Supplemental Digital Content is available in the text
Keywords: bladder cancer, hematological markers, meta
Abstract
Background:
Accumulating emerging studies have demonstrated that systemic inflammation can obviously affect tumor occurrence and progression. Nevertheless, the prognostic value of hematological inflammation biomarkers in bladder cancer is controversial. Thus, we conducted a meta-analysis to evaluate the key hematological biomarkers with various clinical outcomes in bladder cancer.
Methods:
We used online databases PUBMED and EMBASE to search relevant studies published prior to August 2019. After collecting the basic characteristics and prognostic data from the studies included, overall survival (OS), cancer-specific survival (CSS) and progression-free survival (PFS) were used as primary results. Subgroup analyses were performed according to ethnicity, the number of samples, survival outcomes, the value of cut-off, follow-up time and metastasis stage.
Results:
Thirty-three independent studies with 17,087 bladder cancer patients were added in the present analysis. The collected results showed that the increased neutrophil-to-lymphocyte ratio was associated with a poor OS (hazard ratio [HR] = 1.48, 95% confidence interval [CI]: 1.32–1.67, P < .00001), CSS (HR = 1.71, 95%CI: 1.35–2.18, P < .0001) and PFS (HR = 1.59, 95%CI: 1.38–1.83, P < .00001). Additionally, the elevated platelet-to-lymphocyte ratio was related to a poor OS (HR = 1.29, 95% CI: 1.07–1.54, P = .007), CSS (HR = 1.14, 95%CI = 0.98–1.34, P = .02) and PFS (HR = 1.2, 95%CI: 1.08–1.34, P = .0008). Moreover, a decreased lymphocyte-to-monocyte ratio was associated with a poor OS (HR = 0.77, 95% CI: 0.70–0.84, P = .001), CSS (HR = 0.76, 95%CI: 0.70–0.84). An elevated modified Glasgow prognostic score was also associated with a poor OS (HR = 2.71, 95%CI: 1.08–2.82, P = .003), CSS (HR = 1.50, 95%CI: 0.56–4.05) and PFS (HR = 1.52, 95%CI: 1.23–1.88, P = .001).
Conclusions:
Our study indicated that the pretreatment hematological biomarkers (neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and modified Glasgow prognostic score) were predicative biomarkers of prognosis in bladder cancer patients. Further research is needed to conduct further prospective and multicenter studies to confirm our findings.
1. Introduction
Bladder cancer has become one of the most commonly seen malignancies of the urinary system in the United States of America. An American research estimates that 80470 new bladder cancer patients and 1767 deaths in 2019.[1] In China, the mortality and morbidity rate associated with bladder cancer ranked second compared to all other malignancies of urinary system.[2] Bladder cancer is generally divided into 2 types, muscle-invasive bladder cancer (20%–30%) and non-muscle invasive bladder cancer (70%–80%).[3] For muscle-invasive bladder cancer, OS after radical cystectomy is poor and about 50% of patients have distant metastasis and death after radical cystectomy.[4,5] The transurethral resection of bladder tumor (TURBT) marks the foremost step for patients with non-muscle invasive bladder cancer and recurrent tumors are also usually treated by repeat TURBT surgery. However, it is very difficult to sort out the eligible patients because of the weak prognostic value of the traditional TNM staging system. Therefore, finding novel and effective prognostic biomarkers is significant for improving survival rate for patients with bladder cancer.
Accumulating emerging studies have demonstrated that systemic inflammation could obviously affect tumor occurrence and progression,[6,7] like albumin-to-globulin ratio,[8] C-reactive protein/albumin ratio,[9] inflammation-based index,[10] neutrophil-to-lymphocyte ratio (NLR),[11] platelet-to-lymphocyte ratio (PLR),[12] lymphocyte-to-monocyte ratio (LMR)[13] and modified Glasgow prognostic score (mGPS).[14] These novel biomarkers are more easily accessible and inexpensive compared with traditional biomarkers. However, the prognostic function of these aforementioned hematological biomarkers in bladder cancer have not been completely expounded. Thus, we conducted a meta-analysis to evaluate the key hematological biomarkers (NLR, PLR, MLR and mGPS) with various survival outcomes in bladder cancer.
2. Methods
2.1. Search strategy
This meta-analysis was done with regards to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.[15] In August 2019, a systematic literature search was conducted with the help of PubMed and EMBASE. The search terms were as follows: “hematologic biomarkers”, “NLR”, “LMR”, “PLR”, “mGPS”, “prognosis” and “bladder cancer”.
2.2. Studies inclusion and exclusion criteria
We added some studies that met the inclusion criteria given below:
-
(1)
the patients with bladder cancer;
-
(2)
studies with a clear presentation of the main outcomes including at least 1 hematologic biomarker, such as NLR, PLR, LMR, and mGPS;
-
(3)
must contain risk estimates, such as hazard ratio (HR), with 95% confidence intervals (95%CIs).
