Supplemental Digital Content is available in the text.
Keywords: mechanical circulatory support, left ventricular assist device, cost-effectiveness, thoracotomy, minimally invasive, bridge-to-transplant
Abstract
This study reports the first analysis regarding cost-effectiveness of left ventricular assist device (LVAD) implantation via thoracotomy. Cost-effectiveness of LVADs implanted via the traditional surgical approach of sternotomy has been improved through the years because of technological advances, along with understanding the importance of patient selection and postimplant management have on positively affecting outcomes. Given the positive clinical outcomes of the thoracotomy approach, we seek to study the cost-effectiveness of a centrifugal LVAD via this less invasive approach. We developed a Markov model. Survival and quality of life inputs (QALY) for the LVAD arm were based on data from the LATERAL clinical trial. For the Medical Management arm, survival was derived from the Seattle Heart Failure Model. The heart transplant probability was derived from INTERMACS. Survival after heart transplantation used International Society for Heart and Lung Transplantation data. Cost inputs were calculated based on Medicare data and past literature. The incremental cost-effectiveness ratio was found to be $64,632 per quality adjusted life year and $57,891 per life year in the bridge to transplant indication. These results demonstrate further improvement in the overall cost-effectiveness of LVAD therapy and confirm implantation of LVADs via a less invasive approach as being cost-effective.
Worldwide prevalence of heart failure (HF) has been increasing over the last several decades.1 Globally, more than 37.7 million people are living with HF.1 In the United States, there are currently more than 6.5 million people diagnosed with HF,2 with projections showing more than 8 million people will have HF by 2030.2,3 This is mainly because of the growing elderly population and the declining mortality because of improved management of cardiovascular disease.4,5 The increasing number of end-stage HF patients together with the limited availability of suitable organs, and the technological advances in mechanical circulatory support (MCS) devices has increased the number of ventricular assist device (VAD) implantations.6 The number of patients who will need to be supported on VADs for longer periods of time continues to increase.7
Superiority of VADs over optimal medical management (MM) in patients with advanced HF was demonstrated in 2001 in the REMATCH trial.8 Clinical outcomes have vastly improved over time. The traditional surgical approach for LVAD implantation is through a median sternotomy. However, less invasive nonsternotomy approaches have been increasingly utilized with very positive outcomes.9–12 In HVAD LATERAL, the first trial to evaluate LVAD implantation via a thoracotomy approach, freedom from disabling stroke was 98% at 1 year and survival was 87% at 2 years.12 Length of stay was also reduced by 30% for enrolled patients implanted via thoracotomy compared with enrolled patients implanted through sternotomy in the previous HVAD bridge to transplant (BTT) Continued Access Protocol (CAP) trial.12
Economic analysis of less invasive LVAD surgical procedures is limited. To our knowledge, there has been no data reporting cost-effectiveness of LVAD implantation via thoracotomy approach. LVAD cost-effectiveness outcomes have been improving through the years as technology advances. In 2004, they were reported at $804,000/QALY,13 then decreased to approximately $200,000/QALY.14–16 Given the positive clinical outcomes of the thoracotomy implantation approach, we aim to assess the cost-effectiveness of LVADs implanted via thoracotomy compared with optimal MM and heart transplantation in the BTT population.
Materials and Methods
Model Structure
A Markov model was developed to estimate the cost-effectiveness of the thoracotomy approach for implantation of a centrifugal flow LVAD in BTT patients. The model compared LVAD patients implanted via thoracotomy to optimal MM patients; heart transplantation was available for both arms. There were two basic health states, “Alive” and “Dead” (Figure 1). Additionally, there were several poststroke health states reflecting the different modified Rankin Scale (mRS) score strokes. Cycle period was 1 month. Every month, patients alive were exposed to therapy-related adverse events (AEs) and death. The model applied variable mortality rates every cycle up to 10 years postimplantation. A lifetime horizon and payer perspective were employed. The model was built in Microsoft Office Excel 365 ProPlus (version 1902). Finally, costs and benefits were discounted at 3% per annum.
Figure 1.
