Abstract
Variability of blood pressure (BP) is known as a prognostic value for the subsequent target organ damage in hypertensive patients. Arterial stiffness is a risk factor for cardiovascular morbidity and mortality. The relationship between the arterial stiffness and the BP variability has been controversial. The objective of the present study was to investigate the relationship between arterial stiffness and home BP variability in patients with high normal BP and new onset hypertension (HTN).
Four hundred sixty three patients (252 males, 49 ± 12 year-old) with high normal BP or HTN were enrolled. Using radial applanation tonometry, pulse wave analysis (PWA) was performed for evaluation of systemic arterial stiffness. All patients underwent both home BP monitoring (HBPM) and PWA. Home BP variability was calculated as the standard deviation (SD) of 7 measurements of HBPM. Multiple linear regression analysis was performed to estimate and test the independent effects of home BP variability on the arterial stiffness.
Mutivariate analysis showed that both systolic and diastolic morning BP variabilities were correlated with arterial stiffness expressed as augmentation pressure (AP, β-coefficient = 1.622, P = .01 and β-coefficient = 1.07, P = .035). The SDs of systolic and diastolic BP of evening were also associated with AP (β-coefficient = 1.843, P = .001 and β-coefficient = 1.088, P = .036). The SDs of morning and evening systolic BP were associated with augmentation index (AI, β-coefficient = 1.583, P = .02 and β-coefficient = 1.792, P = .001) and heart rate (75 bpm) adjusted AI (β-coefficient = 1.592, P = .001 and β-coefficient = 1.792, P = .001).
In present study, the variability of systolic BP was closely related with arterial stiffness. The home BP variability might be important indicator of arterial stiffness.
Keywords: arterial stiffness, blood pressure variability, home blood pressure monitoring, hypertension, pulse wave analysis
1. Introduction
Independent of mean blood pressure (BP), BP variability has been known to be associated with clinical cardiovascular events.[1,2] In our previous study, we demonstrated the close relationship between visit-to-visit systolic BP (SBP) variability and long-term cardiovascular adverse events in patients with ST elevation myocardial infarction.[3]
Arterial mstiffness is one of well-known risk factors for cardiovascular morbidity and mortality.[4–6] Large elastic arteries stiffen progressively with age, whereas the stiffness of muscular arteries changes little with age. In the load-bearing media of elastic arteries, the orderly arrangement of elastic fibers and laminae is gradually lost over time, and thinning, splitting, fraying, and fragmentation are observed.[7] Arterial stiffness relates to alterations of medial properties leading to reduced distensibility of the arterial wall, and decreasing the buffering capacity of arteries to pulsatile cardiac ejection.[8] Arterial stiffness may reduce baroreceptor sensitivity, which is major determinant of BP variability.[9,10] The relationship between the arterial stiffness and the BP variability has been incompletely understood.[1,11]
The objective of the present study was to investigate the relationship between arterial stiffness by pulse wave analysis (PWA) and home BP variability in patients with high normal BP and hypertension (HTN).
2. Materials and methods
The study included 773 patients (442 males, 48 ± 12 year-old) with high normal BP or HTN, who were consecutively recruited in 11 university hospitals in Korea. We enrolled the patients with firstly diagnosed high normal BP or HTN. As drugs could affect central and peripheral pressure differently,[12] we excluded the patients on any antihypertensive medication at enrollment. Among the study population, 463 patients (252 males, 49 ± 12 year-old), who underwent both home BP monitoring (HBPM) and pulse wave analysis (PWA), were finally enrolled in the present study. The study protocol and informed consent were reviewed and approved by the Institutional Review Board of each participating hospital.
Office BP measurements were taken from both arms 3 times by the study nurse using a validated oscillometric device (Omron HEM 747 mICN BP, Omron Healthcare Co., Kyoto, Japan) after 5 minutes of seated rest and at 2-minute intervals. Using office BP, high normal BP and HTN were defined according to 2013 ESH/ESC practice guidelines (high normal BP as SBP 130 to 139 mm Hg and/or diastolic BP (DBP) 85 to 89 mm Hg and HTN as SBP ≥140 mm Hg and/or DBP ≥90 mm Hg).[13]
Home BP was measured with the same device used in the measurement of office BP. Participants were instructed to measure their BP once every morning within 1 hour of waking and every evening 1 hour before going to bed for 7 consecutive days while seated at a quiet place after more than 2 minutes of rest, as specified by the Japanese guidelines for HBPM.[14] Participants were also instructed to obtain the BP measurement in the morning after micturition and before breakfast. We defined BP variability as the intra-individual standard deviation (SD) of BP across the measurements. Each of home BP variability in morning and evening was calculated as the SD of 7 measurements of HBPM.