The exclusion criteria:
-
(1)
reviews, letters, laboratory studies, case reports and meta-analysis;
-
(2)
studies without survival data, such as overall survival (OS), cancer-specific survival (CSS), disease-specific survival, progression-free survival (PFS), recurrence-free survival, disease-free survival and modified Glasgow prognostic score (mGPS);
-
(3)
article not published in English.
2.3. Data extraction and quality assessment
Data extraction and quality evaluation were independently performed by 2 investigators (Longqing Li, Junxiao Liu). Any disagreements were decided by another author (Lianghao Zhang). The following information was recorded: ethnicity, sample size, survival outcomes, cut-off value, follow-up time, disease stage, HRs and 95%CIs. The quality of the included articles was assessed by the Newcastle-Ottawa Quality Scale (NOS).[16] The NOS includes the following 3 parts:
-
(1)
selection (0–4 points);
-
(2)
comparability (0–2 points); and
-
(3)
outcome (0–3 points).
The maximum score is 9 points and NOS scores ≥ 6 were considered as high-quality studies.
2.4. Statistical analyses
Considering the similar survival outcomes, we combined disease-specific survival and CSS and regarded them as CSS. In addition, recurrence-free survival and PFS were combined as PFS. Meanwhile, pooled HRs and corresponding 95%CIs were used to analyze the association between hematological biomarkers and OS, CSS and PFS for patients with bladder cancer. We measured the heterogeneity among studies was measured by Cochrane Q test and the I2 statistic. A random-effects model (DerSimonian-Laird method) was selected if there was significant heterogeneity (I2 > 50%, P < .05).[17] Otherwise, the fixed-effect model (Mantel-Haenszel method) was adopted. In addition, we performed[18] subgroup analyses to examine the heterogeneity by treatment method, ethnicity, sample size, cut-off and tumor stage of NLR, PLR, and LMR. Publication bias was evaluated by Begg funnel plots and Egger tests.[19] The statistical analyses were conducted using RevMan5.3 (Cochrane Collaboration) and P < .05 was considered statistically significant.
3. Results
The flowchart of the literature selection process was shown in Figure 1. We initially identified 485 potentially relevant articles, after removing 124 duplicates, 361 studies remained. After title and/or abstract examination, 193 papers were excluded and 168 records were evaluated by full-text reading. Among these 168 studies, 43 full text studies were eliminated because of various reasons. Thirty-three studies enrolling 17087 participants met the eligible criteria strictly and were included in the final analysis (Table 1).
Figure 1.
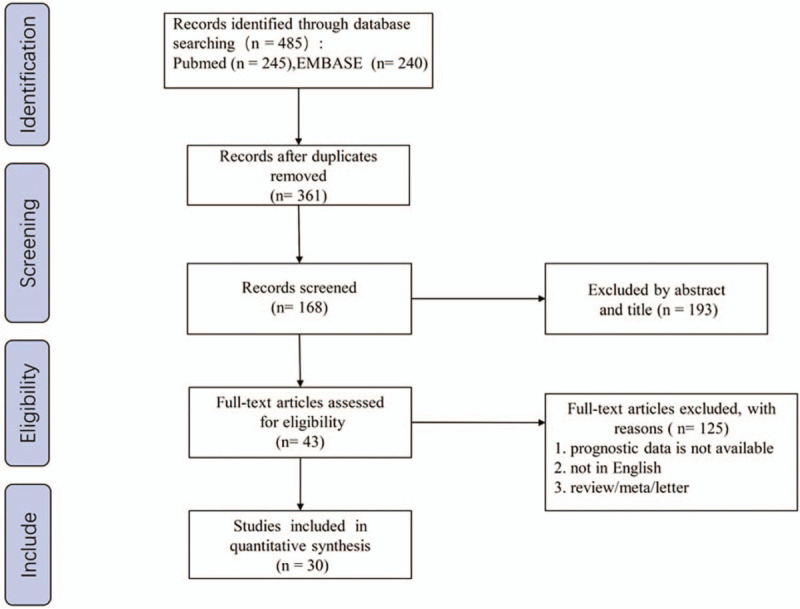
Flow diagram for study selection process.
