Abstract
To evaluate the effect of the severity of spinal stenosis on the peripheral nerves of lower extremities by nerve conduction study (NCS).
One hundred fifteen patients with lumbar spinal stenosis were recruited retrospectively in this study. The grading system for lumbar stenosis was used based on the degree of separation of the cauda equina. The degree of cauda equina damage caused by lumbar central stenosis was assessed by NCS of peripheral nerves. Multiple regression analysis was used to estimate which factors affect peripheral nerve injury, according to the presence of DM, total grading of lumbar central stenosis, and age.
Only age was associated with low amplitude in the tibial and peroneal motor NCS in the multiple regression analysis. The severity of the compression of the cauda equina, caused by spinal stenosis, did not statistically significantly affect the NCS values of nerves on the lower extremities.
In conclusion, the cauda equina is resilient against degenerative lumbar central stenosis. Unlike changes caused by peripheral nerve entrapment, lumbar central stenosis did not affect the findings of NCS on the peripheral nerve of lower extremities.
Keywords: ageing, diabetes mellitus, nerve conduction study, Spinal stenosis
1. Introduction
Lumbar spinal stenosis is a degenerative disease that causes lower back pain and neurogenic claudication in the lower extremities. Diffuse bulging discs, decreased disc height, and hypertrophy of the ligaments decrease the space in the spinal canal; this produces mechanical compression and ischemia in the cauda equina.[1] Spinal stenosis can cause neurogenic claudication due to the lack of oxygen supply to the cauda equina in proportion to the oxygen demand.[2,3] Methods for the diagnosis and grading of spinal stenosis through imaging have been introduced.[4,5] Assessing the dural sac cross-sectional area is a also useful radiologic method to investigate the severity of spinal stenosis.[6] The spinal cross sectional area, however, can be changed by the flexion and extension of the trunk.[7] Moreover, there is no definite correlation between the severity of lumbar spinal stenosis seen on imaging studies and the associated pain.[8,9] Therefore, the measurement of the cross-sectional area, using the static cross section of the lumbar MRI, is clinically limited in the diagnosis and treatment of pain.
Peripheral nerve entrapment is caused by localized compression of the pathway of the peripheral nerves.[10] Mechanical compression of the peripheral nerve can produce axonal injury resulting in denervation in the terminal organs. Nerve conduction study (NCS) is a diagnostic tool used for the evaluation of peripheral nerve injury. NCS can detect abnormal findings in the distal part of a compressed lesion. Central lumbar spinal stenosis compresses the cauda equina; this is similar to peripheral nerve entrapment syndrome. Therefore, severe spinal stenosis has the potential to cause peripheral nerve injury.[11] Thus, if spinal stenosis causes cauda equina injury, NCS of the peripheral nerves of the lower extremities will show abnormal findings consistent with neurological damage. However, there is not much research concerning the relationship between spinal stenosis, according to the degree of cauda equina aggregation, and peripheral nerve injury.
Guen et al introduced a new grading system for lumbar central canal stenosis based on the degree of separation of the cauda equina on T2-weighted axial images.[12] We adopted this grading system to study the effect of central lumbar stenosis on the cauda equina. With this, we investigated the effect of central spinal stenosis on peripheral neuropathy using NCS, according to the spinal stenosis grading.
2. Methods
Approval from the institutional ethics review board of Yeungnam University Hospital (YUMC 2019-11-031) was obtained; the need for obtaining written informed consent from the participants in this study was waived. The inclusion criteria included:
-
1.
the central lumbar stenosis was diagnosed by MRI findings;
-
2.
neurogenic claudication was noted; and
-
3.
NCS was conducted on the lower extremities (e.g., peroneal nerve, tibial nerve, sural nerve).
Exclusion criteria included:
-
1.
unknown peripheral axonal and demyelinating polyneuropathy, except diabetic mellitus (DM); and
-
2.
definite motor weakness of the lower extremities.
We retrospectively recruited 115 patients who visited the outpatient clinic from August 2015 to September 2018. Two physicians agreed and assessed the grade of central lumbar stenosis from L1-2 to L5-S1 levels, based on the grading system.[12] The sum of each grade of individual multi-level lumbar stenosis was calculated. Grading values for each level were from 0 to 3; the maximum total grading value per person was 15. NCS (Carefusion Nicolet EDX with Viking EDX software, UK) was conducted on all patients. The tibial and peroneal motor nerves and the sural nerve were evaluated.
2.1. Statistics
Statistical analysis was performed using SPSS version 22.0 for Windows (SPSS Inc, Chicago, IL). Continuous variables were presented as mean ± standard deviation (SD). The t test and Mann-Whitney U test were used to determine that groups with or without DM had the same age- and stenosis grading distribution. Moreover, the T-test and Mann-Whitney U test were also used to assess the effect of DM on the NCV values. The parameters of the NCS, compound motor action potential (CMAP) and sensory nerve action potential, according to the presence of DM, total grading, and age, were assessed by multiple regression analysis to estimate which factors affect peripheral nerve injury. A P value of less than .05 was considered to be statistically significant.
