Abstract
Patients with infective endocarditis (IE), have high mortality and morbidity, however, its early diagnosis is difficult. Few studies have examined the delayed diagnosis of IE. We aimed to investigate the factors associated with the diagnostic delay of IE.
A retrospective cohort study was conducted for consecutive patients diagnosed with IE in an acute care teaching hospital in Japan from April 2006 to March 2018. Time-to-diagnosis was analyzed using a multivariate Cox hazard model for determining factors associated with days required for IE diagnosis. Factors analyzed in the model included age, gender, activities of daily living, Charlson comorbidity index, presence of internal device, chief complaint, inappropriate antibiotics use, shaking chill, fever >38°C, hypoxemia, serum C-reactive protein (CRP) < 10 mg/dL, Staphylococcus aureus as causative pathogen, findings on first echocardiography, resident as a first contact physician, primary care physician as a first contact doctor, and transport measures to the clinic/hospital.
There were 145 IE patients with a mean age of 70 years and 90 were male (62.1%). The median time to the diagnosis of definite IE was 13 days and median time to consider the diagnosis of IE from first clinic/hospital visit was 6 days. The time to consider IE diagnosis was significantly delayed in patients who had inappropriate prior antibiotic use (hazard ratio [HR], 1.61; 95% confidence interval [CI], 1.01 to 2.57; P = .045), in patients without fever >38°C (HR, 1.80; 95% CI, 1.11 to 2.90; P = .016), in patients with serum CRP level < 10 mg/dL (HR, 1.53; 95% CI, 1.01 to 2.33; P = .046), and in patients who did not use an ambulance for hospital arrival (HR, 3.18; 95% CI, 1.72 to 5.85; P < .001).
Delay in considering IE diagnosis is associated with inappropriate prior antibiotics use, absence of high fever, absence of high CRP level, and use of a hospital arrival vehicle other than an ambulance. For earlier IE diagnosis, inappropriate use of antibiotics should be avoided and IE should not be excluded by relatively low level of temperature or serum CRP.
Keywords: C-reactive protein, delay, diagnosis, fever, inappropriate antibiotics, Infective endocarditis
1. Introduction
Infective endocarditis (IE) is not a common disease, but when it occurs, it is associated with significant mortality and morbidity. A substantial rate of mortality, 9% to 22%, was reported among the patients who received antibiotics for IE.[1–6]
Early diagnosis of IE is essential through investigations including blood culture and echocardiography.[7,8] Early high-dosage antibiotic administration, and surgical intervention, are strongly associated with a reduced risk of in-hospital mortality and morbidity in IE.[9,10]
On the other hand, delay in diagnosis and initiation of therapy lead to complications and unfavorable outcomes.[11] Therefore, delayed diagnosis of IE is a critical issue that needs to be resolved, to save lives and avoid disability. Several studies conducted in IE patients revealed a significant delay in their diagnosis.[2,12] However, to the best of our knowledge, no study has been conducted before that focused on the factors causing delayed IE diagnosis.
Therefore, this study was performed to investigate factors that may have a role in the delayed diagnosis of IE from the day of patients visit to recall diagnosis. We also evaluated the delayed factors of day to definite IE diagnosis from the day of positive blood cultures.
2. Methods
2.1. Study population
Our retrospective cohort study was performed on patients who were diagnosed with IE in Shonan Kamakura General Hospital, Japan from April 2006 to March 2018. The private acute-care teaching hospital provides primary and tertiary care in Kanagawa prefecture, with about 9 million inhabitants. This 619 bedded hospital has departments in Cardiothoracic Surgery, Cardiovascular Medicine, Emergency Medicine, and General Internal Medicine. The hospital receives patients from all quarters, namely, primary care clinics, other hospitals, and directly from the adjoining areas for free access to physicians of various specialties. Our study included hospitalized patients with definite IE according to modified Duke criteria.[13] The consecutive IE patients with nosocomial infections were excluded.
2.2. Data collection
Data on the following patient-related factors were collected: age, gender, alcoholism, activities of daily living (ADL), living place, history of IE, history of cardiac surgery, heart valve disease, valve, device insertion, Charlson comorbidity index,[14] recent history of dental care or dental caries, inappropriate antibiotic use, complaint of fever, complaint related heart failure, complaint related stroke, complaint related osteomyelitis, complaint related other septic embolism, fever >38°C, shaking chill, hypoxemia, quick sequential [Sepsis-related] organ failure assessment (qSOFA) score,[15,16] cardiac murmur, serum C-reactive protein (CRP) level (mg/dL), rhythm on electrocardiogram, ejection fraction >60% on echocardiography, negative finding on first echocardiography, microbiological finding (Staphylococcus aureus and Streptococcus species), impaired valve, and vegetation size. We also collected data on medical facility and physician-related factors like hospital resident on the hospital as a first contact doctor, primary care physician as a first contact doctor, General Internal Medicine physician as a first contact doctor, and whether the patient was transferred to the hospital by ambulance. Moreover, we evaluated data on the following outcome and complications: heart failure, infectious cerebral aneurysm, stroke, osteomyelitis, other septic embolism, surgery, length of stay (day), in-hospital mortality (%), and 90-day mortality (%). For appropriateness of previous antibiotic use, we assessed the chart information. We defined any antibiotics use before obtaining blood cultures as “inappropriate.”
