Abstract
The utility of endovascular thrombectomy for acute occlusion of the distal intracranial artery (A2/A3/M2/M3/P2/P3) is unclear, and aspiration and stent thrombectomy are associated with risk of bleeding. We analyzed patients with acute occlusion of the distal intracranial artery to assess the safety and efficacy of microcatheter-based tirofiban infusion.
We retrospectively reviewed data of the endovascular thrombectomy registry of our center between January 2018 and June 2019. Patients with distal intracranial artery occlusion who underwent endovascular thrombectomy with microcatheter-based infusion of tirofiban were recruited.
Of 13 patients included, 1 presented with anterior cerebral artery occlusion, 2 with posterior cerebral artery occlusion, 2 with posterior inferior cerebellar artery occlusion, and 7 with middle cerebral artery M2 occlusion. The mean National Institute of Health Stroke scale score was 10.1 (3–19). Three patients (23.1%) underwent bridging treatment of intravenous thrombolysis with recombinant plasminogen activator and endovascular thrombectomy. The arithmetic mean onset-to-recanalization time was 696.3 minutes (140–1440) and average operating time was 47.1 minutes (30–80). After treatment, 10 patients (76.9%) underwent revascularization. No operative complications were observed in any case. All patients underwent angiography and were reviewed 7 to 14 days after surgery. Imaging revealed significant improvements in recanalization compared with the immediate postoperative period, with no reoccurrence of occlusion. The mean modified Rankin scale score at the 3-month follow-up was 0.54 (0–2).
Microcatheter-based infusion of bolus-dose tirofiban can result in safe and effective recanalization of acute occlusion of the distal artery in the case of a relatively light thrombotic load.
Keywords: acute ischemic stroke, distal, endovascular procedures, intracranial arterial, tirofiban
1. Introduction
The primary goal of treatment for acute ischemic stroke[1] (AIS) is to restore vascular recanalization and cerebral reperfusion as soon as possible after the onset of stroke.[2] In addition to intravenous thrombolysis, the 2018 guidelines for AIS recommend mechanical thrombolysis within 6 hours of anterior circulation infarction.[2] The DAWN and DEFUSE-3 trials suggested that thrombectomy is effective in selected acute admissions with small cores and large ischemic penumbra within 16 and 24 hours, respectively.[3,4] Nevertheless, there is currently no evidence for the effectiveness of acute mechanical thrombectomy for distal occlusions in the intracranial artery (A2/A3/M2/M3/P2/P3/PICA segments). Studies have shown that traditional antithrombotic therapy is not ideal for patients with anterior cerebral artery and middle cerebral artery M2 segment occlusion.[5–7] This poses the following question: Is there a more effective intervention for patients with distal occlusions in the intracranial artery than intravenous thrombolysis within a 4.5-hour time window? A small number of studies have been published focusing on direct aspiration or stent thrombectomy of the anterior or middle cerebral artery M2 segments. These reports provide evidence suggesting that this therapy is insufficient is some cases and is associated with a considerable risk of operation-related complications such as vasospasm, dissection, and perioperative bleeding.[5,6,8–12] Tirofiban is a reversible, nonpeptide platelet glycoprotein IIb/IIIa receptor antagonist. While it is mainly used to treat unstable angina and in coronary angioplasty, its potential use for acute thrombosis and stent implantation during neuro-interventional surgery has been reported.[13] Use of tirofiban is also suggested to be beneficial during or after surgery for AIS; however, the results are inconsistent and it is possible that tirofiban may increase the risk of bleeding.[14] The present study aimed to answer the following question: For AIS patients who are beyond the 6-hour window, and for patients with simple distal occlusion in the intracranial artery (at the A2/A3/M2/M3/P2/P3/PICA segments) determined by angioplasty, is it safe and effective to microcatheter-based thrombolytic infusion of tirofiban to recanalize occluded vessels? Here, we describe our technique for the procedure and provide the imaging and clinical outcomes of AIS patients.