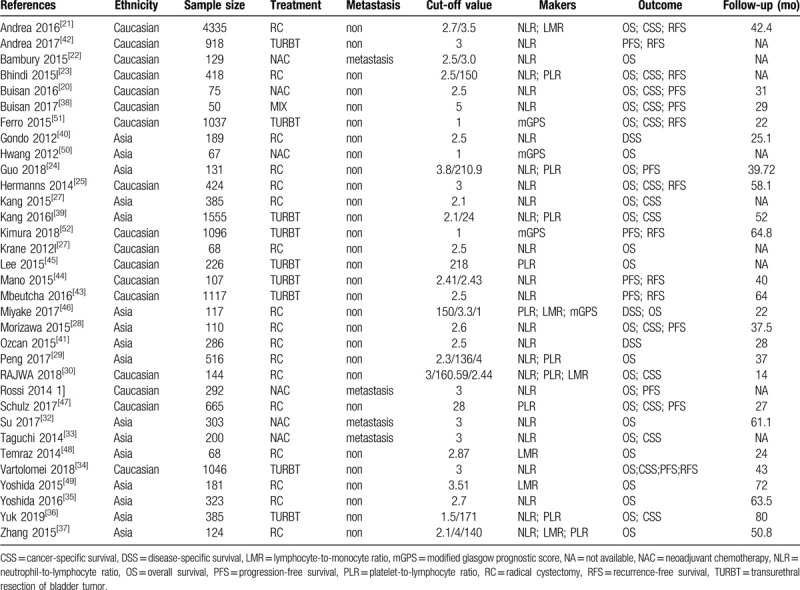
Table 1.
Baseline characteristics of studies included in the meta-analysis.
3.1. The prognostic significance of NLR in bladder cancer
Twenty studies[20–39] comprising 11013 patients provided data for estimating the association between NLR and OS in bladder cancer patients. In these studies, high NLR was significantly correlated with poor OS, HR was 1.48 (95%CI: 1.32–1.67, P < .00001), and had significant heterogeneity (I2 = 81%, P < .00001; Fig. 2).
Figure 2.

Forest plots of NLR for OS, CSS and PFS.
In total, 14 studies,[20,21,23,25,26,28,30,33,34,36,38–41] including 9602 patient, studied in the NLR analysis of CSS. As demonstrated in Figure 2, the higher NLR was correlated with poor CSS and the pooled HR was 1.71 (95%CI: 1.35–2.18, P < .0001) but with moderate heterogeneity (I2 = 65%, P = .003; Fig. 2).
Finally, the association between NLR and PFS was investigated in 13 studies[20,21,23–25,28,29,31,34,38,42–44] involving 9539 bladder cancer patients. NLR had a significant prognostic effect on PFS and the pooled HR was 1.59 (95%CI: 1.38–1.83, P < .00001) and with significant heterogeneity (I2 = 71%, P < .0001; Fig. 2).
The subgroup analysis (Table 2) shown that the significant prognostic value for NLR on OS, CSS and PFS in most subgroups but the CSS TURBT group had no significant prognostic value.
Table 2.
Subgroup analysis of the combination between NLR and OS, CSS, PFS.

3.2. The prognostic significance of PLR in bladder cancer
There were ten researches,[23,24,26,29,30,36,37,45–47] including 4281 patients, providing data for estimating the prognostic effect of PLR on OS in patients with bladder cancer. The pooled analysis illustrated that a high PLR was associated with poor OS, with the pooled HR being 1.29 (95%CI: 1.07–1.54, P = .007) with significant heterogeneity (I2 = 80.0%, P < .00001; Fig. 3).
Figure 3.
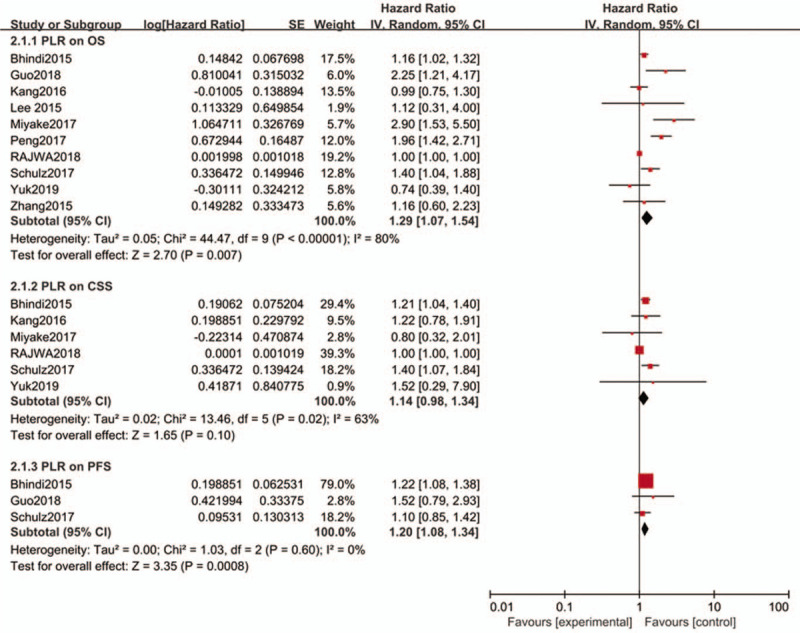
Forest plots of PLR for OS, CSS and PFS.
