Abstract
Background:
Delirium is a frequently encountered complication, which is associated with increased mortality. Suvorexant, an approved agent for the treatment of insomnia, is recently suggested to be also effective for prevention of delirium by some authors. However, a consensus has yet to be reached. The goal of this study was to perform a meta-analysis to overall estimate the effectiveness of suvorexant in preventing delirium and its related consequences.
Methods:
Eligible studies were identified by searching online databases of PubMed, EMBASE, and Cochrane Library. The pooled OR was calculated for binary outcomes (e.g., the incidence of delirium, mortality, or adverse events), while standardized mean difference (SMD) were expressed for continuous outcomes (e.g., time to delirium onset, length of stay in hospital and ICU, time on ventilation).
Results:
Seven studies which comprised 402 suvorexant treatment patients and 487 patients with control treatment were included in this meta-analysis. Overall, pooled analysis indicated the incidence of delirium could be significantly reduced (OR, 0.30; P < .001) and time to delirium onset was significantly lengthened (SMD, 0.44; P = .006) in patients undergoing suvorexant treatment compared with controls. Suvorexant had no beneficial effects on the secondary outcomes [length of stay in hospital (SMD, −0.65; P = .161) and ICU (SMD, 0.34; P = .297), time on ventilation (SMD, 1.09; P = .318), drug-related adverse events (OR, drug-related adverse events (OR, 1.66; P = .319) and mortality (OR, 2.21; P = .261)]. Subgroup analysis also confirmed the benefit of suvorexant on the development of delirium, which was significant in any subgroup.
Conclusion:
Suvorexant should be recommended for the prevention of delirium in clinic.
Keywords: delirium, orexin receptor antagonist, suvorexant
1. Introduction
Delirium is a neuropsychiatric syndrome characterized by acute-onset, fluctuating disturbances in attention, thought, consciousness, perception, behavior, orientation, and memory.[1] Delirium is a frequently encountered complication in patients undergoing various surgical procedures, needing for acute care and hospitalized in intensive care unit (ICU), with the incidence ranging from 5% to 39%.[2–5] Delirium is associated with poorer functional outcomes, hospital re-admissions, prolonged ICU, hospital length of stay, reduced quality of life, and increased mortality,[5–9] which may impose a considerable financial burden to the family and society.[5,10] Therefore, exploring the drugs that prevent the development of delirium is of clinical significance.
Suvorexant, a novel orexin receptor antagonist, is an officially-approved agent in Japan and USA for the treatment of insomnia.[11,12] Extensive evidence has demonstrated pretreatment sleep disruption is one of risk factors associated with posttreatment delirium [odd ratio (OR) = 5.24, 95% confidence interval (CI) = 3.61–7.60; P < .001].[13–15] Thus, theoretically, premedication of suvorexant may be potentially effective to prevent the development of delirium by inducing sleep. This hypothesis has been proved by some scholars. For example, Tamura et al found the incidence of delirium in patients undergoing elective coronary artery bypass grafting was significantly lower in the suvorexant treatment group than that in the control group (2.8% vs 21.2%; P = .008).[16] Hatta et al reported delirium developed less among acute care patients taking suvorexant than those without suvorexant administration (0% vs 17%; P = .025).[17] Kawada et al demonstrated the addition of suvorexant to ramelteon reduced the delirium risk in acute stroke patients (7.0% vs 31%, P < .001).[18] However, some studies supported the negative effects of suvorexant on delirium prevention (Masuyama et al: 43.8% vs 58.8%; P = .149[19]; Azuma et al: 14.7% vs 33.9%; P = .069[20]; Booka et al: 0% vs 11.1%; P = .418[21]) compared with the non-suvorexant group. Hereby, it is necessary to comprehensively evaluate the efficacy of suvorexant for delirium prevention by integrating all relevant evidence.
The goal of this study was to perform a meta-analysis to overall estimate the effectiveness of suvorexant in preventing delirium and its related consequences for all patients that have a risk for the development of delirium.
2. Materials and methods
This analysis was conducted in accordance with the recommendations of Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA). Patient consent and ethical approval were not required since this study is a meta-analysis based on studies published. Two reviewer authors independently performed the literature search, inclusion, data extraction, and quality assessment. Disagreements were resolved by consensus with a third reviewer.
2.1. Data sources and searches
PubMed, EMBASE, and Cochrane Library were searched from inception to December 19, 2019 in order to obtain all relevant articles. The search strategy included: (“suvorexant” or “MK-4305”) AND (“delirium”). Furthermore, reference lists of included studies and previous reviews were manually scanned for other potentially eligible trials.