3. Results
The demographic data are shown in Table 1. Overall, 115 patients (61 males and 54 females) were enrolled (Fig. 1). The mean age was 67.5 ± 9.5 years. Incidence of spinal stenosis at each lumbar level and the accumulated total lumbar central canal stenosis (ALCCS) grading are shown in Table 1. The distribution of the central lumbar stenosis levels was as follows: L1-2: 11 patients (5%), L2-3: 36 (16%), L3-4: 67 (30%), L4-5: 94 (43%), and L5-S1: 13 (6%). The DM group and non-DM groups comprised 25 and 90 patients, respectively.
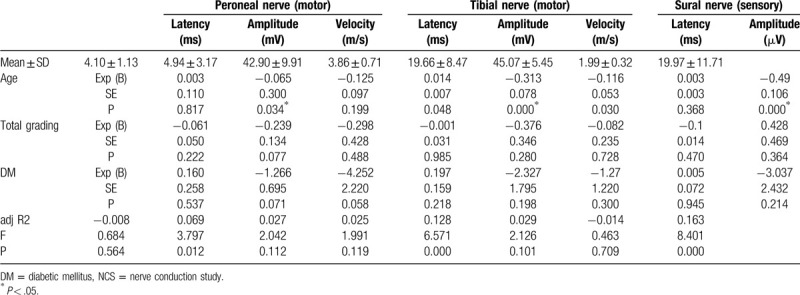
Table 1.
Patient characteristics.

Figure 1.

Flowchart of patient inclusion and exclusion. NCS = nerve conduction study, HLD = herniated lumbar disc.
ALCCS grading for each patient ranged from 1 to 10. The number of patients according to the ALCCS grading is illustrated in Figure 2. The motor nerves (peroneal and tibial) were assessed using latency, amplitude, and velocity. The sural nerve was assessed using latency and amplitude. The amplitude and velocity of the peroneal nerve and the velocity of the tibial nerve showed a significant relationship with the presence of diabetes (Table 2).
Figure 2.

The distribution of total stenosis grading per individual.
Table 2.
NCS values for the nerves in the lower extremities according to the presence of DM.

The mean amplitudes of the motor nerves (peroneal and tibial) were significantly related to age, but not with ALCCS grading, in the multivariate regression analysis (peroneal nerve, P = .03; tibial nerve, P = .00; Fig. 3, Table 3). Moreover, the mean amplitude of the sural nerve also showed a statistically significant relationship with age (P = .00), and not with the ALCCS grading. The relationship between the severity of spinal stenosis, ALCCS grading, and the values of NCS of the nerves of the lower extremities was not statistically significant. Therefore, our results indicated that severe central stenosis with cauda equina aggregates did not cause sustained axonal damage.
Figure 3.

Age showed a statistically significant correlation with the amplitude of motor and sensory nerves in the lower extremities (A, B, C). However, the total grading did not show a statistically significant correlation with the amplitude of motor and sensory nerves in the lower extremities (D, E, F).
Table 3.
Multivariate regression analysis of age, total grading, and DM status according to the NCS findings of the nerves in the lower extremities.
4. Discussion
In our research, the amplitudes of the peripheral motor and sensory nerves in the lower extremities were significantly correlated with age in the multiple regression analysis. DM patients had significantly lower amplitudes and prolonged velocities of the peroneal and tibial nerves in contrast to non-DM patients. However, in the multiple regression analysis, only age was associated with low amplitude in the tibial and peroneal motor NCS. The spinal stenosis grading and DM did not affect the NCS findings of the peripheral nerve in the lower extremities. Therefore, the severity of the compression of the cauda equina, caused by spinal stenosis, did not cause sustained axonal injury to the cauda equine to the extent of injuring the peripheral nerves in the lower extremities.
If older-aged patients with spinal stenosis present with pain or motor weakness in the lower extremities, an electrodiagnostic study may be necessary for a differential diagnosis. As lumbar stenosis is a degenerative disease, older-aged patients are more likely to have a variety of comorbidities.[13] Peripheral neuropathy has multifactorial etiology, involving age, metabolic agents, DM, inflammatory origins, and idiopathic factors.[14] These factors make it difficult to properly interpret electrodiagnostic study findings. Clinically, atypical presentation of peripheral neuropathy can be confused with spinal stenosis.[15] Therefore, we wanted to determine whether severe spinal stenosis that compresses the cauda equina can cause injury of the peripheral nerves of the lower extremities.