The correlation between PLR and CSS was reported in 6 studies[23,26,30,36,46,47] involving 3284 bladder cancer patients. Combined data of these 6 cohorts suggested non-significant prognostic effect of PLR on CSS and HR was 1.14 (95% CI: 0.98–1.34, P = .10; I2 = 63%, P = .02; Fig. 3).
In terms of effect of PLR on PFS, there were 3 studies[23,24,47] presenting it including 1214 bladder cancer patients. The pooled data showed that a high PLR was related to a poor PFS, HR was 1.2 (95%CI: 1.08–1.34, P = .0008), without heterogeneity (I2 = 0%, P = .60; Fig. 3).
The subgroup analysis (Table 3) shown that the OS TURBT group, OS Asian group, OS Sample size ≥200 group had no significant prognostic value.
Table 3.
Subgroup analysis of the combination between PLR and OS, CSS.

3.3. The prognostic significance of LMR in bladder cancer
Six studies[21,30,37,46,48,49] including 4969 patients investigated the association between LMR and OS. The pooled HR was 0.77 (95%CI: 0.70–0.84, P = .0002), which reveal that a high LMR was great connection with favorable OS (I2 = 63%, P = .001; Fig. 4).
Figure 4.
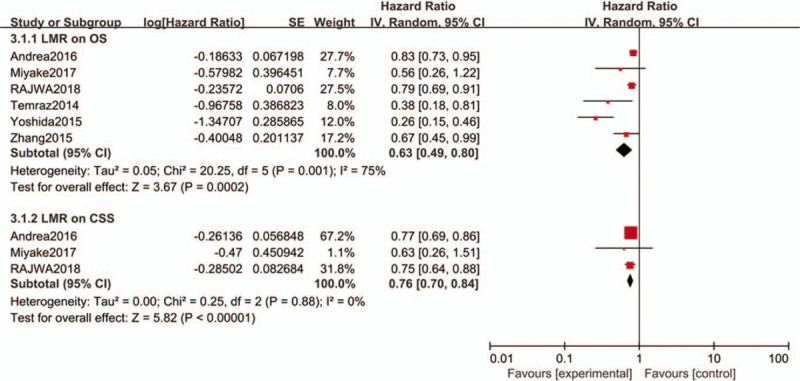
Forest plots of LMR for OS and CSS.
We also investigated the impact of LMR on CSS 21,30,46. The summary HR was 0.76 and the result indicated that a high LMR was related to favorable CSS (95%CI: 0.70–0.84, P < .00001) in a random-effects model for bladder cancer patients. There was no heterogeneity among these studies (I2 = 0%, P = .88; Fig. 4).
The subgroup analysis (Table 4) shown that the significant prognostic value for LMR on OS in most subgroups but the OS cut-off < 3.0 group had no significant prognostic value.
Table 4.
Subgroup analysis of the combination between LMR and OS.

3.4. The prognostic significance of mGPS in bladder cancer
Three studies[46,50,51] presenting data dealing with the effect of GPS on OS among 1221 different patients were observed. The gathered analysis showed that an elevated high GPS was closely associated with a poor OS, HR was 2.71 (95%CI: 1.08–2.82, P = .003), with significant heterogeneity (I2 = 80.0%, P = .007; Fig. 5).
Figure 5.

Forest plots of mGPS for OS, CSS and PFS.
Then we also found 2 studies,[46,51] containing 1154 bladder cancer patients, reported the effect of GPS on CSS. The summary HR was 1.50 (95%CI: 0.56–4.05, P = .42; Fig. 5) in a random-effects model for bladder cancer patients and both 2 cohorts suggested non-significant prognostic effect of mGPS on CSS.
At last, only 2 studies[51,52] including 2133 patients investigated had the association between mGPS and PFS, and the combined HR was 1.52(95%CI: 1.23–1.88, P = .0001) without heterogeneity (I2 = 0%, P = .73; Fig. 5).
3.5. The Association of hematological biomarkers and clinicopathological factors
We analyzed eleven studies which reported the relationship between hematological biomarkers (NLR, PLR, LMR and mGPS) and clinicopathological factors including sex, tumor grade, tumor stage, age and tumor size. As shown in Table 5 and Fig. S1, high PLR was found to be significantly associated with tumor grade G3 (OR = 2.58, 95%CI:1.67–3.99, P < .001).
Table 5.
Meta-analysis results of multiple hematological biomarkers and clinicopathological parameters in patients with bladder cancer.

