Abstract
Background:
Since the outbreak of Novel Coronavirus Pneumonia (NCP), it has swept the world with rapid development. Up to now, there is no effective drug to treat it. Lianhua Qingwen has been used in the treatment of COVID-19 in China, but there is no systematic review about it. This study will systematically evaluate its efficacy and safety in the treatment of COVID-19.
Methods:
We will search electronic database of PubMed, EMBASE, Cochrane library, Web of Science (WOS), China National Knowledge Infrastructure (CNKI), Chinese Biomedical Literature Database (CBM), Chinese Scientific and Journal Database (VIP) and Wan Fang database (Wanfang) for the literature of RCTs of Lianhua Qingwen capsule for coronavirus disease 2019 (COVID-19). We will also search the Chinese Clinical Trial Registry (ChiCTR) and ClinicalTrials.gov (www.ClinicalTrials.gov) for ongoing trials with unpublished data, and the Conference abstracts will be searched manually. We will use the Cochrane Handbook for Systematic Reviews of Interventions to assess the risk of bias. The protocol will be conducted according to the approach and Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P).
Results:
The study results will provide evidence of the efficacy and safety of Lianhua Qingwen (LHQW) for coronavirus disease 2019 (COVID-19).
Conclusion:
The result of the study will be published in a peer-reviewed journal.
PROSPERO registration number:
CRD42020180877.
Keywords: coronavirus disease 2019, COVID-19, Lianhua Qingwen, meta-analysis
1. Introduction
Novel coronavirus pneumonia (SARS-CoV-2) was found in Wuhan, China, in early December 2019,[1] showing a high contagious and rapid transmission characteristic, which could cause severe new crown pneumonia (COVID-19)[2,3] in a certain proportion of patients. According to the WHO report,[4] about 13.8% of the patients are severe patients, 6.1% of them are critical patients, and some of them may develop into critical patients due to poor or delayed treatment. The clinical manifestations[5,6] of COVID-19 are fever, dry cough, dyspnea, muscle or joint pain, diarrhea, and pneumonia, which can lead to respiratory failure or even death in severe cases, COVID-19 is causing a global pandemic.[7,8] Through comprehensive prevention and control, Chinas epidemic has been gradually under control, but at present, CONVID-19 is spreading rapidly outside China. Globally, as of 10:44 pm CEST, May 21, 2020, there have been 4,904,413 confirmed cases of COVID-19, including 323,412 deaths, reported to WHO.[9] Which has become a huge challenge for global public health.[10–12]
Lianhua Qingwen is mainly made of forsythia, honeysuckle, isatis root, menthol, liquorice, agastache, almond, gypsum, ephedra, Rhodiola, houttuynia and other Chinese herbal medicines,[13,14] and it has been used for fighting against the epidemic in China,[15,16] So far, there have been some relevant clinical reports[17,18] on COVID-19 treated with Lianhua Qingwen. Therefore, this study will collect the relevant research, and then review the effectiveness and safety of Lianhua Qingwen for COVID-19.
2. Methods
Preferred reporting items of systematic reviews a meta-analysis protocol (PRISMA-P) 2015[19] will be used as a guideline in performing of the systematic review.
2.1. Inclusion criteria
2.1.1. Types of studies
We will include randomized controlled trials (RCTs) of Lianhua Qingwen for COVID-19 in the treatment groups. There will be no language restrictions
2.1.2. Types of participants
Patients diagnosed with COVID-19 of any age, gender, and the racial group will be included.
2.1.3. Types of interventions
The treatment group with Lianhua Qingwen Capsule or Lianhua Qingwen Granule for COVID-19 and the control group may receive external treatment, placebo, no intervention or other pharmacological intervention will be included.
2.1.4. Types of outcome measures
Primary outcome: severe type conversion rate; secondary outcome: the proportion of participants with fever, Proportion of participants with 1 or more adverse events, Health-related quality of life.