2.2. Inclusion and exclusion criteria
Studies were included if they met all of the following inclusion criteria:
-
1.
comparing suvorexant with other control treatments;
-
2.
prospective randomized controlled trials (RCTs) or retrospective case-control studies;
-
3.
reported at least 1 clinical outcome of interest;
-
4.
the clinical outcomes reported in at least 2 articles; and
-
5.
providing sufficient data for statistical analysis.
Studies were excluded if they were:
-
1.
duplicated publications;
-
2.
case report, reviews or non-human studies;
-
3.
full-text unavailable;
-
4.
not case-control designed;
-
5.
without the outcomes of interest;
-
6.
without detailed data for statistical analysis; and
-
7.
studies written in non-English.
2.3. Data extraction and quality assessment
The following characteristics were extracted from each study: primary author, publication year, country, study design, source of patients, sample size, sex, age, intervention methods, diagnostic criteria of delirium, and several outcome parameters (such as incidence of delirium, time to delirium onset, length of stay in hospital and ICU, time on ventilation, drug-related adverse events, and mortality).
The methodological quality of each study was assessed using the Newcastle–Ottawa Scale (NOS).[22] The total scores obtained from NOS ranged from 0 to 9 and studies with a NOS score more than 7 were considered to be of high quality.
2.4. Statistical analysis
Data were entered into STATA 13.0 software (STATA Corporation, College Station, TX, USA) for this meta-analysis. The pooled OR was calculated for binary outcomes (e.g., the incidence of delirium, mortality, adverse events), while standardized mean difference (SMD) were expressed for continuous outcomes (e.g., time to delirium onset, length of stay in hospital and ICU, time on ventilation). Cochranes Q and I2 statistic tests were used to determine the heterogeneity among studies, with P < .10 and I2 > 50% indicating the presence of heterogeneity. For outcomes with a high heterogeneity among included studies, a random-effect model was used for meta-analysis; otherwise, a fixed-effect model was chosen. Furthermore, a subgroup analysis was also performed according to study design, diagnostic criteria, source of patients, control design, sample size, patients age, and sex. Publication bias was assessed by Egger linear regression test.[23] Sensitivity analysis was performed to evaluate the results stability by omitting each study in turn. P < .05 was considered to be statistically significant.
3. Results
3.1. Study identification
A flow diagram of the study inclusion process is presented in Figure 1. Initially, 638 articles were retrieved by searching the online databases. A total of 459 studies were eliminated following deduplication. After reading titles and abstracts, 170 articles were further removed because they belonged to: case report (n = 7); review (n = 17); animal experiments (n = 14); descriptive studies (n = 39), studies with irrelevant topic (n = 89) or without controls (n = 4). The left 9 studies were reviewed in detail, in which 2 were excluded due to lack of relevant data. Finally, 7 studies (suvorexant treatment group, n = 402; control group, n = 487) were included in our meta-analysis.[16–21,24]
Figure 1.
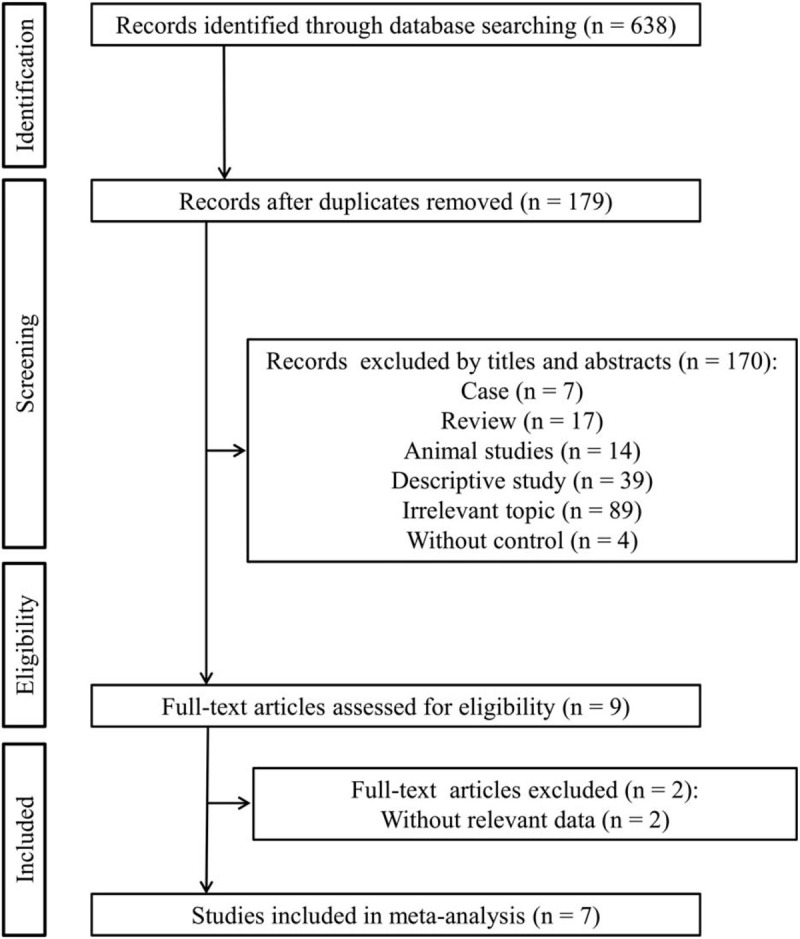
Flow diagram of selection process of the studies.