2.3. Statistical analysis
We aimed to evaluate factors associated with delayed recall diagnosis of IE patients. The primary outcome measure was the duration from the visit to the clinic or hospital and to the recall of the IE diagnosis (Fig. 1). For the day of visit-to-the day of diagnosis, we used the Cox proportional hazard model to analyze factors associated with delaying the diagnosis of IE. In a univariate model, the “patient-related factors” and “medical facility and physician-related factors” were evaluated as possible reasons for the delayed diagnosis of IE, based on log-rank test. Multivariable model was developed using the age, gender, ADL, Charlson comorbidity index, non-device insertion, non-chief complaint related fever, non-chief complaint related heart failure, inappropriate antibiotics use, absence of shaking chill, absence of fever >38°C, hypoxemia, serum CRP level <10 mg/dL, another bacteria of S aureus, negative finding on first echocardiography, resident on the hospital as a first contact physician, primary care physician as a first contact doctor, and transferred by a vehicle other than non-ambulance.
Figure 1.
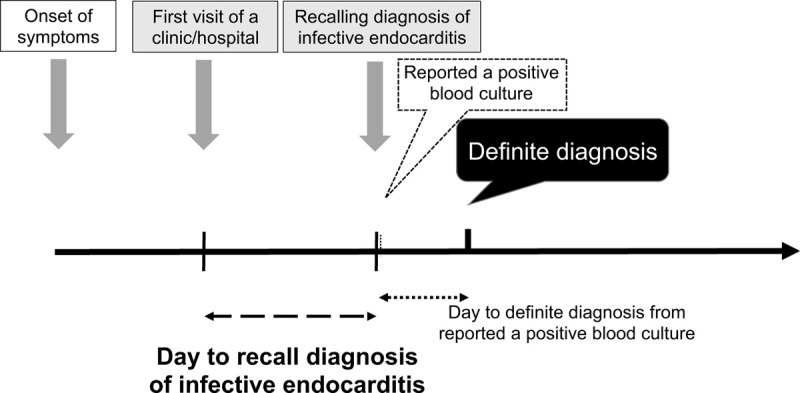
Schematic representation of the diagnosis flow from onset of patient symptoms.
Additionally, we assessed factors associated with the delayed definite diagnosis from reported positive blood cultures among IE patients (Fig. 1). The multivariable model analysis was developed using the multivariate Cox proportional hazard model. We selected the explanatory factors for the age, gender, device insertion, department, only 1 set positive blood culture, negative finding on first echocardiography, negative finding on first echocardiography after reported positive blood cultures, vegetation < 10 mm, S aureus, no complication of stroke, no complication of osteomyelitis, and no complication of septic embolism.
A two-tailed p-value <.05 was considered statistically significant. All statistical tests were undertaken using the SPSS Statistics version 21J (IBM, Tokyo, Japan). This research was approved by the Institutional Review Board of the Shonan Kamakura General Hospital (No. TGE01079-024).
