the remaining outcomes, or not at all. Four trials (Lorenz 1988; Magann 1993; Salama 2016; Springel 2017) used a mix of the co‐intervention with scrubbing; we were unable to assess interventions separately. Therefore, it was not possible to fully address the objectives of this review.
We are unable to draw robust conclusions regarding the different agents and methods of skin preparation for preventing infection following caesarean section. The body of evidence was too small; although we included 13 studies involving 6938 women, the evidence available for each comparison and outcome under investigation reported on too few women and was too limited in scope.
Quality of the evidence
The included studies were subject to some important methodological limitations. Regarding the primary outcome of surgical site infection, most of the studies did not blind outcome assessors, and we could not always be confident in the adequacy of randomisation processes. When we assessed the certainty of the evidence using GRADE criteria, we found it ranged from very low to moderate. We found very‐low certainty of evidence when comparing parachlorometaxylenol with iodine versus iodine alone, due to wide confidence intervals crossing the line of no effect and small sample sizes, and lack of blinding of outcome assessment for surgical site infection and endometritis (Table 1). We found moderate certainty of evidence when comparing chlorhexidine gluconate with povidone iodine, for surgical site infection, low certainty of evidence for endometritis, and very low certainty of evidence for adverse events (maternal ‐ skin irritation or allergic skin reaction), due to wide 95% confidence intervals and lack of blinding for outcome assessment (Table 2). When comparing the use of a drape versus no drape, we found low‐certainty evidence for surgical site infection, very‐low certainty evidence for metritis and moderate‐certainty evidence for length of stay, with downgrading due to wide confidence intervals crossing the line of no effect, small sample sizes, and lack of blinding of outcome assessment (Table 3).
Potential biases in the review process
We believe it is unlikely that we missed any eligible published studies, because of the comprehensive search strategy employed by Cochrane Pregnancy and Childbirth. However, there may be relevant unpublished trials that we were unable to locate. Screening of the studies, data extraction, 'Risk of bias' and GRADE assessments were carried out independently by at least two review authors, therefore ensuring reliable data were available for the review. Professor Hadiati is a named author on Fahmi 2017, but was not involved in the screening process and'Rrisk of bias' assessment.
Agreements and disagreements with other studies or reviews
The Cochrane Review of skin preparation for clean surgery found preoperative skin preparation with 0.5% chlorhexidine in methylated spirits was associated with lower rates of surgical site infections following clean surgery than alcohol‐based povidone iodine, which is consistent with our findings (Dumville 2015). Rather than iodine alone, alternate chlorhexidine might be effective, although the evidence for both comparisons was low certainty of evidence. We need to interpret the results with caution. However, caesarean section is regarded as 'clean‐contaminated' surgery, and so the effect of antiseptic skin preparation may be more evident for this type of surgery because of exposure to both internal and external sources of infection during birth. Because of the limited certainty of the evidence presently available, we were unable to explore this hypothesis further in this review.
Authors' conclusions
Implications for practice.
Moderate‐certainty evidence suggests that preparing the skin with chlorhexidine gluconate before caesarean section is probably slightly more effective at reducing the incidence of surgical site infection in comparison to povidone iodine. For other outcomes examined there was insufficient evidence available from the included randomised controlled trials. Most of the evidence in this review was deemed to be very low or low certainty. This means that for most findings, our confidence in any evidence of an intervention effect is limited, and indicates the need for more high‐quality research. Therefore, it is not yet clear what sort of skin preparation may be most effective for preventing postcaesarean surgical site infection, or for reducing other undesirable outcomes for mother and baby.
We found two studies that are ongoing; we will incorporate the results of these studies in future updates of this review.
Implications for research.
There is a need for high‐quality, well‐designed, randomised controlled trials in this field, with larger sample sizes. Proper randomisation, adequate allocation concealment, blinding of participants, clinicians, outcome assessors (especially where outcomes involve subjective assessment) and data analysts, plus clear attrition policies are essential to ensure appropriate comparisons between groups. The priority comparisons in superiority or non‐inferiority trials could include type of antiseptic (especially iodine versus chlorhexidine), the timing and duration of applying the antiseptic (especially previous night versus day of surgery), and application methods (scrubbing, swabbing, and draping). Various maternal and neonatal outcome measurements could be considered, i.e. length of stay, maternal mortality, repeat surgery, and re‐admission resulting from infection, including surgical site infection, and any adverse events. Furthermore, there is a need for consistency of definitions in future trials for outcomes, such as surgical site infection.
What's new
| Date | Event | Description |
|---|---|---|
| 7 July 2019 | New search has been performed | There is slighlty more evidence to suggest a benefit for chorhexidine gluconate, but for other comparisons the conclusions are largely unchanged. |
| 7 July 2019 | New citation required but conclusions have not changed | Search updated. We included two new studies (Saha 2019; Salama 2016) and excluded four studies (Bianco 2018; Jindal 2019; Lukabwe 2018; NCT00528008). |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 9, 2012
| Date | Event | Description |
|---|---|---|
| 27 November 2017 | New search has been performed | Search updated. We included five new studies (Aworinde 2016; Fahmi 2017; Ngai 2015; Springel 2017; Tuuli 2016) (including one previous ongoing trial: Tuuli 2016) and excluded one new study (Nili 2015). We also identified two new ongoing trials (NCT02402907; NCT02396329). |
| 27 November 2017 | New citation required but conclusions have not changed | Conclusions have not been changed. |
| 26 June 2014 | New citation required but conclusions have not changed | The inclusion of one new trial did not change the conclusions. |
| 26 June 2014 | New search has been performed | Search updated: one new trial added (Kunkle 2015), three new ongoing trials added and three new trials excluded. Methods updated. 'Summary of findings' tables incorporated for this update. |
Acknowledgements
For the first version of this review, the review authors would like to thank staff from the Australasian Cochrane Centre for technical support during the preparation of the review, and sincerely acknowledge Philippa Middleton and Miranda Cumpston for their supportive advice and comments to improve the review. The Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Indonesia provided support for Diah Hadiati and Detty Nurdiati to travel to Australia to finish the first version of this review.
Diah Hadiati wrote the protocol as a part of a SEA‐ORCHID Project Fellowship at the Australasian Cochrane Centre, and wishes to acknowledge the support provided by Steve McDonald and Tari Turner.
For the 2014 update, Erika Ota's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development, and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
This review is supported by funding from the World Health Organization (WHO) and the UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP) to Cochrane Pregnancy and Childbirth (University of Liverpool). HRP supports and coordinates research on a global scale, synthesizes research through systematic reviews of literature, builds research capacity in low‐income countries and develops dissemination tools to make efficient use of ever‐increasing research information. In addition to its cosponsors, the International Planned Parenthood Federation (IPPF) and UNAIDS are both members of HRP’s governing body.
