Abstract
Background:
Water vapor thermal therapy (WVTT) is a minimally invasive procedure for treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia (BPH). There are no known systematic reviews reporting the effectiveness and safety of this increasingly common BPH therapy.
Methods:
We performed a systematic review and meta-analysis of studies utilizing WVTT for symptomatic BPH. The international prostate symptom score (IPSS), IPSS-quality of life (IPSS-QOL), BPH impact index (BPHII), and maximum flow rate (Qmax) were calculated as the weighted mean difference relative to baseline and reported in minimal clinically important difference (MCID) units. MCID thresholds were −3 for IPSS, −0.5 for IPSS-QOL, −0.5 for BPHII, and 2 mL/s for Qmax. The surgical retreatment rate was calculated using life-table methods.
Results:
We identified 5 cohorts treated with WVTT from 4 studies (514 patients; 40% with median lobe obstruction) with 2 years median follow-up (range: 6 months to 4 years). The IPSS, IPSS-QOL, BPHII, and Qmax significantly improved at all intervals between 3 months and 4 years; this benefit ranged from 3.3 to 3.8 MCID units for IPSS, 3.9 to 4.6 MCID units for IPSS-QOL, 6.8 to 8.2 MCID units for BPHII, and 1.5 to 3.0 MCID units for Qmax. The surgical retreatment rate was 7.0% at 4 years of follow-up data. Most adverse events were nonserious and transient; dysuria, urinary retention, and urinary tract infection were most common. No cases of de novo erectile dysfunction occurred.
Conclusions:
WVTT provided improvement in BPH symptoms that exceeded established MCID thresholds, preserved sexual function, and was associated with low surgical retreatment rates over 4 years.
Keywords: benign prostatic hyperplasia, lower urinary tract symptoms, prostate, radiofrequency, Rezum, water vapor thermal therapy
1. Introduction
Benign prostatic hyperplasia (BPH) is a histological diagnosis involving prostatic tissue overgrowth around the urethra that affects most men during their lifetime.[1] In the Olmsted County population-based study of lower urinary tract symptoms (LUTS) secondary to BPH,[2] moderate or severe LUTS were reported in 12% of men aged 40 to 49 years and 29% of men aged 70 to 79 years. Medical management with an alpha blocker and/or a 5-alpha reductase inhibitor may be recommended as a first-line treatment approach for bothersome LUTS secondary to BPH. Use of LUTS/BPH-related medications increases with age, particularly among men aged 40 to 60 years.[3] Yet long-term clinical utility is limited because most patients discontinue these medications within the first year of initiating treatment due to intolerance or inadequate response.[4–6] For patients with bothersome LUTS who fail medical therapy, transurethral resection of the prostate (TURP) is often recommended since this surgery provides clinically meaningful and durable symptom relief.[7,8] However, TURP is associated with several drawbacks such as the need for regional/general anesthesia and hospitalization, high rates of postoperative sexual dysfunction, and increased anesthetic risk in older patients with coexisting medical conditions. Consequently, several minimally invasive surgical therapies have been developed to improve BPH-related quality of life and minimize the risk of iatrogenic complications.
Water vapor thermal therapy (WVTT; Rezum, Boston Scientific, Marlborough, MA) is a promising treatment option for LUTS attributed to BPH in men with or without an obstructive middle lobe, prostate volume under 80 cc, and who wish to preserve sexual function.[7,8] WVTT utilizes convective radiofrequency to create stored thermal energy in the form of steam, which is delivered transurethrally into the transition zone of the prostate to ablate tissue, thereby reducing LUTS. Pivotal trial results of WVTT demonstrated clinically meaningful improvements in LUTS, preservation of sexual function, and low retreatment rates.[9] However, a systematic review of WVTT has not been performed. Dissemination of the benefits and risks of BPH treatments by means of evidence-based approaches including results derived from clinical trials and observational studies is important to facilitate shared decision-making between patients and healthcare providers. Therefore, the objective of this study was to determine the safety and effectiveness of WVTT in the treatment of LUTS attributed to BPH by means of a systematic review and meta-analysis.
2. Methods
2.1. Eligibility criteria
The review protocol was registered at the International Prospective Register of Systematic Reviews public database (CRD#42020150083). Review methods followed the statement on the preferred reporting items for systematic reviews and meta-analyses (PRISMA).[10] Randomized trials, nonrandomized controlled studies, and case series of WVTT for symptomatic BPH were eligible for inclusion in this systematic review. We excluded studies with a sample size of less than 10 patients, studies in which patients were treated with concomitant surgeries, and studies that did not report any prespecified outcome of this review. No date or language restrictions were applied to the searches.
2.2. Search strategy
We performed systematic searches of Medline, Embase, and the Cochrane Central Register of Controlled Trials for potentially eligible studies. The search strategy included combinations of diagnosis-related (benign prostatic hyperplasia, BPH, lower urinary tract symptoms, LUTS) and treatment-specific (convective, radiofrequency, Rezum, thermal therapy, water vapor, WAVE) keywords. Additional searches were conducted in the Directory of Open Access Journals and Google Scholar. We performed manual searches of the reference lists of included papers and relevant meta-analyses. Finally, we requested unpublished data from the manufacturer of WVTT. Two researchers with expertise in systematic reviews independently screened titles and abstracts for eligibility. Full-text manuscripts were obtained for all potentially relevant studies. To account for multiple papers derived from the same primary study or subsamples of the primary study, we maximized the yield of information by mapping all publications to unique studies and collating all available data. Disagreements related to study eligibility were resolved by discussion and consensus. The final search was performed on August 30, 2019.
