Abstract
Objective:
Primary care may be an effective venue for delivering behavioral interventions for sexual safety among HIV-positive men who have sex with men (MSM); however, few studies show efficacy for such an approach. We tested the efficacy of the Treatment Advocacy Program (TAP), a 4-session, primary-care-based, individual counseling intervention led by HIV-positive MSM “peer advocates” in reducing unprotected sex with HIV-negative or unknown partners (HIV transmission risk).
Method:
We randomized 313 HIV-positive MSM to TAP or standard care. HIV transmission risk was assessed at baseline, 6 months, and 12 months (251 participants completed all study waves). We conducted intent-to-treat analyses using general estimating equations to test the interaction of group (TAP vs. standard care) by follow-up period.
Results:
At study completion, TAP participants reported greater transmission risk reduction than did those receiving standard care, χ2(2, N = 249) = 6.6, p = .04. Transmission risk among TAP participants decreased from 34% at baseline to about 20% at both 6 and 12 months: Transmission risk ranged from 23% to 25% among comparison participants.
Conclusions:
TAP reduced transmission risk among HIV-positive MSM, although results are modest. Many participants and peer advocates commented favorably on the computer structure of the program. We feel that the key elements of TAP-computer-based and individually tailored session content, delivered by peers, in the primary care setting-warrant further exploration.
Keywords: HIV prevention, HIV-infected men, behavioral intervention trials
Many HIV-infected men who have sex with men (MSM) continue to engage in unprotected sex. In some cohorts, up to two thirds of HIV-infected MSM have reported unprotected sex with other HIV-positive men, whereas 30%–50% have reported unprotected sex with partners whose status is unknown or HIV seronegative (Patel et al., 2006; van Kesteren, Hospers, & Kok, 2007). This source of risk may account for the increasing sero-incidence of both HIV and other sexually transmitted infections among MSM (Centers for Disease Control and Prevention [CDC], 2005; Wolitski, Valdiserri, Denning, & Levine, 2001). As the life expectancy and quality of life for HIV-infected people have improved (Lohse et al., 2007), the development of effective interventions for HIV risk reduction designed to meet their unique needs has increasingly become a public health priority (CDC, 2003).
A recent meta-analysis suggests that behavioral interventions for sexual safety among HIV-infected individuals can be effective (Crepaz et al., 2006). However, although interventions among HIV-infected individuals may generally be successful, they have proven less so specifically among MSM (Johnson, Carey, Chaudoir, & Reid, 2006). Consistent with this, a large CDC-funded trial of a peer-based counseling intervention for sexual safety among HIV-infected MSM (Seropositive Urban Men’s Intervention Trial [SUMIT]) showed only modest, short-term effects (Wolitski, Parsons, & Gomez, 2004). The effectiveness of the SUMIT may have been limited by its group structure-risky participants may have influenced otherwise safer men-and its inability to modify a key predictor of risk, that of felt responsibility for the protection of partners (O’Leary, Hoff, et al., 2005). In contrast, Morin (2007) found an individual cognitive-behavioral intervention oriented toward personal health to reduce HIV transmission risk in a mixed cohort of HIV-infected men and women. It was proposed that an individual intervention that used cognitive-behavioral and motivational interviewing approaches to enhance coping and self-regulation among HIV-positive MSM would be effective in fostering their sexual safety.
In this article, we report the results of an efficacy trial of the Treatment Advocacy Program (TAP), a peer-based counseling intervention for sexual safety and general coping among MSM infected with HIV. This trial was run in conjunction with a sister project testing a version of the TAP intervention for African American men and women with lower socioeconomic status (Raja, McKirnan, & Glick, 2007). TAP was a randomized controlled trial of a primary-care-based counseling intervention, contrasted with clinic “standard of care.” The use of peer advocates was intended to provide coping models and to decrease the isolation that may accompany an HIV diagnosis. There is considerable evidence of the effectiveness of para-professional peer counseling on health behaviors, such as smoking, anxiety and depressive disorders, or coping with HIV among youths (Bettencourt, Hodgins, Huba, & Pickett, 1998; den Boer, Wiersma, Russo, & van den Bosch, 2005; Malchodi et al., 2003), particularly with structured, manualized approaches (Bright, Baker, & Neimeyer, 1999; Nielsen, 1995). Additionally, Crepaz et al. (2006) noted that effective interventions for HIV-infected people have tended to be delivered in settings where medical or other services are provided and have tended to address a range of health and coping issues.
Our theoretical model drew on basic coping and self-regulation frameworks (Cooper, Agocha, & Sheldon, 2000; Ewart, 1991; Folkman, Lazarus, Gruen, & DeLongis, 1986; Karoly, 1993; Simoni, Frick, & Huang, 2006). A core task in coping with a chronic disease is self-monitoring the disease state and its behavioral requirements (Miller, Rodoletz, Schoreder, Mangan, & Sedlacek, 1996). Remaining self-aware or mindful of difficult behavioral demands-in the case of HIV involving both sexual safety and a treatment regimen-can be emotionally aversive, particularly for those with diminished self-efficacy for coping (McKirnan, Ostrow, & Hope, 1996). The resultant negative affect or cognitive avoidance may compromise both general coping and specific adherence to sexual safety demands. We thus hypothesize that sexual safety among HIV-positive men would be facilitated by self-efficacy and skills for enhancing social support and coping with HIV (O’Leary, Wolitski, et al., 2005), modulating negative affect (Bancroft, Carnes, & Janssen, 2005; Bancroft, Janssen, Strong, & Vukadinovic, 2003), enhancing HIV disclosure (Cole, Kemeny, Taylor, & Visscher, 1996; Semple, Patterson, & Grant, 2004), and enhancing information and motivation specifically around sexuality (Carey et al., 2000; Fisher, Fisher, Amico, & Harman, 2006). Given the importance of alcohol and drug use to sexual risk and avoidant coping generally (McKirnan, Vanable, Ostrow, & Hope, 2001; Parsons, Kutnick, Halkitis, Punzalan, & Carbonari, 2005), TAP included content on substance use harm reduction (Friedman et al., 2007).
In this study, we examined the effects of TAP on sexual HIV transmission risk behavior, including unprotected sex and overall numbers of sexual partners. Given that many HIV-positive men consider sero-concordant sex-even without condoms-a strategy to reduce their risk for transmitting HIV, we hypothesized that overall unprotected anal intercourse [UAI] would lessen only moderately, whereas transmission risk-UAI that may transmit HIV to uninfected partners-would show significant intervention effects. We anticipated that intervention effects would be strongest at the 6-month, follow-up point and would remain significant at 12 months.
Method
Background
The intervention was conducted in three Chicago-area clinics that reflected a range of primary care settings: a well-established gay/lesbian health center (Howard Brown Health Center), a public clinic (Uptown Clinic of the Chicago Department of Health), and a private medical center (Klein, Slotten, & French Medical Associates). The Institutional Review Boards of each participating clinic, the University of Illinois at Chicago, and the CDC reviewed and approved the study.
The intervention consisted of four 60–90-min individual counseling sessions, 3-month “check-in” telephone calls, and 6- and 12-month coping follow-up counseling sessions. The comparison condition was a 12-month waitlist during which participants received standard HIV primary care at their respective clinics. Standard of care for HIV patients was very high at all three clinics in terms of quality of health care and available social supports. Assessments consisted of 45-min interviews in which an audio computer-assisted self-interviewing (ACASI) instrument was used at baseline, 6 months, and 12 months. The primary outcome was self-reported UAI with HIV-negative or HIV-unknown partners (transmission risk). Secondary outcomes were self-reports of UAI over the previous 6 months and overall number of anal sex partners.
Participants
Participants were recruited from a screening pool sample of 945 HIV-infected MSM attending the three target clinics over 61 weeks in May 2004 through July 2005. Enrollment criteria consisted of having received an HIV diagnosis at least 3 months prior to screening, enrollment in primary care at one of the target clinics, and MSM sexual activity within the previous year. Men were excluded if they intended to move within the next year or did not speak English. The target sample size (n = 225 at follow-up) had 90% power (two-tail p < .05) to detect a 15% decrease in the percentage of men in the intervention group who reported UAI at one follow-up wave.
Procedure
Trained research assistants approached all HIV-positive men attending their regular medical provider visits at the target clinics. Project assistants used the same procedures and structured screening form in each of the clinics to assess patients’ interest in the program and the entry criteria. When a patient screened eligible and accepted enrollment, the research assistant scheduled the consent and baseline interview and called a central research office to receive a randomly assigned participant number. The assigned identification number coded the participant as intervention or comparison.
Informed consent and baseline assessments were generally conducted immediately after enrollment, unless time constraints required that a participant come in for a later visit. Participants were introduced to the ACASI in a private interview room by a research assistant. The assistant left the room during the actual interview, although the assistant remained just outside to provide assistance. After the interview, the participant was told his group assignment and was scheduled for his next visit. Participants received $25 for completing the baseline and 6-month visits, and they received $40 for completing the 12-month assessments.
Intervention group participants were scheduled for their TAP sessions at the end of the baseline visit; we attempted to schedule the four TAP visits during the first four to six weeks postenrollment. The mean number of weeks for session completion was 8. We conducted coping follow-up sessions at 6 and 12 months after enrollment, for which participants received $10 each. When possible, we attempted to conduct intervention or follow-up sessions during participants’ regular primary care visit.
