Abstract
Antibacterial activity of nanoparticles has received significant attention worldwide because of their great physical and chemical stability, excellent magnetic properties, and large lattice constant values. These properties are predominate in the food science for enhancing the overall quality, shelf life, taste, flavor, process-ability, etc., of the food. Nanoparticles exhibit attractive antibacterial activity due to their increased specific surface area leading to enhanced surface reactivity. When nanoparticles are suspended in the biological culture, they encounter various biological interfaces, resulting from the presence of cellular moieties like DNA, proteins, lipids, polysaccharides, etc., which helps antibacterial properties in many ways. This paper reviews different methods used for the synthesis of nanoparticles but is specially focusing on the green synthesis methods owing to its non-toxic nature towards the environment. This review highlights their antibacterial application mainly in the food sector in the form of food-nanosensors, food-packaging, and food-additives. The possible mechanism of nanoparticles for their antibacterial behavior underlying the interaction of nano-particles with bacteria, (i) excessive ROS generation including hydrogen peroxide (H2O2), OH− (hydroxyl radicals), and O−22 (peroxide); and (ii) precipitation of nano-particles on the bacterial exterior; which, disrupts the cellular activities, resulting in membranes disturbance. All these phenomena results in the inhibition of bacterial growth. Along with this, their current application and future perspectives in the food sector are also discussed. Nanoparticles help in destroying not only pathogens but also deadly fungi and viruses. Most importantly it is required to focus more on the crop processing and its containment to stop the post-harvesting loss. So, nanoparticles can act as a smart weapon towards the sustainable move.
Keywords: Food safety, Green-biochemical synthesis, Biodegradable polymer, Food additives, Nanosensors, Nanotoxicity
Graphical abstract
1. Introduction
Nanotechnology is the 21st century technological revolution in many research areas due to their remarkable structural, morphological, and magnetic properties. In 1974, Dr. N. Taniguchi (Tokyo University of Science, Japan) is the man behind the word “nanotechnology” but Dr. Richard Phillips Feynman (American physicist) is the person who innovated is latest technology [1]. It is an interesting hotspot, which has attracted attention in recent material sciences because it has beneficial effects on human life and stimulates life sciences, especially biotechnology, biomedical and food technology [2]. Nanotechnology helps in delivering of food nutrients through nano-encapsulation [3,4]. It is widely used in the development of food packaging material [3,[5], [6], [7], [8]] (active packaging [9,10], intelligent packaging [6,10,11], improved packaging [6,12] and biodegradable packaging [[13], [14], [15], [16], [17]], etc.), food additives [6,18,19], food nano-sensors [4,5,20,21]. In particular, this paper will review about nanoparticles (size ≤ a hundred nanometer (nm) [22]) and their application in the bio-science (especially, the food sector). Globally, foodborne diseases affect not only the economy but also human health badly [23]. There are 300 companies across the world that have induced nanotechnology in their food system [24]. In 2011, the Centers for Disease Control and Prevention (CDC) reported that food-borne pathogens worsen the health of approximately 45–48 million people in the United States, of which three thousand die. Therefore, it is important to focus on new technological approaches such as nanosensors for detecting food poisoning bacteria, fungi, and viruses [25]. To overcome this problem, there is a need for novel anti-microbial agents for which there is a requirement to develop a technology, which would help in overcoming this serious issue. Nowadays, the use of nanoparticles in the food sector is increasing, mainly in the food packaging industry where they are used as an antibacterial agent towards food-borne diseases [4,15,16,26]. Proper incorporation of nanoparticles into the packaging materials leads to their interaction with the food-borne pathogens, thereby releasing nanoparticles onto the food surface where they come in contact with bad bacteria and cause bacterial death and/or inhibition, thereby increasing the shelf life of the product. It is important to focus more on processing and its containment to stop the post-harvesting loss. Nanoparticles can act as a smart weapon towards the sustainable move [27].
In the late 70s the demand of food security across India has increased after the green revolution and industrialization, due to which nanotechnology attracted not only the manufacturers but also the farmers to utilize this latest technique [28]. The world's population is increasing every day, and it is important to build a food system that maximizes production without wasting food. According to a report from the United Nations (UN) 2019, the world's population is expected to reach 8.548 billion (bn) by 2030, 9.735 billion by 2050, and 10.874 billion by 2100 [29]. Meeting the overall demand without impacting the environment or human health is a challenge for the agricultural sector. Nanotechnology plays an important role in reducing food waste due to spoilage and thus increases its shelf life. The combination of different metal nanoparticles and different plastic resins (e.g. high-density polyethylene, low-density polyethylene, polyvinyl chloride, polystyrene, and polypropylene, etc.) helps in maintaining the overall quality of the food product [30]. According to Environmental Working Group (EWG) 2008, reported in their article that nearly 9800 food products are circulating across the globe containing nano-scale particles [31]. Nanotechnology helps in food containment, preservation, commercialization, and communication. Nanoparticles such as oxides of zinc, titanium, silicon, and magnesium have been further researched by researchers due to their disinfecting action and their enormous application in the food sector as well as other fields such as the paper, textile, printing, cosmetic, and polymer industries [5]. One of the ethical issues for acceptance of nanotechnology in food-system also reported in various places in Europe questioning incorporation of traceable nano-chips in livestock. The use of nanotechnology in the agricultural sector is increasing day by day due to improved soil fertility, water quality, and better pesticides [3]. Keeping in mind the globalization factor, there must be proper guidelines and regulations made on national and international level for proper application of nanoparticles in the food sector [31]. European Food Safety Authority (EFSA) [31] and Food and Drug Administration (FDA) [30], regulates the Acceptable Daily Intake (ADI) of nanoparticles directly or indirectly from food-system.
Nanoparticles have vast applications in the food sector due to its antimicrobial properties. It not only helps in rupturing the cells of bacteria but also ruptures the cells of fungi and viruses [32]. Knowing that the year 2020 is certainly a bad year for the whole world, this year we are dealing with an invisible enemy called Severe Acute Respiratory Syndrome Corona Virus-2 (SARS CoV-2). The virus has spread since December 2019 and is now pandemic worldwide and the disease caused by the SARS CoV-2 is known as Corona Virus Disease 2019 (COVID-19), termed by virologists [33]. A total of 5,304,772 people were affected across the world by COVID-19 disease, of which 342,029 died, according to a World Health Organization (WHO) report on 25th May (2020) [34]. It is a global emergency, effecting 213 countries and territories. The government of 100 affected countries enforced the partial or full lockdown by the end of March 2020. The situation is getting the worst day by day affecting millions of people. The food sector is now in the limelight because food is necessary for survival even in the pandemic situation. It is a challenging situation over the food producer and the handler to handle the food carefully, through the proper food system. Food security is now top in priority to avoid the spread of this disease (which is highly communicable) [33]. Khezerlou et al. (2018), mentioned the antiviral activity of some nanoparticles, e.g., Gold (Au) nanoparticles against HIV and influenza viruses, Silver (Au) nanoparticles against HIV-1, influenza, monkeypox, respiratory syncytial viruses, Zinc Oxide (ZnO) nanoparticles against herps simplex type-I and type-II, transmissible gastroin-testinal viruses, Titanium Oxide (TiO2) nanoparticles against herps simplex, influenza, zika (mosquitoes as a vector) viruses [32]. These all mentioned nanoparticles are highly utilized in the food sector in many ways and all of them are reviewed in this paper.
2. The demand for nanoparticles in life sciences
Nanoparticles are rich in morphological structures, such as rod-like shape, pyramid structure, micro-flower, etc., having increased surface area in comparison to their counterparts, which allows them to be used in various fields like biosensors, food-packaging, nanomedicine, and bio-nanotechnology [35]. Due to its antiviral activity against pathogenic micro-organisms such as bacteria, fungi, and viruses, nanoparticles are highly used in the food sector [32]. Researchers at the University of Toronto have demonstrated the use of nanoparticles designed to concentrate in a tumor and generate oxygen to increase the effectiveness of the chemotherapy drug doxorubicin. Researchers at the University of Wisconsin have demonstrated a bandage that applies electrical pulses to a wound using electricity produced by nanogenerators worn by the patient [36]. Nanoparticles application in the detection of urea in urine and blood sample makes it easy to detect the proper functioning of the body organs (mainly kidneys and liver). Kaushik et al. (2009) developed a biosensor consists of Fe2O3- Chitosan nanocomposites for urea detection in blood and urine samples [37]. Researchers at Oregon State University are developing nanoparticles that deliver three anti-cancer drugs to the lymph nodes. The intent is to target cancers that use the lymph nodes to spread through the body. Testing of this technique, so far, has been with lab animals. Researchers at IBS are developing a graphene-based device to monitor the glucose level in people with diabetes [36]. Cholesterol is a type of lipids (an organic molecule), which is naturally synthesized in our body tissue (nearly, 75% in liver). The main function of cholesterol is to synthesize lipoprotein and plasma membrane. It acts as a forerunner for many biomolecules (such as, steroid hormones, bile acid, cardiac glycosides, vitamin-D, functional lipids, etc.), which helps in maintaining the metabolic activity of the body [38]. Dietary intake of cholesterol consists of rest 25% which inhibits the functioning of the metabolic activity of the body organs, like, cholesterol in the form of plaque blocks the arteries which trigger heart disease. Nanotechnology helps in detecting the cholesterol level inside the body and thus alert the patient, ZnO containing nanosensors were developed by the researcher to do the same [39]. Researchers have determined that the surface charge of protein filled nanoparticles affects the ability of the nanoparticles to stimulate immune responses. They are thinking that these nanoparticles may be used in inhalable vaccines. The properties of nanoparticles are mostly characterized by their size, composition, crystallinity, and morphology. When the size is reduced to the nanoscale, their chemical, mechanical, electrical, structural, morphological, and optical properties of a material are modified. All these modified properties allow the nanoparticles to interact with cell biomolecules [36]. The transfer of nutritional supplements to the target part of the body organ with the help of nanotechnology is an area of future research [40].
Nanoparticles with their modified properties have great potential to be used as antibacterial agents, as previously mentioned. There are two types of antibacterial agents present i.e., organic (organic acids, bacteriocin, essential oils, etc.) and inorganic (particularly metal oxides). However, organic antibacterial agents possess some problems as they are very sensitive to processing conditions such as high pressure and temperature. Compared to organic ones, inorganic antibacterial agents have good stability at high pressure and temperatures, long shelf life [36,41], and also they show strong antibacterial activity even at low concentrations [42]. The inorganic nanomaterials used in food packaging and storage are transition metals (silver and iron); alkaline earth metals (calcium and magnesium) and non-metals (selenium and silicates). Nano-silver has been used in a number of consumable foods, food contact surfaces, and food packaging. Nano-silver has been used as anti-microbial, anti-odourant, and as a health supplement in different sectors [43]. Similarly, nano-iron also acts as a health supplement, and can be used in the treatment of contaminated water (break down organic pollutants) and is known to kill microbial pathogens [43].
Various metal oxides and nano-ferrites are of great interest as they exhibit varying morphologies and show significant antibacterial activity over a wide range of micro-organisms [32,44]. Metal oxides such as ZnO [45], TiO2 [18,19,46,47], MgO [48,49] and CuO [50] have high reactivity, enhanced bio-availability and bioactivity which possess great concern in food technology. Similarly, metal ferrites such as zinc ferrite, copper ferrite, cobalt ferrite, nickel ferrite etc., have unique magnetic properties, excellent chemical stability, large lattice constant, large antibacterial properties are characterized by strong antibacterial properties [51,52].
Yamamoto (2001), mentioned that there is an increase in the antibacterial activity with decreasing particle size [53]. Among all the nanoparticles, magnetic nanoparticles can be easily manipulated through an external magnetic field, which makes the nano-ferrites a suitable candidate for antimicrobial application.
Nanocomposites film is a thin layer of natural/synthetic polymers containing nanoparticles [54,55]. Mirjalili et al. (2017), has synthesized biodegradable ZnO starch film as a food packaging material. They used various oils, plasticizers, lubricants, and binders to obtain a homogeneous composition of starch solution. The successful synthesis of zinc starch film followed by various characterization techniques such as XRD, SEM, mechanical testing. Their study also showed an interaction between zinc nanoparticles and starch. The tensile strength of the nanocomposite films were increased by adding 0.7 g of ZnO nanoparticles, 0.3 g of carboxy-methyl cellulose, 0.2 g of oil, 1.5 g of lubricant and 3 g of starch. Finally, nanoparticles embedded films had shown the antibacterial activity of about 92% against S. aureus and 98% against E. coli bacterial strain [56]. The functional properties of native films are improved with the incorporation of many compounds such as plasticizers, cross-linking agents, polymers, plant extract, and nanoparticles. Specific Packaging materials and coating material were developed for food such as fruits to meet the specification of that food [57]. To obtain the desired properties for food packaging applications, two or more natural polymers are utilized to fabricate food packaging films using the solvent casting method [58]. With desired food packaging properties, films might be substituted with synthetic packaging materials in the future and due to environmental concerns, several works have been propelled to suggest new biodegradable materials and raw materials for these polymers reached starting from renewable natural resources [59]. To improve the properties of nanocomposites, a uniform dispersion of polymeric material and properly sized and shaped nanoparticles are required. Obtaining a small distribution of nanoparticles usually results in the enhanced properties of the packaging material [60]. However, the main limitations of this approach are the growth of metal nanoparticle agglomerates and the uneven distribution of nanoparticles within the polymer network. This approach usually involves the dissolution of nanoparticles into polymer solution-forming films [[61], [62], [63], [64]]. At present, the green method has been adopted to prepare nanocomposite films whose plant extracts act as stabilizers and reducing agents for nanoparticles inside polymeric materials [65]. The properties of nanoparticles depend on the process of chemical, biochemical, or physical synthesis methods, and this will be discussed further.
3. Various techniques for nanoparticle synthesis
There are many methods known in the literature to synthesize nanoparticles which are grouped into two categories namely bottom-up approach and top-down approach. “ b ottom- Up” (i.e. atoms coalesce together): In this type of approach, atoms and molecules are assembled to form nanoparticles or nanomaterial of required shape and size by controlling deposition or reaction parameters. e.g. Sol-gel method. “ t op-Down” (i.e. to break or disassemble bulk solids into finer particles): In this approach, the atoms and molecules are removed from the bulk material to obtain desired nanoparticles [21,31]. The main disadvantage of the top-down approach is estimated according to the speed and reproducibility. It produces surface defects, results in contamination, and is also responsible for introducing internal stress. In addition to this, the following problems are associated with the top-down process: (a) cost of new machines and cleanroom environment grows exponentially with newer technologies, (b) with conventional materials heat dissipation is a big problem. However, the bottom-up approach has the merits of producing nanomaterials with fewer defects and a more uniform composition [66]. In addition, the bottom-up process can form thin films and the structure is very simple. It is more economical than top-bottom because it doesn't waste material during synthesis. Parameters such as pH, sintering time, and temperature play important roles in controlling the properties of nanoparticles formed by the bottom-up approach [67].