Focal entrapment of the peripheral nerves in the extremities can cause focal axonal injury of the peripheral neuropathy. Focal axonal injury can further affect the findings of NCS in terms of the CMAP and sensory nerve action potential. Local pressure on the intervertebral foramen deteriorates CMAP values in line with the increasing pressure.[16] Although the change is temporary, the amplitude and latency of CMAP can be affected by focal pressure on the dorsal root ganglion. Egli et al reported that 78% of lumbar spinal stenosis patients showed delayed H-reflex and somatosensory evoked potentials and 39% of patients showed pathologic findings with respect to the CMAP amplitude.[17] Thus, NCS findings of nerves within the lower extremities can be used to assess cauda equina injury caused by lumbar stenosis.
However, in our research, even severe spinal stenosis, causing the aggregation of the cauda equina, did not produce significant abnormalities in the amplitude of the peripheral nerves. Moreover, in the multiple regression analysis, the severity of spinal stenosis did not show a significant association with abnormalities in the NCS findings of the nerves of the lower extremities. Unlike the spinal cord, which is vulnerable to spinal canal stenosis,[18] the cauda equine is resilient to mechanical compression by degenerative spinal stenosis. Traumatic compression can damage the cauda equina, resulting in abnormal NCS findings.[19] However, the incidence of neurological deficit is very low when considering the prevalence of degenerative spinal stenosis.[20]
Chronically compressed nerve roots become resistant to acute compression,[21] and nerves have the ability to regenerate after injury.[22] Spinal stenosis causes gradual degeneration rather than progressive degeneration; therefore, spinal stenosis causes gradual damage but this allows the injured nerve to regenerate in time. Therefore, we speculate that these factors affected the results of our research.
Neuropathy is the most common chronic complication of DM. Metabolic processes and ischemic damage contribute to peripheral neuropathy.[23] Meticulous blood sugar control reduces the progression of diabetic neuropathy. DM patients showed significantly abnormal properties of the tibial and peroneal nerves in contrast to non-DM patients. However, in the multivariate regression analysis, DM was not a statistically significant factor. Further, we did not consider other factors affecting diabetic peripheral neuropathy in the analysis, which, as we speculate, may have influenced the results.
Aging is a well-known factor that causes functional and morphological changes in the nervous system.[24] Aging causes decline in the function of nerve regeneration following injury. Further, the deterioration of the myelin sheath and axonal atrophy during aging affects the electrophysiologic properties of the peripheral nerves.[25,26] Consistent with this knowledge, in our study, age correlated with the abnormalities in the electrophysiologic properties of the nerves in the lower extremities.
To assess the relationship between stenosis and peripheral neuropathy, multiple regression analysis was performed with well-known factors that cause peripheral neuropathy. This analysis was aimed to control other variables to determine the extent of the impact of degenerative spinal stenosis. Finally, this study showed that age is a main factor influencing the deterioration of NCS findings. Moreover, our findings suggested that degenerative spinal stenosis is not associated with cauda equina injury and further injury of the peripheral nerves.
Our study has some limitations that warrant mention. First, the study enrolled patients with only subjective symptoms. Second, it was a retrospective study based on an electronic database. Third, we did not consider the disease duration or control of blood sugar in patients with diabetes.
In conclusion, the cauda equine is resilient against degenerative spinal central stenosis. Therefore, if patients with nontraumatic degenerative spinal stenosis show significant abnormalities in NCS findings, it may be more effective to consider causes other than spinal stenosis first.
Author contributions
Jang Seung Wha: first author, writing-original draft, data curation.
Dong Gyu Lee: Corresponding author, data curation, conceptualization, formal analysis, supervision, Writhing, revise & editing.
Footnotes
Abbreviations: ALCCS = the accumulated total lumbar central canal stenosis, CMAP = compound motor action potential, DM = diabetic mellitus, NCS = nerve conduction study.