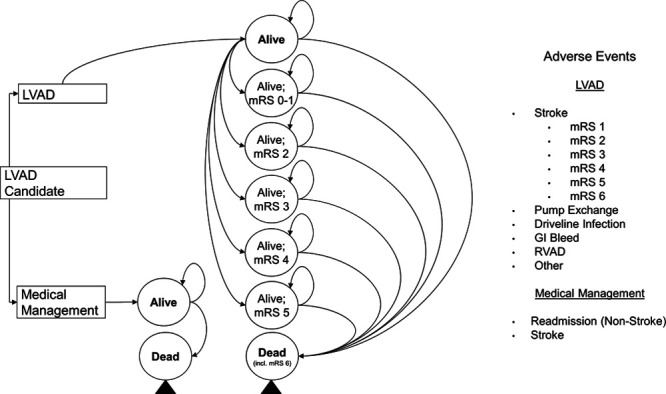
Model schematic. LVAD, left ventricular assist device; mRS, modified Rankin Scale; GI, gastrointestinal; RVAD, right ventricular assist device.
Mortality and Transition Probabilities
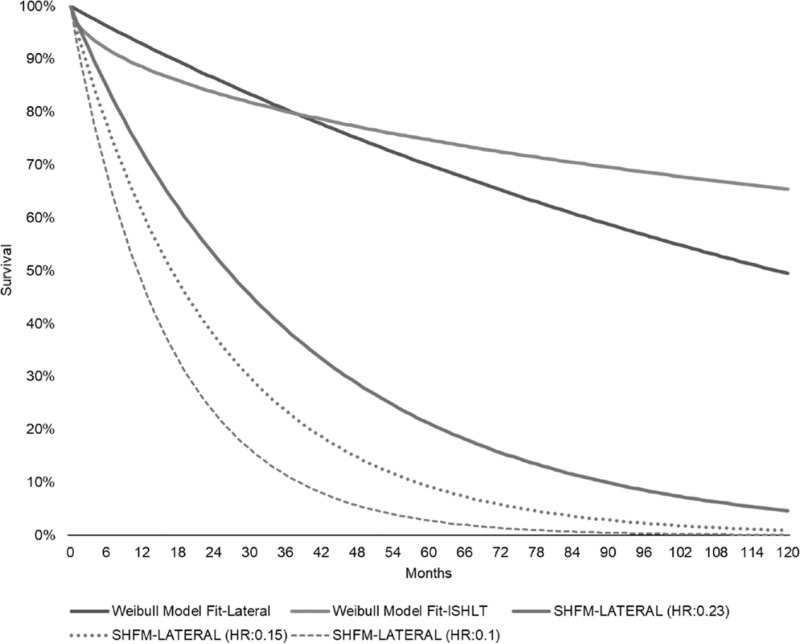
The LATERAL trial was used to populate the survival and AE rates of the LVAD arm.12 In the LATERAL trial, the lateral thoracotomy implant approach was evaluated; mean age was 54.2 (± 11.5) years, 77.1% were male, and 62.5% Caucasian12 (see Table S1, Supplementary Digital Content 1, http://links.lww.com/ASAIO/A510). The Lateral cohort consisted of typical BTT patients similar to historical baseline BTT characteristics. Considering that greater than 80% were in INTERMACS Profile 1-3, along with the inclusion of patients in cardiogenic shock, this is reflective of contemporary candidates for cardiac transplantation.12 Despite the limited access of thoracotomy for concomitant procedures, 6% of the patients underwent a concurrent procedure.12 Individual patient data from the LATERAL12 trial were used to plot time-to-death for the LVAD cohort. The International Society for Heart and Lung Transplantation17 data for continuous flow BTT VAD recipients (years: January 2005–June 2016) were employed for posttransplant survival for both cohorts. Weibull statistical models were fitted and ultimately informed predicted survival. The maximum available follow-up in the trial was used. MM survival was modeled utilizing the Seattle Heart Failure Model (SHFM) by applying the hazard ratio (HR) derived from its MM cohort, 0.23. LVAD AE rates were derived from the LATERAL12 trial and MM AE rates were derived from the literature16 and shown in Tables 1 and 2. LVAD AEs included pump exchange because of pump thrombosis or VAD failure, ischemic and hemorrhagic stroke (mRS ≥ 4 patients became transplant ineligible), driveline infection, gastrointestinal (GI) bleeding, severe right HF (requiring right ventricular assist device), and other AEs that could require hospitalization. Heart transplant rate was sourced by the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS)18 (34% at 12 months). Survival curves used in the model are shown in Figure 2. Actual and modeled LVAD survival curves are shown in Figure S1 (Supplementary Digital Content 2, http://links.lww.com/ASAIO/A510).