3. Results
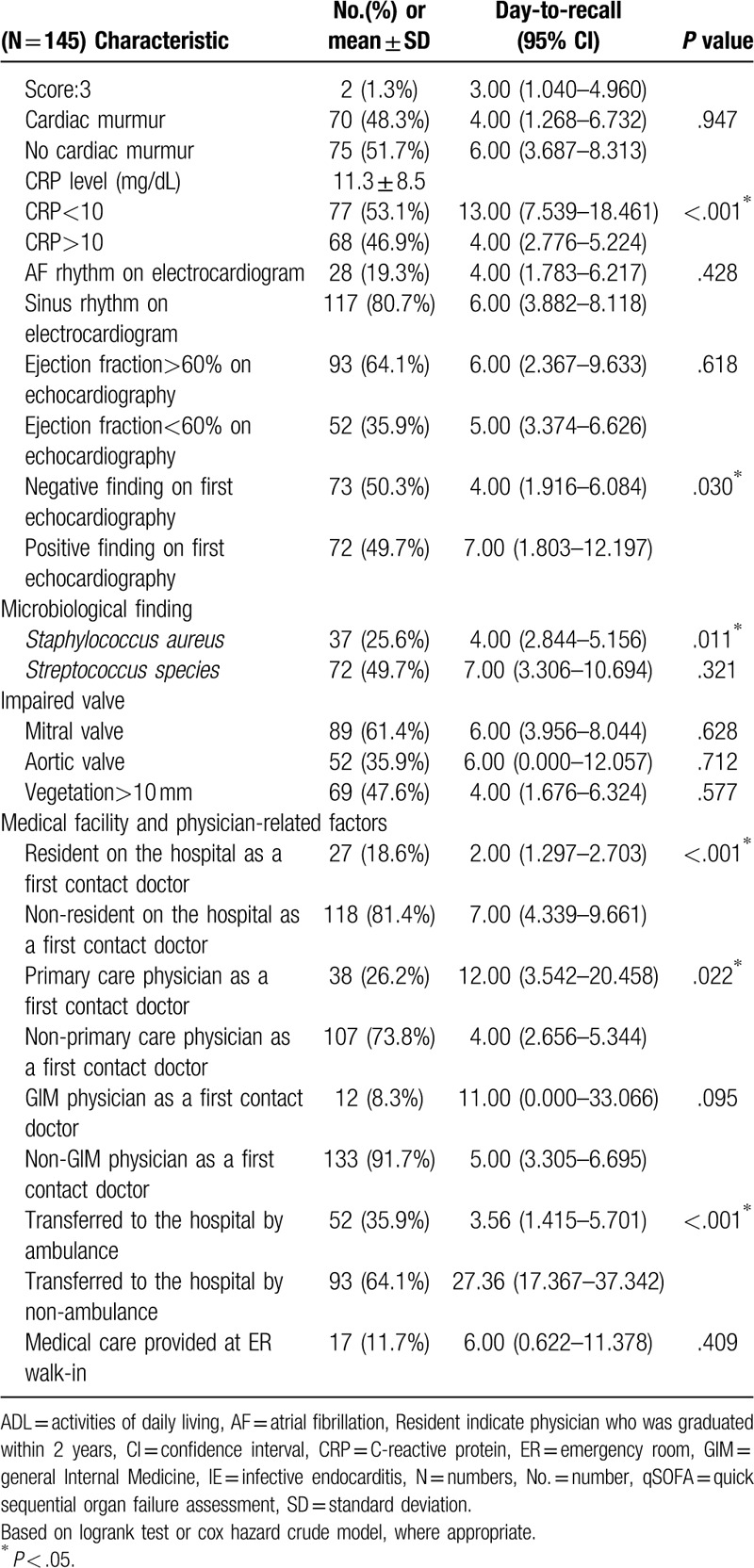
Of the 145 patients diagnosed with definite IE during the study period, the mean age was 69.6 ± 16.1 years with 90 (62.1%) male and 55 (37.9%) female patients (Table 1 ). The various characteristics and univariate analysis in Table 1 shows: the median time from first contact doctor to recall the diagnosis was 6 days (quartile, 2–16 day) (Fig. 2), from reported positive blood culture to definite diagnosis, it took 4 days (quartile, 2–9 day), eighty-one (55.9%) patients obtained blood cultures at the first visit, etiological organism was identified in 138 (95.2%) cases. The organisms associated with IE are seen in Table 1 with Streptococcal species, being the most common (72 cases [49.7%]), followed by Staphylococcal species (44 cases [30.3%]) including S aureus (37 cases [25.5%]). During the hospital stay, 26 (17.9%) patients died in the hospital. Three month and in-hospital morality were 17.9% each. Sixty-eight (46.9%) patients received cardiac surgery for IE treatment. As a complication of IE, 45 (31.0%) patients had heart failure, 2 (1.4%) patients had an infectious cerebral mycotic aneurysm, 63 (43.4%) patients had a stroke, 18 (12.4%) patients had vertebral osteomyelitis, and 59 (40.7%) patients had another septic embolism. The mean length of hospital stay was 46 ± 26 days.
Table 1.
Patients characteristics and univariate analysis associated with delayed recall diagnosis of infective endocarditis.