We contacted all participants in person or by phone at 3 and 9 months to update locator information and to encourage continued participation. Participants were scheduled for full ACASI assessments at 6 and 12 months to assess behavioral risk over the prior 6 months. All assessments were conducted prior to counseling visits by trained research assistants and not by the participants’ treatment advocates. All 12-month follow-up assessments were completed by the end of May 2006.
Measures
ACASI topics included sexual attitudes, alcohol and drug use, sexual risk, and ancillary health areas (exercise, smoking, treatment adherence). All items were from standard instruments in the field and used simple check-boxes or rating scales with appropriate skip patterns. Mean completion time was 41 min (SD = 15.26). This analysis focused primarily on the sexual risk outcomes. ACASI behavioral assessments were used for data collection only; treatment advocates did not have access to participants’ responses.
Demographic and medical characteristics.
Demographic and medical characteristics included standard indicators of ethnicity, age, education, living circumstances, and clinical measures of HIV viral load and CD4 t-cell counts.
Sexual risk.
For sexual risk, participants separately reported the number of HIV-negative, HIV-positive, and unknown sero-status partners they had insertive and receptive anal sex with, both with and without condoms, over the previous 6 months. We focused on partner count rather than sexual occasions because it is the strongest predictor of HIV transmission (Buchbinder et al., 2005). We derived three indices: (a) the total number of partners that participants reported any anal sex with, (b) the number of partners that participants had any UAI with (insertive or receptive, with any status partner), and (c) the number of partners participants reported transmission risk with (defined as insertive or receptive UAI with a partner whose sero-status was unknown or HIV-negative). The latter is our key outcome because it reflects behaviors that are more likely to transmit HIV. Participants also self-reported any diagnosed sexually transmitted infection (i.e., syphilis, gonorrhea, or chlamydia) over the previous year.
We analyzed these risk indices both as binary variables-whether a participant reported, for example, transmission risk with any partner-and as continuous measures, reflecting the number of partners for each index. Continuous measures were highly skewed. To correct skewness for number of UAI partners, we truncated the raw ratings at the 99th percentile (values >35 partners) and performed a square-root transformation, thus lowering skewness from 3.0 to 1.5. Number of transmission risk partners was too skewed to be amenable to a simple square-root procedure, so we transformed the raw values into a five-level variable reflecting 0, 1, 2 or 3, 4–9, or 10+ transmission risk partners, lowering skewness from 4.2 to 1.6. We assessed participants’ overall number of anal sex partners as an index of general sexual activity (truncated at 99%, values >50).
Psychosocial and behavioral mediators.
We assessed four variables hypothesized to mediate the effect of the intervention on sexual risk: substance abuse, self-efficacy for sexual safety, disclosure of HIV status, and depression or negative affect. Substance use was the mean frequency of use of 11 substances over the previous 6 months, ranging from alcohol to methamphetamine. Self-efficacy for sexual safety and HIV treatment represented eight items taken from existing efficacy scales (Katz et al., 2002; Koblin et al., 2003) rated on 5-point “agreement” scales (e.g., “I can have safer sex that is satisfying to me”; “I can take my medications exactly as my doctor tells me to”; α = .82). Disclosure of HIV status was the mean of six items, three addressing the proportion of immediate family members, closer friends, associates that the participant had disclosed to, and three addressing disclosure to HIV negative, positive, and unknown sex partners (α = .83). Negative affect was measured by the mean score on the Center for Epidemiologic Studies-Depression Scale (Radloff, 1977), a widely used 20-item index of depression.
The Intervention Design
Treatment advocates.
Six ethnically diverse, HIV-positive MSM peer counselors (treatment advocates) delivered the intervention at the three clinic sites. Advocates’ education levels varied from high school to postgraduate training, with ages ranging from 24 to 40 years. Treatment advocates were recruited through providers or case managers, and they received 40 hr of training on motivational interviewing and cognitive-behavioral techniques for sexual safety and HIV coping, nonjudgmental communication, confidentiality, research and counseling ethics, and referral resources. Ongoing supervision was provided via weekly meetings with doctoral- and master’s-level licensed therapists. All training and supervision occurred at Howard Brown Health Center. We recorded 20% of sessions to audit them for compliance to key elements of the intervention protocol; protocol compliance averaged over 85% for all advocates.
Intervention approach.
Formative work for the intervention represented a collaboration between university researchers and HIV-infected advocates and medical staff at Howard Brown Health Center, and it included seven focus groups or community meetings, 12 individual interviews or advocate role-plays, and a complete pilot study (McKirnan, Swanson, Tolu-Shams, Ramey, & Flynn, 2001). Common themes that emerged during formative work included the powerful effect of peer delivery, the virtue of delivering behavioral counseling within the clinical environment, and the need for intervention materials to be both highly structured and flexible enough for individual tailoring.
The need for both structure and flexibility initially led us to a complex tabbed paper manual; we then experimented with a computer-driven manual. The strong positive response of both advocates and patients to the computer format during formative work led us to commit to this approach for this and a related intervention trial (Raja et al., 2007).
All counseling sessions were structured by a menu-driven PowerPoint program to maximize stimulus value, to create clear structure for protocol compliance, to individually tailor the sessions to the client, and, eventually, to facilitate program dissemination (Kiene & Barta, 2006). This approach was consistent with recent emphasis on using computer technology to structure and disseminate health behavior interventions within the primary care setting (Forkner-Dunn, 2003). Given that HIV patients typically establish a long-term relationship with a care setting for treatment, primary care is a natural setting for recruitment and retention of counseling participants (Klein, Cruz, O’Connell, Scully, & Birkhead, 2005; Myers et al., 2010).
The intervention consisted of one-to-one sessions with treatment advocates. Advocates and clients met with a computer open on a desk. Advocates clicked through each intervention module using text or images as prompts for information, attitude or motivation change, or skills building. Each slide typically began with a “cardinal” question addressing general motivations and goals (e.g., “How has being infected changed your relationship[s] or sex life?”) and was followed by increasingly structured prompts to facilitate specific behavioral plans. The intervention content combined motivational interviewing and cognitive-behavioral techniques to motivate men to participate in active health behavior change and to inculcate skills and self-efficacy in initiating and maintaining behavioral change (Borrelli, Riekert, Weinstein, & Rathier, 2007).
We attempted to increase motivation by presenting risk reduction in the context of overall HIV coping. Both optimism because of antiretroviral therapy and simple HIV fatigue have led to complacency about HIV risk (Ostrow et al., 2002; Stolte et al., 2006; Vanable, Ostrow, McKirnan, Taywaditep, & Hope, 2000). In contrast, infected men are intrinsically motivated to make their HIV treatment successful (Remien et al., 2003). Skills and self-efficacy were facilitated by tailored goals and plans and by personal feedback (Brug, Steenhuis, vanAssema, & deVries, 1996; Kreuter & Strecher, 1996). Each module concluded with a specific behavioral planning exercise. The intervention comprised eight modules: Three were used during the initial three sessions, described below. During Session 4, the counselor and participant chose one of five “focus” modules. Advocates used structured exercises or probes within each module to “hyperlink” to tailored content within each module or to open one of the focus modules.1
Session 1: HIV coping and basic medication skills.
This module began with information stressing the importance of sexual safety and medication adherence. Advocates then used active dialogue to present the larger intervention model, framing active HIV coping in terms of mindful sexuality and intimacy, drug and alcohol use reduction, regulating negative affect, and social support. The advocate then used cognitive-behavioral techniques to inculcate self-efficacy for basic adherence skills, for example, the use of cue controls, pill boxes and medication monitoring, automaticity and staying mindful of regimen requirements, and communication with the provider.
Session 2: Advanced medication and coping skills.
The advocate helped the participant articulate his values and goals for coping with HIV, assessed current adherence levels, and used cognitive-behavioral strategies to articulate the contexts that challenge adherence goals. Key contexts included periods of negative affect, alcohol or drug use, sexual settings, and challenging social settings. In each context, structured probes assessed the participants’ skills and self-efficacy: Advocates entered responses to, for example, rating scales into the program, which then automatically linked to skills building for “problem” areas. A concluding “coping analysis” was used to develop a written behavioral plan sheet for behavioral rehearsal over the next week.
Session 3: Intimacy and sexuality.
The advocate first presented systematic information about the continuing risks of unprotected sex for HIV infected men. He then conducted a motivational interview to articulate the participants’ sexual values and goals, current satisfactions and dissatisfactions regarding intimacy and sexuality, and commitment to change areas. This led to a cognitive-behavioral analysis of sexual risks vis-à-vis social settings, high-risk partners, moods and feelings, drugs and alcohol, avoidant coping, and communication. “Hot buttons” in each content area linked to skills or coping exercises when appropriate. The advocate and participant then developed a concrete, written behavioral change plan for each target skill area.
Session 4: Focused safety skills.
Session 4 began with an analysis and discussion of behavioral plans: On the basis of previous sessions and the participant’s current plans, the advocate linked to one of five focus modules: (a) HIV transmission information, (b) basic safety skills, (c) HIV communication, (d) alcohol and drug use, and (e) moods and feelings. The substance use module and the moods and feelings module combined motivational enhancement and cognitive-behavioral material consistent with the theoretical approaches underlying Sessions 2 and 3. The most common focus module choices were moods and feelings, followed by substance abuse.
Coping follow-up visits.