Appendices
Appendix 1. Search methods for ClinicalTrials.gov and ICTRP
ClinicalTrials.gov
Advanced search
Type of study: Interventional
Condition/disease: Infection; Cesarean Section
Intervention: Iodine; Chlorhexidine; Anti‐infective
Type of study: Interventional
Condition/disease: Cesarean Section Complications
Other terms: skin preparation; antiseptics; skin cleansing
ICTRP
Each line was run separately
cesarean AND preparation
caesarean AND preparation
cesarean AND prep
caesarean AND prep
cesarean AND cleansing
caesarean AND cleansing
cesarean AND iodine
caesarean AND iodine
cesarean AND chlorhexidine
caesarean AND chlorhexidine
cesarean AND antiseptic(s)
caesarean AND and antiseptic(s)
Data and analyses
Comparison 1. Parachlorometaxylenol with iodine versus iodine alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Surgical site infection | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.99] |
| 1.2 Endometritis | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.56, 1.38] |
Comparison 2. Chlorhexidine gluconate versus povidone iodine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Surgical site infection | 8 | 4323 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.58, 0.91] |
| 2.1.1 Chlorhexidine plus alcohol versus povidone iodine plus alcohol | 4 | 2663 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.45, 0.87] |
| 2.1.2 Chlorhexidine plus alcohol versus povidone iodine | 4 | 1660 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.61, 1.15] |
| 2.2 Endometritis | 3 | 2484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.49, 1.86] |
| 2.3 Re‐admission resulting from infection | 3 | 2484 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.25, 1.02] |
| 2.4 Bacterial growth 18 hours | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.07, 0.70] |
| 2.5 Adverse events (maternal) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.5.1 Any skin reaction | 1 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.32, 1.96] |
| 2.5.2 Erythema | 2 | 1521 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.57, 2.26] |
| 2.5.3 Skin irritation or allergic skin reaction | 3 | 1926 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.28, 1.46] |
Comparison 3. Drape versus no drape.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Surgical site infection | 3 | 1373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.97, 1.71] |
| 3.1.1 Iodine | 1 | 691 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.98, 2.04] |
| 3.1.2 Chlorhexidine | 1 | 603 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.70, 1.76] |
| 3.1.3 Isopropyl alcohol scrub versus iodophor scrub | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.2 Metritis | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.29, 9.16] |
| 3.3 Length of stay | 1 | 603 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.27, 0.46] |
| 3.4 Reduction of skin bacteria colony counts | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.34, 0.48] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aworinde 2016.
| Study characteristics | ||
| Methods | Computer‐generated randomised control trial | |
| Participants | All women (N = 374) who had elective caesarean section with no overt risk for surgical site infection were randomised into 2 groups. The trial was carried out in the Department of Obstetrics and Gynaecology of the Obafemi Awolowo University Teaching Hospitals Complex (OAUTHC), Ile‐Ife, Osun State, Nigeria. |
|
| Interventions | All women had a bath with non‐antiseptic soap before surgery. Shaving of the lower abdomen was done on the surgical table, just before commencing antiseptic skin preparation. For women who fell into the chlorhexidine‐alcohol group (N = 188), skin preparation was done with gauze soaked in Valon® (containing chlorhexidine gluconate 0.3% weight/volume (w/v) and cetrimide 3.0% w/v manufactured by Royal Priesthood Laboratory Ltd), which was diluted with distilled water in a 1:1 ratio. A centrifugal scrubbing motion was used, starting from the area of the intended incision and covered the abdomen from the subcostal margin to the midaxillary line down to the middle of the thigh. This was repeated twice. The area was then dried with a piece of dry gauze in the same centrifugal manner. Moko® (containing Isopropyl alcohol 95% v/v manufactured by New‐Health way Co. Limited) was then applied on the area in the same centrifugal manner and allowed to dry before draping of the area. For women in the povidone‐iodine group (N = 186), Wosan® (containing 10% povidone iodine manufactured by Jawa international limited) was used. The povidone iodine was painted on the aforementioned area and then left to dry completely before draping the area and commencing the surgery. |
|
| Outcomes | Surgical site infections in 30 days after delivery. Bacterial culture for wounds assessed as infected. Development of skin reaction. Definition of surgical site infection: not provided. |
|
| Notes | All women were given prophylactic antibiotic (intravenous cefuroxime 750 mg immediately (stat)), administered after clamping of the cord. The surgery was done under spinal anaesthesia. Pan African Clinical Trials Registry No. PACTR201401000697382 Trial dates: August 2012 to July 2013 Sources of trial funding: Obafemi Awowolowo University Teaching Hospital Complex, Nigeria Trialist declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...computer generated random sequence". |
| Allocation concealment (selection bias) | Low risk | Quote: "...sequentially numbered sealed packets..." and "The sealed envelopes were placed in the labour ward theatre and were drawn from serially by the surgeons just before the procedure". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Blinding both patients and physicians to the antiseptic used for skin preparation (double‐blinding) would have been ideal, however, it was not feasible in this trial". Comment: unlikely that this lack of blinding of participants and personnel could have caused different treatments between groups. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Blinding both patients and physicians to the antiseptic used for skin preparation (double‐blinding) would have been ideal, however, it was not feasible in this trial". Comment: it is possible that this lack of blinding of outcome assessors could account for the different treatment effects between groups, because surgical site infection involves subjective assessment. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Ten were excluded from the analysis due to the fact that they were lost to follow‐up or ended up having a midline scar". Comment: the number of excluded women was similar between intervention groups: chlorhexidine‐alcohol group 4/192 (2.1%) and povidone‐iodine group 6/192 (3.1%). |
| Selective reporting (reporting bias) | Low risk | The same outcomes reported in the trial registry |
| Other bias | Low risk | Other bias was not noticed during review |
Cordtz 1989.
| Study characteristics | ||
| Methods | Randomised multicentre 4‐group trial | |
| Participants | All women undergoing caesarean section (N = 1340) The trial was a Danish multicentre trial in which 8 Danish hospitals participated under supervision of the National Center for Hospital Hygiene, Statens Seruminstitut. |
|
| Interventions | The study had 4 experimental groups: with or without drape and with or without re‐disinfection. For the purpose of this review we only included a comparison of drapes versus no drapes, with standard disinfection, not re‐disinfection. Experimental group (N = 337) incisional plastic drape was applied to the skin after preoperative skin disinfection. Control group (N = 354) no drape was used. All women received preoperative skin disinfection of 2.5% iodine in 70% ethanol. |
|
| Outcomes | Surgical site infection in 14 days after delivery Definition of surgical site infection: (1) Possible wound infection: localised erythema, serous secretion, or both, without presence of pus; (2) Infected: presence of pus, regardless of the results of bacteriological examination. Pus could be classified as superficially or subfascially located. Incision for drainage was also reported. |
|
| Notes | We did not include the re‐disinfection group, as it was defined as disinfection of the skin around the incision shortly before skin closure. Antibiotics for prophylaxis therapy were used in 36 of 337 women (10.7%) in the drape group and 29 of 354 women (8.2%) in the no drape group. Trial dates: September 1985 to May 1986 Type of anaesthesia not described Sources of trial funding: not reported Trialist declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "blocks of eight consecutive patient numbers were labelled... in random order". Comment: although the method of determining random order was not specified, given the numbers in the trial, it is probable that this was generated by computer or random tables. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not described, but is likely that participant and personnel could identify different interventions. Unlikely that this lack of blinding could have caused different treatments between groups. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding stated, and some outcome measurements were subjective. |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data were reported for all women. |
| Selective reporting (reporting bias) | Unclear risk | There was no protocol to access, so we did not know the originally planned outcomes. |
| Other bias | Low risk | Antibiotics for prophylaxis therapy were used in this study. Even though more women in the drape group (10.7%) received antibiotics than in the without drape group (8.2%), but there were more infections in the drape group, so we might underestimate the effect. This effect is likely to be small because of the small difference. |
Fahmi 2017.