3.2. Characteristics of the included studies and risk of bias
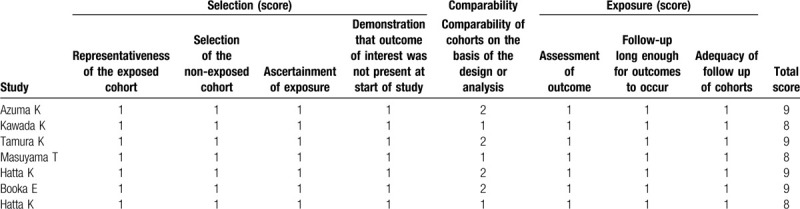
The characteristics of these 7 studies are described in Table 1. The years of publication were between 2017 and 2019 and the sample size involving the cases and controls ranged from 65 to 244. All included studies were conducted for patients in Japan. Three studies were designed as prospective RCTs,[17,20,24] while the other 4 were retrospective case-control studies.[16,18,19,21] Two trials were performed for patients in ICU,[19,20] 4 for non-ICU population[16,18,21,24] and 1 included both of the ICU and non-ICU patients.[17] The average daily dose was 15 or 20 mg. In addition to studies by Hatta et al,[17,24] the control group in the remaining 5 studies underwent treatment with other sleep inducers (such as ramelteon, zolpidem, rilmazafone, etizolam, brotizolam, flunitrazepam, or diazepam). Delirium was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) in 4 studies,[17,20,21,24] Confusion Assessment Method for Intensive Care Unit (CAM-ICU) in 2 studies[18,19] and Intensive Care Delirium Screening Checklist (ICDSC) in 1 study.[16] The total NOS scores varied from 8 to 9, indicating all eligible studies of high-quality (Table 2).
Table 1.
Characteristics of the studies included in the meta-analysis.
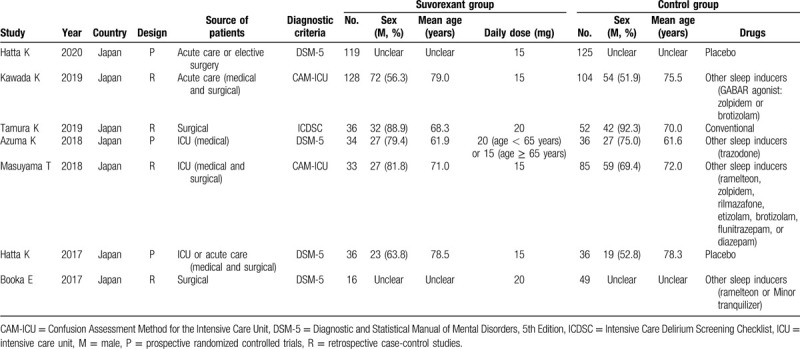
Table 2.
The NOS quality assessment of the included studies.