2.3. Data extraction and outcomes
Researchers independently extracted metadata, study characteristics, patient characteristics, periprocedural outcomes, risk of bias, and main outcomes data from eligible studies using standardized data collection forms. We used the National Institute of Health assessment tool for before-after studies to evaluate the methodological quality of eligible studies.[11] Main outcomes of this review were the international prostate symptom score (IPSS), IPSS quality of life (IPSS-QOL), benign prostatic hyperplasia impact index (BPHII), maximum flow rate (Qmax), surgical retreatments, and adverse events. The IPSS is scored on a 0 to 35 scale with higher scores indicating greater frequency of BPH symptoms.[12] The IPSS-QOL consists of a single question: If you were to spend the rest of your life with your urinary condition just the way it is now, how would you feel about that?, with scores ranging from 0 (delighted) to 6 (terrible).[12] The BPHII is scored on a 0 to 13 scale with higher scores indicating greater health impact of BPH symptoms.[12] Qmax values below 10 mL/s indicate abnormal urinary flow, values between 10 to 15 mL/s are borderline, and values greater than 15 mL/s indicate normal urinary flow. The MCID for change from baseline was defined as −3.0 for IPSS,[12] −0.5 for IPSS-QOL,[13] −0.5 for BPHII,[12] and 2 mL/s for Qmax.[14] Surgical retreatments included any surgical prostate procedure performed for LUTS. A priori, we planned to report the frequency of dysuria, hematuria, urinary tract infection, urethral stricture, pelvic pain, urinary retention, urgency, frequency, incontinence, de novo erectile dysfunction, ejaculatory dysfunction, and any other adverse event reported in more than 5% of patients in any study. For each study, we preferentially extracted data from the paper reporting the longest follow-up duration on the entire cohort and supplemented any missing data using other papers derived from the same study. Data extraction discrepancies between the 2 researchers were resolved by discussion and consensus.
2.4. Statistical analysis
Treatment effects for IPSS, IPSS-QOL, BPHII, and Qmax were calculated as the weighted mean difference (WMD) relative to baseline at distinct follow-up intervals ranging from 3 months to 4 years using a random effects meta-analysis model with inverse variance weighting to account for anticipated heterogeneity among studies. In order to facilitate clinical interpretation of the meta-analysis results, we additionally reported these treatment effects in standardized minimal clinically important difference (MCID) units,[15,16] where the standardized MCID for each outcome was calculated as the WMD divided by the MCID. Treatment effects below 0.5 MCID units indicate that it is unlikely that an appreciable number of patients will show a clinically important benefit, treatment effects between 0.5 and 1 MCID units indicate that a treatment may be beneficial to an appreciable number of patients, and treatment effects above 1 MCID unit indicate that many patients may gain important benefits from treatment.[15,16] The cumulative rate of surgical retreatments through 4 years was calculated with life-table methods by determining the number of patients at risk, the number of surgical retreatments, and the number of censored patients at distinct intervals.[17] Adverse events were reported as weighted event rates. We estimated heterogeneity among studies with the I2 statistic where a value of 0% represented no heterogeneity and larger values represented increasing heterogeneity.[18] We planned to assess the potential for publication bias by visual inspection of funnel plots and with formal analysis using Egger regression test, and to explore potential sources of heterogeneity using metaregression for any outcome reported in at least 10 studies; however, these analyses were not performed due to the limited number of included studies. We performed a 1-study removed analysis in which we iteratively removed 1 study at a time from the meta-analysis to determine whether conclusions for IPSS, IPSS-QOL, BPHII, and Qmax were significantly influenced by any single study; this analysis was performed at the median follow-up duration among all studies to provide a common timepoint for analysis. P-values were 2-sided with a significance level <.05. Analyses were performed using Review Manager v5.3 (The Cochrane Collaboration, Copenhagen, Denmark).
3. Results
3.1. Data ethics
Ethical approval and patient consent were not required because this is a systematic review and meta-analysis of previously published studies. The authors agree to make the raw data from this analysis available upon reasonable request.
3.2. Study selection
We identified 279 papers in our searches and ultimately included 5 cohorts derived from 4 studies of WVTT for LUTS due to BPH. The manufacturer of WVTT additionally provided unpublished 4-year follow-up data from the crossover arm of the pivotal study.[19] Among the 45 full-text papers that were reviewed, 40 were excluded, with review articles (14) and duplicate publications (13) the most common reasons for exclusion (see PRISMA flow diagram Fig. 1).
Figure 1.
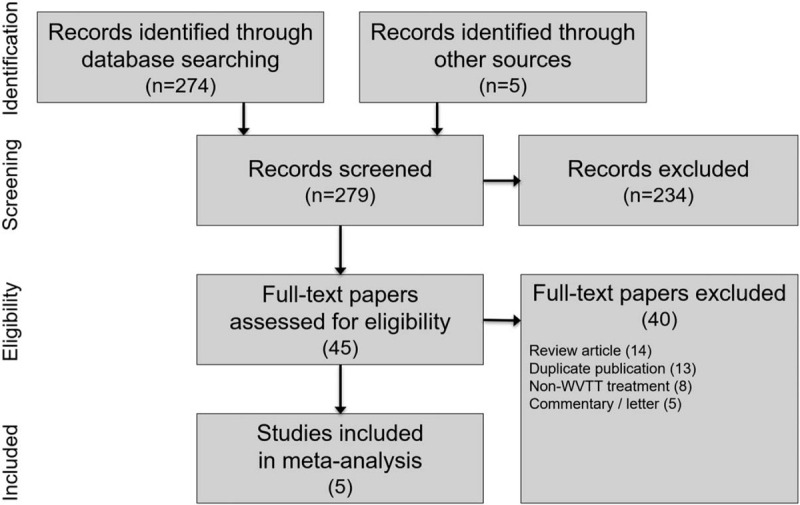
PRISMA study flow diagram. PRISMA = preferred reporting items for systematic reviews and meta-analyses, WVTT = water vapor thermal therapy.
