Abstract
Background
Dysmenorrhoea refers to painful menstrual cramps and is a common gynaecological complaint. Conventional treatments include non‐steroidal anti‐inflammatory drugs (NSAIDs) and oral contraceptive pills (OCPs), which both reduce myometrial activity (contractions of the uterus). A suggested alternative approach is dietary supplements. We used the term 'dietary supplement' to include herbs or other botanical, vitamins, minerals, enzymes, and amino acids. We excluded traditional Chinese medicines.
Objectives
To determine the efficacy and safety of dietary supplements for treating dysmenorrhoea.
Search methods
We searched sources including the Cochrane Gynaecology and Fertility Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, AMED, PsycINFO (all from inception to 23 March 2015), trial registries, and the reference lists of relevant articles.
Selection criteria
We included randomised controlled trials (RCTs) of dietary supplements for moderate or severe primary or secondary dysmenorrhoea. We excluded studies of women with an intrauterine device. Eligible comparators were other dietary supplements, placebo, no treatment, or conventional analgesia.
Data collection and analysis
Two review authors independently performed study selection, performed data extraction and assessed the risk of bias in the included trials. The primary outcomes were pain intensity and adverse effects. We used a fixed‐effect model to calculate odds ratios (ORs) for dichotomous data, and mean differences (MDs) or standardised mean differences (SMDs) for continuous data, with 95% confidence intervals (CIs). We presented data that were unsuitable for analysis either descriptively or in additional tables. We assessed the quality of the evidence using Grading of Recommendations Assessment, Development and Evaluation (GRADE) methods.
Main results
We included 27 RCTs (3101 women). Most included studies were conducted amongst cohorts of students with primary dysmenorrhoea in their late teens or early twenties. Twenty‐two studies were conducted in Iran and the rest were performed in other middle‐income countries. Only one study addressed secondary dysmenorrhoea. Interventions included 12 different herbal medicines (German chamomile (Matricaria chamomilla, M recutita, Chamomilla recutita), cinnamon (Cinnamomum zeylanicum, C. verum), Damask rose (Rosa damascena), dill (Anethum graveolens), fennel (Foeniculum vulgare), fenugreek (Trigonella foenum‐graecum), ginger (Zingiber officinale), guava (Psidium guajava), rhubarb (Rheum emodi), uzara (Xysmalobium undulatum), valerian (Valeriana officinalis), and zataria (Zataria multiflora)) and five non‐herbal supplements (fish oil, melatonin, vitamins B1 and E, and zinc sulphate) in a variety of formulations and doses. Comparators included other supplements, placebo, no treatment, and NSAIDs.
We judged all the evidence to be of low or very low quality. The main limitations were imprecision due to very small sample sizes, failure to report study methods, and inconsistency. For most comparisons there was only one included study, and very few studies reported adverse effects.
Effectiveness of supplements for primary dysmenorrhoea
We have presented pain scores (all on a visual analogue scale (VAS) 0 to 10 point scale) or rates of pain relief, or both, at the first post‐treatment follow‐up.
Supplements versus placebo or no treatment
There was no evidence of effectiveness for vitamin E (MD 0.00 points, 95% CI −0.34 to 0.34; two RCTs, 135 women).
There was no consistent evidence of effectiveness for dill (MD ‐1.15 points, 95% CI −2.22 to −0.08, one RCT, 46 women), guava (MD 0.59, 95% CI −0.13 to 1.31; one RCT, 151 women); one RCT, 73 women), or fennel (MD −0.34 points, 95% CI −0.74 to 0.06; one RCT, 43 women).
There was very limited evidence of effectiveness for fenugreek (MD −1.71 points, 95% CI −2.35 to −1.07; one RCT, 101 women), fish oil (MD 1.11 points, 95% CI 0.45 to 1.77; one RCT, 120 women), fish oil plus vitamin B1 (MD −1.21 points, 95% CI −1.79 to −0.63; one RCT, 120 women), ginger (MD −1.55 points, 95% CI −2.43 to −0.68; three RCTs, 266 women; OR 5.44, 95% CI 1.80 to 16.46; one RCT, 69 women), valerian (MD −0.76 points, 95% CI −1.44 to −0.08; one RCT, 100 women), vitamin B1 alone (MD −2.70 points, 95% CI −3.32 to −2.08; one RCT, 120 women), zataria (OR 6.66, 95% CI 2.66 to 16.72; one RCT, 99 women), and zinc sulphate (MD −0.95 points, 95% CI −1.54 to −0.36; one RCT, 99 women).
Data on chamomile and cinnamon versus placebo were unsuitable for analysis.
Supplements versus NSAIDS
There was no evidence of any difference between NSAIDs and dill (MD 0.13 points, 95% CI −1.01 to 1.27; one RCT, 47 women), fennel (MD −0.70 points, 95% CI −1.81 to 0.41; one RCT, 59 women), guava (MD 1.19, 95% CI 0.42 to 1.96; one RCT, 155 women), rhubarb (MD −0.20 points, 95% CI −0.44 to 0.04; one RCT, 45 women), or valerian (MD points 0.62 , 95% CI 0.03 to 1.21; one RCT, 99 women),
There was no consistent evidence of a difference between Damask rose and NSAIDs (MD −0.15 points, 95% CI −0.55 to 0.25; one RCT, 92 women).
There was very limited evidence that chamomile was more effective than NSAIDs (MD −1.42 points, 95% CI −1.69 to −1.15; one RCT, 160 women).
Supplements versus other supplements
There was no evidence of a difference in effectiveness between ginger and zinc sulphate (MD 0.02 points, 95% CI −0.58 to 0.62; one RCT, 101 women). Vitamin B1 may be more effective than fish oil (MD −1.59 points, 95% CI −2.25 to −0.93; one RCT, 120 women).
Effectiveness of supplements for secondary dysmenorrhoea
There was no strong evidence of benefit for melatonin compared to placebo for dysmenorrhoea secondary to endometriosis (data were unsuitable for analysis).
Safety of supplements
Only four of the 27 included studies reported adverse effects in both treatment groups. There was no evidence of a difference between the groups but data were too scanty to reach any conclusions about safety.
Authors' conclusions
There is no high quality evidence to support the effectiveness of any dietary supplement for dysmenorrhoea, and evidence of safety is lacking. However for several supplements there was some low quality evidence of effectiveness and more research is justified.
Keywords: Female; Humans; Dietary Supplements; Anti‐Inflammatory Agents, Non‐Steroidal; Anti‐Inflammatory Agents, Non‐Steroidal/therapeutic use; Dysmenorrhea; Dysmenorrhea/diet therapy; Dysmenorrhea/therapy; Magnesium; Magnesium/therapeutic use; Phytotherapy; Phytotherapy/methods; Randomized Controlled Trials as Topic; Thiamine; Thiamine/therapeutic use; Vitamin B 6; Vitamin B 6/therapeutic use; Vitamin E; Vitamin E/therapeutic use
Plain language summary
Dietary supplements for pain during menstruation
Review question
Cochrane authors reviewed the evidence of the effect of dietary supplements (e.g. vitamins, minerals, herbs) on period pain (dysmenorrhoea).
Background
Dietary supplements have been used in the treatment of period pain. It is important to explore their benefits and harms. We investigated the effectiveness of dietary supplements compared to other supplements, placebo, no treatment or conventional analgesics (pain relief) in women with either primary dysmenorrhoea (not related to any other diagnosis) or secondary dysmenorrhoea (related to other causes, such as endometriosis). The evidence is current to 23 March 2015.
Study characteristics
We included 27 randomised controlled trials (3101 women). Most participants were students in their late teens or early twenties with primary dysmenorrhoea. Most studies were conducted in Iran. Interventions included 12 different herbal medicines (chamomile, cinnamon, Damask rose, dill, fennel, fenugreek, ginger, guava, rhubarb, uzara, valerian, and zataria), and five non‐herbal supplements (fish oil, melatonin, vitamins B1 and E, and zinc sulphate) in a variety of formulations and doses. Supplements were compared with other supplements, placebo, no treatment, and non‐steroidal anti‐inflammatory drugs (NSAIDs).
Key results
There was no high quality evidence to support the effectiveness of any dietary supplement for dysmenorrhoea, and evidence of safety was lacking. However, for several supplements there was some low quality evidence of effectiveness. Supplements for which there was some very limited evidence to suggest a potential benefit were fenugreek, ginger, valerian, zataria, zinc sulphate, fish oil, and vitamin B1.
There was no strong evidence of benefit for melatonin compared to placebo for dysmenorrhoea secondary to endometriosis.
Quality of the evidence
The evidence was of low or very low quality for all comparisons. The main limitations were imprecision due to very small sample sizes, failure to report study methods, and inconsistency. For most comparisons there was only one included study, and very few included studies reported adverse effects.
Summary of findings
Summary of findings for the main comparison. Dietary supplements versus placebo for primary dysmenorrhoea.
| Dietary supplements versus placebo for moderate or severe primary dysmenorrhoea | ||||||
| Population: women with moderate or severe primary dysmenorrhoea Setting: community Intervention: dietary supplement Comparison: placebo | ||||||
| Outcomes1 | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with dietary supplement (95% CI) | |||||
| Dill seed versus placebo Pain score |
Mean pain score 5.45 (SD 1.41) on a 0‐10 point scale |
The mean pain score in the intervention group was 1.15 points lower (2.22 lower to 0.08 lower) than in the placebo group | — | 95 (1 study) |
⊕⊕⊝⊝ very low2,3,4 | |
| Dill seed versus placebo Pain relief |
391 per 1000 | 304 per 1000 (114 to 598) | OR 0.68 (0.20 to 2.31) | 46 (1 study) |
⊕⊝⊝⊝ very low2,3,4 | |
| Fennel versus placebo Pain score |
Mean pain score 2.18 (SD 0.66) on a 0 to 3 point scale |
The mean pain score in the intervention group was 0.34 points lower (0.74 lower to 0.06 higher) than in the placebo group | — | 43 (1 study) | ⊕⊝⊝⊝ very low2,5 | |
| Fenugreek versus placebo Pain score |
Mean pain score 4.32 (SD 1.5) on a 0 to 10 point scale |
The mean pain score in the intervention group was 1.71 points lower (2.35 lower to 1.07 lower) than in the placebo group | — | 101 (1 study) | ⊕⊕⊝⊝ low2,3 | |
| Ginger versus placebo Pain score |
Mean pain score ranged from 4.81 (SD 1.7) to 6.2 (SD 1.4) on a 0 to 10 point scale |
We did not pool data due to high heterogeneity. However, the direction of effect was consistent, and all studies found a benefit in the intervention group, ranging from mean ‐0.93 points to mean ‐2.30 points | — | 266 (3 studies) | ⊕⊕⊝⊝ low2,6 | |
| Ginger versus placebo Pain relief |
471 per 1000 | 829 per 1000 (615 to 936) | OR 5.44 (1.80 to 16.46) | 69 (1 study) | ⊕⊕⊝⊝ low2,3 | |
| Ginger versus placebo Adverse effects |
44 per 1000 | 43 per 1000 (6 to 248) | OR 0.96 (0.13 to 7.09) | 92 (1 study) | ⊕⊝⊝⊝ very low2,3 | |
| Guava leaf versus placebo Pain score |
Mean pain score ranged from 4.31 (SD 2.12) to 5.13 (SD 2.23) on a 0 to 10 point scale |
The mean pain score in the intervention group was 0.59 points lower (0.13 lower to 1.31 higher) than in the placebo group | — | 151 (1 study) | ⊕⊕⊝⊝ low 2,3 | |
| Valerian versus placebo Pain score |
Mean pain score 2.65 (SD 1.81) on a 0 to 10 point scale |
The mean pain score in the intervention group was 0.76 points lower (1.44 lower to 0.08 lower) than in the placebo group | — | 100 (1 study) | ⊕⊕⊝⊝ low 2,3 | |
| Zataria versus placebo Pain relief |
353 per 1000 | 784 per 1000 (592 to 901) | OR 6.66 (2.66 to 16.72) | 99 (1 study) | ⊕⊕⊝⊝ low2,3 | |
| Fish oil versus placebo Pain score |
Mean pain score 5.22 (SD 1.96) on a 0 to 10 point scale |
The mean pain score in the intervention group was 1.59 points lower (2.25 lower to 0.93 lower) than in the placebo group | — | 120 (1 study) |
⊕⊕⊝⊝ low2,3 | |
| Fish oil + vitamin B1 versus placebo Pain score |
Mean pain score 4.01 (SD 1.2) on a 0 to 10 point scale |
The mean pain score in the intervention group was 2.8 points lower (3.33 lower to 2.27 lower) than in the placebo group | — | 120 (1 study) |
⊕⊕⊝⊝ low2,3 | |
| Vitamin B1 versus placebo Pain score |
Mean pain score 4.11 (SD 1.73) on a 0 to 10 point scale |
The mean pain score in the intervention group was 2.7 points lower (3.32 lower to 2.08 lower) than in the placebo group | — | 120 (1 study) |
⊕⊕⊝⊝ low2,3 | |
| Vitamin E versus placebo Pain score |
Mean pain score 5.4 (SD 2.4) on a 0 to 10 point scale |
The mean pain score in the intervention group was the same (SMD 0.00, 0.34 standard deviations (SDs) lower to 0.34 SDs higher) as in the placebo group7 | — | 135 (2 studies) |
⊕⊕⊝⊝ low2,3 | |
| Zinc sulphate versus placebo Pain score |
Mean pain score 6.18 (SD 1.7) on a 0 to 10 point scale |
The mean pain score in the intervention group was 0.95 points lower (1.54 lower to 0.36 lower) than in the placebo group | — | 99 (1 study) |
⊕⊝⊝⊝ very low2,3 | |
| Zinc sulphate versus placebo Adverse effects |
44 per 1000 | 37 per 1000 (5 to 221) | OR 0.83 (0.11 to 6.12) | 99 (1 study) |
⊕⊝⊝⊝ very low2,3 | |
Abbreviations: OR: odds ratio; SD: standard deviation; SMD: standardised mean difference 1 Outcome at first measurement after treatment commencement. 2 Downgraded one level for serious risk of bias due to inadequate reporting of study methods. 3 Downgraded one level for serious imprecision: single small study and/or results compatible with benefit in one or both groups and with no effect
4 Downgraded one level for serious inconsistency: findings for pain score were inconsistent with findings for rate of pain relief. 5 Downgraded two levels for very serious imprecision: single very small study 6 Downgraded one level for serious inconsistency (I2=78%) 7 Data pooled to calculate standardised mean difference, as the two studies utilised different pain scales.
Background
This Cochrane review is an update of a Cochrane review that was first published in 2001 (Proctor 2001).
Description of the condition
Dysmenorrhoea or painful menstruation is the most common gynaecological complaint in women. According to a recent review of 15 primary studies (19,010 women) published between 2002 and 2011, the prevalence of dysmenorrhoea varies widely with reports ranging from 16% to 91% in women of reproductive age, with 2% to 29% experiencing severe dysmenorrhoea (Ju 2014). A higher prevalence of dysmenorrhoea was generally found in adolescent women, with estimates ranging from 20% to 90% (French 2005). A recent Australian study of senior high school girls, Parker 2010, reported that 93% of 1803 teenagers had pain with menstruation and about 40% reported moderate or severe pain. Dysmenorrhoea interferes with life daily activities and reduces quality of life, with absence from school or work ranging from 13% to 51% (Proctor 2006). The wide variation in reported prevalences are likely due to a difference in study populations, study quality, and length of investigation.
Risk factors for the development of dysmenorrhoea include: younger age at menarche, longer duration of menstruation and heavier menstrual flow, irregular menstrual cycles, depression/anxiety, smoking, and alcohol consumption (French 2005; Osayande 2014; Proctor 2006; Wallace 2010).
Dysmenorrhoea is commonly defined using two subcategories (Lichten 1987; Osayande 2014; Proctor 2006; Wallace 2010). Menstrual pain without organic pathology is considered to be primary dysmenorrhoea, while secondary dysmenorrhoea is associated with an identifiable pathological condition, such as endometriosis or ovarian cysts. The initial onset of primary dysmenorrhoea is at around six to 12 months after menarche, when ovulatory cycles are established. Pain duration is commonly eight to 72 hours and the pain is usually associated with the onset of the menstrual flow. In contrast, secondary dysmenorrhoea is more likely to occur years after the onset of menarche and can occur premenstrually as well as during menstruation. About 10% of adolescents and young adults with dysmenorrhoea have secondary dysmenorrhoea (Harel 2006).
