Abstract
Vitamin B12 (B12), also known as cobalamin, is a water-soluble vitamin. It is a cofactor in DNA synthesis and is involved in the metabolism of every cell of the human body, including the central nervous system. Those with a deficiency of B12 can present with peripheral neuropathy, pernicious anemia, or a cognitive disorder. Previous studies have revealed that a deficiency of B12 is associated with cognitive decline or Alzheimer disease.
The data of 2991 people were evaluated from 2 years of the Korean Frailty and Aging Cohort Study, a nationwide multicenter survey. To assess cognitive function, a short form of the Korean version of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD-K) was used. Of the CERAD-K tests, we included the Mini-Mental State Examination in the Korean version of the CERAD assessment packet (MMSE-KC), the word list: memory/recall/recognition, digit span (forward, backward), trail making test-A, and the frontal assessment battery. B12 concentrations were classified into clinically relevant categories, insufficient (<350 pg/mL) and sufficient (≥350 pg/mL). A linear regression analysis was used to evaluate the relationship between cognitive function and B12 levels.
The mean age of the 2991 participants was 76.4 ± 3.9 years old. Overall, 414 (13.8%) were classified as B12 insufficient, and 2577 (86.2%) as B12 sufficient. The sufficient B12 group performed better in the MMSE-KC, Wordlist: memory, Wordlist: recognition, TMT-A test, digit span, and FAB tests. This was statistically significant (P < .05). However, in the multivariable linear regression analysis, after adjusting for age, sex, education period, marriage, smoking and drinking habits, and comorbidities, the association between the B12 group and cognitive function was not statistically significant.
Although our study does not show that B12 insufficiency is a direct risk factor to cognitive decline, B12 levels could be a contributing factor to cognitive function. Our results suggest that cognition was affected by the B12 levels, along with demographic and sociological variables.
Keywords: cognitive function, geriatrics, Vitamin B12
1. Introduction
Vitamin B12 (B12), also known as cobalamin, is a water-soluble vitamin. It is a cofactor in DNA synthesis and is involved in the metabolism of every cell of the human body.[1] In particular, it has an important role in the synthesis of myelin in the central nervous system. Also, those with a deficiency of B12 can present with peripheral neuropathy, blood cell disorders such as pernicious anemia, and cognitive impairment.[2]
B12 is synthesized by bacteria that inhabit the gastrointestinal tract and is believed to be the primary source of this vitamin in humans.[3] The leading cause of B12 deficiency is impaired absorption due to a loss of gastric intrinsic factor and long-term antacid therapy, such as using proton pump inhibitors or H2 blockers.[3] B12 deficiency is more likely to occur in those over 60-year old and increases in incidence with advancing age. Reduced oral intake and a reluctance to eat meat due to reduced chewing ability may also contribute to the increased prevalence of low B12 levels among the elderly.[4] Besides, other risk factors such as atrophic gastritis, hypoacidity of the stomach, pernicious anemia, and use of the antidiabetic medication, metformin, may contribute to B12 deficiency in the elderly.[5] In previous studies, the prevalence of B12 deficiency has been reported to range from 5.8% to 15.3% among the elderly.[6–8]
Deterioration of cognitive function caused by degenerative changes in the brain increases with age.[9] In Korea, cognitive decline measured using the Korean version of the Consortium to Establish a Registry for Alzheimer's Disease Assessment Packet (CERAD-K) in the elderly community-dwelling population over 65, estimated that cognitive decline was prevalent in 24.1% and overall dementia in 8.1%.[10] Prolonged life expectancy and a growing elderly population require active medical intervention to prevent cognitive decline.
The disease mechanisms that lead to cognitive impairment due to B12 insufficiency are unclear. Previous studies have reported an association between cognition and B12 deficiency, but the results were controversial.[11,12] Also, previous Cochrane systematic reviews of randomized, double-blind trials with B12 supplements found no evidence of the potential benefits of B12 supplements in subjects with cognitive impairment.[13]
The purpose of this study was to investigate the relationship between B12 and cognitive impairment in community-dwelling elderly Korean using baseline data from the Korean Frailty and Aging Cohort Study (KFACS). KFACS is a nationwide, multicenter study, with over 3000 participants recruited from 10 medical and public health centers across Korean urban, agricultural, and countryside communities since 2016. We hypothesize that there is a positive correlation between B12 levels and cognitive decline. In the present study, a cross-sectional design was used. The multivariable-adjusted association between B12 levels and cognitive decline was examined using data from KFACS.