| Study characteristics | ||
| Methods | Multicenter, randomised clinical trial | |
| Participants | All women undergoing scheduled or emergency caesarean section The study was conducted in Dr Sardjito Hospital and 2 affiliated hospital (Saras Husada Hospital and Panembahan Senopati Hospital), Indonesia. |
|
| Interventions | Alcohol‐chlorhexidine group (N = 87) and alcohol‐povidone‐iodine group (N = 87) | |
| Outcomes | Surgical site infections on day 3 and day 7 after caesarean section Definition of surgical site infection: diagnosed based on Centers for Disease Control and Prevention (CDC) criteria |
|
| Notes | We have the full thesis from the author in Indonesian All women received preoperative prophylactic antibiotic cefotaxime 1 hour before skin incision. Type of anaesthesia not described Trial dates: June 2013 to May 2014 Sources of trial funding: not reported Trialist declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...subjects were randomly divided into two groups...". Comment: computer‐generated random sequence (information provided by trial author) |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not described, but is likely that participants and personnel could identify different interventions. Unlikely that this lack of blinding could have caused different treatments between groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers and outcome assessors did not know the type of intervention (information provided by trial author). |
| Incomplete outcome data (attrition bias) | Low risk | All women were followed until the 7th day after caesarean section. |
| Selective reporting (reporting bias) | Unclear risk | There was no protocol to access, so we did not know the originally planned outcomes. |
| Other bias | Low risk | Other bias was not noticed during review. |
Kunkle 2015.
| Study characteristics | ||
| Methods | A single‐centre, randomised controlled trial | |
| Participants | Women aged 18 to 45 years, undergoing scheduled caesarean delivery at 36 gestational weeks or later. The trial was conducted at the University of Southern California Los Angeles County Medical Center, USA. |
|
| Interventions | 60 participants were included: chlorhexidine gluconate group (N = 27) versus povidone‐iodine group (P = 33) | |
| Outcomes | Surgical site infection at 3 days and 2 weeks. Positive bacterial culture rates at 3 minutes and 18 hours Definition of surgical site infection: the presence of purulent drainage, cellulitis, or the need for incision and drainage, or treatment with antibiotics for a clinical diagnosis of infection |
|
| Notes | Type of anaesthesia not described. Clinical Trials.gov. identifier: NCT01975805 Trial dates: January 2010 to March 2012 Sources of trial funding: University of Southern California Trialist declarations of interest: reported, no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The choice of disinfectant was determined by simple randomization where each participant was independently assigned to either PI or CG without any regard for previous patient assignments". Comment: no specific randomisation methods stated |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The operating surgeons could not be blinded because both agents possess distinctly different coloring when applied to the skin". Comment: unlikely that this lack of blinding could have caused different treatments between groups |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding stated, but outcome assessment of surgical site infection was subjective, while bacterial culture rates were objective measurements. |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data were reported for all women. |
| Selective reporting (reporting bias) | Low risk | The same outcomes reported in the trial registry |
| Other bias | Low risk | Other bias was not noticed during review. |
Lorenz 1988.
| Study characteristics | ||
| Methods | Randomised clinical trial using a table of random numbers | |
| Participants | All women undergoing caesarean section. The women were excluded if they were allergic to iodine, younger than 18 years of age, had chorioamnionitis, refused to participate, emergency clinical circumstances prevented adequate informed consent, or no culture plate available. The trial was conducted at Pennsylvania State University, Hersey, USA |
|
| Interventions | A total of 79 women were included. Experimental group (N = 38) received a 1‐minute scrub with 70% isopropyl alcohol followed by application of iodophor‐impregnated adhesive film. Control group (N = 41) received a 5‐minute iodophor scrub followed by application of iodophor solution. |
|
| Outcomes | Metritis, surgical site infection (assessed on 2 separate measurements at least 24 hours postoperatively), and reduction of skin bacteria colony counts. All participants had skin bacterial counts before skin preparation and immediately postoperatively. Definition of surgical site infection: infectious morbidity (body temperature > 38°C on 2 separate measurements at least 24 hours postoperatively) with (1) erythema and tenderness of the wound or separation of skin edges, and (2) uterine tenderness or malodorous, discoloured lochia |
|
| Notes | Type of anaesthesia not described Trial dates: June 1983 to April 1984 Sources of trial funding: not reported Trialist declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each patient was randomly assigned, using a table of random number...". |
| Allocation concealment (selection bias) | Unclear risk | The allocation was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not described, but is likely that participants and personnel could have identified the different interventions. It is unlikely that lack of blinding could have caused different caesarean treatment relevant to the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding stated and some outcome measurements were subjective. |
| Incomplete outcome data (attrition bias) | Low risk | All women were followed up to the end of the study. |
| Selective reporting (reporting bias) | Unclear risk | There was no protocol to access, so we did not know the originally planned outcomes. |
| Other bias | Low risk | Other bias was not noticed during review. |
Magann 1993.
| Study characteristics | ||
| Methods | Randomised trial using a table of random numbers | |
| Participants | All women undergoing caesarean section. The exclusion criteria were chorioamnionitis, emergency for caesarean section for fetal distress with inadequate time for skin preparation, and refusal to participate. The trial was conducted at the University of Mississippi Medical Center, USA. |
|
| Interventions | A total of 100 women were included and divided into 4 subgroups of 25 women. For the purpose of this review the following 2 groups were included. Experimental group (N = 25) received a 5‐minute scrub with parachlorometaxylenol followed by povidone‐iodine solution and normal saline irrigation of the pelvis and subcutaneous tissue at uterine closure and fascial closure. Control group (N = 25) received a povidone‐iodine surgical scrub (7.5%) followed by povidone iodine (10%) and normal saline irrigation of the pelvis and subcutaneous tissue at uterine closure and fascial closure. |
|
| Outcomes | Endometritis and surgical site infection Definition of surgical site infection: hyperemic skin incision and a fluctuant mass, that when opened, contained purulent material |
|
| Notes | Type of anaesthesia: general, spinal or epidural Trial dates: not reported Sources of trial funding: not reported Trialist declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "This randomised study ... with group appointment derived from a random number table". |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Random assignment was achieved by card selection from sealed opaque envelopes ...". Comment: it was unclear what 'card selection' involved, and therefore, whether investigators could have foreseen assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not described, but it is likely that participants and personnel could identify different interventions. It is unlikely that this lack of blinding could have caused different caesarean treatment relevant to the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding stated and some outcome measurements were subjective. |
| Incomplete outcome data (attrition bias) | Low risk | All women were followed up to the end of the study. |
| Selective reporting (reporting bias) | Unclear risk | There was no protocol to access, so we did not know the originally planned outcomes. |
| Other bias | Low risk | Other bias was not noticed during review. |
Ngai 2015.