2.2. Search strategies
Electronic databases of PubMed, EMBASE, Cochrane library, Web of Science(WOS), China National Knowledge Infrastructure (CNKI), Chinese Biomedical Literature Database (CBM), Chinese Scientific and Journal Database (VIP), and Wan Fang database (Wanfang) will be searched to identify literature of RCTs of Lianhua Qingwen for coronavirus disease 2019 (COVID-19). Meanwhile, we will search the Chinese Clinical Trial Registry (ChiCTR) and ClinicalTrials.gov (www.ClinicalTrials.gov) for ongoing trials with unpublished data, and the Conference abstracts will be searched manually. We will search the above databases from inception to 30 Jun 2020 with no language restrictions.
The search strategy of PubMed is listed in Table 1. We will appropriately adjust the search strategy for different databases accordingly.
Table 1.
Search strategy for PubMed.

2.3. Data collections and analysis
2.3.1. Selection of literature
Two reviewers (CF and XQ) will identify the eligibility of the studies independently using inclusion and exclusion criteria by Endnote software (V.x9.0) to eliminating the duplicates, then they will exclude other non RCTs, such as animal experiment, case report or systematic review by reading titles and abstracts. Finally, they will read full papers of the screened articles to decide whether it could be included. If there is any dispute during this period, the 2 reviewers may refer to the third expects (BLS), they would make the decision by voting. All screening and managing processes of the articles will be performed with Endnote software. The screening process of this study will be carried out according to Figure 1
Figure 1.
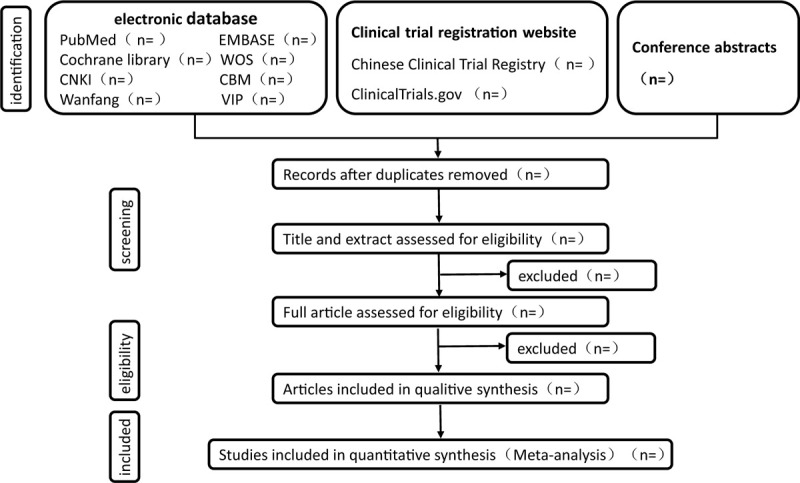
The screening process.
2.3.2. Data extraction and management
Then 2 reviewers (YHS and JNL) will extract title, the first author, publication year, country, language, journal source; information of participants: gender, age, study design, sample size, intervention, type of measures, risk of bias assessment, and findings from include studies with Excel file.
The results will be cross-checked by the 2 reviewers, and any disagreements will be resolved by consensus, with any ongoing differences in opinion being arbitrated by a third reviewer (JNL).
2.3.3. Assessment of risk of bias in included studies
Two independent reviewers (QSZ and FC) will evaluate the quality of the included trials by assessing the risk of bias by using the Cochran Collaboration Network Bias Risk Assessment Tool. The 2 authors will assess the risk of bias of sequence generation, allocation concealment, blinding of participants personnel and outcome assessment, incomplete outcome data, selective outcome reporting, and other bias. If there is disagreement during the assessing process, discuss with the third experts (YFW) to make decisions. The evaluation grades are low, high, and unclear risk of bias.
2.4. Dealing with missing data
If data of the included studies missed, we would contact the author for help. If it does not work, we will turn to follow strategies to evaluate the potential influence of missing data.[20]
-
1.