For evaluating the additional effects of circadian variability, 329 patients (171 males, 49 ± 12 year-old) among the enrolled patients underwent 24-hour ambulatory BP monitoring. The device was set to obtain BP readings at 30-minute intervals during the day (between 6:00 am and 11:59 pm) and at 60-minute intervals during the night (between 12:00 am and 5:59 am). Participants were instructed to continue with their normal daily activities during the day. A valid measurement was defined as valid readings for more than 70% of the total measurement attempts, and at least 14 measurements during the daytime and at least 7 measurements during the nighttime. Each of circadian BP variability in daytime and nighttime was calculated as the standard deviation (SD) of ambulatory BP measurements.
As carotid-femoral pulse wave velocity, considered the gold standard in arterial stiffness, is not convenient to measure,[15] wave reflection analysis, which is more convenient to measure and widely accepted as a marker of arterial stiffness,[16,17] was used. Using commercially available applanation tonometry (SphygmoCor, AtCor Medical, Sydney, Australia), central hemodynamics and parameters of arterial stiffness were assessed with PWA of the radial artery. After 20 sequential waveforms had been acquired, a validated generalized transfer function was used to generate the corresponding central aortic pressure waveform.[18,19] Central systolic and diastolic BP, augmentation pressure (AP) and augmentation index (AI) were derived using the technique of PWA. Augmentation pressure is the difference between the second and the first systolic peaks, and the AI is the ratio of AP to aortic pulse pressure calculated as the difference between respective systolic and diastolic pressure. As AI is influenced by heart rate,[20] an index adjusted for heart rate of 75 bpm (AI@HR75) was also calculated. As the calculated value of AI@HR75 has already been proven as a good measurement for risk prediction,[16,17] AI@HR75 was also used as one of markers representing arterial stiffness in the present study.
SPSS 18.0 statistical software package (SPSS, Chicago, Illinois, USA) was used for all calculations. Data are shown as the mean ± standard deviation for continuous variables and as numbers and percentages for categorical variables. Comparisons were conducted by unpaired Student's t test and ANOVA for continuous variables and Pearson chi-square test for categorical variables. To estimate and test the independent effects of home BP variability and circadian BP variability on the arterial stiffness, multivariate analyses were performed using linear regression. Null hypotheses of no difference were rejected if P values were <.05.
3. Results
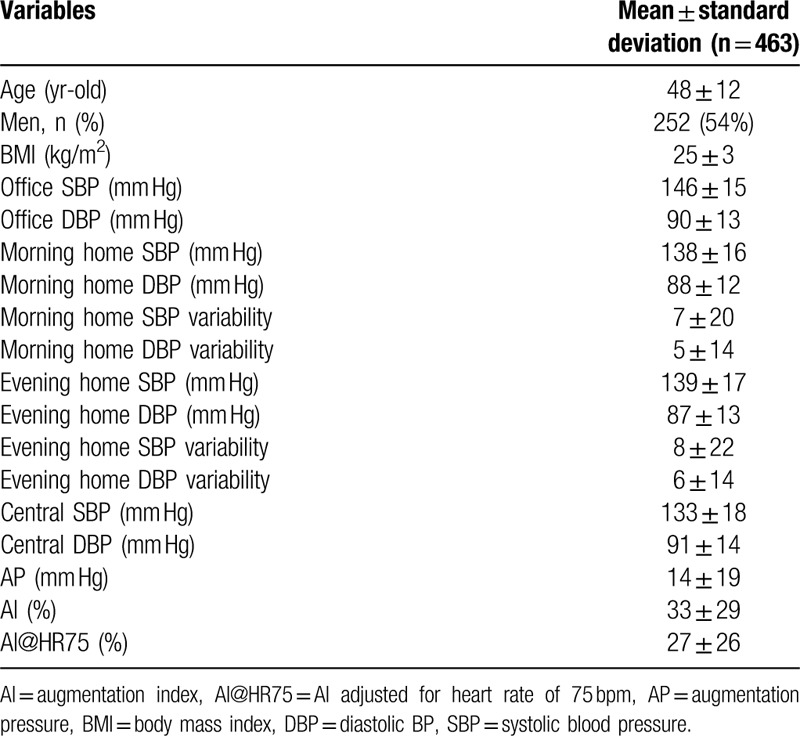
Baseline clinical characteristics are summarized in Table 1. In the morning BP, home systolic BP (SBP) variability was 7 ± 20 and home diastolic BP (DBP) variability was 5 ± 14. In the evening, home SBP variability was 8 ± 22 and home DBP variability was 6 ± 14.