2. Methods
2.1. Data and study population
We used data from KFACS to investigate the relationship between vitamin B12 levels and cognitive function in community-dwelling individuals aged between 70 and 84-year old. KFACS's goal is to identify risks and preventive measures for side effects in frail community dwellers.[14] Among the 3014 participants, 23 participants were excluded. These included those with severe dementia who could not complete the test, those who did not complete the blood analysis, and those who did not give their informed consent. A total of 2991 participants (1416 males and 1575 females) were recruited. Participants were recruited from 10 centers across Korea. In medical institutions, including 8 medical centers and 2 public health centers, participants were individually interviewed and got blood tests. Demographic data and medical history, including age, sex, education, location of residence, current smoker, alcohol use, depression, body mass index, marriage, annual income, and comorbidities, were collected. Current smokers were defined as those participants who smoked more than 1 cigarette per month. Drinkers were defined as participants who drunk alcohol at least once a week. The KFACS protocol was approved by the Institutional Review Board[15] of the Clinical Research Ethics Committee of the Kyung Hee University Medical Center, and all subjects provided written informed consent (IRB number: 2015-12-103).
2.2. Cognitive function
Cognitive function was evaluated using the Korean version of the CERAD-K, which is used for assessing the cognitive status of patients with Alzheimer disease (AD). The Consortium to Establish a Registry for Alzheimer's Disease (CERAD) is originally an English-American version of the clinical and neuropsychological assessment batteries for AD. The CERAD includes 8 tests, as follows: Verbal Fluency, Boston Naming, Mini-Mental State Exam (MMSE), Word List Learning, Constructional Praxis, Word List Recall, Word List Recognition, Constructional Praxis recall. The CERAD has been translated into a Korean version.[16,17] Of the 8 tests, we included the MMSE in the Korean version of the CERAD assessment packet (MMSE-KC), the Wordlist memory/recall/recognition, digit span (forward, backward), trail making test-A (TMT-A), frontal assessment battery for evaluating memory, processing performance, executive, and overall cognitive function.
The MMSE-KC is widely used as a screening test of overall cognitive function, and consists of 5 domains: 10 points for time and place orientation, 6 points for registration and recall memory, 5 points for attention and calculation, 8 points for language ability and comprehension, and 1 point for visual construction. The total score is 30 points, with a higher score reflecting better cognitive status. A score below 24 points is considered to indicate cognitive impairment.[16]
The Word List Memory Test is an immediate recall test for new information. Every 2 seconds, the participant reads 10 common words and instantly remembers as many words as possible in 90 seconds. The total score is 30 points, and a higher score reflects better instant memory. The Word List Recall test evaluates short-term memory. After 15 minutes, the participant retrieves 10 words within the Word List Memory Test. The participants were given 90 seconds, and the total score was 10 points.[18] The Digit Span test is a common way to measure short-term memory and evaluates the patient's working memory and attention. Participants hear the numbers and then recall the numbers forward (range 3–9) and backward (range 2–8).[19] The TMT-A is a neuropsychological test that evaluates visual attention, sequential processing, and task switching. Participants draw lines to connect numbers in ascending order from 1 to 25. The maximum time given to complete the test is 300 seconds, with a faster time indicating better performance.[18] FAB is used to assess the executive function of patients with a neurodegenerative disease. It is used to differentiate between patients with frontotemporal dementia (FTD) and AD. Patients with FTD present with more dysexecutive syndrome than AD. The FAB consists of 6 tasks: conceptualization (similarities task), mental flexibility (lexical fluency), programming (Luria motor tests), sensitivity to interference (conflicting instructions), inhibitory control (Go-No Go), and environmental autonomy (prehension behavior). The total score is a maximum of 18, with higher scores indicating better executive function.[20]
2.3. Vitamin B12
Serum samples were collected. B12 was measured with an Architect vitamin kit (Abbott Diagnostics, Lake Forest, IL). B12 concentrations were divided into the clinically relevant categories, deficient (<200 pg/mL, same as 147.6 pmol/L), subclinically insufficient (200–350 pg/mL, same as 147.6–258.3 pmol/L), and sufficient (≥350 pg/mL, same as 258.3 pmol/L). Then, because less than 2% of the subjects had a B12 level below 200 pg/mL, we categorized insufficient B12 as < 350 pg/mL and sufficient B12 as ≥350 pg/mL. In a previous study, 350 pg/mL was used as it was found to be the protective level of neurogenesis and rules out B12 deficiency in almost all individuals.[21]
2.4. Statistical analysis
The demographic characteristics based on the B12 levels of participants were analyzed using the χ2 test for categorical variables and t test for continuous variables. The results are presented as a mean ± standard deviation (SD) or number (%) according to the characteristics of the variables. Univariable and multivariable analyses were performed using generalized linear regression models. These examined the association between serum B12 levels and cognitive function. Variables were adjusted for multiple correlations between cognitive function and other potential confounder variables such as age, sex, education periods, location of residence, body mass index, alcohol use, current smoker, depression, marriage, annual income, presence of diabetes mellitus, and cardiovascular disease. Pearson's correlation coefficient was used to measure the strength of the association among B12 and each cognitive test. The collected data were analyzed using SPSS version 23.0 (IBM Inc, Chicago, IL). A P value of < .05 was considered statistically significant.