There are large numbers of physical, chemical, biological, and hybrid methods available to synthesize different types of nanoparticles [48]. Physical and chemical methods such as Gas Condensation, Vacuum Deposition and Vaporization, Chemical Vapor Deposition (CVD) method and Chemical Vapor Condensation (CVC) method, Mechanical Attrition, chemical precipitation method, sol-gel techniques [68], are widely used. The nanoparticles can be obtained by these methods just by adjusting or changing parameters such as temperature, pressure, precursors, and their hydrolysis ratio [69]. Recent studies have shown that the properties (chemical and physical) and different morphologies are strongly affected by the experimental conditions, the kinetics of action between the metal ions and the reducing agent, and adsorption of the stabilizing agent with the metal nanoparticles [70].
3.1. Green/biochemical synthesis
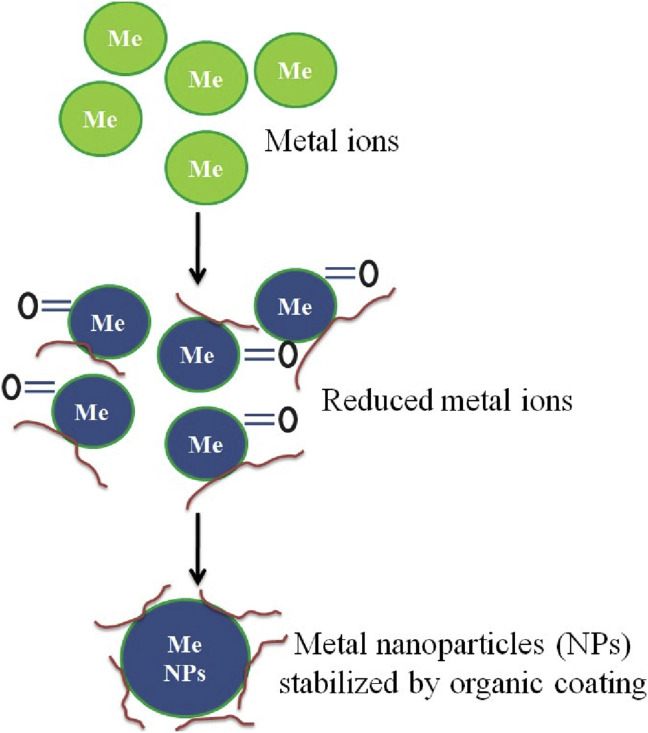
Chemical synthesis processes are very expensive and toxic, posing an adverse effect on the environment. So, today's main focus is to develop an eco-friendly, simple, and clean method to reduce the use and generation of hazardous substances [71]. The green synthesis employs the use of natural sources such as plants, yeast, fungi, and bacteria [72]. Natural sources like plant extract, leaves, seeds, and stems, etc. can act as strong binding and reducing agents. These compounds convert metal ions into nanoparticles [[73], [74], [75]]. The plant extract is mixed with the solution containing metal ions to carry out green synthesis. The biochemical/green synthesis possesses many advantages over physical and chemical methods such as low cost, less time, no use of any harmful chemical (i.e., less harmful risk over the human body), and no need to use high pressure, energy and temperature [76].
Nanoparticles synthesized in the plant extracts already have a functionalized surface containing organic ligands, proteins, polysaccharides, and polyatomic alcohols, which are absent when synthesized by other physical or chemical methods. The presence of these components increases the stability of the particles and facilitates the subsequent attachment of the functional molecules, such as antibodies or DNA to the nanoparticles [77,78]. The reason behind this phenomenon is that the nanoparticles synthesized by using plant extract have a functionalized surface. The presence of a functionalized surface is due to the presence of organic lipids, proteins, polysaccharide, which would otherwise be absent in the case of the chemical and physical method of nanoparticle synthesis [77,78]. Nanoparticles produced from green synthesis can be mono-dispersed easily by controlling some parameters such as pH, temperature, incubation period, and the mixing ratio. The use of micro-organisms for the synthesis is very costly as they require proper conditions for their growth as well as is very long process regarding their growth. Plant forms an attractive platform for nanoparticle synthesis because of its several advantages like low cost of cultivation, short production time, easy availability, safety, and also have the ability to form high production volume [79]. The green synthesis approach of metal nanoparticles is eco-friendly, economic, and more beneficial as compared to the chemical synthesis approach. Incorporation of metal nanoparticles in starch films minimizes the problem of biodegradability and food containment [7].
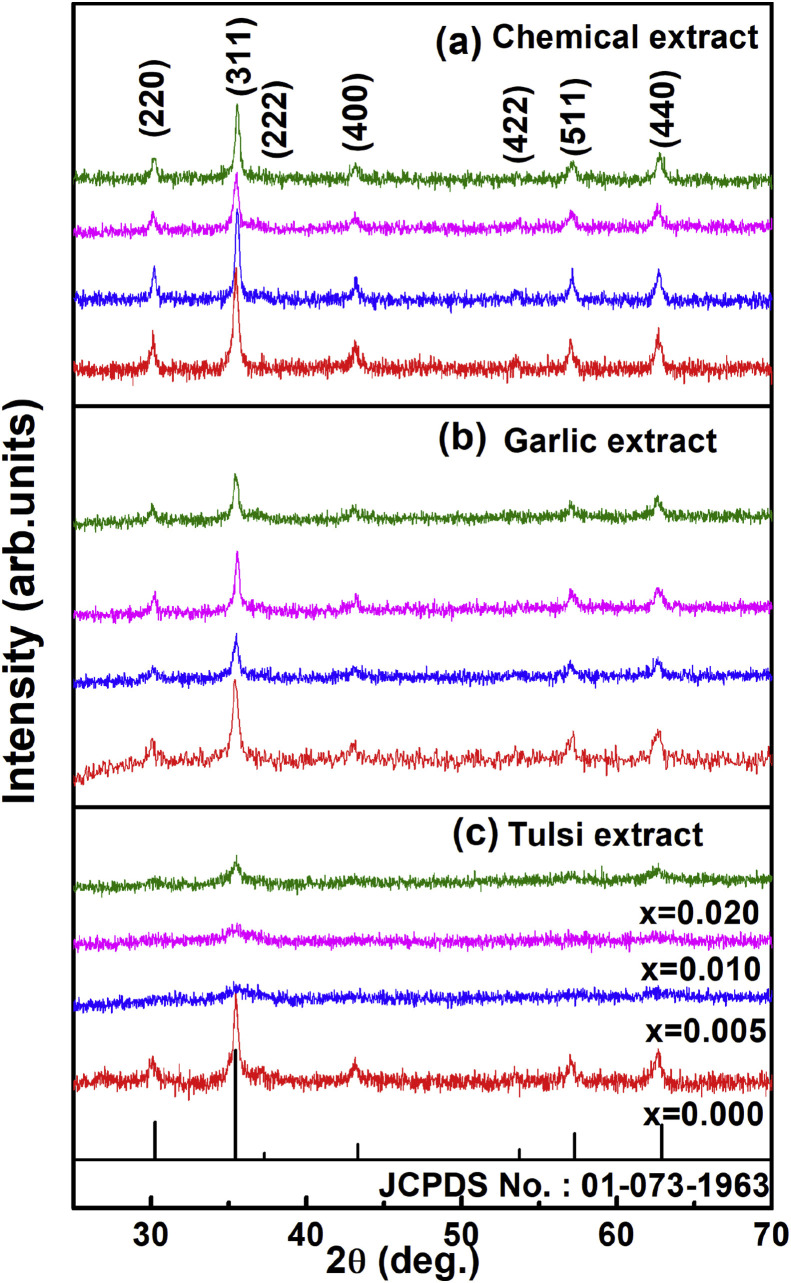
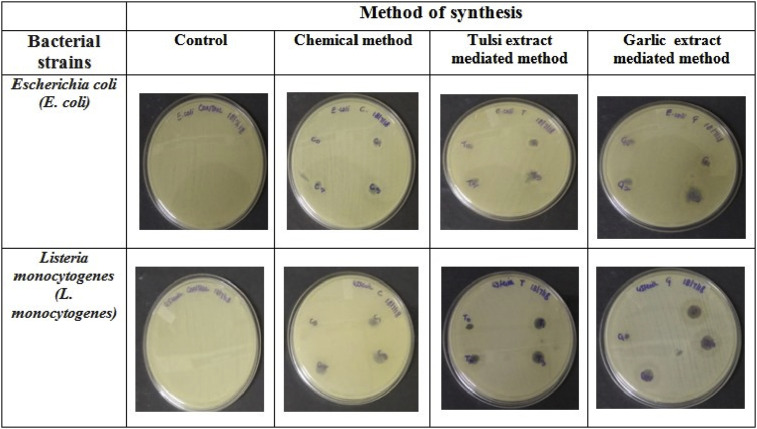
Mahajan et al. (2019), synthesized silver (Ag)-doped cobalt ferrite nanoparticles using the sol-gel auto-combustion technique via chemical and green synthesis approach by using Ocimum sanctum (tulsi) and Allium sativum (garlic). Through different characterization, they confirmed that the prepared samples are crystalline and have a cubic (inverse spinal) structure. The positive antibacterial activity of the prepared sample at different concentration of Ag and Co in a compound (Agx Co1−x Fe2O4 where, x = 0, 0.005, 0.001, 0.02) were tested against gram-positive and gram-negative bacterial strain [80]. Their observations for chemically synthesized CoFe2O4 (cobalt ferrite) nanoparticles (as shown in Fig. 1 (a) ) well matches with the Sanpo et al. observations [51]. In Mahajan's work their team, level up the work and synthesized Ag-doped cobalt ferrite nanoparticles using Ocimum sanctum (tulsi) and Allium sativum (garlic) and obtained the XRD result which is shown in Figs. 1 (b) and 1 (c). Bacterial inhibition zone test is also performed to check the antibacterial property of the synthesized sample against E. coli and L. mnoncytogenes, as shown in Fig. 2 . Gnanasangeetha et al. (2013), demonstrated the formation of metal nanoparticles of size 100–190 nm using Cor riandrum sativum [81]. It was shown using TEM images that spherical nanoparticles were obtained of size 21.12 nm (average) from P. trifoliate extract [82]. As ZnO nanoparticles extracted from Cassia auriculata were analyzed with SEM showed that most of the metal nanoparticles obtained were of spherical shape [26]. The size and shape of the nanoparticles synthesized from Ocimum tenuiorum leaves were analyzed using SEM images which determined the hexagonal shape nano-metals (i.e. ZnO nanoparticles) of size 11–25 nm [83]. So, biochemicals are better synthesizers when compared to the other chemical methods.
Fig. 1.
(a) XRD pattern of silver (Ag)-doped cobalt ferrite nanoparticles using the sol-gel auto-combustion technique via chemical and green synthesis approach by using, (b) Allium sativam (garlic), and (c) Ocimum sanctum (tulsi); at different concentration of Ag and Co in a compound (Agx Co1−x Fe2O4 where, x = 0, 0.005, 0.001, 0.02). The XRD pattern are confirmed with JCPDS No. 01-073-1963 for their spinel crystal structure. Reprinted with permissions from P. Mahajan et al., Vacuum 161 (2019) 389–397, Elsevier copyright 2019 [80]. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Zone of inhibition shown by synthesized Ag-doped cobalt ferrite nanoparticles against bacterial strains. Reprinted with permissions from P. Mahajan et al., Vacuum 161 (2019) 389–397, Elsevier copyright 2019 [80].
3.1.1. Synthesis of nanoparticles using the plant extract
There are three phases of mechanism by which nanoparticles are synthesized in the plant extract [84]:
-
•
Activation phase: In this stage, metal ions get reduced and nucleate to form reduced metal atoms.
-
•
Growth phase: In this stage, small adjacent nanoparticles coalesce to form particles of larger size and the process is referred to as Ostwald ripening. In this phase the stability of the nanoparticles also increases.
-
•
The termination phase: In this stage, nanoparticles acquire favorable final confor-mation.
The mechanism by which the nanoparticles are formed by using sol-gel process via plant extract is depicted in Fig. 3 . The metal ions bind to reducing metabolites and stabilizing nanoparticles. The growth of smaller particles into larger ones occurs by the coarsening process [84]. A variety of plant extracts such as sesame leaves, aloe vera leaves, hibiscus flower/leaves, ginger root, etc., are being widely used for the preparation of the metal oxide nanoparticles [78,85].
Fig. 3.
The typical mechanism for synthesis of metal nanoparticles with the help of green plant extract, is shown in well labeled diagram. Here, phytochemical in plant extract acts as a reducing and stabilizing agent. The complex formed between metal ion and metabolite interaction forms small metal nanoparticles. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.1.2. The role of plant metabolites
Size, shape, and morphology of the metal nanoparticles are strongly influenced by the metabolites in the plant extract, which leads to the formation of nanoparticles of high stability with narrow size distribution [86,87]. Plant metabolites are terpenoids, polyphenols, sugars, alkaloids, phenolic acid, and proteins which play an important role in the binding and reduction of metal ions, thus thereby yielding metal nanoparticles as shown in Fig. 4 . Shankar et al. (2003) suggested that metabolite terpenoids plays an important role in the transformation of metal ions into nanoparticles using extracts from geranium leaves [88].
Fig. 4.
Role of phytochemicals in reduction of metal ions for the synthesis of metal nanoparticles in green leaf extract. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
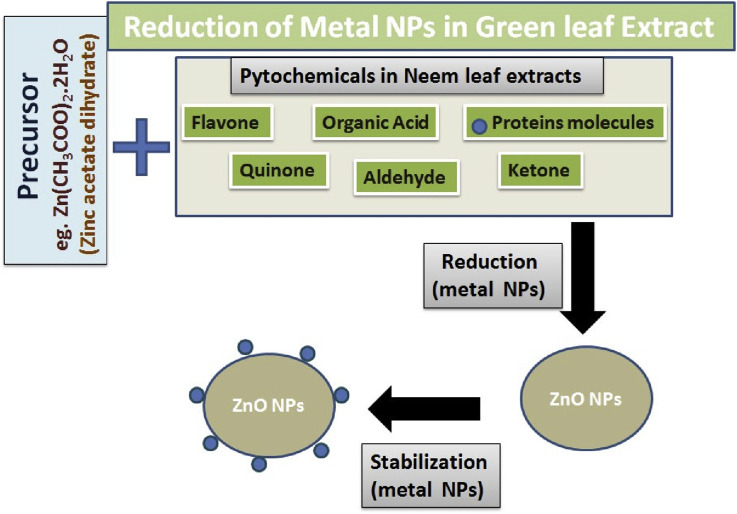
Flavonoids (polyphenolic compounds) comprising several classes as anthocyanins, isoflavonoids, flavonols, chalcones, flavones, and flavanones, actively chelate and reduce metal ions into nanoparticles [48]. Metabolites such as carbohydrates, amino acids, enzymes, etc., act as capping agents, reducing agent, stabilizing agent, and also a chelating agent for capturing metal ions [85]. Sugars present in the plant extracts are also able to induce the formation of metal nanoparticles. The synthesis of Azadirachta indica green leaf extract is shown in Fig. 5 , it is also environmentally friendly and economically favorable as compared to the different chemical processes [49]. Calotropis gigantea was used in synthesis of MgO nanoparticles, which is an environmentally friendly approach, and its toxicity was evaluated in zebra-fish [49]. Bhuyan et al., in 2015 synthesized ZnO nanoparticles from neem (Azardirachta indica) [89]. They mentioned that leaf extract of neem contains phytochemicals such as organic acids, quione, aldehyde, ketone, protein molecules, flavones which helps in the reduction and stabilization of the biochemically synthesized metal nanoparticles. Quione from these biomolecules helps in the reduction of the particle size whereas proteins help in the stabilization of the biosynthesized ZnO nanoparticles. According to the US Food and Drug Administration (FDA), ZnO has been introduced into the pharmaceutical and agricultural sectors and is therefore considered safe for consumption, they added. The antibacterial activity of biosynthesized ZnO nanoparticles was examined against gram-positive and gram-negative bacteria [87]. It seemed that with increasing the concentrations of the nanoparticles (from 20 μg/mL to 100 μg/mL) the antimicrobial activity of the ZnO nanoparticles also gets increased. Consequently, it can be concluded that gram-positive bacteria are more sensitive towards ZnO nanoparticles than the gram-negative bacteria [89].