Table 1.
Baseline characteristics.
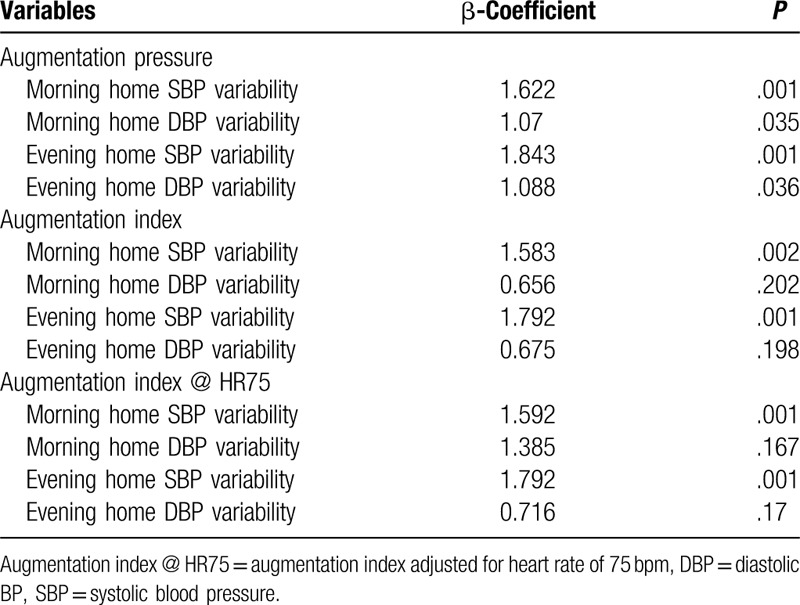
Multivariate logistic regression analysis demonstrated that both home SBP and DBP variabilities in the morning were correlated with arterial stiffness expressed as AP (Table 2, β-coefficient = 1.622, P = .01 and β-coefficient = 1.07, P = .035). Both home SBP and DBP variabilities in the evening were also related with AP (β-coefficient = 1.843, P = .01 and β-coefficient = 1.088, P = .036). The parameters of arterial stiffness, AI and AI@HR75 were correlated with home SBP variabilities, but not with home DBP variabilities. The variabilities of morning and evening SBP were associated with AI (β-coefficient = 1.583, P = .02 and β-coefficient = 1.792, P = .001) and AI@HR75 (β-coefficient = 1.592, P = .001 and β-coefficient = 1.792, P = .001).
Table 2.
Multivariate logistic regression analysis of home blood pressure variability for the arterial stiffness.