Table 1.
Monthly Transition Probabilities—LVAD
| Event | Rate | References |
|---|---|---|
| Stroke | 28 | |
| Ischemic | 0.005 | |
| Hemorrhagic | 0.003 | |
| Pump exchange | 28 | |
| VAD thrombus | 0.002 | |
| VAD failure | 0.001 | |
| Driveline infection | 0.010 | 28 |
| GI bleed | 0.020 | 28 |
| Severe right heart failure (requiring RVAD) | 0.0004 | 28 |
| Other adverse events | 0.005 | 28 |
| Heart transplant rate | 2.83% | 18 |
The values presented at the table are transformed monthly event rates as used in the model.
LVAD, left ventricular assist device; VAD, ventricular assist device; GI, gastrointestinal; RVAD, right ventricular assist device.
Table 2.
Monthly Transition Probabilities—Medical Management
| Event | Rate | References |
|---|---|---|
| Stroke | ||
| Ischemic | 0.002 | 29 |
| Hemorrhagic | 0.001 | 29 |
| Readmission (apart from stroke) | 0.300 | 16 |
| Heart transplant rate | 2.83% | 18 |
The values presented at the table are transformed monthly event rates as used in the model.
Figure 2.
Survival curves in the model. SHFM, Seattle Heart Failure Model; ISHLT, International Society for Heart and Lung Transplantation.
Costs
Administrative claims analyses were conducted to inform the majority of cost inputs in the model. The 100% Medicare limited data set from CY2015 to 16 were used. The Medicare cohort was extracted using the Instant Health Data (IHD) platform (BHE, Boston, MA). Sample selection and creation of analytic variables were performed using the IHD platform (BHE, Boston, MA). Statistical analyses were undertaken with R, version 3.2.1 (R Foundation for Statistical Computing, Vienna Austria) and Microsoft Office Excel 365 Plus. For all costs, hospitalizations involving biventricular assist devices were excluded. Our general costing approach was to identify hospitalizations in LVAD implanted patients and for which the primary diagnostic code matched the AE in question and other studied AE codes were absent. Sole exception was gastrointestinal bleeding for which remainder AE codes were not excluded. Strokes were identified using Medicare Severity Diagnosis Related Groups (MS-DRGs). Stroke costing included the hospitalization, the period to 90 days postevent and longer-term costs. Costs up to 90 days postevent were based on claims and after 90 days, on the literature.19 Claims were also used to assess other AE costs; for these we subtracted the cost associated with each of the explicitly modeled AEs from the total inpatient and outpatient cost over 12 months postdischarge. Outliers were managed by excluding subjects whose costs exceeded 1.96 times the standard deviation of the mean. All costs were adjusted to reflect 2018 prices either on the IHD platform or using the medical care specific CPI from the Bureau of Labor Statistics.20 The CMS 2019 DRGs were used for the LVAD/right ventricular assist device implantation and heart transplantation. Costs used in the model are presented in Table 3.
Table 3.