2. Materials and methods
2.1. Study population
We recruited consecutive patients who presented to our center with AIS between January 2018 and June 2019. Inclusion criteria were as follows: presentation within 24 hours of stroke onset; complete angioplasty evaluation and emergency mechanical thrombectomy; and treatment with microcatheter-based infusion of bolus-dose tirofiban into the thrombus, as per Crockett et al.[15] Exclusion criteria: the cases with incomplete data in Electronic Medical Record, lack of complete imaging data and lack of follow-up data which were excluded. A neurologist obtained a rapid medical history, physical examination, and National Institute of Health Stroke scale (NIHSS) score evaluation[16] for each patient in the emergency department.
2.2. Imaging
Immediately after suspecting AIS, physicians implemented one or more of the standard stroke imaging assessments: head computed tomography (either computed tomography [CT] scan, CT angiography [CTA], or CT perfusion [CTP]), magnetic resonance imaging (including diffusion-weighted imaging, fluid attenuation inversion recovery, time-of-flight MR angiogram, or perfusion-weighted imaging) to assess the need for intravenous thrombolysis or mechanical thrombectomy.[2] Implementation and determination of the dosage of intravenous thrombolytics were performed according to current guidelines.[2] Patients requiring thrombectomy were evaluated using multimodal imaging to identify large-vessel occlusion, and small-core infarction was determined according to DAWN[3] and DEFUSE-3[4] criteria. Large ischemic penumbra, severe clinical symptoms, progressive stroke, and intracranial hemorrhage were excluded by imaging within 24 hours.
2.3. Outcomes
The primary outcome was the restoration of forward flow of occluded vessels and a thrombolysis in cerebral infarction (TICI) score of ≥2b1. The modified Rankin scale (mRS) score 90 days after stroke onset was the secondary outcome, with a good functional outcome defined as mRS ≤2.
2.4. Procedure
After cerebral angiography, the interventional neurosurgeon determined whether distal intracranial artery (A2/A3/M2/M3/P2/P3/PICA) occlusion was present by evaluating the clinical symptoms and imaging results, and therefore decided whether interventional treatment should be performed under local or general anesthesia. Using an 8F or 6F guide catheter, an intermediate catheter was used to provide support where necessary, and a microcatheter guided by a 0.014 guidewire was directed to the proximal and distal regions of blocked vessels to perform angiography. The position and length of the thrombus were determined. A microcatheter was placed in contact with the thrombus and tirofiban was infused at a flow rate of 1 mL/min and maximum dosage of 10 μL/kg. Imaging was performed every 5 minutes to determine whether the blood flow had been restored. If the forward flow was restored, infusion was immediately stopped and a 15-minute observation for re-occlusion was performed. The absence of reocclusion, together with a TICI level of 2b or 3, was taken to indicate success of the operation. However, aspiration or mechanical-stent thrombectomy were considered if blood flow was not restored after the maximum infusion dose of tirofiban had been reached. Following surgery, DynaCT was used to exclude postoperative intracranial hemorrhages.
2.5. Postoperative treatment
All patients were admitted to the neurologic intensive care unit for close observation after the operation, then transferred to the stroke unit as soon as vital signs stabilized. Head CT was re-examined 24 to 48 hours postoperatively or at the time of early neurologic deterioration if this occurred. When clinical symptoms were stable, CTA, MRA, or digital subtraction angiography (DSA) were performed 7 to 14 days after the operation to compare the occluded and open blood vessels. Postoperative NIHSS and mRS scores were assessed by independent stroke neurologists on the ward. At 3 months after onset, patients were followed up by the neurologists via the outpatient service or by telephone, and the mRS score was evaluated.
2.6. Data collection and analysis
We collected data including baseline characteristics (age, gender), medical history (hypertension, diabetes, coronary heart disease, atrial fibrillation, smoking, etc), time of onset of symptoms, and NIHSS score. The times of admission, head CT, thrombolysis, entering the operating room, and blood-flow recanalization were recorded, along with the total length of operation. Blood vessel diameter, occlusion site, and thrombus length were determined by angiography. Angiographic evaluation of vascular recanalization, TICI grading, and complications (contrast extravasation, vascular dissection, and vasospasm) was carried out by experienced neuro-interventional physicians. The presence of cerebral infarction, cerebral hemorrhage, or subarachnoid hemorrhage was determined by routine follow-up imaging.