Fig. 5.
Synthesis of Azadirachta indica (neem) leaf extract which containing both natural stabilizing and reducing agents, which helps in reduction of metal nanoparticles.
Monosaccharides such as glucose, act as a reducing agent. Disaccharides (maltose and lactose) have reducing ability because at least one of their monomer has open chain formation. Whereas in contrast, sucrose has no reducing ability because both monomers, glucose, and fructose, are linked in such a way that open-chain is not formed [84]. Tan et al. analyzed all of the 20 natural α-amino acids to determine their potential for the reduction and binding of metal ions [90]. They found that tryptophan is the strongest reducing agent for gold (Au)-ions, whereas histidine is one of the strongest binding agents for Au-ions. Amino acids bind to metal ions by their amino and carbonyl groups of the main chain or through side chains, such as the carboxyl groups of aspartic and glutamic acid or a nitrogen atom of the imidazole ring of histidine. Thus, these reports indicate that size, shape, and morphology of the metal nanoparticles are strongly influenced by the metabolites in the plant extract [86]. Metabolites (discussed above) play a key role in the overall formation of the eco-friendly nanoparticles. Metal oxide nanoparticles such as ZnO [7,8,27,34,53,63,89], MgO [48,49], CuO [50], TiO2 [18,19,46,47], Graphene [4], etc., have a great application in food system due to its great antimicrobial activity against pathogenic microorganisms. Nanoparticles can be synthesized from a wide variety of biological entities such as actinomycetes, algae, bacteria, fungus, plants, viruses, and yeast. Each biological entity has varying degrees of bio-chemical processing capabilities that can be effectively used to synthesize particular metallic or metallic oxide nanoparticles. Not all biological entities can synthesize nanoparticles due to their enzyme activities and intrinsic metabolic processes. Therefore, careful selection of the appropriate biological entity is necessary, to produce nanoparticles with well-defined properties such as size and morphology. Properties of the plant extract such as its concentration, metal salt concentration, reaction time, reaction solution pH, and temperature significantly influence the quality, size, and morphology of the synthesized nanoparticles [91].
3.2. Chemical synthesis
Choosing the right synthesis method is important for obtaining high-quality nanoparticles. This section describes the preparation of nanoparticles by choosing the most commonly used chemical methods such as chemical co-precipitation, sol-gel, auto-combustion, and hydrothermal methods.
3.2.1. Co-precipitation synthesis
This method is used to control the particle size. The technique avoids the physical changes and aggregation of tiny crystallites. Reaction between constituent materials in a solvent forms the basis of the synthesis. Before the precipitation reaction, the dopant is added to the parent solution. Particles formed are kept separated by the addition of surfactants. The nano-crystal formed are then separated by centrifugation, washed, and then vacuum dried [68]. Anandan et al. (2016), synthesized cobalt doped zinc oxide nanoparticles of single-phase structure with a crystallite size of 31–41 nm. These nanoparticles were tested against gram-positive Bacillis subtilis and gram-negative Klebsiella pneumonia. The antibacterial activity of zinc oxide was found to increase when it was doped with cobalt [92].
3.2.2. Sol-gel synthesis
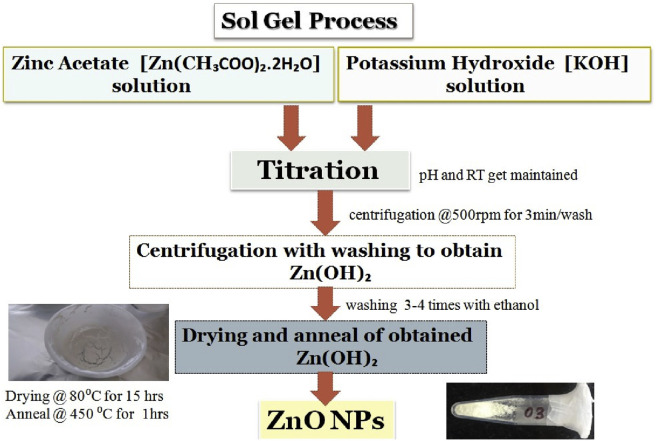
It is a wet-chemical technique requiring the use of low temperature. The process involves the conversion of precursor solution into an inorganic solid through inorganic polymerization reactions induced by water. In general, a precursor can be 1) an inorganic, 2) a metal salt or 3) a metal-organic compound, such as alkoxide. The most commonly used precursors are metal alkoxide as they readily react with water and also with most of the metals. This process involves the formation of inorganic networks through the formation of a sol i.e., colloidal suspension and then gelation of sol to form a gel which is a network in a continuous liquid phase. At last calcination of gel produces oxides [68]. Sharma, V. (2012), synthesized ZnO nanoparticles by this method and tested its antibacterial activity against E. coli in comparison with antibiotics as erythromycin and vancomycin [93]. It was investigated that ZnO nanoparticles were more effective than antibiotics. Fig. 6 represents the synthesized ZnO nanoparticles through sol-gel synthesis technique.
Fig. 6.
Typical synthesis approach of ZnO nanoparticles through sol-gel approach using zinc acetate dihydrate (as precursor) and potassium hydroxide.
3.2.3. Auto-combustion synthesis
It is the most versatile method, also known as self-propagating synthesis route. Metal nitrates are the main raw materials used in this method. There is an exothermic redox reaction between the nitrates and citrate ions to produce the desired product. This technique produces homogeneous and crystalline powders without the requirement of further calcination step [68]. Kooti et al. (2013), synthesized silver-coated cobalt ferrite nanocomposite by combustion route using glycine as a fuel. Their antibacterial efficiency was investigated in comparison with silver nanoparticles and found to be higher when treated with silver-coated cobalt ferrites [94]. Rajendar et al. (2014), studied the antibacterial property of silver doped ZnO nanoparticles, prepared by auto-combustion. The average crystallite size was in the range of 10–40 nm. The study concluded that silver doped ZnO materials are suitable for their anti-bacterial applications [95]. Gautam et al. (2011), synthesized nano ferrites (with average crystallite size of 31–43 nm) of CuxCo1−xFe2O4 (where 0.0 < x < 1.0) by auto-combustion approach and their magnetic properties were studied by X-ray absorption spectroscopy (XAS) [96] and also discussed the widespread applications of nano ferrites in electronics and medicine.
3.2.4. Hydrothermal synthesis
It is based upon the fact that many of the oxides are soluble in an alkali solution so, this method can be defined as a method of synthesis of single crystals depending upon the solubility of the materials, in hot water under high-pressure [97]. Crystal growth is performed in an autoclave, in which nutrients are supplied along with water. Santosh kumar et al. (2016), synthesized nickel oxide (NiO) nanoparticles using nickel sulphate (NiSO4) as a precursor. The average crystallite size was found to be 92 nm, possessing irregular shape. The nanoparticles exhibited a good zone of inhibition against bacterial strains of Bacillus subtilis, S. aureus, E. coli and P. vulgaris [98]. Dinesh et al. (2016), synthesized zinc stannate (Zn2SnO4) nanoparticles by using potassium hydroxide as a mineralizer, with a crystallite size of about 20 nm. It was found that on increasing the concentration of nanoparticles leads to an increase in inhibition zone [99].
3.3. Physical synthesis
There are many methods which fall into this category such as mentioned below:
3.3.1. Inert gas condensation (IGC) method
In this process magnetron plasma sputtering is used [100]. Thermal vaporization source [68] with molecular dynamic [101] simulation at different temperature and pressure helps in the synthesis metal/alloy nanoparticles. Molecular dynamic simulation helps in synthesis of many metal nanoparticles like gold-platinum, gold, gold-copper, etc. Nanoparticles size, its morphology, structure, depends upon the Gas Condensation process at different thermal and pressure treatment. Lotfi et al. (2019), described the synthesis of gold (Au) nanoparticle synthesis by using the IGC method and mentioned in their study that with increasing temperature of the procedure the number of nanoparticles decreases. The sphericity of Au- nanoparticles decreases with a decrease in temperature and vice-versa [101]. Yang et al. (2019), synthesized the (Au–Cu)-nanoparticles with high stability and sphericity [100].
3.3.2. Vacuum Deposition and Vaporization method
In this method, thermal vaporization is used for the deposition of metal, alloy, or compounds in a vacuum chamber [68].
3.3.3. Chemical Vapor Deposition (CVD) and Chemical Vapor Condensation method (CVC)
CVD promoted chemical reaction by deposition of metal, alloy or compounds in a vacuum over a heated surface (above 900 °C) while CVC needs the minimum activation energy to start the procedure [68]. High thermal energy and low pressure is required for vaporization, this is done with the help of sonicator. After this electro magnetic radiation and plasma activation is required to activate the reaction [100]. Huh et al. (2003), synthesized the carbon-nanoparticles using CVD method [102].
3.3.4. Mechanical Attrition method
Coarse particles disintegrated into nano-scale (through ball mill) [103]. There are different types of ball mill processes like attrition ball mill, vibrational ball mill, Low Energy Tumbling Mill (LETM), and High Energy Ball Mill (HEBM). HEBM is high preferable ball milling procedure in industry due to its precision [104]. It helps in the synthesis of metallic, alloy, and compound nanoparticles. The agitator is used in ball mill which can rotate from 500 to 1200 rpm, depends upon the types [68]. There is an inverse relation between nanoparticles size and the attrition time [103]. Lam et al. (2000), has synthesized the silicon nanoparticles using ball milling with the help of silicon dioxide and graphite [105].
4. Antibacterial activity of the nanoparticles
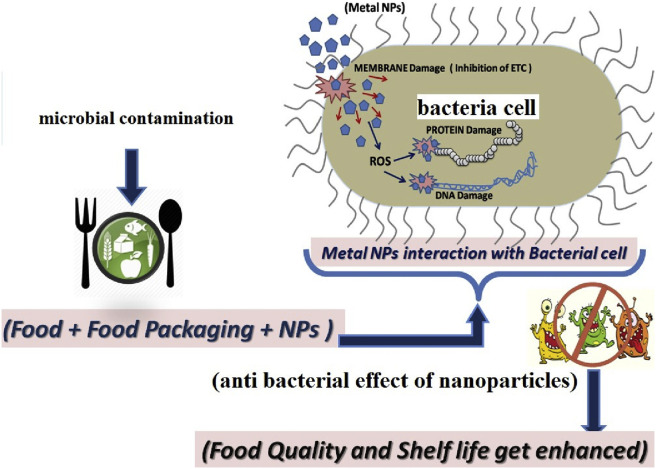
Although there are several methods to assess the antibacterial activity of nanoparticles, the most widely used method is the broth dilution method, which is followed by colony counting to the appropriate environmental conditions containing bacteria and nanoparticles. Antibacterial agents deleteriously affect the metabolic processes of cells and destroy DNA structure. It inhibits protein synthesis and causes cell wall collapse. The bacterial cell wall is composed of teichoic acid, lipoteichoic acid and peptidoglycan in gram-positive bacteria and lipopolysaccharide and a thin layer of peptidoglycan in case of gram-negative bacteria [106,107]. Therefore, the effect of antibacterial agents differs for both the types of bacterial cell because of the presence of antioxidants and antioxidant enzymes, which act as detoxifying agents within the bacterial cells as explained by Applerot et al. (2009) [108]. The antibacterial behavior of the metal nanoparticles is due to the oxidative stress or ROS generation inside the bacterial cell [7].
According to different structures, composition, and function of the bacterial cell wall, bacterial strains can be divided into two main categories: gram-positive bacteria and gram-negative bacteria [107]. There is a thick layer of the peptidoglycan (PG) attached to teichoic acids in the case of gram-positive bacteria where as in gram-negative bacteria its outer membrane is composed of a thin layer of the peptidoglycan (PG). The presence of lipopolysaccharides in the outer membrane increases the negative charge of the cell membrane and is essential for the structural integrity. Thus the bacterial cell wall plays an important role in the functioning of the nanoparticles [109,110]. For instance, many reports have shown that nanoparticles have better antibacterial activity for the gram-positive bacteria as compared to the gram-negative bacteria. The probable mechanism can be due to the difference in the cell membrane structure [110]. Biofilm formation is a significant problem that leads to the failure of the nanoparticles to fight with the bacteria as it covers the bacterial cell, also leading to the development of infectious diseases [111]. Among various nanoparticles, super-paramagnetic iron oxide nanoparticles show effective antibacterial activity against bacterial strains which form biofilms [112].
Gingasu et al. (2016), used self-combustion and wet fertilization methods to obtain cobalt ferrite and silver cobalt ferrite nanoparticles using the aqueous extract of Hibiscus rosa-sinensis flower and leaf. It was found that Ag–CoFe2O4 nanoparticles have much more improved antibacterial activity than CoFe2O4 nanoparticles [113]. A new approach to increase the antibacterial property of ferrite nanoparticles has been suggested by Sapno et al. (2013). They also said that replacing the spinel ferrite with a transition metal can improve the antibacterial capacity of the nanoparticles. Gram-negative bacteria, E. Coli and gram-positive bacteria, Staphylococcus aureus were used to test the antimicrobial activity of transition metal substituted cobalt ferrite nanoparticles [51].
Xavier et al. (2014), used sol-gel technique to synthesize silver substituted cobalt ferrite nanoparticles Co1−xAgxFe2O4 with x of varying concentrations as x = 0.0, 0.025, 0.05, 0.075, 0.1. Gram-negative and gram positive-bacterial species as Escherichia coli, Pseudomonas aeruginosa, Serratia marcescens and Staphylococcus aureus were selected to check the antibacterial activity [114]. Jha et al. (2012), used a low-cost green and reproducible yeast (Sachhromyces cerevisiae) for the production of cobalt ferrite nanoparticles [115]. Manikandan et al. (2015), synthesized spinel copper ferrite (CuFe2O4) using hibiscus flower extract [116]. Gingasu et al. (2016), synthesized cobalt ferrite nanoparticles by sesame seed extract and evaluated their antimicrobial, anti-biofilm, and cytotoxic properties [85]. Gingasu et al. (2017), synthesized cobalt ferrite nanoparticles by employing the use of the aqueous extract of ginger root and cardamom seeds, through self-combustion method [117].