Coping follow-up visits were given at the 6- and 12-month visits, after the behavioral assessment. These visits, led by the treatment advocate, used the same structure and computer approach as the core intervention. Participants responded to structured probes to report recent sexual risks, adherence to medications, social support, alcohol or drug use, negative affect, and general coping with HIV. Responses indicating difficulties in any area linked the advocate and participant to the appropriate intervention content, typically replicating content from the core intervention modules. Men who were coping well typically took 15–20 min; for men who identified areas of continued risk or coping difficulties, follow-up sessions lasted up to 90 min.
Method of Analysis
All intervention effects were tested by the general estimating equation procedure in SAS. We used the Type III Wald chi-square with an autoregressive correlation structure to test the interaction of group (intervention vs. comparison) by follow-up period for each outcome. Interactions were tested with the main effects entered as prior terms in the model. We conducted simple contrasts to test the interactions of group by (a) baseline versus 6 months, (b) baseline versus 12 months, and (c) baseline versus the mean of 6 and 12 months. All these analyses tested linear effects of group differences on risk levels across wave. We also examined quadratic trends to test whether the groups showed different nonlinear trends across waves. For all analyses, we entered clinic, age, ethnicity, income, and education as covariates prior to the terms coding group and follow-up period. Secondary analyses were also conducted to test whether the effect of the intervention was attributable to changes in men’s level of sexual activity (rates of abstinence, number of sexual partners) over the course of the intervention.
Core analyses used an intent-to-treat, listwise missing value procedure, wherein we analyzed all participants who had data for all waves (n = 251 [comparison n = 120, intervention n = 131]; 80% of participants). We determined these analyses to be appropriate by testing whether data were missing completely at random, meaning that the probability of observing a case is independent of the values of any independent or dependent variable. In bivariate analyses, after testing all demographic measures, all study outcomes, a wide range of psychosocial and behavioral measures (e.g., self-efficacy for sexual safety, alcohol and drug use), indicators of clinical health (viral load, CD4 counts, medication status), and intervention versus comparison group membership, the only predictor of loss to follow-up was Hispanic ethnicity (see below). Under these conditions, results with missing data are statistically unbiased, although subject to a loss of power (James, 2006; Little & Rubin, 2002).
We compared these results with analyses in which we imputed missing values among participants with at least one follow-up wave (n = 297; 95% of participants). For continuous outcomes, we used the multiple imputation procedure from SAS; because the data were non-monotonically missing, we used the Markov Chain Monte Carlo procedure. We used all available data regarding demographic status, psychosocial variables, UAI, and transmission risk partners to impute missing values on risk outcomes. Missing data correction for binary measures used the previous wave value. This was very conservative because most missing data were at the 6-month follow-up and were replaced by the baseline value. Mediating analysis used the method prescribed by Baron and Kenny (1986).
Results
Sample Characteristics
The participant flow is given in Figure 1. Of an HIV-infected male patient population of 945, we screened 581 patients (61%), of whom 411 (71% of screening pool) met the eligibility requirements. Of these men, 317 (77%) agreed to enroll and were randomized to the comparison group (n = 151) or intervention group (n = 166). Four participants were dropped from the analyses sample because of death during the study, for a final sample of 313 (intervention n = 163, comparison n = 150). A total of 251 participants had data available for all three assessment waves (intervention n = 131, comparison n = 120; see Figure 1). Intervention and comparison groups did not differ in initial enrollment rate or in retention at any wave.
Figure 1.
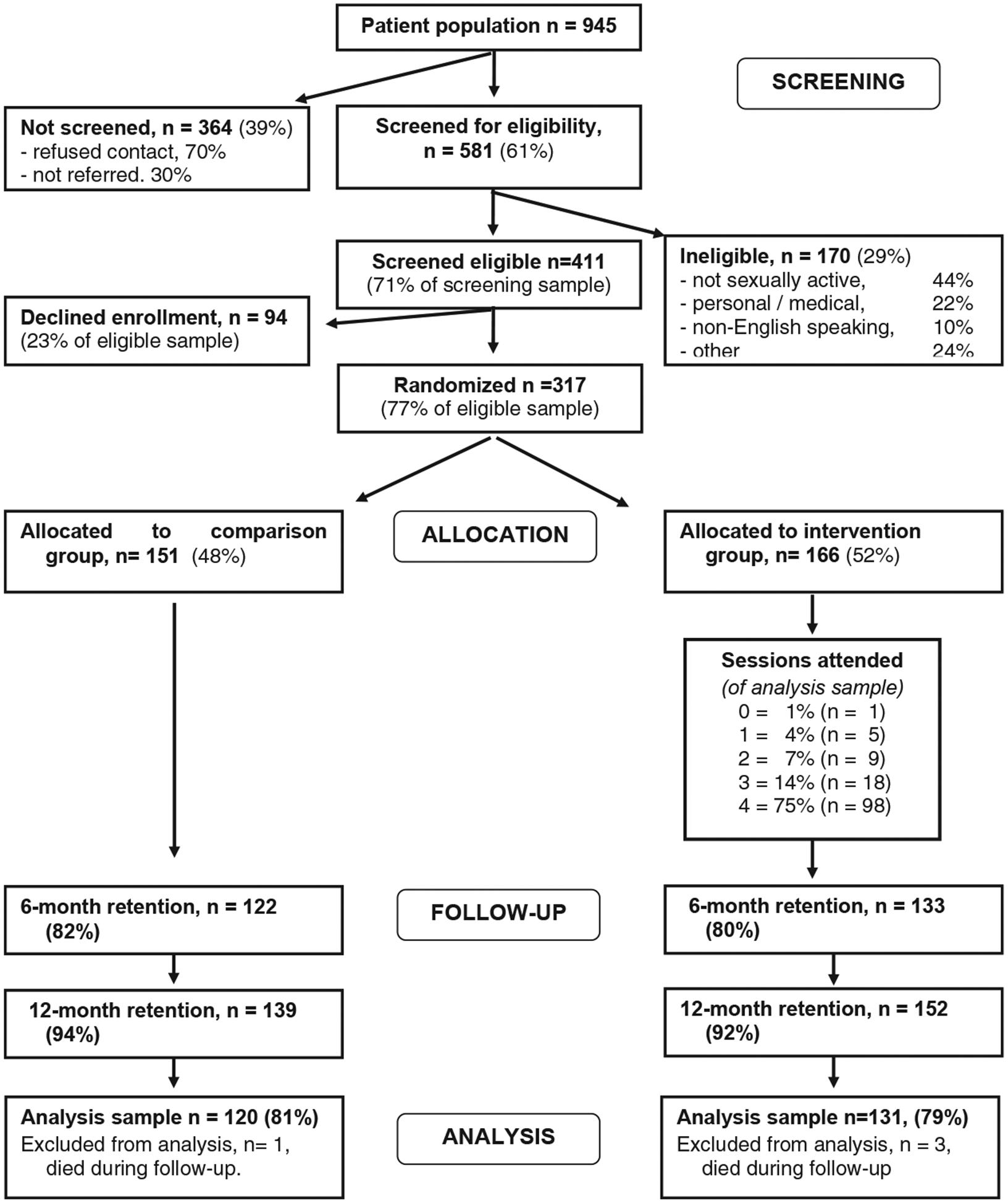
Treatment Advocacy Program trial: Participant recruitment and retention flow.
Demographic and other characteristics are given in Table 1. Of the participants, 90% self-identified as “gay.” The sample had a mean age of 42 years and was diverse in terms of race (32% were African American) and ethnicity (17% were Latino). Income was low, partially because of the high proportion of participants who were on disability (34%). Consistent with the ethnic and educational diversity of the sample, many participants were not “out” as MSM. Participants had been living with HIV for a mean of 8.3 years; 42% had been diagnosed prior to the advent of highly active antiretroviral therapy treatments. Although all participants were in HIV primary care, at baseline, 27% had viral loads greater than log 4.3, and 30% had CD4 counts less than 350.
Table 1.
Treatment Advocacy Program: Demographic Characteristics of the Baseline Sample
| Variable | Complete sample (N = 313) | |
|---|---|---|
| n | % | |
| Age (years) | ||
| 18–29 | 41 | 13 |
| 30–39 | 94 | 30 |
| 40–49 | 137 | 44 |
| 50+ | 41 | 13 |
| Ethnicity | ||
| African American | 98 | 31 |
| Latino | 54 | 17 |
| White | 147 | 47 |
| Asian/other | 14 | 5 |
| Education | ||
| High school/GED or less | 80 | 26 |
| Some college/technical | 121 | 39 |
| College degree | 76 | 24 |
| Any post college | 36 | 11 |
| Income | ||
| <S10,000 | 95 | 30 |
| $10,000–$20,000 | 84 | 27 |
| $21,000–$40,000 | 79 | 25 |
| >$40,000 | 55 | 18 |
| “Out” as MSM to social network | ||
| Half or less | 98 | 31 |
| Most of network | 133 | 43 |
| Completely “out” | 82 | 26 |
| Time since diagnosis | ||
| <3 years | 100 | 32 |
| 4–9 years | 93 | 30 |
| 10+ years | 120 | 38 |
| Clinical status | ||
| On medications | 225 | 72 |
| Discontinued medications | 32 | 10 |
| Medication naive | 56 | 18 |
| CD4 t-cell counts < 350 | 94 | 30 |
Note. GED = General Equivalency Diploma; MSM = men who have sex with men.
Visit completion rates for intervention participants are given in Figure 1; 95% of intervention participants received at least two sessions. The mean time to complete all four sessions was 8 weeks. The intervention and comparison groups did not differ on any demographic or clinical variable at baseline. Mean intervention session length was 65 min (SD = 17, range = 30–120 min); the modal length of follow-up sessions was 20–30 min.