Description of the intervention
There are a range of treatment options available for women with dysmenorrhoea, including non‐steroidal anti‐inflammatory drugs (NSAIDs), oral contraceptive pills (OCPs), COX‐2 (cyclo‐oxygenase‐2) specific inhibitors, and complementary and alternative medicines (CAM) (French 2005; Osayande 2014; Proctor 2006). The goal of treatment is to provide adequate relief of pain and symptoms.
Two Cochrane reviews have suggested the efficacy of conventional treatments (NSAIDs and OCPs) (Marjoribanks 2010; Proctor 2006). Marjoribanks 2010 found that NSAIDs were much more effective than placebo for pain relief in women with primary dysmenorrhoea (OR 4.50, 95% CI 3.85 to 5.27) but with an increased risk of adverse effects such as mild neurological (e.g. headache, drowsiness, dizziness) and gastrointestinal symptoms (e.g. nausea, indigestion). COX‐2 specific inhibitors are effective for dysmenorrhoea but these drugs have been withdrawn from use in many countries due to questions about the cardiovascular and cardioprotective safety (Proctor 2006).
Many consumers are now seeking alternatives to conventional medicine. CAM use has become popular with both consumers and practitioners of conventional medicine. It is thought that up to 38% of adults use some form of CAM to treat a variety of diseases and conditions (Barnes 2008). A Cochrane review, Zhu 2008, provides some support for the use of Chinese herbal medicine for primary dysmenorrhoea, but is limited by the low methodological quality of the included studies.
How the intervention might work
This Cochrane review focuses on dietary supplements and excludes traditional Chinese medicines as these are the subject of another Cochrane review (Zhu 2008). Dietary supplements are defined as vitamins, minerals, herbs or other botanicals, enzymes, and amino acid dietary substances intended to supplement the diet by increasing the total dietary intake, or a concentrate, metabolite, constituent, extract, or combination of any of the aforementioned ingredients. Dietary supplements are often marketed in forms such as tablets, capsules, soft gels, and gel caps (US FDA 2014).
Dietary supplements are a type of CAM. Based on the National Health Interview Survey (NHIS) in 2007, people in the USA spent USD 33.9 billion out‐of‐pocket on CAM over the previous 12 months. A total of 44% of all out‐of‐pocket costs for CAM, or about USD 14.8 billion, was spent on the purchase of non‐vitamin, non‐mineral, natural products (Barnes 2008; Nahin 2009). Herbal and dietary therapies are especially popular as treatments for disorders such as dysmenorrhoea as they can be self‐administered and are often easily available from health shops, pharmacies, and supermarkets. This ease of access, while in some ways beneficial, can in itself create problems with the control of dosage and quality and possible drug interactions (Cupp 1999; Winslow 1998).
A small RCT that investigated the effects of different dietary levels of calcium and manganese showed that an increase in calcium intake reduced the mood and pain symptoms associated with menstruation (Penland 1993). Another finding was that low dietary manganese increased mood and pain symptoms during the premenstrual phase (Penland 1993). An open trial of magnesium as treatment for dysmenorrhoea reported that the menstrual cycles experienced with supplement intake had greatly reduced symptoms compared with the pretreatment control cycles (Benassi 1992). Additional evidence from French 2005 and Proctor 2006 suggested that thiamine, vitamin E, omega‐3 polyunsaturated fatty acids, and a Japanese herb (Toki‐shakuyaku) may be effective compared to placebo.
Why it is important to do this review
As alternative therapies become more widely used, it is important to ensure the safety and efficacy of such interventions. We assessed the efficacy and safety of dietary supplements as treatment for dysmenorrhoea.
Objectives
To determine the efficacy and safety of dietary supplements for treating dysmenorrhoea.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel group or crossover randomised controlled trials (RCTs) of the effectiveness of dietary supplements for pain relief in dysmenorrhoea.
Types of participants
We included:
women of reproductive age;
women with moderate to severe primary dysmenorrhoea (pain that does not respond well to analgesics, affects daily activity or has a high baseline score on a validated pain scale) or women with secondary dysmenorrhoea of identifiable pathology. We included trials where the severity of dysmenorrhoea was not formally assessed if the potential participants had sought medical advice for the perceived pain;
women that experienced dysmenorrhoea during most menstrual cycles.
We excluded:
women with mild dysmenorrhoea (mild pain that responded to analgesics);
women with irregular or infrequent menstrual cycles (outside of the typical range of a 21‐ to 35‐day cycle);
women using an intrauterine contraceptive device (IUD) or taking oral contraceptive pills (OCPs).
Types of interventions
Dietary supplements in the treatment group versus placebo, no treatment, against each other, or any other conventional treatment. We excluded RCTs that reported the use of Chinese medicinal herbs as these are the subject of another Cochrane review (Zhu 2008).
Types of outcome measures
Primary outcomes
Pain (measured either by a visual analogue scale (VAS), other validated scales, or as a dichotomous outcomes);
adverse effects from treatment (incidence and duration of side effects and types of side effects).
Secondary outcomes
Requirements for additional medication (measured as the proportion of women that required analgesics in addition to their assigned treatment);
restriction of daily life activities (measured as the proportion of women who reported activity restriction);
absence from work or school (measured as the proportion of women that reported absences from work or school, and also as hours or days of absence as a more selective measure).
Search methods for identification of studies
For this review update, we searched for RCTs by following a search strategy that we developed in consultation with the Trials Search Co‐ordinator for the Cochrane Gynaecology and Fertility Group (CGF), formerly the Menstrual Disorders and Subfertility Group (MDSG). There was no language restriction in the literature searches.
Electronic searches
We developed all search strategies in consultation with the Trials Search Co‐ordinator for the Cochrane Gynaecology and Fertility Group (CGF), and searched the following electronic sources from inception to 23 March 2015:
the CGF Specialised Register (Appendix 1);
CENTRAL (Appendix 2);
OvidMEDLINE (Appendix 3);
EMBASE (Appendix 4);
PsycINFO (Appendix 5);
AMED (Appendix 6);
Searching other resources
We also searched the reference lists of relevant articles.
Data collection and analysis
Selection of studies
Two review authors (Porjai Pattanittum (PP) and Naowarat Kunyanone (NK), Julie Brown (JB), or Jane Marjoribanks (JM)) initially screened the titles and abstracts of articles retrieved by the searches. We retrieved the full‐text articles of all potentially eligible studies. At least two review authors (PP, NK, JB or JM) independently examined these full‐text articles for compliance with the inclusion criteria and selected studies eligible for inclusion in the review. We resolved any disagreements regarding study eligibility by discussion or consulted a third review author, Ussanee S Sangkomkamhang (US). We have documented the selection process in a PRISMA flow chart (Figure 1).
1.
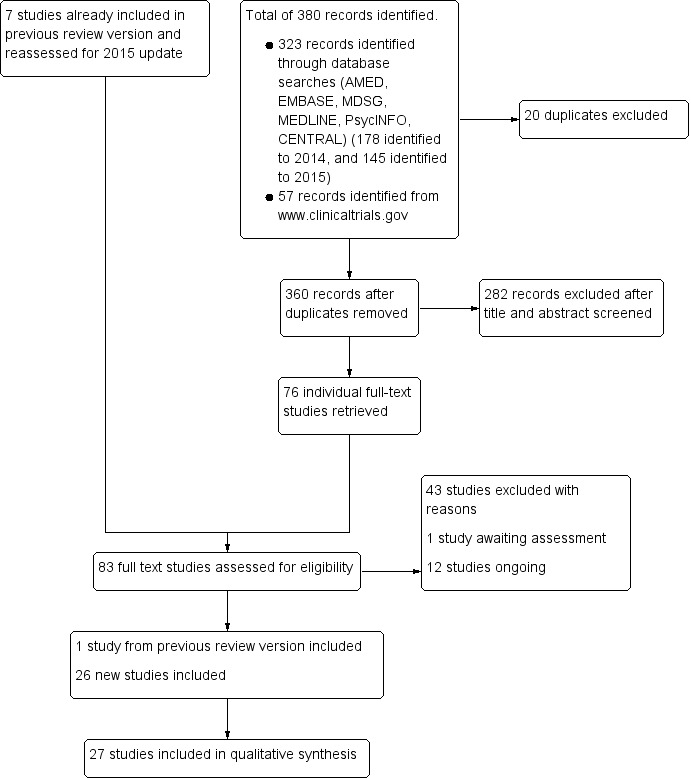
Study flow diagram.
Data extraction and management
Two review authors (PP and NK or JM) independently extracted data from the included studies where the full‐text article was available in English, using a data extraction form that was designed and pilot‐tested by the review authors. Vahid Seyfoddin (VS) extracted data from the Persian studies. We resolved any disagreements by consensus with a third review author (US). Data abstraction included study characteristics, participant characteristics, treatment characteristics, 'Risk of bias' items, and outcomes (see Appendix 7 for the data extraction form details). We resolved disagreements by discussion or by consensus with review author US, or both.
Assessment of risk of bias in included studies
Two review authors (PP and NK or JM) independently assessed the included studies for risk of bias and used the Cochrane 'Risk of bias' assessment tool in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to assess the following: selection bias (random sequence generation and allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessors); attrition bias (incomplete outcome data); reporting bias (selective reporting); and other bias. We assessed each item as at either 'low risk of bias', 'unclear’(uncertain risk of bias), or 'high risk of bias'. We resolved any disagreements by discussion or consulted a third review author (US). We described all our 'Risk of bias' judgements fully and presented the conclusions in the 'Risk of bias' tables, which we incorporated into the interpretation of the review findings.
Measures of treatment effect
For dichotomous data (e.g. number of women with pain), we used the numbers of events in the control and intervention groups of each study to calculate Mantel‐Haenszel odds ratios (ORs), together with their 95% confidence intervals (CIs). For continuous data (e.g. pain score), we reported mean differences (MDs) between the control and intervention groups, with 95% CIs. In addition, we considered the use of standardized mean differences (SMDs) with their 95% CIs for pain score that measured in a different way; VAS 0 to 10, Multi‐dimensional scale 0 to 3.
Unit of analysis issues
The primary analysis was per woman randomised. For studies with multiple intervention groups, we made multiple pair‐wise comparisons between all possible pairs of intervention groups. We counted individual participants in the meta‐analysis only once. For cross‐over trials, we considered only the data from the first phase.
Dealing with missing data
We analysed data on an intention‐to‐treat basis as far as possible. In case of missing or unclear data, we tried to obtain additional information from the study authors.
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed heterogeneity using the I² statistic and the Chi² test with a 10% level of statistical significance. We took an I² statistic value of greater than 50% to indicate moderate heterogeneity, and an I² statistic value greater than 75% to indicate substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
In view of the difficulty in detection and correction for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and duplication of data. When appropriate, we planned to use a funnel plot to assess the possibility of small‐study effects. We planned to construct a funnel plot to assess potential publication bias if sufficient studies (at least 10) reported the same comparison.
Data synthesis
When the included studies were clinically and methodologically sufficiently similar, we combined the data using a fixed‐effect model provided there was no moderate or substantial statistical heterogeneity (I² statistic value of less than 50%). If there was moderate heterogeneity (I² statistic value of 50% to 75%), we applied a random‐effects model. If we detected substantial heterogeneity (I² statistic value greater than 75%), we did not pool the data across studies.
Subgroup analysis and investigation of heterogeneity
We did not plan to perform any subgroup analyses a priori. Where we detected substantial heterogeneity, we planned to consider clinical and methodological differences between the included studies and to conduct exploratory subgroup analyses if possible.
Sensitivity analysis
We planned to perform sensitivity analyses for the primary outcomes to determine whether the findings were robust to arbitrary decisions made regarding the eligibility and analysis. These analyses included consideration of whether the review conclusions would have differed if:
we had restricted eligibility to studies with low risk of bias (i.e. low risk of bias for allocation concealment, less than 10% of data missing for the primary outcomes and no domains with high risk of bias);
we had used a random‐effects model;
risk ratios (RRs) rather than ORs had been used.
Overall quality of the evidence: 'Summary of findings' tables
We evaluated the overall quality of the evidence for the primary review outcomes (pain score (VAS 0 to 10), pain improvement, and adverse effects from treatment) by using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness, and publication bias). We justified, documented and recorded our judgements about evidence quality (high, moderate, low, or very low) into the reporting of results for each outcome. We used the GRADEpro Guideline Development Tool (GDT) (GRADEpro GDT) to create the 'Summary of findings' tables.
Results
Description of studies
We reassessed the seven studies included in the previous version of this Cochrane review (Proctor 2001). We excluded five of the previously included studies with reasons (Fontana 1990; Harel 1996; Kotani 1997; Salazar de Roldan 1993; Seifert 1989) (see the 'Characteristics of excluded studies' section). For Davis 1988, a thesis, we await an interlibrary loan of the full‐text article (which we will add to the next review update). We included one study, Gokhale 1996, in the current review update.
Results of the search
We performed literature searches up to 23 March 2015, and retrieved a total of 360 potentially eligible articles. We retrieved 79 eligible studies, which we examined in full‐text. We assessed seven included studies of the previous review version (as described above).
Of these 86 full‐text articles:
we included 27 studies: one study, Gokhale 1996, from the previous review version, and 26 new studies (Abdelmaeboud 2014; Akbari 2012; Akhavan Amjadi 2009; Bani 2014; Bokaie 2013; Dolation 2010; Doubova 2007; Ghodsi 2014; Heidarifar 2014; Hosseinlou 2014; Iravani 2009; Jenabi 2010; Jenabi 2012; Jenabi 2013; Kashanian 2013; Kashefi 2014; Khorshidi 2003; Modaress 2011; Moslemi 2012; Nasehi 2013; Nayeban 2014; Nazarpour 2007; Rahnama 2010; Rahnama 2012; Rehman 2015; Schwertner 2013) (see the 'Characteristics of included studies' section for further details);
we excluded 46 with reasons (see the 'Characteristics of excluded studies' section);
one study, Davis 1988, from the previous review version is awaiting assessment as we await access to the full‐text article (see the 'Studies awaiting classification' section);
11 studies are ongoing (see the 'Ongoing studies' section).
See Figure 1 for a study flow diagram.
We attempted to contact the authors of completed or ongoing studies for more information. In most cases we did not receive a reply, except from two study authors (Bokaie 2013; IRCT2014120917501N1).
Included studies
Study design and setting
We included 27 randomised controlled trials (RCTs) with a total of 3110 women, of whom we included 2894 (93%) in the analyses. Five were crossover trials with a total of 440 participants (Abdelmaeboud 2014; Bani 2014; Khorshidi 2003; Modaress 2011; Nasehi 2013), and the rest were parallel group trials with a total of 2661 participants.
Twenty‐two studies were conducted in Iran (Akbari 2012; Akhavan Amjadi 2009; Bani 2014; Bokaie 2013; Dolation 2010; Ghodsi 2014; Heidarifar 2014; Hosseinlou 2014; Iravani 2009; Jenabi 2010; Jenabi 2012; Jenabi 2013; Kashanian 2013; Kashefi 2014; Khorshidi 2003; Modaress 2011; Moslemi 2012; Nasehi 2013; Nayeban 2014; Nazarpour 2007; Rahnama 2010; Rahnama 2012), one in Brazil (Schwertner 2013), one in Egypt (Abdelmaeboud 2014), two in India Gokhale 1996, Rehman 2015), and one in Mexico (Doubova 2007).
We translated six studies from Persian (Akhavan Amjadi 2009; Dolation 2010; Iravani 2009; Jenabi 2010; Jenabi 2012; Modaress 2011).
Participants
All studies included women with moderate or severe primary dysmenorrhoea, except for Schwertner 2013 which included women with moderate or severe secondary dysmenorrhoea.
Participants in nearly all studies of primary dysmenorrhoea were university students.
Interventions
The included studies reported a wide range of comparisons. Some reported more than one comparison.
There were 13 comparisons of herbal medicines versus placebo or no treatment (15 RCTs):
chamomile tea (two cups a day) versus no treatment (Jenabi 2010);
cinnamon powder 420 mg versus placebo, five times a day (Akhavan Amjadi 2009);
dill seed (500 mg, powdered) two capsules 12‐hourly versus placebo (Heidarifar 2014);
fennel extract (46 mg) versus placebo every six hours (Moslemi 2012);
fennel capsule 30 mg every four hours versus no treatment (Ghodsi 2014);
fennel oil 1% or 2% (0.3 to 1 mL) versus placebo, as required no more than four‐hourly (Khorshidi 2003);
fennel 20 to 30 drops every four to eight hours versus placebo (Nazarpour 2007);
fenugreek seed powder 900 mg (two to three capsules three times a day) versus placebo (Akbari 2012);
ginger powder 500 mg versus placebo (Jenabi 2013; Rahnama 2010; Rahnama 2012);
ginger powder (250 mg) versus placebo three times a day (Kashefi 2014);
guava leaf extract 3 mg and 6 mg versus placebo, eight‐hourly (Doubova 2007);
valerian root powder 255 mg versus placebo, three times a day (Dolation 2010);
zataria extract (1% or 2%) versus placebo, four‐hourly (Iravani 2009).