3. Results
The baseline characteristics of the participants are presented in Table 1. Out of a total of 3014 KFACS participants, 2991 were included in the current study. There were 1416 male participants and 1565 female participants. The mean age was 76.8 years for males and 76.2 years for females. In total 56 (1.8%) participants had B12 levels below 200 pg/mL, of which 31 (2.2%) were male and 25 (1.6%) were female. Three hundred fifty-eight (11.9%) participants had subclinically insufficient levels between 200 and 350 pg/mL. Baseline characteristics based on B12 levels are shown in Table 2. The mean age of those with B12 levels less than 350 pg/mL was 77.2 (SD = 3.9), which was significantly older than those with sufficient levels (76.4, SD = 3.9) (P < .001). The percentage of male participants with sufficient B12 levels was lower than that of females (P < .001). There was also a difference according to the education period. Those people who had undergone education for longer were more likely to have sufficient B12 levels, to a statistically significant level (P < .003). Besides, there were statistical differences between those with diabetes and different vitamin D levels (P < .001, respectively). Table 3 presents the results of the cognitive function analysis based on the B12 levels. In the t test analysis, the sufficient group better performed in the MMSE-KC, World list-memory, World list-recall, TMT-A test, digit span test, and FAB. This was statistically significant (P < .05). Likewise, participants with sufficient B12 in the unadjusted linear regression model had better cognitive functions in the MMSE-KC, TMT-A test, world catalog memory, world catalog recall, TMT-A test, numerical range test, and FAB. However, in a fully adjusted linear regression model, these associations were attenuated.
Table 1.
Baseline characteristics of the KFACS participants, by sex.
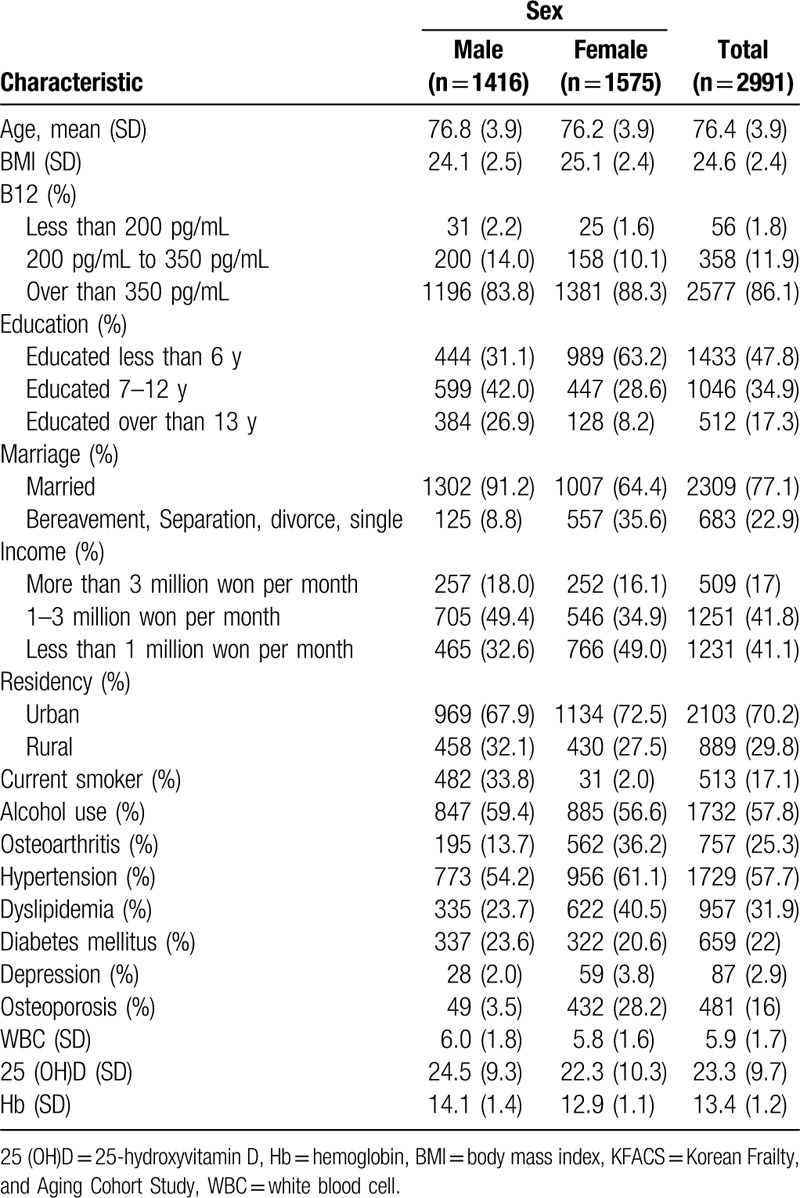
Table 2.