Of the participants, 69% were recruited from Howard Brown Health Center, 18% were recruited from the public clinic, and 13% were recruited from the private clinic. Participants from the three clinics did not differ in sexual risk, HIV medication use, assignment to intervention versus comparison group, or in study retention. Excluding four participants who died during follow-up, retention was 82% at 6 months (122/148 comparison, 133/165 intervention; mean follow-up time = 5.8 months), 93% at 12 months (291/313; mean follow-up time = 11.4 months), and 80% across either 6- or 12-month follow-up (251/313). Neither 6- nor 12-month retention varied by group. The only significant predictor of 6-month dropout was ethnicity: 31.5% of Latino men were lost to follow-up versus 10.8% of African Americans and 18.1% of Caucasians, χ2(2, N = 305) = 10.2, p < .00. No other demographic variable-as well as drug use, psychosocial measures, clinical health, the sexual risk outcome variables, or time since HIV diagnosis-predicted dropout. Retention was not differentially related to any sexual risk index across the intervention and comparison groups.
Over the course of the study-representing 18 months of observation-82% of participants reported at least one instance of UAI; 41% reported any transmission risk (UAI with an HIV-negative or unknown partner); and 29% reported a diagnosis of syphilis, gonorrhea, or chlamydia.
Table 2 shows baseline sexual behaviors assessed over the previous 6 months for the comparison and intervention groups. Differences between groups in baseline UAI and transmission risk were not statistically significant. The strongest demographic predictor of transmission risk was age; younger men were substantially more risky than were older men, Wald χ2(1, N = 249) = 24, p < .001. In general, riskier participants tended to be younger, Caucasian, employed, better educated, and more “out” about their MSM activity (ps < .05).
Table 2.
Treatment Advocacy Program: Overall Sexual Behavior at Study Baseline, by Study Group
| Variable | Complete sample (N = 313) | Intervention group (n = 165) | Comparison group (n = 148) |
|---|---|---|---|
| Any anal sex partner, n (%) | 259 (83) | 140 (85) | 119 (80) |
| Any UAI, n (%) | 150 (48) | 86 (52) | 64 (43) |
| Any transmission risk, n (%) | 91 (29) | 54 (33) | 37 (25) |
| Anal sex partners, M (SD) | 5.7(9.10) | 6.3(9.01) | 5.3 (9.02) |
| UAI partners, M (SD) | 3.3 (6.65) | 3.5 (6.40) | 3.0(6.41) |
| Transmission risk partners, | |||
| M (SD) | 1.5(3.89) | 1.6(3.78) | 1.3(3.78) |
| Any STI (past year), n (%) | 55 (18) | 28 (17) | 27 (18) |
Note. All group comparisons are nonsignificant. All behaviors were reported for a recall period of the past 6 months. UAI = unprotected anal intercourse; STI = sexually transmitted infection.
Intervention Effects on Sexual Risk
Overall UAI.
Participants’ reports of any UAI (i.e., with any sero-status partner) did not show a statistically significant interaction of time by group in the three-wave repeated measures analyses (i.e., baseline-6 months-12-months), χ2(2, N = 249) = 4.5, p = .10. However, there was a significant effect at the 6-month follow-up, χ2(1, N = 249) = 4.02, p = .045, in the predicted direction (see top of Figure 2). These results were similar for participants’ overall number of UAI partners: The overall three-wave intervention effect was nonsignificant, χ2(2, N = 249) = 5.21, p = .074, although the 6-month intervention effect was statistically significant, χ2(1, N = 249) = 5.19, p = .023 (see Figure 3). All these results were similar, although slightly attenuated, when using imputed values for missing observations (data not shown). Thus, intervention participants showed a greater decline in risk levels from baseline to 6 months than did the control participants, although this effect was not carried through the 12-month follow-up.
Figure 2.
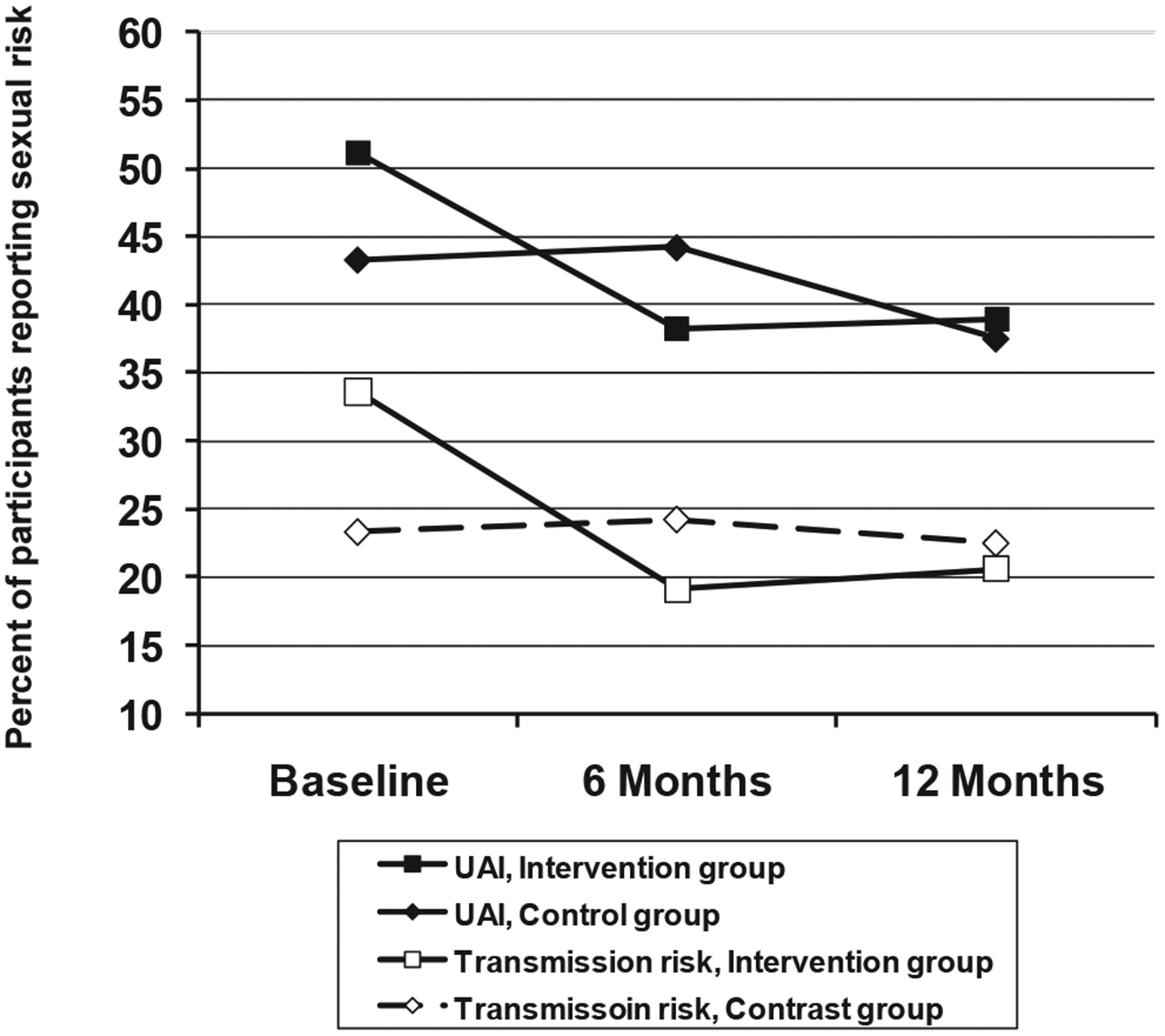
Treatment Advocacy Program: Any unprotected anal intercourse (UAI) and any transmission risk, by group (intervention vs. comparison) and study wave.
Figure 3.