We identified nine comparisons of herbal medicines versus NSAIDs (nine RCTs):
chamomile (400 mg) versus mefenamic acid, four times a day (Modaress 2011);
Damask rose fruit extract 200 mg versus mefenamic acid 250 mg (Bani 2014);
dill seed (500 mg, powdered) two capsules 12‐hourly versus mefenamic acid 250 mg 12‐hourly (Heidarifar 2014);
fennel 20 to 30 drops every four to eight hours versus mefenamic acid 250 mg every six hours (Nazarpour 2007);
fennel 2% versus mefenamic acid 250 mg (Bokaie 2013);
guava extract 3 mg and 6 mg versus ibuprofen 400 mg (Doubova 2007);
rhubarb (420 mg versus mefenamic acid (250 mg), three times a day (Rehman 2015);
uzara root 40 mg versus ibuprofen 400 mg (Abdelmaeboud 2014);
valerian 250 mg versus mefenamic acid 250 mg (Jenabi 2012).
There was one comparison of herbal medicines plus non‐herbal dietary supplement versus NSAIDs:
fennel extract (60 mg capsule) plus vitamin E (150 IU) versus ibuprofen 400 mg, four times a day (Nasehi 2013).
There were three comparisons of herbal medicines versus dietary supplements (three RCTs):
fennel extract (46 mg) versus vitamin E (100 units) every six hours (Moslemi 2012);
fennel extract (60 mg) versus vitamin E (150 IU), four times a day (Nasehi 2013);
ginger powder (250 mg) versus zinc sulphate (220 mg), three times a day (Kashefi 2014).
We noted seven comparisons of non‐herbal dietary supplements versus placebo (six RCTs):
fish oil capsule 500 mg versus placebo, daily (Hosseinlou 2014);
fish oil capsule 500 mg + vitamin B1 100 mg versus placebo, daily (Hosseinlou 2014);
melatonin 10 mg versus placebo (Schwertner 2013);
vitamin B1 100 mg versus placebo, daily (Gokhale 1996; Hosseinlou 2014);
vitamin E (100 units) versus placebo every six hours (Moslemi 2012);
vitamin E 400 IU versus placebo, daily (Kashanian 2013; Moslemi 2012);
zinc sulphate (220 mg) versus placebo, three times a day (Kashefi 2014).
There were four comparisons of non‐herbal supplements versus each other (two RCTs):
fish oil capsule 500 mg versus vitamin B1 100 mg daily (Hosseinlou 2014);
fish oil capsule 500 mg + vitamin 100 mg B1 versus fish oil only, daily (Hosseinlou 2014);
fish oil capsule 500 mg + vitamin 100 mg versus vitamin B1 only, daily (Hosseinlou 2014);
vitamin B1 100 mg daily versus vitamin E 400 units daily (Nayeban 2014).
Outcomes
Reporting of primary outcomes
Pain
All studies reported pain as the primary outcome. Continuous measures included a VAS scale 0 to 10 (0 is pain‐free, 10 is unbearable pain) and a 0 to 3 scale. Dichotomous measures included rate of improvement. Where studies failed to report data that we could analyse, we included their data in an additional table or reported P values (where available) in the text. The most common limitation was failure to report means and standard deviations (SDs) for continuous data.
Adverse effects
Most studies failed to report adverse effects as an outcome, and some reported adverse events only in the intervention group and not in the control group.
Reporting of secondary outcomes
Very few included studies reported any of the secondary outcomes for this Cochrane review.
Excluded studies
The previous version of this Cochrane review excluded 10 studies. We have excluded a further 36 studies, which gives a total of 43 excluded studies. Please see the 'Characteristics of excluded studies' table for reasons for exclusion.
Risk of bias in included studies
Refer to the 'Characteristics of included studies' section and associated 'Risk of bias' tables, and also Figure 2 and Figure 3 for further details.
2.
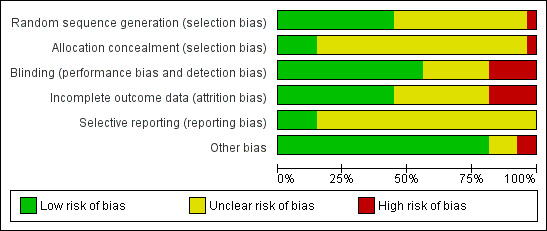
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
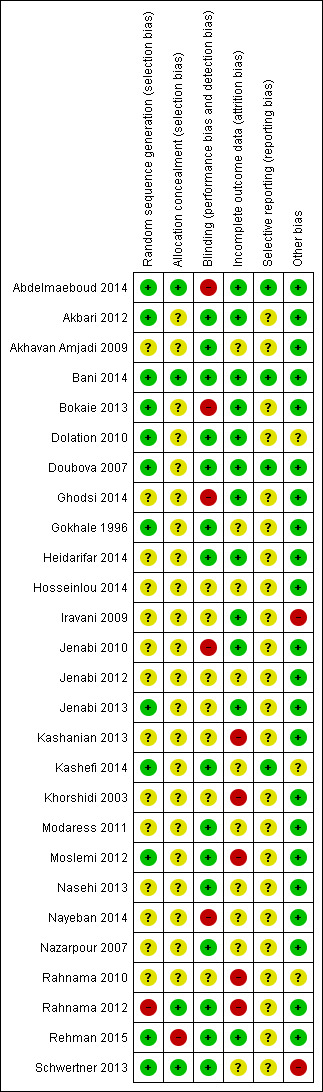
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Sequence generation
We rated 12 studies at low risk of bias in this domain (Abdelmaeboud 2014; Akbari 2012; Bani 2014; Bokaie 2013; Dolation 2010; Doubova 2007; Gokhale 1996; Jenabi 2013; Kashefi 2014; Moslemi 2012; Rehman 2015; Schwertner 2013). These trials used either a random number table or a computer to generate a random number. We considered 14 studies as at unclear risk of bias because they did not give details of sequence generation methods (Akhavan Amjadi 2009; Ghodsi 2014; Heidarifar 2014; Hosseinlou 2014; Iravani 2009; Jenabi 2010; Jenabi 2012; Kashanian 2013; Khorshidi 2003; Modaress 2011; Nasehi 2013; Nayeban 2014; Nazarpour 2007; Rahnama 2010). One study, Rahnama 2012, applied the random number table with a block of two. As use of a block of two made it possible to predict future assignment, we judged it as at high risk of bias.
Allocation concealment
We rated four studies at low risk of bias in this domain (Abdelmaeboud 2014; Bani 2014; Rahnama 2012; Schwertner 2013), and 22 studies at unclear risk because they did not give details of the allocation concealment methods (Akbari 2012; Akhavan Amjadi 2009; Bokaie 2013; Dolation 2010; Doubova 2007; Ghodsi 2014; Gokhale 1996; Heidarifar 2014; Hosseinlou 2014; Iravani 2009; Jenabi 2010; Jenabi 2012; Jenabi 2013; Kashanian 2013; Kashefi 2014; Khorshidi 2003; Modaress 2011; Moslemi 2012; Nasehi 2013; Nayeban 2014; Nazarpour 2007; Rahnama 2010). We considered one trial, Rehman 2015, at high risk of bias because the investigators were not blinded to allocation.
Blinding
We rated 15 studies at low risk of bias related to blinding (Akbari 2012; Akhavan Amjadi 2009; Bani 2014; Dolation 2010; Doubova 2007; Gokhale 1996; Heidarifar 2014; Kashefi 2014; Modaress 2011; Moslemi 2012; Nasehi 2013; Nazarpour 2007; Rahnama 2012; Rehman 2015; Schwertner 2013), and seven at unclear risk (in most cases because methods of blinding were not described in sufficient detail) (Hosseinlou 2014; Iravani 2009; Jenabi 2012; Jenabi 2013; Kashanian 2013; Khorshidi 2003; Rahnama 2010). We considered five studies at high risk of bias, because they did not appear to be blinded (Abdelmaeboud 2014; Bokaie 2013; Ghodsi 2014; Jenabi 2010; Nayeban 2014).
Incomplete outcome data
We considered 12 studies at low risk of attrition bias because all or most of the women randomised were included in analysis(Abdelmaeboud 2014; Akbari 2012; Bani 2014; Bokaie 2013; Dolation 2010; Doubova 2007; Ghodsi 2014; Heidarifar 2014; Iravani 2009; Jenabi 2010; Jenabi 2013; Rehman 2015). We rated 10 studies at unclear risk of attrition bias, in most cases because up to 10% of women were excluded from analysis (Akhavan Amjadi 2009; Gokhale 1996; Hosseinlou 2014; Jenabi 2012; Kashefi 2014; Modaress 2011; Nasehi 2013; Nayeban 2014; Nazarpour 2007; Schwertner 2013). We rated five studies at high risk of attrition bias, in most cases because over 10% of randomised women were excluded from analysis (Kashanian 2013; Khorshidi 2003; Moslemi 2012; Rahnama 2010; Rahnama 2012).
Selective reporting
Regarding selective reporting bias, we rated four studies at low risk of selective reporting bias, as they clearly reported expected outcomes (Abdelmaeboud 2014; Bani 2014; Doubova 2007; Kashefi 2014). We considered all the other studies at unclear risk of this bias, as they did not clearly report adverse events in both groups (Akbari 2012; Akhavan Amjadi 2009; Bokaie 2013; Dolation 2010; Ghodsi 2014; Gokhale 1996; Heidarifar 2014; Hosseinlou 2014; Iravani 2009; Jenabi 2010; Jenabi 2012; Jenabi 2013; Kashanian 2013; Khorshidi 2003; Modaress 2011; Moslemi 2012; Nasehi 2013; Nayeban 2014; Nazarpour 2007; Rahnama 2010; Rahnama 2012; Rehman 2015; Schwertner 2013).
Other potential sources of bias
For other potential sources of bias, we rated 20 studies at low risk of other potential bias (Abdelmaeboud 2014; Akbari 2012; Akhavan Amjadi 2009; Bani 2014; Bokaie 2013; Doubova 2007; Ghodsi 2014; Gokhale 1996; Heidarifar 2014; Hosseinlou 2014; Jenabi 2010; Jenabi 2012; Jenabi 2013; Kashanian 2013; Khorshidi 2003; Moslemi 2012; Nayeban 2014; Nazarpour 2007; Rahnama 2012; Rehman 2015). We considered five studies at unclear risk, due to insufficient reporting of study methods (Dolation 2010; Kashefi 2014; Modaress 2011; Nasehi 2013; Rahnama 2010). Two studies were at high risk of other potential bias, due to baseline imbalance between the groups (Schwertner 2013) and presentation of data in a graphical rather than a numerical form (Iravani 2009).
Effects of interventions
See: Table 1
Regarding data presentation, we have grouped together different formulations or doses of the same medicines.
1. Herbal medicines versus placebo or no treatment (15 RCTs):
chamomile versus no treatment (one RCT);
cinnamon versus placebo (one RCT);
dill versus placebo (one RCT);
fennel versus placebo or no treatment (four RCTs);
fenugreek versus placebo (one RCT);
ginger versus placebo (four RCTs);
guava versus placebo (one RCT);
valerian versus placebo (one RCT);
zataria versus placebo (one RCT).
2. Herbal medicines versus NSAIDs (10 RCTs):
chamomile versus mefenamic acid (one RCT);
Damask rose versus mefenamic acid (one RCT);
dill versus mefenamic acid (one RCT);
fennel versus mefenamic acid (one RCT);
guava versus ibuprofen (one RCT);
rhubarb versus mefenamic acid (one RCT);
uzara versus ibuprofen (one RCT);
valerian versus mefenamic acid (one RCT).
3. Herbal medicines versus dietary supplements (three RCTs):
fennel versus vitamin E (two RCTs);
ginger versus zinc sulphate (one RCT).
4. Non‐herbal dietary supplements versus placebo (seven RCTs):
fish oil versus placebo (one RCT);
fish oil + vitamin B1 versus placebo (one RCT);
melatonin versus placebo (one RCT);
vitamin B1 versus placebo (one RCT);
vitamin E versus placebo (three RCTs);
zinc sulphate versus placebo (one RCT).
5. Non‐herbal dietary supplements versus each other (two RCTs):
fish oil versus vitamin B1 (one RCT);
fish oil + vitamin B1 versus fish oil (one RCT);
fish oil + vitamin B1 versus vitamin B1 (one RCT);
vitamin B1 versus vitamin E (one RCT).
Except where stated below, none of the included studies reported any of our secondary outcomes.
1. Herbal medicines versus placebo or no treatment (15 RCTs)
1.1 Chamomile versus no treatment
One RCT, Jenabi 2010, compared chamomile tea (Matricaria recutita) versus no treatment in 82 women with primary dysmenorrhoea.
Primary outcomes
1.1.1 Pain
Data were skewed and unsuitable for analysis. Pain scores decreased from baseline in both groups, but the reduction was significantly greater in the chamomile tea group than in the control group (P < 0.001; Table 2).
1. Pain.
| Study | Measurement | Intervention (N) | Control (N) | Follow‐up | Intervention gp score (SD) | Control gp score (SD) | P value for difference in change from baseline |
| Jenabi 2010 | McGill Short Form Pain scores (0 to 10) | Chamomile tea bd (40) | No intervention (40) | At baseline | 8.42 (SD 11.88) | 7.35 (SD 11.91) | — |
| 1 month | 7.32 (SD 7.59) | 7.36 (SD 10.73) | P < 0.001 | ||||
| 3 months | 5.94 (SD 6.01) | 7.10 (SD 10.39) | P < 0.001 | ||||
| Schwertner 2013 | VAS 0 to 10 (adjusted mean) | Melatonin (20) | Placebo (20) | Follow‐up | 4.24 (SD 2.61) | 6.84 (SD 2.38) | — |
| Akhavan Amjadi 2009 | 0 to 3 pain scale | Cinnamon powder (26) | Placebo (21) | Baseline | 2.1 | 2.14 | — |
| After 2 cycles | 1.04 | 1.67 | — |
Abbreviations:
N: number of participants
SD: standard deviation
VAS: visual analogue scale
1.1.2 Adverse effects
Adverse effects were not reported as an outcome.
1.2 Cinnamon versus placebo
One study, Akhavan Amjadi 2009, compared cinnamon powder versus placebo in 47 women with primary dysmenorrhoea.
Primary outcomes
1.2.1 Pain
Data were unsuitable for analysis as SDs were not reported. Akhavan Amjadi 2009 measured pain using a 0 to 3 scale, over two cycles. Pain score decreased from baseline in both groups. It was unclear whether there was any difference between the groups (Table 2).
1.2.2 Adverse effects
Adverse effects were not reported as an outcome.
1.3 Dill versus placebo
One study compared dill seed versus placebo (Heidarifar 2014).
Primary outcomes
1.3.1 Pain
Heidarifar 2014 measured pain on a 0 to 10 VAS scale. Pain scores were lower in the dill group in the first cycle (MD −1.15, 95% CI −2.22 to −0.08; one RCT, 46 women) and the second cycle (MD −0.95, 95% CI −1.88 to −0.02; one RCT, 46 women). See Analysis 1.1.
1.1. Analysis.
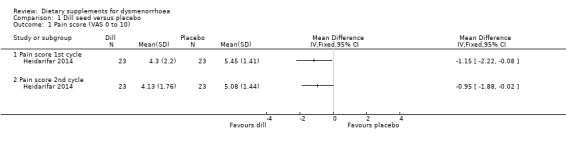
Comparison 1 Dill seed versus placebo, Outcome 1 Pain score (VAS 0 to 10).
The study also measured pain as rates of pain relief. There was no evidence of a difference between the groups in the first cycle (OR 0.68, 95% CI 0.20 to 2.31; one RCT, 46 women) or the second cycle (OR 0.48, 95% CI 0.14 to 1.60; one RCT, 46 women). See Analysis 1.2.
1.2. Analysis.
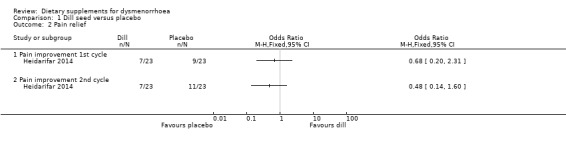
Comparison 1 Dill seed versus placebo, Outcome 2 Pain relief.