Baseline characteristics of KFACS participants, by Vitamin B12 levels.
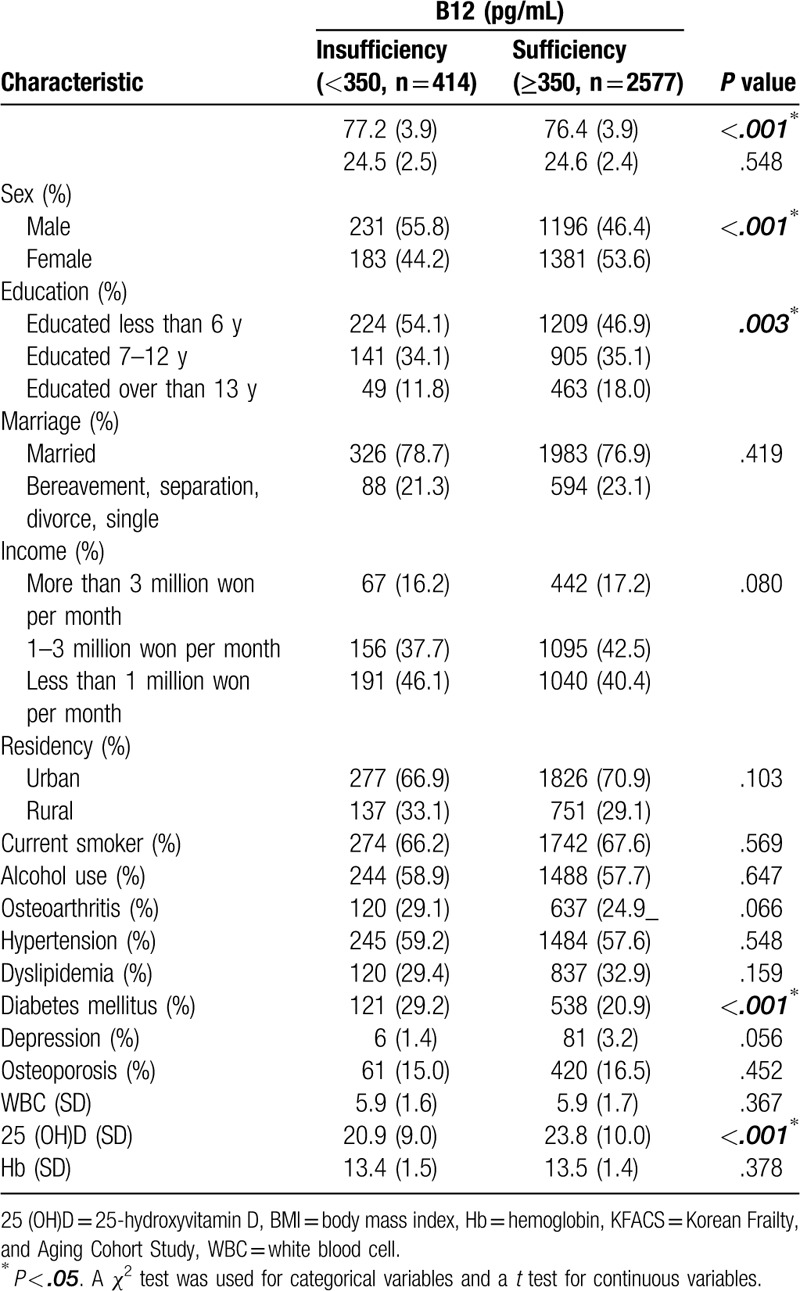
Table 3.
Analysis of cognitive function in KFACS participants, by serum Vitamin B12 levels.