3.3. Characteristics of studies, patients, and procedures
Five cohorts comprising 514 patients were included in this meta-analysis. The cohorts were derived from a prospective pilot study,[20] the prospective treatment arm of the pivotal study,[9,19,21–23] the prospective crossover arm of the pivotal study,[19] a retrospective postmarket study,[24] and a retrospective single-site study[25]; the last study included 25 patients who were also enrolled in the treatment arm of the pivotal study. Thus, the results of this meta-analysis were derived from a cohort of 95% unique patients. The only study that utilized a control group was the pivotal study,[9,19,21–23] a randomized trial of WVTT vs. a sham control. At 3 months post-treatment, 87% of control patients crossed over to receive WVTT. Therefore, comparative results from this study were only available through 3 months, after which data in the WVTT arm were reported descriptively. For this reason, we extracted data only from the WVTT arm of each study for the meta-analysis.
Patient follow-up in each study ranged from 6 to 48 months (median = 24 months) (Table 1). The risk of bias was rated low for 2 studies and moderate for 3 studies (Table 2). The mean patient age in each study ranged from 63 to 71 years (median = 67 years), mean prostate volume ranged from 45 to 53 cc (median = 46 cc), 57% of patients presented with severe BPH symptoms (median IPSS = 20), and urinary flow obstruction was common (median Qmax = 9.9 mL/s) (Table 3). Patients received an average of 5 water vapor injections per procedure, which included 40% of patients who received obstructive median lobe treatment. Anesthetic regimens varied among studies, with most procedures performed under oral or intravenous sedation. All WVTT procedures in each study were successfully completed without serious procedure-related complications (Table 4).
Table 1.
Characteristics of included studies.
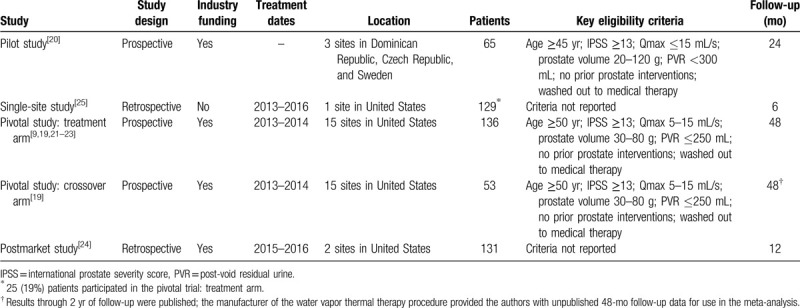
Table 2.
Study quality assessment using the National Institute of Health assessment tool for before-after studies∗.
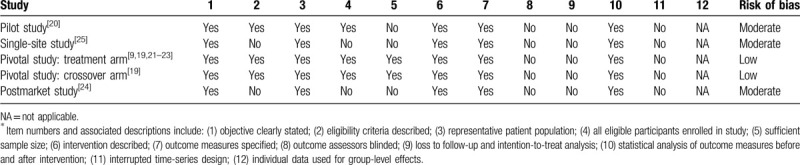
Table 3.
Baseline patient characteristics∗.
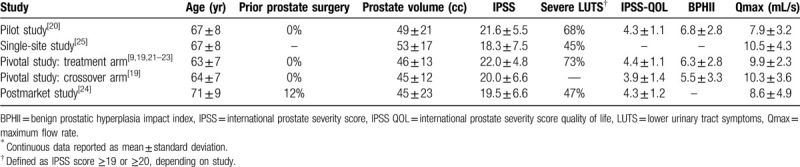
Table 4.
Water vapor thermal therapy procedural details.
3.4. Temporal trends in main outcomes after WVTT
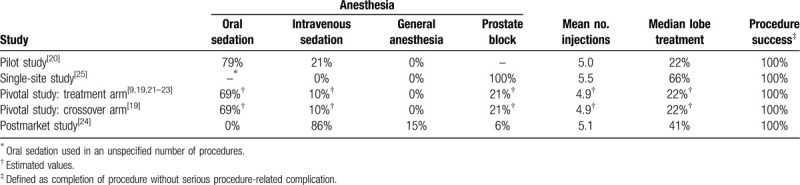
IPSS, IPSS-QOL, BPHII, and Qmax significantly improved following WVTT and the changes from baseline were statistically significant and clinically important at all follow-up intervals. The treatment benefit of WVTT in relation to established MCIDs ranged from 3.3 to 3.8 MCID units for IPSS, 3.9 to 4.6 MCID units for IPSS-QOL, 6.8 to 8.2 MCID units for BPHII, and 1.5 to 3.0 MCID units for Qmax (Fig. 2), indicating for each of these outcomes at each follow-up interval that “many patients may gain important benefits from treatment.”[15,16] Meta-analysis results of IPSS, IPSS-QOL, BPHII, and Qmax at each follow-up interval are provided in Table 5. IPSS scores decreased by 11.2 points at 3 months and this improvement was sustained through 4 years (Fig. 3). IPSS-QOL scores were approximately 2 points below the baseline value at all follow-up intervals through 4 years (Fig. 4). BPHII decreased by 3.7 points by 3 months and remained largely unchanged thereafter over 4 years (Fig. 5). Qmax improved from approximately 10 mL/s at baseline to between 13 and 16 mL/s at each follow-up interval through 4 years (Fig. 6). These conclusions were unchanged in 1-study removed sensitivity analyses performed at 2 years (median) follow-up where the WMD for change from baseline ranged from −11.4 to −10.5 for IPSS, −2.3 to −1.9 for IPSS-QOL, −4.2 to −3.3 for BPHII, and 3.6 to 4.5 mL/s for Qmax (all P < .001).