4. Discussion
Among all the cancers in U.S, bladder carcinoma stands the 4th most commonly diagnosed among men and 10th most commonly diagnosed among women.[1] In previous studies, the role of several promising molecular prognostic biomarkers, like Ki-67 overexpression or fibroblast growth factor receptor 3 mutations, were not convincing. In addition, when combined with standard clinical and pathological parameters, some emerging biomarkers, such as tumor protein 53 mutations or p53 overexpression, have failed to show the clinical value.[53]
Nowadays, serum biomarkers are commonly used in the diagnosis of tumors, of which inflammation biomarkers are the most important. As early as 19th century, Rudolf Virchow observed leucocytes in neoplastic tissues and established the hypothesis about the relationship between inflammation and tumor.[54] Due to the limitations of the times and technology, this speculation has been silent for many years. In recent years, more and more evidence suggest that inflammation of the tumor microenvironment promotes tumorigenesis, progression and metastasis and there is a link between inflammation and tumor.[55–57]
NLR is the most meaningful hematological inflammation biomarker. Studies have shown that tumor infiltrating lymphocytes may limit the metastatic cascade of cancer cells while neutrophils may contribute to tumor cell migration and metastasis by remodeling the extracellular matrix and promotion of angiogenesis.[58–60] Wu et al[61] has conducted a meta-analysis on the clinical use of NLR in bladder cancer patients, and their results are similar to ours. However, we added more latest qualified studies compared to theirs. What's more, we have also used more detailed and in-depth methods, such as sensitivity analysis, “trim and fill” analysis to discover the potential heterogeneity and validate our conclusions.
Platelets can mediate tumor cell growth, angiogenesis, and proliferation by secreting vascular endothelial growth factor, basic fibroblast growth factor, platelet-derived growth factor and other angiogenesis and tumor growth factors, and also protect tumor cells from immune elimination and support tumor metastasis.[62–64] Previous researches have shown the prognostic function of PLR in different cancers, but these studies still have limitations, such as Xingmu Wang et al[65] only reported association between PLR and tumor metastasis for OS. In our study, we included more indicators that are prognostic and rationally combined them. Furthermore, we discovered that the prognostic function of high PLR for poorer OS, PFS and non-significant prognostic effect of PLR for CSS. The prognostic value of PLR could be insignificant as the follow-ups of CSS is comparatively short. Notably, in the research of the correlation between PLR and clinicopathological factors, we found that PLR was significantly associated with tumor grade G3 (P < .001). Tumor differentiation may be related to tumor microenvironment lymphocyte infiltration, however, research on this aspect is still insufficient. Research on tumor lymphocyte infiltration will become a hot spot in the future.
Recently, LMR, as an integrated inflammatory-based prognostic system, it has shown spectacular prognostic value in numerous cancers. Macrophages derived from circulating monocytes might accelerate tumor progression and angiogenesis.[66] In this meta-analysis, we explored the prognostic value of LMR in bladder cancer and focused on more outcome's indicator contain OS and CSS than previous studies.
Moreover, our study included more valuable significant biomarkers. None of these meta-analyses about prognostic significance of hematological biomarkers for bladder cancer concentrated on the value of mGPS until now. Furthermore, we investigated the prognostic value of NLR, PLR, LMR and mGPS in same 1 study for the first time and these biomarkers could show more reliable prognostic value in bladder cancer.
Nevertheless, our study also has following several limitations.
-
(1)
Despite we conducted a lot of subgroup analysis; we still cannot eliminate the significant heterogeneity between several studies. After discussion, we finally believe that the heterogeneity attributes to the grade of bladder cancer, histological type and individual patient difference.
-
(2)
In the studies we included, some did not have multivariate analysis data, so we just included a part of the univariate analysis data.
-
(3)
Most hematological biomarkers have different cut-off value.
-
(4)
Included studies were all retrospective studies.
-
(5)
Several biomarkers, such as C-reactive protein/albumin ratio and plasma fibrinogen have been studied too little to conduct meta-analysis. In the future, large-scale studies about these biomarkers are needed to validate the results.
4.1. Publication bias
Publication bias was insignificant with respect to the prognostic value of NLR on OS (P = .456, Fig. 6A), NLR on CSS (P = .381, Fig. 6B) and PLR on OS (P = .754, Fig. 6C) according to the plots of publication given in Fig. 6. Begg's funnel plots showed notable asymmetry regarding the NLR with PFS (P = .033, Fig. 6D) in bladder cancer patients. Moreover, we performed “trim and fill” analysis and the results suggested that there were 3 unpublished studies assessing NLR on PFS (Fig. 6E). The results of “trim and fill” showed no significant difference in the previous and new HRs (HR = 1.513, 95% CI: 1.310–1.747; P < .001).
Figure 6.

Begg's funnel plots of (A) NLR for OS, (B) NLR for CSS, (C) PLR for PFS and (D) NLR for PFS. Filled funnel plots of (E) NLR for PFS.
5. Conclusions
Our study indicated that the pretreatment hematological inflammation biomarkers could be regarded as 1 of predicative biomarkers in bladder cancer patients. Therefore, hematologic biomarkers are promising and inexpensive biomarkers that can be used in clinical management and foresee survival outcome in bladder carcinoma. However, further prospective and innovative studies are required to validate our conclusions.