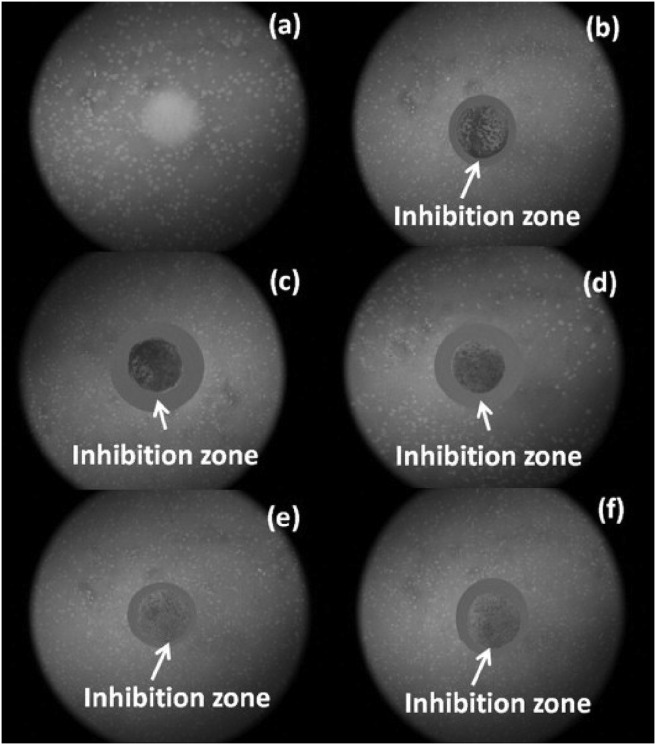
Sanpo et al. (2013), investigated the contact biochemical properties of all ferrite samples prepared using Kirby-Bauer technology. It was found that the diameter of the zone of inhibition for copper substituted cobalt ferrite is larger followed up by zinc, nickel, pure cobalt and manganese [51], shown in Fig. 7 .
Fig. 7.
Zone of inhibition for E. coli obtained by (a) without cobalt ferrite nanoparticles (b) CoFe2O4 (c) Co0.5Cu0.5Fe2O4, (d)Co0.5Zn0.5Fe2O4, (e) Co0.5Mn0.5Fe2O4 and (f) Co0.5Ni0.5Fe2O4. Reprinted with permissions from N. Sanpo et al., Acta Biomater. 9 (2013) 5830–5837. Copyright 2013 Elsevier [51].
Desselberger (2000), found that ZnO nanoparticles doped with manganese (Mn) had a pronounced effect on both gram-positive as well as gram-negative bacteria than undoped ZnO nanoparticles [118]. Gunalan et al. (2011), investigated the antibacterial property of nano ZnO prepared from chemical and green method for pathogens such as Staphylococcus aureus, P. mirabilis, S. marcescens and C. freundii. Their study revealed that green synthesized ZnO nanoparticles were more effective against these bacteria than chemically synthesized [119]. Rani et al. (2014), tested copper oxide nanoparticles against gram-negative bacteria strains K. pneumonia, S. typhimurium, E. aerogenes. They found that the zone of inhibition increased with increasing the minimum inhibitory concentration of copper oxide [120].
It can be concluded that size, shape, morphology, the type of dopant, and their concentration added to the sample can strongly influence their anti-bacterial activity. All the reports show that the nanoparticles can be successfully synthesized by the use of the green synthesis route, resulting in less environmental harm. They are also found to be exhibiting efficient properties required for exhibiting anti-bacterial activity.
5. Antibacterial mechanisms of nanoparticles
The exact mechanism of nanoparticles for bacterial species has not been fully elucidated so far, but their function is primarily due to the induction of oxidative stress due to the formation of free radicals [110]. Below are some steps in which nanoparticles affect bacterial growth:
5.1. Nanoparticles penetration
The nanoparticles penetrate through the holes and pits; present over the cell membrane and thus damage the cell.
5.2. Nanoparticles interaction with bacterial cell
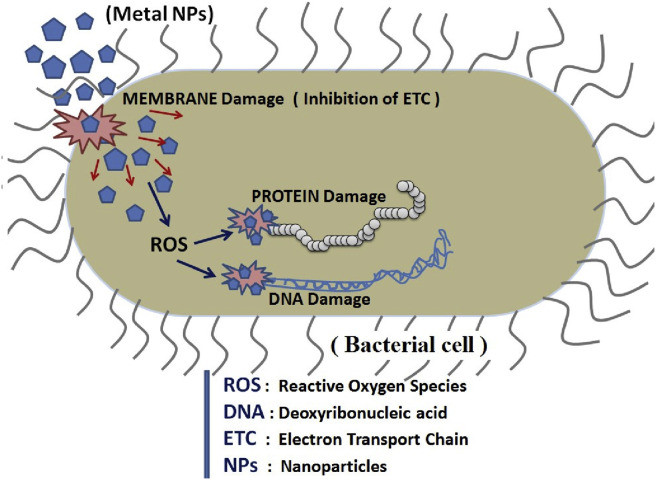
In this, the antiparticles bind to the membranes of micro-organisms which can prolong the lag phase of the growth cycle and increase the generation time of the micro-organisms. Metal nanoparticles attach to the bacterial cell membrane and release metal ions which change the permeability of the cell membrane causing the death of the bacterial cell [7]. The destruction of the cell wall and the extrusion of the content present in the cytoplasm is shown in Fig. 8 .
Fig. 8.
Interaction of metal nanoparticles with bacterial cell, is depicted in the above figure. ROS has been the major factor leading to cell damage. The penetration rate of active oxides through the bacterial cell wall plays an important role in the killing rate of bacteria. These mechanisms lead to the mitochondria weakness, intracellular outflow, and reduced cell division, which causes eventual cell growth inhibition and ultimately cell death.
5.3. Probable mechanisms inside bacterial cell
Metal ions uptake into cells, depletion of cell, disruption of DNA replication, releasing metallic ions and generating ROS, and accumulation and dissolution of NP in the bacterial membrane, are the probable mechanism for the functioning of nanoparticles as an antibacterial agent, is depicted in Fig. 10. The release of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) and super-oxide (O2−) generated from the interaction of metal nanoparticles. ROS induces oxidative stress, impairs cellular signaling, deplete antioxidant enzyme, resulting in the loss of cell wall. Hydrogen peroxide generated from the surface of ceramic nanopowders can easily penetrate the cell wall of bacteria and cause cell destruction [7].
Fig. 10.
Proposed mechanism of antibacterial activity of nanoparticles against pathogenic bacterial cell.
The mechanism and functioning of the nanoparticles depends upon various factors such as surface modification, intrinsic properties, type of bacterial species, and physicochemical properties of the nanoparticles [110]. Properties of nanoparticles include their size, charge, zeta potential, surface morphology, and their crystal structure, as explained next.
6. Factors affecting the functioning of nanoparticles
6.1. Particle size
Reducing particle size to the nanoscale is a reliable and efficient tool for improving the material biocompatibility. Morphology and size of the particles are examined by electron microscopy. It has been found that particle size offer the rate of drug release. Small particles offer fast drug release because of the large surface area [121]. Small size nanoparticles are more stable than the large ones [122]. Yamamoto et al. (1998), mentioned out that particle size affects antibacterial activity and observed an increase in activity with decreasing particle size [123]. The large specific surface area increases the probability of the nanoparticle to come in contact with and pass through the bacterial cell membrane [124]. Zhang et al. (2007), found an increase in the antibacterial activity of ZnO nanoparticle with the decrease in particle size [125]. The bactericidal property of ZnO suspensions of different sizes as 12 nm, 45 nm, and 2 μm was tested against E. coli by Padmavathy et al. (2008). The results showed that ZnO suspension having a size of 12 nm was more effective than the other suspensions having large particle sizes [126].
6.2. Roughness
It has been reported that the increase in roughness of nanoparticle increases, size, and surface area to mass ratio that promotes the adsorption of bacterial protein [127,128].
6.3. Zeta potential
Many studies have reported that zeta potential has a strong influence on the bacterial adhesion. Positive nanoparticles are much more prone to adhere to the bacteria surface which is negatively charged, as compared to negatively charged nanoparticles [129]. Positively charged nanoparticles are also believed to produce more ROS. Negatively charged nanoparticles do not adhere to the bacteria due to the negative charge on both but have shown a certain level of antibacterial activity due to molecular crowding [130].
6.4. Doping modification
It is one of the best methods to control and regulate the interaction between the nanoparticle and bacteria. Doping modification helps in preventing the aggregation of the nanoparticles, allowing them to effectively disperse in the aqueous environment [124]. Doping also results in the production of more ROS. For example, doping of ZnO nanoparticles with the fluorine results in more ROS production and thereby have enhanced antibacterial activity as compared to the ZnO nanoparticles [131,132].
6.5. Surface charge
It plays a key role in maintaining the stability of nanoparticles [133]. Surface charge of the particle can lead to nonspecific interactions with the biological fluids, resulting in undesirable changes [134]. To stabilize nanoparticles, their agglomeration by van der waal's forces should be prevented. This can be obtained by electrostatic repulsion in which like surface charges repel each other [133], resulting in stabilized nanoparticles.
6.6. Minimum inhibitory concentration
It can be defined as the minimum concentration of the nanoparticles required to impede or absolutely prevent the bacterial growth. Cobalt ferrite and substituted cobalt ferrite was analyzed for their inhibition properties against both E. coli and S. aureus. It was found that E. coli killing rate was much higher than the S. aureus. The antibacterial ability was higher when copper and cobalt were substituted than for nickel and manganese substituted cobalt ferrite [51].
7. Safety and biocompatibility of nanoparticles
Use of nanotechnology in the food sector and medicinal field requires an evaluation of its health risk (stated by Mu and Sprando in 2010) [135]. Different degrees of biological effects are believed to be related with their characteristics, because of small size and the large surface area [136]. Toxicology can be defined as an adverse effect of chemical, physical and biological agents on human being, animals and environment. Toxic cellular effects are related to impairment of mitrochondrial activity, leakage of the membrane along with induced morphological changes, and adverse effects on cell viability and metabolic activity [137]. The toxicity of nanoparticles is dependent upon various factors including the amount of dosage, chemical composition and structural properties [138,139]. Synthetic Amorphous Silicase (SAS) nanoparticles were tested on Dutch people by feeding 9.4 mg of silica nanoparticles per kg of body weight and found that there is little effect on human health (like a minimal gastrointestinal problem) [1]. It was observed that silica nanoparticles easily get agglomerates in the stomach (acidic conditions) and then again dispersed in nano-form in its further travel in the intestinal duct. So, there is no proven epigenetic effect on the genetic level inside the human body due to silica nanoparticles [1]. Similarly, TiO2 is used as a coloring and whitening agent [3] in confectionaries, especially sugar-coated chewing gum with EU unique code of E171 and found that it also has no harmful effect on human health (but observed acutely). According to the new study by the researchers, four million TiO2 nanoparticles are being consumed every year across the globe [1].
Some metals, such as gold, are safe in bulk-form, where nano-form is highly toxic [139]. Certain metals like cobalt, nickel, zinc, cadmium, and silver are toxic to biological entities, while others, including titanium and iron oxide are considered to be less toxic [34,[140], [141], [142]]. When the nanoparticles enter the body, it comes in contact with the various biological entities such as proteins, lipids, and enzymes present in the biological fluid. These biomolecules interact with the surface of nanoparticles to form a complex layer called as 'corona', over the nanoparticles [143,144]. The strength of corona either weak or strong, is determined by the physical and chemical properties of the particles. This protein corona is responsible for various toxic outcomes [143]. In ZnO nanoparticles, rod shaped nanoparticles are more toxic than the spherical shape nanoparticles [1]. There is attachment and detachment of protein particles as they move from one biological environment to another. Mahmoudi et al. (2011), mentioned in their studies that the same concentration of iron oxide nanoparticles are more toxic to neuronal and glial cells than to other cell types such as heart and kidney cells [145]. However, further research in this area is needed relating to their dosage to be used in the food related area to provide the best results in their applied application as well as towards the safety regarding their consumption. Several studies are showing that nanoparticles in the environment behave like antidotes in the environment [5]. Nanoremediation, a term coined by research, says that nanoparticles exposed to the environment help clean up soil, water, and sewage faster [5].
8. Application of nanoparticles in the food industry
Applications of nanoparticles in the food sector are growing rapidly. Nanoparticles not only increases the shelf life of food but also improves the overall quality of the food and thus increases the degree of acceptance of the food among the consumers. Food safety and quality are always major concerns. Nanostructures are basically in the form of films, clusters, and wires. Nanoparticles, the simplest form, are the building block of nanostructures [21]. In the food sector, nanotechnology plays an important role in two ways: food additives (depicted as nano-inside) and food packaging (depicted as nano-outside). There are several reports describing the application of nanotechnology in the food sector to improve food safety, prolong shelf life, improve processing, and improve nutritional quality [146]. Besides the antimicrobial property, nanomaterials can be used to detect the food spoilage through nanosensors [21]. Categorization of the application of nanomaterials in the food sector can be done into food packaging, processing, antimicrobials, and food ingredients/additives.
8.1. Food additives
Food additives are the substances normally added in food to enhance its taste, shelf life, nutritional properties or to preserve the original flavor, appearance, and other qualities like biological, physiochemical, sensorial, and rheological properties of the food [6,18,147]. Recently researchers are more concerned about the application of metal nanoparticles as food additives due to its antimicrobial and food quality enhancing properties, like SiO2 and TiO2 [18,19,46,47]. Food additives can be divided into several groups based on CODEX Alimetaruis with a unique identification number. These additives are like, Acidu-lants, Acidity regulators, Anticaking agents, Antioxidants, Bulking agents, Food color en-hancer, Fortifying agents, Color retention agents, Emulsifiers, Flavor enhancers, Glazing agents, Humectants, Tracer gases, Preservatives, Stabilizing agents, Sweeteners, and Thickeners. Many countries regulate the food additives used in food because of its toxic effects on the consumers [18,19]. For example Boric acid (H3BO3), it was widely used as preservatives in food industries between 1807 and 1920s but was banned after World War I due to its toxic effects on both human and animals and during World War II its demand again increases due to its cheap cost and easiness of its availability in the market but was finally banned nearly in 1950s. Silver nanoparticles are used as anti-odor and antibacterial agent in many food items [1].
Food industries nowadays using nanoencapsulation techniques in the manufacturing of food additives to enhance the food quality by improving its overall physical and chemical stability. Nanoencapsulation of essential oils and plant extracts like cinnamon leaf, garlic, tulsi, lemon, etc. not only gives biological stability but also improves the overall quality and shelf life of the food [12]. Amorphous silica (SiO2) nanoparticles are an approved food additive in the United States and European Union with unique code E551, which is used as an anti-caking agent [47] and it also improves the overall biological stability of the targeted food products. SiO2 nanoparticles have antimicrobial property against the gram-positive and gram-negative bacteria [46]. According to the study, SiO2 is non-toxic for human consumption in a short interval of time [1]. Lee et al. (2017), alters the toxicity of nanoparticles and their interaction with living cells above acceptable levels [18]. Antifungal and antibacterial activity of the SiO2 nanoparticles increased when doped with Zn, using deposition precipitation method [12]. Similarly, Dorier et al. (2018), studied that TiO2 nanoparticles also used as food additives as whitening agent with unique code E171, in cakes and pastries. The effects of TiO2 nanoparticles, on human cells are also well studied. They specifically mentioned that TiO2 and TiO2 nanoparticles increases the intercellular ROS (i.e. Reactive Oxygen Species) level and also not much effect the cell stability like damaging cell's DNA or damaging of the cell's growth or damaging the endoplasmic reticulum structure [19]. There is no suitable method or guide to assess the toxicity of these nanoparticles used in food additives [1]. Nano-encapsulation of food additives (generally containing nanoparticles) helps deliver nanoparticles to the targeted body organ or body part. In Germany, nano-encapsulation technique is used to encapsulate the selenium (which helps in proper functioning of the body metabolites) from green-tea leaves with the help of canola oil and phytosterol to minimize the absorption of cholesterol [3]. TiO2 used as a coloring agent in processed finished food with negative toxicity effects [10].