Figure 2.
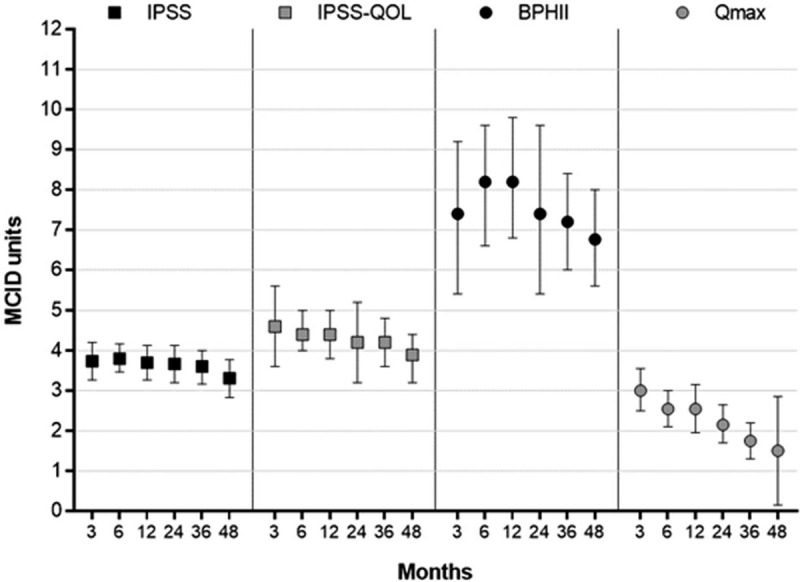
Improvement in BPH symptoms from baseline following water vapor thermal therapy reported in standardized MCID units with 95% CI. The MCID is −3.0 for IPSS,[12] −0.5 for IPSS-QOL,[12] −0.5 for BPHII,[12] and 2 mL/s for Qmax.[14] Treatment effects below 0.5 MCID units indicate that it is unlikely that an appreciable number of patients will show a clinically important benefit, treatment effects between 0.5 and 1 MCID units indicate that a treatment may be beneficial to an appreciable number of patients, and treatment effects above 1 MCID unit indicate that many patients may gain important benefits from treatment.[15,16] BPH = benign prostatic hyperplasia, BPHII = benign prostatic hyperplasia impact index, CI = confidence interval, IPSS = international prostate severity score, IPSS QOL = International prostate severity score quality of life, MCID = minimal clinically important difference, Qmax = maximum flow rate.
Table 5.
Pooled estimates of outcome measures following water vapor thermal therapy.
Figure 3.
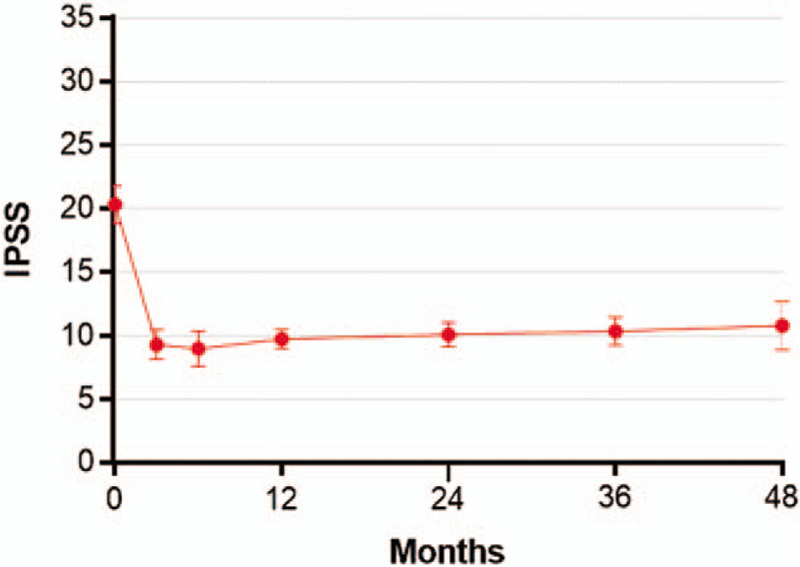
Temporal trends in IPSS scores following water vapor thermal therapy. The IPSS is scored on a 0 to 35 scale with higher scores indicating greater frequency of BPH symptoms. Plotted data are weighted mean and 95% confidence interval. BPH = benign prostatic hyperplasia, IPSS = international prostate severity score.
Figure 4.
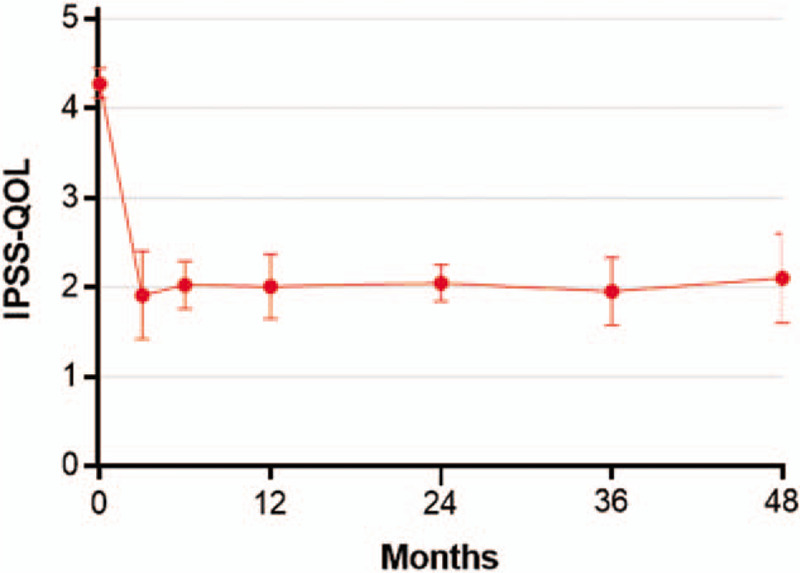
Temporal trends in IPSS-QOL scores following water vapor thermal therapy. IPSS-QOL is a single question: If you were to spend the rest of your life with your urinary condition just the way it is now, how would you feel about that?, with scores ranging from 0 (delighted) to 6 (terrible). Plotted data are weighted mean and 95% confidence interval. IPSS QOL = international prostate severity score quality of life.