Author contributions
Lianghao Zhang collected and analyzed the data and wrote the paper. Longqing Li, Junxiao Liu assisted in collecting the data and participated in the writing. Jiange Wang, Yafeng Fan, Biao Dong assisted in the design of this study. Zhaowei Zhu and Xuepei Zhang are responsible for the integrity of the data and the accuracy of the data analysis. All authors critically revised the manuscript.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CSS = cancer-specific survival, HR = hazard ratio, mGPS = modified Glasgow prognostic score, NLR = neutrophil-to-lymphocyte ratio, OS = overall survival, PFS = progression-free survival, PLR = platelet-to-lymphocyte ratio, TURBT = transurethral resection of bladder tumor.
How to cite this article: Zhang L, Li L, Liu J, Wang J, Fan Y, Dong B, Zhu Z, Zhang X. Meta-analysis of multiple hematological biomarkers as prognostic predictors of survival in bladder cancer. Medicine. 2020;99:30(e20920).
LZ, LL, and JL are co-first authors of this manuscript.
Funding: This work was supported by grant from The National Natural Science Foundation of China (No. 81702503).
Availability of data and materials: Please contact author for data requests.
Ethics approval and consent to participate: Not applicable
Consent for publication: Yes
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- [2].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [3].van Rhijn BW, Burger M, Lotan Y, et al. Recurrence and progression of disease in non-muscle-invasive bladder cancer: from epidemiology to treatment strategy. Eur Urol 2009;56:430–42. [DOI] [PubMed] [Google Scholar]
- [4].Ploussard G, Xylinas E, Lotan Y, et al. Conditional survival after radical nephroureterectomy for upper tract carcinoma. Eur Urol 2015;67:803–12. [DOI] [PubMed] [Google Scholar]
- [5].Hautmann RE, de Petriconi RC, Pfeiffer C, et al. Radical cystectomy for urothelial carcinoma of the bladder without neoadjuvant or adjuvant therapy: long-term results in 1100 patients. Eur Urol 2012;61:1039–47. [DOI] [PubMed] [Google Scholar]
- [6].Gakis G, Todenhöfer T, Stenzl A. The prognostic value of hematological and systemic inflammatory disorders in invasive bladder cancer. Curr Opin Urol 2011;21:428–33. [DOI] [PubMed] [Google Scholar]
- [7].Schepisi G, Santoni M, Massari F, et al. Urothelial cancer: inflammatory mediators and implications for immunotherapy. BioDrugs 2016;30:263–73. [DOI] [PubMed] [Google Scholar]
- [8].Oh TK, Choi YR, Cho JY, et al. The high-sensitivity c-reactive protein/albumin ratio predicts long-term oncologic outcomes after curative resection for hepatocellular carcinoma. J Clin Med 2018;7(6): [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fujikawa H, Toiyama Y, Inoue Y, et al. Prognostic impact of preoperative albumin-to-globulin ratio in patients with colon cancer undergoing surgery with curative intent. Anticancer Res 2017;37:1335–42. [DOI] [PubMed] [Google Scholar]
- [10].Gkika E, Bettinger D, Krafft L, et al. The role of albumin-bilirubin grade and inflammation-based index in patients with hepatocellular carcinoma treated with stereotactic body radiotherapy. Strahlenther Onkol 2018;194:403–13. [DOI] [PubMed] [Google Scholar]
- [11].Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- [12].Zhou X, Du Y, Huang Z, et al. Prognostic value of PLR in various cancers: a meta-analysis. PLoS One 2014;9:e101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nishijima TF, Muss HB, Shachar SS, et al. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev 2015;41:971–8. [DOI] [PubMed] [Google Scholar]
- [14].McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534–40. [DOI] [PubMed] [Google Scholar]
- [15].Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group,. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [17].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [18].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [19].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [20].Buisan O, Orsola A, Oliveira M, et al. Role of inflammation in the perioperative management of urothelial bladder cancer with squamous-cell features: impact of neutrophil-to-lymphocyte ratio on outcomes and response to neoadjuvant chemotherapy. Clin Genitourin Cancer 2017;15:e697–697. [DOI] [PubMed] [Google Scholar]
- [21].D’Andrea D, Moschini M, Gust KM, et al. Lymphocyte-to-monocyte ratio and neutrophil-to-lymphocyte ratio as biomarkers for predicting lymph node metastasis and survival in patients treated with radical cystectomy. J Surg Oncol 2017;115:455–61. [DOI] [PubMed] [Google Scholar]
