Abstract
Background
This is an updated version of the original Cochrane review published in Issue 6, 2012.
Epilepsy is one of the most common chronic neurological disorders. Despite the plethora of antiepileptic drugs (AEDs) currently available, 30% of people continue having seizures. This group of people requires a more aggressive treatment, since monotherapy, the first choice scheme, fails to control seizures. Nevertheless, polytherapy often results in a number of unwanted effects, including neurological disturbances (somnolence, ataxia, dizziness), psychiatric and behavioural symptoms, and metabolic alteration (osteoporosis, inducement or inhibition of hepatic enzymes, etc.). The need for better tolerated AEDs is even more urgent in this group of people. Reports have suggested an antiepileptic role of melatonin with a good safety profile.
Objectives
To assess the efficacy and tolerability of melatonin as add‐on treatment for epilepsy.
Search methods
For the latest update, we searched the Cochrane Epilepsy Group's Specialized Register (12 January 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO, 12 January 2016), and MEDLINE (Ovid, 11 January 2016). We searched the bibliographies of any identified study for further references. We handsearched selected journals and conference proceedings. We applied no language restrictions. In addition, we contacted melatonin manufacturers (i.e. Nathura) and original investigators to identify any unpublished studies.
Selection criteria
Randomized controlled trials; double, single, or unblinded trials; parallel group or cross‐over studies. People with epilepsy regardless of age and gender, including children and adults with disabilities. Administration of melatonin as add‐on treatment to any AED(s) compared to add‐on placebo or no add‐on treatment.
Data collection and analysis
Review authors independently selected trials for inclusion according to pre‐defined criteria, extracted relevant data, and evaluated the methodological quality of trials. We assessed the following outcomes: at least 50% seizure reduction, seizure freedom, adverse events, and quality of life.
Main results
We included six publications, with 125 participants (106 aged under 18 years). Two different comparisons were available: melatonin versus placebo and melatonin 5 mg versus melatonin 10 mg. Despite our primary intention, due to insufficient information on outcomes, we were unable to perform any meta‐analyses, but summarized data narratively. Four studies were randomized, double‐blind, cross‐over, placebo‐controlled trials and two were randomized, double‐blind, parallel, placebo‐controlled trials. Only two studies provided the exact number of seizures during the trial compared to the baseline: none of the participants with seizures during the trial had a change in seizure frequency compared with the baseline. Two studies systematically evaluated adverse effects (worsening of headache was reported in a child with migraine under melatonin treatment). Only one study systematically evaluated quality of life, showing no statistically significant improvement in quality of life in the add‐on melatonin group.
Authors' conclusions
Included studies were of poor methodological quality, and did not systematically evaluate seizure frequency and adverse events, so that it was impossible to summarize data in a meta‐analysis. It is not possible to draw any conclusion about the role of melatonin in reducing seizure frequency or improving quality of life in people with epilepsy.
Keywords: Adolescent; Adult; Child; Child, Preschool; Humans; Infant; Young Adult; Anticonvulsants; Anticonvulsants/therapeutic use; Drug Therapy, Combination; Drug Therapy, Combination/methods; Epilepsy; Epilepsy/drug therapy; Melatonin; Melatonin/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
The use of melatonin as an adjunctive treatment for epilepsy
Background
Epilepsy is one of the most common long‐term disorders of the nervous system, and despite several antiepileptic drugs being available, 30% of people continue having seizures (fits). Reports have suggested that melatonin can work in epilepsy with a good safety profile. Melatonin is produced by the body and is prescribed by doctors to treat sleep disorders and problems such as jet lag.
Study characteristics
We searched medical databases for clinical trials of melatonin added to another antiepileptic drug (add‐on treatment) compared with antiepileptic drug plus add‐on pretend treatment (placebo) or add‐on no treatment in people with epilepsy. The participants were of any age or sex and included children and adults with disabilities. The studies measured reduction of seizure frequency by half, proportion of people with no seizures (seizure freedom), side effects, and improvement in quality of life.
Key results
We found six trials representing 125 participants for the present review. They reported two different comparisons: melatonin versus placebo and melatonin 5 mg versus melatonin 10 mg.
Included trials did not evaluate seizure frequency, seizure freedom, and adverse events in a methodical way. Only one study reported seizure frequency and none of the participants had a change in frequency occurring during the trial compared to before the trial. Only one trial evaluated the effect of melatonin on quality of life and found no improvement with add‐on melatonin compared with add‐on placebo.
Quality of the evidence
The included trials were of poor methodological quality and it was not possible to draw any definitive conclusions about the role of melatonin in reducing seizure frequency or improving the quality of life in people with epilepsy.
The evidence was current to January 2016.
Background
This review is an update of a previously published review in The Cochrane Database of Systematic Reviews (Issue 6, 2012) on 'Melatonin as add‐on treatment for epilepsy' (Brigo 2012).
Epilepsy is defined as the occurrence of at least two unprovoked epileptic seizures (Commission ILAE 1989). It is one of the most common neurological disorders: in Western countries the incidence in adults is 50/100,000 population per year (Hauser 1998) with a prevalence of 5/1000 to 10/1000 people (Goodridge 1983; McDonald 2000). In children from birth to 15 years of age, the incidence is 5/10,000 to 7/10,000 children per year and prevalence 5/1000 children, the differences being mainly due either to benign epilepsy syndromes that remit spontaneously or to severe pathologies with neurological involvement causing death.
Despite the plethora of antiepileptic drugs (AEDs) developed since the introduction of phenobarbital in 1912, 30% of people continue having seizures (Annegers 1979; Camfield 1996; Cockerell 1995; Elwes 1984; Goodridge 1983; McDonald 2000; Shorvon 1982). This group of people requires a more aggressive treatment, since monotherapy, the first choice scheme, fails to control seizures. Nevertheless, polytherapy often results in a number of unwanted effects, including neurological disturbances (somnolence, ataxia, dizziness), psychiatric and behavioural symptoms, and metabolic alteration (osteoporosis, inducement or inhibition of hepatic enzymes, etc.). The need for better tolerated AEDs is even more urgent in this group of people.
Research has suggested an antiepileptic role of melatonin, an indolamine synthesized from tryptophan in the pineal gland and released in a circadian pattern (Brzezinski 1997). In clinical practice, melatonin is used to treat sleep‐wake cycle disorders, mainly jet lag syndrome and shift worker disturbances (Herxheimer 2003; Herxheimer 2005; Revell 2006), as well as for the treatment of sleep disorders in children with neurological and developmental problems (Cortesi 2010; Weiss 2010).
Both in vitro and in vivo studies have suggested an antiepileptic activity of melatonin (Anton‐Tay 1974; Je 1996; Molina‐Carballo 1997; Mevissen 1998; Fauteck 1999), mediated by an antioxidant effect (Kabuto 1998), an increase in γ‐aminobutyric acid (GABA) concentration (Niles 1987) and GABA receptor affinity (Acuna‐Castroviejo 1986), or a reduction of the N‐methyl‐D‐aspartate (NMDA) excitatory effect (Munoz‐Hoyos 1998). In contrast, one author has reported a proconvulsant effect of melatonin (Sheldon 1998). Although studies investigating the long‐term effects of melatonin are still lacking, high melatonin doses have so far proved safe (Seabra 2000).
Objectives
To evaluate the efficacy and tolerability of melatonin as add‐on treatment for epilepsy.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Double, single, or unblinded trials.
Parallel group or cross‐over studies.