3.3. Efficacy assessments of suvorexant
All 7 studies evaluated the prevention effects of suvorexant on the incidence of delirium[16–21,24] (Table 3). A fixed-effect model was used for the meta-analysis because there was no heterogeneity present among the studies (I2 = 15.9%, P = .311). Pooled analysis from these 7 trials indicated that the incidence of delirium could be significantly reduced after the use of suvorexant (OR, 0.30; 95% CI, 0.21–0.44, P < .001) (Fig. 2). This benefit of suvorexant was also confirmed in subgroup analyses according to study design (prospective: OR, 0.39; 95% CI, 0.22–0.67, P = .001; retrospective: OR, 0.25; 95% CI, 0.15–0.42, P < .001), diagnostic criteria (DSM-5: OR, 0.36; 95% CI, 0.21–0.62, P < .001; CAM-ICU: OR, 0.28; 95% CI, 0.16–0.49, P < .001; ICDSC: OR, 0.11; 95% CI, 0.01–0.87, P = .036), source of patients (ICU: OR, 0.45; 95% CI, 0.23–0.88, P = .019; non-ICU/mixed: OR, 0.26; 95% CI, 0.16–0.41, P < .001), mean age (>65 years: OR, 0.23; 95% CI, 0.10–0.54, P = .001; unclear: OR, 0.45; 95% CI, 0.24–0.85, P = .015), male percentage (<65%: OR, 0.16; 95% CI, 0.07–0.34, P = .005; >65%: OR, 0.39; 95% CI, 0.21–0.75, P < .001; unclear: OR, 0.45; 95% CI, 0.24–0.85, P = .015), control design (sleep inducers: OR, 0.29; 95% CI, 0.16–0.55, P < .001; others: OR, 0.24; 95% CI, 0.07–0.89, P = .033) and sample size (<100: OR, 0.29; 95% CI, 0.16–0.55, P < .001; >100: OR, 0.24; 95% CI, 0.07–0.89, P = .033) (Table 4).
Table 3.
Study outcomes of individual studies.
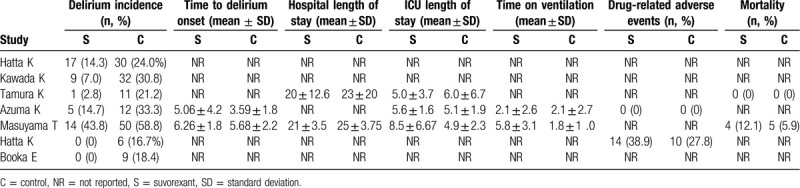
Figure 2.
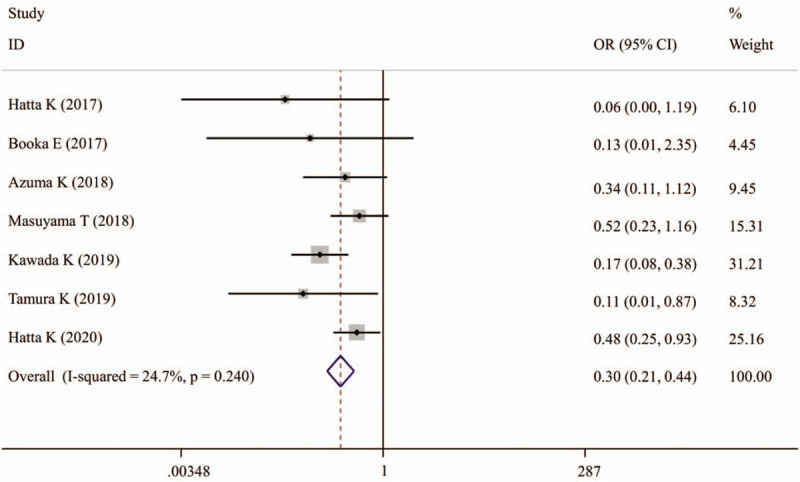
Forest plots showing the preventative effects of suvorexant on the incidence of delirium. CI = confidence interval, OR = odd ratio.
Table 4.
Subgroup analysis.
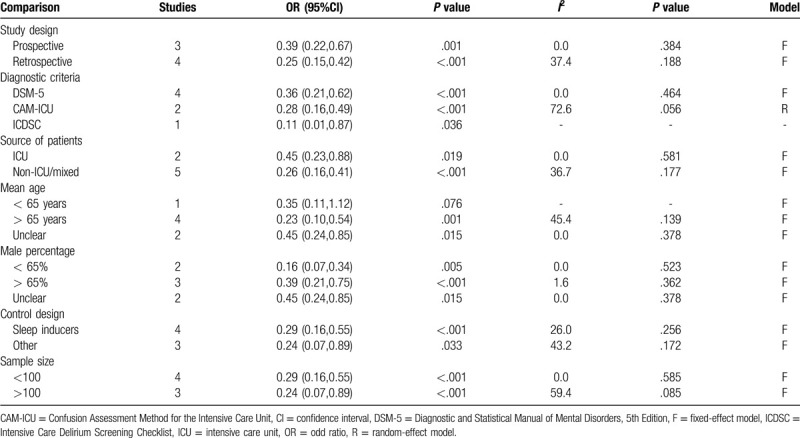
Time to delirium onset was recorded in 2 studies (Table 3). A fixed-effect model was selected for the pooled analysis because no evidence of heterogeneity was observed among these 2 studies (I2 = 0%, P = .414). The meta-analysis results demonstrated that time to delirium onset was significantly longer in patients undergoing suvorexant treatment than those without suvorexant treatment (SMD, 0.44; 95% CI, 0.13–0.74, P = .006) (Fig. 3).
