Abstract
Objective:
Menopause represents a period in which bone deterioration is accelerated; thus, primary prevention strategies to address age-related bone loss are crucial. Dairy products contain more than a dozen essential nutrients, including calcium, phosphorus, vitamin D, and high-quality protein, as well as bioactive compounds that may promote bone mineralization. However, the relationship between dairy consumption and bone health across the menopause transition remains largely unknown. The purpose of this analysis was to estimate the change in lumbar spine and femoral neck bone mineral density and the risk of bone fracture by the frequency of dairy intakes among women across the menopausal transition using the publicly available data from the Study of Women's Health Across the Nation.
Methods:
We analyzed total dairy foods in four categories of <0.5, 0.5 to <1.5, 1.5 to <2.5, and ≥2.5 servings/d or <1.5 and ≥1.5 servings/d. A general linear model was used to estimate the association of dairy intake with the 10-year bone mineral density loss rate and a linear mixed model was used to estimate the annualized bone mineral density loss rate of the femoral neck and lumbar spine. A Cox proportional hazard model was applied to calculate hazard ratios and 95% confidence intervals of the nontraumatic fractures. Poisson regression was used to determine the relative risks and 95% confidence intervals of the nontraumatic fractures. The models were controlled for race/ethnicity, age, height, weight, smoking status, physical activity, alcohol consumption, calcium use, menopausal status, and total caloric intake.
Results:
No significant differences in bone mineral density change were observed, regardless of baseline menopausal status. No significant differences in the risk of nontraumatic fracture were observed.
Conclusions:
In this group of US women undergoing the menopausal transition, dairy food intake was neither associated with femoral and spine bone mineral density loss nor the risk of fractures.
Keywords: Bone density, Dairy, Fracture, Menopause, Women's health
Osteoporosis is a nonreversible, age-related progressive degenerative skeletal disease and is associated with increased susceptibility to bone fracture.1,2 Age and sex are the most proximal risk factors for osteoporosis; indeed, in the United States, 30% of women older than age 50 years have osteoporosis.3,4 Thus, modifiable risk factors for disease have become increasingly important, especially in the context of population aging.5 There is broad scientific consensus that high bone mineral density (BMD) at peak bone mass is associated with a decreased risk of osteoporotic fractures later in life.6 However, understanding strategies beyond this to reduce loss of BMD in adulthood is especially salient, especially during and after the menopause transition.7,8 The Study of Women's Health Across the Nation (SWAN) is a multicenter, multiethnic, community-based longitudinal cohort designed to examine the health of women during their middle years (ie, menopause transition)9 that has advanced our understanding of the impact of the menopause transition and midlife aging on health and well-being in women.10 In SWAN, the use of calcium dietary supplements was associated with less annualized loss of femoral neck BMD (−0.0032 vs −0.0040 g/cm2/y; P < 0.001) and lumbar spine BMD (−0.0046 vs−0.0053 g/cm2/y, P = 0.021) over a decade.11 This BMD effect was largely driven by menopausal status; women who were premenopausal at baseline were significantly protected, but there was no association among perimenopausal women. Unfortunately, no associations were observed in the risk of bone fracture in any women, regardless of menopausal status. Thus, the exploration for potential dietary factors to mitigate the risk of bone fracture and BMD loss across the menopausal transition continues.
Foods such as dairy products are universally preferred by nutrition scientists over supplements as a source of calcium because they represent complex matrices of many micronutrients that all have the potential to optimize bone.12 Dairy products provide more bone-beneficial nutrients (eg, calcium, magnesium, potassium, protein, vitamin D, etc.) per unit of energy than any other food group.13,14 The 2015 to 2020 Dietary Guidelines for Americans (DGA) recommend that adults consume three servings per day of low- or nonfat dairy products or alternatives (eg, fortified soymilk).15 However, the relevance of dairy product consumption for long-term bone health has resurged as some observational studies have suggested consumption to be associated with an increased risk of fractures.16 The recently updated Canadian Food Guide now groups milk and milk alternatives with other proteins, instead of recommending discrete servings per day.17 Given the absence of long-term clinical trial data on premenopausal women, the objective of this study was to examine dairy intake relative to bone health outcomes in the SWAN data.