1.3.2 Adverse effects
Data on adverse events were unsuitable for analysis as the denominator was unclear. In the dill group, two women reported increased menstrual bleeding and one reported gastrointestinal discomfort. In the placebo group, each of the mentioned side‐effects was only observed in one woman.
1.4 Fennel versus placebo or no treatment
Four studies compared fennel versus either placebo (Khorshidi 2003; Moslemi 2012; Nazarpour 2007) or no treatment (Ghodsi 2014).
Primary outcomes
1.4.1 Pain
Moslemi 2012 measured pain on a 0 to 3 scale. There was no evidence of a difference between the groups in the first cycle (MD −0.34, 95% CI −0.74 to 0.06; one RCT, 43 women) but the score was lower in the fennel group in the second cycle (MD −0.65, 95% CI −1.05 to −0.25; one RCT, 43 women). See Analysis 2.1.
2.1. Analysis.
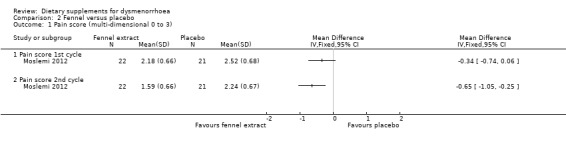
Comparison 2 Fennel versus placebo, Outcome 1 Pain score (multi‐dimensional 0 to 3).
Nazarpour 2007 used a 0 to 10 pain scale. Data were unsuitable for analysis as the study authors did not present mean and SD values. They reported that pain scores were significantly lower in the fennel group after the first and the second treatment (P < 0.05).
Ghodsi 2014 reported that they used the McGill Short form pain questionnaire, and stated that pain was mild in 85% of the intervention group by the third cycle. However, the study authors did not present any comparative data on pain scores.
1.4.2 Adverse effects
Adverse effects were not reported as an outcome in any of these studies.
1.5 Fenugreek versus placebo
One study compared fenugreek versus placebo in women with primary dysmenorrhoea (Akbari 2012).
Primary outcomes
1.5.1 Pain
Pain was measured on a 0 to 10 VAS scale (where zero is pain‐free and 10 is unbearable pain). There was evidence of reduced pain intensity in the fenugreek group compared to the placebo group in both the first cycle (MD −1.71, 95% CI −2.35 to −1.07; one RCT, 101 women) and the second cycle (MD −2.71, 95% CI −3.33 to −2.09; one RCT, 101 women). See Analysis 3.1.
3.1. Analysis.
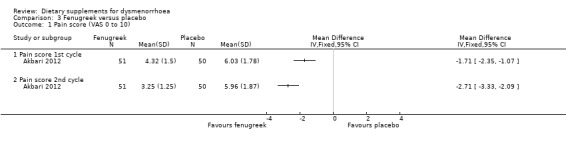
Comparison 3 Fenugreek versus placebo, Outcome 1 Pain score (VAS 0 to 10).
1.5.2 Adverse effects
The study authors stated that no side effects were observed in the fenugreek group. No data were reported on adverse effects in the placebo group.
Secondary outcomes
1.5.3 Requirement for additional medication
The study authors did not present statistical data but stated that the mean number of sedative tablets needed in the treatment group decreased significantly in the intervention groups compared to the placebo group.
1.6 Ginger versus placebo
Four studies compared ginger to placebo in women with primary dysmenorrhoea (Jenabi 2013; Kashefi 2014; Rahnama 2010; Rahnama 2012).
Primary outcomes
1.6.1 Pain
The studies measured pain on a 0 to 10 VAS scale (where zero is pain‐free and 10 is unbearable pain). In the three studies with data suitable for analysis, participants in the ginger groups had lower pain intensity in the first cycle and the second cycle. We did not pool data due to high heterogeneity. However, the direction of effect was consistent, and all studies found a benefit in the intervention group. See Analysis 4.1.
4.1. Analysis.
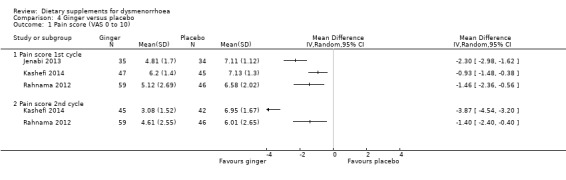
Comparison 4 Ginger versus placebo, Outcome 1 Pain score (VAS 0 to 10).
An earlier study by the same authors, Rahnama 2010, reported that administration of ginger powder decreased the severity of dysmenorrhoea compared to placebo (P < 0.01), among 78 university students. Only the study abstract was available, which reported no data suitable for analysis.
A third study reported the number of participants who got better or much better in terms of improvement in their symptoms (Jenabi 2013). Rates of pain relief were significantly higher in the ginger group (OR 5.44, 95% CI 1.80 to 16.46; one RCT, 69 women; Analysis 4.2).
4.2. Analysis.
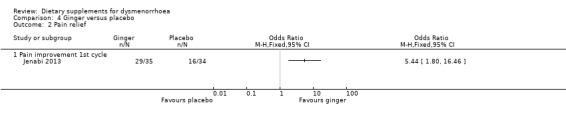
Comparison 4 Ginger versus placebo, Outcome 2 Pain relief.
1.6.2 Adverse effects
Two studies reported adverse effects (Kashefi 2014; Rahnama 2012). There was no evidence of a difference between the groups in cycle 1 (OR 0.96, 95% CI 0.13 to 7.09; one RCT, 92 women) or cycle 2 (OR 2.15, 95% CI 0.47, 9.77; two RCTs 182 women). See Analysis 4.3.
4.3. Analysis.
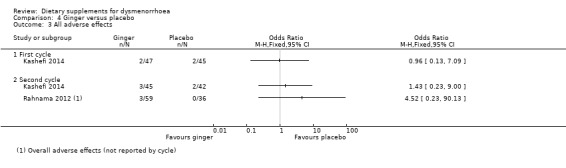
Comparison 4 Ginger versus placebo, Outcome 3 All adverse effects.
Jenabi 2013 noted that there were no adverse effects in the ginger group but no data were reported for the control group. Rahnama 2010 did not report this outcome.
1.7 Guava leaf versus placebo
One study compared guava leaf (Psidii guajavae folium extract) to placebo (Doubova 2007).
Primary outcomes
1.7.1 Pain
Doubova 2007 measured pain on a 0 to 10 VAS scale. There was no evidence of a difference between the groups in the first cycle (MD 0.59, 95% CI −0.13 to 1.31; one RCT, 151 women; Analysis 5.1), the second cycle (MD 0.69, 95% CI −0.05 to 1.44; one RCT, 151 women; Analysis 5.2), or the third cycle (MD 0.66, 95% CI −0.11 to 1.42; one RCT, 151 women; Analysis 5.3). 0.59
5.1. Analysis.
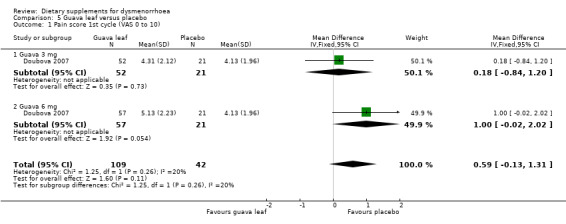
Comparison 5 Guava leaf versus placebo, Outcome 1 Pain score 1st cycle (VAS 0 to 10).
5.2. Analysis.
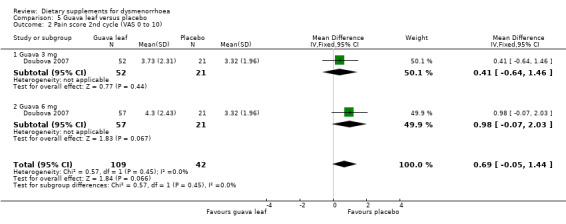
Comparison 5 Guava leaf versus placebo, Outcome 2 Pain score 2nd cycle (VAS 0 to 10).
5.3. Analysis.
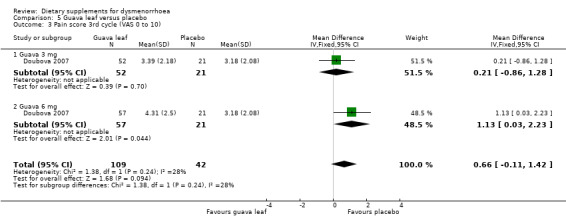
Comparison 5 Guava leaf versus placebo, Outcome 3 Pain score 3rd cycle (VAS 0 to 10).
1.7.2 Adverse effects
No comparative data were reported on adverse effects.
1.8 Valerian versus placebo
One study compared valerian root to placebo (Dolation 2010).
Primary outcomes
1.8.1 Pain
Dolation 2010 measured pain on a 0 to 10 VAS scale. There was evidence of reduced pain in the valerian group in the first cycle (MD −0.76, 95% CI −1.44 to −0.08; one RCT, 100 women) and the second cycle (MD −2.42, 95% CI −3.05 to −1.79; one RCT, 100 women). See Analysis 6.1.
6.1. Analysis.
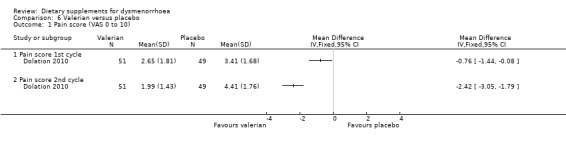
Comparison 6 Valerian versus placebo, Outcome 1 Pain score (VAS 0 to 10).
1.8.2 Adverse effects
The study authors noted that there were no adverse effects of treatment in the valerian group. No data were reported on adverse effects in the placebo group.
1.9 Zataria versus placebo
One study compared zataria extract (1% or 2%) to placebo (Iravani 2009).
Primary outcomes
1.9.1 Pain
This study measured pain on a categorical scale, defined as moderate or severe according to VAS ratings. There was evidence of a higher rate of pain relief (pain absent or mild) among participants in the zataria groups, compared to those who received placebo (OR 6.66, 95% CI 2.66 to 16.72; one RCT, 99 women; Analysis 7.1).
7.1. Analysis.
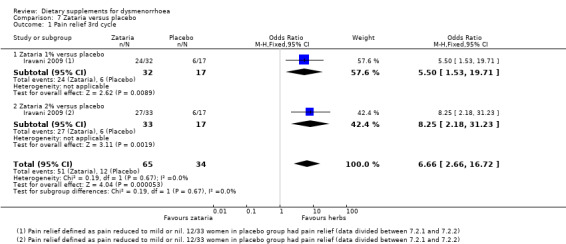
Comparison 7 Zataria versus placebo, Outcome 1 Pain relief 3rd cycle.
1.9.2 Adverse effects
Adverse effects were not reported as an outcome.
Secondary outcomes
Iravani 2009 did not report any of our secondary outcomes in a form in which we could extract data for the women with moderate or severe pain.
2. Herbal medicines versus NSAIDs
2.1 Chamomile versus mefenamic acid
One study compared German chamomile versus mefenamic acid (Modaress 2011).
Primary outcomes
2.1.1 Pain
This study measured pain on a 0 to 10 VAS scale. Pain scores were lower in the chamomile group in both the first cycle (MD 1.42, 95% CI −1.69 to −1.15; one RCT, 160 women) and the second cycle (MD −3.73, 95% CI −4.23 to −3.23; one RCT, 160 women). See Analysis 8.1.
8.1. Analysis.
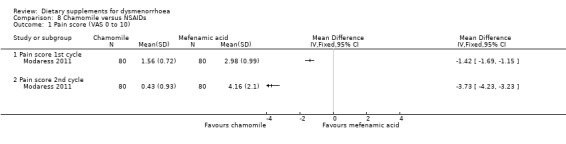
Comparison 8 Chamomile versus NSAIDs, Outcome 1 Pain score (VAS 0 to 10).
2.1.2 Adverse effects
Adverse effects were not reported as an outcome.
2.2 Dill versus mefenamic acid
One study compared dill seed versus mefenamic acid (Heidarifar 2014).
Primary outcomes
2.2.1 Pain
Heidarifar 2014 measured pain on a 0 to 10 VAS scale. There was no evidence of a difference between the groups in pain score in the first cycle (MD 0.13, 95% CI −1.01 to 1.27; one RCT, 47 women) or the second cycle (MD 0.35, 95% CI −0.56 to 1.26; one RCT, 47 women). See Analysis 9.1.
9.1. Analysis.
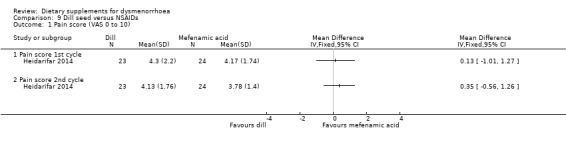
Comparison 9 Dill seed versus NSAIDs, Outcome 1 Pain score (VAS 0 to 10).
The study authors also reported rates of pain relief. There was no evidence of a difference between the groups in rates of pain relief in the first cycle (OR 22.27, 95% CI 1.19 to 417.10; one RCT, 47 women) or the second cycle (OR 3.06, 95% CI 0.68 to 13.74; one RCT, 47 women). See Analysis 9.2.
9.2. Analysis.
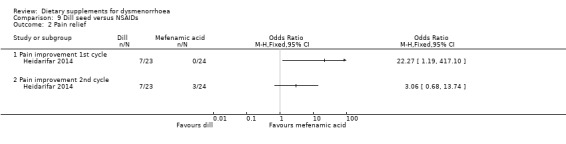
Comparison 9 Dill seed versus NSAIDs, Outcome 2 Pain relief.
2.2.2 Adverse effects
Data on adverse events were unsuitable for analysis as the denominator was unclear. In the dill group, two women reported increased menstrual bleeding and one reported gastrointestinal discomfort. In the mefenamic acid group, one woman reported increased menstrual bleeding and two reported gastrointestinal discomfort.
2.3 Fennel versus NSAIDs
Two studies compared fennel versus mefenamic acid (Bokaie 2013; Nazarpour 2007).
Primary outcomes
2.3.1 Pain
Bokaie 2013 used a 0 to 10 VAS pain scale. There was no evidence of a difference between the groups (MD −0.70, 95% CI −1.81 to 0.41; one RCT, 59 women; Analysis 10.1).
10.1. Analysis.
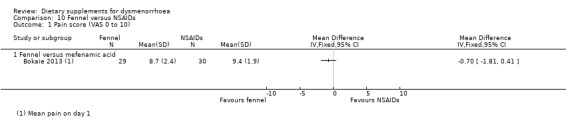
Comparison 10 Fennel versus NSAIDs, Outcome 1 Pain score (VAS 0 to 10).
Nazarpour 2007 also used a 0 to 10 pain scale. Data were unsuitable for analysis as the study authors did not present mean and SD values. They reported that there was no significant difference between fennel and mefenamic acid in either the first or the second cycle (P > 0.05).
2.3.2 Adverse effects
Bokaie 2013 reported no comparative data on adverse events, but noted that many volunteers in the fennel group complained of side effects, such as nausea, due to the unpleasant smell and taste of fennel drops, and that one participant had severe menstruation after taking fennel drops.
Nazarpour 2007 did not report adverse effects.
2.4 Guava leaf versus NSAIDs
One study compared guava leaf (Psidii guajavae folium extract) versus ibuprofen (Doubova 2007).
Primary outcomes
2.4.1 Pain
Doubova 2007 measured pain on a 0 to 10 VAS scale. Pain was higher in the guava leaf group in the first cycle (MD 1.17, 95% CI 0.42 to 1.96; one RCT, 155 women; Analysis 11.1) and the second cycle (MD 1.01, 95% CI 0.30 to 1.73; one RCT, 155 women; Analysis 11.2), but there was no evidence of a difference between the groups in the third cycle (MD 0.62, 95% CI −0.12 to 1.35; one RCT, 155 women; Analysis 11.3).
11.1. Analysis.
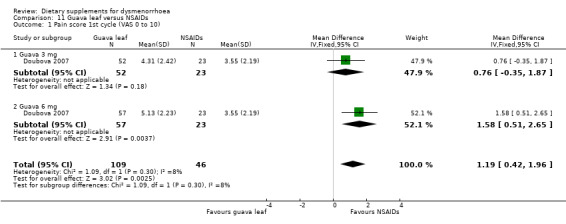
Comparison 11 Guava leaf versus NSAIDs, Outcome 1 Pain score 1st cycle (VAS 0 to 10).