Figure 3.
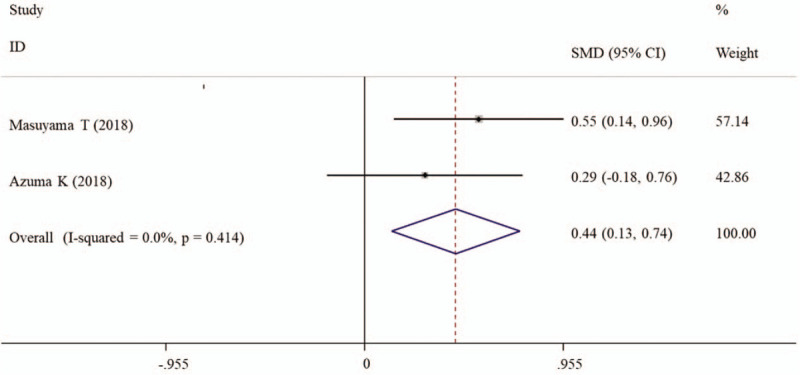
Forest plots showing the overall effects of suvorexant on time to delirium onset. CI = confidence interval. SMD = standardized mean difference.
Two or 2 studies explored the effects of suvorexant on the secondary outcomes, including length of stay in hospital (n = 2) and ICU (n = 3), time on ventilation (n = 2), drug-related adverse events (n = 2), and mortality (n = 2) (Table 3). The pooled results showed that suvorexant seemed to have no beneficial effects on these outcomes compared with other drugs [length of stay in hospital (I2 = 92.3%, P = .001; SMD, −0.65; 95% CI, −1.57–0.26, P = .161) and ICU (I2 = 84.0%, P = .002; SMD, 0.34; 95% CI, −0.30–0.97, P = .297), time on ventilation (I2 = 97.5%, P < .001; SMD, 1.09; 95% CI, −1.05 to 3.22, P = .318), drug-related adverse events (OR, 1.66; 95% CI, 0.62–4.46, P = .319) and mortality (OR, 2.21; 95% CI, 0.55–8.79, P = .261)].
3.4. Publication bias and sensitivity analyses
Publication bias analysis was performed for clinical outcomes reported in more than 2 studies by Egger test. The results showed there was no evidence of publication bias for the incidence of delirium (P = .172) and length of stay in ICU (P = .855). The sensitivity analyses also demonstrated the robust stability of the results (Fig. 4).
Figure 4.
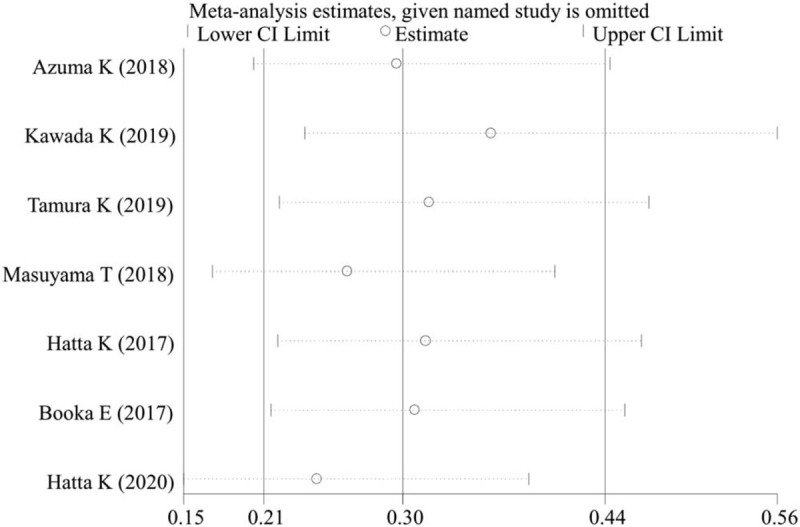
Sensitivity analysis for the incidence of delirium. CI = confidence interval.