2.7. Ethical statement
This study was conducted in accordance with the principles of the Helsinki Declaration. This study and all of the protocols were approved by the Institutional Review Board of The 940th Hospital of Joint Logistic Support Force of the PLA (approval number: 2019XYLL057). The written informed contents were obtained from all the patients.
2.8. Statistical analysis
For continuous variables, those conforming to a normal distribution and a non-normal distribution are expressed as mean (standard deviation) and median (interquartile range), respectively. Data analysis was performed using the SPSS version 19.0 software.
3. Results
3.1. Study population
From January 2018 to June 2019, 414 patients presenting to our clinic met the eligibility criteria. Of these, 95 underwent complete angiographic evaluation and emergency mechanical thrombectomy, 13 of whom were treated with microcatheter-based thrombolytic infusion of bolus-dose tirofiban. These 13 patients all underwent thrombectomy of distal occlusion in the intracranial artery (A2/A3/M2/M3/P2/P3/PICA) and were enrolled in the study.
3.2. Operative data and outcome measurements
Baseline characteristics and outcome indicators of the enrolled patients are summarized in Tables 1 and 2. In 2 patients (15.4%), stroke developed while they were hospitalized (case 1, Fig. 1 and case 6, Fig. 2), and 3 (23.1%; cases 6, 7, and 13) received intravenous thrombolysis with tissue plasminogen activator and bridging for intravascular intervention. For all patients, tirofiban was applied to the thrombus via microcatheter that was placed in advance at the location of the thrombus, to achieve recanalization of the occluded vessel. The mean dose of tirofiban was 10 mL (9–12). There were 3 cases of failure (23.1%; cases 3, 7, and 13) that required stent-retriever thrombectomy Solitaire FR (ev3/Covidien, Irvine, CA, USA) and Trevo PorVue (Stryker, Fremont, CA, USA). Vascular recanalization (TICI 2b) was achieved in all cases, 10 (76.9%; cases 1, 2 [Fig. 3], 4–6, 8–12) achieved vascular recanalization after microcatheterization of tirofiban. The lengths of thrombi were 2 to 5 mm in all patients other than cases 3, 7, and 13, where the thrombus complex was large (10 mm in length). The mRS score before stroke was 0 in all except 1 case, where postoperative immobilization of lower-limb fracture influenced the score. Vascular imaging at 7 to 14 days postoperatively indicated significant improvements in segment reocclusion, without new infraction or reocclusion. The median NIHSS score 7 days after surgery was 7 points lower compared with before surgery. The mean mRS score at 3 months after onset was 0.54 (0.776), and the clinical prognosis of all patients was improved.
Table 1.
Baseline clinical characteristics.
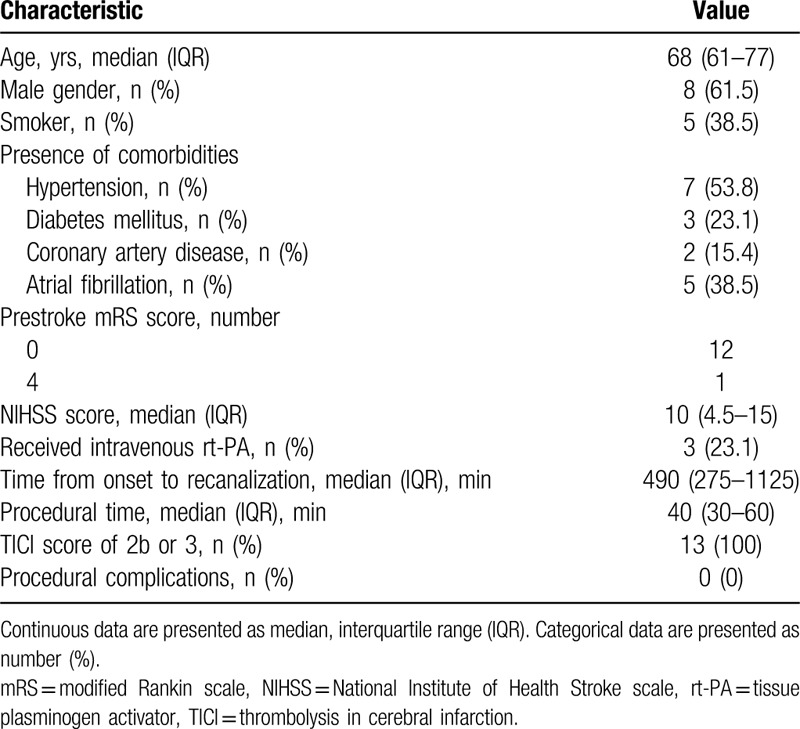
Table 2.