Worst-case scenario analysis: All participants with missing data counted as failures.
-
2.
Extreme worst-case/best-case scenario analysis: Participants with missing outcome data in the exercise arm counted as failures and in the control arm as success and vice versa.
2.5. Assessment of heterogeneity
Standard I2 test will be used for assessing the statistical heterogeneity, the significance level of I2 ≥ 50% indicates significant, otherwise, it means insignificant.
2.6. Assessment of reporting bias
If more than 10 studies are included, a funnel plot will be used to assess reporting bias, and if less than 10studies are included, P value will be used.
2.7. Data synthesis
Meta-analyses will be conducted when at least 2 studies included. We will conduct Statistical analyses using the RevMan software (version 5.3.5) Risk ratio (RR) with 95% CIs will be used to investigate dichotomous data. Standard mean difference (SMD) with 95% CIs or a weighted mean difference (WMD) will be used to analyze continuous data. The WMD will be used for the same scale or the same assessment instrument; SMD will be used for different assessment tools. The fixed-effect model will be utilized if the heterogeneity test indicates that there is no significant heterogeneity (I2 < 50%; P > .1); otherwise, the random-effects model will be used.
2.8. Subgroup analysis and investigation of heterogeneity
Subgroup analysis will be performed to determine the potential heterogeneity and inconsistency clinically and statistically, if enough data are extracted. Subgroup analysis based on: gender, age, whether patients in the included trials have basic diseases or not
2.9. Sensitivity analysis
We will conduct sensitivity analysis to assess the robustness and reliability of the pooled results. If the results are unstable, of high-risk bias studies may be excluded, otherwise, we will check the processing method of missing data (Worst-case scenario analysis: All participants with missing data counted as failures; Extreme worst-case/best-case scenario analysis: Participants with missing outcome data)
2.10. Grading the quality of evidence
We will make use of GRADE to assess the quality of evidence.
2.11. Ethics and dissemination
Ethical approval is not necessary for this study, for there is no information related to the individual patient. The systematic review will be conducted according to PRISMA guidelines, and we will show the assessment of the effect and safety of LHQW for COVID-19, and we will publish it in a peer-reviewed journal.
3. Discussion
LHQW Formula is a traditional Chinese herbal medicine prescription, and it is used for Influenza in China before, which also shows good clinical efficacy in treating Novel Coronavirus Pneumonia (NCP) resulted from SARS-CoV-2. LHQW was also composed into the Diagnosis and Treatment Programs of 2019 New Coronavirus Pneumonia (from fourth to seventh editions) formulated by the National Health Commission of China. Aiming to prevent and treat viral influenza.[21] On April 12, 2020, the National Medical Products Administration of China issued that: the functional indications item of Lianhua Qingwen Capsule and Lianhua Qingwen Granule added “In the treatment of Novel Coronavirus Pneumonia, it can be used for fever, cough, and fatigue caused by light and common types”[22] So far there is still no systematic review and meta-analysis on efficiency and safety of LHQW for COVID-19. We conduct this study, aim to provide evidence and guide for clinical decision making. We plan to publish this review within 2 months since the protocol published, then we will update it every 3 years.
Author contributions
QSZ and BLS conceived and designed the protocol, QSZ, FC, and YHS registered the protocol review in the Prospero database and drafted the manuscript. YFW and XHX designed the search strategy. QSZ and XQ draft the protocol, QSZ, FC, YFW, YHS, XHX, JNL, XQ, SQS, GCJ, and BLS contribute to and approved the final manuscript of the protocol review.
Conceptualization: Qiongshuai Zhang, Bailin Song
Data curation: Guangcheng Ji, Xun Qi, and Jiannan Li.
Funding acquisition: Bailin Song
Methodology: Yihan Sun and Shaoqian Sun.
Original draft: Qiongshuai Zhang and Xun Qi.
Project administration: Yufeng Wang.