11.2. Analysis.
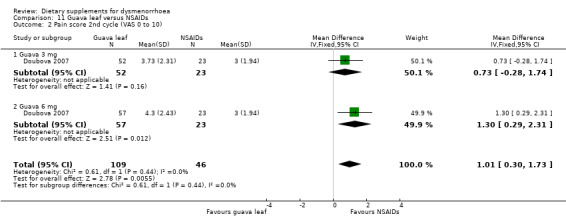
Comparison 11 Guava leaf versus NSAIDs, Outcome 2 Pain score 2nd cycle (VAS 0 to 10).
11.3. Analysis.
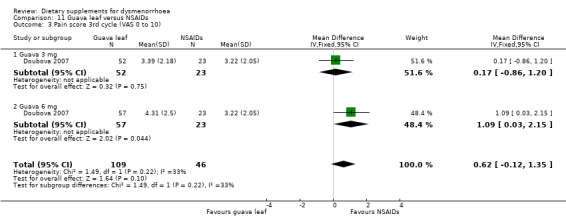
Comparison 11 Guava leaf versus NSAIDs, Outcome 3 Pain score 3rd cycle (VAS 0 to 10).
2.4.2 Adverse effects
There was no evidence of a difference between the groups in the incidence of abdominal pain or nausea, or both (OR 0.62, 95% CI 0.10 to 3.86; one RCT, 155 women; Analysis 11.4).
11.4. Analysis.
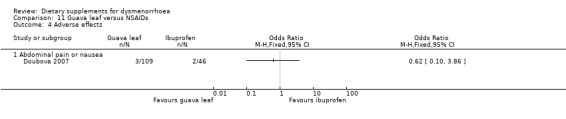
Comparison 11 Guava leaf versus NSAIDs, Outcome 4 Adverse effects.
2.5 Rhubarb versus mefenamic acid
One study compared rhubarb versus mefenamic acid (Rehman 2015).
Primary outcomes
2.5.1 Pain
The study authors measured pain on a 0 to 10 VAS scale. There was no evidence of a difference between the groups in the first cycle (MD −0.20, 95% CI −0.44 to 0.04; one RCT, 45 women) but pain scores were lower in the mefenamic acid group in the second cycle (MD 0.64, 95% CI 0.37 to 0.91; one RCT, 45 women) and the third cycle (MD 0.50, 95% CI 0.25 to 0.75; one RCT, 45 women). See Analysis 12.1.
12.1. Analysis.
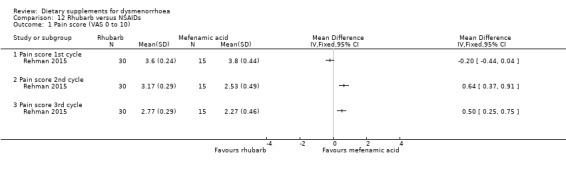
Comparison 12 Rhubarb versus NSAIDs, Outcome 1 Pain score (VAS 0 to 10).
2.5.2 Adverse effects
It was unclear whether data on adverse effects were collected systematically in both groups. The study authors stated that six (20%) women reported mild side effects in the rhubarb group, which comprised of two cases of bloating and four cases of diarrhoea.
2.6 Damask rose versus mefenamic acid
One study compared Damask rose versus mefenamic acid (Bani 2014).
Primary outcomes
2.6.1 Pain
Bani 2014 measured pain on a 0 to 10 VAS scale, at eight time points ranging from one hour to 71 hours after starting treatment. Findings at the various time points were highly inconsistent and the direction of effect varied. See Analysis 13.1. The study authors reported that there was no significant difference between the average of pain intensity in two groups (P = 0.35).
13.1. Analysis.
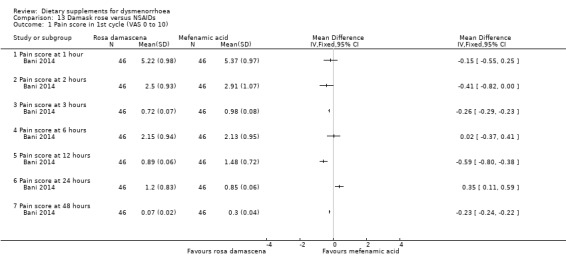
Comparison 13 Damask rose versus NSAIDs, Outcome 1 Pain score in 1st cycle (VAS 0 to 10).
2.6.2 Adverse effects
Bani 2014 reported that no participants experienced any adverse effects.
2.7 Uzara versus ibuprofen
One study (Abdelmaeboud 2014) compared uzara root to NSAIDS (ibuprofen). This was a crossover study and no first phase data were available for analysis.
Primary outcomes
2.7.1 Pain
Abdelmaeboud 2014 measured effectiveness by the number of women whose VAS pain measure fell to 3/10 or lower. The study authors reported that rates were similar in the two groups: 78% (47/60) versus 52/60 (87%).
2.7.2 Adverse effects
The study authors reported that participants tolerated uzara well with 0% (0/60) side effects compared to 8.3% (5/60) in the ibuprofen group (P < 0.05). All reported side‐effects were gastrointestinal.
Secondary outcomes
2.7.3 Requirement for additional medication
The study authors reported that there was no evidence of a difference between the groups in the need for a rescue drug (81.7% versus 90%, P = 0.295).
2.7.4 Restriction of daily life activities
Abdelmaeboud 2014 did not report this outcome.
2.7.5 Absence from work or school
The study authors reported that school absence rates were comparable in the two groups, being 11.7% (7/60) for uzara and 13.3% (8/60) for ibuprofen.
2.8 Valerian versus mefenamic acid
One study compared valerian to mefenamic acid (Jenabi 2012).
Primary outcomes
2.8.1 Pain
Jenabi 2012 measured pain on a 0 to 10 scale (where zero is pain‐free and 10 is unbearable pain). There was no evidence of a difference between the groups after two cycles (MD 0.62, 95% CI 0.03 to 1.21; one RCT, 99 women; Analysis 14.1).
14.1. Analysis.
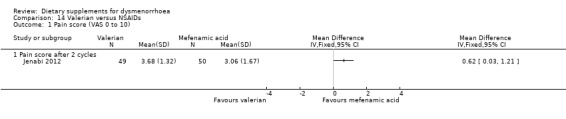
Comparison 14 Valerian versus NSAIDs, Outcome 1 Pain score (VAS 0 to 10).
2.8.2 Adverse effects
Jenabi 2012 reported that no participants in the valerian group experienced any adverse effects, but did not report whether there were any adverse effects in the mefenamic acid group.
3. Herbal medicines versus dietary supplements
3.1 Fennel versus vitamin E
One study compared fennel versus vitamin E (Moslemi 2012).
Primary outcomes
3.1.1 Pain
Pain was measured on a 0 to 3 scale. There was no evidence of a difference between the groups in the first cycle (MD −0.37, 95% CI −0.84 to 0.10; one RCT, 42 women), but pain scores were lower in the fennel group in the second cycle (MD −0.56, 95% CI −1.05 to −0.07; one RCT, 42 women). See Analysis 15.1.
15.1. Analysis.
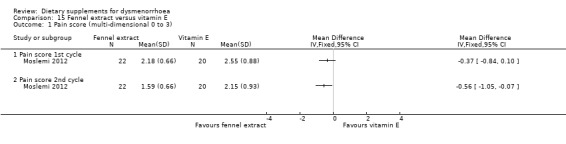
Comparison 15 Fennel extract versus vitamin E, Outcome 1 Pain score (multi‐dimensional 0 to 3).
3.1.2 Adverse effects
Adverse effects were not reported.
3.2 Ginger versus zinc sulphate
One study compared ginger versus zinc sulphate (Kashefi 2014).
Primary outcomes
3.2.1 Pain
Pain was measured on a 0 to 10 VAS scale. There was no evidence of a difference between the groups in the first cycle (MD 0.02, 95% CI −0.58 to 0.62; one RCT, 101 women) or the second cycle (MD −0.04, 95% CI −0.59 to 0.51; one RCT, 98 women). See Analysis 16.1.
16.1. Analysis.
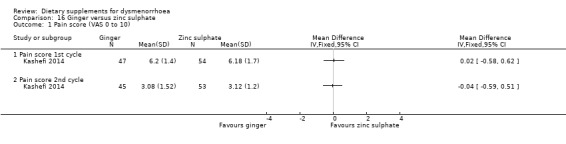
Comparison 16 Ginger versus zinc sulphate, Outcome 1 Pain score (VAS 0 to 10).
3.2.2 Adverse effects
There was no evidence of a difference between groups in the incidence of adverse effects in cycle 1 (OR 1.16, 95% CI 0.16 to 8.54; one RCT, 101 women) or cycle 2 (OR 0.88, 95% CI 0.19 to 4.13; one RCT, 98 women). See Analysis 16.2.
16.2. Analysis.
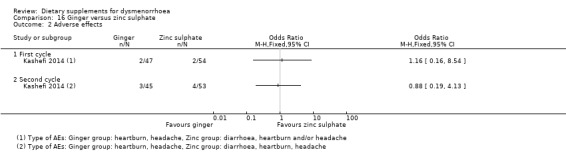
Comparison 16 Ginger versus zinc sulphate, Outcome 2 Adverse effects.
4. Herbal medicines plus non‐herbal dietary supplement versus NSAIDs
4.1 Fennel plus vitamin E versus NSAIDS
One study compared fennel plus vitamin E versus ibuprofen (Nasehi 2013).
Primary outcomes
4.1.1 Pain
The study authors measured pain on a 0 to 10 VAS scale at 1, 2, 3, 6, and 48 hours follow‐up. Data were unsuitable for analysis, as the study authors did not report mean and SD values. They reported that there was no evidence of a difference between the groups in pain scores over follow‐up except in the first two hours, when pain scores were lower in the fennel and vitamin E group (P < 0.04).
4.1.2 Adverse effects
Adverse effects were not reported.
5. Non‐herbal dietary supplements versus placebo
5.1 Fish oil versus placebo
Hosseinlou 2014 compared fish oil versus placebo.
Primary outcomes
5.1.1 Pain
Hosseinlou 2014 measured pain on a 0 to 10 VAS scale. Pain scores were lower in the fish oil group in the first cycle (MD −1.59, 95% CI −2.25 to −0.93; one RCT, 120 women) and the second cycle (MD −4.14, 95% CI −4.87 to −3.41; one RCT, 120 women). See Analysis 17.1.
17.1. Analysis.
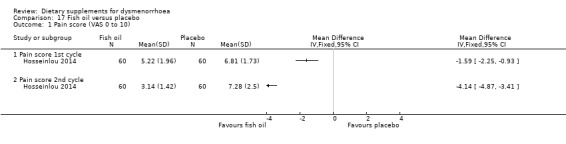
Comparison 17 Fish oil versus placebo, Outcome 1 Pain score (VAS 0 to 10).
5.1.2 Adverse effects
Adverse effects were not reported as an outcome.
5.2 Fish oil + vitamin B1 versus placebo
One study compared fish oil plus vitamin B1 versus placebo (Hosseinlou 2014).
Primary outcomes
5.2.1 Pain
The study authors measured pain on a 0 to 10 VAS scale. Pain scores were lower in the fish oil plus vitamin B1 group in the first cycle (MD −2.80, 95% CI −3.33 to −2.27; one RCT, 120 women) and the second cycle (MD −4.99, 95% CI −5.76 to −4.22; one RCT, 120 women). See Analysis 18.1.
18.1. Analysis.
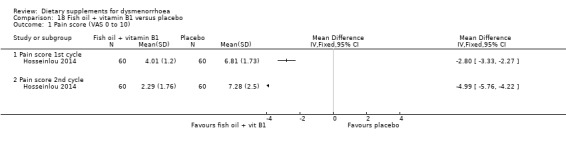
Comparison 18 Fish oil + vitamin B1 versus placebo, Outcome 1 Pain score (VAS 0 to 10).
5.2.2 Adverse effects
Adverse effects were not reported as an outcome.
5.3 Melatonin versus placebo
One study compared melatonin versus placebo in women with secondary dysmenorrhoea (Schwertner 2013).
Primary outcomes
5.3.1 Pain
This study presented data as adjusted mean and SD values in a post‐hoc analysis, and were unsuitable for analysis (see Table 3). The study authors reported that melatonin reduced dysmenorrhoea.
2. Outcome: number of sedative tablets.
| Study | Cycle | Herbs (Valerian) | Placebo | ||
| Dolation 2010 | N | mean | N | mean | |
| First | 51 | 1.29 | 49 | 1.59 | |
| Second | 51 | 0.55 | 49 | 1.31 | |
Abbreviations:
N: number of participants
5.3.2 Adverse effects
Adverse effects were not reported as an outcome.
Secondary outcomes
5.3.3 Requirement for additional medication
There was no evidence of a difference between the groups for this outcome (OR 0.34, 95% CI 0.08 to 1.44; one RCT, 36 women, Analysis 19.1).
19.1. Analysis.
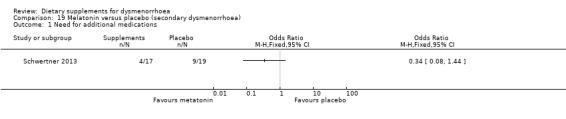
Comparison 19 Melatonin versus placebo (secondary dysmenorrhoea), Outcome 1 Need for additional medications.
5.4 Vitamin B1 versus placebo
One cross‐over RCT, Gokhale 1996, and one parallel‐group RCT, Hosseinlou 2014, compared vitamin B1 (thiamine) versus placebo. Participants in the cross‐over study received 60 days active treatment followed by 90 days placebo, or 60 days placebo followed by 90 days active treatment. Women in parallel‐group study took vitamin B1 at the beginning days of menses for two consecutive months.
Primary outcomes
5.4.1 Pain
In Gokhale 1996, data were unsuitable for analysis. At 60 days follow‐up, 55% of the intervention group (N = 277) and none of the placebo group (N = 279) reported a cure (complete disappearance of pain and other symptom), but at 150 days follow‐up, findings were similar in the 'treatment first' group and the 'placebo first' group, with 86% to 88% of participants reporting cure.
Hosseinlou 2014 measured pain on a 0 to 10 VAS scale. Pain scores were lower in the vitamin B1 group in both the first cycle (MD −2.70, 95% CI −3.32 to −2.08; one RCT, 120 women) and the second cycle (MD −4.90, 95% CI −5.64 to −4.16; one RCT, 120 women). See Analysis 20.1.
20.1. Analysis.
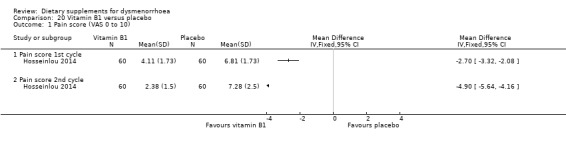
Comparison 20 Vitamin B1 versus placebo, Outcome 1 Pain score (VAS 0 to 10).
5.4.2 Adverse effects
Adverse effects were not reported as an outcome.
5.5 Vitamin E versus placebo
Two studies compared vitamin E versus placebo (Kashanian 2013; Moslemi 2012).
Primary outcomes
5.5.1 Pain
Kashanian 2013 measured pain on a 0 to 10 VAS scale, and Moslemi 2012 on a 0 to 3 scale. There was no evidence of a difference between the two groups in pain scores after one cycle (SMD 0.00, 95% CI −0.34 to 0.34; two RCTs, 135 women) or two cycles (SMD −0.25, 95% CI −0.59 to 0.09; two RCTs, 135 women). See Analysis 21.1.
21.1. Analysis.
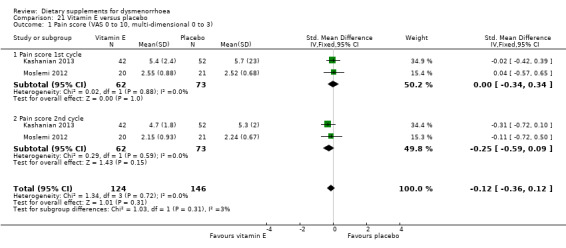
Comparison 21 Vitamin E versus placebo, Outcome 1 Pain score (VAS 0 to 10, multi‐dimensional 0 to 3).
5.5.2 Adverse effects
Neither study reported adverse effects as an outcome.
5.6 Zinc sulphate versus placebo
Kashefi 2014 compared zinc sulphate versus placebo.
Primary outcomes
5.6.1 Pain
Kashefi 2014 measured pain on a 0 to 10 scale. Pain scores were lower in the zinc sulphate group in both the first cycle (MD −0.95, 95% CI −1.54 to −0.36; one RCT, 99 women) and the second cycle (MD −3.83, 95% CI −4.43 to −3.23; one RCT, 95 women). See Analysis 22.1.