METHODS
Study sample
The SWAN cohort is composed of community based, multiethnic women across the menopause transition. SWAN is one of few longitudinal data sets available and has been extensively described.9 Briefly, the SWAN bone sub-study began baseline data collection in 1996, with 3,302 pre- or early perimenopausal women aged between 42 and 53 years who had an intact uterus, at least one ovary, and no hormone usage in the last 3 months prior to screening from 5 clinical sites in the United States (Los Angeles, California; Boston, Massachusetts; Detroit, Michigan; Oakland, California; and Pittsburgh, Pennsylvania). After enrollment, women were followed annually for collection of information on demographic, clinical, and anthropometric data.
Among 2,335 women with complete data on femoral neck and/or lumbar spine BMD at baseline, those who had osteoporosis (n = 31 from self-report18; n = 17 based on BMD T-scores < 2.5 standard deviations from the referent group),1 diabetes (n = 113 from self-report; n = 37 based on fasting glucose level ≥126 mg/dL),19 and cancer (n = 47 from self-report)20 were excluded (Fig. 1). In addition, 135 women with missing or unknown menopausal status (n = 19), dairy intake (n = 99), physical activity (n = 6), or smoking status (n = 11) at baseline were also excluded. Therefore, the sample size for the annualized rate of BMD loss and fracture analyses was 1,955. To obtain the 10-year femoral neck BMD loss rate ([BMD at Visit 10 – BMD at baseline]/(BMD at baseline)×100), women who were missing data on femoral neck BMD measurement at baseline (n = 7) and at Visit 10 (n = 587), and final menstrual period (n = 252) were excluded from the 10-year BMD loss of femoral neck analysis, leaving 1,109 women. Similarly, 1,097 women were included in the 10-year BMD analysis for lumbar spine.
FIG. 1.

Flowchart of the analysis sample at baseline. BMD, bone mineral density; SWAN, Study of Women's Health Across the Nation.
Assessment of BMD and fracture
Dual-energy x-ray absorptiometry scans were performed on the Hologic QDR 2000 Bone Densitometer (Hologic Inc., Bedford, MA) for the Pittsburgh and Oakland sites and on the QDR 4500A for the other three sites to obtain BMD on both the femoral neck and lumbar spine at each annual visit. From Visit 8, the two sites that had the QDR 2000 used the QDR 4500A.21 The machine change calibration correction factors have been applied to the BMD scores. During each of 10 annual follow-up visits, the investigators used a standardized interviewer-administered questionnaire to ask participants about the number of fractures, the site for bone fractures, and the causes of fractures. Fractures at Visits 1 to 6 were completely self-reported absent of ascertainment, but fractures at Visits 7 to 10 were confirmed by review of radiology reports in medical records. Traumatic fractures and fractures that are not typically associated with osteoporosis (eg, fractures of the toe, digit, or face) were excluded from the analyses. Fractures were considered as traumatic if they occurred due to the following reasons: (1) a fall from a height >6 inches; (2) a motor vehicle accident; (3) moving fast, like running or bicycling; (4) playing sports; or (5) because something heavy fell on or struck the participant.22 Fracture history since age 20 years was also self-reported by the participants at baseline.
Dietary assessment
A modified block food frequency questionnaire (FFQ) was administered at baseline, Visit 5, and Visit 9 to collect eating habits and average use over the past year for 137 food items, including milk, cheese, yogurt, and so forth.23 The individual FFQ food subgroup variables are not publicly available, but a derived composite variable of all dairy intake is provided. The average number of dairy servings per day and total caloric intake variables were used in the analyses, and any missing FFQ data were imputed using the last observation carried forward method.24 The number of dairy servings and total caloric intakes were cumulatively averaged over follow-up.25 In detail, the number of dairy servings at baseline was used as the number of dairy servings at Visit 1 to Visit 4. An average of the number of dairy servings at baseline and Visit 5 was used at Visit 5 to Visit 8. The average of baseline, Visit 5, and Visit 9 was used at Visit 9 and Visit 10. Women were classified into 4 dairy groups based on this cumulative average dairy intake25 (<0.5 serving, between 0.5 and 1.5 servings, between 1.5 and 2.5 servings, and ≥2.5 servings). To test the robustness of the dairy exposure construct, sensitivity analyses were conducted using the average of diary intakes and total caloric intakes at baseline, Visit 5, and Visit 9 and compared to the cumulative exposure method.
