Abstract
In a hepatitis C virus (HCV)/HIV-positive Brazilian cohort, evaluate the safety and efficacy of HCV DAAs, the frequency of resistance substitutions in the HCV NS5A and NS5B genes and identify predictors of treatment failure.
Retrospective multicenter study of HCV/HIV patients treated with sofosbuvir (SOF)-based regimens at 10 reference centers in Brazil.
Clinical and virological data were collected. Genetic diversity in the NS5A and NS5B genes was assessed by direct nucleotide sequencing. The primary outcome was sustained virological response (SVR) 12 weeks after DAA completion.
Of 643 HCV/HIV patients analyzed, 74.7% were male, median CD4+ T cell count was 617 cells/mm3, 90% had an undetectable HIV viral load. HCV genotype 1 was detected in 80.2%, and 60% were taking at least 1 medication other than antiretroviral drugs during their DAA therapy. Cirrhosis was present in 42%. An SOF/daclatasvir (DCV) regimen was used in most patients (98%). The frequency of NS5A polymorphisms associated with clinically relevant resistance to DCV was 2%; no relevant NS5B variants were identified. The SVR12 rate was 92.8% in an intention to treat (ITT) analysis and 96% in a modified ITT (m-ITT) analysis. AE occurred in 1.6% of patients. By multivariate analysis, therapeutic failure was associated, in the m-ITT analysis, with concomitant use of anticonvulsant drugs (P = .001), age (P = .04), and female gender (P = .04).
SOF/DCV regimens were associated with a high SVR rate in an HCV/HIV population. The use of concurrent anticonvulsant drugs and DAAs decreases the chances of achieving an SVR.
Keywords: direct acting antivirals, effectiveness, hepatitis C treatment, hepatitis C-HIV coinfection, safety
1. Introduction
Hepatitis C virus (HCV) and HIV coinfection is a major global public health problem.[1,2] According to the World Health Organization (WHO), there are currently around 71 million individuals chronically infected with HCV, 31 million with HIV, and more than 2 million with HCV-HIV coinfection.[2,3] Since HCV and HIV infections share the same routes of transmission, coinfections are often found with an estimated prevalence of 25% to 30% in the general population of people living with HIV/AIDS. These rates are even higher in specific groups, such as in injection drug users.[4,5] Coinfection adversely affects the natural course of hepatitis C infection, accelerating the progression of liver fibrosis and increasing the risk of hepatic decompensation and hepatocellular carcinoma in cirrhotic patients.[6–8] Currently, end-stage liver disease is one of the leading causes of death in the HCV/HIV population.[5,9,10]
The advent of direct-action antivirals (DAAs) has substantially changed the HCV therapy scenario, including in the HIV-infected population.[11–13] In the interferon (IFN) treatment era, a minority of individuals could be treated due to the occurrence of significant adverse effects and low sustained virological response (SVR) rates.[12,14,15] With DAAs, especially IFN-free regimens, HCV therapy has been simplified and coinfected patients have achieved high cure rates.[11,12] Randomized clinical trials and real-life studies in different parts of the world have shown SVR rates greater than 90% in patients with HCV/HIV, similar to that reported for patients without HIV coinfection.[16–21]
However, conflicting results regarding the impact of HIV and immunosuppression on SVR rate in these patients have been reported.[22–24] In some studies, HIV coinfection has been associated with worse clinical outcomes.[22–25] In addition, for this population, adherence to therapy, the high frequency of comorbidities, the concomitant use of medications with potential drug–drug interactions (DDI) with DAAs, and the impact of the presence of resistance-associated substitutions (RASs) in the HCV genome are additional significant challenges that could interfere with the chances of treatment success.[11,26–29]
In Brazil, the public health system has provided free-of-charge treatment with IFN-free DAAs regimens beginning in 2015 to all HCV/HIV coinfected patients, irrespective of the degree of liver fibrosis.[30] We now present results of a real-life study in this population with the following objectives: to estimate the real-life safety and efficacy of sofosbuvir (SOF)-based treatment regimens in an HCV/HIV Brazilian cohort; to investigate the frequency of baseline RASs in HCV NS5A and NS5B genes, and to identify predictors of treatment failure.
2. Methods
2.1. Study design and population
We conducted an observational, retrospective, and multicenter study of patients at 10 Brazilian reference centers specialized in the treatment of HCV. These centers are located in 3 Brazilian states: São Paulo, Rio Grande do Sul, and Rio de Janeiro.
Eligible subjects were all patients with HCV/HIV coinfection who started HCV therapy with SOF-based regimens between July 2015 and August 2017, according to the Brazilian Ministry of Health's Therapeutic Guidelines.[30] Inclusion criteria were: age ≥ 18 years, having received at least 1 dose of the prescribed treatment regimen, and no history of previous therapy with SOF and/or daclatasvir (DCV) or with any other NS5A antiviral regimen. Patients were not excluded based on the presence of comorbidities.
According to the Brazilian Therapeutic Guidelines, the following treatment regimens were administered for the different HCV genotypes (GT): for HCV GT 1 and GT4: SOF and DCV; for GT2: SOF-ribavirin (RBV); for GT3: SOF-DCV combination or SOF plus pegIFN-RBV.[30] The choice of concomitant administration of RBV and/or the treatment of HCV GT3 patients was made at the discretion of the attending physician.
This study was approved by the Ethics Committee for the Analysis of Research Projects (Comissão de Ética para Análise de Projetos de Pesquisa—CAPPesq—Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo—HC-FMUSP) under protocol no. 46221015.0.0000.0068. All patients provided written informed consent prior to inclusion in the study.