Summary of clinical characteristics and outcomes of patients with distal intracranial arterial occlusion.
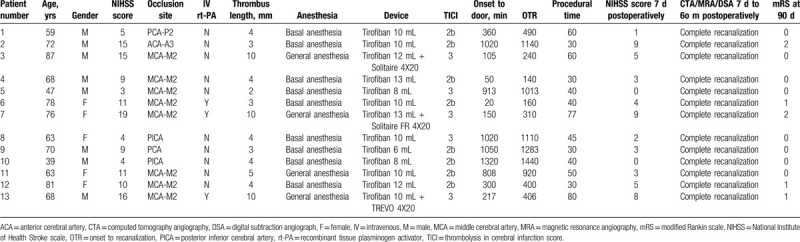
Figure 1.
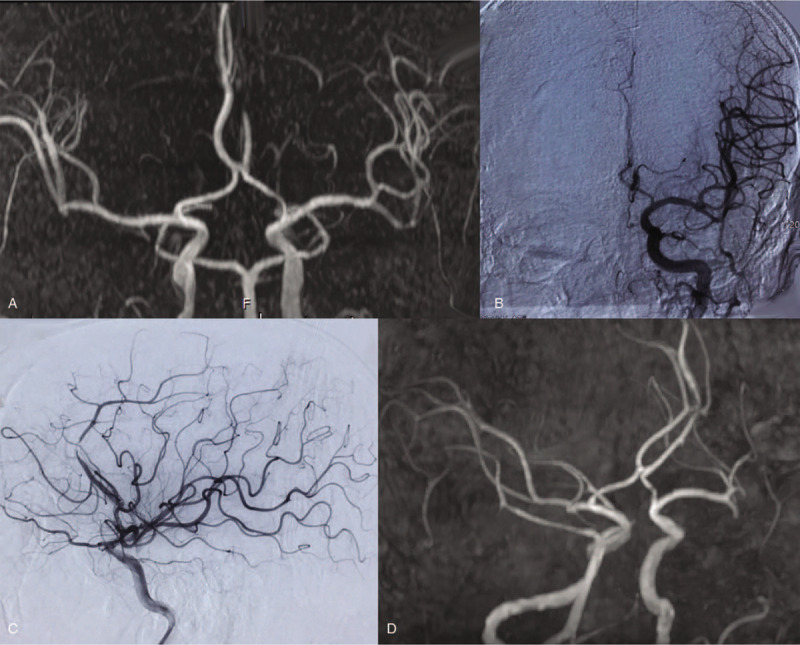
Imaging findings from a 59-year-old man with vertigo, right-side facial numbness, and locomotor ataxia for 6 hours (case 1). (A) Computed tomography angiography (CTA) revealed the right posterior cerebral artery P2 segment to be occluded. (B) Digital subtraction angiography (DSA) confirmed CTA findings. (C) About 10 mL of tirofiban was infused into the occluded vessel through the microcatheter; however, the right posterior cerebral artery P2 was only partially recanalized. (D) After 2 weeks, reexamination of DSA revealed complete recanalization of the right posterior cerebral artery P2.
Figure 2.
