Figure 1. Sampling and volunteer information for proteome microarray studies.
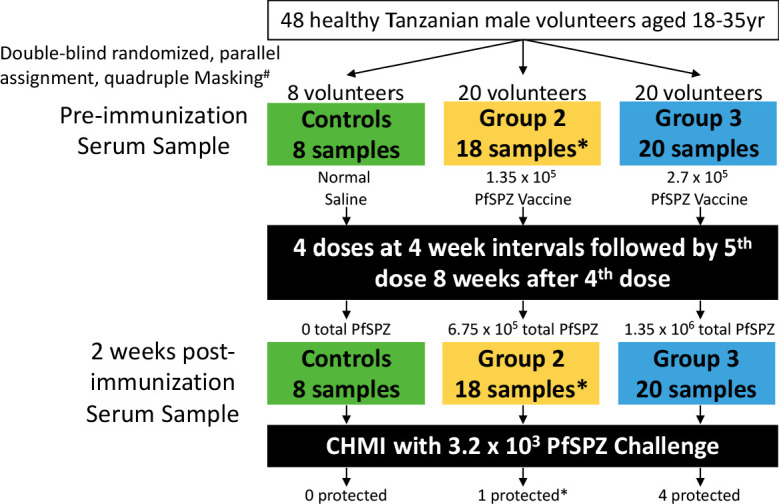
Three arms of a randomized, double-blind Phase 1 trial of PfSPZ Vaccine were selected for antibody profiling on Pf whole proteome microarrays: normal saline controls, a lower dose (group 2, 1.35 × 105 PfSPZ Vaccine/dose) and a higher dose (group 3, 2.7 × 105 PfSPZ Vaccine/dose). Serum samples were collected before immunization and 2 weeks after the final immunization. Information on the protection status of the volunteers after a 3 week post-immunization CHMI is provided. #Masking included participant, care provider, investigator and outcome assessor. *Samples were unavailable for protein array screening from two group 2 volunteers, one did not receive the 5th immunization dose and one left the country before CHMI (Jongo et al., 2019). All volunteers in the clinical trial who received 5 doses of immunization and who underwent CHMI 3 weeks after last immunization dose were included in the current analysis.