Figure 5.
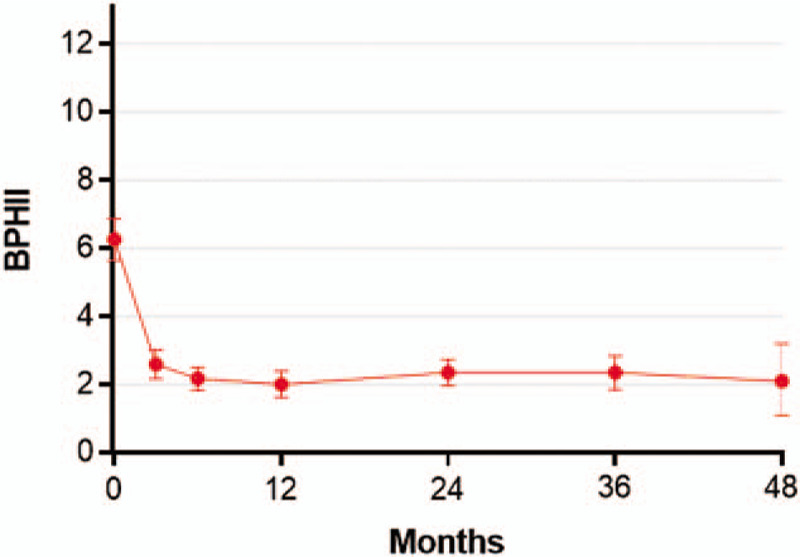
Temporal trends in BPHII scores following water vapor thermal therapy. The BPHII is scored on a 0 to 13 scale with higher scores indicating greater health impact of BPH symptoms. Plotted data are weighted mean and 95% confidence interval. BPH = benign prostatic hyperplasia, BPHII = benign prostatic hyperplasia impact index.
Figure 6.
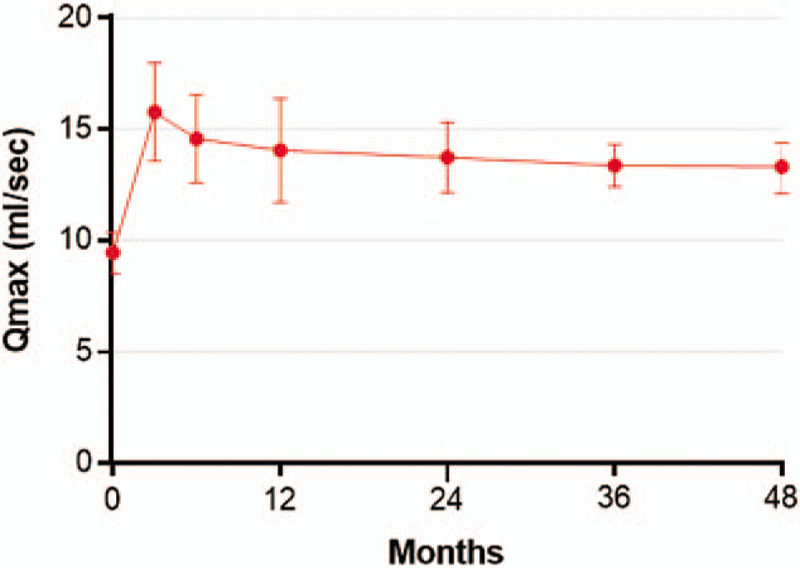
Temporal trends in Qmax (mL/s) following water vapor thermal therapy. Qmax values below 10 mL/s indicate abnormal urinary flow, values between 10 and 15 mL/s are borderline, and values greater than 15 mL/s indicate normal urinary flow. Plotted data are weighted mean and 95% confidence interval. Qmax = maximum flow rate.
3.5. Surgical retreatments and complications with WVTT
The cumulative surgical retreatment rate was 2.4% at 1 year, 5.3% at 2 years, 6.3% at 3 years, and 7.0% at 4 years of follow-up (Fig. 7). The surgical procedures included repeat WVTT (13), TURP (8), open prostatectomy (2), and photovaporization (1). The most common adverse events with WVTT were dysuria (16.2%), urinary retention (11.2%), and urinary tract infection (10.9%). There were no reports of de novo ED (Fig. 8). Qualitatively, most reported adverse events were nonserious and transient. However, adverse events were reported with insufficient detail overall to objectively determine temporal trends or relatedness to the WVTT procedure.
Figure 7.