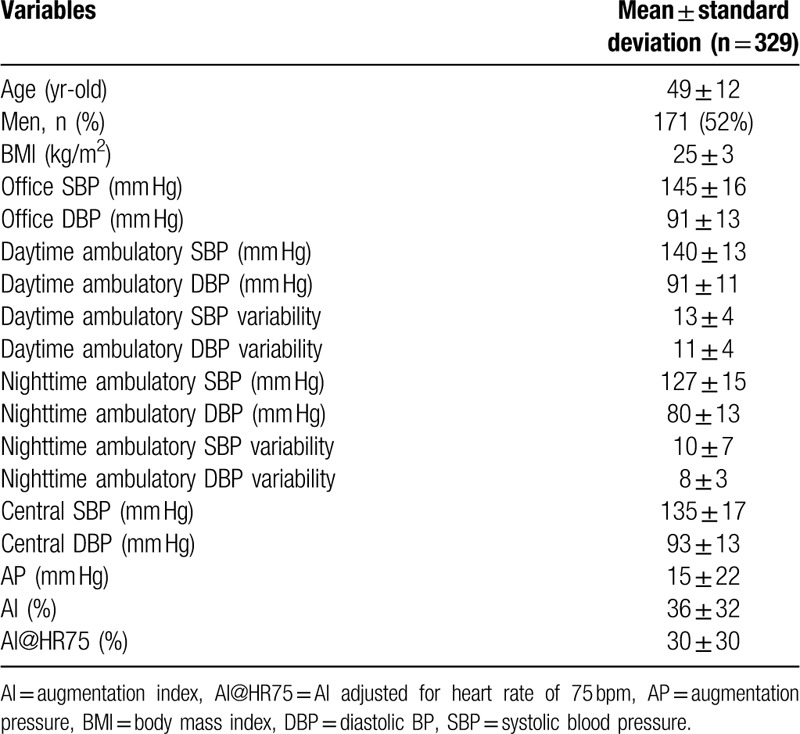
For evaluating the additional effects of circadian variability, 329 patients (171 males, 49 ± 12 year-old) among the enrolled patients underwent 24-hour ambulatory BP monitoring. Baseline clinical characteristics of these are summarized in Table 3. Circadian BP variabilities included daytime ambulatory SBP and DBP variabilities and nighttime ambulatory SBP and DBP variabilities. Daytime ambulatory SBP variability was 13 ± 4 and daytime ambulatory DBP variability was 11 ± 4. Nighttime ambulatory SBP variability was 10 ± 7 and nighttime ambulatory variability was 8 ± 3.
Table 3.
Baseline characteristics of 329 patients who underwent additional ambulatory blood pressure monitoring.
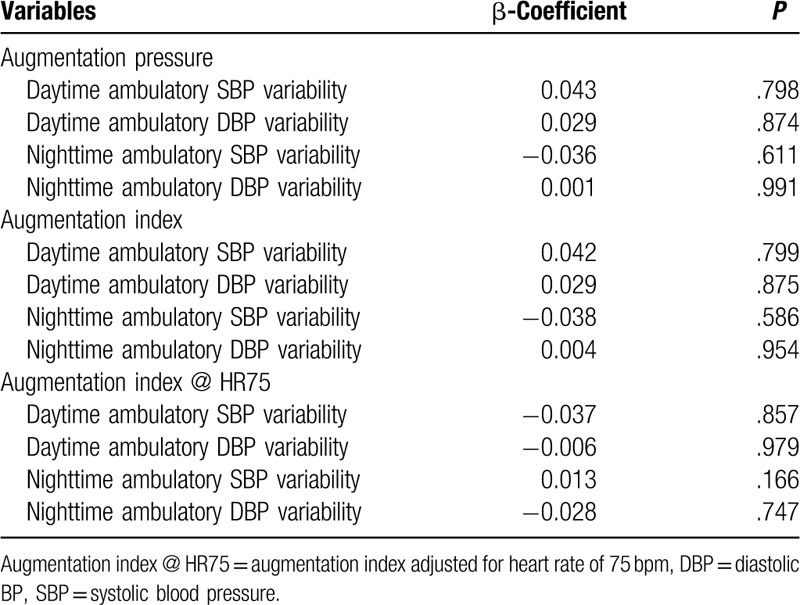
Multivariate logistic regression analyses of circadian BP variabilities for the arterial stiffness are listed in Table 4. There was no statistical significance between circadian BP variabilities and parameters of arterial stiffness.
Table 4.
Multivariate logistic regression analysis of circadian blood pressure variability for the arterial stiffness.
4. Discussion and conclusions
The present study demonstrated the significant relationship between home BP variability and arterial stiffness in patients with high normal BP or HTN. Especially, the variability of SBP measured by HBPM was closely related with arterial stiffness. However, there was no relationship between circadian BP variability measured by ambulatory BP monitoring and arterial stiffness.