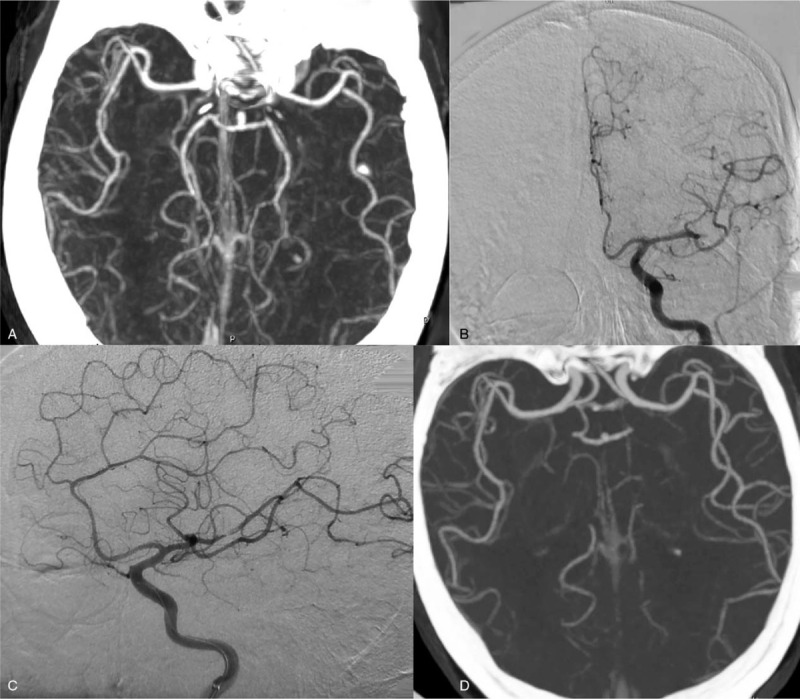
Imaging findings from a 78-year-old woman with global motor aphasia and right limb hemiplegia for 2 hours and 13 minutes (case 6). (A) Head computed tomography angiography (CTA) revealing occlusion of the left middle cerebral artery, M2 segment. (B) Digital subtraction angiography (DSA) confirming CTA findings. (C) After 10 mL of tirofiban was infused into the occluded vessel, DSA revealed the left middle cerebral artery M2 to be recanalized; however, stenosis remained. (D) After 2 weeks, repeat head CTA revealed residual stenosis to have disappeared completely.
Figure 3.
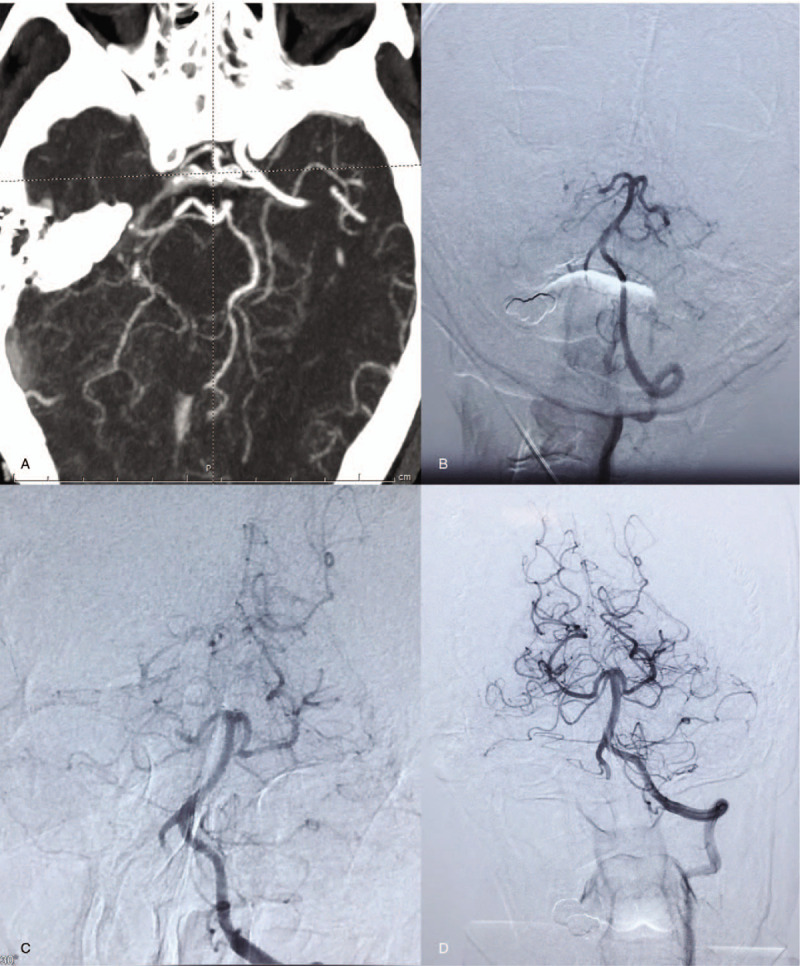
Imaging findings from a 72-year-old man (case 2) with partial motor aphasia and right-limb hemiplegia for 17 hours. (A) Magnetic resonance angiography (MRA) revealed an occluded left anterior cerebral artery A3 segment. (B) Digital subtraction angiography confirmed the MRA findings. (C) After 10 mL of tirofiban was infused into the occluded vessel, the left anterior cerebral artery A3 was recanalized; however, stenosis remained. (D) After 1 week, repeat head MRA revealed that residual stenosis of left intracranial artery A3 had disappeared completely.
3.3. Complications
No intraoperative bleeding, vascular spasm, mezzanine, or other operation-related complications were observed in any patient. Neither subarachnoid hemorrhages nor parenchymal hemorrhages were found in any of the postoperative CT images.
4. Discussion
The benefits of thrombectomy for intracranial large vessel occlusion are widely known. Improvements in instrumentation, multimodal imaging methods, and expertise of surgeons mean that the potential time window for thrombectomy following stroke onset has been increased, and more sites of thrombectomy can be used. There is evidence to suggest that thrombectomy has beneficial effects for embolism from the distal intracranial arteries (A2/A3/M2/M3/P2/P3).[5,10] However, because of the circuit in which the distal artery is included and the small vascular diameter, it is difficult for existing techniques, including aspiration (even via aspiration catheter), to reach the desired location and provide effective therapy.