Types of participants
People with epilepsy, defined as with at least two unprovoked seizures, diagnosed by a physician, regardless of age, sex, ethnicity, and diagnosis, including children and adults with disabilities.
Types of interventions
Administration of melatonin as add‐on treatment to any AED(s) compared to add‐on placebo or no add‐on treatment.
Types of outcome measures
Primary outcomes
At least a 50% reduction in frequency of seizures of any type
The proportion of people with a 50% or greater reduction in seizure frequency during the treatment period compared to the pre‐randomization baseline period.
Seizure freedom
The proportion of people with cessation of seizures during the treatment period.
Adverse events
The proportions of people with adverse events.
Secondary outcomes
Improvement in quality of life
The proportion of people reported to have a better quality of life according to validated questionnaires (e.g. Quality of Life in Childhood Epilepsy (QOLCE), United States Quality of life in Childhood Epilepsy (USQOLCE), 36‐item Short Form (SF‐36) health profile including quality of life, etc.)
Search methods for identification of studies
Electronic searches
Searches were run for the original review in March 2008. Subsequent searches were run in May 2011, May 2012, July 2014, and January 2016.
For the latest update, we searched the following databases:
Cochrane Epilepsy Group Specialized Register (12 January 2016) using the search strategy outlined in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO, 12 January 2016) using the search strategy outlined in Appendix 2;
MEDLINE (Ovid, 1946 to 11 January 2016) using the search strategy outlined in Appendix 3.
Searching other resources
We searched the bibliographies of any included studies for further references. We handsearched selected journals and conference proceedings. We applied no language restrictions. In addition, we contacted melatonin manufacturers (i.e. Nathura) and original investigators to identify any unpublished study.
Data collection and analysis
Selection of trials
Both review authors (FB and SCI) independently assessed trials for inclusion and resolved any disagreements by discussion.
Assessment of methodological quality
Both review authors (FB and SCI) independently assessed the methodological quality of all the included studies and recorded the findings. We noted the following methodology aspects: study design, type of control, method of allocation, concealment, and completeness of follow‐up. We used preprinted selection forms to evaluate methodological quality.
Data extraction
One review author (FB) extracted the data onto a pre‐specified data extraction form, and the other review author (SCI) independently checked the data. We pilot tested the data collection forms to improve reliability. Data reported by published sources were used in this trial.
Data analysis
We extracted the following data for trials meeting our inclusion criteria.
Methodological/trial design
Method of generation of random list.
Method of concealment of randomization.
Blinding methods.
Participants' covariates
Age.
Sex.
Seizure type.
Epileptic syndrome.
Presence of neurological signs/intellectual disabilities.
Electroencephalogram.
Neuroradiology (computed tomography, magnetic resonance imaging).
Duration of disease prior to treatment.
Monotherapy versus polytherapy before randomizations.
Outcomes data
Fifty per cent or greater reduction in seizure frequency: proportion of participants with at least 50% or greater reduction in seizure frequency at the end of the study (numerator)/number of participants at pre‐randomization baseline period (denominator).
Seizure freedom: proportion of participants achieving cessation of seizures (numerator)/number of participants at pre‐randomization baseline period (denominator).
Incidence of adverse events of any type: number of adverse events (numerator)/total number of participants at pre‐randomization baseline period (denominator).
Improvement in quality of life as assessed by validated and reliable rating scales (quality of life rating scores).
Data analysis plan
We sought data on the number of participants in the treatment groups and with each outcome, irrespective of compliance or completeness of follow‐up, in the articles to undertake an intention‐to‐treat analysis.
Despite our primary intention, due to insufficient information on outcomes, we were unable to perform any meta‐analyses.
Therefore, we planned to:
extract data from the trials;
summarize efficacy (seizure frequency and seizure freedom) data narratively;
document tolerability (incidence of individual adverse events) narratively;
summarize quality of life data narratively.
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
See Figure 1.
1.
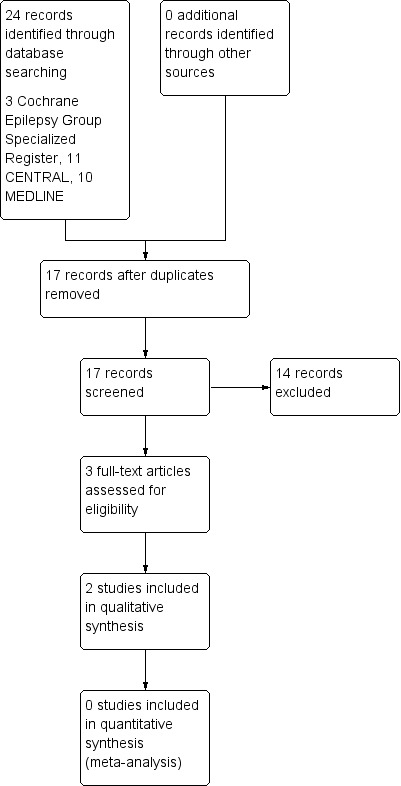
Study flow diagram. This diagram refers only to the updated version of the review.
The update of searches for this review yielded 24 results (three from Cochrane Epilepsy Group Specialized Register, 11 from CENTRAL, and 10 from MEDLINE). After removing seven duplicates, we identified 17 articles for possible inclusion. After careful evaluation of titles and abstracts, we excluded 14 articles. We added two studies (Goldberg‐Stern 2012; Jain 2015), to the four studies already included in the previous version of this review (Coppola 2004; Gupta 2004a; Gupta 2004b; Hancock 2005).
Hence, six publications with 125 participants (mostly children) fulfilled the inclusion criteria of the present review. Four publications were randomized, double‐blind, cross‐over, placebo‐controlled trials (Coppola 2004; Hancock 2005; Goldberg‐Stern 2012; Jain 2015), and two studies were randomized, double‐blind, parallel, placebo‐controlled trials (Gupta 2004a; Gupta 2004b).
Included studies
See: Characteristics of included studies table.
Coppola 2004
Coppola 2004 was a randomized, double‐blind, cross‐over, placebo‐controlled trial conducted in people aged over 12 months, with mental retardation with/without seizures, and diagnosed with wake‐sleep disorders. The study enrolled 32 participants, seven participants (28%) were lost to the study. Twenty‐five participants (16 males, nine females), aged from 3.6 to 26 years (mean 10.5 years), mostly (18/25) with epileptic seizures, completed both melatonin and placebo phases. Participants were randomized to oral synthetic fast‐release melatonin or placebo. Phase one (melatonin or placebo) lasted four weeks and, after a cross‐over period of one week, each participant entered phase two (melatonin or placebo), which lasted four weeks. Melatonin was initiated at a daily dose of 3 mg, at nocturnal bedtime. In case of inefficacy, melatonin dose could be titrated up to 9 mg during the following two weeks in increments of 3 mg/week, unless the participant was unable to tolerate it. The dose of pre‐existing medication was maintained throughout the trial. At the end of phase two, responders to melatonin entered an open‐label phase of two months. The study reported seizure freedom and seizure frequency without clearly specifying the exact number of seizures; authors stated only that out of the 11 seizure‐free participants before starting the study, nine remained unchanged on melatonin treatment. There was no overall significant change with regards to seizure control with melatonin. Melatonin was well tolerated in all participants and no adverse effects were reported.