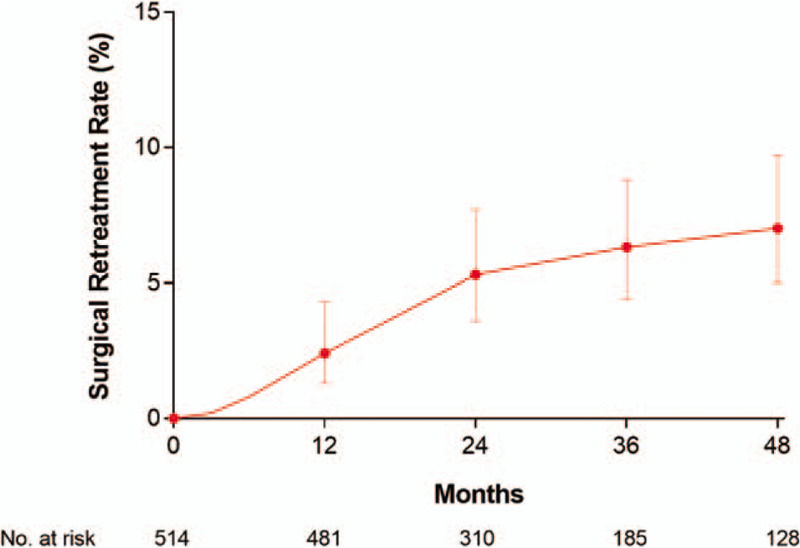
Cumulative surgical retreatment rate following water vapor thermal therapy. Plotted data are cumulative event rate and 95% confidence interval. At 4 yr follow-up, the surgical retreatment rate was 7.0% (95% confidence interval = 5.0%–9.7%).
Figure 8.
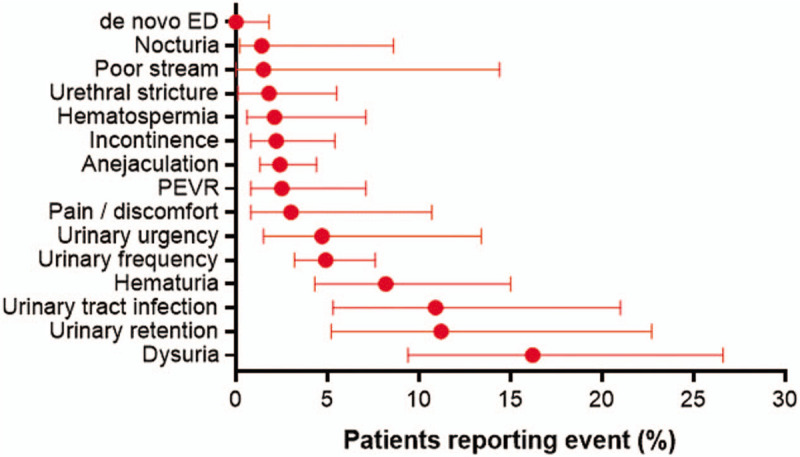
Frequency of adverse events following water vapor thermal therapy. Plotted values are weighted event frequency and 95% confidence interval. ED = erectile dysfunction, PEVR = perceived ejaculate volume reduction.
4. Discussion
LUTS secondary to BPH is an undertreated condition that adversely impacts quality of life in many men.[26] Men with severe symptoms may be reluctant to undergo surgery due to concerns regarding anesthetic risk and sexual side effects. WVTT has been used with increasing frequency to treat men with bothersome BPH symptoms who wish to avoid invasive surgery while preserving sexual function. We performed the first known systematic review and meta-analysis of WVTT for LUTS due to BPH. Our review demonstrated that WVTT was successfully delivered to all patients, including those with an obstructive median lobe. Following treatment, symptom improvement exceeded published MCIDs by several-fold over 4 years of follow-up. The rate of surgical retreatments was low, the safety profile of the procedure was acceptable as most adverse events were nonserious and transient, and no cases of de novo erectile dysfunction were reported. These results suggest that WVTT may be a valuable addition to the urological armamentarium for older men who are seeking a less invasive, yet durable treatment for bothersome BPH symptom that preserves sexual function.
It is plausible that the effectiveness of WVTT may be influenced by patient characteristics. Results of WVTT categorized by baseline symptom severity and presence of an obstructive median lobe were reported in some studies, but with insufficient detail to pool the results in the meta-analysis. Dixon et al[20] reported that IPSS scores decreased by 54% in patients with moderate LUTS and by 54% in those with severe LUTS over 2 years after WVTT. Mollengarden et al[25] reported that no significant differences were noted between those with moderate vs. severe LUTS or with versus without median lobe treatment; however, detailed results were not available. Darson et al[24] reported IPSS improvements of 41% and 50% over 1 year in patients with moderate and severe LUTS, respectively. They also reported a higher likelihood of achieving a 3-point decrease in IPSS in patients with vs. without obstructive median lobe treatment (92% vs 75%). McVary et al[23] reported an 11.8-point decrease in IPSS among those with a treated median lobe versus a 9.9-point decrease in those with no median lobe present (P = .61 for subgroup comparison). Further, IPSS improved by 56% and 38% for patients with severe and moderate LUTS, respectively. There was also evidence in this study that an untreated obstructive median lobe may be a risk factor for surgical retreatment following WVTT since 4 of 6 surgical retreatments were performed in those with identified but untreated median lobes. Overall, these results suggest that WVTT provides clinically meaningful improvements in LUTS that is independent of baseline severity or obstructive median lobe treatment. However, the risk of surgical retreatment following WVTT may be higher in patients with an untreated obstructive median lobe.
In the American Urological Association guidelines for surgical management of LUTS attributed to BPH,[7,8] 2 less invasive alternatives to TURP were recommended for well-selected men who desired preservation of sexual function—WVTT and PUL. While no study has directly compared WVTT to PUL, results from previous studies are available to draw indirect comparisons between these treatments. In a systematic review of PUL outcomes at 1 year, IPSS decreased by 8.0 points, IPSS-QOL decreased by 2.2 points, and Qmax improved by 3.8 mL/s.[27] Over this same period in our analysis, IPSS decreased by 11.1 points, IPSS-QOL decreased by 2.2 points, and Qmax improved by 5.1 mL/s. Longer term indirect comparisons of these therapies are facilitated by review of the pivotal study results for each treatment. Over 5 years of follow-up, 32 of 140 (22.9%) patients treated with PUL underwent surgical retreatment (19 for LUTS and 13 for implant removal)[28] while over 4 years, 6 of 135 (4.4%) patients treated with WVTT underwent surgical retreatment (all for LUTS).[9] Additionally, the percentage of patients who restarted BPH medications was 9.3% with PUL and 5.2% with WVTT. When the combination of treatment effectiveness, retreatment rate, and cost was included in a cost effectiveness analysis, PUL was more expensive and less effective than WVTT.[29] Thus, the clinical and cost utility of WVTT appears favorable in relation to PUL. Importantly, the above results were derived from indirect comparisons and should be viewed as hypothesis-generating. Prospective studies that directly compare these treatments while adequately controlling for potential confounding variables are encouraged to derive more definite conclusions regarding the comparative risks and benefits of each therapy.