[6] Recently, micro-ADAPT technology was developed.[15] Although this technology provides a rapid and effective technique for thrombus extraction from the A3, M3, and P3 segments, it is difficult for finer vascular aspiration catheters to be positioned in contact with the thrombus for suction. This therapy also has limitations for distal vessels, and the outcome may be disastrous if bleeding occurs. Studies have shown that neurologic deficits are aggravated following anterior cerebral artery or middle cerebral artery M2 occlusion[8]; therefore, the development of new methods of intervention for these distal vessels is crucial.
In the present study, all 13 patients were 1st treated with bolus-dose tirofiban via microcatheter infusion directly to the thrombus. In 10 patients (76.9%), forward blood flow was achieved; in three cases, use of the Solitaire FR 4/20-mm stent and Trevo ProVue 4/20 mm were required for thrombectomy and recanalization. In a retrospective study of 30 patients with cerebral artery occlusion,[5] treatment failed in 5 patients because the microwire and microcatheter could not be put in place. Stent thrombectomy was successful in 22 (88%) of the remaining 25 patients; however, 3 (10%) suffered vasospasm and 1 (3.3%) dissection was reported. Bhogal et al[6] reviewed 585 cases of middle cerebral artery thrombectomy, including 106 cases of M2 segment occlusion. Successful recanalization of blood vessels was achieved in 90.5% of patients; however, the bleeding rate was 26.4%. Similarly, a meta-analysis conducted by Saber et al,[10] including 1080 patients with M2 segment occlusion from 12 studies, reported the rates of recanalization, mortality, and symptomatic intracranial hemorrhage to be 81%, 16%, and 10%, respectively, after mechanical thrombolysis by aspiration or stent. In our study, we found microcatheter infusion of bolus-dose tirofiban for AIS due to occlusion of distal intracranial arterial resulted in a recanalization rate of 76.9%, with no deaths or symptomatic intracranial hemorrhages.