Gupta 2004a
Gupta 2004a was a randomized, double‐blind, parallel, placebo‐controlled trial. It was conducted in children with epilepsy aged 3 to 12 years on carbamazepine monotherapy to evaluate the effects of add‐on melatonin administration on quality of life using a parental questionnaire (Sleep Behavior Questionnaire). Children had to be seizure‐free for at least the last six months before enrolment. Of the 31 children enrolled, 13 (mean age 8.3 years) randomly received add‐on melatonin, and 15 (mean age 8.1 years) received add‐on placebo. Two children in the placebo group and one child in the melatonin group were lost to follow‐up. The questionnaire was administered before add‐on melatonin/placebo and four weeks after (28 to 32 days). No data on seizure freedom or seizure frequency were reported. No adverse events warranting discontinuation of the therapy were reported.
Gupta 2004b
Gupta 2004b was a subsequent study performed by the same investigators (Gupta 2004b). It was a randomized, double‐blind, placebo‐controlled trial. It was also conducted in children with an epilepsy, aged 3 to 12 years on valproate monotherapy to evaluate the effect of add‐on melatonin on quality of life using a parental questionnaire (QOLCE). Children had to be seizure‐free for six months before enrolment. Of the 31 children enrolled, 16 (mean age 7.4 years) randomly received add‐on melatonin, and 14 (mean age 6.6 years) received add‐on placebo. One child in the placebo group was lost to follow‐up. The questionnaire was administered before add‐on melatonin/placebo and four weeks after (28 to 32 days). No data on seizure freedom or seizure frequency were reported. No adverse events warranting discontinuation of therapy were reported.
Hancock 2005
Hancock 2005 was a randomized, double‐blind, controlled, cross‐over trial investigating the response to oral melatonin using two dose regimens in people with sleep disorders associated with tuberous sclerosis complex (Hancock 2005). Eight outpatients with tuberous sclerosis complex and sleep disorder (aged 18 months to 31 years) received either 5 or 10 mg of melatonin. All participants had epilepsy and received concurrent AEDs, and all had mental retardation and behavioural difficulties. The trial consisted of following phases: an initial two‐week baseline period to confirm the sleep disorder and familiarize the participant or carer with the requirements of the trial with no treatment; a two‐week period of treatment with either 5 or 10 mg of melatonin; a two‐week washout period with no treatment; and a two‐week period of treatment with the alternative dose of melatonin (i.e. 5 mg after 10 mg or 10 mg after 5 mg). Sleep latency, total sleep time, number of awakenings, and seizure frequency were recorded in sleep and seizure diaries. There was no evidence of a dose effect between 5 and 10 mg with respect to any outcome measure. The exact number of seizures occurring during the trial, compared to baseline, was given. None of the children who had seizures during the trial had a change in seizure frequency compared with the baseline period before melatonin treatment at either dose. Trial duration (including follow‐up) was six weeks. During the study, three out of seven participants who completed the study remained seizure‐free. There were no adverse effects reported or observed. Quality of life was not considered as an outcome.
Goldberg‐Stern 2012
Goldberg‐Stern 2012 was a randomized, double‐blind, cross‐over, placebo‐controlled trial conducted in people aged four years or over with drug‐resistant epilepsy, defined as failure of three or more AEDs to control seizures. The inclusion criteria were no changes in AEDs dosage (therapeutic stability) for two months prior to the study and four or more seizures in the three weeks prior to the initiation of the study. Twelve participants (five male, seven female) aged between 9 and 32 years were included. The analysis excluded two participants because of lack of compliance in completing the diaries to assess seizure occurrence, and behaviour and sleep features. Five out of the 10 participants analyzed were younger than 18 years. All adults had childhood‐onset epilepsy. Participants were randomly assigned to receive treatment with melatonin (10 mg daily at bedtime) or placebo for three weeks (stage one). After a one‐week washout period, participants were switched to the other group (either placebo or melatonin) for three weeks (stage two). Trial duration (including follow‐up) was seven weeks. The mean number of diurnal seizures was 7.75 during placebo treatment and 4.6 during melatonin treatment (P value = 0.034). Three participants showed a decrease of 50% or greater in diurnal seizures during melatonin treatment compared to placebo. There were no adverse effects reported or observed. Quality of life was not considered as an outcome.
Jain 2015
Jain 2015 was a randomized, double‐blind, cross‐over study conducted on 11 children aged 6 to 11 years with epilepsy and without developmental delay. Participants were randomized to receive placebo or a 9 mg sustained release melatonin formulation given about 30 minutes before bedtime for four weeks, followed by a one‐week washout and a four‐week cross‐over condition. Seizure frequency was assessed by seizure diary reported by parents or carers. Thirteen participants were enrolled, 11 were randomized, and 10 (91%) completed the entire study and served the cohort for the analysis. Adherence was assessed at greater than 93% by counting tablets remaining at each treatment visit. Data were available for all 10 participants.
Four participants reported adverse events while taking melatonin (increased severity of headache in a child with history of migraine, bronchitis and ear infection, agitation, and increased urinary frequency) as compared to two participants taking placebo (agitation and increased urinary frequency).
Regarding antiepileptic efficacy, only two participants had ongoing epilepsy, although they had 50% reduction in seizure frequency with melatonin. Eight participants who were seizure‐free remained seizure‐free during the study. There was no increase in seizure frequency or difference in epilepsy frequency between melatonin and placebo.
Participants
We included six studies with 125 participants (106 participants aged under 18 years). One study was conducted in children, adolescents, and young adults with wake‐sleep disorder and mental retardation, most of them receiving chronic AED therapy (Coppola 2004). The principal investigator of Coppola 2004 indicated by mail (December 2011) that 21/25 participants who completed the study were aged under 18 years. The same group of investigators conducted two studies on children (Gupta 2004a; Gupta 2004b). When contacted by mail (29 December 2011), authors of one trial that also included adults specified that all participants enrolled (included participants lost to follow‐up) except one were aged under 18 years old (Hancock 2005). One study was conducted in children and adults with drug‐resistant epilepsy (Goldberg‐Stern 2012). One study was conducted in prepubertal children aged 6 to 11 years (Jain 2015).
One study was conducted in participants with or without epilepsy (Coppola 2004). We included this study but we analyzed only data from people with epilepsy. One study was conducted in people with tuberous sclerosis complex and sleep disorder (children and adults) (Hancock 2005). In this trial, all participants had epilepsy and were on concurrent AEDs.
Five studies compared the efficacy of melatonin versus placebo (Coppola 2004; Gupta 2004a; Gupta 2004b; Goldberg‐Stern 2012; Jain 2015), whereas Hancock 2005 compared two different doses of melatonin (5 mg versus 10 mg).
Adverse events
Only one study systematically reported adverse events (Hancock 2005).
Quality of life
Based on the protocol's requirement and the data collected from the included studies, the only validated quality‐of‐life scale adopted in the included studies was the QOLCE (Gupta 2004b). One study included the evaluation of sleep quality as an outcome; however, we excluded this trial for this outcome as the questionnaire used assessed sleep quality alone, and not the overall quality of life (Gupta 2004a).
Excluded studies
None of the seven articles obtained by the search strategy and eventually excluded (see Results of the search) appeared to meet the eligibility criteria. For studies excluded by the previous version of this systematic review please see: Brigo 2012.
Risk of bias in included studies
See: Characteristics of included studies table.
2.