| Study characteristics | ||
| Methods | Computer‐generated randomisation through www.radomisation.com with alternating block sizes of 6 and 12 | |
| Participants | All women at 37 weeks of gestation, based on best obstetric estimate, who were undergoing scheduled or non‐emergent caesarean delivery. Women were excluded if they had a urogenital tract infection within 2 weeks of delivery and if they were younger than 18 years old. The trial was conducted at 2 Montefiore Medical Center, Einstein Medical College, NY, USA. |
|
| Interventions | The 1404 participants were enrolled in a 1:1:1 fashion to 1 of 3 groups: povidone iodine with alcohol group (PA group: N = 462), chlorhexidine with alcohol group (CA group: N = 467), and combination of povidone iodine with alcohol, and chlorhexidine with alcohol used together (BOTH group: N = 465). For the purpose of this review, we compared the PA group and CA group. |
|
| Outcomes | Surgical site infections on 2 and 6 weeks postcaesarean Definition of surgical site infection: according to Horan and colleagues. and the Centers for Disease Control and Prevention. A surgical site infection outcome was defined as the patient reporting the requirement of antibiotic use for a wound infection, or documented wound infection in the medical record at the outpatient visit within 30 days of discharge. |
|
| Notes | All women received preoperative prophylactic antibiotics within 1 hour of skin incision. Surgical drapes were placed after a minimum of 4 minutes drying time. All women had regional anaesthesia, a spinal, epidural, or combined spinal and epidural. ClinicalTrials.gov Identifier: NCT01870583 Trial dates: January 2013 to July 2014 Sources of trial funding: the study provided no support for a particular method of skin preparation Trialist declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was computer‐generated through www.randomization.com with alternating block sizes of six and 12". |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization allocation was concealed in identical, opaque, sequentially numbered, sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding was not described. but it is likely that participants and personnel could identify different interventions. It was unlikely that this lack of blinding could have caused different treatments between groups. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding stated, but it is possible that this lack of blinding could account for the different treatment effects between groups, because surgical site infection involves subjective assessment. |
| Incomplete outcome data (attrition bias) | Low risk | Postrandomisation exclusions were reported in the study due to protocol breach or loss to follow‐up: PA group 8/463 (1.7%), CA group 18/474 (3.8%), and BOTH group 11/467 (2.4%). It is unlikely that this number of missing participants was enough to affect the outcome of surgical site infection. |
| Selective reporting (reporting bias) | Low risk | The same outcomes reported in the trial registry. |
| Other bias | Low risk | Other bias was not noticed during review. |
Pello 1990.
| Study characteristics | ||
| Methods | Open comparative randomised trial | |
| Participants | Women with male fetus undergoing caesarean section. The trial was conducted in Paris, France. |
|
| Interventions | Skin preparation with chlorhexidine 0.5% was compared with 70% alcohol plus drape (IOBAN 2). A total of 22 women were randomised, but the number allocated to each group was not stated. | |
| Outcomes | Newborn exposure to iodine, measured from cord blood iodine concentration, 48‐hour urine iodine excretion, and thyroid stimulating hormone blood concentration on the 5th day. | |
| Notes | Only abstract available Type of anaesthesia: not described Trial dates: not reported Sources of trial funding: not reported Trialist declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Twenty‐two women had the skin preparation randomly allocated...". |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding stated. Evaluation of outcome was diagnosed by objective signs. |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of women in each group not reported |
| Selective reporting (reporting bias) | Unclear risk | Only abstract available, so we did not know the originally planned outcomes. The key outcome for this review was not reported (i.e. surgical site infection) by this study, however the abstract made it clear that the study examined the effect of iodine on newborns, so the lack of data reporting on women was not deemed to be of concern. |
| Other bias | Unclear risk | There was too little information provided to exclude other bias. |
Saha 2019.
| Study characteristics | ||
| Methods | Randomised trial using computer‐generated randomisation. | |
| Participants | All women (N = 311) undergoing scheduled or emergency caesarean section. The trial was conducted in Chandigarh, India. | |
| Interventions | Chlorhexidine 2% and isopropyl alcohol 70% group (N = 153) Povidone‐iodine 10% group (N = 158) |
|
| Outcomes | Surgical site infection (a period of 30 days to monitor) The definition of SSI is followed from the CDC. |
|
| Notes | Only abstract available Type of anaesthesia: not reported Trial dates: start July 2016, end date not reported Sources of trial funding: primary sponsor Dr Luwang (principle investigator) Trialist declarations of interest: not reported Clinical Trials Registry: CTRI/2018/05/014294. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated randomization". |
| Allocation concealment (selection bias) | High risk | Quote: "An open list of random numbers". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was not described. |
| Incomplete outcome data (attrition bias) | Unclear risk | Number of women in each group not reported. |
| Selective reporting (reporting bias) | Unclear risk | More outcomes reported in the trial registry. The abstract may reported limited outcome. |
| Other bias | Unclear risk | There was not enough information provided to exclude other bias. |
Salama 2016.
| Study characteristics | ||
| Methods | Randomised, prospective, controlled clinical trial using computer‐generated randomisation. | |
| Participants | All women (N = 390) undergoing scheduled or emergency caesarean section The trial was carried out in Ain Shams University Maternity Hospital and Manshiet ElBakry General Hospital in Cairo, Egypt. |
|
| Interventions | Chlorhexidine and 70% alcohol group (N = 196) were prepared similarly by 3 applications of 2% chlorhexidine solution followed by drying with a sterile towel after 30 seconds and 3 applications of 70% alcohol (Alkanol; Hikma Pharmaceutical Industries, Cairo, Egypt). Povidone‐iodine and 70% alcohol group (N = 194) were scrubbed preoperatively with an applicator that contained 10% povidone‐iodine scrub solution (3 consecutive applications), followed by drying with a sterile towel after 1 minute and 3 application of 10% povidone‐iodine in 70% alcohol (sBetadine; Nile Company for Pharmaceuticals and Chemical Industries, Cairo, Egypt). |
|
| Outcomes | Surgical site infection within 1 week after surgery (as evidenced by pain, tenderness, swelling, redness, heat, purulent discharge from the incision, or deliberate reopening of the surgical wound), endometritis, hospital readmission due to sepsis, allergic reactions, post sterilisation skin cultures. | |
| Notes | All patients in both groups had swabs for skin cultures using sterile cotton swabs taken from their skin at the site of the caesarean section immediately before and after skin sterilization to assess the rate of skin decontamination. All patients in both groups received a single dose of an intravenous third generation cephalosporin (ceftriaxone) 1 g as a prophylactic antibiotic 1 hour before the operation. The area scrubbed in all patients in both groups was from the xiphoid to the knee, reaching the midaxillary line laterally. Type of anaesthesia: not described Trial date: June 2014 to December 2014 Sources of trial funding: Ain Shams Maternity Hospital Trialist declarations of interest: reported, no conflict of interest clinical trials.gov protocol registration system under the number NCT02396329 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...study were randomized by an independent statistician using a computer‐generated randomization sheet into two groups in the ratio 1: 1". |
| Allocation concealment (selection bias) | Low risk | Quote: "Another research coordinator who was not involved in assessing the outcome assigned the participants to the interventions". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Each woman in the study received a closed envelope that contained a random number corresponding to which group she will be enrolled to in the randomization table. The envelope was opened by the surgeon immediately before the operation". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A third research coordinator who was blinded to the interventions in either groups assessed the outcomes and followed up the patients". |
| Incomplete outcome data (attrition bias) | Low risk | Postrandomisation exclusions were reported in the study due to withdrawn consent or loss to follow‐up: chlorhexidine and 70% alcohol group 8/204 (3.9%), Povidone‐iodine and 70% alcohol group 7/201 (3.5%), and BOTH group 15/405 (3.7%). It is unlikely that this number of missing participants was enough to affect the outcome of surgical site infection. |
| Selective reporting (reporting bias) | High risk | More outcomes reported in the trial registry. |
| Other bias | Low risk | Other bias was not noticed during review. |
Springel 2017.