2.2. Data collection
Demographic, clinical, and laboratory data were collected. Data was obtained from medical files after the end of treatment, by using a standard clinical questionnaire. For staging of liver disease, the results obtained by liver biopsy were considered according to the classification of the METAVIR Co-operative Study Group.[31] Liver elastography was also used for liver fibrosis staging. The corresponding transient elastography cutoff points (FibroScan, Echosens, France) for Metavir were less than 5.5 kPa = F0, 5.6 to 7.0 = F1, 7.1 to 9.4 kPa = F2, 9.5 to 12.4 kPa = F3, and ≥ 12.5 kPa = F4.[32] Cirrhosis was defined by liver biopsy or noninvasive method of liver fibrosis, according to the cut-off values mentioned above, or by clinical signs of portal hypertension. When biopsy or elastography were not available, those with APRI score ≥ 2 and FIB score ≥ 3.25 were also classified as cirrhotic.[33–35] Comorbidity was defined as the presence of any clinical condition that required clinical or pharmacological intervention by the medical team. Known DDI between DAAs and other concomitant medications were identified from the website www.hep-druginteractions.org.
2.3. Outcomes
The primary efficacy endpoint was the proportion of patients with SVR, defined as an HCV RNA level below the limit of quantification (HCV RNA PCR <12 IU/mL; Abbot Molecular, Des Plaines, IL) following 12 weeks of treatment among all those who received at least 1 dose of the study treatment (intention-to-treat analysis (ITT)).
We also analyzed SVR, in a modified intention-to-treat analysis (m-ITT), in which we excluded cases such as loss to clinical follow-up, treatment interruptions, and/or deaths not associated with DAA adverse events.
An adverse event (AE) was defined as any event during treatment that required a dose change or discontinuation of a drug from HCV therapy regimen or other drug intervention. Serious adverse events (SAE) were defined as those that led to discontinuation of DAA therapy or death.
2.4. Baseline RASs
We analyzed for RASs in pretreatment samples. HCV RNA extraction was performed from serum using the QIAamp Viral RNA Kit (Qiagen, Hilden, Germany), followed by amplification using the SuperScript III / Platinum Taq High Fidelity One-Step enzyme (InvitrogenTM, Thermo Fisher Brand, Carlsbad, USA), following the manufacturer's protocol. The amplified samples were submitted to a sequencing reaction (bidirectional) derived from the Sanger methodology.[36] The sequences obtained were initially analyzed using the Electropherogram quality analysis program (http://asparagin.cenargen.embrapa.br/phph/). By aligning the amino acid sequences of each sample with reference sequences, NS5A inhibitor RASs were screened at positions 28, 30, 31, 58, 92, and 93; and for NS5B inhibitors at positions 282, 316, and 321.[29,37,38] In addition, all consensus sequences were submitted to Geno2pheno [hcv] 0.92 (https://hcv.geno2pheno.org/) for confirmation of the results.[39]
2.5. Statistical analysis
We used bivariate analyses followed by logistic regression to identify factors associated with lack of a SVR. Categorical variables were compared using χ2 or Fisher exact tests and continuous variables were compared using the Student t or Mann–Whitney test. Variables with P < .10 were selected for logistic regression analysis. Variables with P < .05 in the multiple analysis were retained in the final model. Odds ratios (OR) of each variable were estimated with the corresponding confidence interval (95% CI) at the 5% significance level. IBM-SPSS for Windows version 20.0 software was used to perform the analysis.
3. Results
3.1. Baseline characteristics
The main baseline characteristics of the 643 HCV/HIV coinfected patients who started DAA therapy are presented in Table 1. The majority were male (74.7%), median age 50, 90% with an undetectable HIV viral load and a median CD4 + T cell count of 617/mm3. Liver cirrhosis was identified in 42% of patients, mostly Child-Pugh A (85.3%) and Child-Pugh B (14.7%). There were no subjects with Child-Pugh C cirrhosis in our cohort. About 60% of patients had at least 1 comorbidity, with an average of 2 medical conditions per patient (range 1–9), including extrahepatic manifestations and hepatitis B virus coinfection. One hundred sixteen patients (18%) had 3 or more comorbidities. The most frequent comorbidities observed were: systemic arterial hypertension (n = 150), psychiatric disorders (n = 142), diabetes mellitus (n = 79), and dyslipidemia (n = 72). Twelve patients had hepatitis B virus coinfection (HBsAg positive).
Table 1.
Baseline characteristics of 643 Brazilian HCV-HIV patients.