Costs
| Event | Cost ($) | References |
|---|---|---|
| LVAD implantation | 154,565* | |
| LVAD monthly outpatient | 3,187 | 16 |
| MM monthly outpatient | 3,515 | 16 |
| Living with LVAD > 10 years—annual | 18,377† | |
| Living on MM > 10 years—annual | 9,005† | |
| Heart transplantation | 154,565* | |
| Living after HT—annual | 16,807‡ | |
| Stroke | ||
| First 90 days | 27,904‡ | |
| Follow-up | 19 | |
| mRS 0—monthly | 956 | |
| mRS 1—monthly | 984 | |
| mRS 2—monthly | 1,138 | |
| mRS 3—monthly | 1,955 | |
| mRS 4—monthly | 3,956 | |
| mRS 5—monthly | 5,816* | |
| Pump exchange | 154,565* | |
| Driveline infection | 13,681‡ | |
| GI bleed | 9,990‡ | |
| RVAD | 78,676§ | |
| Other adverse events | 9,220‡ | |
| MM readmission (apart from stroke) | 12,934 | 16 |
All costs were adjusted to reflect 2018 prices either on the IHD platform or using the medical care–specific CPI from the Bureau of Labor Statistics.20
*CMS 2019 DRGs (i.e., 91.5% DRG 001 and 8.5% DRG 002).
†DRG 291 for cost estimation; event rate post 18-month resource use in Smedira.30
‡Medicare claims analysis.
§CMS 2019 DRG 215.
LVAD, left ventricular assist device; MM, medical management; HT, heart transplantation; mRS, modified Rankin Scale; GI, gastrointestinal; RVAD, right ventricular assist device.
Utilities
Individual patient data from contemporary HeartWare HVAD trials were used to calculate VAD-specific and thoracotomy-specific utilities.12,21–24 LATERAL,12 ADVANCE BTT+CAP,21,24 and ENDURANCE22 used EQ-5D-3L and ENDURANCE Supplemental23 used EQ-5D-5L as instruments to measure quality-of-life improvements in the trials. “Living with LVAD” utility was calculated as the average across all available timepoints in nonmajor AE patients from the LATERAL12 trial. “Living on MM” utility was based on the preimplant measurement from the ADVANCE BTT+CAP21,24 trial. AE decrements used the average before–after score difference by patient utilizing the ADVANCE BTT+CAP,21,24 ENDURANCE,22 and ENDURANCE Supplemental23 questionnaires. Utilities are presented in Table 4.
Table 4.
Utilities
| Event | Utility |
|---|---|
| Living with LVAD | 0.795 |
| Living on medical management | 0.591 |
| Living after heart transplantation | 0.795 |
| Stroke | |
| mRS 0—monthly | 0.795 |
| mRS 1—monthly | 0.795 |
| mRS 2—monthly | 0.697 |
| mRS 3—monthly | 0.691 |
| mRS 4—monthly | 0.573 |
| mRS 5—monthly | 0.573 |
| Pump exchange | |
| VAD thrombus | 0.755 |
| VAD failure | 0.559 |
| Driveline infection | 0.795 |
| GI bleed | 0.752 |
| RVAD | 0.786 |
| Other adverse events | 0.795 |
| MM readmission (apart from stroke) | 0.591 |
Sensitivity and Scenario Analyses
Besides the base–case analysis, we ran several sensitivity and scenario analyses to test uncertainty in results. One-way sensitivity analysis was performed for the major LVAD AEs including pump exchange, stroke, driveline infection, and GI bleed (minimum was 0, maximum was +100% increase from the base case values). Additionally, a probabilistic sensitivity analysis (PSA) was run where key inputs of the model were varied ±25% and 1,000 simulations were performed. For the scenario analyses, first the MM survival was lowered by using lower HRs. Second, the heart transplant rate was lowered in the model because of the recent changes on the United Network for Organ Sharing (UNOS) donor allocation criteria. Stable LVAD patients have a lower status under the new allocation system. And third, the AE rates of the LATERAL12 thoracotomy trial was substituted with the ADVANCE BTT + CAP7,21 sternotomy trial.
RESULTS
Base Case
The base case results were $ 64,632/QALY and $ 57,891 per life-year (LY). LVAD patients had higher lifetime costs and higher lifetime benefits. Total cost for the LVAD arm was $551,934 and for MM $334,117. Total LYs were 12.31 vs. 8.55 for LVAD bridged to a transplant and MM cohorts who proceeded to transplant without a LVAD, respectively. The QALYs accrued by the LVAD patients bridged to transplant were 9.77 vs. 6.40 by the MM patients who proceeded to transplant without a LVAD. Results are presented in Table 5.
Table 5.