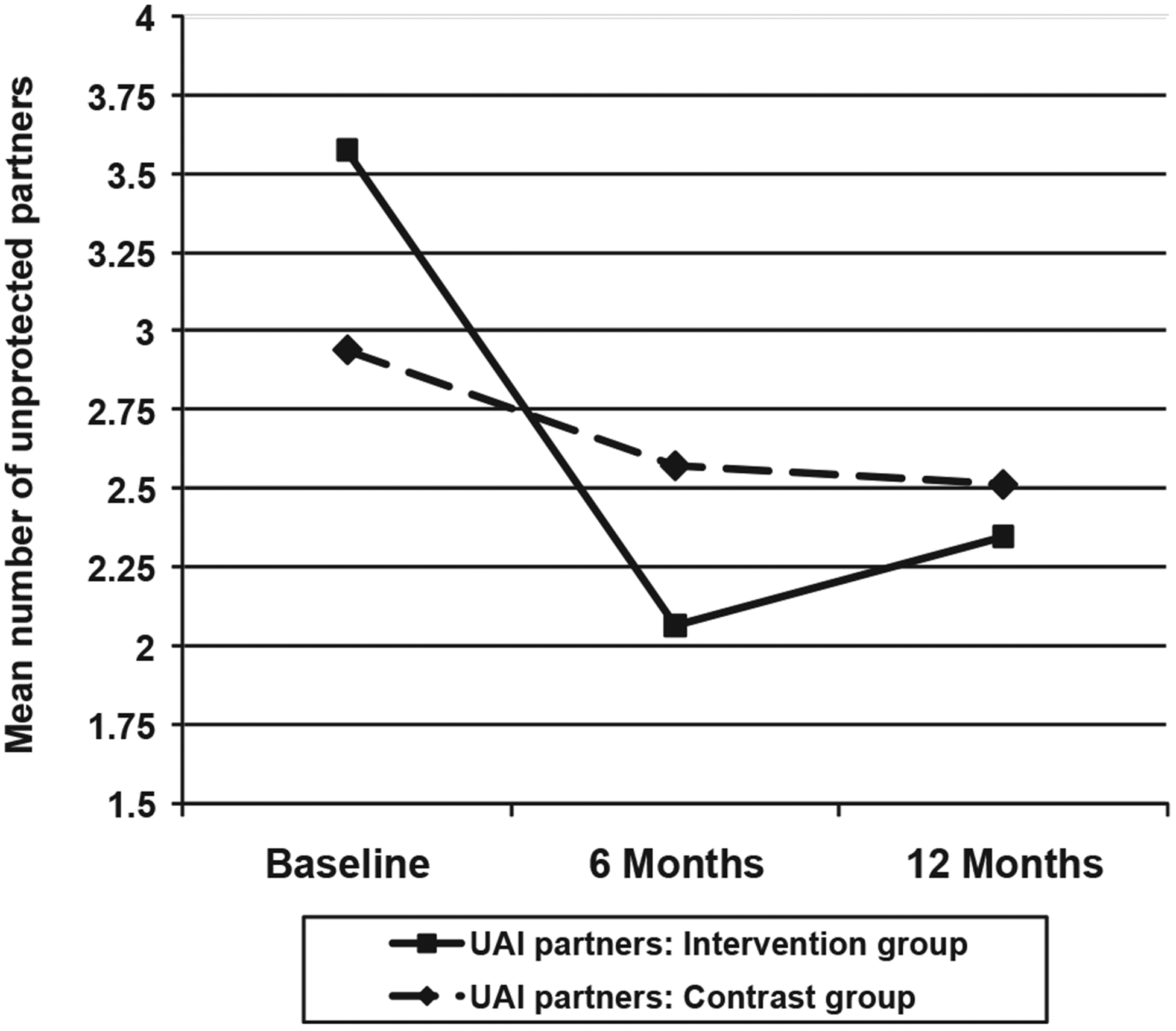
Treatment Advocacy Program: Mean number of unprotected anal intercourse (UAI) partners, by intervention group and study wave.
Transmission risk.
The key variable for our analyses was transmission risk behavior-UAI with HIV-negative or unknown status partners. Results for the binary coding of this variable are given in the bottom of Figure 2, in which there was a significant interaction of intervention group by study wave across the 12 months of follow-up, χ2(2, N = 249) = 6.59, p = .037. The rate of transmission risk among intervention participants went from 33.6% at baseline to about 20% at both 6 and 12 months, whereas transmission risk remained almost constant at approximately 23% among comparison participants.
Intervention effects on transmission risk were reflected in simple contrasts. The intervention group showed a significantly greater decline in risk than did controls from baseline to 6 months and from baseline to the mean of 6 and 12 months, χ2(1, N = 249) = 6.57, p = .01, and χ2(1, N = 249) = 5.47, p = .019, respectively, although the shift from baseline to 12 months was not statistically significant, χ2(1, N = 249) = 2.55, p = .11. In sum, the intervention group showed a significantly greater linear decrease in transmission risk over waves, with the effect remaining significant over 6 and 12 months. These results were slightly attenuated with missing values imputed (data not shown). Intervention effects were not reflected in mean differences between groups at either 6 or 12 months. Rather, the groups differed in their patterns of change over the follow-up periods.
Results for the number of transmission risk partners replicated those for the binary measure, given in Figure 4. Across the three waves, mean transmission risk partners among comparison group participants went from 0.55 at baseline to 0.43 at 12 months, whereas intervention participants reported a decline of 0.74 to 0.42, χ2(2, N = 249) = 7.16, p = .008. In simple contrasts, the groups differed in changes in mean partners from baseline to 6 months and from baseline to the mean of 6 and 12 months, χ2(1, N = 249) = 7.01, p = .008, and χ2(1, N = 249) = 6.3, p = .012, respectively, although the shift from baseline to 12 months was not statistically significant, χ2(1, N = 249) = 3.21, p = .073. As with other analyses, these results were essentially the same for data with imputed means.
Figure 4.
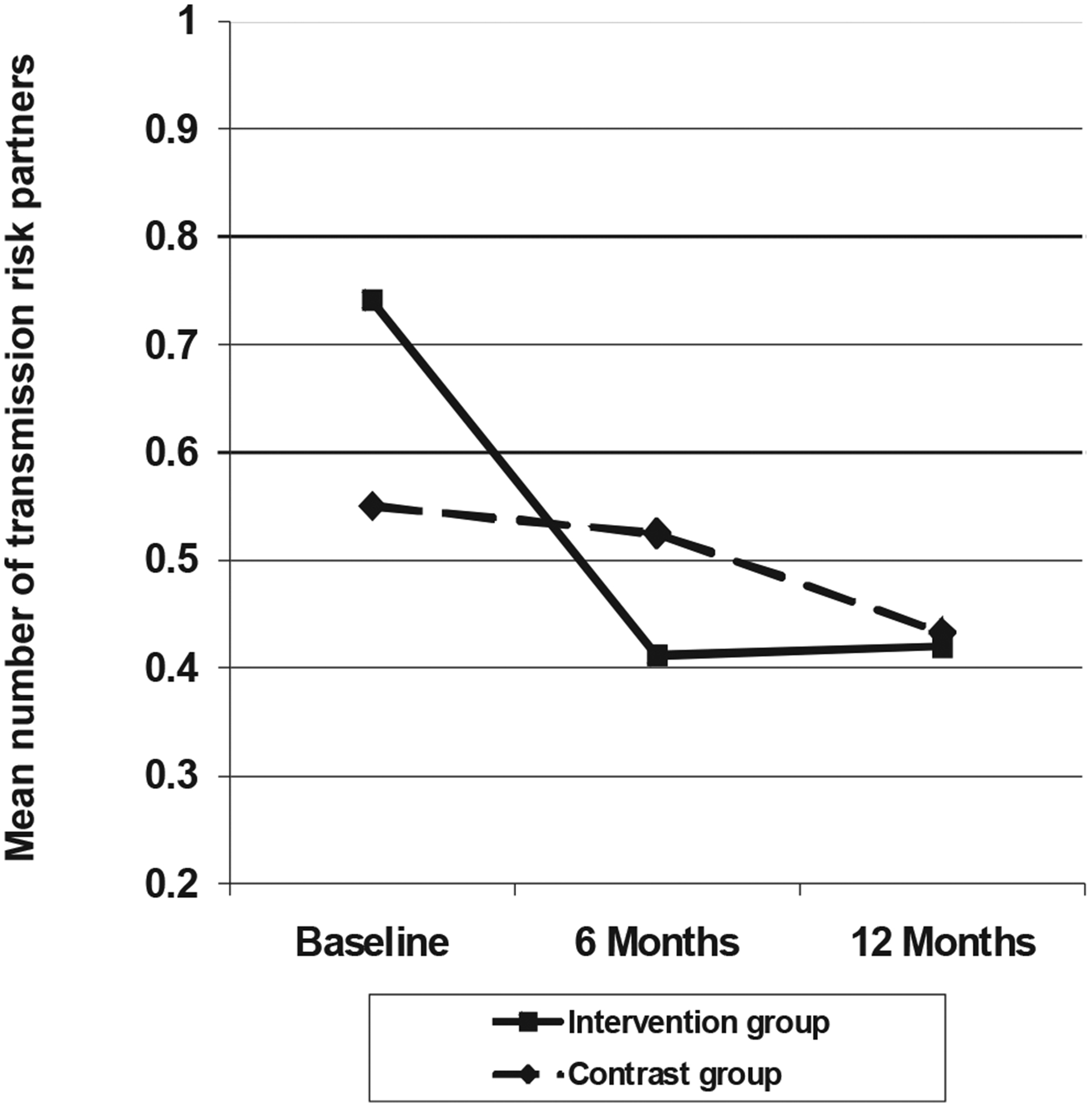
Treatment Advocacy Program: Mean number of transmission risk partners, by intervention group and study wave.
The relatively weaker effects of the intervention on UAI versus transmission risk may be partially explained by a trend toward sero-sorting: At baseline, all participants were significantly more likely to report HIV-positive than HIV-negative or unknown partners (53% vs. 39%, p < .01). Anecdotally, many participants considered UAI in a mutually HIV-positive relationship to be “safe,” thus UAI was less responsive to our behavioral intervention than was transmission risk.
Nonlinear trends.
We examined group differences in quadratic trends to empirically test the general finding that short-term shifts in risk produced by behavioral interventions do not persist over time (Koblin, Chesney, Coates, & EXPLORE Study Team, 2004). For both UAI and transmission risk, the comparison and intervention groups differed in quadratic trends. Thus, in Figure 2, the intervention group showed a noticeable decline in UAI from baseline to 6 months that leveled out during the 6- to 12-month interval. This contrasted with a more linear trend in the comparison group, χ2(1, N = 249) = 4.31, p = .038. This effect was replicated in the results for transmission risk (see the bottom of Figure 2), χ2(1, N = 249) = 4.61, p = .032. These significant quadratic trends indicate that intervention participants’ rate of decline lessened over the follow-up intervals, despite an overall decrease in transmission risk from baseline to 12 months.