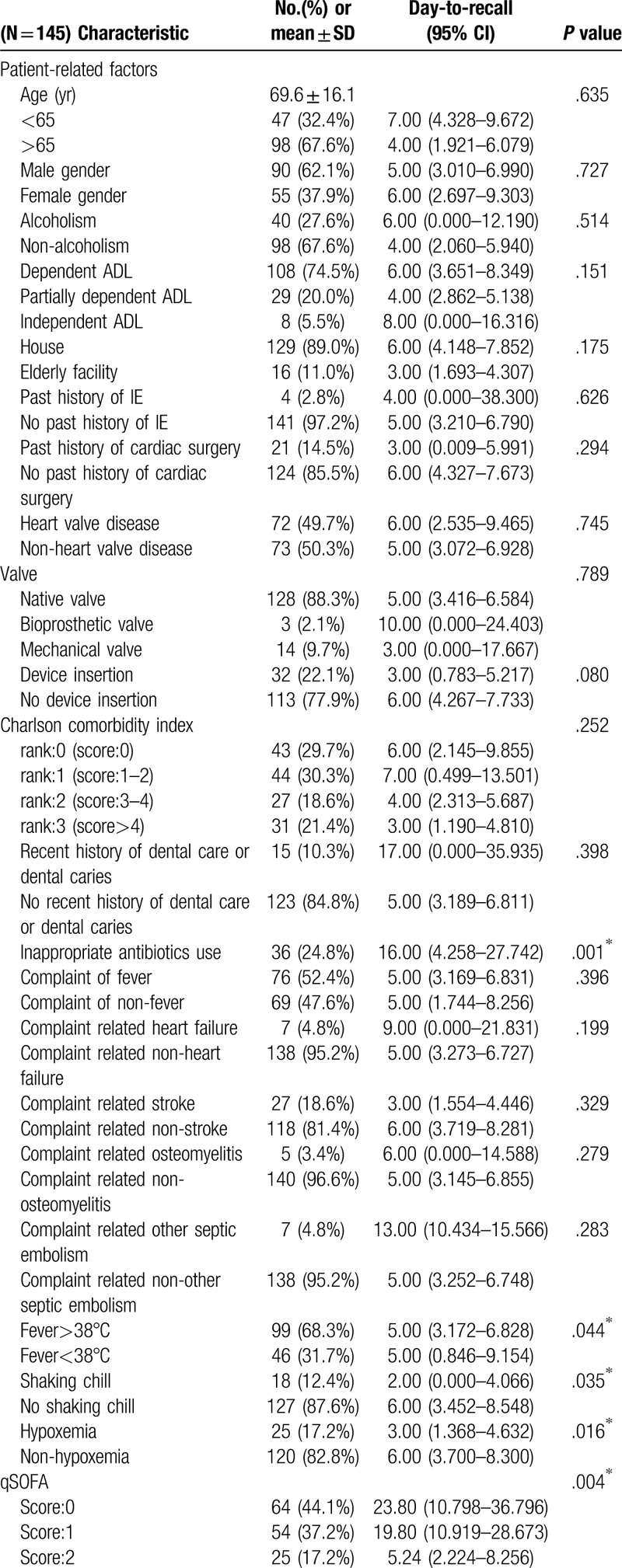
Figure 2.
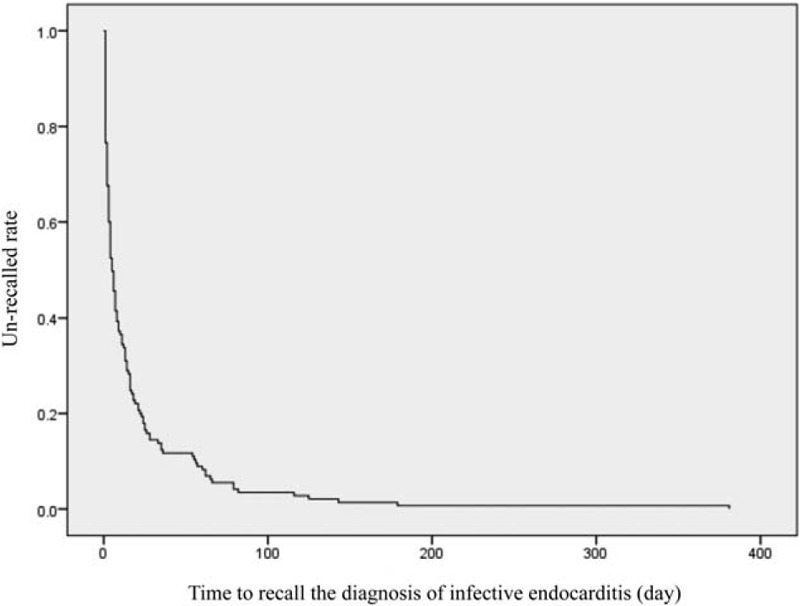
Kaplan-Meier curve of the time to recall the diagnosis of infective endocarditis (day).
The median days for various events as shown in Figure 3 include, patient symptoms to the first visit to a clinic/hospital was 3 days, to obtain blood cultures was 6 days, to admit in the hospital was 7 days, to report a positive blood culture was 7 days, to recall diagnosis of IE was 9 days, and to diagnose definite IE was 13 days, respectively.
Figure 3.