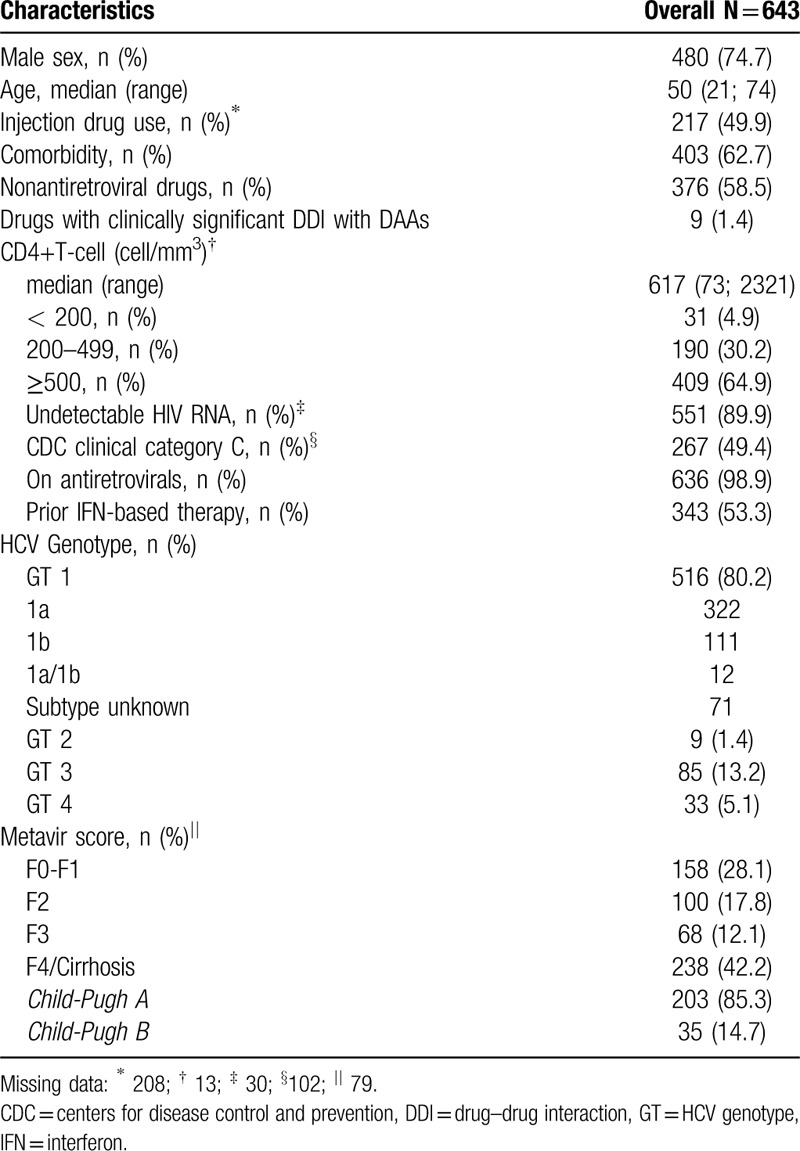
Almost all patients (n = 636) were taking antiretroviral medication. Antiretroviral treatment therapy (ART) was done according to Brazilian Guidelines. ART was documented and no combination was excluded. Among these patients, 250 (38.9%) had their medication modified prior to initiating DAA to prevent drug–drug interactions. During HCV treatment with DAAs, 376 patients (58.5%) reported the concomitant use of other medications (an average of 3 drugs per patient) that were unrelated to HIV or HCV treatment. Antihypertensive drugs, psychotropic drugs, and those used to treat diabetes were the most commonly used medication classes. Although anticonvulsant medications are generally contraindicated during SOF and DCV treatment due to the risk of adverse interactions, these drugs were prescribed in 9 (1.4%) cases, as follows: carbamazepine (n = 4), phenobarbital (n = 2), phenytoin (n = 2), and oxcarbazepine (n = 1).
3.2. Treatment regimens
Table 2 presents the DAAs regimens used in this cohort, according to HCV genotypes.
Table 2.
Treatment regimens and sustained virologic response rate according HCV genotype.
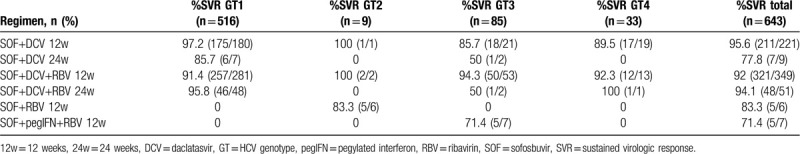
3.3. Response to treatment
The overall rate of SVR in the ITT analysis was 92.8% (n = 597; 95% CI 90.9–94.8). Sixteen subjects (2.5%) did not achieve a virological response. For 30 patients (4.7%) no SVR data was available: 9 subjects (1.4%) discontinued DAAs treatment due to SAE and 21 patients (3.3%) were lost to follow-up, interrupted treatment or died due to different causes not associated with HCV or the use of DAAs. In the m-ITT analysis, the SVR rate was 96% (95% CI 94.4%–97.5%) (Fig. 1).
Figure 1.
DAA = direct-acting antiviral, SVR = sustained virological response, AE = adverse event.
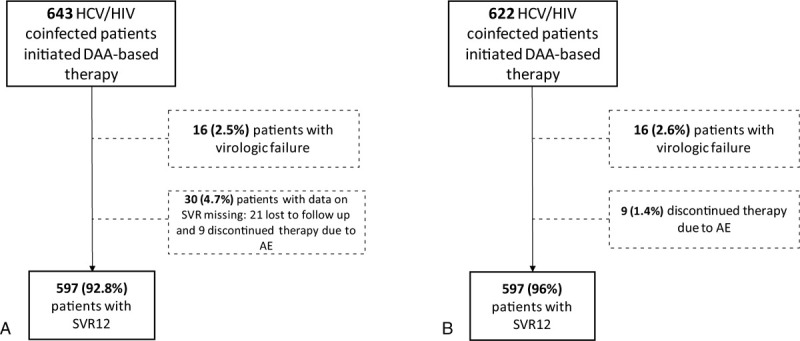
(A) = intention-to-treat approach, (B) = modified intention-to-treat approach.
Cirrhotic patients achieved lower SVR rates when compared with noncirrhotic patients, but with no significant difference in both analyses (P = .21 and P = .44 in the ITT and m-ITT approaches, respectively). Child-Pugh B cirrhotic patients (N = 35) achieved SVR rates of 88.6% in the ITT analysis and 91.1% in the m-ITT analysis.