- [22].Bambury RM, Benjamin DJ, Chaim JL, et al. The safety and efficacy of single-agent pemetrexed in platinum-resistant advanced urothelial carcinoma: a large single-institution experience. Oncologist 2015;20:508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bhindi B, Hermanns T, Wei Y, et al. Identification of the best complete blood count-based predictors for bladder cancer outcomes in patients undergoing radical cystectomy. Br J Cancer 2016;114:207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Guo Y, Cai K, Mao S, et al. Preoperative C-reactive protein/albumin ratio is a significant predictor of survival in bladder cancer patients after radical cystectomy: a retrospective study. Cancer Manag Res 2018;10:4789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hermanns T, Bhindi B, Wei Y, et al. Pre-treatment neutrophil-to-lymphocyte ratio as predictor of adverse outcomes in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Br J Cancer 2014;111:444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kang M, Jeong CW, Kwak C, et al. Preoperative neutrophil-lymphocyte ratio can significantly predict mortality outcomes in patients with non-muscle invasive bladder cancer undergoing transurethral resection of bladder tumor. Oncotarget 2017;8:12891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Krane LS, Richards KA, Kader AK, et al. Preoperative neutrophil/lymphocyte ratio predicts overall survival and extravesical disease in patients undergoing radical cystectomy. J Endourol 2013;27:1046–50. [DOI] [PubMed] [Google Scholar]
- [28].Morizawa Y, Miyake M, Shimada K, et al. Neutrophil-to-lymphocyte ratio as a detection marker of tumor recurrence in patients with muscle-invasive bladder cancer after radical cystectomy. Urol Oncol 2016;34:257.e11–7. [DOI] [PubMed] [Google Scholar]
- [29].Peng D, Gong YQ, Hao H, et al. Preoperative prognostic nutritional index is a significant predictor of survival with bladder cancer after radical cystectomy: a retrospective study. BMC Cancer 2017;17:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rajwa P, Życzkowski M, Paradysz A, et al. Evaluation of the prognostic value of LMR, PLR, NLR, and dNLR in urothelial bladder cancer patients treated with radical cystectomy. Eur Rev Med Pharmacol Sci 2018;22:3027–37. [DOI] [PubMed] [Google Scholar]
- [31].Rossi L, Santoni M, Crabb SJ, et al. High neutrophil-to-lymphocyte ratio persistent during first-line chemotherapy predicts poor clinical outcome in patients with advanced urothelial cancer. Ann Surg Oncol 2015;22:1377–84. [DOI] [PubMed] [Google Scholar]
- [32].Su YL, Hsieh MC, Chiang PH, et al. Novel inflammation-based prognostic score for predicting survival in patients with metastatic urothelial carcinoma. PLoS One 2017;12:e0169657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Taguchi S, Nakagawa T, Matsumoto A, et al. Pretreatment neutrophil-to-lymphocyte ratio as an independent predictor of survival in patients with metastatic urothelial carcinoma: a multi-institutional study. Int J Urol 2015;22:638–43. [DOI] [PubMed] [Google Scholar]
- [34].Vartolomei MD, Ferro M, Cantiello F, et al. Validation of neutrophil-to-lymphocyte ratio in a multi-institutional cohort of patients With T1G3 non-muscle-invasive bladder cancer. Clin Genitourin Cancer 2018;16:445–52. [DOI] [PubMed] [Google Scholar]
- [35].Yoshida T, Kinoshita H, Yoshida K, et al. Perioperative change in neutrophil-lymphocyte ratio predicts the overall survival of patients with bladder cancer undergoing radical cystectomy. Jpn J Clin Oncol 2016;46:1162–7. [DOI] [PubMed] [Google Scholar]
- [36].Yuk HD, Jeong CW, Kwak C, et al. Elevated neutrophil to lymphocyte ratio predicts poor prognosis in non-muscle invasive bladder cancer patients: initial intravesical bacillus calmette-guerin treatment after transurethral resection of bladder tumor setting. Front Oncol 2018;8:642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang GM, Zhu Y, Luo L, et al. Preoperative lymphocyte-monocyte and platelet-lymphocyte ratios as predictors of overall survival in patients with bladder cancer undergoing radical cystectomy. Tumour Biol 2015;36:8537–43. [DOI] [PubMed] [Google Scholar]
- [38].Buisan O, Orsola A, Areal J, et al. Low pretreatment neutrophil-to-lymphocyte ratio predicts for good outcomes in patients receiving neoadjuvant chemotherapy before radical cystectomy for muscle invasive bladder cancer. Clin Genitourin Cancer 2017;15:145–51e2. [DOI] [PubMed] [Google Scholar]
- [39].Kang M, Jeong CW, Kwak C, et al. The prognostic significance of the early postoperative neutrophil-to-lymphocyte ratio in patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Ann Surg Oncol 2016;23:335–42. [DOI] [PubMed] [Google Scholar]
- [40].Gondo T, Nakashima J, Ohno Y, et al. Prognostic value of neutrophil-to-lymphocyte ratio and establishment of novel preoperative risk stratification model in bladder cancer patients treated with radical cystectomy. Urology 2012;79:1085–91. [DOI] [PubMed] [Google Scholar]