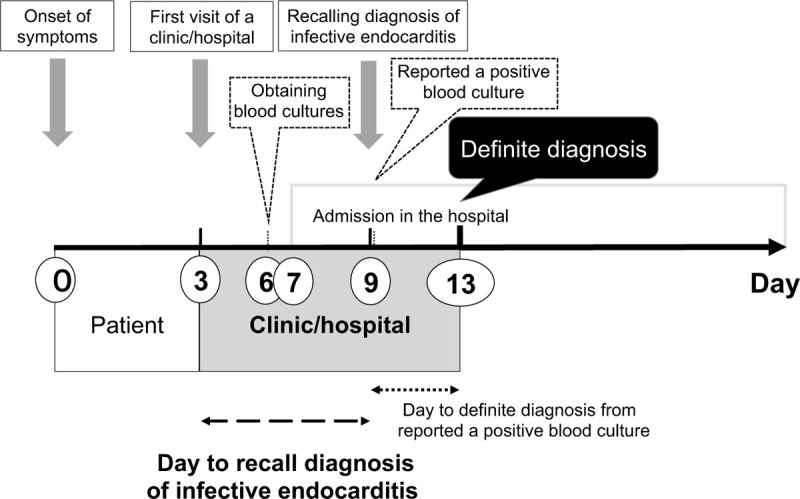
Day until definite diagnosis of infective endocarditis from appearance of patient symptoms.
Univariate analysis description revealed, inappropriate antibiotics use (P = .001), fever (P = .044), no-shaking chill (P = .035), non-hypoxemia (P = .016), qSOFA score (P = .004), serum CRP <10 mg/dL (P <.001), negative-finding on first echocardiography (P = .03), S aureus (p = .011), resident in the hospital as a first contact doctor (P < .001), primary care physician as a first contact doctor (P = .022), and transfer to the hospital by ambulance (P < .001) were significant factors associated with a delayed diagnosis of IE (Table 1 ).
On multivariate analysis using Cox hazard model, inappropriate antibiotics use (HR, 1.61; 95% CI, 1.01 to 2.57; P = .045), absence of fever >38°C (HR, 1.80; 95% CI, 1.11 to 2.90; P = .016), serum CRP level <10 mg/dL (HR, 1.53; 95% CI, 1.01 to 2.33; P = .046), and transfer to hospital by a vehicle other than an ambulance (HR, 3.18; 95% CI, 1.72 to 5.85; P < .001) were significantly associated with delayed recall diagnosis of IE (Table 2)
Table 1 (Continued).
Patients characteristics and univariate analysis associated with delayed recall diagnosis of infective endocarditis.
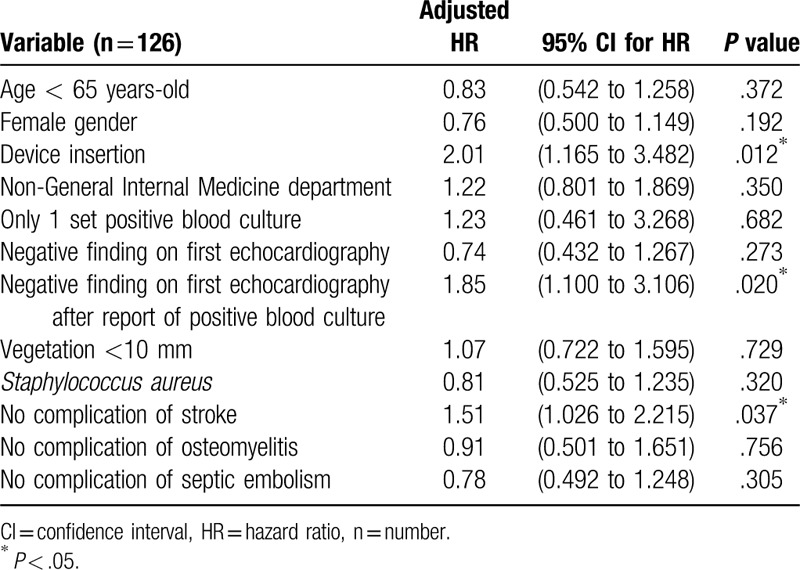
Out of the 126 patients evaluated for the delayed definite diagnosis from reported positive blood cultures among IE patients, 19 patients were excluded. The 7 among excluded, were negative for blood culture and the 12 were diagnosed with IE before reported positive blood culture. On additional multivariate analysis, Cox hazard model revealed, device insertion (HR, 2.01; 95% CI, 1.17 to 3.48; P = .012), negative finding on first echocardiography after report of positive blood culture (HR, 1.85; 95% CI, 1.10 to 3.11; P = .020), no complication of stroke (HR, 1.51; 95% CI, 1.03 to 2.22; P = .037); all these factors were significantly associated with delayed definite diagnosis of IE after a reported positive blood culture (Table 3).
Table 2.
Factors associated with delayed recall diagnosis of infective endocarditis.