SVR rates also did not differ significantly among HCV GTs or between Child-A or Child-B patients. Although cirrhotic patients positive for GT3 had a lower SVR rate (74.1%), this was not significantly different from cirrhotic patients with other HCV genotypes (P = .90).
3.4. Safety
One hundred three patients experienced an AE during DAA therapy (AE rate: 16%; 95% CI 13.2–18.9). The most frequent AE was anemia, reported for 90 patients, all of whom were using RBV. The diagnosis of anemia secondary to the use of ribavirin was made by the attending physician, based on a decrease in hemoglobin levels during hepatitis C therapy compared with baseline hemoglobin values. None of the cases with anemia resulted in discontinuation of HCV treatment.
SAE occurred in 10 individuals (1.6%; 95% CI 0.6–2.5), of which 2 died due to liver decompensation; both were Child-Pugh B cirrhotic. The other SAE reported were as follows: decompensation of psychiatric disorder (n = 1), elevation of alanine aminotransferase, and aspartate aminotransferase to a level 10 times the upper limit of normal (n = 1) and gastrointestinal intolerance (n = 6).
Among the 10 patients with SAE, 1 individual with gastrointestinal intolerance completed 8 weeks of therapy and achieved SVR. The other patients did not achieve SVR.
3.5. HCV gene polymorphisms
We analyzed for polymorphisms in the HCV NS5B and NS5BA genes in 261 (40.6%) and 254 (39.5%) patients, respectively. In the analysis of the HCV NS5A region, genetic variants were found in 52 (20.5%) patients, In 5 of these individuals (2.0%) the substitutions conferred clinically relevant resistance to DCV.[40] The amino acid variants identified were: L31 M in 1 patient with HCV GT 1a, Y93C in 1 patient with HCV GT 1a, and Y93H in 3 HCV GT 1b-positive patients.
3.6. Predictors of treatment failure
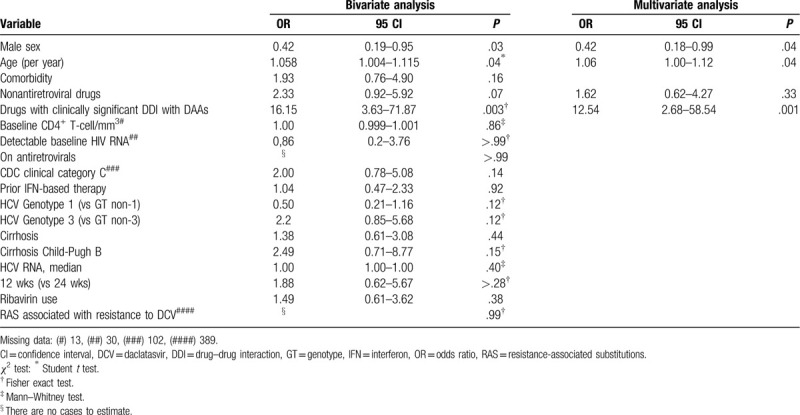
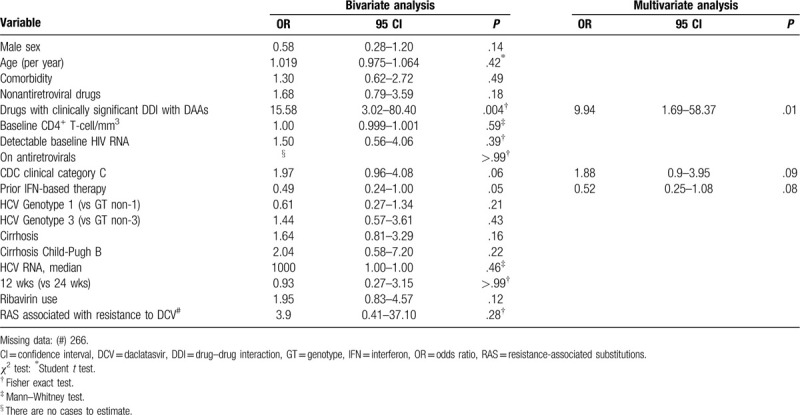
In the final logistic regression model, in both the ITT and m-ITT analyses, the use of drugs with relevant DDI with DAAs was significantly associated with non-SVR (P = .004 and P = .001, respectively). In the ITT analysis, the absence of prior IFN therapy was also associated with therapeutic failure (P = .04), whereas in the m-ITT analysis, female gender and age (> 50 years old) were also predictive of lack of SVR (P = .04 and P = .04, respectively). The m-ITT analysis results are summarized in Table 3.
Table 3.
Results of bivariate and multivariate analyses of factors associated with lack of SVR in a modified intention-to-treat approach—643 HCV-HIV coinfected patients.
To evaluate whether characteristics associated with HIV infection could interfere with the likelihood of obtaining SVR, we performed additional analyses on patients with known CD4 + T lymphocyte counts, HIV viral load, and a CDC clinical classification for AIDS. In bivariate and multivariate analysis of these 520 HCV/HIV coinfected individuals, only the use of drugs with significant DDI was associated with treatment failure (P = .01). The results are presented in Table 4.
Table 4.
Results of bivariate and multivariate analyses of factors associated with lack of SVR for 520 HCV-HIV coinfected patients with complete data on HIV infection.
4. Discussion
In a cohort of 643 HCV-HIV coinfected patients from 10 centers in Brazil, the SVR rate was 96% following SOF and DCV-based therapy. Using a modified-ITT analysis, therapeutic failures were associated with the concomitant use of anticonvulsant drugs (P = .001), age above 50 years old (P = .04), and female gender (P = .04). Regarding drug safety, less than 2% of patients discontinued therapy due to AE. Factors related to HIV immunosuppression were not associated with lower rates of a therapeutic response. Lastly, the frequency of baseline mutations in HCV associated with clinical resistance to DAAs was infrequent, around 2%, and did not impair the SVR rate.