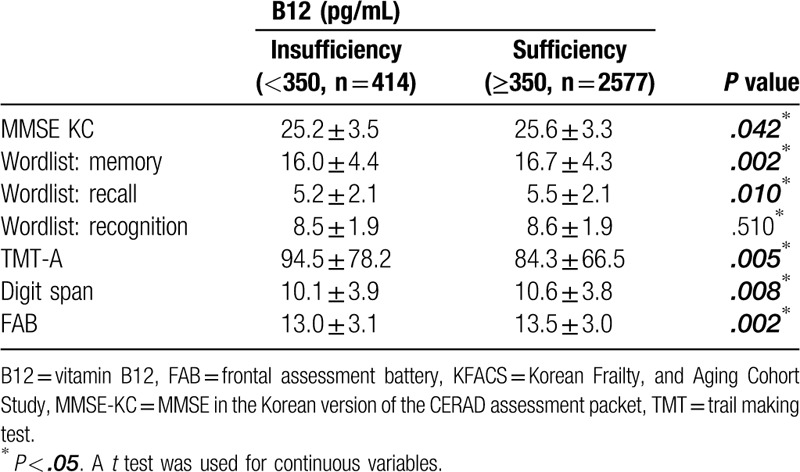
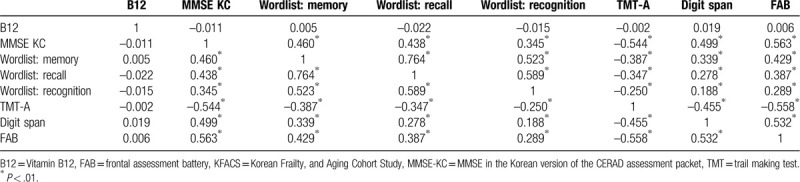
These results were not statistically significant following adjustments for age, sex, education periods, location of residence, body mass index, alcohol use, smoking, depression, marriage, annual income, presence of diabetes mellitus, and cardiovascular disease (Table 4). There was a statistically significant correlation between cognitive function test scores, but no correlation between the concentration degree of B12 levels and each cognitive function test score observed in the Pearson's correlation analysis (Table 5). This means that higher or lower B12 concentration levels do not improve or worsen cognitive function.
Table 4.
Linear regression model analysis of cognitive function in KFACS, by serum Vitamin B12 levels.
Table 5.
Pearson's correlation analysis of Vitamin B12 and cognitive function in KFACS participants, by serum B12 levels.
4. Discussion
The relationship of B12 to neurogenesis is widely known.[22] As B12 is essential for the development of myelination, it is necessary for the development and maintenance of cranial, peripheral nerves as well as the central nervous system such as the spinal cord.[23] B12 also affects the small vessels of the brain, which are associated with lacunar infarcts and white matter lesions (WML).[24] Conditions affecting the small vessels of the brain are thought to be caused by a disorder of the blood–brain barrier (BBB). If B12, which plays an essential role in maintaining BBB, is deficient, the BBB can be affected.[25] In a cross-sectional study, Pieters et al[26] reported that periventricular WML was related to low B12 levels in patients with lacunar stroke. In another study that had a 2-year follow-up period, the progression of periventricular WML relates to low levels of B12 in lacunar stroke patients.[27] These suggest that B12 deficiency may be closely related to brain degeneration and possibly associated with cognitive function.
Indeed, many previous studies have examined the association between B12 and cognitive function. In particular, articles studying the relationship between AD and B12 have reported that B12 deficiency increased the incidence of AD. Whyte et al[28] studied B12 levels in 643 AD patients and found that lower serum B12 showed lower performance on the MMSE, Dementia rating scale, and digit span tests. Stuerenburg et al studied B12 levels in 241 AD patients and used the MMSE. In this, the average was 4.3 points lower in the lowest 10% B12 group than that of in the highest 10% B12 group.[29] According to these studies, B12 might be associated with the development of AD.
In studies involving community-dwelling elderly, Clarke et al[30] reported in a 10-year prospective study of 1648 people aged 65 years or older with high blood holotranscobalam levels, an active fraction of B12, a 30% slower cognitive decline rate. Also, there was a report that in 549 elderly patients with B12 levels below 350 pg/mL, cognitive impairment developed faster after 8 years.[7] In another cross-sectional study, Goodwin et al[31] reported lower results in the Wechsler memory test when B12 was lower than 300 pg/mL in a study of 260 patients. As such, low B12 levels have been shown to increase the possibility of cognitive decline as well as current cognitive function.
In this study, a large cohort study of the elderly Korean population was conducted in order to identify their B12 levels and correlate these levels with cognitive function. When the variables were not adjusted, B12 based on 350 pg/ mL significantly correlated with cognitive function. In contrast there was no statistical significance in the fully adjusted model when potential confounder variables were adjusted. Besides, when analyzed by Pearson's correlation coefficient, there was no linear correlation between B12 levels and cognitive function. These results suggest that a higher B12 level does not mean higher cognitive function and indicates that it does not affect cognitive function if it is above a certain B12 level.