8.2. Nanosensors
Food quality monitoring is very necessary to control the deterioration of food. Nanosensors can be used in the food sector to monitor the food pathogens (which changes the quality of food with time) and this leads to the development of smart packaging. Several nanosensors have been developed by researchers around the world to monitor the overall quality of the food. Some of these nanosensors are shown below:
-
•
Canadian Wheat Board Centre for Grain Storage Research, University of Manitoba, Canada; using conducting polymer nanoparticles, which detect the source and the type of spoilage conducted by the type bacteria, fungus, viruses, insects or rodents [20].
-
•
Electronic tongue was developed by Ruengruglikit et al. in 2004 to detect the freshness of the food by detecting the gases coming from the spoilage food. Smart package also ensures the integrity of the food product [148].
-
•
Nanosensors have been reported to detect toxins present or developed in the food products. They can also track the history of various parameters of the food and food package such as time, temperature, and expiration date [21]. Nanosensors are most importantly used in food packaging and food transport, ensuring that the food received by the consumers fresh and free from any type of deterioration [149].
-
•
Fu et al. (2008), developed a biosensor in which fluorescent dye particles attached to anti-salmonella antibodies with a silicon-gold nanorod array. When the salmonella bacteria interact with the nanosized dye particles then the dye on the sensor becomes visible as an indicator [150].
-
•
Neethirajan et al. (2011), produced the novel food containing bioactive nanoparticles which directly binds with the biomolecular structures of the Campylobacter jejuni. As Campylobacter jejuni results in abdominal cramps and diarrhea [20].
-
•
Agromicron Ltd. (Plexus Institute, Hong Kong in 2006) has developed a low-cost Nano Bioluminescent Spray, which can react with the bacterial strain on food and produce a visual glow for easy identification. In this sensor, the intensity of the glow depends upon the number of the nano-particles (which is present in spray) interacting with the bacterial strain [20].
-
•
Oxygen calorimetric indicator containing TiO2, SnO2 nanoparticles, were synthesized due to their photosensitize property. DNA based nanosensors would be a revolutionary technology, which is still under progress [5].
-
•
Ag & Au based nanosensor was developed fruitfully to detect residual pesticides, which works on the Raman spectroscopy principle [4].
8.3. Food packaging
As per environmental and food security concerns, the demand for biodegradable and smart food packaging material increasing day by day in the market. In the food sector researchers are now focused on the packaging materials which having both biodegradable and antimicrobial properties. Biodegradable properties of food packaging materials enhanced by using polysaccharides such as cellulose, pullulan, agarose, starch and chitosen [151] and antimicrobial properties of the food packaging materials enhanced by adding metal nanoparticles into it. It is considered that the application of nanoparticles going to be increased by 25% by 2020, which includes three trillion US dollar businesses across the world [25]. Bradley et al. (2011), explained that nano-fibers, nano-rods, cellulose, and nano-tubes, nano-clays, etc. can be used inside packaging materials and are reported to provide physical strength and rigidity to the packaging materials. By incorporating nanoparticles into the traditional polymer layer of the packaging material, the barrier properties of the packaging material can be enhanced e.g., the vacuum deposited Al-coating over polyethylene films [9]. European Food Safety Authority (EFSA) in 2012 limited the use of Titanium nanoparticles in food packaging material with polyethylene terephthalate (PET) bottles by not more than 20 mg/kg [30]. Some industries involved in this 21st century technology are Honeywell (USA based) [5], Nestle [6], A-Do Global (Korea based) [30], Nanocor (USA based) [5], Quan Zhou Hu Zeng Nano Technology Co., Ltd. (China based) [30], Bayer (USA based) [5], Nano-Prix [6], Baby-Dream (Korea based) [30], Aqua Nova (Germany based) [30], etc.
Nanotechnology provides food safety in terms of packaging to ensure a longer shelf life of food products by avoiding loss of nutrients [21]. Researchers are now more focusing on the nanoparticles embedded food packaging materials due to its high antimicrobial properties. Nanoparticles embedded food packaging materials not only enhance the shelf life of food but also minimize food spoilage, ensures food safety, and also indicate the food history: as in terms of the intelligent packaging system. FresherlongerT M nano-composite containing Ag nanoparticles and PP (polypropylene) helps in the inhibition of pathogens. Montmorillonite, a natural clay (extracted form, volcanic ash, or other rocks) used as the nanoparticles in the highly advanced packaging system [31]. Huang et al. (2015), discussed the application of nanoparticles, such as Ag, Zn, Cu and TiO2, in manufacturing of food packaging material, e.g., fruit (like apple, apple slices, fresh-cut melon, orange, strawberry, and kiwi), fruit juice (like, apple, orange and pineapple), meat product (like, fresh cut beef) [31]. Nanotechnology-based food packaging can be divided into three categories: first is the Improved packaging system, second is the Active packaging system and third is the Intelligent packaging system [6,31], explained below:
8.3.1. Improved packaging system
In this packaging system the gas, temperature, humidity barrier properties get improved with an addition of nanoparticles in polymer [6,12]. Nanocomposites have a great potential of improving mechanical strength, increasing resistance to heat, reducing weight, and improving barrier properties (against carbon dioxide, moisture, oxygen, and volatiles present inside food) of the food packaging material [12]. Several studies have been conducted in order to determine the interaction of the nanoparticles with the foodborne pathogens. The biodegradable starch film containing ZnO nanoparticles is shown in Fig. 9 , at various angles. Bactericidal activity of the zinc oxide has been found against various pathogens mainly E. coli and S. aureus. ZnO nanoparticles incorporated into the polymeric matrix permits the interaction of food with the nanoparticles present inside the nanocomposites, which ultimately protect the food from the pathogens by destroying the DNA of bacterial cells [8]. Japan is a leader in nanoparticles based packaging material, and the market value of its nano-packaging is increasing by 13% each year [5]. Commercially there are a huge application of metal and metal oxide nanoparticles in the food system (also discussed above), e.g. Silver nanoparticles are used in baby bottles, nano-clays are utilized in manufacturing beer bottles by many breweries due to which filled beer's expiry date increases to thirty weeks, carbon nano-tubes utilized in food packaging material because it provides mechanical strength to the package and also shows the antimicrobial property, ZnO nanoparticles embedded food packaging and wrapping material [31].
Fig. 9.
Starch-based nanocomposite (bio-films) containing ZnO nanoparticles are displayed in different angles and backgrounds. It also demonstrated the translucent nature of starch-based bio-films [16].
8.3.2. Active packaging system
In this packaging system, the unwanted components are eliminated from the food environment inside the package. Active packages are like, oxygen scavengers, carbon-dioxide scavengers, C2H2 scavengers, ethanol emitters, moisture absorber [9,10], etc. It has dynamic nature on the food packaging system for its (food) preservation. Oxygen accelerates the oxidative degradation of packaged foods, and oxygen scavengers (containing nanoparticles e.g., TiO2) slow this possibility. Ag, Au, ZnO nanoparticles are chemically more stable than other metal nanoparticles and also have antifungal and antibacterial properties [5].
Active food packaging has emerged as an effective approach in food packaging and has largely replaced traditional packaging systems. Active packaging protects the food from environmental factors by providing the barrier to the external conditions [10]. The probable mechanism of antibacterial activity of nanoparticles against the bacterial strains is shown in Fig. 10 , this demonstrates that how nanoparticles help in protecting the food against the contamination. Another potential application of nanoparticles into the food packaging is the degradation of ethylene (ripening gas). The idea behind this application is to insert the active nanoparticles into the polymer matrices that could lead to a two-fold advantage by improving performances of food packaging material, enhancing the prolongation of the shelf life of the packaged food product [43]. Pathakoti et al. (2017), mentioned the liberation of the nanoparticles through the packaging system, halts the microbial growth and thus saves the food from spoilage. Thus, this type of packaging is also known as antimicrobial packaging [21]. They include the use of various metal and metal oxide nanoparticles for their antimicrobial behavior, in the form of nanocomposites for food packaging sector. Copper, copper oxide [120], zinc oxide [7,8,26,27,53,63,89,106], titanium oxide [19] and silver [41,94,112] based nanofillers are widely used [11]. Silver nanoparticles [41,48,77,94,112,114] are used to preserve the food products for longer time periods by killing microbes in just 6 min [152,153].
8.3.3. Intelligent packaging system
In this packaging system, the embedded nanoparticles senses any biological or chemical changes inside or outside the food. This kind of packaging system known as the smart packaging system. Currently Nestle, Nano Prix supermarket, etc. are using chemical nanosensors which easily get detects the color change [6]. Intelligent packaging helps in tracing, fraudulent [5], and tracking and protection of the brand name. Intelligent packaging in the form of strips is developed by Oxonica (Oxford, United Kingdom) in which gold, silver, and platinum are utilized for tracing, tracing authenticity of the food product. NanoInk, Skokie, USA has discovered a Dip Pen Nanolithography technique to encrypt the food information [20]. Recently, pH indicator with silicon nanoparticles were introduced [5].
Types of intelligent packaging system are [6,10,11,154]:
-
•
To improve the overall food quality: Quality indicator (QI), temperature indicator (TI), time-temperature indicator (TTI) e.g., TimeSrips [5], ThermoTrace [30], TEM XRD ICP-MS [30], etc., gas concentration indicator (GC) etc.
-
•
Provide more convenience : Thermo chromic inks, Microwave doneness indicator Radio Frequency Identification (RFID) etc.
-
•
Protection against counterfeiting, theft, and tampering of the packages.
8.3.4. Biodegradable nanocomposites as packaging materials
Starch-based food packaging material with embedded nanoparticles attracted the attention of all researchers across the world due to its antibacterial and biodegradable properties [16]. Starch-based food packaging materials are alternates to synthetic polymers in food packaging application revolution. Petroleum-based synthetic polymers are non-biodegradable in nature and its wide application (as food packaging material) increases the attention of the researchers towards environmental waste disposal crisis [13,15]. Researchers focus on the synthesis of biomass-based food packaging materials based on agricultural-based products that are alternatives to synthetic polymers. Biomass-based food packaging materials are cheap, easily available, eco-friendly, and abundant in nature. Biodegradable starch films, as food packaging materials, are eatable due to the presence of starch and other plant-based byproducts like lipids, proteins, and polysaccharides [14,17,155]. Many researchers are developing bio-composites from renewable resources, producing more sustainable and environmental friendly materials has gained attention at the international level. Synthetic based food packaging materials as solid waste creates a severe environmental problem due to its non-biodegradability property. Plastic packaging is one of the major causes of sea pollution as over 67 million tons of polymeric packaging waste has resulted in environmental issues [156]. Globally, there are special rules and regulations to dispose of synthetic polymeric waste (like plastic) but less than 5% of the whole get recycled [157].
The consideration of people towards the biodegradable material can reduce the environmental pressure [158]. The development of biomaterials holds great promise to mitigate many of the sustainability problems, offering the potential of renewability, biodegradation, and a path away from harmful additives. Packaging films and coatings from biopolymers such as polysaccharides, proteins, and fats had significantly growing to enhance the shelf life, the overall quality, and stability of the packaged food. Among polysaccharides, starch has been studied as substitute biomaterial for packaging due to its polymeric properties [16,59,159]. Hence, the demand of the bio-based polymer materials is increasing rapidly in order to solve the waste disposal issues to the controlled magnitude [151,160]. In recent years, researchers have seen that nanotechnology playing an important role in filling the gap of innovative development in food packaging material [126]. In 2018, researchers from Yonsei University, Republic of Korea, has successfully developed an antimicrobial paper-based packaging material coated with chitosan:starch-AgNPs [161]. Nanocomposites biodegradable films are composed of metal/non-metal nanoparticles [162].
9. Challenges in combining nanoparticles with food-system
Nanoparticles have many beneficial properties, as reported above by many researchers, but there are many challenges on which researchers are focussed, like nanoparticles type, morphologies, surface chemistry, and concentrations, which can be a deciding factor for its future in the food sector. The large surface area and attractiveness of active surface chemistry can be problematic due to the fact that they are always active against unwanted chemical reactions. Few or negligible research on nanoparticle waste is a major concern for researchers, making it difficult to use nanoparticles in the food sector [9]. The United States Food Drug and Administration (U.S. F.D.A.) on August 5, 2015 has issued a guideline for the use of nano-based technology in the food-system [47]. The European commission in 2011 regulates a directive 85/572/EEC with a regulation no. October 2011 for the migration of nanoparticles into food products (contact directly or indirectly with nanoparticles) and decided that it should not exceed the limit of 10 mg/dm2 [30]. Therefore, it is very important to update food regulations depending on the use of nanoparticles in food systems. Food additives (like, coloring agents (TiO2 and Al2O3) [47], anti-caking agents (SiO2) [46,47], whitening agents (TiO2) [19,47], etc.) are more in direct contact with internal body organs and could accumulated slowly in body e.g., TiO2 form the sugar-coated chewing gum enters the body with saliva and gets accumulated inside body [47]. Therefore, the intestinal epithelium is a likely target for these types of food additives.
Nanoparticles have a large surface to volume ratio due to which there is a limitation in their large scale use in food industries due to the active chemical and bio-reactivity and potential toxicity towards human health, but it is less reported [1]. Awareness is very important among consumers and there must be the proper transparency between consumers, producers (farmers), and manufacturers, consumers need to know exactly what they are eating. In the case of Genetically Modified (GM) food, people were initially very scared because of the alteration, due to which they were banned (still it is banned in many countries, e.g., Russia and cultivation is still banned in many EU countries but their import is not banned) but in the case of nanotechnology there is no such alteration of genes are doing so far due to which it is the 21st century technology [3]. Consumer shift to greener, eco-friendly, and chemical-free foods is really putting pressure on the food industries [4,44,49]. Research shows that in developing countries, people devouring 10 trillion nanoparticles each day [4]. Nanoparticles effects on genetic level are poorly studied. Lim et al. (2017), explained the epigenetic effect of nanoparticles interaction with RNA strands [1]. So, it is necessary to research more about its toxicity. Most of the population across the globe having a smartphone and it is easy for food traceability from farm to the dinning table, through this nanotechnology.