4. Discussion
To our knowledge, this is the first meta-analysis to comprehensively evaluate the pharmaceutical effects of suvorexant for the prevention of delirium and its related consequences. In present study, 7 case-control studies were included and both of the overall and subgroup analyses showed that suvorexant could reduce the incidence of delirium compared with other sleep inducers or placebo. Also, overall analysis indicated that time to delirium onset was significantly lengthened in patients undergoing suvorexant treatment. However, suvorexant had no beneficial effects on any of the secondary outcomes, such as hospital mortality, adverse events, length of ICU stay, length of hospital stay, and time on ventilation. These findings were not consistent with some of the individual studies (such as the incidence of delirium, P = .149, .069 and P = .418 in the study of Masuyama et al[19]; Azuma et al,[20] and Booka et al,[21] respectively; length of hospital stay, P = .019 and P = .035 in the study of Kawada et al[18] and Tamura et al[16]; length of ICU stay, P < .001 and P = .001 in the study of Masuyama et al[19] and Tamura et al[16]), demonstrating the necessity and superiority of meta-analysis by increasing the sample size and statistical power.
Suvorexant is a selective dual antagonist for orexin-A and orexin-B receptor. Orexin-A and -B are neuropeptides secreted by perifornical and lateral hypothalamic neurons.[25] By binding to the corresponding receptor, orexin can mediate the excitability of neurons via regulating various ion (potassium, calcium) channels.[26,27] Thus, block of the orexin-receptor pathway may be potentially effective for reducing the excitability of brain neurons.[28] Similar to insomnia, delirium is also a neuroexcitatory symptom. Also, the plasma levels (roughly reflecting the cerebrospinal fluid) of orexin-A, were found to be relatively higher in patients with delirium than those without, although no significant difference was observed.[29] Theoretically, suvorexant may be effective for prevention and treatment of delirium, like insomnia. This hypothesis was proved by previous studies which investigated the improvement in sleep and delirium simultaneously.[18,24,30] In addition, orexin was reported to increase sympathetic nerve activity by promoting expression of pro-inflammatory cytokines [interleukin (IL)-1β, 2.7-fold; IL-6, 1.7-fold; tumor necrosis factor-α, 1.5-fold][31]; while there was also a strong relationship between inflammatory factors and delirium incidence (such as high C-reactive protein: relative risk, 3.0; 95% CI, 1.4–6.7[32]; IL-6: OR, 1.61; 95%CI, 1.00–2.58, P = .04[33]). Hereby, suvorexant may also exert preventative effects for delirium by inhibiting the inflammatory pathways.
This meta-analysis has several limitations. First, suvorexant was approved only in a few countries (Japan and USA), which led to all the included studies performed in Japan. Whether it is effective for the patients of other countries needs subsequent follow-up reports. Second, the treatment duration was not clearly reported in several studies. Thus, we could not evaluate this was a long-term or short-term effect, which may result in an overestimation or underestimation of the effectiveness of suvorexant on delirium prevention. Third, the sample size was small. This may be also another one reason to influence the efficacy estimation for suvorexant. Furthermore, the safety of suvorexant use in clinic remained indefinite by our analysis because only 2 articles recorded the adverse events. Our conclusion showing no significant difference in the incidence of adverse drug reactions between suvorexant and control groups was different from the previous study of suvorexant during treatment of insomnia.[34] Fourth, more than half of the included studies were retrospective in nature. This may cause unavoidable bias in selection of the patients. Fifth, although our subgroup revealed suvorexant may be more effective for treatment of elderly patients (>65 years), the present limitation was that we divided the articles into 2 age groups only based on the mean age. No included studies performed the effect evaluation independently in the population with age >65 or <65 years.[35] Likewise, sex stratification analysis (male or female) was also not conducted.[35] Sixth, there was no evidence to compare suvorexant with other delirium preventative therapeutic agents (such as haloperidol,[36] melatonin,[37] dexmedetomidine, olanzapine, and risperidone[38]). Accordingly, prospective RCTs studies with larger sample size, more control groups (especially other delirium preventative drugs) and specific population (age >65 or <65 years; female and male) should be designed in the future to further validate the clinical value of suvorexant for delirium.