Other measurements
Self-reported race/ethnicity was categorized into Black/African American, Chinese/Chinese American, Japanese/Japanese American, Non-Hispanic White, and Hispanic. No Hispanics were recruited in the SWAN bone sub-study. Age was calculated by the SWAN team based on the date of birth and the interview completion date, rounded to the next lowest integer. Self-reported recreational physical activity in comparison with other women of their own age at baseline was categorized as much less, somewhat less, the same, somewhat more, and much more. Self-reported smoking status at baseline was categorized as never smoked, former smoker, and current smoker. Height (in centimeters) and weight (in kilograms) were measured at each visit using standardized protocols; missing height and weight at baseline were imputed using the screening data. Missing weight in the follow-up visits was imputed using the last observation carried forward method.
Menopausal status was based on annual questions about bleeding patterns, current hormone use, pregnancy, breastfeeding, hysterectomy, and oophorectomy and was categorized as premenopause (bleeding in the past 3 months with the same pattern since last year), early perimenopause (bleeding in the past 3 months with decreased menstrual regularity in the past year), late perimenopause (no bleeding for 3-11 months), and postmenopause (no bleeding in the past 12 months). Final menstrual period was defined as the last menstrual date reported at the visit prior to be classified as postmenopausal.21 The cumulative days spent in the post-menopause period were constructed based on final menstrual period and the BMD measurement date at Visit 10. If a woman did not transition to postmenopause until Visit 10, then 0 was assigned to the cumulative day. Information on current use of dietary supplements containing calcium and alcohol consumption was obtained as part of a standardized annual follow-up interview questionnaire except at baseline when this information was only collected as part of FFQ. At baseline, calcium supplement use was dichotomized into nonuser (<300 mg from the FFQ data) and user (>300 mg from the FFQ data); the 300-mg criterion was selected because it represents what is likely achieved from a serving of milk or a dairy product, not a multivitamin mineral supplement that contains a typical dose of approximately 130 mg of calcium. At time points other than baseline and Visit 5, self-reported calcium supplement use was categorized into nonuser (not taking any) and user (≥1 d per week) based on the standardized questionnaire. Visit 5 supplement use was imputed using data from Visit 4 and/or Visit 6 (when Visit 4 data were missing). Self-reported alcohol consumption was categorized into consumer (self-reported drink any beer, wine, liquor, or mixed drinks from interview questionnaire or average daily servings > 0 from FFQ data) or non-consumer (self-reported did not drink in interview questionnaire or average daily servings = 0 in the FFQ data).
Statistical analysis
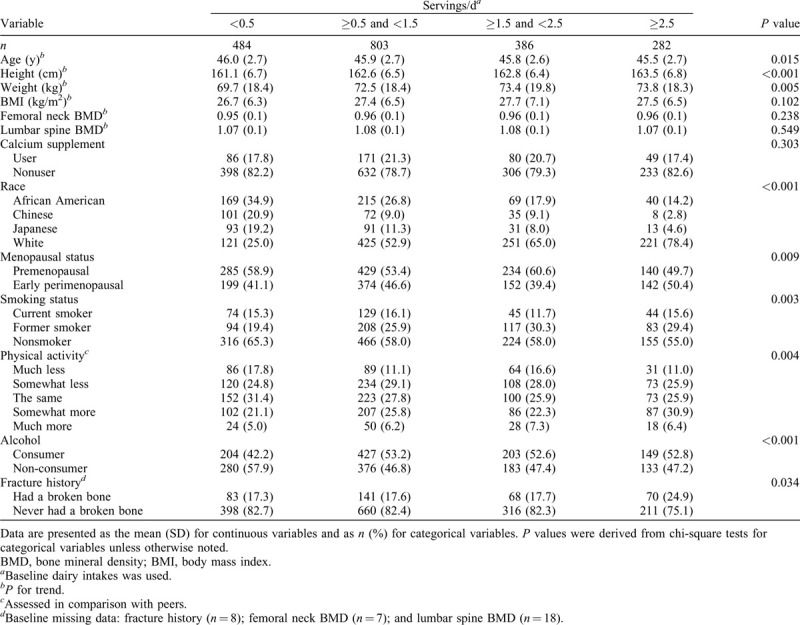
All statistical analyses were conducted using SAS 9.4 software (SAS Institute Inc., Cary, NC). Statistical significance was set conservatively at P < 0.01. Differences in participant characteristics by baseline dairy groups were tested using the linear trend tests for continuous variables (eg, age, height, weight, body mass index [BMI], and BMD) and chi-square tests for categorical variables (Table 1).