Many studies demonstrated that higher BP variability was one of predictors of cardiovascular outcome over and beyond BP level.[1–3,21] Higher BP variability could attenuate hemodynamic homeostasis, cause end-organ damage, and have negative impacts on the vascular system, leading to cardiovascular mortality.[22,23] Especially SBP variability has been known to be linked to macro-, such as myocardial infarction, stroke, and peripheral artery disease, and microvascular complications, such as nephropathy and retinopathy.[2,24,25] Excessive SBP variability might create stress on vascular system that in turn accelerate cholesterol plaque deposition. In high risk patients, shear stress from the exaggerated variability of blood pressure could trigger rupture of advanced vulnerable plaque and diminish attenuation of the pulse transmitted to the peripheral arteries, resulting in the onset of cardiovascular events.[26,27]
In the present study, the variability of SBP measured by HBPM was closely related with arterial stiffness. Circadian BP variabilities calculated as the SD of ambulatory BP measurements were not significantly related with arterial stiffness. Our previous study demonstrated that arterial stiffness was closely related with the pattern of non-dipping in young patients with HTN and high normal BP.[28] The underlying mechanisms, clinical significance, and prognostic implications might differ between types of BP variability.[29] When interpreting BP variability, the method and time interval of its measurement should be taken into account. Although HBPM does not assess BP during sleep or at work, it provides measurements over a longer period than 24-hour ambulatory BP monitoring. The BP variability of relatively longer period, compared with 24-hour ambulatory BP monitoring, might be more closely related with arterial stiffness.
Compared to 24-hour ambulatory BP monitoring, HBPM is cheaper, more widely available, and more convenient for patients. The higher practicality of HBPM over ambulatory BP measurement is widely recognized.[30] Additionally, HBPM might have the advantage of better prediction of prognosis than 24-hour ambulatory BP monitoring. Several studies already demonstrated the significant association between home SBP variability and cardiovascular or stroke death.[31,32] Especially in patients with end stage renal disease, that is, in high risk hypertensive patients, relative long term SBP variability was strongly related with adverse cardiovascular outcomes.[33] Close relationship between home SBP variability and arterial stiffness might be potential underlying mechanism of these phenomena. Despite the present study did not evaluate the cardiovascular events, close relationship between home SBP variability and arterial stiffness might suggest potential future risk. The results of the present study suggest that home SBP variability could be useful predictor not only for high risk hypertensive patients.
This study has several limitations. First, home BP variability could be influenced by methodological problems. There is possibility of artifacts and errors at the time of self-BP measurements by the patients, given that the quality of HBPM was not checked any longer after an initial training. To lessen this methodological errors, all the participants were strictly instructed to measure their BP in accordance with the Japanese guidelines for HBPM. Nevertheless, a possible interference of artifactual measurements with the quantification of home BP variability cannot be completely excluded. Second, we examined the relationship between home SBP variability and arterial stiffness, suggesting prognostic significance of home SBP variability, and did not evaluate whether the home SBP variability could be therapeutic target for reducing adverse cardiovascular outcomes. Reducing home SBP variability might affect the clinical outcomes. Further studies would be needed to demonstrate the beneficial effect of reducing home SBP variability on cardiovascular outcomes. Third, the present study could not demonstrate the effect of comorbidities, such as diabetes or dyslipidemia, on BP variability, due to the relatively small number of patients with comorbidities. Owing to increased oxidative stress and decreased endothelial function, hypertensives with diabetes had higher BP variability.[34,35] Further studies would be needed to demonstrate the effect of comorbidities on BP variability.
In conclusion, the variability of SBP measured by HBPM was closely related with arterial stiffness in patients with high normal BP or HTN. The HBPM might have addictive value in predicting future cardiovascular events.
Author contributions
Conceptualization: Jin-Sun Park, Joon-Han Shin.
Data curation: Dong-Ju Choi, Ho-Joong Youn, Chang-Gyu Park, Jun Kwan, Dong-Woon Kim, Seung-Woo Park.
Formal analysis: Chang-Gyu Park, Jun Kwan, Seung-Woo Park.
Investigation: Dong-Ju Choi, Ho-Joong Youn, Youngkeun Ahn, Jidong Sung, Jang-Ho Bae.
Methodology: Jeong-Bae Park, Jun Kwan, Dong-Woon Kim, Se-Joong Rim, Jidong Sung.
Resources: Jidong Sung.