| Study characteristics | ||
| Methods | Open‐label, parallel‐design, unmasked randomised controlled trial | |
| Participants | All women undergoing caesarean section, who met eligibility criteria: caesarean delivery, age 18 to 65 years, and ability to consent in English or Spanish Exclusion criteria were: inability or unwillingness to consent to study participation in English or Spanish, current incarceration, pre‐operative diagnosis of chorioamnionitis, perceived inability to complete follow‐up for data collection, or any prior known allergy or adverse reaction to either study preparation The trial was conducted in an urban tertiary care institution (MetroHealth Medical Center, Cleveland, OH), USA |
|
| Interventions | 932 women were randomly assigned to either chlorhexidine‐isopropyl alcohol (CA group, N = 461) or povidone‐iodine scrub and paint (PI group, N = 471). Both interventions were applied to the skin pre‐operatively for surgical site anti‐sepsis. | |
| Outcomes | Surgical site infection (composite SSI) occurring within 30 days, endometritis, re‐admission to hospital for management of SSI, non‐SSI wound complications Definition of surgical site infection: > 1 of superficial, deep, and organ space (endometritis in the case of caesarean delivery) infection as defined by the US National Healthcare Safety Network (NHSN) of the CDC |
|
| Notes | General anaesthesia for some women ClinicalTrials.gov Identifier: NCT02202577 Trial dates: February 2013 to May 2016 Sources of trial funding: Edward Henry Springel, MD Trialist declarations of interest: reported, no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "... subjects were ... randomized ..." Comment: random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Masking: none (open‐label)". Comment: blinding of participants and personnel was not done, but it is unlikely that this lack of blinding could have led to different treatments between groups. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Masking: none (open‐label)". Blinding of outcome assessment was not done, and it was possible that this lack of blinding could account for the different treatment effects between groups, because assessment of surgical site infection involved subjective assessment. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Rates of follow‐up for evaluation after 30 days were 97% in the CA group and 96% in the PI group". Comment: the loss to follow‐up was balanced between the 2 intervention groups |
| Selective reporting (reporting bias) | Low risk | The same outcomes reported in the trial registry. |
| Other bias | Low risk | Other bias was not noticed during review. |
Tuuli 2016.
| Study characteristics | ||
| Methods | Single‐centre, randomised, controlled trial | |
| Participants | This study recruited pregnant women undergoing scheduled or unscheduled caesarean delivery at Washington University Medical Center in St. Louis, USA. Women who had known allergy to chlorhexidine, alcohol, iodine, or shellfish, or who had a skin infection adjacent to the operative site were excluded. |
|
| Interventions | 1147 women were randomly assigned to receive preoperative skin preparation with either chlorhexidine–alcohol (N = 572 women) or iodine–alcohol (N = 575). Chlorhexidine–alcohol combination contained 2% chlorhexidine gluconate with 70% isopropyl alcohol, and iodine–alcohol combination contained 8.3% povidone–iodine with 72.5% isopropyl alcohol. | |
| Outcomes | Primary outcome: superficial or deep surgical site infection within 30 days after caesarean delivery Prespecified secondary outcomes: length of hospital stay, physician office visits and hospital readmissions for infection‐related complications, endometritis, positive wound culture, skin irritation, and allergic reaction Posthoc secondary outcomes: other wound complications (including skin separation, seroma, haematoma, and cellulitis), emergency department visits for wound complications, additional wound surgery, use of home health services or services of a wound clinic, and duration of wound care Definition of surgical site infection: on the basis of the National Healthcare Safety Network definitions of the Centers for Disease Control and Prevention |
|
| Notes | Women also received standard infection‐prevention measures, including body weight–based preoperative antibiotic prophylaxis. Type of anaesthesia not described ClinicalTrials.gov Identifier: NCT01472549 Trial dates: September 2011 to June 2015 Sources of trial funding: supported by a Women’s Reproductive Health Research Career Development grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (1K12HD063086‐01, to Drs Tuuli and Macones), and the Department of Obstetrics and Gynecology, Washington University School of Medicine in St. Louis Trialist declarations of interest: reported. Dr Tuuli reported grant support from the National Institutes of Health during the conduct of the study. All other authors declared no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "... computer‐generated random sequence produced by the study statistician". |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants and personnel, but it is unlikely that this lack of blinding could have caused different treatments between groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The diagnosis of surgical‐site infection within 30 days after cesarean delivery "... was made by the treating physician and verified by means of chart review by the principal investigator, who was unaware of the study‐group assignments". |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "A similar number of participants in each group — 34 (5.9%) in the chlorhexidine–alcohol group and 31 (5.4%) in the iodine–alcohol group — were lost to follow‐up". Comment: reason for loss to follow‐up were 'did not have postoperative follow‐up' or 'discontinued study'. Numbers in each group, and for each reason, were similar between the 2 interventions. |
| Selective reporting (reporting bias) | High risk | Compared to the trial registration, more outcomes were reported in the final report, and some predefined outcomes, such as proportion of women with skin contamination after skin prep or cost savings were not reported. |
| Other bias | Low risk | Other bias was not noticed during review. |
Ward 2001.
| Study characteristics | ||
| Methods | Randomisation using a table of random numbers | |
| Participants | All women undergoing caesarean section The trial was conducted at Livingston Hospital, Eastern Province of South Africa. |
|
| Interventions | All women (N = 605) were prepared before the incision by washing the abdomen and perineum with 4% chlorhexidine soap. On the operating table, the abdomen was swabbed with 0.5% chlorhexidine in 80% alcohol solution for 30 seconds. After the preparation: the experimental group (N = 305) received incisional plastic drape; the control group (N = 300) did not receive a drape. |
|
| Outcomes | Surgical site infection and length of hospital stay Definition of surgical site infection: diagnosed if 2 of 3 features were present: (1) erythematous cellulitis, (2) seropurulent discharge from the wound, (3) positive swab culture (organisms and leucocytes). |
|
| Notes | All women received preoperative antibiotics administration of 1 g cephazolin intravenously, unless antibiotics were already being administered. In addition, a 1 g metronidazole suppository was inserted preoperatively, and repeated after 12 hours. Women received general anaesthesia Trial dates: August 1992 to January 1993 Sources of trial funding: not reported Trialist declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...taken from a random number table". |
| Allocation concealment (selection bias) | Low risk | Quote: "On deciding that a woman was to undergo caesarean section, the surgeon removed an opaque unmarked envelope from a box of identical envelopes. Inside was a card, inscribed with an integer taken from a random number table. If the number was even, a drape was to be used and if odd, then no drape was used. The card was then resealed in the envelope and stapled to the patient’s folder, and opened only when she left the study". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...patients received general anesthesia as part of normal hospital practice and the drape was applied after induction and removed before extubation". Comment: although not explicitly stated in the study, we assumed that the medical personnel were not blinded, as they could see the different interventions. It was unlikely that this lack of blinding could have caused different caesarean treatment relevant to the outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Assessor bias was avoided because postoperative care was conducted by staff unrelated to surgery". |
| Incomplete outcome data (attrition bias) | Low risk | Postrandomisation exclusions were reported in the study, 15 (2.4%) in total were excluded due to critical data missing, 2 additional women from the control group were excluded: 1 due to ruptured appendix and another who requested early discharge on day 2 after caesarean section. Based on a sensitivity analysis, it was unlikely that this number of missing participants was enough to affect the outcome of surgical site infection. |
| Selective reporting (reporting bias) | Unclear risk | Selective reporting was not noticed during review, but we were unable to consult the trial registry. |
| Other bias | Low risk | Other bias was not noticed during review. |
CDC: Centers for Disease Control; SSI: Surgical site infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bianco 2018 | Intervention was different. This study focused on preoperative washing with a chlorhexidine gluconate cloth by the surgical team. |
| Brown 1984 | Trial compared antiseptics for general surgery that included cases of caesarean section, but the trial did not report separate results for the caesarean section cases. |
| Jindal 2019 | Trial compared the effectiveness of not only antiseptic agent but also use of closing pans. |
| Kosus 2010 | Intervention was different. Rifamycin is an antibiotic, not an antiseptic agent. |
| Lukabwe 2018 | Intervention was different. This study focused on preoperative bathing with chloroxylenol. |
| NCT00528008 | The study was stopped following interim analysis. The details of the result of interim analysis is unclear and we could not contact the authors. |
| NCT01700803 | Intervention was different. This study focused on preoperative handwashing by the surgical team. |
| NCT02027324 | Intervention was different. This study focused on preoperative handwashing by the surgical team. |
| Nili 2015 | This is not a randomised controlled trial but a cohort study. |
| Robins 2005 | Trial compared the effectiveness of chlorhexidine spray and single‐use sachets for skin preparation before regional nerve blockade, not for preparation of the incision site before caesarean section. |
Characteristics of ongoing studies [ordered by study ID]
NCT02396329.