TABLE 1.
Baseline characteristics by frequency of dairy intakes (n = 1,955)
A general linear model was used to estimate the association of dairy intake with the 10-year BMD loss rate from baseline to Visit 10 [(BMD at Visit 10 – BMD at baseline)/(BMD at baseline) × 100]. Three models were constructed: the unadjusted model, model 1 (adjusted for race, baseline height, baseline age, baseline activity, baseline smoking status, baseline weight, baseline menopausal status, baseline alcohol use, baseline calcium use, baseline scanner mode, percentage weight change from baseline, and total caloric intake), and model 2 (adjusted for the cumulative days spent in the postmenopause period and all other covariates included in model 1). Log transformation was used for total caloric intake to address the right skewness of the distribution.
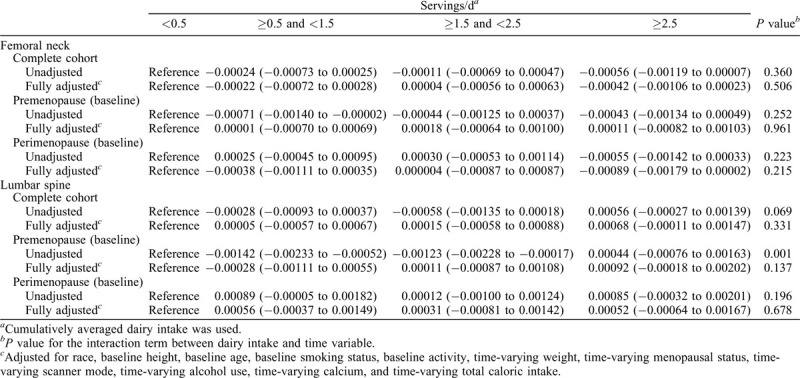
A linear mixed model for repeated measures was used to estimate the annualized BMD loss rate of the femoral neck and lumbar spine from baseline to Visit 10. The length of time in years between BMD scan date and baseline scan date was used as the time variable in the models. The interaction term between time and dairy intake groups estimates the difference in annualized BMD loss among the groups. The fully adjusted models controlled for race, baseline height, baseline age, baseline smoking status, baseline activity, time-varying weight, time-varying menopausal status, time-varying scanner mode, time-varying alcohol use, time-varying calcium use, and time-varying total caloric intake. Participants were stratified into premenopause and early perimenopause based on their baseline menopausal status. The same mixed models were applied to the stratified samples. Participants who became pregnant, were breastfeeding, entered postmenopause due to bilateral salpingo-oophorectomy, or had diabetes, cancer, or overactive/underactive thyroid or were still in pre- or early perimenopause at Visit 5 and beyond were censored from the time of report until the end of the study.
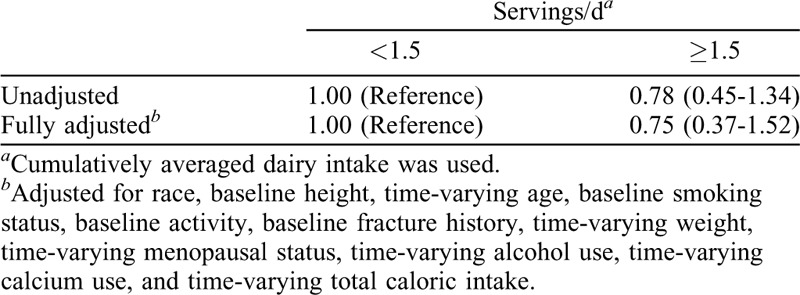
A Cox proportional hazard model was used to calculate hazard ratios (HRs) and 95% confidence intervals (95% CIs) of the first nontraumatic fractures. The survival time (in days) was defined as the fracture date or the last interview date (if no broken bone) across all of the visits from the baseline interview day. Because the exact fracture date was not recorded, it was imputed using the midpoint between the visit that reported fracture and the previous visit.26 We built three models with the same covariates as the general linear models except for excluding scanner mode and including fracture history as a covariate in model 1 and model 2. All of the covariates met the proportional hazards assumption that the HR is constant over time. Due to the small numbers of fractures, we compared the HR between the two groups (<1.5 dairy serving per day and ≥1.5 dairy serving per day)25 using the same models. In addition, a Poisson regression model with a log link function was used to determine the relative risks and 95% CIs for the nontraumatic fractures. The fully adjusted model used the same covariates as the ones in the mixed model with the exceptions that scanner mode and baseline age were excluded; and time-varying age and fracture history were included. For fracture analyses, women who became pregnant, were breastfeeding, entered postmenopause due to bilateral salpingo oophorectomy, or had diabetes, cancer, or overactive/underactive thyroid were censored from the time of report until the end of the study.11
RESULTS
Baseline characteristics of SWAN participants for BMD and fracture analyses are illustrated in Table 1. At baseline, those who consumed higher amounts of dairy at baseline were more likely to be taller, heavier, nonsmoker, alcohol consumer, and in premenopausal status at baseline and report to do “somewhat more” physical activity compared to their peers. Non-Hispanic white individuals were more likely to consume higher amounts of dairy compared to African American, Chinese, and Japanese individuals. No significant differences were observed for baseline age, BMI, femoral neck and lumbar spine BMD, calcium supplement use, or fracture history by dairy intake groups.