Supervision: Joon-Han Shin, Jeong-Bae Park, Dong-Woon Kim, Se-Joong Rim, Jang-Ho Bae.
Validation: Youngkeun Ahn, Se-Joong Rim, Seung-Woo Park.
Writing – original draft: Jin-Sun Park.
Footnotes
Abbreviations: AI = augmentation index, AI@HR75 = heart rate (75 bpm) adjusted augmentation index, AP = augmentation pressure, BP = blood pressure, DBP = diastolic blood pressure, HBPM = home blood pressure monitoring, HTN = hypertension, PWA = pulse wave analysis, SBP = systolic blood pressure, SD = standard deviation.
How to cite this article: Park JS, Shin JH, Park JB, Choi DJ, Youn HJ, Park CG, Kwan J, Ahn Y, Kim DW, Rim SJ, Park SW, Sung J, Bae JH. Relationship between arterial stiffness and variability of home blood pressure monitoring. Medicine. 2020;99:30(e21227).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Nagai M, Kario K. Visit-to-visit blood pressure variability, silent cerebral injury, and risk of stroke. Am J Hypertens 2013;26:1369–76. doi: 10.1093/ajh/hpt167. [DOI] [PubMed] [Google Scholar]
- [2].Muntner P, Whittle J, Lynch AI, et al. Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: a cohort study. Ann Intern Med 2015;163:329–38. doi: 10.7326/M14-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Soh MS, Park JS, Seo KW, et al. Visit-to-visit systolic blood pressure variability in patients with ST-elevation myocardial infarction predicts long-term cardiovascular outcomes. J Hum Hypertens 2019;33:259–66. doi: 10.1038/s41371-019-0176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Willum-Hansen T, Staessen JA, Torp-Pedersen C, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 2006;113:664–70. [DOI] [PubMed] [Google Scholar]
- [5].Laurent S, Katsahian S, Fassot C, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke 2003;34:1203–6. [DOI] [PubMed] [Google Scholar]
- [6].Park JS, Choi UJ, Lim HS, et al. The Relationship between coronary artery calcification as assessed by multi-detector computed tomography and arterial stiffness. Clin Exp Hypertens 2011;33:501–5. [DOI] [PubMed] [Google Scholar]
- [7].Benetos A, Laurent S, Hoeks A, et al. Arterial alterations with aging and high blood pressure: a noninvasive study of carotid and femoral arteries. Arterioscler Thromb 1993;13:90–7. [DOI] [PubMed] [Google Scholar]
- [8].Alberto, Avolio Arterial Stiffness. Pulse 2013;1:14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Omboni S, Parati G, Di Rienzo M, et al. Blood pressure and heart rate variability in autonomic disorders: a critical review. Clin Auton Res 1996;6:171–82. [DOI] [PubMed] [Google Scholar]
- [10].Kingwell BA, Cameron JD, Gillies KJ, et al. Arterial compliance may influence baroreflex function in athletes and hypertensives. Am J Physiol 1995;268(1 Pt 2):H411–8. [DOI] [PubMed] [Google Scholar]
- [11].Shimbo D, Shea S, McClelland RL, et al. Associations of aortic distensibility and arterial elasticity with long-term visit-to-visit blood pressure variability: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Hypertens 2013;26:896–902. doi: 10.1093/ajh/hpt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Morgan T, Lauri J, Bertram D, et al. Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens 2004;17:118–23. [DOI] [PubMed] [Google Scholar]
- [13].ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens 2013;31:1925–38. [DOI] [PubMed] [Google Scholar]
- [14].Imai Y, Kario K, Shimada K, et al. The Japanese Society of Hypertension guidelines for self-monitoring of blood pressure at home (second edition). Hypertens Res 2012;35:777–95. [DOI] [PubMed] [Google Scholar]
- [15].Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588–605. [DOI] [PubMed] [Google Scholar]
- [16].Weber T, Auer J, O’Rourke MF, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 2004;109:184–9. [DOI] [PubMed] [Google Scholar]
- [17].Weber T, Auer J, O’rourke MF, et al. Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions. Eur Heart J 2005;26:2657–63. [DOI] [PubMed] [Google Scholar]