The main strengths of this review were prospectively defined methodology, adherence to PRISMA guidelines, excellent generalizability of results to real-world clinical practice given the inclusion of clinical trials as well as real-world observational studies, and robust conclusions that were unchanged in sensitivity analyses. There were also several limitations pertaining to the quality of the included studies that may have influenced conclusions. First, there was less precision in the results with extended follow-up since fewer studies with longer term data were available. Additional studies would improve the reliability of the meta-analysis estimates. Second, the number of included studies was insufficient to evaluate publication bias or sources of heterogeneity. Third, patients in the included studies typically presented with a prostate volume no larger than 80 cc and, therefore, the safety and effectiveness of WVTT in larger prostates are unclear. A clinical trial of WVTT for prostate sizes between 80 and 150 cc is ongoing (ClinicalTrials.gov Identifier: NCT03605745), but results are not yet unavailable. Fourth, retrospective enrollment, unclear inclusion criteria, and limited follow-up duration were aspects of certain studies that may limit interpretability of results. Fifth, adverse event reporting was inconsistent among studies and it is unclear if complication under-reporting may have affected the accuracy of our estimates. Development of consistent and comprehensive adverse event reporting standards for use in future trials of minimally invasive BPH therapies is warranted. Finally, although 1 study utilized a sham control, most control patients elected to crossover to WVTT at 3 months due to insufficient symptom relief. Aside from this 3-month period in a single study, direct comparative data with a control group or other BPH treatments are not available. Therefore, comparisons of results with WVTT versus treatments such as PUL or TURP should be interpreted cautiously.
5. Conclusion
The WVTT procedure provides improvement in BPH symptoms that exceeds established MCID thresholds, preserves sexual function, and is associated with low surgical retreatment rates over 4 years of follow-up. These results suggest that the WVTT procedure may be a valuable addition to the urological armamentarium to treat LUTS in men with BPH.
Acknowledgments
The authors thank David Fay, PhD for assistance with literature review and data extraction, and Boston Scientific for providing unpublished extended follow-up data from the crossover arm of the WVTT pivotal study.
Author contributions
Conceptualization: Larry E. Miller, Bilal Chughtai, Kevin McVary, Ricardo R. Gonzalez, Sirikan Rojanasarot, Kyle DeRouen, Samir Bhattacharyya.
Formal analysis: Larry E. Miller.
Funding acquisition: Samir Bhattacharyya.
Investigation: Larry E. Miller, Bilal Chughtai, Kevin McVary, Ricardo R. Gonzalez, Sirikan Rojanasarot, Kyle DeRouen, Samir Bhattacharyya.
Methodology: Larry E. Miller, Bilal Chughtai, Kevin McVary, Ricardo R. Gonzalez, Sirikan Rojanasarot, Kyle DeRouen, Samir Bhattacharyya.
Project administration: Larry E. Miller, Bilal Chughtai, Kevin McVary, Ricardo R. Gonzalez, Sirikan Rojanasarot, Kyle DeRouen, Samir Bhattacharyya.
Resources: Samir Bhattacharyya.
Supervision: Samir Bhattacharyya.
Validation: Larry E. Miller, Bilal Chughtai, Kevin McVary, Ricardo R. Gonzalez, Sirikan Rojanasarot.
Visualization: Larry E. Miller.
Writing – original draft: Larry E. Miller.
Writing – review & editing: Bilal Chughtai, Kevin McVary, Ricardo R. Gonzalez, Sirikan Rojanasarot, Kyle DeRouen, Samir Bhattacharyya.
Footnotes
Abbreviations: BPH = benign prostatic hyperplasia, BPHII = benign prostatic hyperplasia impact index, IPSS = international prostate symptom score, IPSS-QOL = international prostate symptom score quality of life, LUTS = lower urinary tract symptoms, MCID = minimal clinically important difference, PRISMA = preferred reporting items for systematic reviews and meta-analyses, Qmax = maximum flow rate, TURP = transurethral resection of the prostate, WMD = weighted mean difference, WVTT = water vapor thermal therapy.
How to cite this article: Miller LE, Chughtai B, McVary K, Gonzalez RR, Rojanasarot S, DeRouen K, Bhattacharyya S. Water vapor thermal therapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia: systematic review and meta-analysis. Medicine. 2020;99:30(e21365).
Larry Miller discloses consultancy with Boston Scientific. Bilal Chughtai discloses consultancy with Boston Scientific and Olympus. Kevin McVary discloses consultancy and PI for Astellas, Boston Scientific, NIDDK, Merck, UroNext, Olympus, and SRS Medical Systems. Ricardo Gonzalez discloses consultancy and was an investigator for Boston Scientific, MediTate, Procept BioRobotics, Medeon Bio, and Allergan. Sirikan Rojanasarot, Kyle DeRouen, and Samir Bhattacharyya disclose employment with Boston Scientific.