The mean of 10-year BMD loss rate of femoral neck and lumbar spine by dairy intake frequency is shown in Table 2. There was no significant differences across four cumulative averaged dairy intake groups, regardless of adjustment for potential confounding variables. Sensitivity analysis using the simple average of dairy intakes at baseline, Visit 5, and Visit 9 produced similar results (Supplemental Table 1). Table 3 shows the annualized BMD loss rate of femoral neck and lumbar spine from baseline to Visit 10. No significant differences were noted across four dairy intake groups.
TABLE 2.
Mean of 10-year percentage loss (95% confidence interval) of femoral neck and lumbar spine bone mineral density by frequency of dairy intakes

TABLE 3.
Annualized rate of femoral neck and lumbar spine bone mineral density loss (in g/cm/y) (95% confidence interval) by frequency of dairy intakes
During 10 years of follow-up, 64 women experienced a total of 72 nontraumatic fractures of bone related to osteoporosis. No differences in the HR and relative risks of nontraumatic fractures were observed by the frequency of cumulative averaged dairy intakes in fully adjusted models (Tables 4 and 5). In addition, no differences in HR of nontraumatic fractures were noted when using the simple average of dairy intakes (ie, the average of dairy intakes at baseline, Visit 5, and Visit 9 (Supplemental Table 2).
TABLE 4.
Hazard ratio (95% confidence interval) of non-traumatic fractures by frequency of dairy intakes
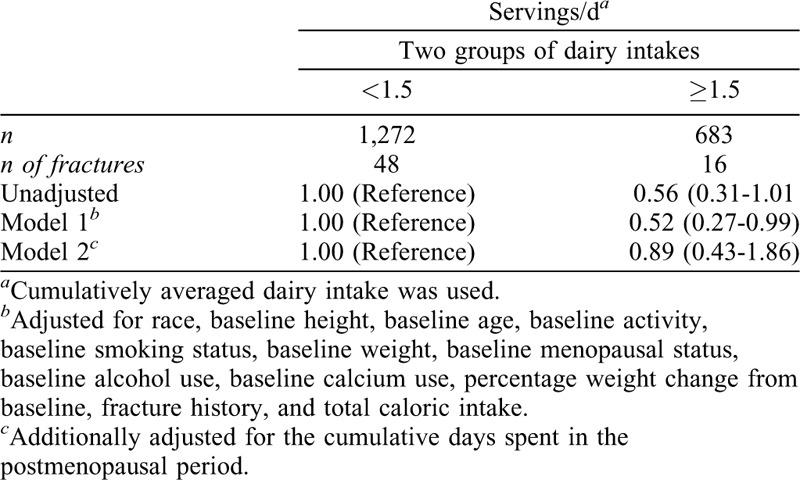
TABLE 5.