- [41].Ozcan C, Telli O, Ozturk E, et al. The prognostic significance of preoperative leukocytosis and neutrophil-to-lymphocyte ratio in patients who underwent radical cystectomy for bladder cancer. Can Urol Assoc J 2015;9:E789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].D’Andrea D, Moschini M, Gust K, et al. Prognostic role of neutrophil-to-lymphocyte ratio in primary non-muscle-invasive bladder cancer. Clin Genitourin Cancer 2017;15:e755–64. [DOI] [PubMed] [Google Scholar]
- [43].Mbeutcha A, Shariat SF, Rieken M, et al. Prognostic significance of markers of systemic inflammatory response in patients with non-muscle-invasive bladder cancer. Urol Oncol 2016;34:483e17–24. [DOI] [PubMed] [Google Scholar]
- [44].Mano R, Baniel J, Shoshany O, et al. Neutrophil-to-lymphocyte ratio predicts progression and recurrence of non-muscle-invasive bladder cancer. Urol Oncol 2015;33:67e1–7. [DOI] [PubMed] [Google Scholar]
- [45].Lee SM, Russell A, Hellawell G. Predictive value of pretreatment inflammation-based prognostic scores (neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and lymphocyte-to-monocyte ratio) for invasive bladder carcinoma. Korean J Urol 2015;56:749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Miyake M, Morizawa Y, Hori S, et al. Integrative assessment of pretreatment inflammation-, nutrition-, and muscle-based prognostic markers in patients with muscle-invasive bladder cancer undergoing radical cystectomy. Oncology 2017;93:259–69. [DOI] [PubMed] [Google Scholar]
- [47].Schulz GB, Grimm T, Buchner A, et al. Prognostic value of the preoperative platelet-to-leukocyte ratio for oncologic outcomes in patients undergoing radical cystectomy for bladder cancer. Clin Genitourin Cancer 2017;15: e915-915e921. [DOI] [PubMed] [Google Scholar]
- [48].Temraz S, Mukherji D, Farhat ZA, et al. Preoperative lymphocyte-to-monocyte ratio predicts clinical outcome in patients undergoing radical cystectomy for transitional cell carcinoma of the bladder: a retrospective analysis. BMC Urol 2014;14:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Yoshida T, Kinoshita H, Yoshida K, et al. A novel risk stratification model, involving preoperative lymphocyte-monocyte ratio and standard pathological factors, for overall survival in patients with bladder cancer undergoing radical cystectomy. Jpn J Clin Oncol 2015;45:1162–7. [DOI] [PubMed] [Google Scholar]
- [50].Hwang EC, Hwang IS, Yu HS, et al. Utility of inflammation-based prognostic scoring in patients given systemic chemotherapy first-line for advanced inoperable bladder cancer. Jpn J Clin Oncol 2012;42:955–60. [DOI] [PubMed] [Google Scholar]
- [51].Ferro M, De Cobelli O, Buonerba C, et al. Modified Glasgow Prognostic Score is associated with risk of recurrence in bladder cancer patients after radical cystectomy: a multicenter experience. Medicine (Baltimore) 2015;94:e1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kimura S, D’ Andrea D, Soria F, et al. Prognostic value of modified Glasgow Prognostic Score in non-muscle-invasive bladder cancer. Urol Oncol 2019;37:179e19–28. [DOI] [PubMed] [Google Scholar]
- [53].Malats N, Bustos A, Nascimento CM, et al. P53 as a prognostic marker for bladder cancer: a meta-analysis and review. Lancet Oncol 2005;6:678–86. [DOI] [PubMed] [Google Scholar]
- [54].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow. Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- [55].Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- [56].Grivennikov SI, Greten FR, Karin M. Immunity, inflammation,;1; and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res 2004;10:4895–900. [DOI] [PubMed] [Google Scholar]
- [59].Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol 2015;15:73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 2013;13:759–71. [DOI] [PubMed] [Google Scholar]
- [61].Wu S, Zhao X, Wang Y, et al. Pretreatment neutrophil-lymphocyte ratio as a predictor in bladder cancer and metastatic or unresectable urothelial carcinoma patients: a pooled analysis of comparative studies. Cell Physiol Biochem 2018;46:1352–64. [DOI] [PubMed] [Google Scholar]
- [62].Goubran HA, Burnouf T, Radosevic M, et al. The platelet-cancer loop. Eur J Intern Med 2013;24:393–400. [DOI] [PubMed] [Google Scholar]
- [63].Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer 2011;11:123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sullivan LA, Brekken RA. The VEGF family in cancer and antibody-based strategies for their inhibition. MAbs 2010;2:165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wang X, Ni X, Tang G. Prognostic role of platelet-to-lymphocyte ratio in patients with bladder cancer: a meta-analysis. Front Oncol 2019;9:757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 2002;196:254–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