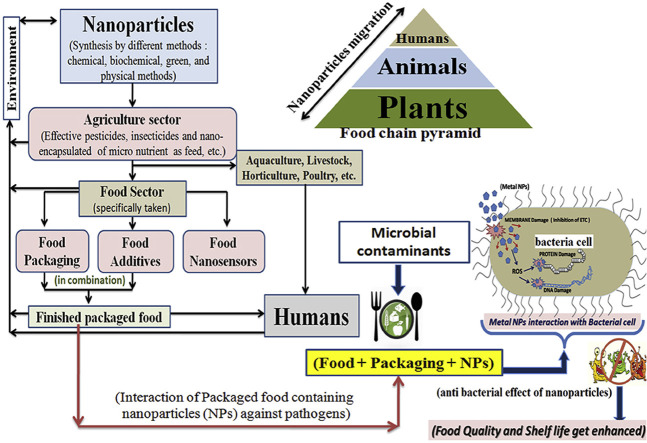
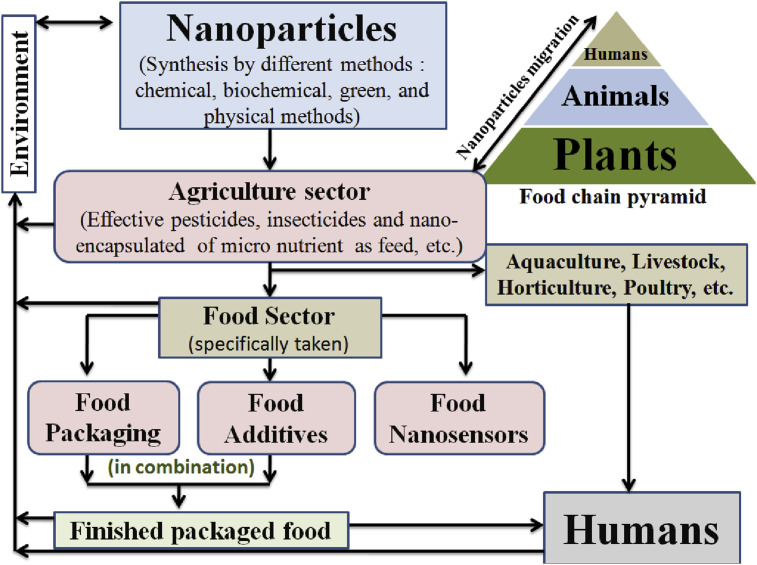
Migration of metal nanoparticles from packaging material to food depends upon the type of food, its nature, and the type of polymer (used specifically for packaging). The migration of nanoparticles from its synthesis (usually through, chemical, physical, biochemical/green synthesis method; which is also mentioned in this review paper) to humans is shown in Fig. 11 . Humans are at the top of the food chain, and nanoparticles flow bi-directionally, from humans to plants and from plants to humans, and so on. In the agriculture sector, nanoparticles are migrated in the form of advanced pesticides, insecticides, and many encapsulated nutritive feed and this feed used in aquaculture, livestock production, horticulture, poultry, etc. Nanoparticles in a food chain are finally accumulated in the human body through finished food. There is also the deposition of nanoparticles in the environment, which comes from the synthesis unit, agriculture sector, packaging, and also from human excreta; Fig. 11 [24,44]. Leaching of nanoparticles depends upon various factors such as packaged food initial concentration, temperature and environmental, packaged material thickness, swelling index, solubility, tensile strength, biodegradability, water vapor permeability, the particle size of embedded nanoparticles, nature, morphology of embedded particles and last but not the least, time and storage of the packaged food. The solubility of nano-metals elevated with the increase in temperature and decrease in pH value due to which migration also accelerated in the packaging system [31]. Silver nanoparticles used in food products have some extent of toxicity on the consumer health due to the fact that it affects the T-cell badly [1]. The European Commission (regulation no. 10/2011) regulates the migration of toxic components from the packaging material, it may be Bisphenol-A from plastic and nanoparticles from the nanocomposite polymer. Time and temperature must be used for tracking of the migration of the nanopartices, according to the EU council's Directive 97/48/EC. Mathematical modeling (depending upon the diffusion and partition coefficient) can be used to measure the concentration of the migrated nanoparticles from packaging material to the containing food [31]. The use of nanotechnology has not been thoroughly studied till so far, in the context of its toxicity. The big challenge is combining nanoparticles with the food-system. Mathematical models or response surface methodology can be utilized in a smart way to measure the toxicity of the nanoparticles used in a food-system. Risk assessment of nanotechnology applications in the food sector should be done.
Fig. 11.
A proposed typical cycle of migration of nanoparticles from their source of synthesis to the agriculture sector, and then finally accumulate inside the human body through the finished packaged food.
10. Conclusion and future perspective
Nanoparticles are promising antibacterial agent due to its wide activity against both gram-positive and gram-negative bacterial cells. Many methods such as sol-gel, hydrothermal, co-precipitation, auto-combustion, and green/biochemical synthesis methods have been used to prepare nanoparticles. Green/biochemical synthesis provides an attractive platform for nanoparticle synthesis by virtue of its several advantages like low cost of synthesis, short production time, easy availability, eco-friendly, economic (have the ability to form high production volume), and is more beneficial as compared to the chemical synthesis approach. The incorporation of metal nanoparticles in starch films minimizes the problem of biodegradability and food containment. Natural sources like plant extracts, leaves, seeds, stems, etc. can act as strong binding and reducing agents. Nanoparticles synthesized in the plant extracts already have a functionalized surface containing organic ligands, proteins, polysaccharides, and polyatomic alcohols, which are absent when synthesized by other physical or chemical methods. The antibacterial activity of nanoparticles is size as well as concentration-dependent and can be enhanced by doping with transition metals. The exact mechanism regarding antibacterial activity is although not established yet but the three possible mechanisms are the formation of ROS, their interaction with bacteria damaging the cell wall, and the release of the metal ions. In the food sector, nanoparticles are used as food additives, food packaging material, different bio-nanosensors, and biodegradable polymers. It not only enhances the overall quality of the food but also increases the shelf life and helps in traceability and communication of the food product. Nanoparticles used as antimicrobial agents show good antibacterial activity when used in the food packaging material. This is why in terms of the environment, green packaging and the incorporation of metal nanoparticles has become a more important subject of scientific research. The transfer of nutritional supplements with the help of nano-encapsulation in a target organ is an area of future research. A major obstacle to the application of nanotechnology in the food sector is minimal research on their potential toxicity (towards environment and consumer). Further research needs to be done to study the appropriate mechanism of the antibacterial activity of nanoparticles in the future.
Credit author statement
S.Gautam contributed in conceptualization, methodology, revisions, and supervision, R. Kaur & P. Mahajan contributed in data curation, draft writing, Prakash Kumar contributed in methodology, and original draft preparation, revisions and critic discussions.
Declaration of Competing Interest
All contributing authors declare no conflicts of interest.
Acknowledgments
SG acknowledges the UGC-DAE CSR research project(CSR-IC/BL-11/CRS-108/2019- 20/204) and TEQIP-III (MHRD, Govt. of India) R&D grant. Dr. Sandeep Kumar (Guru Jambheshwar University of Science & Technology, Hisar, India) is duly acknowledged for ZOI measurements and discussions. We show our huge gratitude towards all warriors, who helped in this global medical emergency, the medical staff (doctors, nurses, and researchers), hygiene workers, police, food manufacturers, and many more. We dedicate this paper to the COVID-19 warriors.
References
- 1.Lim J.P., Gyeong H.B., Dinesh Kumar S., Thameem D.S., Boon Huat B. Potential adverse effects of engineered nanomaterials commonly used in food on the mirnome. Food Chem. Toxicol. 2017;109:771–779. doi: 10.1016/j.fct.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 2.Khan I., Saeed K., Khan I. Nanoparticles: properties, applications and toxicities. Arab. J. Chem. 2017;12(7):908–931. doi: 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- 3.Coles D., Frewer L.J. Nanotechnology applied to european food production-a review of ethical and regulatory issues. Trends Food Sci. Technol. 2013;34:32–43. [Google Scholar]
- 4.Eleftheriadou M., Pyrgiotakis G., Demokritou P. Nanotechnology to the rescue: using nano-enabled approaches in microbiological food safety and quality. Curr. Opin. Biotechnol. 2017;44:87–93. doi: 10.1016/j.copbio.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silvestre C., Duraccio D., Cimmino S. Food packaging based on polymer nanomaterials. Prog. Polym. Sci. 2011;36:1766–1782. [Google Scholar]
- 6.Pal M. Nanotechnology: a new approach in food packaging. J. Food Hyg. Saf. 2017;2:1–2. [Google Scholar]
- 7.Sirelkhatim A., Mahmud S., Seeni A., Kaus N.H.M., Ann L.C., Bakhori S.K.M., Hasan H., Mohamad D. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015;7:219–242. doi: 10.1007/s40820-015-0040-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naveed Ul Haq A., Nadhman A., Ullah I., Mustafa G., Yasinzai M., Khan I. Synthesis approaches of zinc oxide nanoparticles: the dilemma of ecotoxicity. J. Nanomater. 2017;2017 [Google Scholar]
- 9.Bradley E.L., Castle L., Chaudhry Q. Applications of nanomaterials in food packaging with a consideration of opportunities for developing countries. Trends Food Sci. Technol. 2011;22:604–610. [Google Scholar]
- 10.Dudefoi W., Villares A., Peyron S., Moreau C., Ropers M.-H., Gontard N., Cathala B. Nanoscience and nanotechnologies for biobased materials, packaging and food applications: new opportunities and concerns. Innov. Food Sci. Emerg. 2018;46:107–121. [Google Scholar]
- 11.Lee K.T. Quality and safety aspects of meat products as affected by various physical manipulations of packaging materials. Meat Sci. 2010;86:138–150. doi: 10.1016/j.meatsci.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 12.Zanetti M., Carniel T.K., Dalcanton F., dos Anjos R.S., Riella H.G., de Aräıo P.H., de Oliveira D., Fiori M.A. Use of encapsulated natural compounds as antimicrobial additives in food packaging: a brief review. Trends Food Sci. Technol. 2018;81:51–60. [Google Scholar]
- 13.Napierala D.M., Stangierski J. Tensile properties of wheat starch film with the addition of sorbitol and albumin. Acta Chromatogr. 2007;9:123–133. [Google Scholar]
- 14.Liu L. Bioplastics in food packaging: innovative technologies for biodegradable packaging. San Jose State University Packaging Engineering. 2006;13:1348–1368. [Google Scholar]
- 15.Sharlina M.E., Yaacob W., Lazim A.M., Fazry S., Lim S.J., Abdullah S., Noordin A., Kumaran M. Physicochemical properties of starch from dioscorea pyrifolia tubers. Food Chem. 2017;220:225–232. doi: 10.1016/j.foodchem.2016.09.196. [DOI] [PubMed] [Google Scholar]
- 16.Kumar P., Gautam S. arXiv e-prints; 2019. Developing ZnO Nanoparticle Embedded Antimicrobial Starch Biofilm for Food Packaging. arXiv:1909.05083. [Google Scholar]
- 17.Adetunji C., Fawole O., Arowora K., Nwaubani S., Oloke J., Adepoju A., Adetunji J., Ajani A. Performance of edible coatings from carboxymethylcellulose (CMC) and corn starch (CS) incorpo- rated with moringa oleifera extract on citrus sinensis stored at ambient temperature. Agros (pelotas) 2013;13:77–86. [Google Scholar]
- 18.Lee J.-A., Kim M.-K., Song J.H., Jo M.-R., Yu J., Kim K.-M., Kim Y.-R., Oh J.-M., Choi S.-J. Biokinetics of food additive silica nanoparticles and their interactions with food components. Colloids Surf., B. 2017;150:384–392. doi: 10.1016/j.colsurfb.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Dorier M., Tisseyre C., Dussert F., B¨ıal D., Arnal M.-E., Douki T., Valdiglesias V., Laffon B., Fraga S., Brand¨ıo F., Herlin-Boime N., Barreau F., Rabilloud T., Carriere M. Toxicological impact of acute exposure to E171 food additive and TiO2 nanoparticles on a co-culture of Caco-2 and HT29-MTX intestinal cells. Mutat. Res-Gen Tox. En. 2018;845 doi: 10.1016/j.mrgentox.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Neethirajan S., Jayas D.S. Nanotechnology for the food and bioprocessing industries. Food Bioprocess Technol. 2011;4:39–47. doi: 10.1007/s11947-010-0328-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pathakoti K., Manubolu M., Hwang H.-M. Nanostructures: current uses and future applications in food science. J. Food Drug Anal. 2017;25:245–253. doi: 10.1016/j.jfda.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buzea C., Pacheco I.I., Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2:MR17–MR71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert P., Collier P., Brown M. Influence of growth rate on susceptibility to antimicrobial agents: biofilms, cell cycle, dormancy, and stringent response. Antimicrob. Agents Chemother. 1990;34:1865. doi: 10.1128/aac.34.10.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jafari S.M., Katouzian I., Akhavan S. Nanoencap- Sulation Technologies for the Food and Nutraceutical Industries. Elsevier; 2017. Safety and regulatory issues of nanocapsules; pp. 545–590. [Google Scholar]
- 25.Duncan T.V. Applications of nanotechnology in food packaging and food safety: barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011;363:1–24. doi: 10.1016/j.jcis.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramesh P., Rajendran A., Meenakshisundaram M. Green syntheis of zinc oxide nanoparticles using flower extract cassia auriculata. J. Nanosci. Nanotechnol. 2014;2:41–45. [Google Scholar]
- 27.Nafchi A.M., Alias A.K., Mahmud S., Robal M. Antimicrobial, rheological, and physicochemical properties of sago starch films filled with nanorod-rich zinc oxide. J. Food Eng. 2012;113:511–519. [Google Scholar]
- 28.Sastry R.K., Rashmi H., Rao N. Nanotechnology for enhancing food security in India. Food Pol. 2011;36:391–400. [Google Scholar]
- 29.David W. The City of Grace. Springer; 2020. Population, globalization, the market and the environment; pp. 1–12. [Google Scholar]
- 30.Hannon J.C., Kerry J., Cruz-Romero M., Morris M., Cummins E. Advances and challenges for the use of engineered nanoparticles in food contact materials. Trends Food Sci. Technol. 2015;43:43–62. [Google Scholar]
- 31.Huang J.-Y., Li X., Zhou W. Safety assessment of nanocomposite for food packaging application. Trends Food Sci. Technol. 2015;45:187–199. [Google Scholar]
- 32.Khezerlou A., Alizadeh-Sani M., Azizi-Lalabadi M., Ehsani A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses, Microb. Pathogens. 2018;123:505–526. doi: 10.1016/j.micpath.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Galanakis C.M. The food systems in the era of the coronavirus (COVID-19) pandemic crisis. Foods. 2020;9:523. doi: 10.3390/foods9040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coronavirus Disease COVID-19, Situation Report-126. World Health Organization; 2020. https://www.who.int/docs/default-source/coronaviruse/ URL: [Google Scholar]
- 35.Nikalje A.P. Nanotechnology and its applications in medicine. Med. Chem. 2015;5:185–189. [Google Scholar]