There are several reasons for these results. First, the exact deficiency levels of B12 are not precise, so the criteria for dividing patients into deficient or sufficient groups are not clear. In previous studies, such grouping had a sensitivity of 65% to 95% at <200 pg/mL, while the specificity was relatively high at 50% to 60%. In case the cutoff value was increased to <350 pg/mL, sensitivity was 90%, and specificity was 25%, which was a relatively low specificity.[23] Future studies will need to establish the exact B12 levels that affect cognitive function. Our results suggest that elderly Koreans have less B12 deficiency than Western countries. In this study, those with levels <200 pg/mL were 1.8%, which is lower than previous research. Those with levels <350 pg/ml were also lower than previous research. In the United States of America, 8.8% had levels < 200 pg/mL and 30.5% had levels <350 pg /mL. In the case of Australia, 6.3% had levels <170 pg/mL and 29% had levels <300 pg /mL.[32,33] In a previous study that measured B12 levels in 195 Koreans, B12 deficiency (<150 pg / mL) was 2.0% and 1.0% in males and females, respectively. These results show that Koreans have relatively less B12 deficiency than Western countries.[34] These differences are considered due to food, lifestyle, and racial disparities.[23] Despite these differences, this study compared those with sufficient B12 levels and those with insufficient B12 levels to analyze the effects of B12 on cognitive function. There were significant differences seen in the univariable results, but not in the multivariable results. This could indicate that cognitive impairment is multifactorial and that while B12 is a factor that affects cognition, it alone does not determine the presence of cognitive impairment. As mentioned before, the major causes of cognitive dysfunction are age,[35] education,[36] annual income,[37] smoking,[38] alcohol consumption,[39] depression,[40] diabetes,[41] and cardiac diseases such as hypertension,[42] blood pressure variability,[43] and atrial fibrillation.[44] Therefore, to evaluate the cause of cognitive decline, multifactorial assessments must be performed. Additionally, the analysis of baseline characteristics found that several factors were associated with B12 deficiency. This indicates that cognitive function and B12 levels are affected by demographic factors. Although B12 levels and cognitive function appear to be correlated, when variables are adjusted for, no direct correlation was observed.
There are a few limitations to this study. First, this is a cross-sectional study of B12 levels and cognitive function in community-dwelling populations. As mentioned earlier, since cognitive function is affected by various confounding factors, it is necessary to evaluate the effects of B12 levels on cognitive function using prospective studies or randomized controlled trials. Second, this study did not evaluate other types of vitamin B or homocysteine. Deficiencies of other types of vitamin B, including folic acid (vitamin B9), can lead to hyperhomocysteinemia. Elevated homocysteine levels are a risk factor for vascular disease and have been reported to cause DNA damage in the brain.[45,46] The relationship between B12, folate, homocysteine, and cognitive function has been reported to be somewhat correlated in previous studies.[47,48] A further large cohort study is required to study these correlations. Third, participants were not asked whether they took B12 supplements. As many commercially available multivitamin supplements contain B12, there may be differences in B12 levels between those who take them and those who do not. This study did not identify whether participants took supplements when the blood samples were collected. Fourth, the cohort study excluded patients with severe AD or those who could not finish the questionnaire, so this study did not consider the relationship between B12 and severe cognitive impairment.
This is the first large, population-based study of almost 3 thousand community-dwelling elderly participants in Korea that investigates the relationship between B12 and cognitive function. Previous studies conducted included subjects with AD or mild cognitive impairment.[49] In our study, B12 insufficiency was not significantly associated with cognitive decline when possible confounders were adjusted for. This is a large community-dwelling elderly population cohort study, and the data contains various demographic and cognitive information. Therefore, our results are meaningful for understanding the relationship between B12 levels and cognition.
5. Conclusion
In this study, the group with sufficient B12, performed better in the MMSE-KC, Wordlist: memory, Wordlist: recognition, TMT-A test, digit span, and FAB tests, to a statistically significant degree. However, when all possible confounder variables were adjusted for, no direct correlation between B12 insufficiency and cognitive impairment was observed. Although our study does not indicate that B12 is a direct risk factor of cognitive decline, B12 levels could be a contributing factor. Further prospective studies or randomized controlled trials are needed to evaluate the changes in cognitive function according to B12 levels, and examine the effect that supplements have on B12 levels.
Acknowledgments
This manuscript acquired the editorial certificate by “Editage by cactus (https://online.editage.co.kr/).”
Author contributions
Conceptualization: Yunsoo Soh, Do Hun Lee
Data curation: Chang Won Won.
Interpretation of Data: Yunsoo Soh
Investigation: Yunsoo Soh
Methodology: Yunsoo Soh, Do Hun Lee
Supervision: Yunsoo Soh, Chang Won Won
Writing – original draft: Yunsoo Soh
Writing – review & editing: Yunsoo Soh
Footnotes
Abbreviations: AD = Alzheimer disease, B12 = vitamin B12, CERAD-K = Korean version of the Consortium to Establish a Registry for Alzheimer Disease, FAB = frontal assessment battery, KFACS = Korean Frailty and Aging Cohort study, MMSE-KC = Mini-Mental State Examination in the Korean version of the CERAD assessment packet, TMT = trail making test.
How to cite this article: Soh Y, Lee DH, Won CW. Association between Vitamin B12 levels and cognitive function in the elderly Korean population. Medicine. 2020;99:30(e21371).