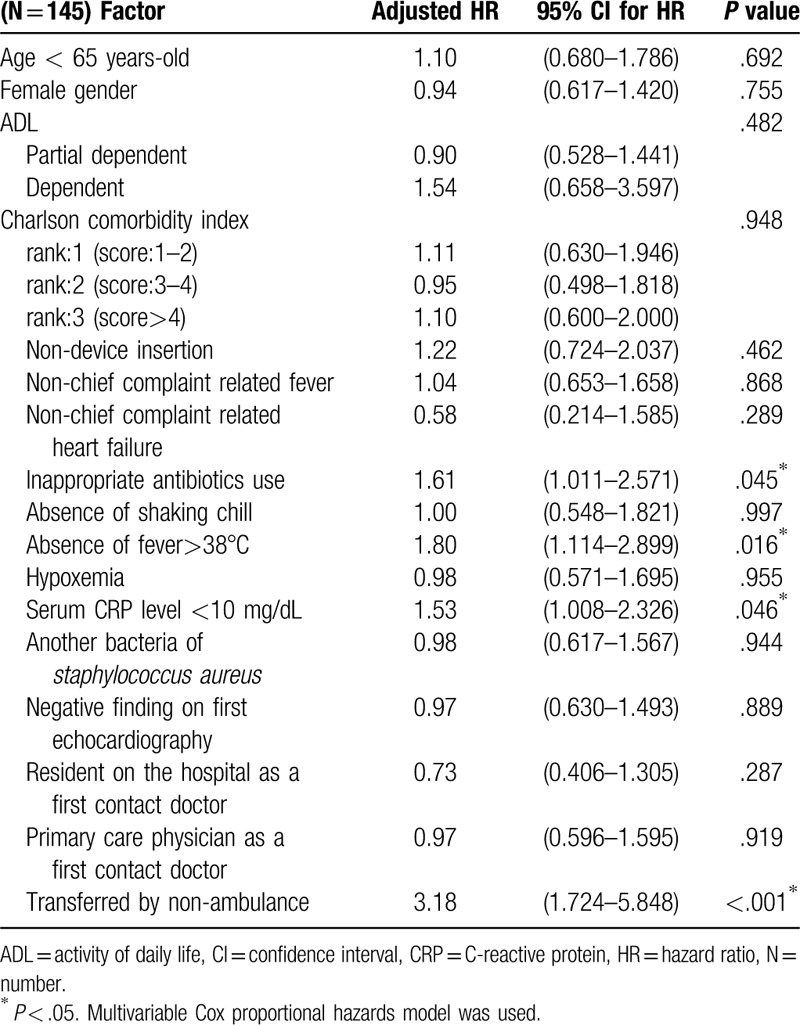
Table 3.
Factors related with delayed definite diagnosis of infective endocarditis after a reported positive blood culture.
4. Discussion
In present retrospective cohort study, we found that inappropriate antibiotic use, non-ambulance transfer to a hospital, absence of fever >38°C, and serum CRP level <10 mg/dL were associated with delayed diagnosis of IE. Device insertion; negative echocardiography finding after reporting a positive blood culture, and no complication of stroke were factors related to a delayed definite diagnosis, after a reported positive blood culture.
The duration between the first visit to a clinic/hospital and the definite diagnosis of IE (10 days), was more than the duration between the development of patient symptoms to the first visit of a clinic/hospital (3 days) (Fig. 3). The result show that diagnosing IE as early as possible is more important than the duration taken for the first visit to a clinic/hospital, to improve its mortality and morbidity. It is, therefore, important to promote medical education and improve the diagnostic methods for IE, to shorten the duration for its diagnosis.
In one of the earlier studies, univariate analysis has shown the tendency of the relationship about inappropriate antibiotics use as an associate factor for the delayed diagnosis of IE, but the multivariate analysis did not show any relationship, probably due to small sample size used.[2] Although high body temperature predicts bacteremia,[17,18] patients without fever >38°C may be overlooked bacteremia such as for a diagnosis of IE. Fever may be absent in 20% to 30% of elderly patients being affected with a serious infection.[19] Therefore, physicians can’t exclude the diagnosis of IE even in the absence of a fever. We speculate, physicians may delay obtaining blood cultures in patients with lower CRP level, similar to delay in diagnosis of vertebral osteomyelitis related to lower CRP level.[20] Physicians may attribute either viral infections or some other non-bacterial causes in patients with the lower CRP level. Moreover, our research (data not shown in the part of Results) showed that patients with IE attributed to streptococcus species had lower CRP levels. In patients with serum CRP level <10 mg/dL (n = 77), S aureus were involved in 13.0% (n = 10) and Streptococcus species were involved in 54.5% (n = 42). On the other hand, in patients with serum CRP level >10 mg/dL (n = 68), S aureus were involved in 39.7% (n = 27) and Streptococcus species were involved in 44.1% (n = 30). Therefore, physicians should take care of delay diagnosis of Streptococcal IE patients with lower CRP level. It is easy to obtain blood cultures in emergency room (ER), especially patients using ambulance are obtained due to available access. While the diagnosis of IE was delayed in patients using non-ambulance services for hospital visit due to difficulty access to obtain blood cultures. Therefore, delayed recall diagnosis of IE, due to inappropriate antibiotics use, in patients transferred by non-ambulance vehicular services, in patients without high fever, and in patients with lower CRP level can be improved significantly through educating physicians and improving the diagnostic methods.