Base–Case Results
| Costs | Life Years | QALYs | |
|---|---|---|---|
| LVAD | 551,934 | 12.31 | 9.77 |
| Medical management | 334,117 | 8.55 | 6.40 |
| Difference | 217,817 | 3.76 | 3.37 |
| ICER ($/LY) | 57,891 | ||
| ICER ($/QALY) | 64,632 |
LVAD, left ventricular assist device; ICER, incremental cost-effectiveness ratio; LY, life years; QALY, quality adjusted life years.
Sensitivity and Scenario Analyses
One-way sensitivity analysis.
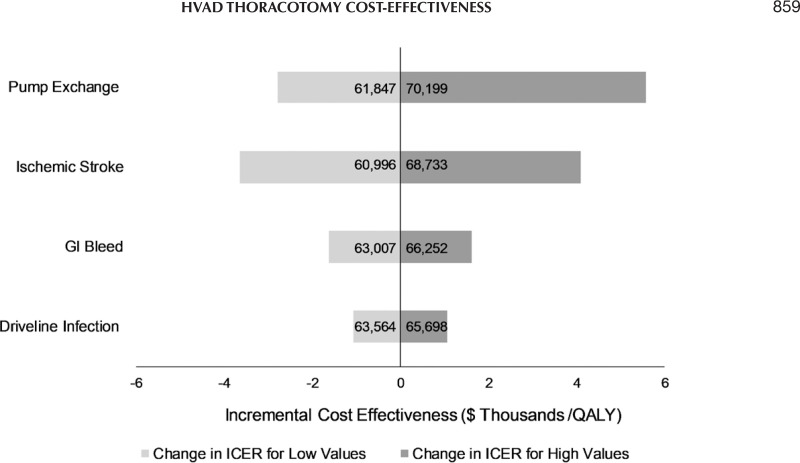
One-way sensitivity analysis was run on the main LVAD AEs. The ICER was most sensitive to pump exchange and stroke rates however less sensitive to GI bleed and driveline infections. Overall the changes in the ICER were rather low with the values varying from 6% to 9%. Results are presented in Figure 3.
Figure 3.
Tornado diagram—one-way sensitivity analysis. GI, gastrointestinal; QALY, quality adjusted life years; ICER, incremental cost-effectiveness ratio.
Probabilistic sensitivity analysis.
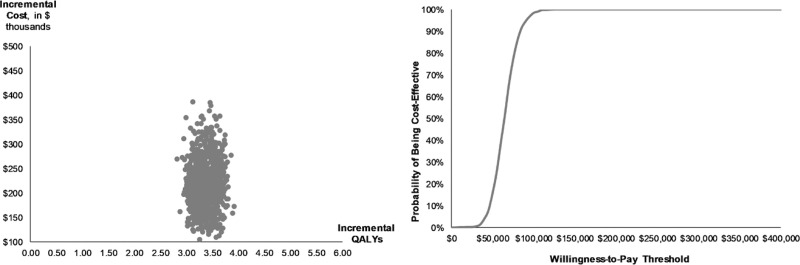
The PSA found a probabilistic ICER of $64,915/QALY (95% CI, $35,609–$94,221/QALY). ICERs were less than $50,000/QALY in 15.5% of simulations and in 98.4% less than $100,000/QALY (Figure 4).
Figure 4.
Incremental cost-effectiveness ratio scatter plot and cost-effectiveness acceptability curve (CEAC). QALY, quality adjusted life years.
Scenario analyses.
Three different scenario analyses were run. The first scenario was to run the model with lower LVAD HRs (0.15 and 0.1) than the base–case (0.23) for the MM survival. The HR of 0.15 resulted in an ICER of $61,336/QALY and the HR of 0.1 resulted in an ICER of $59,527/QALY (see Table S2–S3, Supplementary Digital Content http://links.lww.com/ASAIO/A510).
The second scenario was to lower the heart transplant rate to better capture the current situation under the revised UNOS allocation system. As the transplant rate was decreasing, the ICER was decreasing, approaching the $50,000/QALY threshold. At a transplant rate of 20% (decreased from 34% of the base-case), the ICER dropped to $58,545/QALY and at 10%, to $54,856 (see Table S4–S5, Supplementary Digital Content http://links.lww.com/ASAIO/A510).