This research was supported by a grant from the Korea Health Technology R&D Project through the Korean Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI15C3153).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Stover PJ. Physiology of folate and vitamin B 12 in health and disease. Nutr Rev 2004;62: suppl_1: S3–12. [DOI] [PubMed] [Google Scholar]
- [2].Langan RC, Zawistoski KJ. Update on Vitamin B 12 deficiency. Am Fam Physician 2011;83:1425–30. [PubMed] [Google Scholar]
- [3].Stevens CE, Hume ID. Contributions of microbes in vertebrate gastrointestinal tract to production and conservation of nutrients. Physiol Rev 1998;78:393–427. [DOI] [PubMed] [Google Scholar]
- [4].Andrès E, Loukili NH, Noel E, et al. Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ 2004;171:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Moore EM, Watters DA, Ames D, et al. Vitamin B12 and cognitive impairment. In: Diet and Nutrition in Dementia and Cognitive Decline Amsterdam: Elsevier; 2015;637–48. [Google Scholar]
- [6].Lindenbaum J, Rosenberg IH, Wilson P, et al. Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr 1994;60:2–11. [DOI] [PubMed] [Google Scholar]
- [7].Angela Garcia M, Alicia Paris-Pombo M, Evans L, et al. Is low-dose oral cobalamin enough to normalize cobalamin function in older people? J Am Geriatr Soc 2002;50:1401–4. [DOI] [PubMed] [Google Scholar]
- [8].Van Asselt D, de Groot LC, van Staveren WA, et al. Role of cobalamin intake and atrophic gastritis in mild cobalamin deficiency in older Dutch subjects. Am J Clin Nutr 1998;68:328–34. [DOI] [PubMed] [Google Scholar]
- [9].Drachman DA. Aging of the brain, entropy, and Alzheimer disease. Neurology 2006;67:1340–52. [DOI] [PubMed] [Google Scholar]
- [10].Kim KW, Park JH, Kim M-H, et al. A nationwide survey on the prevalence of dementia and mild cognitive impairment in South Korea. J Alzheimers Dis 2011;23:281–91. [DOI] [PubMed] [Google Scholar]
- [11].Meng H, Li Y, Zhang W, et al. The relationship between cognitive impairment and homocysteine in a B12 and folate deficient population in China: a cross-sectional study. Medicine 2019;98:e17970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ellinson M, Thomas J, Patterson A. A critical evaluation of the relationship between serum vitamin B, folate and total homocysteine with cognitive impairment in the elderly. J Hum Nutr Diet 2004;17:371–83. [DOI] [PubMed] [Google Scholar]
- [13].Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev 2003;Cd004326. [DOI] [PubMed] [Google Scholar]
- [14].Won CW, Lee Y, Choi J, et al. Starting construction of frailty cohort for elderly and intervention study. Ann Geriatr Med Res 2016;20:114–7. [Google Scholar]
- [15].Senanarong V, Vannasaeng S, Poungvarin N, et al. Endogenous estradiol in elderly individuals: cognitive and noncognitive associations. Arch Neurol 2002;59:385–9. [DOI] [PubMed] [Google Scholar]
- [16].Lee JH, Lee KU, Lee DY, et al. Development of the Korean Version of the Consortium to Establish a Registry for Alzheimer's Disease Assessment Packet (CERAD-K) clinical and neuropsychological assessment batteries. J Gerontol B Psychol Sci Soc Sci 2002;57:47–53. [DOI] [PubMed] [Google Scholar]
- [17].Chandler MJ, Lacritz LH, Hynan LS, et al. A total score for the CERAD neuropsychological battery. Neurology 2005;65:102–6. [DOI] [PubMed] [Google Scholar]
- [18].Beeri M, Schmeidler J, Sano M, et al. Age, gender, and education norms on the CERAD neuropsychological battery in the oldest old. Neurology 2006;67:1006–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Baddeley A. Working memory. Science 1992;255:556–9. [DOI] [PubMed] [Google Scholar]
- [20].Dubois B, Slachevsky A, Litvan I, et al. The FAB: a Frontal Assessment Battery at bedside. Neurology 2000;55:1621–6. [DOI] [PubMed] [Google Scholar]
- [21].Werder SF. Cobalamin deficiency, hyperhomocysteinemia, and dementia. Neuropsychiatr Dis Treat 2010;6:159–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Healton EB, Savage DG, Brust JC, et al. Neurologic aspects of cobalamin deficiency. Medicine 1991;70:229–45. [DOI] [PubMed] [Google Scholar]
- [23].Stabler SP. Vitamin B12 deficiency. N Engl J Med 2013;368:2041–2. [DOI] [PubMed] [Google Scholar]
- [24].Wardlaw JM. Blood-brain barrier and cerebral small vessel disease. J Neurol Sci 2010;299:66–71. [DOI] [PubMed] [Google Scholar]
- [25].Scalabrino G. The multi-faceted basis of vitamin B12 (cobalamin) neurotrophism in adult central nervous system: lessons learned from its deficiency. Prog Neurobiol 2009;88:203–20. [DOI] [PubMed] [Google Scholar]