As device insertions, including cardiac pacemaker and implantable cardioverter-defibrillator were increased worldwide,[21] device-related infective endocarditis also increased.[22] The diagnosis of cardiac device infections, particularly device-related endocarditis, is challenging.[23] Thus, patients with device insertion may be taken longer days from reported a positive blood culture to definite IE diagnosis, consistent with the results of our additional analysis. After reporting a positive blood culture, transthoracic echocardiography (TTE) is usually performed. Most guidelines recommend transesophageal echocardiography (TEE) in cases of suspected IE, particularly when an initial TTE is negative.[24,25] Thus, those patients with false negative TTE will have their diagnosis delayed. False negative TTE may confer a more benign prognosis among patients ultimately diagnosed with IE.[8,26,27] In the hospital, there is a department of neuro-endovascular surgery. After diagnosed the stroke in the department of ER, the neuro-endovascular surgeon is referred as soon as possible and assesses the etiology of stroke. Therefore, IE complicated stroke may be early diagnosed after reporting a positive blood culture. The factors of device insertion, negative echocardiography, and no complication of stroke depend on the IE patients, does not depend on steps taken for diagnostic improvement.
To the best of our knowledge, our research is the first study that focuses on factors associated with delayed diagnosis of IE. The noteworthy strength of this study is that the data were collected from a relatively large-scale sample. Moreover, we followed real data from patient symptoms through clinic/hospital visit to definite diagnosis of IE (Fig. 3). The results show that the duration between the patient's clinic/hospital visit and the day of obtaining blood cultures or the day recalling diagnosis of IE needs a lot to improve, during the period to a definite diagnosis.
This study has several limitations. Our research was based on a single-center cohort design. The clinical ability of physicians was variable, depending on their level of training and experience, which may have led to selection bias. Also, we are unable to establish a causal relationship in this retrospective observational study.
Future research needs to be directed at understanding the factors associated with a delayed diagnosis of IE; this information may help with earlier diagnosis of IE patients and improve patient outcomes. Therefore, multi-center, prospective studies, by using a multi-level analysis are required, across different population groups to support the evidence of our findings.
5. Conclusions
We revealed the process for a definite diagnosis of IE, from a time of its initial symptoms. Physicians can improve the diagnostic delay of IE, by shortening the duration to obtain blood cultures or recalling the IE diagnosis. Diagnosis of IE was delayed in patients with inappropriate antibiotics use, patients transferred by non-ambulance vehicular services, patients without high fever, and patients with lower CRP level. Therefore, physicians need to take care of appropriate antibiotic use and consider obtaining blood cultures in patients who are transferred by other means of transportation than in ambulances, patients without fever >38°C, and patients with lower CRP levels for earlier IE diagnosis.
Author contributions
Conceptualization: Sho Nishiguchi, Koichi Nishino, Izumi Kitagawa, Yasuharu Tokuda.
Data curation: Sho Nishiguchi, Koichi Nishino.
Formal analysis: Sho Nishiguchi, Yasuharu Tokuda.
Supervision: Izumi Kitagawa, Yasuharu Tokuda.
Writing – original draft: Sho Nishiguchi.
Writing – review & editing: Yasuharu Tokuda.
Footnotes
Abbreviations: ADL = activities of daily living, CI = confidence interval, CRP = C-reactive protein, ER = emergency room, HR = hazard ratio, IE = infective endocarditis, qSOFA = quick Sequential (Sepsis-related) Organ Failure Assessment, TEE = transesophageal echocardiography, TTE = transthoracic echocardiography.