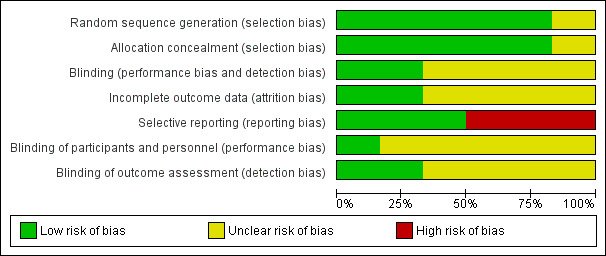
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
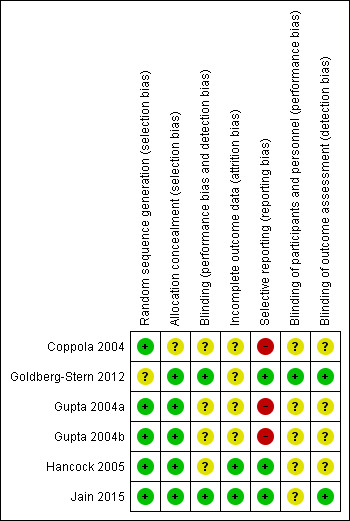
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Three trials explicitly reported and adequately performed sequence generation (Gupta 2004a; Gupta 2004b; Jain 2015). In all three studies, a statistician, not connected to the study, prepared randomization code lists and the studies used computer‐generated permutation of code numbers for the treatment groups. In three publications, the authors did not explicitly state how they created sequence generation (Coppola 2004; Goldberg‐Stern 2012; Hancock 2005). However, after contact (by mail, 29 December 2011), the principal investigators of Hancock 2005 and Coppola 2004 indicated that allocation sequence was computer generated. We also contacted the principal investigator of Goldberg‐Stern 2012 (by mail, 27 April 2015), but he provided no further information.
Allocation concealment was adequate in five trials (Gupta 2004a; Gupta 2004b; Goldberg‐Stern 2012; Hancock 2005; Jain 2015), whereas in one study it was unclear due to lack of information (Coppola 2004). In four studies, the placebo tablets were identical in shape, size, colour, and packaging (Gupta 2004a; Gupta 2004b; Goldberg‐Stern 2012; Jain 2015). In Hancock 2005, participants received identical capsules (principal investigator specified that the placebo was made by the company who supplied the melatonin). We contacted the principal investigator of the fourth study by mail (29 December 2011), but he provided no additional information to evaluate allocation concealment (Coppola 2004). All studies except Jain 2015 reported reasons for loss of follow‐up, although no study performed an intention‐to‐treat analysis. Only two studies explicitly reported or systematically evaluated the presence of adverse events (Hancock 2005; Jain 2015). In two trials, the completeness of outcome reporting was unclear: each participant received a daily diary with the instruction to record any adverse events or unusual symptoms observed immediately; however, in the results sections, the authors stated only that no adverse events warranting discontinuation of the therapy were observed (Gupta 2004a; Gupta 2004b).
Effects of interventions
There were two different comparisons available (melatonin versus placebo; melatonin 5 mg versus melatonin 10 mg), but only the melatonin versus placebo comparison included more than one study.
Comparison 1: melatonin (any dose) versus placebo
Three trials evaluated melatonin versus placebo (Coppola 2004; Gupta 2004a; Gupta 2004b). The melatonin dose varied between trials. Despite our primary intention, due to insufficient information on each outcome, we were unable to perform a meta‐analysis.
Primary outcomes
Fifty per cent or greater reduction in seizure frequency
In one study, the authors stated that seizures occurred, without further specifying the exact number of seizures occurring in each group (melatonin/placebo) (Coppola 2004). We contacted the principal investigator of this study by mail (29 December 2011), but no additional information was provided. In one trial, which selected only participants who were seizure‐free for at least six months before the beginning of the trial, all participants (13/13 receiving add‐on melatonin, 16/16 receiving add‐on placebo) remained seizure‐free, so that a 50% seizure reduction rate during the treatment period from baseline could not be calculated (Gupta 2004a). One study gave no data regarding seizure reduction (Gupta 2004b); this trial selected only participants who were seizure‐free for at least six months before the beginning of the trial, so that 50% seizure reduction during the treatment period from baseline could not be calculated. In one cross‐over placebo‐controlled study conducted in 12 participants, the mean number of diurnal seizures was 7.75 during placebo treatment and 4.6 during melatonin treatment (P value = 0.034) (Goldberg‐Stern 2012). In this trial, three participants showed a decrease of 50% or greater in diurnal seizures during melatonin treatment compared to placebo. In one study conducted in prepubertal children with epilepsy without developmental delay, only two children had ongoing seizures, although had 50% reduction in seizure frequency with melatonin (Jain 2015). There was no increase in seizure frequency or difference in epilepsy frequency observed between melatonin and placebo.
The duration of trials (including follow‐up) varied from four weeks (Gupta 2004a) to nine weeks (Coppola 2004; Jain 2015).
Seizure freedom
Three studies reported data regarding seizure freedom (Coppola 2004; Gupta 2004a; Jain 2015). We requested, but did not receive, additional data on seizure freedom by mail for the fourth included study (Gupta 2004b). In one trial, all participants (13/13 receiving add‐on melatonin, 15/15 receiving add‐on placebo) remained seizure‐free at eight week' follow‐up (Gupta 2004a). One study described seizure freedom and seizure frequency without clearly specifying the exact number of seizures; authors stated only that out of the 11 seizure‐free participants before starting the study, nine remained unchanged on melatonin treatment (Coppola 2004). They did not report the number on placebo (if any) who remained seizure free. We contacted the principal investigator of this study by mail (29 December 2011), but he provided no additional information to clarify such an aspect (Coppola 2004). In the study by Jain 2015, eight participants who were seizure‐free remained seizure‐free during the study.
Incidence of adverse events of any type
Only one study reported adverse events systematically (Jain 2015). In two trials, no adverse effects were reported (Coppola 2004; Goldberg‐Stern 2012). In two trials, the completeness of outcome reporting was unclear (Gupta 2004a; Gupta 2004b). The trial provided a daily diary for each participant with the instruction to record any adverse events or unusual symptoms observed immediately. However, in the results section the authors stated only that no adverse events warranting discontinuation of the therapy were observed. In the study by Jain 2015, four children reported adverse events while taking melatonin (increased severity of headache in a child with history of migraine, bronchitis and ear infection, agitation, and increased urinary frequency) as compared to two participants taking placebo (agitation and increased urinary frequency); the authors of the study considered only the worsening of headache in the child with migraine to be related to melatonin treatment.
Secondary outcomes
Improvement in quality of life
Only one study systematically evaluated quality of life, showing no statistically significant improvement in quality of life in either group (valproic acid plus melatonin group: intragroup P value = 0.08; valproic acid plus placebo group: intragroup P value = 0.16) (Gupta 2004b). The authors performed no intergroup statistical evaluation between valproic acid plus melatonin and valproic acid plus placebo.
One study evaluated sleep quality alone, without focusing on global quality of life (Gupta 2004a). One study did not provide quantitative or validated data (Coppola 2004). The authors stated only that "half the parents/caregivers preferred improved behavior and alertness in children who appeared more quiet and better disposed to rehabilitation treatment; familial environment improved concomitantly, as a consequence of a better quality of night time" (Coppola 2004).
Comparison 2: melatonin 5 mg versus melatonin 10 mg
One study evaluated melatonin 5 mg versus melatonin 10 mg (Hancock 2005).