The method presented here may offer an option that is safer than aspiration or stent thrombectomy, possibly because the microcatheter has less impact on the blood vessels, meaning that there will be no rupture of the perforator vessel due to the stent pulling on the blood vessels, and there is no risk of vascular displacement or vasospasm caused by the large aspiration catheter. The risk of reperfusion hemorrhage is also lower because of the partial restoration of forward flow, after which the thrombus is gradually dissolved into the circulation. Reexamination of vascular MRA, CTA, and DSA results suggested that the thrombus was significantly smaller or had disappeared, and the scope of infarction was not larger following infusion of tirofiban. Immediate clinical symptoms of intraoperative blood flow recanalization were significantly improved in cases 1 and 8, and postoperative muscle strength was significantly improved in case 5. The average NIHSS score of patients was lower by 6 points at discharge compared with those at admission, and the mRS score at 3 months was ≤2 for all patients. Although the clinical symptoms of some patients with AIS are relatively mild, this method could improve prognosis.
The average operative time in the present study was not lower than that of aspiration or stent thrombectomy[5,8]; this may be related to the proficiency of the surgeons. It was nevertheless shorter than the average time for thrombectomy at our center. Although injection of tirofiban requires time; overall, it reduces the time of procedure as it removes the need for stenting and the subsequent 5-minute wait, thereby allowing rapid recanalization and recovery of brain-tissue perfusion. Nevertheless, in the present study, mechanical thrombectomy was performed again in three patients (cases 3, 7, and 13), resulting in prolonged operation time and increased recanalization time.
There are many factors that influence the success of the procedure, including the nature and length of the embolus. Of all the patients in this study, 5 presented with atrial fibrillation, which were considered to be cardiogenic strokes, and 7 with nonatrial fibrillation-related stroke, which were considered to be arterial embolisms. There appeared to be no correlation between embolism type and recanalization; the pathology of the embolus could not be assessed because the thrombi were not removed. The small sample size of this study means that the association between the nature of the embolus and recanalization cannot be confirmed.
In cases 3, 7, and 13, where the thrombus complex was large (length = 10 mm), infusion of tirofiban was unsuccessful, and the vessel was recanalized after removal of the stent. The forward blood flow during the operation and re-examination of MRA, CTA, or DSA 7 to 14 days after the operation indicated that recanalization of the blood vessel was significantly improved compared with recanalization during the operation in all other patients. This suggests that the length of the thrombus may affect the recanalization of occluded vessels by the present method.
The present study had some limitations. First, this was a retrospective study with a small number of cases, and no control group. This means that the data are likely to suffer from selection bias. Second, the use of tirofiban was off-label. Although good outcomes were observed among this small sample group, the safety of the drug requires further investigation. Finally, the method of tirofiban infusion may not be applicable for occlusions in the internal carotid artery, middle cerebral artery M1 segment, or basilar artery due to the relatively heavy thrombotic load.
5. Conclusion
Introduction of tirofiban through a microcatheter can result in safe and effective recanalization of acute occlusions of the intracranial distal arterial that have a relatively light thrombotic load. Future studies with larger sample sizes as well as prospective randomized control trials are needed to further assess the safety and efficacy of tirofiban infusion in this context.
Acknowledgments
The authors thank Editage (https://app.editage.com/?type=individual) for its linguistic assistance during the preparation of this manuscript.
Author contributions
Conceptualization: Rong Yin.
Data curation: Shao Ju Shao, Long Zhao, Hong Bin Ma.
Formal analysis: Guo Zhen Zhang, Zhi Qi Yang, Rong Yin.
Funding acquisition: Rong Yin.
Investigation: Shaoju Shao, Guo Zhen Zhang, Fa Rong Huo.
Methodology: Rong Yin.
Resources: Ling Zhu.
Project administration: Rong Yin.
Supervision: Rong Yin.
Visualization: Zhi Qi Yang.
Writing – original draft: Rong Yin, Zhi Qi Yang.
Writing – review & editing: Zhi Qi Yang, Rong Yin.