How to cite this article: Nishiguchi S, Nishino K, Kitagawa I, Tokuda Y. Factors associated with delayed diagnosis of infective endocarditis: a retrospective cohort study in a teaching hospital in Japan. Medicine. 2020;99:30(e21418).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Takayama Y, Okamoto R, Sunakawa K. Definite infective endocarditis: clinical and microbiological features of 155 episodes in one Japanese university hospital. J Formosan Med Assoc 2010;109:788–99. [DOI] [PubMed] [Google Scholar]
- [2].Fukuchi T, Iwata K, Ohji G. Failure of early diagnosis of infective endocarditis in Japan--a retrospective descriptive analysis. Medicine 2014;93:e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Koeda C, Tashiro A, Itoh T, et al. Mild renal dysfunction on admission is an important prognostic predictor in patients with infective endocarditis: a retrospective single-center study. Intern Med 2013;52:1013–8. [DOI] [PubMed] [Google Scholar]
- [4].Hanai M, Hashimoto K, Mashiko K, et al. Active infective endocarditis: management and risk analysis of hospital death from 24 years’ experience. Circul J 2008;72:2062–8. [DOI] [PubMed] [Google Scholar]
- [5].Alkhawam H, Sogomonian R, Zaiem F, et al. Morbidity and mortality of infective endocarditis in a hospital system in New York City serving a diverse urban population. J Invest Med 2016;64:1118–23. [DOI] [PubMed] [Google Scholar]
- [6].Shih CJ, Chu H, Chao PW, et al. Long-term clinical outcome of major adverse cardiac events in survivors of infective endocarditis: a nationwide population-based study. Circulation 2014;130:1684–91. [DOI] [PubMed] [Google Scholar]
- [7].Steckelberg JM, Murphy JG, Ballard D, et al. Emboli in infective endocarditis: the prognostic value of echocardiography. Ann Intern Med 1991;114:635–40. [DOI] [PubMed] [Google Scholar]
- [8].Heriot GS, Newcomb A, Darby J, et al. Early transthoracic echocardiography has useful prognostic value in left-sided native valve endocarditis despite limited diagnostic performance. Eur J Clin Microbiol Infect Dis 2019;38:1569–75. [DOI] [PubMed] [Google Scholar]
- [9].Lalani T, Cabell CH, Benjamin DK, et al. Analysis of the impact of early surgery on in-hospital mortality of native valve endocarditis: use of propensity score and instrumental variable methods to adjust for treatment-selection bias. Circulation 2010;121:1005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kang DH, Kim YJ, Kim SH, et al. Early surgery versus conventional treatment for infective endocarditis. N Engl J Med 2012;366:2466–73. [DOI] [PubMed] [Google Scholar]
- [11].Lodise TP, McKinnon PS, Swiderski L, et al. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis 2003;36:1418–23. [DOI] [PubMed] [Google Scholar]
- [12].Naderi HR, Sheybani F, Erfani SS. Errors in diagnosis of infective endocarditis. Epidemiol Infect 2018;146:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000;30:633–8. [DOI] [PubMed] [Google Scholar]
- [14].Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- [15].Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Asai N, Shiota A, Ohashi W, et al. The SOFA score could predict the severity and prognosis of infective endocarditis. J Infect Chemother 2019;25:965–71. [DOI] [PubMed] [Google Scholar]
- [17].Bates DW, Cook EF, Goldman L, et al. Predicting bacteremia in hospitalized patients. A prospectively validated model. Ann Intern Med 1990;113:495–500. [DOI] [PubMed] [Google Scholar]
- [18].Coburn B, Morris AM, Tomlinson G, et al. Does this adult patient with suspected bacteremia require blood cultures? JAMA 2012;308:502–11. [DOI] [PubMed] [Google Scholar]
- [19].Norman DC, Yoshikawa TT. Fever in the elderly. Infect Dis Clin North Am 1996;10:93–9. [DOI] [PubMed] [Google Scholar]
- [20].Jean M, Irisson JO, Gras G, et al. Diagnostic delay of pyogenic vertebral osteomyelitis and its associated factors. Scand J Rheumatol 2017;46:64–8. [DOI] [PubMed] [Google Scholar]
- [21].Mond HG, Irwin M, Ector H, et al. The world survey of cardiac pacing and cardioverter-defibrillators: calendar year 2005 an International Cardiac Pacing and Electrophysiology Society (ICPES) project. Pacing Clin Electrophysiol 2008;31:1202–12. [DOI] [PubMed] [Google Scholar]
- [22].Jedrzejczyk-Patej E, Mazurek M, Kowalski O, et al. Device-related infective endocarditis in cardiac resynchronization therapy recipients - Single center registry with over 2500 person-years follow up. Int J Cardiol 2017;227:18–24. [DOI] [PubMed] [Google Scholar]
- [23].Chen W, Sajadi MM, Dilsizian V. Merits of FDG PET/CT and functional molecular imaging over anatomic imaging with echocardiography and CT angiography for the diagnosis of cardiac device infections. JACC Cardiovasc Imaging 2018;11:1679–91. [DOI] [PubMed] [Google Scholar]
- [24].Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a Scientific statement for healthcare professionals from the American Heart Association. Circulation 2015;132:1435–86. [DOI] [PubMed] [Google Scholar]
- [25].Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075–128. [DOI] [PubMed] [Google Scholar]
- [26].Fowler VG, Jr, Sanders LL, Kong LK, et al. Infective endocarditis due to Staphylococcus aureus: 59 prospectively identified cases with follow-up. Clin Infect Dis 1999;28:106–14. [DOI] [PubMed] [Google Scholar]
- [27].Sekar P, Johnson JR, Thurn JR, et al. Comparative sensitivity of transthoracic and transesophageal echocardiography in diagnosis of infective endocarditis among veterans with staphylococcus aureus bacteremia. Open Forum Infect Dis 2017;4:ofx035. [DOI] [PMC free article] [PubMed] [Google Scholar]