22.1. Analysis.
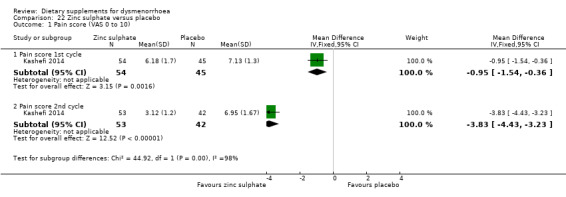
Comparison 22 Zinc sulphate versus placebo, Outcome 1 Pain score (VAS 0 to 10).
5.6.2 Adverse effects
There was no evidence of a difference between the groups in the incidence of adverse effects in cycle 1 (OR 0.83, 95% CI 0.11 to 6.12; one RCT, 99 women) or cycle 2 (OR 1.43, 95% CI 0.23 to 9.00; one RCT, 87 women). See Analysis 22.2.
22.2. Analysis.
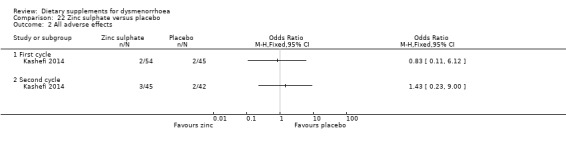
Comparison 22 Zinc sulphate versus placebo, Outcome 2 All adverse effects.
6. Non‐herbal dietary supplements versus each other
6.1 Fish oil versus vitamin B1
One study compared fish oil versus vitamin B1 (Hosseinlou 2014).
Primary outcomes
6.1.1 Pain
Hosseinlou 2014 measured pain on a 0 to 10 VAS scale. Findings favoured vitamin B1, as pain scores were higher in the fish oil group at cycle 1 (MD 1.11, 95% CI 0.45 to 1.77; one RCT, 120 women) and cycle 2 (MD 0.76, 95% CI 0.24 to 1.28; one RCT, 120 women). See Analysis 23.1.
23.1. Analysis.
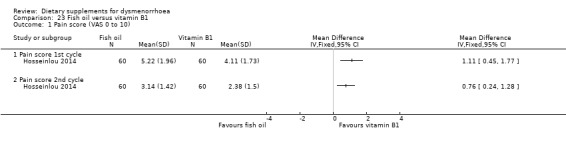
Comparison 23 Fish oil versus vitamin B1, Outcome 1 Pain score (VAS 0 to 10).
6.1.2 Adverse effects
Adverse effects were not reported as an outcome.
6.2 Fish oil + vitamin B1 versus fish oil alone
6.2.1 Pain
This study measured pain on a 0‐10 VAS scale. Pain scores were higher in the fish oil plus vitamin B1 group at cycle 1 (MD −1.21 , 95% CI −1.79 to −0.63; one RCT, 120 women) and cycle 2 (MD −0.85, 95% CI −1.42 to −0.28; one RCT, 120 women). See Analysis 24.1.
24.1. Analysis.
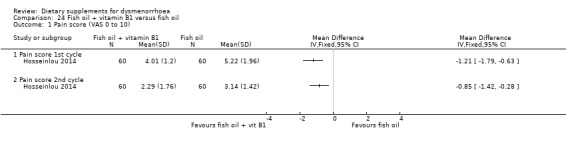
Comparison 24 Fish oil + vitamin B1 versus fish oil, Outcome 1 Pain score (VAS 0 to 10).
6.2.2 Adverse effects
Adverse effects were not reported as an outcome.
6.3 Fish oil + vitamin B1 versus vitamin B1
One study compared fish oil plus vitamin B1 versus vitamin B1 alone (Hosseinlou 2014).
Primary outcomes
6.3.1 Pain
Hosseinlou 2014 measured pain on a 0 to 10 VAS scale. There was no evidence of a difference between the groups at cycle 1 (MD −0.10, 95% CI −0.63 to 0.43; one RCT, 120 women) or cycle 2 (MD −0.09, 95% CI −0.68 to 0.50; one RCT, 120 women). See Analysis 25.1.
25.1. Analysis.
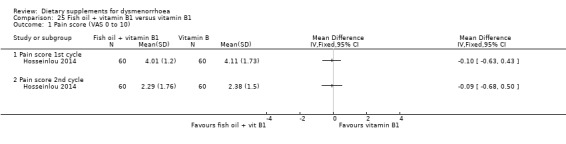
Comparison 25 Fish oil + vitamin B1 versus vitamin B1, Outcome 1 Pain score (VAS 0 to 10).
6.3.2 Adverse effects
Adverse effects were not reported as an outcome.
6.4 Vitamin B1 versus vitamin E
One study, Nayeban 2014, compared vitamin B1 versus vitamin E.
Primary outcomes
6.4.1 Pain
This study measured pain on a 0 to 100 VAS scale. Data were unsuitable for analysis as the study authors did not report SD values. They stated that there was no statistically significant difference between the groups in pain scores, though both groups improved significantly from baseline.
6.4.2 Adverse effects
Adverse effects were not reported as an outcome.
Reporting bias
Because of the small numbers of included studies for each outcome, we were unable to use a funnel plot to assess the possibility of small‐study effects.
Sensitivity analysis
There was an insufficient number of studies (only two RCTs) to conduct the planned sensitivity analysis by study quality. The sensitivity analyses by choice of statistical model and effect measure did not substantially change any of the review findings.
Discussion
Summary of main results
For treating primary dysmenorrhoea, there was no evidence of effectiveness for vitamin E, and no consistent evidence of effectiveness for dill, guava, or fennel. There was very limited evidence of effectiveness for fenugreek, fish oil, fish oil plus vitamin B1, ginger, valerian, vitamin B1 alone, zataria, and zinc sulphate.
When supplements were compared to NSAIDs, there was no evidence of a difference between dill, fennel, guava, rhubarb, and valerian and NSAIDs. There was no consistent evidence of a difference between Damask rose and NSAIDs, but there was some very limited evidence that chamomile was more effective than NSAIDs.
When we compared supplements head‐to‐head, there was no evidence of a difference in effectiveness between ginger and zinc sulphate, but vitamin B1 may be more effective than fish oil.
For treating dysmenorrhoea secondary to endometriosis, there was no strong evidence of benefit for melatonin.
With respect to the safety of supplements, only four of the 27 included studies reported adverse effects in both treatment groups. There was no evidence of a difference between the groups but data were too scanty to reach any conclusions about safety.
Overall completeness and applicability of evidence
Few data were available for any of our comparisons of interest and most analyses included only one small study. Very few studies made head‐to‐head comparisons of dietary supplements and most failed to systematically report adverse effects. Only one study assessed secondary dysmenorrhoea (Schwertner 2013).
Most included trials of primary dysmenorrhoea recruited university students and all included studies were conducted in low and middle‐income countries, predominantly in Iran. The applicability of the evidence to women in other contexts is uncertain.
Quality of the evidence
The evidence was of low or very low quality. The main limitations were imprecision due to very small sample sizes, failure to report study methods, and inconsistency. For most comparisons there was only one included study, and very few studies reported adverse effects. The studies were heterogeneous with respect to the type of intervention and the timing of the intervention, and we could not pool data for most analyses. The overall quality of the evidence presented in this Cochrane review, as assessed by the GRADE approach, was low or very low for all comparisons.
Potential biases in the review process
We attempted to identify and include all eligible studies. However, despite our attempts to contact study authors, references to some studies proved irretrievable and for others we were unable to ascertain whether the participants' level of pain severity met our criteria.
We excluded Chinese herbal medicines from this Cochrane review, as this intervention is covered in another Cochrane review (Zhu 2008). However it is difficult to draw a firm boundary between CAM interventions, and we have included in this review two studies of ginger powder, which is commonly used in China.
Several studies required translation from Persian. One (Persian‐speaking) review author extracted the data from these studies, which we did not double‐check.
Agreements and disagreements with other studies or reviews
A previous systematic review, Terry 2011, investigated the effect of ginger for treating pain. However, as it did not include any RCTs, comparisons with this current review do not appear relevant. Another systematic review, Mirabi 2014, included RCTs of medicinal herbs for dysmenorrhoea. In common with this current review, Mirabi 2014 found promising evidence for the use of medicinal herbs, but noted that the evidence was limited by methodological flaws.
Authors' conclusions
Implications for practice.
There is no high quality evidence to support the effectiveness of any dietary supplement for dysmenorrhoea, and evidence of safety is lacking. However for several supplements there was some low quality evidence of effectiveness and more research is justified.
Participants in the included studies may be unrepresentative of all populations of women with dysmenorrhoea. Also, data were too scanty to reach any conclusions about the safety of supplements. Therefore the results of this Cochrane review should be viewed with caution.
Implications for research.
Further research is needed on the short‐term and long‐term effectiveness and safety of dietary supplements for treatment of primary and secondary dysmenorrhoea.
What's new
| Date | Event | Description |
|---|---|---|
| 24 February 2016 | New search has been performed | This Cochrane review was published in 2001 as Herbal and dietary therapies for primary and secondary dysmenorrhoea (Proctor 2001) and included seven studies. In this review update, we changed the title to Dietary supplements for dysmenorrhoea. We revised the rationale and background, and also reviewed and updated the definition of dietary supplement. We included 26 new studies, excluded five of the previously included studies, and added a 'Summary of findings' table. |
| 24 February 2016 | New citation required and conclusions have changed | This is an update of a previously published Cochrane review. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 3 May 2011 | New search has been performed | The MDSG performed a new search on 3 May 2011. |
| 7 April 2010 | New search has been performed | We updated the search and identified five new studies in June 2009; subsequently we reran the search in April 2010 and identified three new studies. We excluded three studies from the original review due to their study design. |
| 12 November 2008 | Amended | We converted to a new review format. |
| 13 February 2001 | New citation required and conclusions have changed | We made a substantive amendment to the review. |
Acknowledgements
For this 2016 update, we thank Rana Taghipouran from the University of Auckland who very kindly helped with the Persian translations to help us ascertain study eligibility.
We acknowledge Michelle Proctor and Patricia A Murphy as authors of the original version of this Cochrane review, Rachel Heslop for her kind assistance in accessing one study, and the other review authors before we adopted this Cochrane review.
We are grateful to the Cochrane Gynaecology and Fertility Group for its support, especially Marian Showell, Trials Search Co‐ordinator, and Helen Nagels, Managing Editor.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group Specialised Register search strategy
MDSG search strings for MW524 23.03.15
Keywords CONTAINS "dysmenorrhea" or "Dysmenorrhea‐Symptoms" or "dysmenorrhoea" or "pelvic pain" or "Pain‐abdominal" or "pain‐dysmenorrhea" or "pain‐dyspareunia" or "pain‐pelvic" or "menstrual cramps" or "menstrual distress" or "menstrual pain" or "dyspareunia" or Title CONTAINS"dysmenorrhea" or "Dysmenorrhea‐Symptoms" or "dysmenorrhoea" or "pelvic pain" or "Pain‐abdominal" or "pain‐dysmenorrhea" or "pain‐dyspareunia" or "pain‐pelvic" or "menstrual cramps" or "menstrual distress" or "menstrual pain" or "dyspareunia"
AND
Keywords CONTAINS "herba epimedii" or "herbal preparations" or "herbal remedy" or "herbal supplement" or "herbal supplements" or "plant extracts" or "diet" or "Diet Supplementation" or "diet therapy" or "dietary supplement" or "dietary intervention" or "complementary therapy" or "Homeopathy" or "phytotherapy" or "Magnesium" or "fatty acids" or "cimicifuga" or "Cimicifuga racemosa" or "black cohosh" or "Vitex agnus castus" or "vitamin" or "vitamin E" or "chasteberry " or "antioxidant"or "antioxidants" or Title CONTAINS"herba epimedii" or "herbal preparations" or "herbal remedy" or "herbal supplement" or "herbal supplements" or "plant extracts" or "diet" or "Diet Supplementation" or "diet therapy" or "dietary supplement" or "dietary intervention" or "complementary therapy" or "Homeopathy" or "phytotherapy" or "Magnesium" or "fatty acids" or "cimicifuga" or "Cimicifuga racemosa" or "black cohosh" or "Vitex agnus castus" or "vitamin" or "vitamin E" or "chasteberry " or "alternative therapy"
Appendix 2. CENTRAL search strategy
Searched 23 March 2015 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp pelvic pain/ or exp dysmenorrhea/ (582) 2 (pelvi$ adj3 pain).tw. (618) 3 dysmenorrh$.tw. (793) 4 (pain$ adj5 menstrua$).tw. (250) 5 (pain$ adj5 period$).tw. (2345) 6 (menstrua$ adj3 pain$).tw. (211) 7 (menstrua$ adj3 cramp$).tw. (26) 8 or/1‐7 (3770) 9 exp Homeopathy/ (178) 10 homoeopath$.tw. (117) 11 herbal medicine$.tw. or exp Medicine, Herbal/ (689) 12 herbal therapy.tw. or exp Phytotherapy/ (3003) 13 medicinal plant$.tw. or exp Plants, Medicinal/ (898) 14 naturopathy.tw. or exp Naturopathy/ (26) 15 phytotherapy.tw. (50) 16 Magnesium/ or magnesium.tw. (3381) 17 manganese.tw. or Manganese/ (153) 18 dietary supplement$.tw. or exp Dietary Supplements/ (7358) 19 exp Vitamins/ or nutritional supplement$.tw. (11957) 20 (protein or fatty acids).tw. (27199) 21 Cimicifuga racemosa.tw. or Cimicifuga/ (56) 22 black cohosh.tw. (56) 23 Centaurea cyanus.tw. (0) 24 echinacea.mp. or Echinacea/ (98) 25 Vitex agnus.tw. (22) 26 Plant Extracts/ or Vitex agnus.tw. (2551) 27 chaste tree.tw. or exp Plant Preparations/ (7417) 28 Viscum album.tw. or Viscum album/ (33) 29 mistletoe.tw. or Mistletoe/ (69) 30 Origanum majorana.mp. or Origanum/ (5) 31 majoram.mp. (0) 32 Potentilla/ or Potentilla anserina.mp. (0) 33 Ruta graveolens.mp. or Ruta/ (5) 34 rue.mp. (4) 35 Lamium album.mp. (2) 36 vitamin$.tw. (11481) 37 exp antioxidants/ or exp free radical scavengers/ (10287) 38 antioxidant$.tw. (4598) 39 exp Diet/ or exp Diet Therapy/ or exp Diet, Vegetarian/ or exp Diet, Macrobiotic/ (11929) 40 diet$.tw. (27498) 41 or/9‐40 (83637) 42 randomized controlled trial.pt. (351235) 43 controlled clinical trial.pt. (84610) 44 randomized.ab. (220247) 45 placebo.tw. (141732) 46 clinical trials as topic.sh. (33063) 47 randomly.ab. (110679) 48 trial.ti. (134981) 49 (crossover or cross‐over or cross over).tw. (48224) 50 or/42‐49 (585556) 51 exp animals/ not humans.sh. (4) 52 50 not 51 (585554) 53 8 and 41 and 52 (216) 54 limit 53 to yr="2014 ‐Current" (18)