Adjusted relative risk (95% confidence interval) of nontraumatic fractures by frequency of dairy intakes
DISCUSSION
The menopause transition is a major health milestone for women, with influences that extend far beyond reproduction. Few data are available to fully appreciate whether early nutritional prevention strategies can mitigate bone loss longitudinally across the menopause transition into the postmenopausal state. Our previous work indicated a potential premenopausal critical window in regard to the effectiveness of calcium supplements.11 We did not find similar associations in this study of dairy intake. We also conducted sensitivity analyses adjusting for hormone use (eg, using birth control, estrogen, progesterone, and estrogen/progesterone pills and having estrogen injected), although hormone use was highly correlated with menopausal status, and the results remain unchanged. However, several factors should be considered when interpreting these results. First, dairy intake was low among SWAN participants, with 65% reporting consumption of <1.5 servings per day. Dairy intake was particularly low among women of races other than Non-Hispanic white; this racial disparity has been consistently suggested in the US population and could be partly attributable to lower rates of lactose intolerance among non-Hispanic whites as compared to other racial groups.27-29 Given that there were no significant differences in calcium supplement use across dairy intake groups, it is likely that dairy intakes across SWAN participants did not influence total calcium intake of participants sufficiently enough to impact overall femoral neck and lumbar spine BMD outcomes.
The benefit of dairy consumption for preserving BMD or preventing fractures has not been established. A meta-analysis of prospective cohort studies concluded that there was no significant association between milk consumption and risk of hip fracture in women.30 In a more recent meta-analysis where men and women were combined, total dairy product consumption was not significantly associated with hip fracture risk, yogurt and cheese consumption was associated with a lower risk of hip fracture, and no consistent evidence was found on the effect of milk consumption on the risk of hip fracture.31 More recently, Feskanich et al25 reported that each serving of total dairy food intake was associated with a 7% lower risk of hip fracture in women and a 6% lower risk of hip fracture in women and men combined using data from the Nurse's Health Study and the Health Professionals Follow-up Study. Although we did not find significant associations between dairy food intake and any fractures even using the same methods to calculate cumulative average dairy consumption, some differences in our study and the study by Feskanich et al25 are worth noting. Feskanich et al25 followed a large number of postmenopausal women older than age 50 years and, thus, had more statistical power to detect an association. We further excluded fractures of the toe, digit, or face that are not typically associated with osteoporosis in addition to traumatic fractures as in other SWAN bone sub-study analyses.32 Lastly, information about milk intake during teenage years that was available in the Nurse's Health Study (ie, the study by Feskanich et al25) was not available in the SWAN data.
Strengths and limitations
The major strength of this analysis is that the cohort was designed to assess changes in BMD and fractures, among other outcomes.9 Multiple clinic visits and information on many confounding variables also improved the strength of this analysis. Mean dairy intake was similar to reported intake among adult women in the US National Health and Nutrition Examination Survey.29 However, one of the limitations of this analysis is the low proportion (7%) of individuals who meet the DGA recommendations for dairy intake, as in the entire US population aged 51 to 70 years (∼2%).33 We were not able examine different types of dairy foods (eg, milk, cheese, and yogurt) separately because only a derived composite variable of total dairy intake was publicly available, and it should be noted that nutrient contents can vary between different types of dairy foods. Lack of knowledge regarding lactose intolerance among participants is also a limiting factor, as is the low number of total fractures in the cohort, making it difficult to truly assess the impact of dairy intake. Furthermore, a relatively small number of fractures were available within the SWAN cohort when compared with similar age groups at the national level using data from the National Health and Nutrition Examination Survey.34 Public-use data only extended to Visit 10 and did not include information on the study site women were attending, which could modify the results. Fractures were confirmed by medical record review starting at Visit 6, but fractures from baseline to Visit 5 were completely self-reported absent of ascertainment; however, previous comparison has shown self-reported fractures to yield a false positive in <5% of cases.35 The exact date of fracture was not asked, so it was imputed using the midpoint between the visit that reported fracture and the previous visit.36 Dietary intake (including supplemental intakes) was also self-reported by FFQ and thus may contain reporting errors; the rate of under-reporting with a FFQ was ∼28% but varied by personal characteristics such as BMI and age,37 and this FFQ information was only collected at baseline, Visit 5, and Visit 9. Although we adjusted for many possible confounders, residual confounding and missing data could have influenced the results.38 Lastly, these data do not include and may not be applicable to women of Hispanic origin or men.
CONCLUSIONS
In the SWAN longitudinal cohort, there was no evidence on beneficial effects of dairy intake on annualized rates of femoral neck and lumbar spine BMD loss or risk of fractures among middle-aged women, regardless of baseline menopausal status or method used to classify dairy intake.