Primary outcomes
Fifty per cent or greater reduction in seizure frequency
The study reported the exact number of seizures occurring during the trial, compared to the baseline. None of the four participants who had seizures during the trial (on either melatonin 5 mg or 10 mg) had a 50% seizure reduction during the treatment (on either melatonin 5 mg or 10 mg) period compared to baseline. The duration of the trial (including follow‐up) was six weeks.
Seizure freedom
During the study three out of seven participants who completed the study remained seizure‐free (on either melatonin 5 mg or 10 mg).
Incidence of adverse events of any type
The study authors asked the carers to record any illness the child had or any possible adverse effects experienced during the trial period. No adverse events were reported.
Secondary outcomes
Improvement in quality of life
The study did not report improvement in quality of life.
Discussion
This systematic review included six trials (two studies (Goldberg‐Stern 2012; Jain 2015) were added to the four RCTs already included in the previous version of the review (Brigo 2012)). They did not systematically evaluate seizure frequency and adverse events, so it was impossible to summarize data in a meta‐analysis. Only two studies systematically examined seizure frequency occurring during the trial, compared to baseline (Hancock 2005; Jain 2015). With the exception of two trials (Hancock 2005; Jain 2015), none of the included trials systematically evaluated seizure freedom and adverse events. Two trials selected only people who were seizure‐free for at least six months before the beginning of the trial, so that 50% seizure reduction during the treatment period from baseline could not be calculated (Gupta 2004a; Gupta 2004b). From a clinical point of view, the reported follow‐up duration of the included studies was not long enough to evaluate the antiepileptic efficacy of add‐on melatonin, maybe because none of the trials was primarily designed to evaluate seizure freedom/reduction. Nevertheless, the relative high number of seizure‐free participants reported in three included studies may be attributed to the fact that a large proportion of them was already recruited as seizure‐free (three out of seven participants on either melatonin 5 mg or 10 mg in Hancock 2005, and all participants in Gupta 2004a and Gupta 2004b). Conversely, in one study conducted in 12 people with drug‐resistant epilepsy only three people experienced a decrease of at least 50% in diurnal seizures and no person was reported to be seizure free during melatonin treatment compared to placebo (Goldberg‐Stern 2012). Only two studies explicitly reported the presence of adverse events (Hancock 2005, none was reported; Jain 2015), and only one trial evaluated the direct influence of melatonin on quality of life showing to significant improvement (Gupta 2004b).
Five out of six studies included in the present review were primarily aimed to evaluate the effect of melatonin on wake‐sleep disorders, thus not focusing on efficacy and tolerability of add‐on melatonin as treatment for epilepsy. The fact that primary outcomes evaluated in most studies did not focus on seizure control is responsible for the lack of information regarding the efficacy of melatonin as add‐on treatment for epilepsy. As a consequence of this lack of data, it was not possible to draw definite conclusions concerning efficacy and tolerability of melatonin as add‐on treatment for epilepsy. Furthermore, it was impossible to establish whether a possible beneficial role of melatonin in epilepsy treatment was direct or indirect (i.e. seizure reduction as a consequence of an improvement in sleep quality).
Authors' conclusions
Implications for practice.
It was not possible to draw any conclusions about the role of add‐on melatonin in reducing seizure frequency or improving the quality of life or about the safety profile of this drug in people with epilepsy.
Implications for research.
Further studies, especially large, well‐conducted, randomized clinical trials with adequate follow‐up, are required before reaching a definite conclusion concerning the efficacy and tolerability of melatonin as add‐on treatment in people with epilepsy. Studies should systematically evaluate and report adverse events.
What's new
| Date | Event | Description |
|---|---|---|
| 11 August 2016 | Amended | Authorship amended. |
| 12 January 2016 | New search has been performed | Searches were updated on 12 January 2016. |
| 12 January 2016 | New citation required but conclusions have not changed | Two new studies have been added (Goldberg‐Stern 2012; Jain 2015); conclusions remain unchanged. |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 6, 2012
| Date | Event | Description |
|---|---|---|
| 31 July 2014 | New search has been performed | Searches were updated on 31 July 2014 |
| 31 July 2014 | New citation required but conclusions have not changed | One new study added; conclusions remain unchanged. |
Acknowledgements
We wish to acknowledge the hard and valuable work that went in to the original version of the review by Alessandra del Felice (Brigo 2012).
Appendices
Appendix 1. Cochrane Epilepsy Group Specialized Register search strategy
#1 MeSH DESCRIPTOR Melatonin Explode All
#2 melatonin*
#3 MT6 OR "Mela‐T" OR Melatol OR Melatonex OR Melovine OR Regulin OR (Night NEXT Rest) OR Circadin
#4 #1 OR #2 OR #3
#5 >30/07/2014:CRSCREATED
#6 #4 AND #5
Appendix 2. CENTRAL via CRSO search strategy
#1 ("5 methoxy n acetyltryptamine" OR circadin OR MT6 OR "mela t" OR melatol OR Melatonex OR Melovine OR "n 2 5 methoxyindol 3 yl ethyl acetamide" OR "n acetyl 5 methoxytryptamine" OR "nature s harmony" OR (Night NEXT Rest) OR Regulin OR "revital melatonin" OR "rx balance" OR "sleep right" OR vivitas):TI,AB,KY
#2 MESH DESCRIPTOR Melatonin EXPLODE ALL TREES
#3 melatonin*:TI,AB,KY
#4 #1 OR #2 OR #3
#5 (epilep* OR seizure* OR convuls*):TI,AB,KY
#6 MESH DESCRIPTOR Epilepsy EXPLODE ALL TREES
#7 MESH DESCRIPTOR Seizures EXPLODE ALL TREES
#8 #5 OR #6 OR #7
#9 #4 AND #8
#10 30/06/2014 TO 29/02/2016:DL
#11 #9 AND #10
Appendix 3. MEDLINE (Ovid) search strategy
This strategy was based on the Cochrane Highly Sensitive Search Strategy for identifying randomized trials (Lefebvre 2011).
1. exp Melatonin/
2. melatonin$.tw.
3. (MT6 or Mela‐T or Melatol or Melatonex or Melovine or Regulin or (Night adj1 Rest) or Circadin).tw.
4. 1 or 2 or 3
5. exp Epilepsy/
6. exp Seizures/
7. (epilep$ or seizure$ or convuls$).tw.
8. 5 or 6 or 7
9. exp *Pre‐Eclampsia/ or exp *Eclampsia/
10. 8 not 9
11. (randomized controlled trial or controlled clinical trial).pt. or (randomi?ed or placebo or randomly).ab.
12. clinical trials as topic.sh.
13. trial.ti.
14. 11 or 12 or 13
15. exp animals/ not humans.sh.