Appendix 3. Ovid MEDLINE search strategy
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to 23 March 2015> ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp pelvic pain/ or exp dysmenorrhea/ (6651) 2 (pelvi$ adj3 pain).tw. (6958) 3 dysmenorrh$.tw. (4295) 4 (pain$ adj5 menstrua$).tw. (1235) 5 (pain$ adj5 period$).tw. (4747) 6 (menstrua$ adj3 pain$).tw. (940) 7 (menstrua$ adj3 cramp$).tw. (145) 8 or/1‐7 (17874) 9 exp Homeopathy/ (4148) 10 homoeopath$.tw. (656) 11 herbal medicine$.tw. or exp Medicine, Herbal/ (9147) 12 herbal therapy.tw. or exp Phytotherapy/ (31175) 13 medicinal plant$.tw. or exp Plants, Medicinal/ (57992) 14 naturopathy.tw. or exp Naturopathy/ (997) 15 phytotherapy.tw. (888) 16 Magnesium/ or magnesium.tw. (86217) 17 manganese.tw. or Manganese/ (36008) 18 dietary supplement$.tw. or exp Dietary Supplements/ (50445) 19 exp Vitamins/ or nutritional supplement$.tw. (270922) 20 (protein or fatty acids).tw. (1971310) 21 Cimicifuga racemosa.tw. or Cimicifuga/ (502) 22 black cohosh.tw. (367) 23 Centaurea cyanus.tw. (19) 24 echinacea.mp. or Echinacea/ (992) 25 Vitex agnus.tw. (116) 26 Plant Extracts/ or Vitex agnus.tw. (73726) 27 chaste tree.tw. or exp Plant Preparations/ (152684) 28 Viscum album.tw. or Viscum album/ (549) 29 mistletoe.tw. or Mistletoe/ (1088) 30 Origanum majorana.mp. or Origanum/ (486) 31 majoram.mp. (2) 32 Potentilla/ or Potentilla anserina.mp. (137) 33 Ruta graveolens.mp. or Ruta/ (239) 34 rue.mp. (276) 35 Lamium album.mp. (10) 36 vitamin$.tw. (155044) 37 exp antioxidants/ or exp free radical scavengers/ (359391) 38 antioxidant$.tw. (120169) 39 exp Diet/ or exp Diet Therapy/ or exp Diet, Vegetarian/ or exp Diet, Macrobiotic/ (208881) 40 diet$.tw. (402399) 41 or/9‐40 (3109887) 42 randomized controlled trial.pt. (387707) 43 controlled clinical trial.pt. (88879) 44 randomized.ab. (312629) 45 placebo.tw. (163685) 46 clinical trials as topic.sh. (171490) 47 randomly.ab. (226095) 48 trial.ti. (134758) 49 (crossover or cross‐over or cross over).tw. (63083) 50 or/42‐49 (964381) 51 exp animals/ not humans.sh. (4003250) 52 50 not 51 (888169) 53 8 and 41 and 52 (210) 54 (2014$ or 2015$).ed. (1166108) 55 (2014$ or 2015$).dp. (1244597) 56 54 or 55 (1773742) 57 53 and 56 (40)
Appendix 4. EMBASE search strategy
Database: Embase <1980 to 2015 Week 12> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 dysmenorrh$.tw. or exp DYSMENORRHEA/ (9282) 2 (pain$ adj4 menstrua$).tw. (1393) 3 (pain$ adj4 period$).tw. (4576) 4 or/1‐3 (14469) 5 exp Homeopathic Agent/ or exp Homeopathy/ or homeopath$.tw. (10086) 6 herbal medicine$.tw. or exp Herbal Medicine/ (21915) 7 exp Plant Extract/ or exp Herbaceous Agent/ or herbal therap$.tw. (181611) 8 medicinal plant$.tw. or exp Medicinal Plant/ (161807) 9 naturopath$.tw. (1106) 10 (magnesium or manganese).tw. (73663) 11 diet$.tw. or exp Diet Supplementation/ (508358) 12 nutritional supplement$.tw. or exp Vitamin/ (480563) 13 vitamin$.tw. (186051) 14 (protein or fatty acids).tw. (2178665) 15 exp Cimicifuga Racemosa/ or exp Cimicifuga/ or exp Cimicifuga Racemosa Extract/ (1119) 16 Cimicifuga.tw. (531) 17 black cohosh.tw. (535) 18 Centaurea cyanus.tw. (26) 19 exp ECHINACEA ANGUSTIFLORA EXTRACT/ or exp ECHINACEA PURPUREA/ or exp ECHINACEA/ or exp ECHINACEA PALLIDA EXTRACT/ or exp ECHINACEA EXTRACT/ or exp ECHINACEA PURPUREA EXTRACT/ (2347) 20 echinacea.tw. (1345) 21 Vitex agnus.tw. (235) 22 exp plant extract/ (152040) 23 Plant Extract$.tw. (10170) 24 phytotherap$.tw. or exp PHYTOTHERAPY/ (16165) 25 chaste tree.tw. (50) 26 [or/5‐38] (0) 27 [or/41‐58] (0) 28 [or/60‐62] (0) 29 dysmenorrh$.tw. or exp DYSMENORRHEA/ (9282) 30 (pain$ adj4 menstrua$).tw. (1393) 31 (pain$ adj4 period$).tw. (4576) 32 or/29‐31 (14469) 33 exp Homeopathic Agent/ or exp Homeopathy/ or homeopath$.tw. (10086) 34 herbal medicine$.tw. or exp Herbal Medicine/ (21915) 35 exp Plant Extract/ or exp Herbaceous Agent/ or herbal therap$.tw. (181611) 36 medicinal plant$.tw. or exp Medicinal Plant/ (161807) 37 naturopath$.tw. (1106) 38 (magnesium or manganese).tw. (73663) 39 diet$.tw. or exp Diet Supplementation/ (508358) 40 nutritional supplement$.tw. or exp Vitamin/ (480563) 41 vitamin$.tw. (186051) 42 (protein or fatty acids).tw. (2178665) 43 exp Cimicifuga Racemosa/ or exp Cimicifuga/ or exp Cimicifuga Racemosa Extract/ (1119) 44 Cimicifuga.tw. (531) 45 black cohosh.tw. (535) 46 Centaurea cyanus.tw. (26) 47 exp ECHINACEA ANGUSTIFLORA EXTRACT/ or exp ECHINACEA PURPUREA/ or exp ECHINACEA/ or exp ECHINACEA PALLIDA EXTRACT/ or exp ECHINACEA EXTRACT/ or exp ECHINACEA PURPUREA EXTRACT/ (2347) 48 echinacea.tw. (1345) 49 Vitex agnus.tw. (235) 50 exp plant extract/ (152040) 51 Plant Extract$.tw. (10170) 52 phytotherap$.tw. or exp PHYTOTHERAPY/ (16165) 53 chaste tree.tw. (50) 54 exp Plant Medicinal Product/ (1047268) 55 exp Viscum Album/ (1480) 56 Viscum album.tw. (757) 57 mistletoe.tw. or Mistletoe/ (1986) 58 Origanum majorana.tw. or Origanum/ (280) 59 majoram.tw. (5) 60 Potentilla/ or Potentilla anserina.tw. (208) 61 Ruta graveolens.tw. or Ruta/ (320) 62 rue.tw. (362) 63 Lamium album.tw. (18) 64 exp antioxidant/ (107597) 65 antioxidant$.tw. (154719) 66 exp diet/ (212209) 67 or/33‐66 (4047228) 68 32 and 67 (1692) 69 Clinical Trial/ (838864) 70 Randomized Controlled Trial/ (362270) 71 exp randomization/ (65232) 72 Single Blind Procedure/ (19622) 73 Double Blind Procedure/ (118316) 74 Crossover Procedure/ (41811) 75 Placebo/ (252691) 76 Randomi?ed controlled trial$.tw. (111049) 77 Rct.tw. (16193) 78 random allocation.tw. (1380) 79 randomly allocated.tw. (21730) 80 allocated randomly.tw. (1995) 81 (allocated adj2 random).tw. (719) 82 Single blind$.tw. (15348) 83 Double blind$.tw. (147829) 84 ((treble or triple) adj blind$).tw. (424) 85 placebo$.tw. (209475) 86 prospective study/ (279059) 87 or/69‐86 (1429105) 88 case study/ (30774) 89 case report.tw. (274945) 90 abstract report/ or letter/ (915984) 91 or/88‐90 (1215577) 92 87 not 91 (1390368) 93 68 and 92 (491) 94 (2014$ or 2015$).em. (1974963) 95 (2014$ or 2015$).dp. (201946) 96 94 or 95 (1977129) 97 93 and 96 (65)
Appendix 5. PsycINFO search strategy
Database: PsycINFO <1806 to March Week 3 2015> ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp dysmenorrhea/ (178) 2 (pelvi$c adj3 pain).tw. (450) 3 dysmenorrh$.tw. (335) 4 (pain$ adj5 menstrua$).tw. (221) 5 (pain$ adj5 period$).tw. (780) 6 (menstrua$ adj3 pain$).tw. (174) 7 (menstrua$ adj3 cramp$).tw. (19) 8 or/1‐7 (1700) 9 homeopath$.tw. (359) 10 herbal medicine$.tw. (413) 11 herbal therap$.tw. (68) 12 medicinal plant$.tw. (161) 13 naturopathy.tw. (36) 14 magnesium.tw. (1015) 15 manganese.tw. (522) 16 dietary supplement$.tw. (691) 17 nutritional supplement$.tw. (411) 18 (protein or fatty acids).tw. (47340) 19 Cimicifuga.tw. (7) 20 black cohosh.tw. (24) 21 Centaurea cyanus.tw. (0) 22 Echinacea.tw. (17) 23 Vitex agnus.tw. (13) 24 chaste tree.tw. (4) 25 Viscum album.tw. (1) 26 mistletoe.tw. (21) 27 Origanum majorana.tw. (2) 28 majoram.tw. (1) 29 Potentilla anserina.tw. (0) 30 Ruta graveolens.tw. (0) 31 rue.tw. (88) 32 Lamium album.tw. (0) 33 exp Diets/ or exp Dietary Supplements/ or exp Lipids/ or exp Carbohydrates/ (21243) 34 exp food/ or exp nutrition/ or exp weight control/ (20077) 35 exp "medicinal herbs and plants"/ or exp "plants (botanical)"/ or exp hypericum perforatum/ (2478) 36 exp vitamins/ or exp antioxidants/ or exp vitamin therapy/ (5530) 37 or/9‐36 (89651) 38 8 and 37 (41) 39 limit 38 to yr="2014 ‐Current" (4)
Appendix 6. AMED search strategy
Database: AMED (Allied and Complementary Medicine) <1985 to March 2015> ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 exp dysmenorrhea/ (106) 2 (pelvi$c adj3 pain).tw. (185) 3 dysmenorrh$.tw. (169) 4 (pain$ adj5 menstrua$).tw. (44) 5 (pain$ adj5 period$).tw. (435) 6 (menstrua$ adj3 pain$).tw. (37) 7 (menstrua$ adj3 cramp$).tw. (12) 8 or/1‐7 (797) 9 exp homeopathy/ or exp naturopathy/ (12969) 10 exp Antioxidants/ or exp Vitamins/ or exp Dietary supplements/ or exp Nutrition/ or exp Plants medicinal/ or exp Minerals/ (26042) 11 homeopath$.tw. (16076) 12 herbal medicine$.tw. (1184) 13 herbal therap$.tw. (100) 14 medicinal plant$.tw. (1744) 15 naturopathy.tw. (1100) 16 magnesium.tw. (235) 17 manganese.tw. (28) 18 dietary supplement$.tw. (1358) 19 nutritional supplement$.tw. (207) 20 (protein or fatty acids).tw. (2907) 21 Cimicifuga.tw. (84) 22 black cohosh.tw. (49) 23 Centaurea cyanus.tw. (1) 24 Echinacea.tw. (222) 25 Vitex agnus.tw. (29) 26 chaste tree.tw. (6) 27 Viscum album.tw. (222) 28 mistletoe.tw. (180) 29 Origanum majorana.tw. (12) 30 majoram.tw. (1) 31 Potentilla anserina.tw. (1) 32 Ruta graveolens.tw. (33) 33 rue.tw. (16) 34 Lamium album.tw. (3) 35 or/9‐34 (45487) 36 8 and 35 (81) 37 limit 36 to yr="2014 ‐Current" (3)
Appendix 7. Data extraction form
MW524 Data collection form
Notes: [Note here whether study eligible for inclusion. If excluded, note reason for exclusion]
1. General Information
Name/date of person extracting data
Study funding sources/COIs
(including role of funders)
Trial reg no
2. Study Eligibility
Review eligibility criteria:
Check whether study meets these criteria Yes/no Location in text (optional)
Type of study RCT
Participants
Inclusion criteria:
• women of reproductive age;
• women with moderate to severe primary dysmenorrhoea (pain that does not respond well to analgesics, affects daily activity or has a high baseline score on a validated pain scale) or women with secondary dysmenorrhoea of identifiable pathology. Trials where the severity of dysmenorrhoea was not formally assessed were included if the potential participants had sought medical advice for the perceived pain;
• women experiencing dysmenorrhoea in the majority of menstrual cycles.
Exclusion criteria:
• women with mild dysmenorrhoea (mild pain that responded to analgesics);
• women with irregular or infrequent menstrual cycles (outside of the typical range of a 21 to 35‐day cycle);
• women using an intrauterine contraceptive device (IUD) or taking oral contraceptive pills (OCPs).
Types of intervention
Included
· Dietary supplements in the treatment group versus placebo or no treatment; or against each other or any other conventional treatment.
Excluded
RCTs reporting the use of Chinese medicinal herbs
Types of outcome measure
Primary
1. Pain (measured either by a visual analogue scale (VAS), other validated scales, or as a dichotomous outcomes)
2. Adverse effects from treatment (incidence and duration of side effects and types of side effects)
Secondary
3. Requirements for additional medication
4. Restriction of daily life activities
5. Absence from work or school
Symptoms of dysmenorrhoea (might add as outcome in future)
3. Study characteristics
1. Design: crossover or parallel group
2. Publication status
3. Country
4. Primary or secondary dysmenorrhoea
5. Comparison
Location in text (pg & ¶/fig/table)
Notes: This is brief info to go in main text under Included studies
4. Risk of Bias assessment
See Chapter 8 of the Cochrane Handbook
Domain Risk of bias Justification
Random sequence generation:
Was the allocation sequence adequately generated?
(selection bias)
Allocation concealment:
Was allocation adequately concealed?
(selection bias)
Blinding (performance bias and detection bias)
Incompleteness of outcome data: State number randomised, and number included in analysis
Selective outcome reporting: Are reports of the study free of suggestion of selective outcome reporting?