Review & editing: Yufeng Wang and. Bailin Song
Software: Shaoqian Sun, Jiannan Li
Footnotes
Abbreviations: CIs = credible intervals, GRADE = Recommendations Assessment, Development and Evaluation Reliability Study, LHQW = Lianhua Qingwen, MDs = mean differences, PRISMA-P = Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols, RCTs = Randomized Clinical Trails, RR = relative risk, SMDs = standard mean differences.
How to cite this article: Zhang Q, Cao F, Ji G, Xu X, Sun Y, Li J, Qi X, Sun S, Wang Y, Song B. The efficacy and safety of Lianhua Qingwen (LHQW) for coronavirus disease 2019 (COVID-19): A protocol for systematic review and meta analysis. Medicine. 2020;99:30(e20979).
This study is supported by the National Key R&D Program of China (2019YFC1709900), which is supported by the Ministry of science and technology of the People's Republic of China.
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis 2020;ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020;579:265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].F.Q, J.Z Pedicle torsion of ovarian cyst and acupuncture--a case report. Acupunct Med 2006;24:134–6. [DOI] [PubMed] [Google Scholar]
- [5].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Grech V. Unknown unknowns - COVID-19 and potential global mortality. Early Hum Dev 2020;144:105026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [8].Barlow A, Landolf KM, Barlow B, et al. Review of emerging pharmacotherapy for the treatment of coronavirus disease 2019. Pharmacotherapy 2020;40:416–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].WHO. coronavirus disease outbreak(COVID-19). WHO. https://covid19.who.int/. Published 2020. Accessed [Google Scholar]
- [10].Li YK, Peng S, Li LQ, et al. Clinical and transmission characteristics of covid-19 - a retrospective study of 25 cases from a single thoracic surgery department. Curr Med Sci 2020;40:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Aminian A, Safari S, Razeghian-Jahromi A, et al. COVID-19 outbreak and surgical practice: unexpected fatality in perioperative period [published online ahead of print, 2020 Mar 26]. Ann Surg 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Meng L, Hua F, Bian Z. Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J Dent Res 2020;99:481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang CH, Zhong Y, Zhang Y, et al. A network analysis of the Chinese medicine Lianhua-Qingwen formula to identify its main effective components. Mol Biosyst 2016;12:606–13. [DOI] [PubMed] [Google Scholar]
- [14].Wang SH, Liu JF, Zhang YL, et al. Systematic review of efficacy and safety of Lianhua Qingwen Capsules in treatment of viral influenza. Zhongguo Zhong Yao Za Zhi 2019;44:1503–8. [DOI] [PubMed] [Google Scholar]
- [15].Fan T, Chen Y, Bai Y, et al. Analysis of medication characteristics of traditional Chinese medicine in treating coronavirus disease-19 based on data mining. Zhejiang Da Xue Xue Bao Yi Xue Ban 2020;49:0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xiong Y, Liu Y, Cao L, et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg Microbes Infect 2020;9:761–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lv R, Wang W, Li X. Novel coronavirus pneumonia suspected cases treated with Lianhua Qingwen Decoction: a clinical observation of 63 cases. J Trad Chin Med 2020. [Google Scholar]
- [18].Yao K, Liu M, li X. Retrospective analysis of novel coronavirus pneumonia treated with Lianhua Qingwen. Chin J Exp Trad Med Formulae 2020. [Google Scholar]
- [19].Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- [20].Aamann L, Dam G, Rinnov AR, et al. Physical exercise for people with cirrhosis. Cochrane Database Syst Rev 2018;12:Cd012678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ye C-h, Gao M-n, Lin W-q, et al. Theoretical study of the anti-NCP molecular mechanism of traditional Chinese medicine Lianhua-Qingwen Formula (LQF). ChemRxiv 2020. [Google Scholar]
- [22].Zhao P. Two Chinese patent medicines officially approved for the treatment of new coronary pneumonia. Beijing Daily 2020;12. [Google Scholar]