Boston Scientific provided funding for this study.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Berry SJ, Coffey DS, Walsh PC, et al. The development of human benign prostatic hyperplasia with age. J Urol 1984;132:474–9. [DOI] [PubMed] [Google Scholar]
- [2].Chute CG, Panser LA, Girman CJ, et al. The prevalence of prostatism: a population-based survey of urinary symptoms. J Urol 1993;150:85–9. [DOI] [PubMed] [Google Scholar]
- [3].Welliver C, Feinstein L, Ward JB, et al. Trends in lower urinary tract symptoms associated with benign prostatic hyperplasia, 2004 to 2013: the urologic diseases in America project. J Urol 2019;203:171–8. [DOI] [PubMed] [Google Scholar]
- [4].Cindolo L, Pirozzi L, Fanizza C, et al. Drug adherence and clinical outcomes for patients under pharmacological therapy for lower urinary tract symptoms related to benign prostatic hyperplasia: population-based cohort study. Eur Urol 2015;68:418–25. [DOI] [PubMed] [Google Scholar]
- [5].Koh JS, Cho KJ, Kim HS, et al. Twelve-month medication persistence in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia. Int J Clin Pract 2014;68:197–202. [DOI] [PubMed] [Google Scholar]
- [6].Zabkowski T, Saracyn M. Drug adherence and drug-related problems in pharmacotherapy for lower urinary tract symptoms related to benign prostatic hyperplasia. J Physiol Pharmacol 2018;69:639–45. [DOI] [PubMed] [Google Scholar]
- [7].Foster HE, Dahm P, Kohler TS, et al. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline amendment 2019. J Urol 2019;202:592–8. [DOI] [PubMed] [Google Scholar]
- [8].Foster HE, Barry MJ, Dahm P, et al. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline. J Urol 2018;200:612–9. [DOI] [PubMed] [Google Scholar]
- [9].McVary KT, Rogers T, Roehrborn CG. Rezum water vapor thermal therapy for lower urinary tract symptoms associated with benign prostatic hyperplasia: 4-year results from randomized controlled study. Urology 2019;126:171–9. [DOI] [PubMed] [Google Scholar]
- [10].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65–94. [DOI] [PubMed] [Google Scholar]
- [11].National Institute of Health. Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. [Accessed June 2, 2019]. [Google Scholar]
- [12].Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol 1995;154:1770–4. [DOI] [PubMed] [Google Scholar]
- [13].Barry MJ, Cantor A, Roehrborn CG, et al. Relationships among participant international prostate symptom score, benign prostatic hyperplasia impact index changes and global ratings of change in a trial of phytotherapy in men with lower urinary tract symptoms. J Urol 2013;189:987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].National Institute for Health and Care Excellence. Lower Urinary Tract Symptoms in Men: Management; Clinical Guideline [CG97]. 2010. Available from: https://www.nice.org.uk/guidance/CG97. [Accessed August 21, 2019]. [Google Scholar]
- [15].Johnston BC, Patrick DL, Thorlund K, et al. Patient-reported outcomes in meta-analyses-part 2: methods for improving interpretability for decision-makers. Health Qual Life Outcomes 2013;11:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Johnston BC, Thorlund K, Schunemann HJ, et al. Improving the interpretation of quality of life evidence in meta-analyses: the application of minimal important difference units. Health Qual Life Outcomes 2010;8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Roehrborn CG, Gange SN, Gittelman MC, et al. Convective thermal therapy: durable 2-year results of randomized controlled and prospective crossover studies for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. J Urol 2017;197:1507–16. [DOI] [PubMed] [Google Scholar]
- [20].Dixon CM, Cedano ER, Pacik D, et al. Two-year results after convective radiofrequency water vapor thermal therapy of symptomatic benign prostatic hyperplasia. Res Rep Urol 2016;8:207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McVary KT, Gange SN, Gittelman MC, et al. Erectile and ejaculatory function preserved with convective water vapor energy treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: randomized controlled study. J Sex Med 2016;13:924–33. [DOI] [PubMed] [Google Scholar]
- [22].McVary KT, Gange SN, Gittelman MC, et al. Minimally invasive prostate convective water vapor energy ablation: a multicenter, randomized, controlled study for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol 2016;195:1529–38. [DOI] [PubMed] [Google Scholar]
- [23].McVary KT, Roehrborn CG. Three-year outcomes of the prospective, randomized controlled Rezum system study: convective radiofrequency thermal therapy for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Urology 2018;111:1–9. [DOI] [PubMed] [Google Scholar]
- [24].Darson MF, Alexander EE, Schiffman ZJ, et al. Procedural techniques and multicenter postmarket experience using minimally invasive convective radiofrequency thermal therapy with Rezum system for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Res Rep Urol 2017;9:159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mollengarden D, Goldberg K, Wong D, et al. Convective radiofrequency water vapor thermal therapy for benign prostatic hyperplasia: a single office experience. Prostate Cancer Prostatic Dis 2018;21:379–85. [DOI] [PubMed] [Google Scholar]
- [26].Speakman M, Kirby R, Doyle S, et al. Burden of male lower urinary tract symptoms (LUTS) suggestive of benign prostatic hyperplasia (BPH) - focus on the UK. BJU Int 2015;115:508–19. [DOI] [PubMed] [Google Scholar]
- [27].Perera M, Roberts MJ, Doi SA, et al. Prostatic urethral lift improves urinary symptoms and flow while preserving sexual function for men with benign prostatic hyperplasia: a systematic review and meta-analysis. Eur Urol 2015;67:704–13. [DOI] [PubMed] [Google Scholar]
- [28].Roehrborn CG, Barkin J, Gange SN, et al. Five year results of the prospective randomized controlled prostatic urethral L.I.F.T. study. Can J Urol 2017;24:8802–13. [PubMed] [Google Scholar]
- [29].Ulchaker JC, Martinson MS. Cost-effectiveness analysis of six therapies for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. Clinicoecon Outcomes Res 2018;10:29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]