16. 14 not 15
17. 4 and 10 and 16
18. remove duplicates from 17
19. limit 18 to ed=20140714‐20160111
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Coppola 2004.
| Methods | Randomized, double‐blind, cross‐over, placebo‐controlled trial | |
| Participants | Inclusion criteria: mental retardation with/without epileptic seizures; aged > 12 months; diagnosis of sleep disorder, defined according to DSM‐IV criteria as the circadian rhythm sleep disorder; exclusion of medical issues such as gastro‐oesophageal reflux, pain, or epileptic seizures mimicking sleep disorders; persisting sleep disturbances despite maintaining appropriate sleep hygiene; informed consent by parents, carers, or both Exclusion criteria: progressive neurological, systemic, or both, diseases; aged < 12 months; poor compliance from parents/carers with the study requirements before trial entry N: 32 (7 lost to follow‐up); 25 completed the trial M: 16; F: 9 Age: 3.6‐26 years (mean age 10.5 years) 18/25 participants had epilepsy and on concurrent AEDs Type of seizures: complex partial (8), with secondary generalization (5), tonic‐clonic (4), tonic (3), drop‐attacks (2), atypical absences (1), myoclonic seizures (2) Type of epilepsy: partial epilepsy (9); generalized symptomatic (5) or cryptogenic (1) epilepsy; multifocal epileptic encephalopathy (3). A genetic syndrome was diagnosed in 5 participants (20%); they were the following: Angelman syndrome; Saethre‐Chotzen syndrome; 11p13 microdeletion; Leber amaurosis; CHARGE syndrome Concurrent AED treatment: monotherapy (11), bi‐therapy (2;), and tri‐therapy (5) Seizure frequency: seizure‐free (7); sporadic (3); 1‐3/month (3); > 1/week (2); > 1/day (3) Duration of the trial (including follow‐up): 9 weeks; phase 1 (melatonin or placebo): 4 weeks, cross‐over period: 1 week, phase 2 (placebo or melatonin): 2 weeks. |
|
| Interventions | Melatonin (fast release) compared with placebo (see text for more details) | |
| Outcomes | Seizure freedom/seizure frequency: out of the 11 seizure‐free participants before starting the study, 9 remained unchanged on melatonin; in the other 2, seizures re‐appeared after 1 month (1 Lennox‐Gastaut syndrome; 1 partial seizures). Soon after discontinuing melatonin, seizures stopped in both these participants. Among the 7 participants with uncontrolled seizures during the baseline phase, 1 became seizure‐free (1 secondarily generalized partial seizure), 2 partially improved (1 partial seizure; 1 myoclonic seizure), and the other 2 were unchanged; the remaining 2 showed a seizure worsening 1 month after starting melatonin phase (1 cryptogenic generalized seizure; 1 secondary generalized partial seizure). In the latter participants, seizures decreased soon after melatonin withdrawal Adverse events: none reported Quality of life: not systematically evaluated. Authors stated that "half the parents/caregivers referred improved behavior and alertness in children who appeared more quiet and better disposed to rehabilitation treatment; familial environment improved concomitantly, as a consequence of a better quality of night time" |
|
| Notes | Not all participants had epilepsy Seizure diaries were used to monitor the frequency and type of seizures Reasons for lost to follow‐up (7 participants) were reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated permutation of code numbers for the treatment groups (information provided directly from the principal Investigator) |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not explicitly reported (insufficient information to permit judgement) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported missing to follow‐up. No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Authors stated that seizures occurred, without further specifying the exact number of seizures. Adverse events not systematically evaluated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not explicitly reported (insufficient information to permit judgement) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not explicitly reported (insufficient information to permit judgement) |
Goldberg‐Stern 2012.
| Methods | Randomized, double‐blind, cross‐over, placebo‐controlled trial | |
| Participants | Inclusion criteria: aged ≥ 4 years; failure of ≥ 3 different AEDs to control seizures; therapeutic stability for 2 months prior to the study; (iv) ≥ 4 seizures in the 3 weeks prior to the initiation of the study Exclusion criteria: children with vasculitis, cardiovascular anomalies, or blindness N: 12 M: 5; F: 7 Age: 9‐32 years (range) All participants had epilepsy and received AEDs Seizure type: symptomatic generalized (4); symptomatic partial (1); symptomatic partial with secondary generalization (1); cryptogenic partial with secondary generalization (3); idiopathic partial (1) Duration of the trial (including follow‐up): 7 weeks |
|
| Interventions | Melatonin (10 mg daily at bedtime) compared with placebo (see text for more details) | |
| Outcomes | Seizure freedom/seizure frequency: mean number of diurnal seizures was 7.75 during placebo treatment and 4.6 during melatonin treatment. Three participants showed a decrease of ≥ 50% in diurnal seizures during melatonin treatment compared to placebo Adverse events: none reported Quality of life: not systematically evaluated |
|
| Notes | 5/10 participants analyzed were aged < 18 years. All adults had childhood‐onset epilepsy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors did not specify how randomization was performed. Randomization was made by a pharmacist, not connected to the study |
| Allocation concealment (selection bias) | Low risk | Placebo and melatonin tablets identical in size and appearance |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo and melatonin tablets were identical in size and appearance |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants were excluded from the analysis because of lack of compliance in completing the diaries to assess seizure occurrence, and behaviour and sleep features. No further information on these 2 participants were reported |
| Selective reporting (reporting bias) | Low risk | All data regarding outcomes of interest for this systematic review were reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo and melatonin tablets were identical in size and appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo and melatonin tablets were identical in size and appearance |
Gupta 2004a.
| Methods | Randomized, double‐blind, parallel, placebo‐controlled trial | |
| Participants | Inclusion criteria: children of either sex, aged 3‐12 years, carbamazepine monotherapy, confirmed diagnosis of epilepsy according to the International Classification of Epileptic Seizures, seizure‐free for ≥ 6 months Exclusion criteria: children with a history of psychiatric or other progressive neurological disorder, or a chronic haematological, cardiac, hepatic, renal, or thyroid disorder N: 31 (3 lost to follow‐up) M: 21; F: 7 Type of seizures: complex partial seizures (19); generalized tonic‐clonic seizures (6); simple partial seizures (3) Concurrent AED treatment: carbamazepine monotherapy Seizure frequency: all participants were seizure free for ≥ 6 months Duration of the trial (including follow‐up): 8 weeks |
|
| Interventions | Melatonin (fast release) compared with placebo (see text for more details) | |
| Outcomes | Seizure freedom: participants were followed up clinically for 8 weeks during which all participants remained seizure free Adverse events: authors stated that "no adverse event warranting discontinuation of the therapy was observed" Quality of life: not evaluated systematically. Sleep quality after add‐on melatonin administration was assessed by a parental questionnaire, the SBQ |
|
| Notes | Reasons for lost to follow‐up (3 participants) were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization code list prepared by a statistician, not connected to the study. Computer‐generated permutation of code numbers for the treatment groups |
| Allocation concealment (selection bias) | Low risk | Placebo and melatonin tablets were identical in shape, size, colour, and packaging |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not explicitly reported (insufficient information to permit judgement) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing to follow‐up reported (the reason for 2 lost to follow‐up not reported). No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Each participant had a daily diary to record any adverse events or unusual symptoms observed immediately. In results section, authors only stated that "no adverse event warranting discontinuation of the therapy was observed" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not explicitly reported (insufficient information to permit judgement) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not explicitly reported (insufficient information to permit judgement) |
Gupta 2004b.