Supplementary Material
Footnotes
Funding/support: Funding for this study was provided through an investigator-initiated educational grant from the National Dairy Council to Think Healthy Group. The funder had no role in the design, analysis, interpretation, or presentation of the data and results. The authors and sponsor strictly adhered to the American Society for Nutrition guiding principles for private funding for food science and nutrition research.
Financial disclosure/conflicts of interest: TCW previously served on the Scientific Advisory Board of The Vitamin Shoppe and has received research support from the National Dairy Council and scientific consulting fees from several food companies. CMW is on the scientific advisory boards of the Yogurt in Nutrition Initiative (YINI) and the US Food and Drug Administration and serves on the Board of Trustees of the International Life Sciences Institute (ILSI). She has also received grants from Tate & Lyle and DMI. RLB has served as a consultant in the past to the National Institutes of Health Office of Dietary Supplements, Nestlé, the General Mills Bell Institute, RTI International, and Nutrition Impact; and is a trustee of the International Food Information Council and a board member of ILSI North America. In the past, RLB has received travel support to present her research on dietary supplements. SJ, PZ, GPM, BAC, and JAC have no conflicts of interest to disclose.
REFERENCES
- 1.Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ, 3rd, Khaltaev N. A reference standard for the description of osteoporosis. Bone 2008; 42:467–475. [DOI] [PubMed] [Google Scholar]
- 2.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res 1994; 9:1137–1141. [DOI] [PubMed] [Google Scholar]
- 3.Wright NC, Saag KG, Dawson-Hughes B, Khosla S, Siris ES. The impact of the new National Bone Health Alliance (NBHA) diagnostic criteria on the prevalence of osteoporosis in the USA. Osteoporos Int 2017; 28:1225–1232. [DOI] [PubMed] [Google Scholar]
- 4.Siris ES, Adler R, Bilezikian J, et al. The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int 2014; 25:1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent GK, Velkoff VA. The Next Four Decades: The Older Population in the United States: 2010 to 2050. Washington, DC: US Bureau of the Census; 2010. [Google Scholar]
- 6.Weaver CM, Gordon CM, Janz KF, et al. The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int 2016; 27:1281–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002; 359:1761–1767. [DOI] [PubMed] [Google Scholar]
- 8.Ensrud KE. Epidemiology of fracture risk with advancing age. J Gerontol A Biol Sci Med Sci 2013; 68:1236–1242. [DOI] [PubMed] [Google Scholar]
- 9.Sowers MFR, Crawford SL, Sternfeld B. Lobo R, Kelsey J, Marcus R, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. Menopause: Biology and Pathobiology. San Diego, CA: Academic Press; 2000. 175–188. [Google Scholar]
- 10.El Khoudary SR, Greendale G, Crawford SL, et al. The menopause transition and women's health at midlife: a progress report from the Study of Women's Health Across the Nation (SWAN). Menopause 2019; 26:1213–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey RL, Zou P, Wallace TC, et al. Calcium supplement use is associated with less bone mineral density loss, but does not lessen the risk of bone fracture across the menopause transition: data from the Study of Women's Health Across the Nation. JBMR Plus 2019; 4:e10246.Published ahead of print October 19, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mozaffarian D. Dairy foods, obesity, and metabolic health: the role of the food matrix compared with single nutrients. Adv Nutr 2019; 10:917S–923S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heaney RP. Dairy and bone health. J Am Coll Nutr 2009; 28:82S–90S. [DOI] [PubMed] [Google Scholar]
- 14.Heaney RP. Calcium, dairy products and osteoporosis. J Am Coll Nutr 2000; 19: Suppl: 83S–99S. [DOI] [PubMed] [Google Scholar]
- 15.US Department of Health and Human Services. 2015-2020 Dietary guidelines for Americans (8th ed). Available at: https://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- 16.Michaelsson K, Wolk A, Langenskiold S, et al. Milk intake and risk of mortality and fractures in women and men: cohort studies. BMJ 2014; 349:g6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Health Canada. Welcome to Canada's food guide. Available at: https://food-guide.canada.ca/en/. [Google Scholar]