- 36.Bouwmeester H., Dekkers S., Noordam M.Y., Hagens W.I., Bulder A.S., de Heer C., ten Voorde S.E., Wijnhoven S.W., Marvin H.J., Sips A.J. Review of health safety aspects of nanotechnologies in food production. Regul. Toxicol. Pharmacol. 2009;53:52–62. doi: 10.1016/j.yrtph.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Kaushik A., Solanki P.R., Ansari A.A., Sumana G., Ahmad S., Malhotra B.D. Iron oxide-chitosan nanobiocomposite for urea sensor. Sensor. Actuator. B Chem. 2009;138:572–580. [Google Scholar]
- 38.Chen L., Remondetto G.E., Subirade M. Food protein-based materials as nutraceutical delivery systems. Trends Food Sci. Technol. 2006;17:272–283. [Google Scholar]
- 39.Solanki P.R., Kaushik A., Ansari A.A., Malhotra B. Nanostructured zinc oxide platform for choles- terol sensor. Appl. Phys. Lett. 2009;94:143901. [Google Scholar]
- 40.Chellaram C., Murugaboopathi G., John A., Sivakumar R., Ganesan S., Krithika S., Priya G. Sig- nificance of nanotechnology in food industry. APCBEE procedia. 2014;8:109–113. [Google Scholar]
- 41.Rai M., Yadav A., Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Daniel S.K., Vinothini G., Subramanian N., Nehru K., Sivakumar M. Biosynthesis of Cu, ZVI, and Ag nanoparticles using dodonaea viscosa extract for antibacterial activity against human pathogens. J. Nano Res. 2013;15:1319. [Google Scholar]
- 43.Sekhon B.S. Food nanotechnology-an overview. Nanotechnol. Sci. Appl. 2010;3:1. [PMC free article] [PubMed] [Google Scholar]
- 44.Rossi M., Cubadda F., Dini L., Terranova M.L., Aureli F., Sorbo A., Passeri D. Scientific basis of nanotechnology, implications for the food sector and future trends. Trends Food Sci. Technol. 2014;40(2):127–148. doi: 10.1016/j.tifs.2014.09.004. [DOI] [Google Scholar]
- 45.Espitia P.J.P., Soares N.d.F.F., Te´ofilo R.F., dos Reis Coimbra J.S., Vitor D.M., Batista R.A., Ferreira S.O., de Andrade N.J., Medeiros E.A.A. Physical–mechanical and antimicrobial properties of nanocomposite films with pediocin and ZnO nanoparticles. Carbohydr. Polym. 2013;94:199–208. doi: 10.1016/j.carbpol.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Huang Z., Zheng X., Yan D., Yin G., Liao X., Kang Y., Yao Y., Huang D., Hao B. Toxicological effect of ZnO nanoparticles based on bacteria. Langmuir. 2008;24:4140–4144. doi: 10.1021/la7035949. [DOI] [PubMed] [Google Scholar]
- 47.He X., Hwang H.-M. Nanotechnology in food science: functionality, applicability, and safety assess- ment. J. Food Drug Anal. 2016;24:671–681. doi: 10.1016/j.jfda.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gudikandula K., Charya Maringanti S. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016;11:714–721. [Google Scholar]
- 49.Verma S.K., Nisha K., Panda P.K., Patel P., Kumari P., Mallick M., Sarkar B., Das B. Green synthesized MgO nanoparticles infer biocompatibility by reducing in vivo molecular nanotoxicity in embryonic zebrafish through arginine interaction elicited apoptosis. Sci. Total Environ. 2020;713:136521. doi: 10.1016/j.scitotenv.2020.136521. [DOI] [PubMed] [Google Scholar]
- 50.Mohammadinejad R., Karimi S., Iravani S., Varma R.S. Plant-derived nanostructures: types and applications. Green Chem. 2016;18:20–52. [Google Scholar]
- 51.Sanpo N., Berndt C.C., Wen C., Wang J. Transition metal-substituted cobalt ferrite nanoparticles for biomedical applications. Acta Biomater. 2013;9:5830–5837. doi: 10.1016/j.actbio.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 52.Phua L., Xu F., Ma Y., Ong C. Structure and magnetic characterizations of cobalt ferrite films prepared by spray pyrolysis. Thin Solid Films. 2009;517:5858–5861. [Google Scholar]
- 53.Yamamoto O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 2001;3:643–646. [Google Scholar]
- 54.Abreu A.S., Oliveira M., de S´a A., Rodrigues R.M., Cerqueira M.A., Vicente A.A., Machado A. Antimicrobial nanostructured starch based films for packaging. Carbohydr. Polym. 2015;129:127–134. doi: 10.1016/j.carbpol.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 55.Ali A., Xie F., Yu L., Liu H., Meng L., Khalid S., Chen L. Preparation and characterization of starch-based composite films reinfoced by polysaccharide-based crystals. Compos. B Eng. 2018;133:122–128. [Google Scholar]
- 56.Mirjalili F., Yassini Ardekani A. Preparation and characterization of starch film accompanied with ZnO nanoparticles. J. Food Process. Eng. 2017;40 [Google Scholar]
- 57.Sharma P., Kehinde B.A., Kaur S., Vyas P. Application of edible coatings on fresh and minimally processed fruits: a review. Nutr. Food Sci. 2019;49(4):713–738. doi: 10.1108/NFS-08-2018-0246. [DOI] [Google Scholar]
- 58.Fakhouri F.M., Martelli S.M., Caon T., Velasco J.I., Mei L.H.I. Edible films and coatings based on starch/gelatin: film properties and effect of coatings on quality of refrigerated red crimson grapes. Postharvest Biol. Technol. 2015;109:57–64. [Google Scholar]
- 59.Wang K., Wang W., Ye R., Liu A., Xiao J., Liu Y., Zhao Y. Mechanical properties and solubility in water of corn starch-collagen composite films: effect of starch type and concentrations. Food Chem. 2017;216:209–216. doi: 10.1016/j.foodchem.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 60.Kanmani P., Rhim J.-W. Properties and characterization of bionanocomposite films prepared with various biopolymers and ZnO nanoparticles. Carbohydr. Polym. 2014;106:190–199. doi: 10.1016/j.carbpol.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 61.Ma J., Zhu W., Tian Y., Wang Z. Preparation of zinc oxide-starch nanocomposite and its application on coating. Nanoscale Res. Lett. 2016;11:200. doi: 10.1186/s11671-016-1404-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hasnidawani J., Azlina H., Norita H., Bonnia N., Ratim S., Ali E. Synthesis of ZnO nanostructures using sol-gel method. Procedia. Chem. 2016;19:211–216. [Google Scholar]
- 63.Ko-lodziejczak-Radzimska A., Jesionowski T., Krysztafkiewicz A. Obtaining zinc oxide from aqueous solutions of KOH and Zn(CH3COO)2. Physicochem. Probl. Mi. 2010;44:93–102. [Google Scholar]
- 64.Nagavarma B., Yadav H.K., Ayaz A., Vasudha L., Shivakumar H. Different techniques for preparation of polymeric nanoparticles-a review. Asian J. Pharmaceut. Clin. Res. 2012;5:16–23. [Google Scholar]
- 65.Chaudhuri S.K., Malodia L. Biosynthesis of zinc oxide nanoparticles using leaf extract of calotropis gigantea: characterization and its evaluation on tree seedling growth in nursery stage. Appl. Nanosci. 2017;7:501–512. [Google Scholar]
- 66.Hsieh P.Y.-H., Ofori J.A. Innovations in food technology for health, Asia Pac. J. Clin. Nutr. 2007;16:65–73. [PubMed] [Google Scholar]
- 67.Moraru C.I., Panchapakesan C.P., Huang Q., Takhistov P., Liu S., Kokini J.L. Nanotechnology: a new frontier in food science. Food Technol. Mag. 2003;57(12) [Google Scholar]
- 68.Rajput N. Methods of preparation of nanoparticles-a review. Int. J. Adv. Eng. Technol. 2015;7:1806. [Google Scholar]
- 69.Wu W., Wu Z., Yu T., Jiang C., Kim W.-S. Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015;16 doi: 10.1088/1468-6996/16/2/023501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghorbani H.R. A review of methods for synthesis of Al nanoparticles. Orient. J. Chem. 2014;30:1941–1949. [Google Scholar]
- 71.Salam H.A., Sivaraj R., Venckatesh R. Green synthesis and characterization of zinc oxide nanoparti- cles from ocimum basilicum l. var. purpurascens benth.-lamiaceae leaf extract. Mater. Lett. 2014;131:16–18. [Google Scholar]
- 72.Yuvakkumar R., Suresh J., Nathanael A.J., Sundrarajan M., Hong S. Novel green synthetic strategy to prepare ZnO nanocrystals using rambutan (nephelium lappaceum l.) peel extract and its antibac- terial applications. Mater. Sci. Eng. C. 2014;41:17–27. doi: 10.1016/j.msec.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 73.Machado S., Pinto S., Grosso J., Nouws H., Albergaria J.T., Delerue-Matos C. Green production of zero-valent iron nanoparticles using tree leaf extracts. Sci. Total Environ. 2013;445:1–8. doi: 10.1016/j.scitotenv.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 74.Pattanayak M., Nayak P. Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Azadirachta indica (Neem) World J. Nano Sci. Technol. 2013;2:6–9. [Google Scholar]
- 75.Verma S.K., Jha E., Panda P.K., Thirumurugan A., Suar M. Advanced Nanostructured Materials for Environmental Remediation. Springer; 2019. Biological effects of green-synthesized metal nanoparticles: a mechanistic view of antibacterial activity and cytotoxicity; pp. 145–171. [Google Scholar]
- 76.Parveen K., Banse V., Ledwani L. AIP Conference Proc. vol. 1724. AIP Publishing; 2016. Green synthesis of nanoparticles: their advantages and disadvan- tages; p. 20048. 1. [Google Scholar]
- 77.Javad S., Obaid B., Tariq A., Iqbal Z., Ghaffar N. Evaluation of efficiency of different plants to develop silver nanoparticles. Pakistan J. Bot. 2017;49:283–287. [Google Scholar]
- 78.Paul P., Pattnaik Y., Panda P.K., Jha E., Verma S.K., Suar M. Green Methods for Wastewater Treatment. Springer; 2020. Green synthesized metal oxide nanomaterials photocatalysis in combating bacterial infection; pp. 73–86. [Google Scholar]
- 79.Prasad R. Synthesis of silver nanoparticles in photosynthetic plants. J. Nanotechnol. 2014;2014 [Google Scholar]
- 80.Mahajan P., Sharma A., Kaur B., Goyal N., Gautam S. Green synthesized (ocimum sanctum and allium sativum) Ag-doped cobalt ferrite nanoparticles for antibacterial application. Vacuum. 2019;161:389–397. [Google Scholar]
- 81.Gnanasangeetha D., SaralaThambavani D. One pot synthesis of zinc oxide nanoparticles via chemical and green method. Res. J. Mater. Sci. 2013;2320:6055. [Google Scholar]
- 82.Nagajyothi P., An T.M., Sreekanth T., Lee J.-i., Lee D.J., Lee K. Green route biosynthesis: characterization and catalytic activity of ZnO nanoparticles. Mater. Lett. 2013;108:160–163. [Google Scholar]
- 83.Sagar Raut D.P., Thorat R.T. Green synthesis of zinc oxide (ZnO) nanoparticles using ocimum tenuiflorum leaves. Int. J. Sci. Res. 2015:2319–7064. [Google Scholar]
- 84.Makarov V., Love A., Sinitsyna O., Makarova S., Yaminsky I., Taliansky M., Kalinina N. Green nanotechnologies: synthesis of metal nanoparticles using plants. Acta Naturae. 2014;6 [PMC free article] [PubMed] [Google Scholar]
- 85.Gingasu D., Mindru I., et al. Synthesis of nanocrystalline cobalt ferrite through soft chemistry methods: a green chemistry approach using sesame seed extract. Mater. Chem. Phys. 2016;182:219–230. [Google Scholar]
- 86.Kavyashree D., Kumari R.A., Nagabhushana H., Sharma S., Vidya Y., Anantharaju K., Prasad B.D., Prashantha S., Lingaraju K., Rajanaik H. Orange red emitting Eu(3+) doped zinc oxide nanophos- phor material prepared using guizotia abyssinica seed extract: structural and photoluminescence studies. J. Lumin. 2015;167:91–100. [Google Scholar]
- 87.Verma S.K., Jha E., Panda P.K., Das J.K., Thirumurugan A., Suar M., Parashar S. Molecular aspects of core-shell intrinsic defect induced enhanced antibacterial activity of ZnO nanocrystals. Nanomedicine. 2018;13:43–68. doi: 10.2217/nnm-2017-0237. [DOI] [PubMed] [Google Scholar]
- 88.Shankar S.S., Ahmad A., Pasricha R., Sastry M. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J. Mater. Chem. 2003;13:1822–1826. [Google Scholar]
- 89.Bhuyan T., Mishra K., Khanuja M., Prasad R., Varma A. Biosynthesis of zinc oxide nanoparticles from azadirachta indica for antibacterial and photocatalytic applications, Mat. Sci. Semicon. Proc. 2015;32:55–61. [Google Scholar]
- 90.Tan Y.N., Lee J.Y., Wang D.I. Uncovering the design rules for peptide synthesis of metal nanopar- ticles. J. Am. Chem. Soc. 2010;132:5677–5686. doi: 10.1021/ja907454f. [DOI] [PubMed] [Google Scholar]
- 91.Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638–2650. [Google Scholar]
- 92.Anandan M., Dinesh S., Krishnakumar N., Balamurugan K. Influence of Co doping on combined photocatalytic and antibacterial activity of ZnO nanoparticles. Mater. Res. Express. 2016;3:115009. [Google Scholar]
- 93.Sharma V. Sol-gel mediated facile synthesis of Zinc-Oxide nanoaggregates, their characterization and antibacterial activity. J. Appl. Chem. 2012;2:52–55. [Google Scholar]
- 94.Kooti M., Saiahi S., Motamedi H. Fabrication of silver-coated cobalt ferrite nanocomposite and the study of its antibacterial activity. J. Magn. Magn Mater. 2013;333:138–143. [Google Scholar]
- 95.Rajendar V., Dayakar T., Chakra C., Rao K.V. Systematic approach on the fabrication of Ag doped ZnO nanoparticles by novel auto combustion method for antibacterial applications. Nanomed- Nanotechnol. 2015;2:21–27. [Google Scholar]
- 96.Gautam S., Muthurani S., Balaji M., Thakur P., Padiyan D.P., Chae K., Kim S., Asokan K. Electronic structure studies of nanoferrite CuxCo1−xFe2O4 by X-ray absorption spectroscopy. J. Nanosci. Nanotechnol. 2011;11:386–390. doi: 10.1166/jnn.2011.3249. [DOI] [PubMed] [Google Scholar]
- 97.Hayashi H., Hakuta Y. Hydrothermal synthesis of metal oxide nanoparticles in supercritical water. J. Mater. Sci. 2010;3:3794–3817. doi: 10.3390/ma3073794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Santhoshkumar A., Kavitha H.P., Suresh R. Hydrothermal synthesis, characterization and antibac- terial activity of NiO nanoparticles. J. Adv. Chem. Sci. 2016:230–232. [Google Scholar]
- 99.Dinesh S., Barathan S., Premkumar V., Sivakumar G., Anandan N. Hydrothermal synthesis of zinc stannate (Zn2SnO4) nanoparticles and its application towards photocatalytic and antibacterial activity. J. Mater. Sci. Mater. 2016;27:9668–9675. [Google Scholar]
- 100.Yang Q., Danaie M., Young N., Broadley V., Joyce D.E., Martin T.L., Marceau E., Moody M.P., Bagot P.A. Atom probe tomography of Au-Cu bimetallic nanoparticles synthesized by inert gas condensation. J. Phys. Chem. C. 2019;123:26481–26489. [Google Scholar]