The observed high SVR rate is consistent with results of prior studies on smaller numbers of subjects from outside Brazil that employed the same therapeutic regimen.[40–43] Other studies, however, have associated the presence of HIV coinfection with a higher failure rate of anti-HCV DAA.[20,22–24] Berenguer et al[22] observed in a cohort of more than 2000 coinfected individuals that CD4+ T lymphocyte counts below 200/mm3 and a history of opportunistic disease were associated with therapeutic failure. In contrast, ARV therapy was successful in the majority of patients (98.9%) in our study and less than 5% had a CD4+ T cell count below 200/mm3. It is likely that this effective control of HIV infection contributed to the observed high positive rates of SVR and negated our ability to evaluate the possible impact of variables associated with immunodepression on success rates.
Other factors, such as infection with HCV GT3, liver cirrhosis, or baseline RASs, have also been related to reduced SVR.[19,37,44–46] RBV use and the duration of therapy have also been reported to interfere with SVR rates in specific populations.[20,40,45] However, these variables were not associated with a lack of SVR in our cohort. In our study, patients who received a regimen of SOF+DVC 24 weeks (n = 9; 7 HCV GT1 and 2 HCV GT3) and SOF+pegIFN+RBV (n = 7; all HCV GT3; 50% with cirrhosis) had relatively low SVR rates (77.8% and 71.4%, respectively). We did not find a statistical difference in the SVR rates between HCV genotypes 1 and 3 or duration of treatment (12 versus 24 weeks). It is important to highlight that the number of patients receiving these 2 therapy regimens was small (n = 16) and according to national guidelines, the choice of concomitant administration of RBV and/or the treatment of HCV GT3 positive patients with IFN-based regimen was made at the discretion of the attending physician.
In our cohort, patients with liver cirrhosis had slightly lower SVR rates than those observed in the noncirrhotic population, but without statistical significance (P = .21 in ITT analysis and P = .44 in m-ITT analysis). When analyzing only cirrhotic patients (N = 238), those positive for HCV GT3 achieved the lowest SVR rate (74.1%), but this also was not statistically significant when compared with cirrhotic individuals with other HCV genotypes (P = .90).
Our analysis identified that anticonvulsant medications predicted a reduction in the SVR rate (OR = 12.54 in m-ITT analysis). Nine patients (1.4%) were prescribed anticonvulsant medications to control chronic neurological or psychiatric disorders. Anticonvulsant medications are reported to be potent inducers of P-glycoprotein polypeptide and cytochrome P450-CYP3A4 enzymes capable of decreasing sofosbuvir and daclatasvir plasma concentrations, respectively, leading to reduced therapeutic effect of these antivirals.[47–50] It is important to emphasize that in our series, the use of anticonvulsant medications was associated with therapeutic failure in all analyses performed, whether in the multivariate analysis of the 643 patients (by IIT or m-ITT) or in the separate analysis of the 520 coinfected patients, for whom we had all the clinical information related to HIV immunosuppression.
In our study, female gender and age were also significantly associated with a reduction in the SVR (P = .04 and P = .04 with modified-ITT analysis). It is known that with IFN-based anti-HCV therapy, gender (specifically male), and advanced age are predictive of nonresponse to treatment.[51,52] With the advent of DAAs, these variables have become of less concern, as high rates of SVR have been achieved, and most studies have not reported their association with therapeutic failure.[16–19] In the few studies that identified an impact of gender and age on SVR in the DAAs era, the results have been conflicting. Berenguer et al found that males had a higher rate of virological failure in a Spanish HCV/HIV cohort, while Bischoff reported in a German cohort that males aged over 50 years had a higher SVR rate.[20,22] Our sample consisted predominantly of men (74.7%) and individuals over 40 years of age (89.9%). We found that the prevalence of comorbidities, psychiatric disorders, and the use of non-ARV medications were higher in females than in men in our cohort. These variables, either individually or in combination, may have contributed to the observed female association with therapeutic failure.
The impact of comorbidities on DAAs treatment outcomes has been described previously.[53–56] A recent Brazilian study with mono-infected patients showed that having a higher number of comorbidities was significantly associated with therapeutic failure using SOF-based regimens.[55] In the cohort of HCV/HIV coinfected patients described by Cachay et al,[56] the only factor identified as associated with a lower SVR was the presence of psychiatric disease. Considering the evidence that individuals with HIV infection have a higher prevalence and number of comorbidities, especially psychiatric disorders, it is essential in this population to evaluate all possible comorbidities, medication usage, known drug interactions, and patient adherence to achieve an optimal outcome with HCV therapy.[26,53,56,57]
Regarding substitutions in the HCV genome associated with clinical resistance to DAAs, these were identified in less than 2% of our patients analyzed. The frequency of RASs in HCV NS5A genes in our study was lower than observed in other studies,[38,58–60] and their presence did not impair SVR rates in our population. An explanation for our results is that RASs, especially in the HCV NS5A gene, appear to impact the treatment response mainly in patients infected with HCV GT 1a and GT 3a.[37,38,58] In addition, we highlight that in the present study, RASs were not found at baseline in HCV GT3-positive patients (N = 32). This contrasts with results of other Brazilian studies where amino acid substitutions at positions 30 and 93 were identified.[61,62]
Limitations in our study need to be acknowledged. Its retrospective nature did not allow us to obtain totally comprehensive data for all included patients nor was it possible to adequately assess adherence to treatment, relying only on data from medical records. In addition, the high SVR rates observed made it difficult to identify predictors of treatment failure, resulting in a reduction in the statistical power of these analyses. We acknowledge that, although the SOF-DCV regimen is not currently a first-line regimen in the Americas, Europe, and Brazil, it remains one of the preferred regimens according to WHO guidelines.[33] Despite these limitations, this is one of the largest series of subjects to be evaluated using SOF-DCV combination therapy in people living with HIV as well as possibly the largest case series of HCV/HIV coinfected patients in Latin America.