Footnotes
Abbreviations: ACA = anterior cerebral artery, AIS = acute ischemic stroke, CT = computed tomography, CTA = computed tomography angiography, CTP = computed tomography perfusion, DSA = digital subtraction angiography, MCA = middle cerebral artery, mRS = modified Rankin scale, NIHSS = National Institute of Health Stroke scale, OTR = onset to recanalization, PICA = posterior inferior cerebral artery, rt-PA = recombinant tissue plasminogen activator, TICI = thrombolysis in cerebral infarction.
How to cite this article: Shao SJ, Zhang GZ, Zhao L, Huo FR, Ma HB, Zhu L, Yang ZQ, Yin R. Microcatheter infusion of bolus-dose tirofiban for acute ischemic stroke due to distal intracranial artery occlusion. Medicine. 2020;99:30(e21366).
SJS, GZZ, and LZ contributed equally to the work.
The authors have no funding and conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013;44:2650–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018;49:e46–110. [DOI] [PubMed] [Google Scholar]
- [3].Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- [4].Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pfaff J, Herweh C, Pham M, et al. Mechanical thrombectomy of distal occlusions in the anterior cerebral artery: recanalization rates, periprocedural complications, and clinical outcome. AJNR Am J Neuroradiol 2016;37:673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bhogal P, Bucke P, AlMatter M, et al. A comparison of mechanical thrombectomy in the m1 and m2 segments of the middle cerebral artery: a review of 585 consecutive patients. Interv Neurol 2017;6:191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dorn F, Lockau H, Stetefeld H, et al. Mechanical thrombectomy of m2-occlusion. J Stroke Cerebrovasc Dis 2015;24:1465–70. [DOI] [PubMed] [Google Scholar]
- [8].Uno J, Kameda K, Otsuji R, et al. Mechanical thrombectomy for acute anterior cerebral artery occlusion. World Neurosurg 2018;120:e957–61. [DOI] [PubMed] [Google Scholar]
- [9].Chen CJ, Wang C, Buell TJ, et al. Endovascular mechanical thrombectomy for acute middle cerebral artery m2 segment occlusion: a systematic review. World Neurosurg 2017;107:684–91. [DOI] [PubMed] [Google Scholar]
- [10].Saber H, Narayanan S, Palla M, et al. Mechanical thrombectomy for acute ischemic stroke with occlusion of the m2 segment of the middle cerebral artery: a meta-analysis. J Neurointerv Surg 2018;10:620–4. [DOI] [PubMed] [Google Scholar]
- [11].Salahuddin H, Ramaiah G, Slawski DE, et al. Mechanical thrombectomy of m1 and m2 middle cerebral artery occlusions. J Neurointerv Surg 2018;10:330–4. [DOI] [PubMed] [Google Scholar]
- [12].Gory B, Lapergue B, Blanc R, et al. Contact aspiration versus stent retriever in patients with acute ischemic stroke with m2 occlusion in the aster randomized trial (contact aspiration versus stent retriever for successful revascularization). Stroke 2018;49:461–4. [DOI] [PubMed] [Google Scholar]
- [13].Zhao W, Che R, Shang S, et al. Low-dose tirofiban improves functional outcome in acute ischemic stroke patients treated with endovascular thrombectomy. Stroke 2017;48:3289–94. [DOI] [PubMed] [Google Scholar]
- [14].Wu Y, Yin C, Yang J, et al. Endovascular thrombectomy. Stroke 2018;49:2783–5. [DOI] [PubMed] [Google Scholar]
- [15].Crockett MT, Phillips TJ, Chiu AHY. Dual suction Headway27 microcatheter thrombectomy for the treatment of distal intracranial arterial occlusion strokes: initial experience with the micro-ADAPT technique. J Neurointerv Surg 2019;11:714–8. [DOI] [PubMed] [Google Scholar]
- [16].Meyer BC, Hemmen TM, Jackson CM, et al. Modified national institutes of health stroke scale for use in stroke clinical trials: prospective reliability and validity. Stroke 2002;33:1261–6. [DOI] [PubMed] [Google Scholar]