Finally, the third scenario was to substitute the AE rates of the thoracotomy trial (LATERAL)12 with AE rates from a sternotomy trial (ADVANCE BTT + CAP)7,21 and compare the two cohorts (thoracotomy vs. sternotomy AE profile). Results showed thoracotomy was dominant over sternotomy. Patients with the thoracotomy AE rates gained more QALYs (9.77 vs. 9.42) and accrued lower costs ($551,934 vs. $572,871) than patients with the sternotomy AE rates (see Table S6, Supplementary Digital Content, http://links.lww.com/ASAIO/A510)
Discussion
This report is the first cost-effectiveness analysis of the thoracotomy approach to LVAD implantation. Previous studies have evaluated the traditional implantation surgical approach of sternotomy, with outcomes showing a positive trend in cost-effectiveness. ICERs started from $802,700/QALY in 200413 using data from the REMATCH trial8 [1998–2001, destination therapy (DT) indication] and then decreased to $198,184/QALY in 201214 using HeartMate II trial data26 (2005–2007, DT indication). In 2014, values remained similar with $201,600/QALY for the DT indication and $226,300/QALY for the BTT indication using INTERMACS data from the period 2006 to 2012.15 In 2017, there was an assessment for ambulatory patients (INTERMACS 4–7) with DT indication resulting in an ICER of $209,400/QALY (INTERMACS data 2009 to 2014).16 The current study reconfirms the positive trend and demonstrated an even lower ICER of $64,632/QALY for the thoracotomy approach in the BTT indication using HVAD LATERAL trial data (2015–2016, BTT thoracotomy).12
Drivers of these results include the use of contemporary LVAD and MM data. In the modern era, LVAD survival has been improving. The LATERAL trial demonstrated very high survival (1-year 89%, 2-year 87%) and an improved clinical profile especially in bleeding and avoidance of severe right HF complications.12 Patients were also discharged earlier from the ICU. A potential mechanism of protecting RV function is that the lateral approach reduces the distortion of cardiac geometry: The pericardium is only partially opened, and thereby position and geometry of the right ventricle (RV) are fully maintained. The potential benefit of preserving RV geometry and function leads to a limited ability to perform concomitant procedures. Aortic valve replacement can be performed via a thoracotomy approach. Mitral, tricuspid, or patent foramen ovale procedures can potentially be accomplished via an upper hemisternotomy or right thoracotomy but are more challenging. There were two aortic valve replacements, and one mitral valve repair performed in the LATERAL patient population, as well as an additional six other concomitant procedures. In addition, evolving patient management recommendations have an important role in the improved outcomes and decreased resource utilization. For example, improved blood pressure management is associated with a reduced stroke rate.23 These improved clinical outcomes lead to better economic outcomes because of lower ICU and total length of stay and/or less readmissions. Lastly, utilizing inputs from the SHFM data for the MM survival arm allows a more accurate and contemporary assessment.
The HR from SHFM was used to model survival in the MM arm. Although the most appropriate input may have been a value from a randomized clinical trial, current data are unavailable as the last clinical trial in the U.S. comparing LVAD and MM patients was REMATCH which enrolled patients ineligible for heart transplantation from 1998 to 2001.8 In the modern era, there is no longer clinical equipoise to randomize advanced HF patients to medical therapy; hence, more contemporary MM data will not likely be available. Even though a number of studies use HR ratios from REMATCH for the MM cohort survival,14,15 we decided to use a more contemporaneous HR, this of SHFM because of improved LVAD survival with the newest generation devices.