Potential Mediators of Intervention Effects
Psychosocial mediators.
We tested four potential mediators of intervention effects: negative affect, HIV disclosure, drug use, and self-efficacy for sexual safety. Each of these is generally important to unsafe sex and was targeted in the intervention. We tested the effect of these variables on participants’ number of transmission risk partners by framing them as time-varying covariates. Greater negative affect across waves predicted more transmission risk partners, χ2(2, N = 249) = 10.8, p = .001, as did less disclosure of HIV status, drug use, and lower sexual self-efficacy, χ2s(2, N = 249) > 24, ps = .001.
Negative affect decreased over waves, Wald χ2(2, N = 249) = 16.4, p < .001, and HIV disclosure increased, Wald χ2(2, N = 249) = 7.6, p < .001, although neither showed an effect of the intervention: intervention group by wave, Wald χ2s(2, N = 249) = 0.17 and 2.35, ns, respectively. Substance use decreased over wave, Wald χ2(2, N = 249) = 27.6, p < .001, and tended to decrease more in the intervention group than the comparison group from baseline to 6 months, Wald χ2(2, N = 249) = 3.63, p < .06. Sexual self-efficacy increased over wave, Wald χ2(2, N = 249) = 24.4, p < .001, and showed a greater increase in the intervention than in the comparison group from baseline to 12 months and from baseline to the mean of 6 and 12 months, Wald χ2s(2, N = 249) > 4.5, ps < .05. Thus, intervention effects on substance use and self-efficacy were consistent with our mediating hypothesis.
The intervention effect on transmission risk partners was χ2(2, N = 249) = 7.16, p = .008. Mediating analyses testing sexual self-efficacy and drug use showed each to lessen this effect to χ2(2, N = 249) = 3.93, p = .14, and to χ2(2, N = 249) = 3.74, p = .15, respectively. Each of these represented a significant trend toward mediation, Δχ2s(1, N = 249) = 3.22 and 3.42, ps = .07, respectively. The joint effect of drug use and sexual self-efficacy significantly mediated the effect of the intervention on number of transmission risk partners to χ2(2, N = 249) = 2.94, p = .23; Δχ2(1, N = 249) = 4.22, p = .04. Thus, sexual self-efficacy and drug use showed significant intervention effects, and simple mediating analyses showed them to partially underlie the effect of the intervention on participants’ number of transmission risk partners.
Sexual activity as a mediator.
We also explored whether the effect of the intervention was attributable to simple changes in men’s level of sexual activity. Rates of abstinence (no reported anal sex partners over the previous 6 months) did increase over wave, Wald χ2(2, N = 249) = 15.6, p = .001, and participants’ number of anal sex partners significantly decreased, Wald χ2(2, N = 249) = 26.8, p = .001. However, neither abstinence nor number of anal sex partners showed any intervention effect, Wald χ2s(2, N = 249) < 0.3, ns, or mediated the effect of the intervention on HIV transmission risk.
Discussion
Complacency or “burnout” over sexual risk appears to be contributing to recent increases in HIV infections among MSM (CDC, 2005; Stockman et al., 2004; Valdiserri, 2004; Vanable, Ostrow, & McKirnan, 2003). We attempted to counter these attitudes by framing sexual safety within a larger coping intervention for HIV-infected MSM. The TAP intervention provided promising evidence that a peer-led, computerized, and tailored intervention for HIV-positive MSM may reduce HIV transmission behaviors. Intervention effects were partially mediated by a decrease in drug abuse and an increase in self-efficacy for sexual safety, suggesting two important foci for further development of the intervention. These effects were not simply a matter of lessened overall sexual activity and, by being more pronounced for actual transmission risk, suggested that participants were specifically modifying their most risky behaviors.
Key features of TAP were its delivery by HIV-positive peer counselors and its use of a computer-driven protocol. Peer-based counseling for HIV risk reduction has shown modest effects among MSM, particularly when using a structured approach (Morin, 2007; Wolitski et al., 2004). Our peer treatment advocates were full- or part-time employees who received systematic training and supervision by a doctoral- or master’s-level clinical psychologist within a gay and lesbian primary care setting. This both provided a high-skill level and helped prevent advocate burnout from dealing with treatment or sexual issues shared with their clients. Participants’ positive responses to the peer advocates both here and in a sister TAP project targeting African American men and women (Raja et al., 2007) suggests that peer involvement should be integral to these interventions.
The resources we devoted to training and supervision may exceed those available in many community or primary care settings. This may limit potential dissemination. However, the computer format can be easily disseminated and tailored to specific settings or populations (Raja et al., 2007), and the counseling approaches we used are standard in any clinical or counseling psychology training program. Thus, university or medical school collaborations may provide a mechanism for program implementation in even resource-poor community settings. Further, the National Institutes of Health, the CDC, and a variety of private funding agencies emphasize “technology transfer” or “capacity building” as core funding areas.
This is the first study to our knowledge in which peers delivered a computer-based intervention for HIV secondary prevention. The computer protocol was designed to be both highly structured-which increases treatment fidelity and may be particularly important for peer counselors-and flexible enough to be individually tailored to each participant (e.g., Crits-Christoph et al., 1998; Haug, Sorensen, Gruber, Lollo, & Roth, 2006; Scaturo, 2001). The computer protocol allowed us to include a range of theory-based intervention content, including overall HIV-coping, specific safety techniques, negative affect, drug use, and disclosure skills, tailored to the participant via hot-button links within each module. Advocates uniformly reported positive responses to the computer protocol and showed a high level of intervention fidelity within the TAP sessions.
We delivered TAP in the primary care environment. Both the primary care basis and the session content attempted to weave secondary prevention into more general HIV coping, including medication adherence and medical communication. We hoped that this approach would capitalize on participants’ motivation for successful HIV treatment. Although this setting did prove to be effective for recruitment, it may have selected for less risky men: Patients who regularly attend their HIV care visits may be more likely to also adhere to sexual safety standards.
We attempted to integrate a treatment advocate session with each primary care visit. However, we found this approach to take substantial clinic cooperation, and many men had moved toward a biannual or even annual primary care visit schedule, which may be too sparse for HIV prevention needs. As a consequence, most follow-up visits were “free-standing” rather than part of a primary care visit, and we could not test the efficacy of true integration of prevention into primary care. Although primary care is an important venue for prevention, community recruitment and follow-up may be important adjuncts for broader scale secondary prevention.
There are significant limitations both to our findings and, potentially, to this intervention approach. One issue is that behavioral interventions may be most effective if begun early after diagnosis. We did not have sufficient statistical power to test that hypothesis here, but anecdotal reports have suggested that participants who had been living with HIV-and having characteristic sexual patterns-for many years may have been more resistant to change.
Our range of clinics and patients also limits these results. Our participants were not randomly sampled and were therefore prey to unmeasurable sampling bias. In particular, our ability to show intervention effects may have been suppressed by lower than expected baseline risk behavior. In addition, the standard of care at the participating clinics was very high, potentially higher than in HIV clinics more generally. Thus, we may have seen stronger effects among riskier, more recently diagnosed men who were being compared with men receiving a more typical standard of HIV care. Future studies need to directly address these sampling issues.
There was a trend for the intervention group participants to report more risk at baseline than did the comparison group. Although this group difference was not statistically significant, it does raise the prospect that some of the observed behavioral change in the intervention group was due to a regression to the mean. The randomization procedure was rigorously followed by screening staff and was unlikely to have been biased. Nonetheless, these baseline differences warrant caution in consideration of these results, as the groups could have differed at baseline by chance in ways that were not captured by our randomization checks. Concern about differences at baseline is partially offset by our finding that drug use and self-efficacy partially mediated the intervention effects. This suggests that the outcomes were more than simply a regression to the mean in the intervention group.
The analyses addressed group differences in trajectories of behavior across waves: Simple mean risk levels for intervention versus comparison participants did not significantly differ. Finally, the comparison group was “standard of care” at each clinic. Although these standards were high, they consisted of less patient contact time than in the intervention group.
Maintenance of initial gains is obviously important yet is often inadequately addressed in health-behavior interventions (Wing, 2000), including HIV prevention (Herbst et al., 2007). The relatively quick “decay” of behavioral intervention effects that are typically found were replicated here vis-à-vis significant quadratic effects-relatively strong effects at 6 months attenuated by the 12-month follow-up. We hoped to enhance maintenance by adding preventive counseling to patients’ ongoing primary care; it is possible that our inability to fully articulate the follow-up sessions into the primary care schedule limited the effectiveness of these visits. Of course it is also possible that brief follow-up sessions are not adequate to maintain behavioral changes induced by an initial, more intensive intervention.
Computer assessment and screening tools have been routinely used to collect sensitive sexual and drug-related information (Tideman et al., 2007), and the Internet is increasingly relevant to health promotion and risk reduction among MSM (Bowen, Horvath, & Williams, 2007; Bull, Lloyd, Rietmeijer, & McFarlane, 2004; Rhodes, 2004). Internet-based behavioral interventions have shown efficacy in areas as diverse as cardiac risk (Kuhl, Sears, & Conti, 2006), diabetes management (Albisser, 2005), and adolescent smoking (McDaniel & Stratton, 2006). Programs that combine “live” interactions with computer-based protocols, such as TAP, may be particularly appealing and familiar to high-risk MSM and may be easily adapted to the Internet. As a follow-up to treatment advocate sessions, an ongoing Internet relationship may be efficient in maintaining contact with men with varying treatment schedules and may assist in longer term maintenance of behavioral changes. Thus, the combination of live and computer-based intervention elements may represent an important area for future health promotion research (Griffiths, Lindenmeyer, Powell, Lowe, & Thorogood, 2006).