| Study name | Chlorhexidine versus povidone‐iodine antisepsis for reduction of post caesarean section surgical site infection |
| Methods | Single‐blinded (participants) randomised controlled trial conducted in Egypt |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Chlorhexidine versus povidone iodine |
| Outcomes | Primary outcome: surgical site infection within 1 week after surgery (time frame: 1 week). Secondary outcomes: surgical site infection within 30 days after surgery (time frame: 30 days), long hospital stay, more than 5 days (time frame: 30 days), hospital readmission (time frame: 30 days) due to sepsis, febrile morbidity (time frame: 10 days) with an oral temperature of 38.0 degree celsius or more, on any 2 of the first 10 days postpartum, exclusive of the first 24 hours |
| Starting date | June 2014 |
| Contact information | AMR HELMY YEHIA, Ain Shams Maternity Hospital |
| Notes | NCT Number; NCT02396329 |
NCT02402907.
| Study name | STRIPES Study: Study To Reduce Infection Post caEsarean Section |
| Methods | Masked randomised controlled trial conducted in the USA |
| Participants | Women (18 years or older) > 24 weeks' gestation, scheduled for a primary or repeat caesarean section. Exclusion criteria: allergy to chlorhexidine, unplanned or emergency caesarean section |
| Interventions | 2% chlorhexidine gluconate cloth versus placebo cloth |
| Outcomes | Primary outcomes: rate of infectious morbidity (time frame: up to 6 weeks) Secondary outcomes: incidence of neonatal intensive care unit admissions (time frame: up to 6 weeks), maternal length of stay (time frame: up to 6 weeks), incidence of maternal readmissions (time frame: up to 6 weeks) |
| Starting date | April 2015 |
| Contact information | Contact: Brittany Noel Robles, MD |
| Notes | NCT Number; NCT02402907 |
Differences between protocol and review
The methods have been updated to reflect the latest Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and Cochrane Pregnancy and Childbirth's methodological guidelines. We added two secondary outcomes; reduction of skin bacteria colony counts and adverse effects. We used GRADE to assess the certainty of the evidence and included 'Summary of findings' tables.
In the 2018 update, we added a co‐author (Katharina da Silva Lopes). We also added a search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
We also assessed the certainty of the evidence for two new outcomes, using GRADE criteria.
Length of stay
Adverse events (maternal or neonatal)
We assessed the certainty of the evidence, and added the two outcomes to the 'Summary of findings' table because length of stay indicates the severity of the infection, which prolongs the hospital stay after caesarean section, and adverse events are important outcomes for women's decision making.
In this update, we provided additional information about the included studies: trial dates, sources of trial funding, and trial authors' declarations of interest.
In the 2019 update, we added a co‐author (Yuko Masuzawa).
We also added a post hoc subgroup analysis:
To assess the effect of the addition of alcohol to povidone iodine we performed a post hoc subgroup analysis comparing 'chlorhexidine plus alcohol versus povidone iodine plus alcohol' versus 'chlorhexidine plus alcohol versus povidone iodine alone' in comparison 2, Analysis 2.1. We added this subgroup in response to a query from the guideline development team at the World Heatlh Organization (WHO).
Contributions of authors
Diah Hadiati wrote the first draft of the protocol. Detty Nurdiati and Hakimi Mohammad contributed to defining the selection criteria and commented on the draft. All authors contributed to data extraction, preparation of results, and finalisation of the report.
For the 2014 update, Erika Ota prepared the first draft, incorporated the results of the additional new study, and prepared the 'Summary of findings' tables. All authors approved the final version of the update for publication.
For the 2018 update, Diah Hadiati, Mohammad Hakimi, and Detty S Nurdiati incorporated results of additional new studies. Katharina da Silva Lopes extracted data and assessed risk of bias for newly added studies, incorporated the results of the additional studies, and prepared the manuscript. Erika Ota rechecked extracted data and risk of bias assessment, prepared the 'Summary of finding' tables, and edited the review text. All authors approved the final version of the update for publication.
For the 2019 update, Yuko Masuzawa and Katharina da Silva Lopes screened, extracted data and assessed risk of bias for newly added studies. Yuko Masuzawa incorporated the results of the additional studies. Yuko Masuzawa and Katharina da Silva Lopes updated the manuscript. Erika Ota checked the analyses, prepared the 'Summary of finding' tables, and edited the review text. All authors approved the final version of the update for publication.
Sources of support
Internal sources
Universitas Gadjah Mada, Indonesia
St.Luke's International University, Japan
External sources
-
World Health Organization (WHO) and the UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Switzerland
This review is supported by funding to Cochrane Pregnancy and Childbirth (University of Liverpool)
Declarations of interest
Diah R Hadiati: Diah Hadiati is a named author on Fahmi 2017, but was not involved in the screening process and 'Risk of bias' assessment.
Mohammad Hakimi: none known.
Detty S Nurdiati: none known.
Erika Ota: none known.
Katharina da Silva Lopes: none known.
Yuko Masuzawa: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aworinde 2016 {published data only}
- Aworinde O, Olufemi-Aworinde K, Fehintola A, Adeyemi B, Owonikoko M, Adeyemi AS. Antiseptic skin preparation for preventing surgical site infection at caesarean section. Open Journal of Obstetrics and Gynecology 2016;6:246-51. [Google Scholar]
- PACTR201401000697382. Comparative study of antiseptic skin preparation for preventing surgical site infection at caesarean section: a randomised control trial. pactr.org/ATMWeb/appmanager/atm/atmregistry?dar=true&tNo=PACTR201401000697382 (first received 5 November 2013).
Cordtz 1989 {published data only}
- Cordtz T, Schouenborg L, Laursen K, Daugaard HO, Buur K, Munk Christensen B, et al. The effect of incisional plastic drapes and redisinfection of operation site on wound infection following caesarean section. Journal of Hospital Infection 1989;13(3):267-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fahmi 2017 {published data only}
- Fahmi MN, Hadiati DR, Widad S. Comparison of skin preparation with alcohol-chlorhexidine versus alcohol-povidone iodine on surgical site infection following caesarean section. Journal of Obstetrics and Gynaecology Research 2017;43:38. [Google Scholar]
Kunkle 2015 {published data only}
- Kunkle CM, Marchan J, Safadi S, Whitman S, Chmait RH. Chlorhexidine gluconate versus povidone iodine at cesarean delivery: a randomized controlled trial. Journal of Maternal-Fetal & Neonatal Medicine 2015;28(5):573-7. [DOI: 10.3109/14767058.2014.926884] [DOI] [PubMed]
- Murray C, Marchan J, Safadi S, Opper N, Yedigarova L, Chmait R. Efficacy of chlorhexidine gluconate versus povidone iodine for skin disinfection at cesarean section: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S152-3. [Google Scholar]
- NCT01975805. Chlorhexidine gluconate versus povidone iodine at cesarean delivery: a randomized controlled trial. In: clinicaltrials.gov/ct2/show/NCT01975805. (first received 5 November 2013).