How to cite this article: Jang SW, Lee DG. Can the severity of central lumbar stenosis affect the results of nerve conduction study?. Medicine. 2020;99:30(e21466).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Truumees E. Orthopaedic Knowledge Update: Spine. Fifth ed.Rosemont, IL: American Academy of Orthopaedic Surgeons; 2018. [Google Scholar]
- [2].Baramki HG, Steffen T, Schondorf R, et al. Motor conduction alterations in patients with lumbar spinal stenosis following the onset of neurogenic claudication. Eur Spine J 1999;8:411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kobayashi S. Pathophysiology, diagnosis and treatment of intermittent claudication in patients with lumbar canal stenosis. World J Orthop 2014;5:134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hamanishi C, Matukura N, Fujita M, et al. Cross-sectional area of the stenotic lumbar dural tube measured from the transverse views of magnetic resonance imaging. J Spinal Disord 1994;7:388–93. [PubMed] [Google Scholar]
- [5].Laurencin CT, Lipson SJ, Senatus P, et al. The stenosis ratio: a new tool for the diagnosis of degenerative spinal stenosis. Int J Surg Investig 1999;1:127–31. [PubMed] [Google Scholar]
- [6].Lonne G, Odegard B, Johnsen LG, et al. MRI evaluation of lumbar spinal stenosis: is a rapid visual assessment as good as area measurement? Eur Spine J 2014;23:1320–4. [DOI] [PubMed] [Google Scholar]
- [7].Inufusa A, An HS, Lim TH, et al. Anatomic changes of the spinal canal and intervertebral foramen associated with flexion-extension movement. Spine (Phila Pa 1976) 1996;21:2412–20. [DOI] [PubMed] [Google Scholar]
- [8].Athiviraham A, Yen D, Scott C, et al. Clinical correlation of radiological spinal stenosis after standardization for vertebral body size. Clin Radiol 2007;62:776–80. [DOI] [PubMed] [Google Scholar]
- [9].Lohman CM, Tallroth K, Kettunen JA, et al. Comparison of radiologic signs and clinical symptoms of spinal stenosis. Spine (Phila Pa 1976) 2006;31:1834–40. [DOI] [PubMed] [Google Scholar]
- [10].Daroff RB, Jankovic J, Mazziotta JC, et al. Bradley's Neurology in Clinical Practice E-book. London, New York, Oxford, Philadelphia, St. Louis, Sydney, and Toronto: Elsevier Health Sciences; 2015. [Google Scholar]
- [11].Storm PB, Chou D, Tamargo RJ. Lumbar spinal stenosis, cauda equina syndrome, and multiple lumbosacral radiculopathies. Phys Med Rehabil Clin N Am 2002;13:713–33. ix. [DOI] [PubMed] [Google Scholar]
- [12].Lee GY, Lee JW, Choi HS, et al. A new grading system of lumbar central canal stenosis on MRI: an easy and reliable method. Skeletal Radiol 2011;40:1033–9. [DOI] [PubMed] [Google Scholar]
- [13].Laughlin RS, Dyck PJ, Melton LJ, 3rd, et al. Incidence and prevalence of CIDP and the association of diabetes mellitus. Neurology 2009;73:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hanewinckel R, van Oijen M, Ikram MA, et al. The epidemiology and risk factors of chronic polyneuropathy. Eur J Epidemiol 2016;31:5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jung DY, Cho KT, Lee SC. Atypical guillain-barre syndrome misdiagnosed as lumbar spinal stenosis. J Korean Neurosurg Soc 2013;53:245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Morishita Y, Hida S, Naito M, et al. Measurement of the local pressure of the intervertebral foramen and the electrophysiologic values of the spinal nerve roots in the vertebral foramen. Spine (Phila Pa 1976) 2006;31:3076–80. [DOI] [PubMed] [Google Scholar]
- [17].Egli D, Hausmann O, Schmid M, et al. Lumbar spinal stenosis: assessment of cauda equina involvement by electrophysiological recordings. J Neurol 2007;254:741–50. [DOI] [PubMed] [Google Scholar]
- [18].Kadanka Z, Kerkovsky M, Bednarik J, et al. Cross-sectional transverse area and hyperintensities on magnetic resonance imaging in relation to the clinical picture in cervical spondylotic myelopathy. Spine (Phila Pa 1976) 2007;32:2573–7. [DOI] [PubMed] [Google Scholar]
- [19].Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 2001;26: 24 Suppl: S2–12. [DOI] [PubMed] [Google Scholar]
- [20].Johnsson KE, Sass M. Cauda equina syndrome in lumbar spinal stenosis: case report and incidence in Jutland, Denmark. J Spinal Disord Tech 2004;17:334–5. [DOI] [PubMed] [Google Scholar]
- [21].Kikuchi S, Konno S, Kayama S, et al. Increased resistance to acute compression injury in chronically compressed spinal nerve roots. An experimental study. Spine (Phila Pa 1976) 1996;21:2544–50. [DOI] [PubMed] [Google Scholar]
- [22].Menorca RM, Fussell TS, Elfar JC. Peripheral nerve trauma: mechanisms of injury and recovery. Hand Clin 2013;29:317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].R Miranda-Massari J, J Gonzalez M, J Jimenez F. Metabolic correction in the management of diabetic peripheral neuropathy: improving clinical results beyond symptom control. Curr Clin Pharmacol 2011;6:260–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Verdú E, Ceballos D, Vilches JJ, et al. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst 2000;5:191–208. [DOI] [PubMed] [Google Scholar]
- [25].Palve SS, Palve SB. Impact of aging on nerve conduction velocities and late responses in healthy individuals. J Neurosci Rural Pract 2018;9:112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Senthilkumari K, Umamaheswari BM, Bhaskaran M. A study on median nerve conduction velocity in different age groups. Int J Res Med Sci 2015;3:3313–7. [Google Scholar]