- 18.Kubota T, Namba N, Kurotobi S, et al. Beneficial effect of oral bisphosphonate treatment on bone loss induced by chronic administration of furosemide without alteration of its administration and urinary calcium loss. Clin Pediatr Endocrinol 2006; 15:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalil N, Sutton-Tyrrell K, Strotmeyer ES, et al. Menopausal bone changes and incident fractures in diabetic women: a cohort study. Osteoporos Int 2011; 22:1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guise TA. Bone loss and fracture risk associated with cancer therapy. Oncologist 2006; 11:1121–1131. [DOI] [PubMed] [Google Scholar]
- 21.Nagaraj N, Boudreau RM, Danielson ME, et al. Longitudinal changes in hip geometry in relation to the final menstrual period: Study of Women's Health Across the Nation (SWAN). Bone 2019; 122:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang PY, Gold EB, Cauley JA, et al. Triglyceride levels and fracture risk in midlife women: Study of Women's Health Across the Nation (SWAN). J Clin Endocrinol Metab 2016; 101:3297–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang MH, Norris J, Han W, et al. Development of an updated phytoestrogen database for use with the SWAN food frequency questionnaire: intakes and food sources in a community-based, multiethnic cohort study. Nutr Cancer 2012; 64:228–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCullough ML, Willett WC. Evaluating adherence to recommended diets in adults: the Alternate Healthy Eating Index. Public Health Nutr 2006; 9 (1a):152–157. [DOI] [PubMed] [Google Scholar]
- 25.Feskanich D, Meyer HE, Fung TT, Bischoff-Ferrari HA, Willett WC. Milk and other dairy foods and risk of hip fracture in men and women. Osteoporos Int 2018; 29:385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishii S, Cauley JA, Greendale GA, et al. Pleiotropic effects of obesity on fracture risk: the Study of Women's Health Across the Nation. J Bone Miner Res 2014; 29:2561–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicklas TA, Qu H, Hughes SO, et al. Self-perceived lactose intolerance results in lower intakes of calcium and dairy foods and is associated with hypertension and diabetes in adults. Am J Clin Nutr 2011; 94:191–198. [DOI] [PubMed] [Google Scholar]
- 28.Bailey RK, Fileti CP, Keith J, Tropez-Sims S, Price W, Allison-Ottey SD. Lactose intolerance and health disparities among African Americans and Hispanic Americans: an updated consensus statement. J Natl Med Assoc 2013; 105:112–127. [DOI] [PubMed] [Google Scholar]
- 29.Beydoun MA, Gary TL, Caballero BH, Lawrence RS, Cheskin LJ, Wang Y. Ethnic differences in dairy and related nutrient consumption among US adults and their association with obesity, central obesity, and the metabolic syndrome. Am J Clin Nutr 2008; 87:1914–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bischoff-Ferrari HA, Dawson-Hughes B, Baron JA, et al. Milk intake and risk of hip fracture in men and women: a meta-analysis of prospective cohort studies. J Bone Miner Res 2011; 26:833–839. [DOI] [PubMed] [Google Scholar]
- 31.Bian S, Hu J, Zhang K, Wang Y, Yu M, Ma J. Dairy product consumption and risk of hip fracture: a systematic review and meta-analysis. BMC Public Health 2018; 18:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishii S, Cauley JA, Greendale GA, et al. C-reactive protein, bone strength, and nine-year fracture risk: data from the Study of Women's Health Across the Nation (SWAN). J Bone Miner Res 2013; 28:1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krebs-Smith SM, Guenther PM, Subar AF, Kirkpatrick SI, Dodd KW. Americans do not meet federal dietary recommendations. J Nutr 2010; 140:1832–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Looker AC, Sarafrazi Isfahani N, Fan B, Shepherd JA. FRAX-based estimates of 10-year probability of hip and major osteoporotic fracture among adults aged 40 and over: United States, 2013 and 2014. Natl Health Stat Report 2013; 2017:1–16. [PubMed] [Google Scholar]
- 35.Cauley JA, Ruppert K, Lian Y, et al. Serum sex hormones and the risk of fracture across the menopausal transition: Study of Women's Health Across the Nation. J Clin Endocrinol Metab 2019; 104:2412–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Law CG, Brookmeyer R. Effects of mid-point imputation on the analysis of doubly censored data. Stat Med 1992; 11:1569–1578. [DOI] [PubMed] [Google Scholar]
- 37.Freedman LS, Commins JM, Moler JE, et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am J Epidemiol 2014; 180:172–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey RL, Sahni S, Chocano-Bedoya P, et al. Best practices for conducting observational research to assess the relation between nutrition and bone: an international working group summary. Adv Nutr 2019; 10:391–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