Lorenz 1988 {published data only}
- Lorenz RP, Botti JJ, Appelbaum PC, Bennett N. Skin preparation methods before cesarean section. Journal of Reproductive Medicine 1988;33(2):202-4. [PubMed] [Google Scholar]
Magann 1993 {published data only}
- Magann EF, Dodson MK, Ray MA, Harris RL, Martin JN, Morrison JC. Preoperative skin preparation and intraoperative pelvic irrigation: impact on endometritis following Cesarean delivery. In: 41st Annual Clinical Meeting of the American College of Obstetricians and Gynecologists; 1993 May 3-6; Washington DC, USA. 1993:11.
- Magann EF, Dodson MK, Ray MA, Harris RL, Martin JN, Morrison JC. Preoperative skin preparation and intraoperative pelvic irrigation: impact on post-cesarean endometritis and wound infection. Obstetrics and Gynecology 1993;81(6):922-5. [PMID: ] [PubMed] [Google Scholar]
Ngai 2015 {published data only}
- Maiwald M. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstetrics and Gynecology 2017;129(4):750-1. [DOI] [PubMed] [Google Scholar]
- NCT01870583. Comparison of surgical skin preps during cesarean deliveries. clinicaltrials.gov/ct2/show/record/NCT01870583 (first received 6 June 2013).
- Ngai I, Govindappagari S, Van Arsdale A, Judge NE, Neto N, Bernstein J, et al. Skin preparation in cesarean birth for prevention of surgical site infection (SSI): a prospective randomized clinical trial. American Journal of Obstetrics and Gynecology 2015;212(1 Suppl 1):S424. [DOI] [PubMed] [Google Scholar]
- Ngai IM, Van Arsdale A, Govindappagari S, Judge NE, Neto NK, Bernstein J, et al. Skin preparation for prevention of surgical site infection after cesarean delivery: a randomized controlled trial. Obstetrics and Gynecology 2015;126(6):1251-7. [DOI] [PubMed] [Google Scholar]
Pello 1990 {published data only}
- Pello JY, Pons G, Leger FA, Moisson-Tardieu MT, Dailhe-Dupont D, Francoual C, et al. Ioban 2 for cesarean section operative field: study of innocuity for the newborn. Therapie 1990;45:85. [Google Scholar]
Saha 2019 {published data only}
- Luwang AL, CTRI/2018/05/014294. Comparison of efficacy of chlorhexidine-alcohol versus povidone-iodine as preoperative antiseptic skin preparation for prevention of SSI after Cesarean section. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=19954 (31 May 2018).
- Saha PK, Luwang AL, Rohilla M, Sikka P, Saha L, Gautam V. Chlorhexidine-alcohol versus povidone-iodine as preoperative skin antisepsis for prevention of surgical site infection in caesarean section. BJOG: an international journal of obstetrics and gynaecology 2019;126(S2):162. [Google Scholar]
Salama 2016 {published data only}
- Salama F, Yehia AH, Wahba KA, Abdelmoniem RM. Efficacy and safety of chlorhexidine versus povidone-iodine skin antisepsis in reducing surgical site infection in cesarean sections: a randomized controlled trial. Evidence Based Women's Health Journal 2016;6:32-6. [Google Scholar]
Springel 2017 {published data only}
- NCT02202577. Chlorhexidine-alcohol versus povidone-iodine for surgical site antisepsis prior to cesarean delivery. clinicaltrials.gov/ct2/show/NCT02202577 (first received 29 July 2014).
- Springel EH, Sarfoh V, Stetzer B, Weight S, Mercer B, Wang XY. A randomized controlled trial of chlorhexidine-alcohol versus povidone-iodine for cesarean antisepsis. American Journal of Obstetrics and Gynecology 2017;216(1 Suppl):S30, Abstract no: 42. [NCT02202577] [DOI] [PubMed] [Google Scholar]
- Springel EH, Wang XY, Sarfoh VM, Stetzer BP, Weight SA, Mercer BM. A randomized open-label controlled trial of chlorhexidine-alcohol versus povidone-iodine for cesarean antisepsis: the CAPICA trial. American Journal of Obstetrics and Gynecology 2017;217:463.e1-8. [DOI] [PubMed] [Google Scholar]
Tuuli 2016 {published data only}
- NCT01472549. Antiseptic skin preparation for preventing surgical site infection at cesarean delivery: a randomized comparative effectiveness trial. clinicaltrials.gov/show/NCT01472549 (first received November 2011).
- Stout MJ, Martin S, Cahill AG, Macones GA, Tuuli MG. Impact of chlorhexidine-alcohol versus iodine-alcohol skin antisepsis on methicillin-resistant staphylococcus aureus infection after cesarean. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S119, Abstract no: 194. [NCT01472549] [Google Scholar]
- Temming LA, Raghuraman N, Carter EB, Stout MJ, Rampersad RM, Macones GA, et al. Impact of evidence-based interventions on wound complications after cesarean delivery. American Journal of Obstetrics & Gynecology 2017;217(4):449.e1-9. [DOI] [PMC free article] [PubMed]
- Temming LA, Raghuraman N, Stout MJ, Cahill AG, Macones GA, Tuuli MG. Impact of evidence based interventions on wound complications after cesarean. American Journal of Obstetrics and Gynecology 2017;216(1):S106, Abstract no: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuuli MG, Liu J, Stout MJ, Martin S, Cahill AG, Colditz G, et al. Chlorhexidine-alcohol compared with iodine-alcohol for preventing surgical-site infection at cesarean: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S3, Abstract no: 4. [Google Scholar]
- Tuuli MG, Liu J, Stout MJ, Martin S, Cahill AG, Odibo AO, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. New England Journal of Medicine 2016;374(7):647-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuuli MG, Woolfolk C, Stout MJ, Temming L, Cahill AG, Macones GA. Does the relative efficacy of chlorhexidine-alcohol versus iodine-alcohol antisepsis differ between unscheduled and scheduled cesareans? American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S120, Abstract no: 196. [NCT01472549] [Google Scholar]
Ward 2001 {published data only}
- Ward HR, Jennings OG, Potgieter P, Lombard CJ. Do adhesive plastic drapes prevent post caesarean wound infection? Journal of Hospital Infection 2001;47:230-4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bianco 2018 {published data only}
- Bianco A, NCT03423147. A randomized trial to determine if a pre-operative wash with a chlorhexidine gluconate cloth and chlorhexidine gluconate vaginal scrub reduces infectious morbidity in patients undergoing cesarean section after labor. https://clinicaltrials.gov/ct2/show/NCT03423147 (first received 6 February 2018).
Brown 1984 {published data only}
- Brown TR, Ehrlich CE, Stehman FB, Golichowski AM, Madura JA, Eitzen HE. A clinical evaluation of chlorhexidine gluconate spray as compared with iodophor scrub for preoperative skin preparation. Surgery, Gynecology and Obstetrics 1984;158(4):363-6. [PubMed] [Google Scholar]
Jindal 2019 {published data only}
- Jindal M, CTRI/2019/01/017110. Evaluation of surgical site infection bundle to decrease postoperative infectious morbidity in women undergoing emergency lower segment caesarean section. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=28772 (16 January 2019).