- [18].Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 2001;38:932–7. [DOI] [PubMed] [Google Scholar]
- [19].Chen CH, Nevo E, Fetics B, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation 1997;95:1827–36. [DOI] [PubMed] [Google Scholar]
- [20].Wilkinson IB, MacCallum H, Flint L, et al. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol 2000;525(Pt 1):263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Johansson JK, Niiranen TJ, Puukka PJ, et al. Prognostic value of the variability in home-measured blood pressure and heart rate: the Finn-Home Study. Hypertension 2012;59:212–8. doi: 10.1161/hypertensionaha.111.178657. [DOI] [PubMed] [Google Scholar]
- [22].Diaz KM, Veerabhadrappa P, Kashem MA, et al. Relationship of visit-to-visit and ambulatory blood pressure variability to vascular function in African Americans. Hypertens Res 2012;35:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mancia G, Parati G. The role of blood pressure variability in end-organ damage. J Hypertens Suppl 2003;21:S17–23. [DOI] [PubMed] [Google Scholar]
- [24].Hata J, Arima H, Rothwell PM, et al. ADVANCE Collaborative Group. Effects of visit-to-visit variability in systolic blood pressure on macrovascular and microvascular complications in patients with type 2 diabetes mellitus: the ADVANCE trial. Circulation 2013;128:1325–34. doi: 10.1161/circulationaha.113.002717. [DOI] [PubMed] [Google Scholar]
- [25].Sohn MW, Epstein N, Huang ES, et al. Visit-to-visit systolic blood pressure variability and microvascular complications among patients with diabetes. J Diabetes Complications 2017;31:195–201. doi: 10.1016/j.jdiacomp.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kario K. Prognosis in relation to blood pressure variability: pro side of the argument. Hypertension 2015;65:1163–9. doi: 10.1161/hypertensionaha.115.04800. [DOI] [PubMed] [Google Scholar]
- [27].Manios E, Stamatelopoulos K, Tsivgoulis G, et al. Time rate of blood pressure variation: a new factor associated with coronary atherosclerosis. J Hypertens 2011;29:1109–14. doi: 10.1097/hjh.0b013e3283454ff4. [DOI] [PubMed] [Google Scholar]
- [28].Park JS, Shin JH, Park JB, et al. Relationship between arterial stiffness and circadian pattern of blood pressure. Medicine (Baltimore) 2019;98:e14953.doi: 10.1097/MD.0000000000014953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Parati G, Ochoa JE, Lombardi C, et al. Assessment and management of blood-pressure variability. Nat Rev Cardiol 2013;10:143–55. doi: 10.1038/nrcardio.2013.1. [DOI] [PubMed] [Google Scholar]
- [30].Imai Y, Obara T, Asamaya K, et al. The reason why home blood pressure measurements are preferred over clinic or ambulatory blood pressure in Japan. Hypertens Res 2013;36:661–72. [DOI] [PubMed] [Google Scholar]
- [31].Hashimoto T, Kikuya M, Ohkubo T, et al. Home blood pressure level, blood pressure variability, smoking, and stroke risk in Japanese men: the Ohasama study. Am J Hypertens 2012;25:883–91. doi: 10.1038/ajh.2012.62. [DOI] [PubMed] [Google Scholar]
- [32].Ohkubo T, Imai Y, Tsuji I, et al. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens 1998;16:971–5. [DOI] [PubMed] [Google Scholar]
- [33].Rossignol P, Cridlig J, Lehert P, et al. Visit-to-visit blood pressure variability is a strong predictor of cardiovascular events in hemodialysis: insights from FOSIDIAL. Hypertension 2012;60:339–46. doi: 0.1161/hypertensionaha.111.190397. [DOI] [PubMed] [Google Scholar]
- [34].Ohara M, Kohata Y, Nagaike H, et al. Association of glucose and blood pressure variability on oxidative stress in patients with type 2 diabetes mellitus and hypertension: a cross-sectional study. Diabetol Metab Syndr 2019;11:29.doi: 10.1186/s13098-019-0425-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schoina M, Loutradis C, Minopoulou I, et al. Ambulatory blood pressure trajectories and blood pressure variability in diabetic and non-diabetic chronic kidney disease. Am J Nephrol 2020;1–0. doi: 10.1159/000507416. [DOI] [PubMed] [Google Scholar]