Under the revised heart allocation system since 2018, LVAD patients are status 4 and are anticipated to stay longer on the waiting list as they are lower relative priority compared with the prior allocation system.25 Newer, longitudinal data on the rate of heart transplantation under the current UNOS heart allocation system are not available yet. As such, the transplant rate used in the model is the UNOS heart allocation in place before the revision implemented in October 2018. For that reason, the sensitivity analyses included scenarios where the heart transplant rate was variably decreased. It was found that the more the transplant rate of LVAD patients decreased, the more cost-effective the LVAD implantation via thoracotomy became. This difference is amplified significantly by the superior survival demonstrated in the LATERAL trial.12
One last analysis compared two LVAD cohorts; the thoracotomy cohort and a second cohort, in which sternotomy AE rates were substituted for the thoracotomy AE rates and all other inputs were unchanged. Since less invasive implantation techniques have been associated with improved clinical outcomes, this comparison was an opportunity to define the impact this difference could have on cost-effectiveness of thoracotomy compared with sternotomy. Results showed the thoracotomy cohort was far more cost-effective and dominant over the sternotomy AE profile cohort. The thoracotomy patients accrued higher benefits (QALYs) at a lower cost. However, it is noted that the ADVANCE BTT + CAP21 trial is a much earlier trial than LATERAL12 and the model cannot account for the learning curve, technical improvements in the device or improved MM techniques.
Current changes in heart allocation were not included in the paper. It is speculated though that the new allocation system favors the use of short-term devices as BTT. Early analyses show an increase in temporary MCS device use and possible worse posttransplant clinical outcomes.25,27 Although temporary MCS is historically associated with permanent hospitalization, the costs per patient likely exceed by far the amount calculated here for LVAD therapy. More data under the new allocation rules are needed.
In this study, the cost-effectiveness of thoracotomy as an implantation strategy for a centrifugal LVAD was assessed for the first time. Progress in surgical technique as well as patient management led to greatly improved outcomes. The results show that thoracotomy BTT VAD is a cost-effective strategy. From the standpoint of survival, as well as an economic point of view, there is strong evidence to use durable LVADs as the default BTT strategy in properly selected advanced HF patients.
ACKNOWLEDGMENT
The authors would like to acknowledge Mary V. Jacoski and Alexandra Dedrick of Medtronic for their support in this project.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML and PDF versions of this article on the journal’s Web site (www.asaiojournal.com).
LATERAL, ADVANCE, ENDURANCE and ENDURANCE Supplemental (ClinicalTrials.gov Registration Numbers NCT02268942, NCT00751972, NCT01166347, NCT01966458) and this analysis were funded by Medtronic. Dr. Mahr is a consultant and investigator for Medtronic, Abbott and Abiomed, Syncardia and consultant for Carmat. Dr. Silvestry is a consultant for Medtronic and Abbott. Dr. McGee is a surgical proctor for Medtronic. Dr. Cheung is a consultant and investigator for Medtronic. Dr. Mokadam is a consultant and investigator for Medtronic, Abbott, Syncardia, and consultant for Carmat. Dr. Strueber is a consultant for Medtronic. Dr. Danter is a consultant and investigator for Medtronic, Abbott, and Atricure. Dr. Levy is a consultant for Medtronic and Abbott. Mrs. Beckman is a consultant for Medtronic, Abbott, Abiomed, and Syncardia. Damian May, Eleni Ismyrloglou, and Stelios Tsintzos are employed by Medtronic. Dr. Slaughter has no relationship with the industry.