- [26].Pieters B, Staals J, Knottnerus I, et al. Periventricular white matter lucencies relate to low vitamin B12 levels in patients with small vessel stroke. Stroke 2009;40:1623–6. [DOI] [PubMed] [Google Scholar]
- [27].van Overbeek EC, Staals J, van Oostenbrugge RJ. Vitamin B12 and progression of white matter lesions. A 2-year follow-up study in first-ever lacunar stroke patients. PLoS One 2013;8:e78100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Whyte EM, Mulsant BH, Butters MA, et al. Cognitive and behavioral correlates of low vitamin B12 levels in elderly patients with progressive dementia. Am J Geriatr Psychiatry 2002;10:321–7. [PubMed] [Google Scholar]
- [29].Stuerenburg HJ, Mueller-Thomsen T, Methner A. Vitamin B 12 plasma concentrations in Alzheimer disease. Neuro Endocrinol Lett 2004;25:176–7. [PubMed] [Google Scholar]
- [30].Clarke R, Birks J, Nexo E, et al. Low vitamin B-12 status and risk of cognitive decline in older adults. Am J Clin Nutr 2007;86:1384–91. [DOI] [PubMed] [Google Scholar]
- [31].Goodwin JS, Goodwin JM, Garry PJ. Association between nutritional status and cognitive functioning in a healthy elderly population. JAMA 1983;249:2917–21. [PubMed] [Google Scholar]
- [32].Tucker KL, Rich S, Rosenberg I, et al. Plasma vitamin B-12 concentrations relate to intake source in the Framingham Offspring study. Am J Clin Nutr 2000;71:514–22. [DOI] [PubMed] [Google Scholar]
- [33].Flood VM, Smith WT, Webb KL, et al. Prevalence of low serum folate and vitamin B12 in an older Australian population. Aust N Z J Public Health 2006;30:38–41. [DOI] [PubMed] [Google Scholar]
- [34].Lim HS, Heo YR. Plasma total homocysteine, folate, and vitamin B12 status in Korean adults. J Nutr Sci Vitaminol (Tokyo) 2002;48:290–7. [DOI] [PubMed] [Google Scholar]
- [35].Murman DL. The impact of age on cognition. Paper presented at: Seminars in hearing, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yen C-H, Yeh C-J, Wang C-C, et al. Determinants of cognitive impairment over time among the elderly in Taiwan: results of the national longitudinal study. Arch Gerontol Geriatr 2010;50:S53–7. [DOI] [PubMed] [Google Scholar]
- [37].Rosso AL, Flatt JD, Carlson MC, et al. Neighborhood socioeconomic status and cognitive function in late life. Am J Epidemiol 2016;183:1088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ott A, Andersen K, Dewey M, et al. Effect of smoking on global cognitive function in nondemented elderly. Neurology 2004;62:920–4. [DOI] [PubMed] [Google Scholar]
- [39].Anttila T, Helkala E-L, Viitanen M, et al. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. BMJ 2004;329:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord 2009;119:1–8. [DOI] [PubMed] [Google Scholar]
- [41].Cholerton B, Baker LD, Montine TJ, et al. Type 2 diabetes, cognition, and dementia in older adults: toward a precision health approach. Diabetes Spectrum 2016;29:210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Elias M, Elias P, Sullivan L, et al. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord 2003;27:260–8. [DOI] [PubMed] [Google Scholar]
- [43].Lattanzi S, Brigo F, Vernieri F, et al. Visit-to-visit variability in blood pressure and Alzheimer's disease. J Clin Hypertens (Greenwich) 2018;20:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kim D, Yang P-S, Yu HT, et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a population-based cohort. Eur Heart J 2019;40:2313–23. [DOI] [PubMed] [Google Scholar]
- [45].Irizarry MC, Gurol ME, Raju S, et al. Association of homocysteine with plasma amyloid beta protein in aging and neurodegenerative disease. Neurology 2005;65:1402–8. [DOI] [PubMed] [Google Scholar]
- [46].Kruman II, Kumaravel TS, Lohani A, et al. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer's disease. J Neurosci 2002;22:1752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Duthie SJ, Whalley LJ, Collins AR, et al. Homocysteine B vitamin status, and cognitive function in the elderly. Am J Clin Nutr 2002;75:908–13. [DOI] [PubMed] [Google Scholar]
- [48].Tucker KL, Qiao N, Scott T, et al. High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study. Am J Clin Nutr 2005;82:627–35. [DOI] [PubMed] [Google Scholar]
- [49].Kim H, Kim G, Jang W, et al. Association between intake of B vitamins and cognitive function in elderly Koreans with cognitive impairment. Nutr J 2014;13:118. [DOI] [PMC free article] [PubMed] [Google Scholar]