- 101.Lotfi S., Abbaspour M. Investigation of temperature and pressure effects on thermodynamics and structural properties of gold nanoparticles formed during the gas condensation procedure. J. Mol. Liq. 2019;281:39–47. [Google Scholar]
- 102.Huh Y., Lee J.Y., Cheon J., Hong Y.K., Koo J.Y., Lee T.J., Lee C.J. Controlled growth of carbon nanotubes over cobalt nanoparticles by thermal chemical vapor deposition. J. Phys. Chem. 2003;13:2297–2300. [Google Scholar]
- 103.Koch C. The synthesis and structure of nanocrystalline materials produced by mechanical attrition: a review. J. Nanostruct. 1993;2:109–129. [Google Scholar]
- 104.DeCastro C.L., Mitchell B.S. 2002. Nanoparticles from Mechanical Attrition, Synthesis, Functionalization, and Surface Treatment of Nanoparticles 5. [Google Scholar]
- 105.Lam C., Zhang Y., Tang Y., Lee C., Bello I., Lee S. Large-scale synthesis of ultrafine Si nanoparticles by ball milling. J. Cryst. Growth. 2000;220:466–470. [Google Scholar]
- 106.Espitia P.J.P., Soares N.d.F.F., dos Reis Coimbra J.S., de Andrade N.J., Cruz R.S., Medeiros E.A.A. Zinc oxide nanoparticles: synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012;5:1447–1464. [Google Scholar]
- 107.Jiang W., Saxena A., Song B., Ward B.B., Beveridge T.J., Myneni S.C. Elucidation of functional groups on gram-positive and gram-negative bacterial surfaces using infrared spectroscopy. Langmuir. 2004;20:11433–11442. doi: 10.1021/la049043+. [DOI] [PubMed] [Google Scholar]
- 108.Applerot G., Lipovsky A., Dror R., Perkas N., Nitzan Y., Lubart R., Gedanken A. Enhanced an- tibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv. Funct. Mater. 2009;19:842–852. [Google Scholar]
- 109.Scott J.R., Barnett T.C. Surface proteins of gram-positive bacteria and how they get there. Annu. Rev. Microbiol. 2006;60:397–423. doi: 10.1146/annurev.micro.60.080805.142256. [DOI] [PubMed] [Google Scholar]
- 110.Hajipour M.J., Fromm K.M., Ashkarran A.A., de Aberasturi D.J., de Larramendi I.R., Rojo T., Serpooshan V., Parak W.J., Mahmoudi M. Antibacterial properties of nanoparticles. Trends Biotech- nol. 2012;30:499–511. doi: 10.1016/j.tibtech.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 111.Landini P., Antoniani D., Burgess J.G., Nijland R. Molecular mechanisms of compounds affecting bacterial biofilm formation and dispersal. Appl. Microbiol. Biotechnol. 2010;86:813–823. doi: 10.1007/s00253-010-2468-8. [DOI] [PubMed] [Google Scholar]
- 112.Mahmoudi M., Serpooshan V. Silver-coated engineered magnetic nanoparticles are promising for the success in the fight against antibacterial resistance threat. ACS Nano. 2012;6:2656–2664. doi: 10.1021/nn300042m. [DOI] [PubMed] [Google Scholar]
- 113.Gingasu D., Mindru I., Patron L., et al. Green synthesis methods of CoFe2O4 and Ag-CoFe2O4 nanoparticles using hibiscus extracts and their antimicrobial potential. J. Nanomater. 2016;2016:2106756–2106767. [Google Scholar]
- 114.Xavier S., Cleetus H., Nimila P., Thankachan S., Sebastian R., Mohammed E. Structural and an- tibacterial properties of silver substituted cobalt ferrite nanoparticles. J. pharm. biol. chem. sci. 2014;5:364–371. [Google Scholar]
- 115.Jha A.K., Prasad K. Biological synthesis of cobalt ferrite nanoparticles. Nanotechnol. Dev. 2012;2:9. [Google Scholar]
- 116.Manikandan A., Durka M., Antony S.A. Hibiscus rosa-sinensis leaf extracted green methods, magneto-optical and catalytic properties of spinel CuFe2O4 nano-and microstructures. J. Inorg. Organomet. Polym. Mater. 2015;25:1019–1031. [Google Scholar]
- 117.Ginga,su D., Mˆındru I., Preda S., Calderon-Moreno J.M., Daniela C.C., Patron L., Diamandescu L. Green synthesis of cobalt ferrite nanoparticles using plant extracts. Rev. Roum. Chem. 2017;62:645–653. [Google Scholar]
- 118.Desselberger U. Emerging and re-emerging infectious diseases. J. Infect. 2000;40:3–15. doi: 10.1053/jinf.1999.0624. [DOI] [PubMed] [Google Scholar]
- 119.Gunalan S., Sivaraj R., Rajendran V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. 2012;22:693–700. [Google Scholar]
- 120.Rani R., Kumar H., Salar R., Purewal S. Antibacterial activity of copper oxide nanoparticles against gram negative bacterial strain synthesized by reverse micelle technique. Int. J. Pharmaceut. Res. Dev. 2014;6:72–78. [Google Scholar]
- 121.Singh R., Lillard J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009;86:215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Karuppusamy C., Venkatesan P. Role of nanoparticles in drug delivery system: a comprehensive review. J. Pharmaceut. Sci. Res. 2017;9:318. [Google Scholar]
- 123.Yamamoto O., Hotta M., Sawai J., Sasamoto T., Kojima H. Influence of powder characteristic of ZnO on antibacterial activity. J. Ceram. Soc. Japan. 1998;106:1007–1011. [Google Scholar]
- 124.Wang L., Hu C., Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomed. 2017;12:1227. doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang L., Jiang Y., Ding Y., Povey M., York D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles ZnO nanofluids. J. Nano Res. 2007;9:479–489. [Google Scholar]
- 126.Padmavathy N., Vijayaraghavan R. Enhanced bioactivity of ZnO nanoparticle antimicrobial study. Sci. Technol. Adv. Mater. 2008;9 doi: 10.1088/1468-6996/9/3/035004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ben-Sasson M., Zodrow K.R., Genggeng Q., Kang Y., Giannelis E.P., Elimelech M. Surface func- tionalization of thin-film composite membranes with copper nanoparticles for antimicrobial surface properties. Environ. Sci. Technol. 2013;48:384–393. doi: 10.1021/es404232s. [DOI] [PubMed] [Google Scholar]
- 128.Sukhorukova I., Sheveyko A., Kiryukhantsev-Korneev P.V., Zhitnyak I., Gloushankova N., Denisenko E., Filippovich S.Y., Ignatov S., Shtansky D. Toward bioactive yet antibacterial sur- faces. Colloids Surf., B. 2015;135:158–165. doi: 10.1016/j.colsurfb.2015.06.059. [DOI] [PubMed] [Google Scholar]
- 129.Pan X., Wang Y., Chen Z., Pan D., Cheng Y., Liu Z., Lin Z., Guan X. Investigation of antibacterial activity and related mechanism of a series of nano-Mg(OH)2. ACS Appl. Mater. Interfaces. 2013;5:1137–1142. doi: 10.1021/am302910q. [DOI] [PubMed] [Google Scholar]
- 130.Arakha M., Pal S., Samantarrai D., Panigrahi T.K., Mallick B.C., Pramanik K., Mallick B., Jha S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015;5:14813. doi: 10.1038/srep14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Podporska-Carroll J., Myles A., Quilty B., McCormack D.E., Fagan R., Hinder S.J., Dionysiou D.D., Pillai S.C. Antibacterial properties of F-doped ZnO visible light photocatalyst. J. Hazard Mater. 2017;324:39–47. doi: 10.1016/j.jhazmat.2015.12.038. [DOI] [PubMed] [Google Scholar]
- 132.Guo B.-L., Han P., Guo L.-C., Cao Y.-Q., Li A.-D., Kong J.-Z., Zhai H.-F., Wu D. The antibacterial activity of Ta-doped ZnO nanoparticles. Nanoscale Res. Lett. 2015;10:336. doi: 10.1186/s11671-015-1047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pfeiffer C., Rehbock C., Hühn D., Carrillo-Carrion C., de Aberasturi D.J., Merk V., Barcikowski S., Parak W.J. Interaction of colloidal nanoparticles with their local environment: the (ionic) nanoen- vironment around nanoparticles is different from bulk and determines the physico-chemical properties of the nanoparticles. J. R. Soc. Interface. 2014;11:20130931. doi: 10.1098/rsif.2013.0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guarnieri D., Guaccio A., Fusco S., Netti P.A. Effect of serum proteins on polystyrene nanoparticle uptake and intracellular trafficking in endothelial cells. J. Nano Res. 2011;13:4295. [Google Scholar]
- 135.Mu L., Sprando R.L. Application of nanotechnology in cosmetics. Pharm. Res. (N. Y.) 2010;27:1746–1749. doi: 10.1007/s11095-010-0139-1. [DOI] [PubMed] [Google Scholar]
- 136.Oberd¨orster G., Oberd¨orster E., Oberd¨orster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005;113:823. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Singh N., Jenkins G.J., Asadi R., Doak S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (spion) Nanotechnol. Rev. 2010;1:5358. doi: 10.3402/nano.v1i0.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang D.-M., Chung T.-H., Hung Y., Lu F., Wu S.-H., Mou C.-Y., Yao M., Chen Y.-C. Internalization of mesoporous silica nanoparticles induces transient but not sufficient osteogenic signals in human mesenchymal stem cells. Toxicol. Appl. Pharmacol. 2008;231:208–215. doi: 10.1016/j.taap.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 139.Markides H., Rotherham M., El Haj A. Biocompatibility and toxicity of magnetic nanoparticles in regenerative medicine. J. Nanomater. 2012;2012:13. [Google Scholar]
- 140.Mahmoudi M., Simchi A., Imani M., Shokrgozar M.A., Milani A.S., H¨afeli U.O., Stroeve P. A new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticles. Colloids Surf., B. 2010;75:300–309. doi: 10.1016/j.colsurfb.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 141.Berry C.C. Possible exploitation of magnetic nanoparticle-cell interaction for biomedical applications. J. Mater. Chem. 2005;15:543–547. [Google Scholar]
- 142.Hofmann-Amtenbrink M., Hofmann H., Montet X. Superparamagnetic nanoparticles–a tool for early diagnostics. Swiss Med. Wkly. 2010;140 doi: 10.4414/smw.2010.13081. [DOI] [PubMed] [Google Scholar]
- 143.Lundqvist M., Stigler J., Cedervall T., Bergg¨ırd T., Flanagan M.B., Lynch I., Elia G., Dawson K. The evolution of the protein corona around nanoparticles: a test study. ACS Nano. 2011;5:7503–7509. doi: 10.1021/nn202458g. [DOI] [PubMed] [Google Scholar]
- 144.Mahmoudi M., Lynch I., Ejtehadi M.R., Monopoli M.P., Bombelli F.B., Laurent S. Protein- nanoparticle interactions: opportunities and challenges. Chem. Rev. 2011;111:5610–5637. doi: 10.1021/cr100440g. [DOI] [PubMed] [Google Scholar]
- 145.Mahmoudi M., Laurent S., Shokrgozar M.A., Hosseinkhani M. Toxicity evaluations of superpara- magnetic iron oxide nanoparticles: cell “vision” versus physicochemical properties of nanoparticles. ACS Nano. 2011;5:7263–7276. doi: 10.1021/nn2021088. [DOI] [PubMed] [Google Scholar]
- 146.Lennern¨as H., Abrahamsson B. The use of biopharmaceutic classification of drugs in drug discovery and development: current status and future extension. J. Pharm. Pharmacol. 2005;57:273–285. doi: 10.1211/0022357055263. [DOI] [PubMed] [Google Scholar]
- 147.Brody A.L., Bugusu B., Han J.H., Sand C.K., McHugh T.H. Scientific status summary: innovative food packaging solution. J. Food Sci. 2008;73:R107–R116. doi: 10.1111/j.1750-3841.2008.00933.x. [DOI] [PubMed] [Google Scholar]
- 148.Ruengruglikit C., KIm H.C., Miller R.D., Huang Q.R. Abstracts of papers of the American Chemical Society. Vol. 227. Amer Chemical Soc; 1155 16TH ST, NW, WASHINGTON, DC 20036 USA: 2004. Fabrication of nanoporous oligonucleotide microarray for pathogen detection and identification; p. U464. [Google Scholar]
- 149.Omanovi´c-Mikliˇcanina E., Maksimovi´c M. Nanosensors applications in agriculture and food industry, Glas. hem. tehnol. Bosne Herceg. 2016;47:59–70. [Google Scholar]
- 150.Fu J., Park B., Siragusa G., Jones L., Tripp R., Zhao Y., Cho Y.-J. An Au/Si hetero-nanorod-based biosensor for Salmonella detection. Nanotechnology. 2008;19(15):155502. doi: 10.1088/0957-4484/19/15/155502. [DOI] [PubMed] [Google Scholar]
- 151.Noorbakhsh-Soltani S., Zerafat M., Sabbaghi S. A comparative study of gelatin and starch-based nano-composite films modified by nano-cellulose and chitosan for food packaging applications, Car- bohydr. Polymer. 2018;189:48–55. doi: 10.1016/j.carbpol.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 152.Degant O., Schwechten D. German Patent DE10107885A1; 2002. Wheat Flour with Increased Water Binding Capacity and Process and Equipment for its Manufacture. [Google Scholar]
- 153.Sherman L.M. Chasing nanocomposites. Polym-Plast Technol. 2004;50:56–61. [Google Scholar]
- 154.Brody A.L., Bugusu B., Han J.H., Sand C.K., Mchugh T.H. Innovative food packaging solutions. J. Food Sci. 2008;73:107–116. doi: 10.1111/j.1750-3841.2008.00933.x. [DOI] [PubMed] [Google Scholar]
- 155.Jim´enez A., Fabra M.J., Talens P., Chiralt A. Edible and biodegradable starch films: a review. Food Bioprocess Technol. 2012;5:2058–2076. [Google Scholar]
- 156.Rands M.R., Adams W.M., Bennun L., Butchart S.H., Clements A., Coomes D., Entwistle A., Hodge I., Kapos V., Scharlemann J.P., et al. Biodiversity conservation: challenges beyond. Science. 2010;329(2010):1298–1303. doi: 10.1126/science.1189138. [DOI] [PubMed] [Google Scholar]
- 157.Espitia P.J.P., Du W.-X., de Jesu´s Avena-Bustillos R., Soares N.d.F.F., McHugh T.H. Edible films from pectin: physical mechanical and antimicrobial properties-a review. Food Hydrocolloids. 2014;35:287–296. [Google Scholar]
- 158.Nawab A., Alam F., Haq M.A., Lutfi Z., Hasnain A. Mango kernel starch-gum composite films: physical, mechanical and barrier properties. Int. J. Biol. Macromol. 2017;98:869–876. doi: 10.1016/j.ijbiomac.2017.02.054. [DOI] [PubMed] [Google Scholar]
- 159.Sorrentino A., Gorrasi G., Vittoria V. Potential perspectives of bio-nanocomposites for food packaging applications. Trends Food Sci. Technol. 2007;18:84–95. [Google Scholar]
- 160.Caz´on P., Velazquez G., Ramirez J.A., V´azquez M. Polysaccharide based films and coatings for food packaging: a review. Food Hydrocolloids. 2017;68:136–148. [Google Scholar]
- 161.Jung J., Kasi G., Seo J. Development of functional antimicrobial papers using chitosan/starch-silver nanoparticles. Int. J. Biol. Macromol. 2018;112:530–536. doi: 10.1016/j.ijbiomac.2018.01.155. [DOI] [PubMed] [Google Scholar]
- 162.Rana S., Kalaichelvan P. Antibacterial activities of metal nanoparticles. Adv. Biotech. 2011;11:21–23. [Google Scholar]