REFERENCES
- 1.Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol 2016.13: 368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics—2018 update: A report from the American Heart Association. Circulation 2018137: e67–e492. [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States. Circ Hear Fail 2013.6: 606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011.8: 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016.37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 6.McMurray JJV, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart. Eur Heart J 2012.33: 1787–1847. [DOI] [PubMed] [Google Scholar]
- 7.Aaronson KD, Silvestry SC, Maltais S, et al. Patients awaiting heart transplantation on HVAD support for greater than 2 years. ASAIO J 2016.62: 384–389. [DOI] [PubMed] [Google Scholar]
- 8.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med 2001.345: 1435–1443. [DOI] [PubMed] [Google Scholar]
- 9.Schmitto JD, Molitoris U, Haverich A, Strueber M. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: upper hemisternotomy combined with anterolateral thoracotomy. J Thorac Cardiovasc Surg 2012.143: 511–513. [DOI] [PubMed] [Google Scholar]
- 10.Popov AF, Hosseini MT, Zych B, Simon AR, Bahrami T. HeartWare left ventricular assist device implantation through bilateral anterior thoracotomy. Ann Thorac Surg 2012.93: 674–676. [DOI] [PubMed] [Google Scholar]
- 11.Umakanthan R, Haglund NA, Stulak JM, et al. Left thoracotomy HeartWare implantation with outflow graft anastomosis to the descending aorta. ASAIO J 2013.59: 664–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGee E, Danter M, Strueber M, et al. Evaluation of a lateral thoracotomy implant approach for a centrifugal-flow left ventricular assist device: The LATERAL clinical trial. J Hear Lung Transplant 2019.38: 344–351. [DOI] [PubMed] [Google Scholar]
- 13.Special report: cost-effectiveness of left-ventricular assist devices as destination therapy for end-stage heart failure Technol Eval Cent Assess Program Exec Summ 2004. 19: 1, https://pubmed.ncbi.nlm.nih.gov/15314825/ [PubMed] [Google Scholar]
- 14.Rogers JG, Bostic RR, Tong KB, Adamson R, Russo M, Slaughter MS. Cost-effectiveness analysis of continuous-flow left ventricular assist devices as destination therapy. Circ Hear Fail 20125: 10–16. [DOI] [PubMed] [Google Scholar]
- 15.Long EF, Swain GW, Mangi AA. Comparative survival and cost-effectiveness of advanced therapies for end-stage heart failure. Circ Hear Fail 2014.7: 470–478. [DOI] [PubMed] [Google Scholar]
- 16.Baras Shreibati J, Goldhaber-Fiebert JD, Banerjee D, Owens DK, Hlatky MA. Cost-effectiveness of left ventricular assist devices in ambulatory patients with advanced heart failure. JACC Hear Fail 2017.5: 110–119. [DOI] [PubMed] [Google Scholar]
- 17.ISHTL. Adult Heart Transplantation Statistics. Available at: https://ishltregistries.org/registries/slides.asp?year=2018. Accessed June 4, 2019.
- 18.Kirklin JK, Pagani FD, Kormos RL, et al. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J Hear Lung Transplant 2017.36: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 19.Shireman TI, Wang K, Saver JL, et al. Cost-effectiveness of solitaire stent retriever thrombectomy for acute ischemic stroke. Stroke 2017.48: 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Statistics USB of L: U.S. Bureau of Labor Statistics Home Page. Available at: https://www.bls.gov/. Accessed September 27 2019.
- 21.Slaughter MS, Pagani FD, McGee EC, et al. HeartWare ventricular assist system for bridge to transplant: Combined results of the bridge to transplant and continued access protocol trial. J Hear Lung Transplant 2013.32: 675–683. [DOI] [PubMed] [Google Scholar]
- 22.Rogers JG, Pagani FD, Tatooles AJ, et al. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med 2017.376: 451–460. [DOI] [PubMed] [Google Scholar]
- 23.Milano CA, Rogers JG, Tatooles AJ, et al. HVAD: The ENDURANCE supplemental trial. JACC Hear Fail 2018.6: 792–802. [DOI] [PubMed] [Google Scholar]
- 24.Aaronson KD, Slaughter MS, Miller LW, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012.125: 3191–3200. [DOI] [PubMed] [Google Scholar]
- 25.Cogswell R, John R, Estep JD, et al. An early investigation of outcomes with the new 2018 donor heart allocation system in the United States. J Hear Lung Transplant 2020.39: 1–4. [DOI] [PubMed] [Google Scholar]
- 26.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med 2009.361: 2241–2251. [DOI] [PubMed] [Google Scholar]
- 27.Trivedi JR, Slaughter MS. “Unintended” consequences of changes in heart transplant allocation policy: Impact on practice patterns. ASAIO J 2020.6: 125–127. [DOI] [PubMed] [Google Scholar]
- 28.Internal Medtronic Data on File.
- 29.Starling RC, Estep JD, Horstmanshof DA, et al. Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: The ROADMAP study 2-Year results. JACC Hear Fail 2017.5: 518–527. [DOI] [PubMed] [Google Scholar]
- 30.Smedira NG, Hoercher KJ, Lima B, et al. Unplanned hospital readmissions after HeartMate II implantation. JACC Hear Fail 2013.1: 31–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.