We did not have space to discuss this in the article, but the design was a waitlist roll-over structure in which the control group was invited to participate in the intervention after the formal trial was concluded. Virtually every control group participant came back for the full intervention. Thus, despite the limitations of this study, we feel that the feasibility or acceptability of this intervention approach is clear and that the initial efficacy shown in these data justifies further research and development.
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. This research was funded by Centers for Disease Control and Prevention Grant PA 01190. The study is registered on www.clinicaltrials.gov, Trial Number NCT0016433. We thank the many HIV-positive individuals who participated in this research, all the research staff for their dedication and effort, and the staff of the participating medical clinics for their support.
Footnotes
The complete PowerPoint intervention materials are available at http://www.uic.edu/depts/psch/tap/index.html.
Contributor Information
David J. McKirnan, Department of Psychology, University of Illinois at Chicago, and Department of Research, Howard Brown Health Center, Chicago, Illinois
Marina Tolou-Shams, Bradley Hasbro Children’s Research Center, Brown University.
Cari Courtenay-Quirk, Division of HIV/AIDS Prevention, National Center for HIV, STD, and TB Prevention, Centers for Disease Control and Prevention, Atlanta, Georgia.
References
- Albisser AM (2005). A graphical user interface for diabetes management that integrates glucose prediction and decision support. Diabetes Technology & Therapeutics, 7, 264–273. [DOI] [PubMed] [Google Scholar]
- Bancroft J, Carnes L, & Janssen E (2005). Unprotected anal intercourse in HIV-positive and HIV-negative gay men: The relevance of sexual arousability, mood, sensation seeking, and erectile problems. Archives of Sexual Behavior, 34, 299–305. [DOI] [PubMed] [Google Scholar]
- Bancroft J, Janssen E, Strong D, & Vukadinovic Z (2003). The relation between mood and sexuality in gay men. Archives of Sexual Behavior, 32, 231–242. [DOI] [PubMed] [Google Scholar]
- Baron RM, & Kenny DA (1986). The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51, 1173–1182. [DOI] [PubMed] [Google Scholar]
- Bettencourt T, Hodgins A, Huba GJ, & Pickett G (1998). Bay area young positives-A model of a youth-based approach to HIV/AIDS services. Journal of Adolescent Health, 23, 28–36. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Riekert KA, Weinstein A, & Rathier L (2007). Brief motivational interviewing as a clinical strategy to promote asthma medication adherence. Journal of Allergy and Clinical Immunology, 120, 1023–1030. [DOI] [PubMed] [Google Scholar]
- Bowen AM, Horvath K, & Williams ML (2007). A randomized control trial of Internet-delivered HIV prevention targeting rural MSM. Health Education Research, 22, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright JI, Baker KD, & Neimeyer RA (1999). Professional and paraprofessional group treatments for depression: A comparison of cognitive-behavioral and mutual support interventions. Journal of Consulting and Clinical Psychology, 67, 491–501. [DOI] [PubMed] [Google Scholar]
- Brug J, Steenhuis I, vanAssema P, & deVries H (1996). The impact of a computer-tailored nutrition intervention. Preventive Medicine, 25, 236–242. [DOI] [PubMed] [Google Scholar]
- Buchbinder SP, Vittinghoff E, Heagerty PJ, Celum CL, Seage GR, Judson FN, … Koblin BA (2005). Sexual risk, nitrite inhalant use, and lack of circumcision associated with HIV seroconversion in men who have sex with men in the United States. Journal of Acquired Immune Deficiency Syndromes, 39, 82–89. [DOI] [PubMed] [Google Scholar]
- Bull SS, Lloyd L, Rietmeijer C, & McFarlane M (2004). Recruitment and retention of an online sample for an HIV prevention intervention targeting men who have sex with men: The Smart Sex Quest Project. AIDS Care-Psychological and Socio-Medical Aspects of AIDS/HIV, 16, 931–943. [DOI] [PubMed] [Google Scholar]
- Carey MP, Braaten LS, Maisto SA, Gleason JR, Forsyth AD, Durant LE, & Jaworski BC (2000). Using information, motivational enhancement, and skills training to reduce the risk of HIV infection for low-income urban women: A second randomized clinical trial. Health Psychology, 19, 3–11. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2003). Incorporating HIV prevention into the medical care of persons living with HIV: Recommendations of CDC, the Health Resources and Services Administration, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR, 52(No. RR-12), 1–24. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2005, July). HIV and AIDS among gay and bisexual men [CDC Fact Sheet]. Retrieved from http://www.cdc.gov/nchhstp/newsroom/docs/FastFacts-MSM-FINAL508COMP.pdf [Google Scholar]
- Cole SW, Kemeny ME, Taylor SE, & Visscher BR (1996). Elevated physical health risk among gay men who conceal their homosexual identity. Health Psychology, 15, 243–251. [DOI] [PubMed] [Google Scholar]
- Cooper M, Agocha V, & Sheldon MS (2000). A motivational perspective on risky behaviors: The role of personality and affect regulatory processes. Journal of Personality, 68, 1059–1088. [DOI] [PubMed] [Google Scholar]
- Crepaz N, Lyles CM, Wolitski RJ, Passin WF, Rama SM, Herbst JH, … HIV/AIDS Prevention Research Synthesis (PRS) Team. (2006). Do prevention interventions reduce HIV risk behaviours among people living with HIV? A meta-analytic review of controlled trials. AIDS, 20, 143–157. [DOI] [PubMed] [Google Scholar]
- Crits-Christoph P, Siqueland L, Chittams J, Barber JP, Beck AT, Frank A, … Woody G. (1998). Training in cognitive, supportive-expressive, and drug counseling therapies for cocaine dependence. Journal of Consulting and Clinical Psychology, 66, 484–492. [DOI] [PubMed] [Google Scholar]
- den Boer P, Wiersma D, Russo S, & van den Bosch RJ (2005). Paraprofessionals for anxiety and depressive disorders. Retrieved from Cochrane Database of Systematic Reviews (CD004688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart CK (1991). Social action theory for a public health psychology. American Psychologist, 46, 931–946. [DOI] [PubMed] [Google Scholar]
- Fisher JD, Fisher WA, Amico KR, & Harman JJ (2006). An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychology, 25, 462–473. [DOI] [PubMed] [Google Scholar]
- Folkman S, Lazarus RS, Gruen RJ, & DeLongis A (1986). Appraisal, coping, health status, and psychological symptoms. Journal of Personality and Social Psychology, 50, 571–579. [DOI] [PubMed] [Google Scholar]
- Forkner-Dunn J (2003). Internet-based patient self-care: The next generation of health care delivery. Journal of Medical Internet Research, 5, e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman SR, De Jong W, Rossi D, Touze G, Rockwell R, Des Jarlais DC, & Elovich R (2007). Harm reduction theory: Users’ culture, micro-social indigenous harm reduction, and the self-organization and outside-organizing of users’ groups. International Journal of Drug Policy, 18, 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths F, Lindenmeyer A, Powell J, Lowe P, & Thorogood M (2006). Why are health care interventions delivered over the Internet? A systematic review of the published literature. Journal of Medical Internet Research, 8(2), e12. doi: 10.2196/jmir.8.2.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug NA, Sorensen JL, Gruber VA, Lollo N, & Roth G (2006). HAART adherence strategies for methadone clients who are HIV-positive-A treatment manual for implementing contingency management and medication coaching. Behavior Modification, 30, 752–781. [DOI] [PubMed] [Google Scholar]
- Herbst JH, Beeker C, Mathew A, McNally T, Passin WF, Kay LS, … Task Force on Community Preventive Services. (2007). The effectiveness of individual-, group-, and community-level HIV behavioral risk-reduction interventions for adult men who have sex with men: A systematic review. American Journal of Preventive Medicine, 32(Suppl. 1), S38–S67. [DOI] [PubMed] [Google Scholar]
- James XS (2006). Inference methods for saturated models in longitudinal clinical trials with incomplete binary data. Pharmaceutical Statistics, 5, 295–304. [DOI] [PubMed] [Google Scholar]
- Johnson BT, Carey MP, Chaudoir SR, & Reid AE (2006). Sexual risk reduction for persons living with HIV-Research synthesis of randomized controlled trials, 1993 to 2004. Journal of Acquired Immune Deficiency Syndromes, 41, 642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karoly P (1993). Mechanisms of self regulation: A systems view. Annual Review of Psychology, 44, 23–52. [Google Scholar]
- Katz MH, Schwarcz SK, Kellogg TA, Klausner JD, Dilley JW, Gibson S, & McFarland W (2002). Impact of highly active antiretroviral treatment on HIV seroincidence among men who have sex with men: San Francisco. American Journal of Public Health, 92, 388–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiene SM, & Barta WD (2006). A brief individualized computer-delivered sexual risk reduction intervention increases HIV/AIDS preventive behavior. Journal of Adolescent Health, 39, 404–410. [DOI] [PubMed] [Google Scholar]