Considering the epidemiological setting and the impact of HCV/HIV coinfection and its outcomes on global public health, the results regarding the HCV treatment response in this population may contribute to the implementation of improved global strategies for HCV elimination. In addition, it is important to note that HCV treatment in this population goes beyond SVR. Management of the risk for reinfection and the need for hepatocarcinoma surveillance, especially in patients with advanced liver disease, are priorities in the clinical follow-up of this population. It is also necessary to mention that subgroups of coinfected patients continue to have specific barriers to effective care. Comorbidities, especially psychiatric issues, dependency on alcohol, and other drugs, difficulties to access health services and even situations of incarceration reduce access to HCV treatment.[26,28,63] Implementation of health programs involving multidisciplinary teams could improve the overall clinical management of this population.
In summary, the high cure rate of HCV/HIV coinfected individuals after SVR in our multicenter population confirms the efficacy and safety of this protocol and supports its universal distribution.
Author contributions
SMM and MCMC developed the original idea to conduct this study. They were responsible for the data analysis, wrote the first draft and final revision of the manuscript.
AGV, AGL, ACMD, PRAF, DCJ, SBT, CEBM, MPG, FS, KDP, DVN: participated in the study design, in the patient cohort recruitment and data collection and revision of the manuscript.
GLN and RSA were responsible for data analysis
JRRP and FMM were responsible for laboratory analysis
SSW was responsible for the study concept and revision of the article.
All authors assessed, reviewed, and edited the manuscript and approved the final version for publication.
Footnotes
Abbreviations: AE = adverse event, DAAs = direct-action antivirals, DCV = daclatasvir, HCV = hepatitis C virus, ITT = intention to treat, SOF = sofosbuvir, SVR = sustained virological response.
How to cite this article: Machado SM, Vigani AG, Leite AG, Diaz AC, Ferreira PR, Carnaúba-Júnior D, Tenore SB, Brandão-Mello CE, Gonzalez MP, Siroma F, Prado KD, Nunes DV, Lisboa-Neto G, Pinho JR, Malta FM, Azevedo RS, Witkin SS, Mendes-Correa MC. Effectiveness of direct-acting antivirals for hepatitis C virus infection in hepatitis C/HIV co-infected individuals: A multicenter study. Medicine. 2020;99:30(e21270).
LIM 52, Institute of Tropical Medicine, São Paulo.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Global Hepatitis Report, 2017. Geneva: World Health Organization, 2017. Available at: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/. [Google Scholar]
- [2].Stepanova M, Younossi ZM. Economic burden of hepatitis C infection. Clin Liver Dis 2017;21:579–94. [DOI] [PubMed] [Google Scholar]
- [3].Global AIDS Update, 2016. Geneva: United Nations Programme on HIV/AIDS (UNAIDS). Available at: http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf. [Google Scholar]
- [4].Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol 2006;44: 1 suppl: S6–9. [DOI] [PubMed] [Google Scholar]
- [5].Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016;16:797–808. [DOI] [PubMed] [Google Scholar]
- [6].Rockstroh JK, Mohr R, Behrens G, et al. Liver fibrosis in HIV: which role does HIV itself, long-term drug toxicities and metabolic changes play? Curr Opin HIV AIDS 2014;9:365–70. [DOI] [PubMed] [Google Scholar]
- [7].Soto B, Sánchez-Quijano A, Rodrigo L, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol 1997;26:1–5. [DOI] [PubMed] [Google Scholar]
- [8].Ingiliz P, Rockstroh JK. Natural history of liver disease and affect of hepatitis C virus on HIV disease progression. Curr Opin HIV AIDS 2015;10:303–8. [DOI] [PubMed] [Google Scholar]
- [9].Smith CJ, Ryom L, Weber R, et al. D:A:D Study Group. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet 2014;384:241–8. [DOI] [PubMed] [Google Scholar]
- [10].Gjærde LI, Shepherd L, Jablonowska E, et al. Trends in incidences and risk factors for hepatocellular carcinoma and other liver events in HIV and hepatitis C virus-coinfected individuals from 2001 to 2014: a multicohort study. Clin Infect Dis 2016;63:821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Barreiro P, Fernandez-Montero JV, de Mendoza C, et al. Towards hepatitis C eradication from the HIV-infected population. Antiviral Res 2014;105:1–7. [DOI] [PubMed] [Google Scholar]
- [12].Sulkowski MS. Interferon-containing and Interferon-free HCV Therapy for HIV-infected Patients. Semin Liver Dis 2014;34:72–8. [DOI] [PubMed] [Google Scholar]