| Methods | Randomized, double‐blind, parallel, placebo‐controlled trial | |
| Participants | Inclusion criteria: children of either sex, aged 3‐12 years, valproate monotherapy, confirmed diagnosis of epilepsy according to the International Classification of Epileptic Seizures, seizure free for ≥ 6 months Exclusion criteria: children with a history of psychiatric or other progressive neurological disorder, or a chronic haematological, cardiac, hepatic, renal, or thyroid disorder N: 31 (1 lost to follow‐up) M: 18; F: 12 Type of seizures: absence (8); complex partial (5); generalized tonic‐clonic seizures (14); Lennox‐Gastaut syndrome (3) Concurrent AED treatment: valproate monotherapy. Participant on sodium valproate (10 mg/kg/day) monotherapy for the last 6 months, and, at the time of inclusion in the study, with serum blood levels in the range 75‐125 μg/mL Seizure frequency: all participants were seizure free for at ≥ 6 months Duration of the trial (including follow‐up): 8 weeks |
|
| Interventions | Melatonin (fast release) compared with placebo (see text for more details) | |
| Outcomes | Adverse events: authors state that "no adverse event warranting discontinuation of the therapy was observed" Quality of life: assessed by the QOLCE questionnaire. Valproic acid + melatonin group: intragroup P value = 0.08; valproic acid + placebo group: intragroup P value = 0.16 |
|
| Notes | Reasons for lost to follow‐up (1 participant) were reported Authors performed no intergroup statistical evaluation between valproic acid + melatonin and valproic acid + placebo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization code list prepared by a statistician, not connected to the study. Computer‐generated permutations of code numbers for the treatment groups |
| Allocation concealment (selection bias) | Low risk | Placebo and melatonin tablets were identical in shape, size, colour, and packaging |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not explicitly reported (insufficient information to permit judgement) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing to follow‐up reported. No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Each participant has a daily diary to record any adverse events or unusual symptoms observed immediately. In results section, authors only state that "no adverse event warranting discontinuation of the therapy was observed" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not explicitly reported (insufficient information to permit judgement) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not explicitly reported (insufficient information to permit judgement) |
Hancock 2005.
| Methods | Randomized, double‐blind, cross‐over, controlled trial | |
| Participants | Inclusion criteria: confirmed diagnosis of tuberous sclerosis complex and sleep problems (Quine Sleep Index score of ≥ 6 out of a possible 8) Exclusion criteria: situational sleep disorder (a higher Quine score at home with a score of < 6 elsewhere) N: 8 (1 lost to follow‐up) M: 4; F: 3 Age: 18 months to 31 years (median age 9 years) All participants had epilepsy and on concurrent AEDs; 2 participants were well controlled All had mental retardation and behavioural difficulties Duration of the trial (including follow‐up): 6 weeks |
|
| Interventions | Melatonin 5 mg vs. melatonin 10 mg (see text for more details) | |
| Outcomes | Seizure frequency: 4 participants had seizures during the trial, without change in the frequency (or type) of seizures compared with the baseline period before melatonin treatment at either dose Seizure freedom: 3 participants with well‐controlled epilepsy remained seizure‐free during the trial Adverse events: none reported |
|
| Notes | Seizure type was not reported Concurrent AEDs not reported Seizure diaries were used to monitor the frequency and type of seizures Reasons for lost to follow‐up (1 participant) reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation made by the pharmacy using random number sequences |
| Allocation concealment (selection bias) | Low risk | Participants received identical capsules |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not explicitly reported (insufficient information to permit judgement) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All data regarding outcomes of interest for this systematic review reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not explicitly reported (insufficient information to permit judgement) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not explicitly reported (insufficient information to permit judgement) |
Jain 2015.
| Methods | Randomized, double‐blind, cross‐over, placebo‐controlled trial. | |
| Participants | Inclusion criteria: prepubertal (Tanner stage I) 6‐ to 11‐year‐old children with epilepsy, with normal development based on school placement (in appropriate grade based on age) and developmental history or intelligence quotient (IQ) > 70. Combined score of 30 or more on sleep fragmentation, parasomnia, and daytime drowsiness subscales assessed with a sleep behaviour questionnaire Exclusion criteria: history of loud snoring, diagnosis of obstructive sleep apnoea (obstructive apnoea‐hypopnoea index > 2/hour), or PLM disorder (PLM index > 5/hour) on polysomnography; people with vagus nerve stimulator, history of a major psychiatric disease, pervasive developmental disorder, severe neurodevelopmental disabilities, immune disorders, or lymphoproliferative disorders. Concurrent use of hypnotics, stimulants, systemic corticosteroids or other immunosuppressants, or a history of using slow‐release melatonin N: 11 (7 lost to follow‐up); 10 completed the trial M: 7; F: 3 Age (mean ± standard deviation): 8.4 ± 1.3 years All participants had epilepsy and on concurrent AEDs Type of epilepsy: focal epilepsy (7, 3 had benign epilepsy with centrotemporal spikes); generalized (2, both with childhood absence epilepsy); undetermined (1). Duration of the trial: 9 weeks |
|
| Interventions | Melatonin 9 mg sustained‐release compared with placebo (see text for more details) | |
| Outcomes | Seizure freedom/seizure frequency: only 2 participants had ongoing, although had 50% reduction in seizure frequency on melatonin. 8 participants who were seizure‐free remained seizure‐free during the study. No increase in seizure frequency or difference in epilepsy frequency was observed between melatonin and placebo Adverse events: 4 participants reported adverse events while taking melatonin (increased severity of headache in a child with history of migraine, bronchitis and ear infection, agitation, increased urinary frequency) as compared to 2 participants (reporting agitation, increased urinary frequency) on placebo Quality of life: not systematically evaluated |
|
| Notes | Seizure diaries used to monitor frequency and type of seizures Reasons for lost to follow‐up (1 participant) not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number generators used |
| Allocation concealment (selection bias) | Low risk | Over‐encapsulation of both melatonin and placebo used to have the same appearance |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and people involved in the study (except for the pharmacy and the statistician) were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant lost to follow‐up for not reported reasons |
| Selective reporting (reporting bias) | Low risk | All data regarding outcomes of interest for this systematic review were reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and people involved in the study (except for the pharmacy and the statistician) were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and people involved in the study (except for the pharmacy and the statistician) were blinded |
AED: antiepileptic drug; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, 4th edition; F: female; M: male; N: number; PLM: periodic limb movement; QOLCE: Quality of Life in Childhood Epilepsy; SBQ: Sleep Behavior Questionnaire.
Contributions of authors
Francesco Brigo and Stanley C Igwe were responsible for this update.
Both review authors assessed the studies for inclusion and extracted the data from the individual studies.