- Klein SJ, Cruz H, O’Connell DA, Scully MA, & Birkhead GS (2005). A public health approach to “prevention with positives”: The New York State HIV/AIDS service delivery system. Journal of Public Health Management and Practice, 11, 7–17. [DOI] [PubMed] [Google Scholar]
- Koblin B, Chesney M, Coates T, & EXPLORE Study Team. (2004, July 3–9). Effects of a behavioural intervention to reduce acquisition of HIV infection among men who have sex with men: The EXPLORE randomised controlled study. Lancet, 364, 41–50. [DOI] [PubMed] [Google Scholar]
- Koblin BA, Chesney MA, Husnik MJ, Bozeman S, Celum CL, Buchbinder S, … EXPLORE Study Team. (2003). High-risk behaviors among men who have sex with men in 6 U.S. cities: Baseline data from the EXPLORE Study. American Journal of Public Health, 93, 926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuter MW, & Strecher VJ (1996). Do tailored behavior change messages enhance the effectiveness of health risk appraisal? Results from a randomized trial. Health Education Research, 11, 97–105. [DOI] [PubMed] [Google Scholar]
- Kuhl EA, Sears SF, & Conti JB (2006). Internet-based behavioral change and psychosocial care for patients with cardiovascular disease: A review of cardiac disease-specific applications. Heart & Lung: The Journal of Acute and Critical Care, 35, 374–382. [DOI] [PubMed] [Google Scholar]
- Little RJA, & Rubin DB (2002). Statistical analysis with missing data (2nd ed.). New York, NY: Wiley. [Google Scholar]
- Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, … Obel N. (2007). Survival of persons with and without HIV infection in Denmark, 1995–2005. Annals of Internal Medicine, 146, 87–95. [DOI] [PubMed] [Google Scholar]
- Malchodi CS, Oncken C, Dornelas EA, Caramanica L, Gregonis E, & Curry SL (2003). The effects of peer counseling on smoking cessation and reduction. Obstetrics and Gynecology, 101, 504–510. [DOI] [PubMed] [Google Scholar]
- McDaniel AM, & Stratton RM (2006). Internet-based smoking cessation initiatives-Availability, varieties, and likely effects on outcomes. Disease Management & Health Outcomes, 14, 275–285. [Google Scholar]
- McKirnan DJ, Ostrow DG, & Hope B (1996). Sex, drugs and escape: A psychological model of HIV-risk sexual behaviours. AIDS Care, 8, 655–669. [DOI] [PubMed] [Google Scholar]
- McKirnan D, Swanson F, Tolu-Shams M, Ramey B, & Flynn J (2001, March). Effectiveness of a general coping approach to HIV adherence Paper presented at the 21st annual meeting of the Society of Behavioral Medicine, Seattle, WA. [Google Scholar]
- McKirnan DJ, Vanable PA, Ostrow DG, & Hope B (2001). Expectancies of sexual “escape” and sexual risk among drug and alcohol-involved gay and bisexual men. Journal of Substance Abuse, 13, 137–154. [DOI] [PubMed] [Google Scholar]
- Miller SM, Rodoletz M, Schoreder CM, Mangan CE, & Sedlacek TV (1996). Applications of the monitoring process model to coping with severe long-term medical threats. Health Psychology, 15, 216–225. [DOI] [PubMed] [Google Scholar]
- Morin SF (2007). Effects of a behavioral intervention to reduce risk of transmission among people living with HIV-The Healthy Living Project randomized controlled study. Journal of Acquired Immune Deficiency Syndromes, 44, 213–221. [DOI] [PubMed] [Google Scholar]
- Myers JJ, Shade SB, Rose CD, Koester K, Maiorana A, Malitz FE, … Morin SF (2010). Interventions delivered in clinical settings are effective in reducing risk of HIV transmission among people living with HIV: Results from the Health Resources and Services Administration (HRSA)’s Special Projects of National Significance initiative. AIDS and Behavior, 14, 483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen BA (1995). Paraprofessionals: They can be competent, and there is more good news. Journal of Psychological Practice, 1, 133–140. [Google Scholar]
- O’Leary A, Hoff CC, Purcell DW, Gomez CA, Parsons JT, Hardnett F, … Lyles CM (2005). What happened in the SUMIT trial? Mediation and behavior change. AIDS, 19(Suppl. 1), S111–S121. [DOI] [PubMed] [Google Scholar]
- O’Leary A, Wolitski RJ, Remien RH, Woods WJ, Parsons JT, Moss S, … Lyles CM (2005). Psychosocial correlates of transmission risk behavior among HIV-seropositive gay and bisexual men. AIDS, 19(Suppl. 1), S67–S75. [DOI] [PubMed] [Google Scholar]
- Ostrow DE, Fox KJ, Chmiel JS, Silvestre A, Visscher BR, Vanable PA, … Strathdee SA (2002). Attitudes towards highly active antiretroviral therapy are associated with sexual risk taking among HIV-infected and uninfected homosexual men. AIDS, 16, 775–780. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Kutnick AH, Halkitis PN, Punzalan JC, & Carbonari JP (2005). Sexual risk behaviors and substance use among alcohol abusing HIV-positive men who have sex with men. Journal of Psychoactive Drugs, 37, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P, Taylor MM, Montoya JA, Hamburger ME, Kerndt PR, & Holmberg SD (2006). Circuit parties: Sexual behaviors and HIV disclosure practices among men who have sex with men at the White Party, Palm Springs, California, 2003. AIDS Care-Psychological and Socio-Medical Aspects of AIDS/HIV, 18, 1046–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. [Google Scholar]
- Raja S, McKirnan D, & Glick N (2007). The Treatment Advocacy Program-Sinai: A peer-based HIV prevention intervention for working with African American HIV-infected persons. AIDS and Behavior, 11, S127–S137. [DOI] [PubMed] [Google Scholar]
- Remien RH, Hirky AE, Johnson MO, Weinhardt LS, Whittier D, & Le GM (2003). Adherence to medication treatment: A qualitative study of facilitators and barriers among a diverse sample of HIV+ men and women in four U.S. cities. AIDS and Behavior, 7, 61–72. [DOI] [PubMed] [Google Scholar]
- Rhodes SD (2004). Hookups or health promotion? An exploratory study of a chat room-based HIV prevention intervention for men who have sex with men. AIDS Education and Prevention, 16, 315–327. [DOI] [PubMed] [Google Scholar]
- Scaturo DJ (2001). The evolution of psychotherapy and the concept of manualization: An integrative perspective. Professional Psychology-Research and Practice, 32, 522–530. [Google Scholar]
- Semple SJ, Patterson TL, & Grant I (2004). Psychosocial characteristics and sexual risk behaviours of HIV+ men who have anonymous sex partners. Psychology and Health, 19, 71–87. [Google Scholar]
- Simoni JA, Frick PA, & Huang B (2006). A longitudinal evaluation of a social support model of medication adherence among HIV-positive men and women on antiretroviral therapy. Health Psychology, 25, 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman JK, Schwarez SK, Butler LM, de Jong B, Chen SY, Delgado V, & McFarland W (2004). HIV prevention fatigue among high-risk populations in San Francisco [Letter]. Journal of Acquired Immune Deficiency Syndromes, 35, 432–434. [DOI] [PubMed] [Google Scholar]
- Stolte IG, de Wit JB, Kolader M, Fennema H, Coutinho RA, & Dukers NH (2006). Association between “safer sex fatigue” and rectal gonorrhea is mediated by unsafe sex with casual partners among HIV-positive homosexual men. Sexually Transmitted Diseases, 33, 201–208. [DOI] [PubMed] [Google Scholar]
- Tideman RL, Chen MY, Pitts MK, Ginige S, Slaney M, & Fairley CK (2007). A randomised controlled trial comparing computer-assisted with face-to-face sexual history taking in a clinical setting. Sexually Transmitted Infections, 83, 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdiserri RO (2004). Mapping the roots of HIV/AIDS complacency: Implications for program and policy development [Proceedings paper]. AIDS Education and Prevention, 16, 426–439. [DOI] [PubMed] [Google Scholar]
- Vanable PA, Ostrow DG, & McKirnan DJ (2003). Viral load and HIV treatment attitudes as correlates of sexual risk behavior among HIV-positive gay men. Journal of Psychosomatic Research, 54, 263–269. [DOI] [PubMed] [Google Scholar]
- Vanable PA, Ostrow DG, McKirnan DJ, Taywaditep KJ, & Hope BA (2000). Impact of combination therapies on HIV risk perceptions and sexual risk among HIV-positive and HIV-negative gay and bisexual men. Health Psychology, 19, 134–145. [DOI] [PubMed] [Google Scholar]
- van Kesteren NMC, Hospers HJ, & Kok G (2007). Sexual risk behavior among HIV-positive men who have sex with men: A literature review. Patient Education and Counseling, 65, 5–20. [DOI] [PubMed] [Google Scholar]
- Wing RR (2000). Cross-cutting themes in maintenance of behavior change. Health Psychology, 19, 84–88. [DOI] [PubMed] [Google Scholar]
- Wolitski RJ, Parsons JT, & Gomez CA (2004). Prevention with HIV-seropositive men who have sex with men-Lessons from the Seropositive Urban Men’s Study (SUMS) and the Seropositive Urban Men’s Intervention Trial (SUMIT). Journal of Acquired Immune Deficiency Syndromes, 37, S101–S109. [DOI] [PubMed] [Google Scholar]
- Wolitski RJ, Valdiserri RO, Denning PH, & Levine WC (2001). Are we headed for a resurgence of the HIV epidemic among men who have sex with men? American Journal of Public Health, 91, 883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]