- [13].Webster DP, Klenerman P, Dusheiko GM, et al. Lancet 2015;385:1124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vogel M, Rockstroh JK. The treatment of chronic hepatitis C virus infection in HIV co-infection. Eur J Med Res 2009;14:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Beisel C, Heuer M, Otto B, et al. German cohort of HCV mono-infected and HCV/HIV co-infected patients reveals relative under-treatment of co-infected patients. AIDS Res Ther 2014;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Luetkemeyer AF, McDonald C, Ramgopal M, et al. 12 weeks of daclatasvir in combination with sofosbuvir for HIV-HCV coinfection (ALLY-2 Study): efficacy and safety by HIV combination antiretroviral regimens. Clin Infect Dis 2016;62:1489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rockstroh JK, Orkin C, Viani RM, et al. Safety and efficacy of ombitasvir, paritaprevir with ritonavir ± dasabuvir with or without Ribavirin in patients with human immunodeficiency virus-1 and hepatitis c virus genotype 1 or genotype 4 coinfection: TURQUOISE-I Part 2. Open Forum Infect Dis 2017;4: ofx154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bhattacharya D, Belperio PS, Shahoumian TA, et al. Effectiveness of all-oral antiviral regimens in 996 human immunodeficiency virus/hepatitis C virus genotype 1-coinfected patients treated in routine practice. Clin Infect Dis 2017;64:1711–20. [DOI] [PubMed] [Google Scholar]
- [19].Sogni P, Gilbert C, Lacombe K, et al. All-oral direct-acting antiviral regimens in HIV/hepatitis C virus-coinfected patients with cirrhosis are efficient and safe: real-life results from the prospective ANRS CO13-HEPAVIH cohort. Clin Infect Dis 2016;63:763–70. [DOI] [PubMed] [Google Scholar]
- [20].Bischoff J, Mauss S, Cordes C, et al. Rates of sustained virological response 12 weeks after the scheduled end of direct-acting antiviral (DAA)-based hepatitis C virus (HCV) therapy from National German HCV registry: does HIV coinfection impair the response to DAA combination therapy? HIV Med 2018;19:299–307. [DOI] [PubMed] [Google Scholar]
- [21].Milazzo L, Lai A, Calvi E, et al. Direct-acting antivirals in hepatitis C virus (HCV)-infected and HCV/HIV-coinfected patients: real-life safety and efficacy. HIV Med 2017;18:284–91. [DOI] [PubMed] [Google Scholar]
- [22].Berenguer J, Gil-Martin Á, Jarrin I, et al. All-oral direct-acting antiviral therapy against hepatitis C virus (HCV) in human immunodeficiency virus/HCV-coinfected subjects in real-world practice: Madrid coinfection registry findings. Hepatology 2018;68:32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Neukam K, Morano-Amado LE, Rivero-Juárez A, et al. HIV-coinfected patients respond worse to direct-acting antiviral-based therapy against chronic hepatitis C in real life than HCV-monoinfected individuals: a prospective cohort study. HIV Clin Trials 2017;18:126–34. [DOI] [PubMed] [Google Scholar]
- [24].Arias A, Aguilera A, Soriano V, et al. Rate and predictors of treatment failure to all-oral HCV regimens outside clinical trials. Antivir Ther 2017;22:307–12. [DOI] [PubMed] [Google Scholar]
- [25].Boesecke C, Ingiliz P, Berger F, et al. Liver cirrhosis as a risk factor for direct-acting antiviral therapy failure in real-life hepatitis C virus/human immunodeficiency virus coinfection. Open Forum Infect Dis 2017;4:ofx158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cachay E, Soriano V. Is HIV still a special population for the treatment of hepatitis C? AIDS 2016;30:2001–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Collins LF, Chan A, Zheng J, et al. Direct-acting antivirals improve access to care and cure for patients with HIV and chronic HCV infection. Open Forum Infect Dis 2017;5:ofx264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sikavi C, Chen PH, Lee AD, et al. Hepatitis C and human immunodeficiency virus coinfection in the era of direct-acting antiviral agents: no longer a difficult-to-treat population. Hepatology 2018;67:847–57. [DOI] [PubMed] [Google Scholar]
- [29].Wyles DL. Resistance to DAAs: when to look and when it matters. Curr HIV/AIDS Rep 2017;14:229–37. [DOI] [PubMed] [Google Scholar]
- [30].Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de DST, 535 Aids e Hepatites Virais. Protocolo Clínico e Diretrizes Terapêuticas para Hepatite Viral 536 C e Coinfecções, 2015. Available at: http://bvsms.saude.gov.br/bvs/publicacoes/protocolo_clinico_diretrizes_hepatite_co_coinfeccoes.pdf. [Google Scholar]
- [31].Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996;24:289–93. [DOI] [PubMed] [Google Scholar]
- [32].Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005;128:343–50. [DOI] [PubMed] [Google Scholar]
- [33].Guidelines for the screening care and treatment of persons with chronic hepatitis C infection. Genebra: World Health Organization [Internet]. 2016 [update 2016 apr; cited 2019]. Available at: http://apps.who.int/iris/bitstream/10665/205035/1/9789241549615_eng.pdf?ua=1. [PubMed] [Google Scholar]
- [34].Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med 2013;158:807–20. [DOI] [PubMed] [Google Scholar]
- [35].Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis patients with HIV/HCV co-infection. Hepatology 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- [36].Sanger F, Coulson AR. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol 1975;94:441–8. [DOI] [PubMed] [Google Scholar]
- [37].Lontok E, Harrington P, Howe A, et al. Hepatitis C virus drug resistance-associated substitutions: state of the art summary. Hepatology 2015;62:1623–32. [DOI] [PubMed] [Google Scholar]
- [38].European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C. J Hepatol 2017;66:153–94. [DOI] [PubMed] [Google Scholar]
- [39].Kalaghatgi P, Sikorski AM, Knops E, et al. Geno2pheno[HCV]—a web-based interpretation system to support hepatitis C treatment decisions in the era of direct-acting antiviral agents. PLoS One 2016;11:e0155869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wyles DL, Ruane PJ, Sulkowski MS, et al. Daclatasvir plus Sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 2015;373:714–25. [DOI] [PubMed] [Google Scholar]