5. Conclusion
This current meta-analysis findings support the use of suvorexant could prevent the development of delirium in all hospitalized patients. Its impacts on hospital mortality, adverse events, length of ICU stay, length of hospital stay, and time on ventilation need additional well-designed prospective RCTs to confirm.
Author contributions
SX, YYC and PLW participated in conception and design of this study. SX and YYC performed acquisition of data and the statistical analyses; JHS was involved in interpretation of data. SX and YYC drafted the manuscript. PLW revised the manuscript for important intellectual content. All authors read and approved the final manuscript.
Footnotes
Abbreviation: CAM-ICU = Confusion Assessment Method for Intensive Care Unit, CI = confidence interval, DSM-5 = the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, ICDSC = Intensive Care Delirium Screening Checklist, ICU = intensive care unit, IL = interleukin, NOS = Newcastle–Ottawa Scale, OR = odd ratio, RCTs = randomized controlled trials, SMD = standardized mean difference.
How to cite this article: Xu S, Cui Y, Shen J, Wang P. Suvorexant for the prevention of delirium: a meta-analysis. Medicine. 2020;99:30(e21043).
SX and YC contributed equally to this work.
The authors report no conflicts of interest in this work.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Mattoo SK, Grover S, Gupta N. Delirium in general practice. Indian J Med Res 2010;131:387–98. [PubMed] [Google Scholar]
- [2].Kanova M, Sklienka P, Roman K, et al. Incidence and risk factors for delirium development in ICU patients - a prospective observational study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2017;161:187–96. [DOI] [PubMed] [Google Scholar]
- [3].Aitken SJ, Blyth FM, Naganathan V. Incidence, prognostic factors and impact of postoperative delirium after major vascular surgery: a meta-analysis and systematic review. Vasc Med 2017;22:387–97. [DOI] [PubMed] [Google Scholar]
- [4].Habeeb-Allah A, Alshraideh JA. Delirium post-cardiac surgery: incidence and associated factors. Nurs Crit Care 2019;doi: 10.1111/nicc.12492. [DOI] [PubMed] [Google Scholar]
- [5].Maria S, Roger S, Soenke B, et al. A hospital-wide evaluation of delirium prevalence and outcomes in acute care patients - a cohort study. BMC Health Serv Res 2018;18:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Koster S, Hensens AG, Schuurmans MJ, et al. Consequences of delirium after cardiac operations. Ann Thorac Surg 2012;93:705–11. [DOI] [PubMed] [Google Scholar]
- [7].Lee H, Ju JW, Oh SY, et al. Impact of timing and duration of postoperative delirium: a retrospective observational study. Surgery 2018;164:137–43. [DOI] [PubMed] [Google Scholar]
- [8].Bagienski M, Kleczynski P, Dziewierz A, et al. Incidence of postoperative delirium and its impact on outcomes after transcatheter aortic valve implantation. Am J Cardiol 2017;120:1187–92. [DOI] [PubMed] [Google Scholar]
- [9].Crocker E, Beggs T, Hassan A, et al. Giles-Smith L: long-term effects of postoperative delirium in patients undergoing cardiac operation: a systematic review. Ann Thorac Surg 2016;102:1391–9. [DOI] [PubMed] [Google Scholar]
- [10].Pezzullo L, Streatfeild J, Hickson J, et al. Economic impact of delirium in Australia: a cost of illness study. BMJ Open 2019;9:e027514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexant—a novel dual orexin receptor antagonist. J Neurogenet 2011;25:52–61. [DOI] [PubMed] [Google Scholar]
- [12].Kuriyama A, Tabata H. Suvorexant for the treatment of primary insomnia: a systematic review and meta-analysis. Sleep Med Rev 2016;35:1–7. [DOI] [PubMed] [Google Scholar]
- [13].Leung JM, Sands LP, Newman S, et al. Preoperative sleep disruption and postoperative delirium. J Clin Sleep Med 2015;11:907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang S, Sigua NL, Manchanda S, et al. Preoperative STOP-BANG scores and postoperative delirium and coma in thoracic surgery patients. Ann Thorac Surg 2018;106:966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fadayomi AB, Ibala R, Bilotta F, et al. A Systematic review and meta-analysis examining the impact of sleep disturbance on postoperative delirium. Crit Care Med 2018;46:e1204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tamura K, Maruyama T, Sakurai S. Preventive effect of suvorexant for postoperative delirium after coronary artery bypass grafting. Ann Thorac Cardiovasc Surg 2019;25:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hatta K, Kishi Y, Wada K. Preventive effects of suvorexant on delirium a randomized placebo-controlled trial. J Clin Psychiatry 2017;78:e970–9. [DOI] [PubMed] [Google Scholar]