Kosus 2010 {published data only}
- Kosus A, Kosus N, Guler A, Capar M. Rifamycin SV application to subcutaneous tissue for prevention of post-cesarean surgical site infection [Sezaryen sonrasi kesi yeri enfeksiyonunu onlemek icin ciltalti rifamisin SV uygulanmasi]. European Journal of General Medicine 2010;7(3):269-76. [Google Scholar]
Lukabwe 2018 {published data only}
- Lukabwe H, NCT03544710. The impact of preoperative bathing with chloroxylenol on the incidence of post caesarean section surgical site infection at Mbarara Regional Referral Hospital: a randomized controlled trial. https://clinicaltrials.gov/ct2/show/NCT03544710 (4 June 2018). [DOI] [PMC free article] [PubMed]
NCT00528008 {published data only}
- NCT00528008. A comparison of surgical preparations and wound infection rates for elective cesarean sections. clinicaltrials.gov/ct2/show/NCT00528008 (first received 11 September 2007).
NCT01700803 {published data only}
- NCT01700803. Povidone iodine and cesarean section wound infections. clinicaltrials.gov/ct2/show/NCT01700803 (first received 4 October 2012).
NCT02027324 {published data only}
- NCT02027324. Prevention of surgical site infection after cesarean delivery (CAPISSI). clinicaltrials.gov/ct2/show/NCT02027324 (first received 6 Janury 2014).
Nili 2015 {published data only}
- IRCT201204289568N1. Studying the relationship between neonatal hypothyroidism and using iodine and non iodine containing disinfectants before caesarian section in icteric neonates that refer to clinic of neonates in Valiasr hospital. en.irct.ir/trial/10126 (first registered 07 July 2012).
- Nili F, Hantoushzadeh S, Alimohamadi A, Shariat M, Rezaeizadeh G. Iodine-containing disinfectants in preparation for caesarean section: impact on thyroid profile in cord blood. Postgraduate Medical Journal 2015;91:681-4. [DOI] [PubMed] [Google Scholar]
Robins 2005 {published data only}
- Robins K, Wilson R, Watkins EJ, Columb MO, Lyons G. Chlorhexidine spray versus single use sachets for skin preparation before regional nerve blockade for elective caesarean section: an effectiveness, time and cost study. International Journal of Obstetric Anesthesia 2005;14(3):189-92. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02396329 {published data only}
- NCT02396329. Chlorhexidine versus povidone-iodine antisepsis for reduction of post cesarean section surgical site infection rate:a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT02396329 (first received 1 March 2015).
NCT02402907 {published data only}
- NCT02402907. A randomized trial to determine if a pre-operative wash with a chlorhexidine cloth reduces infectious morbidity in patients undergoing cesarean section. clinicaltrials.gov/ct2/show/NCT02402907 (first received 30 March 2015).
Additional references
AORN 2002
- Association of Operating Room Nurses. Recommended practices for skin preparation of patients. AORN Journal 2002;75:184-7. [DOI] [PubMed] [Google Scholar]
Boerma 2018
- Boerma T, Ronsmans C, Melesse DY, Barros AJD, Barros FC, Juan L, et al. Global epidemiology of use of and disparities in caesarean sections. Lancet 2018;392(10155):1341-8. [DOI: 10.1016/S0140-6736(18)31928-7. ] [DOI] [PubMed] [Google Scholar]
CDC 2005
- Centers for Disease Control and Prevention. Data and statistics for surgical site infections. www.cdc.gov/ncidod/dhqp/dpac_ssi_data.html (accessed: 5 August 2008).
CDC 2017
- Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surgery 2017;152(8):784-91. [DOI] [PubMed] [Google Scholar]
Cunningham 2018
- Cunningham FG, Leveno KJ, Bloom SL, Spong CY, Dashe JS, Hoffman BL, et al. Puerperal complications. In: Hoffman BL, editors(s). Williams Obstetrics. 25th edition. McGraw-Hill Education, 2018. [Google Scholar]
Dumville 2015
- Dumville JC, McFarlane E, Edwards P, Lipp A, Holmes A, Liu Z. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD003949.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE Handbook
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group 2013.
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed prior to 26 September 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.Available at gradepro.org.
Haas 2020
- Haas DM, Morgan S, Contreras K, Kimball S. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database of Systematic Reviews 2020, Issue 4. [DOI: 10.1002/14651858.CD007892.pub7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hardin 1997
- Hardin W, Nichols R. Handwashing and patient skin preparation. In: Malangoni MA, editors(s). Critical Issues in Operating Room Management. Philadelphia: Lippincott-Raven, 1997:133-44. [Google Scholar]
Henderson 1995
- Henderson E, Love EJ. Incidence of hospital-acquired infections associated with caesarean section. Journal of Hospital Infection 1995;29:245-55. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Johansson 2007
- Johansson I, Ponisseril S. Handbook for Cleaning/Decontamination of Surfaces. Elsevier Science, 2007. [Google Scholar]
Larson 1988
- Larson E. Guideline for use of topical antimicrobial agents. American Journal of Infection Control 1988;16:253-66. [DOI] [PubMed] [Google Scholar]
Leaper 2001
- Leaper DJ, Orr C, Maung Z, White A. Inflammation and Infection: STEP 2000 Module II. Royal College of Surgeons of England: Blackwell Science, 2001. [Google Scholar]
Leclair 1990
- Leclair J. A review of antiseptics. Cleansing agents. Todays OR Nurse 1990;12(10):25-8. [PubMed] [Google Scholar]
Lewis 2013
- Lewis SS, Moehring RW, Chen LF, Sexton DJ, Anderson DJ. Assessing the relative burden of hospital-acquired infections in a network of community hospitals. Infection Control and Hospital Epidemiology 2013;34(11):1229-30. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loudon 2002
- Loudon I. Ignaz Phillip Semmelweis' studies of death in childbirth. www.jameslindlibrary.org (accessed: 5 August 2008). [DOI] [PMC free article] [PubMed]
Mangram 1999
- Mangram JA, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection. Infection Control and Hospital Epidemiology 1999;20:247-78. [DOI] [PubMed] [Google Scholar]
Martin 2015
- Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: Final Data for 2015. National Vital Statistics Report 2017;66(1):1. [PMID: ] [PubMed] [Google Scholar]
Mathai 2013
- Mathai M, Hofmeyr GJ, Mathai NE. Abdominal surgical incisions for caesarean section. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD004453.pub3] [DOI] [PubMed] [Google Scholar]
Normand 2001
- Normand MC, Damato EG. Postcesarean infection. JOGNN - Journal of Obstetric, Gynecologic, & Neonatal Nursing 2001;30(6):642-8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2009
- Schünemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391-400. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Thomas 2001
- Thomas J, Paranjothy S, Royal College of Obstetricians and Gynaecologists Clinical Effectiveness Support Unit. National Sentinel Caesarean Section Audit Report. London: RCOG Press, 2001. [Google Scholar]
WHO 2015
- World Health Organization. WHO Statement on Caesarean Section Rates. www.who.int/reproductivehealth/publications/maternal_perinatal_health/cs-statement/en/ 2015.
References to other published versions of this review
Hadiati 2008
- Hadiati DR, Hakimi M, Nurdiati DS. Skin preparation for preventing infection following caesarean section. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007462] [DOI] [PubMed] [Google Scholar]
Hadiati 2012
- Hadiati DR, Hakimi M, Nurdiati DS. Skin preparation for preventing infection following caesarean section. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD007462.pub2] [DOI] [PubMed] [Google Scholar]
Hadiati 2014
- Hadiati DR, Hakimi M, Nurdiati DS, Ota E. Skin preparation for preventing infection following caesarean section. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD007462.pub3] [DOI] [PubMed] [Google Scholar]
Hadiati 2018
- Hadiati DR, Hakimi M, Nurdiati DS, da Silva Lopes K, Ota E. Skin preparation for preventing infection following caesarean section. Cochrane Database of Systematic Reviews 2018, Issue 10. [DOI: 10.1002/14651858.CD007462.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