Francesco Brigo wrote the text of the final review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding for the Epilepsy Group. The views and opinions expressed therein are those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Coppola 2004 {published data only}
- Coppola G, Iervolino G, Mastrosimone M, Torre G, Ruiu F, Pascotto A. Melatonin in wake‐sleep disorders in children, adolescents and young adults with mental retardation with or without epilepsy: a double‐blind, cross‐over, placebo‐controlled trial. Brain Development 2004;26(6):373‐6. [DOI] [PubMed] [Google Scholar]
Goldberg‐Stern 2012 {published data only}
- Goldberg‐Stern H, Oren H, Peled N, Garty BZ. Effect of melatonin on seizure frequency in intractable epilepsy: a pilot study. Journal of Child Neurology 2012;27(12):1524‐8. [DOI] [PubMed] [Google Scholar]
- Goldberg‐Stern H, Oren H, Shuper A, Peled N, Garty BZ. Melatonin in intractable epilepsy: a possible positive effect. Epilepsia 2010;51(Suppl 4):100. [Google Scholar]
Gupta 2004a {published data only}
- Gupta M, Gupta YK, Aneja AS, Kohli K. Effects of add‐on melatonin on sleep in epileptic children on carbamazepine monotherapy: a randomized placebo controlled trial. Sleep and Biological Rhythms 2004;2:215‐9. [Google Scholar]
Gupta 2004b {published data only}
- Gupta M, Aneja S, Kohli K. Add‐on melatonin improves quality of life in epileptic children on valproate monotherapy: a randomized, double‐blind, placebo‐controlled trial. Epilepsy & Behavior 2004;5:316‐21. [DOI] [PubMed] [Google Scholar]
Hancock 2005 {published data only}
- Hancock E, O'Callaghan F, Osborne JP. Effect of melatonin dosage on sleep disorder in tuberous sclerosis complex. Journal of Child Neurology 2005;20(1):78‐80. [DOI] [PubMed] [Google Scholar]
Jain 2015 {published data only}
- Jain S, Horn P, Simakajornboon N, Holland K, Glauser T. Melatonin improves sleep in children with epilepsy: results from a randomized, double‐blind, placebo‐controlled, cross‐over study. Epilepsy Currents 2014;14(Suppl 1):442. [Google Scholar]
- Jain SV, Horn PS, Simakajornboon N, Beebe DW, Holland K, Byars AW, et al. Melatonin improves sleep in children with epilepsy: a randomized, double‐blind, crossover study. Sleep Medicine 2015;16(5):637‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Acuna‐Castroviejo 1986
- Acuna‐Castroviejo D, Lowenstein PR, Rosenstein R, Cardinali DP. Diurnal variations of benzodiazepine binding in rat cerebral cortex: disruption by pinealectomy. Journal of Pineal Research 1986;3(2):101‐9. [DOI] [PubMed] [Google Scholar]
Annegers 1979
- Annegers JF, Hauser WA, Elveback LR. Remission of seizures and relapse in patients with epilepsy. Epilepsia 1979;20(6):729‐37. [DOI] [PubMed] [Google Scholar]
Anton‐Tay 1974
- Anton‐Tay F. Melatonin: effects on brain function. Advances in Biochemical Psychopharmacology 1974;11:315‐24. [PubMed] [Google Scholar]
Brzezinski 1997
- Brzezinski A. Melatonin in humans. New England Journal of Medicine 1997;336(3):186‐95. [DOI] [PubMed] [Google Scholar]
Camfield 1996
- Camfield PR, Camfield CS. Antiepileptic drug therapy: when is epilepsy truly intractable?. Epilepsia 1996;37(Suppl 1):S60‐5. [DOI] [PubMed] [Google Scholar]
Cockerell 1995
- Cockerell OC, Sander JW, Shorvon SD. Remission of epilepsy. The NGPS. National General Practice Study of Epilepsy. Lancet 1995;346(8984):1228. [DOI] [PubMed] [Google Scholar]
Commission ILAE 1989
- Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989;30(4):389‐99. [DOI] [PubMed] [Google Scholar]
Cortesi 2010
- Cortesi F, Giannotti F, Ivanenko A, Johnson K. Sleep in children with autistic spectrum disorder. Sleep Medicine 2010;11(7):659‐64. [DOI] [PubMed] [Google Scholar]
Elwes 1984
- Elwes RD, Johnson AL, Shorvon SD, Reynolds EH. The prognosis for seizure control in newly diagnosed epilepsy. New England Journal of Medicine 1984;311(15):944‐7. [DOI] [PubMed] [Google Scholar]
Fauteck 1999
- Fauteck J, Schmidt H, Lerchl A, Kurlemann G, Wittkowski W. Melatonin in epilepsy: first results of replacement therapy and first clinical results. Biol Signals Recept 1999;8(1‐2):105‐110. [DOI] [PubMed] [Google Scholar]
Goodridge 1983
- Goodridge DM, Shorvon SD. Epileptic seizures in a population of 6000. II: Treatment and prognosis. BMJ (Clinical Research Edition) 1983;287(6393):645‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hauser 1998
- Hauser WA, Rich SS, Lee JR, Annegers JF, Anderson VE. Risk of recurrent seizures after two unprovoked seizures. New England Journal Medicine 1998;338(7):429‐34. [DOI] [PubMed] [Google Scholar]
Herxheimer 2003
- Herxheimer A, Waterhouse J. The prevention and treatment of jet lag. BMJ 2003;326(7384):296‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herxheimer 2005
- Herxheimer A. Jet lag. Clinical Evidence 2005;13:2178‐83. [PubMed] [Google Scholar]
Je 1996
- Je J, O'Donnell ME. Use of melatonin in the treatment of paediatric sleep disorders. Journal of Pineal Research 1996;21(4):193‐9. [DOI] [PubMed] [Google Scholar]
Kabuto 1998
- Kabuto H, Yokoi I, Ogawa N. Melatonin inhibits iron‐induced epileptic discharges in rats by suppressing perioxidation. Epilepsia 1998;39(3):237‐43. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
McDonald 2000
- McDonald BK, Johnson AL, Goodridge DM, Cockerell OC, Sander JW, Shorvon SD. Factors predicting prognosis of epilepsy after presentation with seizures. Annals of Neurology 2000;48(6):833‐41. [PubMed] [Google Scholar]
Mevissen 1998
- Mevissen M, Ebert U. Anticonvulsant effects of melatonin in amygdala‐kindled rats. Neuroscience Letters 1998;257(1):13‐6. [DOI] [PubMed] [Google Scholar]
Molina‐Carballo 1997
- Molina‐Carballo A, Muñoz‐Hoyos A, Reiter RJ, Sánchez‐Forte M, Moreno‐Madrid F, Rufo‐Campos M, et al. Utility of high doses of melatonin as adjunctive anticonvulsant therapy in a child with severe myoclonic epilepsy: two years' experience. Journal of Pineal Research 1997;23(2):97‐105. [DOI] [PubMed] [Google Scholar]
Munoz‐Hoyos 1998
- Munoz‐Hoyos A, Sanchez‐Forte M, Molina‐Carballo A, Escames G, Martin‐Medina E, Reiter RJ, et al. Melatonin's role as an anticonvulsant and neuronal protector: experimental and clinical evidence. Journal of Child Neurology 1998;13(10):501‐9. [DOI] [PubMed] [Google Scholar]
Niles 1987
- Niles LP, Pickering DS, Arciszewski MA. Effects of chronic melatonin administration on GABA and diazepam binding in rat brain. Journal of Neural Transmission 1987;70(1‐2):117‐24. [DOI] [PubMed] [Google Scholar]
Revell 2006
- Revell VL, Burgess HJ, Gazda CJ, Smith MR, Fogg LF, Eastman CI. Advancing human circadian rhythms with afternoon melatonin and morning intermittent bright light. Journal of Clinical Endocrinology & Metabolism 2006;91(1):54‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seabra 2000
- Seabra ML, Bignotto M, Pinto LR Jr, Tufik S. Randomized, double‐blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. Journal of Pineal Research 2000;29(4):193‐200. [DOI] [PubMed] [Google Scholar]
Sheldon 1998
- Sheldon SH. Pro‐convulsant effects of oral melatonin in neurologically disabled children. Lancet 1998;351(9111):1254. [DOI] [PubMed] [Google Scholar]
Shorvon 1982
- Shorvon SD, Reynolds EH. Early prognosis of epilepsy. BMJ (Clinical Research Edition) 1982;285(6356):1699‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2010
- Weiss MD, Salpekar J. Sleep problems in the child with attention‐deficit hyperactivity disorder: defining aetiology and appropriate treatments. CNS Drugs 2010;24(10):811‐28. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Brigo 2008
- Felice A, Guaraldi P. The use of melatonin as an adjunctive treatment for epilepsy. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006967] [DOI] [Google Scholar]
Brigo 2012
- Brigo F, Felice A. Melatonin as add‐on treatment for epilepsy. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD006967.pub2] [DOI] [PubMed] [Google Scholar]