- [18].Kawada K, Ohta T, Tanaka K, et al. Addition of suvorexant to ramelteon therapy for improved sleep quality with reduced delirium risk in acute stroke patients. J Stroke Cerebrovasc Dis 2019;28:142–8. [DOI] [PubMed] [Google Scholar]
- [19].Masuyama T, Sanui M, Yoshida N, et al. Suvorexant is associated with a low incidence of delirium in critically ill patients: a retrospective cohort study. Psychogeriatrics 2018;18:209–15. [DOI] [PubMed] [Google Scholar]
- [20].Azuma K, Takaesu Y, Soeda H, et al. Ability of suvorexant to prevent delirium in patients in the intensive care unit: a randomized controlled trial. Acute Med Surg 2018;5:362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Booka E, Tsubosa Y, Matsumoto T, et al. Postoperative delirium after pharyngolaryngectomy with esophagectomy: a role for ramelteon and suvorexant. Esophagus 2017;14:229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [23].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hatta K, Kishi Y, Wada K, et al. Real-world effectiveness of ramelteon and suvorexant for delirium prevention in 948 patients with delirium risk factors. J Clin Psychiatry 2020;81:19m12865. [DOI] [PubMed] [Google Scholar]
- [25].van den Top M, Nolan MF, Lee K, et al. Orexins induce increased excitability and synchronisation of rat sympathetic preganglionic neurones. J Physiol 2003;549:809–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hoang QV, Zhao P, Nakajima S, et al. Orexin (hypocretin) effects on constitutively active inward rectifier K+ channels in cultured nucleus basalis neurons. J Neurophysiol 2004;92:3183–91. [DOI] [PubMed] [Google Scholar]
- [27].Larsson KP, Peltonen HM, Bart G, et al. Orexin-A-induced Ca 2 + Entry. J Biol Chem 2005;280:1771–81. [DOI] [PubMed] [Google Scholar]
- [28].Han Y, Yuan K, Zheng Y, et al. Orexin receptor antagonists as emerging treatments for psychiatric disorders. Neurosci Bull 2020;36:432–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nakashima H, Umegaki H, Yanagawa M, et al. Plasma orexin-A levels in patients with delirium. Psychogeriatrics 2019;19:628–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hanazawa T, Kamijo Y. Effect of suvorexant on nocturnal delirium in elderly patients with Alzheimer's disease: a case-series study. Clin Psychopharmacol Neurosci 2019;17:547–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fan Y, Jiang E, Hahka T, et al. Orexin A increases sympathetic nerve activity through promoting expression of proinflammatory cytokines in Sprague-Dawley rats. Acta Physiol 2018;222:e12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vasunilashorn SM, Ngo LH, Inouye SK, et al. Apolipoprotein E genotype and the association between C-reactive protein and postoperative delirium: Importance of gene-protein interactions. Alzheimers Dement 2020;16:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kowalska K, Klimiec E, Weglarczyk K, et al. Reduced ex vivo release of pro-inflammatory cytokines and elevated plasma interleukin-6 are inflammatory signatures of post-stroke delirium. J Neuroinflammation 2018;15:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kishi T, Matsunaga S, Iwata N. Suvorexant for primary insomnia: a systematic review and meta-analysis of randomized placebo-controlled trials. PLoS One 2015;10:e0136910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Herring WJ, Connor KM, Snyder E, et al. Suvorexant in Patients with Insomnia: pooled analyses of three-month data from phase-3 randomized controlled clinical trials. J Clin Sleep Med 2016;12:1215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen Z, Chen R, Zheng D, et al. Efficacy and safety of haloperidol for delirium prevention in adult patients: an updated meta-analysis with trial sequential analysis of randomized controlled trials. J Clin Anesth 2020;61:109623. [DOI] [PubMed] [Google Scholar]
- [37].Campbell AM, Axon DR, Martin JR, et al. Melatonin for the prevention of postoperative delirium in older adults: a systematic review and meta-analysis. BMC Geriatr 2019;19:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liu Y, Li XJ, Liang Y, et al. Pharmacological prevention of postoperative delirium: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med 2019;2019:9607129. [DOI] [PMC free article] [PubMed] [Google Scholar]