- [41].Mandorfer M, Schwabl P, Steiner S, et al. Interferon-free treatment with sofosbuvir/daclatasvir achieves sustained virologic response in 100% of HIV/hepatitis C virus-coinfected patients with advanced liver disease. AIDS 2016;30:1039–47. [DOI] [PubMed] [Google Scholar]
- [42].Rockstroh JK, Ingiliz P, Petersen J, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, in real-world patients with HIV-HCV coinfection and advanced liver disease. Antivir Ther 2017;22:225–36. [DOI] [PubMed] [Google Scholar]
- [43].Lacombe K, Fontaine H, Dhiver C, et al. Real-world efficacy of daclatasvir and sofosbuvir, with and without ribavirin, in HIV/HCV coinfected patients with advanced liver disease in a French early access cohort. J Acquir Immune Defic Syndr 2017;75:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li G, De Clercq E. Current therapy for chronic hepatitis C: the role of direct-acting antivirals. Antiviral Res 2017;142:83–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nelson DR, Cooper JN, Lalezari JP, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology 2015;61:1127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Leroy V, Angus P, Bronowicki JP, et al. Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: a randomized phase III study (ALLY-3þ). Hepatology 2016;63:1430–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bonora S, Puoti M. Use of daclatasvir in HCV/HIV-coinfected patients in a real-life setting. AIDS Rev 2017;19:24–34. [PubMed] [Google Scholar]
- [48].Garimella T, You X, Wang R, et al. A review of daclatasvir drug–drug interactions. Adv Ther 2016;33:1867–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Roncero C, Villegas JL, Martínez-Rebollar M, et al. The pharmacological interactions between direct-acting antivirals for the treatment of chronic hepatitis c and psychotropic drugs. Expert Rev Clin Pharmacol 2018;11:999–1030. [DOI] [PubMed] [Google Scholar]
- [50].Smolders EJ, Smit C, de Kanter C, et al. Management of drug interactions with direct-acting antivirals in Dutch HIV/hepatitis C virus-coinfected patients: adequate but not perfect. HIV Med 2018;19:216–26. [DOI] [PubMed] [Google Scholar]
- [51].Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975–82. [DOI] [PubMed] [Google Scholar]
- [52].Bourlière M, Ouzan D, Rosenheim M, et al. Pegylated interferon-α2a plus ribavirin for chronic hepatitis C in a real-life setting: the Hepatys French cohort. Antivir Ther 2012;17:101–10. [DOI] [PubMed] [Google Scholar]
- [53].Goulet JL, Fultz SL, McGinnis KA, et al. Relative prevalence of comorbidities and treatment contraindications in HIV-mono-infected and HIV/HCV-co-infected veterans. AIDS 2005;19: suppl 3: S99–105. [DOI] [PubMed] [Google Scholar]
- [54].Nagaty A, Abd El-Wahab EW. Real-life results of sofosbuvir based therapy in chronic hepatitis C-naïve and -experienced patients in Egypt. PLoS One 2017;12:e0184654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Miotto N, Mendes LC, Zanaga LP, et al. All-oral direct antiviral treatment for hepatitis C chronic infection in a real-life cohort: the role of cirrhosis and comorbidities in treatment response. PLoS One 2018;13:e0199941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cachay E, Mena A, Benitez L, et al. Predictors of Lack of Hepatitis C Eradication Using Direct-Acting Antivirals [Poster 609]. 25th Conference on Retroviruses and opportunistic infections (CROI) 2018. [Google Scholar]
- [57].Fuller BE, Loftis JM, Rodriguez VL, et al. Psychiatric and substance use disorders comorbidities in veterans with hepatitis C virus and HIV coinfection. Curr Opin Psychiatry 2009;22:401–8. [DOI] [PubMed] [Google Scholar]
- [58].Hernandez D, Zhou N, Ueland J, et al. Natural prevalence of NS5A polymorphisms in subjects infected with hepatitis C virus genotype 3 and their effects on the antiviral activity of NS5A inhibitors. J Clin Virol 2013;57:13–8. [DOI] [PubMed] [Google Scholar]
- [59].Chen ZW, Li H, Ren H, et al. Global prevalence of pre-existing HCV variants resistant to direct-acting antiviral agents (DAAs): mining the GenBank HCV genome data. Sci Rep 2016;6:203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Caudai C, Materazzi A, Saladini F, et al. Natural NS5A inhibitor resistance associated substitutions in hepatitis C virus genotype 1 infected patients from Italy. Clin Microbiol Infect 2018;24:308.e5–8. [DOI] [PubMed] [Google Scholar]
- [61].Malta F, Gaspareto KV, Lisboa-Neto G, et al. Prevalence of naturally occurring NS5A resistance-associated substitutions in patients infected with hepatitis C virus subtype 1a, 1b, and 3a, co-infected or not with HIV in Brazil. BMC Infect Dis 2017;17:716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Costa VD, Brandão-Mello CE, Nunes EP, et al. Treatment of chronic HCV infection with DAAs in Rio de Janeiro/Brazil: SVR rates and baseline resistance analyses in NS5A and NS5B genes. PLoS One 2019;14:e0216327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Collins LF, Chan A, Zheng J, et al. Direct-acting antivirals improve access to care and cure for patients with HIV and chronic HCV infection. Open Forum Infect Dis 2018;5:ofx264. [DOI] [PMC free article] [PubMed] [Google Scholar]