(reporting bias)
Other bias e.g. baseline imbalance
Notes
5. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
Description as stated in report/paper Location in text (pg & ¶/fig/table)
Total no. randomised
Participant inclusion criteria Inclusion Exclusion
Age
Source
Location
Notes: This info to go in Characteristics of included studies table
6. Intervention groups
Copy and paste table for each intervention and comparison group
Description as stated in report/paper Location in text (pg & ¶/fig/table)
Description of intervention(s) and control
Group 1:
Group 2:
Dosing regimen:
Total daily dose
Notes: This info to go in Characteristics of included studies table
7. Outcomes & Results
Outcome name: 1a Pain (continuous)
Results
Group 1 Mean SD No participants; Group 2 Mean SD No participants
Data not suitable for analysis (e.g. p value, data missing standard deviations)
Notes/definitions: Define measure here
Outcome name: 1b Pain (dichotomous)
Results
Group 1 Mean SD No participants; Group 2 Mean SD No participants
Data not suitable for analysis (e.g. p value, findings reported only in text)
Notes/definitions: Define measure
Outcome name: 2. Adverse effects (specify type here)
Results
Group 1 Mean SD No participants; Group 2 Mean SD No participants
Data not suitable for analysis (e.g. p value, findings reported only in text)
Notes/definitions: Copy and paste as required
Outcome name (study definition): 3. Requirement for additional medication
Results
Group 1 Mean SD No participants; Group 2 Mean SD No participants
Data not suitable for analysis (e.g. p value, findings reported only in text)
Notes/definitions: Define measure here
Outcome name (study definition): 4. Restriction of daily life activities
Results
Group 1 Mean SD No participants; Group 2 Mean SD No participants
Data not suitable for analysis (e.g. p value, findings reported only in text)
Notes/definitions:
Outcome name (study definition): 5. Absence from home or school
Results
Group 1 Mean SD No participants; Group 2 Mean SD No participants
Data not suitable for analysis (e.g. p value, findings reported only in text)
Notes/definitions:
Outcome name: 6a. Symptoms of dysmenorrhoea (continuous)
NB This is not currently an outcome, but suggest collect data anyway in case it is added
Results Group 1 Mean SD No participants; Group 2 Mean SD No participants
Data not suitable for analysis (e.g. p value, data missing standard deviations)
Notes/definitions: Copy and paste as required
Outcome name: 6b. Symptoms of dysmenorrhoea (dichotomous)
NB This is not currently an outcome, but suggest collect data anyway in case it is added
Results
Group 1 Mean SD No participants; Group 2 Mean SD No participants
Data not suitable for analysis (e.g. p value, findings reported only in text)
Notes/definitions: Copy and paste as required
Data and analyses
Comparison 1. Dill seed versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain score 1st cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain score 2nd cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain relief | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Pain improvement 1st cycle | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Pain improvement 2nd cycle | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Fennel versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (multi‐dimensional 0 to 3) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain score 1st cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain score 2nd cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Fenugreek versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain score 1st cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain score 2nd cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Ginger versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10) | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Pain score 1st cycle | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain score 2nd cycle | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain relief | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Pain improvement 1st cycle | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 All adverse effects | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 First cycle | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Second cycle | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Guava leaf versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score 1st cycle (VAS 0 to 10) | 1 | 151 | Mean Difference (IV, Fixed, 95% CI) | 0.59 [‐0.13, 1.31] |
| 1.1 Guava 3 mg | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.84, 1.20] |
| 1.2 Guava 6 mg | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐0.02, 2.02] |
| 2 Pain score 2nd cycle (VAS 0 to 10) | 1 | 151 | Mean Difference (IV, Fixed, 95% CI) | 0.69 [‐0.05, 1.44] |
| 2.1 Guava 3 mg | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐0.64, 1.46] |
| 2.2 Guava 6 mg | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 0.98 [‐0.07, 2.03] |
| 3 Pain score 3rd cycle (VAS 0 to 10) | 1 | 151 | Mean Difference (IV, Fixed, 95% CI) | 0.66 [‐0.11, 1.42] |
| 3.1 Guava 3 mg | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.86, 1.28] |
| 3.2 Guava 6 mg | 1 | 78 | Mean Difference (IV, Fixed, 95% CI) | 1.13 [0.03, 2.23] |
Comparison 6. Valerian versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain score 1st cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain score 2nd cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Zataria versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief 3rd cycle | 1 | 99 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.66 [2.66, 16.72] |
| 1.1 Zataria 1% versus placebo | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.5 [1.53, 19.71] |
| 1.2 Zataria 2% versus placebo | 1 | 50 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.25 [2.18, 31.23] |
Comparison 8. Chamomile versus NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain score 1st cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain score 2nd cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Dill seed versus NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain score 1st cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain score 2nd cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Pain relief | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Pain improvement 1st cycle | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Pain improvement 2nd cycle | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Fennel versus NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Fennel versus mefenamic acid | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 11. Guava leaf versus NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score 1st cycle (VAS 0 to 10) | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | 1.19 [0.42, 1.96] |
| 1.1 Guava 3 mg | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.76 [‐0.35, 1.87] |
| 1.2 Guava 6 mg | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.58 [0.51, 2.65] |
| 2 Pain score 2nd cycle (VAS 0 to 10) | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | 1.01 [0.30, 1.73] |
| 2.1 Guava 3 mg | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.73 [‐0.28, 1.74] |
| 2.2 Guava 6 mg | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [0.29, 2.31] |
| 3 Pain score 3rd cycle (VAS 0 to 10) | 1 | 155 | Mean Difference (IV, Fixed, 95% CI) | 0.62 [‐0.12, 1.35] |
| 3.1 Guava 3 mg | 1 | 75 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.86, 1.20] |
| 3.2 Guava 6 mg | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.09 [0.03, 2.15] |
| 4 Adverse effects | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Abdominal pain or nausea | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12. Rhubarb versus NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain score 1st cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain score 2nd cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Pain score 3rd cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 13. Damask rose versus NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score in 1st cycle (VAS 0 to 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain score at 1 hour | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain score at 2 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Pain score at 3 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Pain score at 6 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Pain score at 12 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Pain score at 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Pain score at 48 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 14. Valerian versus NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain score after 2 cycles | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 15. Fennel extract versus vitamin E.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (multi‐dimensional 0 to 3) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain score 1st cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain score 2nd cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 16. Ginger versus zinc sulphate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain score 1st cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain score 2nd cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse effects | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 First cycle | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Second cycle | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 17. Fish oil versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain score 1st cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain score 2nd cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 18. Fish oil + vitamin B1 versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain score 1st cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain score 2nd cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 19. Melatonin versus placebo (secondary dysmenorrhoea).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Need for additional medications | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 20. Vitamin B1 versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain score 1st cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain score 2nd cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 21. Vitamin E versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10, multi‐dimensional 0 to 3) | 2 | 270 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.36, 0.12] |
| 1.1 Pain score 1st cycle | 2 | 135 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.34, 0.34] |
| 1.2 Pain score 2nd cycle | 2 | 135 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.59, 0.09] |
Comparison 22. Zinc sulphate versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Pain score 1st cycle | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐1.54, ‐0.36] |
| 1.2 Pain score 2nd cycle | 1 | 95 | Mean Difference (IV, Fixed, 95% CI) | ‐3.83 [‐4.43, ‐3.23] |
| 2 All adverse effects | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 First cycle | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Second cycle | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 23. Fish oil versus vitamin B1.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain score 1st cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain score 2nd cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 24. Fish oil + vitamin B1 versus fish oil.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain score 1st cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain score 2nd cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 25. Fish oil + vitamin B1 versus vitamin B1.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain score (VAS 0 to 10) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Pain score 1st cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pain score 2nd cycle | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdelmaeboud 2014.
| Methods | Cross‐over trial (a pilot phase III) | |
| Participants | Included: women aged between 18 and 28 years, regular cycles (21 to 35 days) with duration of 3 to 7 days, a history of at least 6 consecutive months of moderate to severe primary dysmenorrhoea as determined by the verbal rating scale (VRS, a 4‐point self‐rated verbal score: 0, none; 1, mild; 2, moderate; and 3, severe menstrual pain), with the pain lasting for at least 2 days and who required analgesia in each of the last 3 consecutive cycles, preceding study participation. Excluded: women who were planning to get married during the study; known or suspected secondary dysmenorrhoea and other causes of chronic pelvic pain, other use of drugs, other medical conditions (listed in publication) Age: median = 23 years; range: 25.5 to 24 years Source: Faculty of Medicine, Ain Shams University Location: Egypt 2011 to 2012 |
|
| Interventions | Group 1: 40 mg uzara tablets (roots of the South‐African uzara plant,Xysmalobium undulatum), 2 tablets per 8 hours, then 1 tablet per 8 hours beginning 2 days before the expected start of menstruation (N = 30) Group 2: 400 ibuprofen tablets, 1 tablet per 6 hours beginning 2 days before the expected start of menstruation (N = 30) Both groups continued for 5 days, and stopped treatment when there was mild or no pain 6 to 8 hours from the last dose. |
|
| Outcomes |
Primary
Secondary
|
|
| Notes | No first phase data were available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomisation sequence was computer generated and kept concealed by the first author who played no role in patients’ recruitment.” |
| Allocation concealment (selection bias) | Low risk | "The randomisation sequence was computer generated and kept concealed by the first author who played no role in patients’ recruitment." “Other co‐authors ‐indulged in patients’ recruitment and consenting to the study‐ were continuously updated regarding number of participants with assigned sequence. They collaborated together to allocate the next available number to each participant in order of her enrolment in the study. Subsequently, the first author was contacted and asked to release the sequence (order of drug intake, uzara/ibuprofen or vice versa).” |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: the study authors did not mention blinding. In addition, blinding might not be accomplished as the difference of dose (2 tablets versus 1 tablets) and administrator. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol was available. Adverse effects were reported in both groups. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Akbari 2012.
| Methods | Randomised controlled trial (RCT) | |
| Participants | Included: students, being unmarried; with moderate or severe dysmenorrhoea according to the McGill pain rating scale; not known to have chronic diseases; had regular intervals of 21 to 35 days for menstrual periods; no history of myoma, pelvic tumour, endometriosis, and PID; no symptoms such as burning, itching, and abnormal discharge during the study; no use of special drugs; no history of allergy to fenugreek or other plants, and not taking herbal medicines during 3 months before intervention. Excluded: allergic to fenugreek seed during intervention; incorrectly taking the capsules; taking any other herbal medicine during intervention and taking less than 4 capsules daily. Students who had irregular menstrual cycles, endometriosis, history of medication usage, experienced acute stress, and/or had vaginal symptoms (burning, irritation, itching, or discharge) Age: mean age of fenugreek group = 19.86 years; mean age of placebo group = 20.0 years Source: Shahid Beheshty University of Medical Sciences Location: Iran |
|
| Interventions | Group 1: fenugreek seeds capsule content of 900 mg seed powder prescribed (N = 53, 51 analysed) Group 2: placebo starch (similar capsule with same recipe content starch; 500 mg) (N = 53, 50 analysed) The intervention group took 2 to 3 capsules 3 times per day, for the first 3 days of their menstruation for two consecutive menstrual cycles (a daily dose of 5400 mg to 8100 mg). |
|
| Outcomes |
|
|
| Notes | There were 2 sources information for this study: a conference abstract (poster presentation) and the full‐text article published in 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Computer generated random numbers were used to divide participants into two groups for receiving fenugreek or placebo.” |
| Allocation concealment (selection bias) | Unclear risk | “Participants and researchers were kept blinded to treatment allocation” Comment: there were insufficient details on how to keep participants and researchers blinded to treatment allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “Fenugreek seed's capsule content of 900 mg seed powder prescribed 3 times a day and 2‐ 3 capsules duration first three days of menstrual period and for persons in control group prescribed placebo (similar capsule with same recipe content starch) in two cycles.” “The capsules were similar with respect to shape, colour, and packaging.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Unmarried Students were randomly assigned to two groups who received fenugreek (n=51) or placebo (n=50).” “106 individuals were enrolled in the study. The final analysis involved 101 students, 51 of whom received fenugreek and 50 received placebo.” |
| Selective reporting (reporting bias) | Unclear risk | “Systemic symptoms of dysmenorrhoea (fatigue, headache, nausea, vomiting, lack of energy, syncope) decreased in the fenugreek seed group (p<0.05). No side effects were reported in the fenugreek group.” Comment: it is unclear whether or not the study authors collected data on adverse effects systematically in both groups. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Akhavan Amjadi 2009.
| Methods | Parallel‐group RCT 47 women analysed, unclear how many randomised |
|
| Participants | Included: single women with moderate to severe primary dysmenorrhoea. Regular periods cycles and with medium to severe pain Excluded: women with any known disease, signs of vaginal infection, history of pelvic inflammatory disease, myoma or tumour, drug or plant allergies, stressful incidence (parental divorce, or relative deaths) in the past 6 months or during the treatment. Incomplete questionnaire or missed a dose Age: 18 to 30 years (mean 20.96) Source: student population Location: Iran, Gilan University |
|
| Interventions | Group 1: cinnamon powder (26 analysed) Group 2: placebo (21 analysed) Dosing regimen: 420 mg Total daily dose: 5 capsules per day up to 3 days after pain started for 2 consecutive menstrual cycles |
|
| Outcomes |
|
|
| Notes | The review author Vahid Seyfoddin (VS) translated this article from Persian to English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the method was unclear (study authors reported a formula). |
| Allocation concealment (selection bias) | Unclear risk | Comment: the method of allocation concealment was not reported. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: triple blinded (patients, clinicians, and statisticians were all blinded). The placebo and cinnamon capsule were completely identical and were manufactured by the same company. They were packed in an identical package and were distinguishable by a code on the packets. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the study authors did not state the initial number of participants randomised. The final number analysed was 47 (26 in cinnamon treated group and 21 in placebo). |
| Selective reporting (reporting bias) | Unclear risk | Comment: adverse effects were not reported. |
| Other bias | Low risk | Comment: we did not identify any other likely sources of bias. |
Bani 2014.
| Methods | Cross‐over trial | |
| Participants | Included: volunteer students with primary dysmenorrhoeal, age between 18 and 24, single, BMI = 19 to 25, a pain intensity score of 5 to 8 in the VAS, filled written consent, no history of abdominopelvic surgery, not prohibited from taking herbs and non‐steroidal anti‐inflammatory drugs (NSAIDs) (e.g. renal, hepatic, or gastrointestinal disease) Excluded: occurrence of a stressful event (e.g. bereavement), using drugs that might interact with NSAIDs, using oral contraceptive pills and lack of compliance Age: mean age of intervention group = 22.2 years; mean age of control group = 22.1 years Source: dormitories of Tabriz University of Medical Sciences Location: Iran |
|
| Interventions | Group 1: Rosa damascena extract capsule* 200 mg (N = 46) Group 2: mefenamic acid capsule (NSAIDs) 250 mg (N = 46) The participants in both groups were supposed to take one capsule every 6 hours during the first 3 days of menstruation (2 cycles) *The dried fruits of Rosa damascena were changed to powder by a mechanical grinder and the extraction was performed with ethanol 70% using the method of maceration |
|
| Outcomes |
|
|
| Notes | No wash out period We extracted data from the first phase (before cross‐over) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The random allocation was done by www. Random.org site with blocking method (size of blocks: 4 and 6).” |
| Allocation concealment (selection bias) | Low risk | “The assistant put the drugs of each number in an envelope with the respective number written on the envelope and 92 envelopes were given to the researcher to pass them to the corresponding participants.” |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “The capsules of Rosa damascena extract were produced with the same appearance, colour and odour as Mefenamic acid capsules” “During the study, the participants and the researcher were unaware of the drug” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “In the present study, two groups of 46 persons participated (92 persons). All of them entered the statistical analysis and no sample loss happened.” |
| Selective reporting (reporting bias) | Low risk | Comment: the study authors reported a priori outcomes. Adverse effects were reported in both groups. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Bokaie 2013.
| Methods | RCT | |
| Participants | Included: 18 to 25 years of age, living in a dormitory, non‐smoker, no systemic disease, not taking an oral contraceptive pill (OCP) and other hormonal and herbal drugs prior to and during the menstrual cycle, regular menstruation, and suffering from moderate to severe primary dysmenorrhoea Excluded: intolerance to fennel drop, no desire to take any of the treatments, and taking other NSAIDs during the study Age: mean age of fennel drop group = 21.07 years; control = 21.17 years Source: Roghaye female student dormitory of Shahid Sadoughi University of Medical Sciences Location: Iran |
|
| Interventions | Group 1: 25 drops of fennelin (Foeniculum vulgare) 2% every 6 hours (each 1 mL drop contained 15.5 mg antole), mefenamic acid capsule 250 mg every 6 hours, if necessary (N = 30, 29 analysed) Group 2: mefenamic acid capsule 250 mg every 6 hours, if necessary (N =30) Students were followed up for two menstruation cycles |
|
| Outcomes |
Primary
Secondary
|
|
| Notes | “Twenty students (70%) in the study group avoided trying this protocol because fennel essence had a bad taste and mefenamic was more comfortable to use” Comment: it is unclear what this means. The study author responded by personal communication, and it appears that 70% of participants did not want to continue using fennel after completion of the study due to the taste |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Every 18‐25‑year‑old student who suffered from primary dysmenorrhoea with moderate to severe intensity was randomly assigned to each of the two study groups by use of a random number table.” |
| Allocation concealment (selection bias) | Unclear risk | Comment: the study authors did not describe allocation concealment methods. |
| Blinding (performance bias and detection bias) All outcomes | High risk | “oral fennel drop”. Comment: blinding was not mentioned. In addition, it is not possible to blind the participant to treatment (liquid forms (drop) versus capsule). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Sixty college students suffering from primary dysmenorrhoea were randomly assigned to two groups”. “Twenty‑nine students in the study group and 30 students in the control group were followed up. One student discontinued the trial due to malodor and bad taste of fennel drop.” Comment: the dropout rate was 3.33% (1/30). |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study authors did not prespecify adverse events as an outcome. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Dolation 2010.
| Methods | RCT | |
| Participants | Included: 106 single female students living in a dormitory at Islamic Azad University, Zanjan, Iran, from 14 January to 21 June 21 2009, who experienced moderate–severe dysmenorrhoea Excluded: students with mild dysmenorrhoea (pain score 1 to 3), or who had a chronic medical condition, used medications, experienced acute stress, had irregular menstrual cycles, or had vaginal symptoms including burning, irritation, itching, or discharge Age: mean age of intervention group = 20.9 years; control group = 21.0 years Source: Islamic Azad University, Zanjan, Iran Location: Iran |
|
| Interventions | Group 1: valerian capsules that contained 255 mg powder of valerian root (N = 51 analysed) Group 2: placebo capsules that contained starch (N = 49 analysed) Both groups took the treatment 3 times daily for 3 days from the first day of menstruation. The intervention continued for 2 menstrual cycles (2 cycles) |
|
| Outcomes |
|
|
| Notes | This study was published in Persian in 2010 and in English in 2011. One review author, VS, translated the Persian version | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Computer generated random numbers were used to allocate the participants to receive either valerian or placebo.” |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment methods were not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “In a double‐blind, randomised, placebo controlled trial, 100 students were randomly assigned to receive valerian (n=49) or placebo (n=51).” “The present study was a double‐blind, randomised clinical trial.” “The participants and the researchers were blind as to who received valerian.” “The valerian capsules contained 255 mg powder of valerian root, whereas the placebo capsules contained starch. The capsules were similar in shape and packaging.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Allocated to receive valerian (n=53), Analyzed (n=51). Withdrawals: 1 loss to follow‐up – changed student dormitory, and 1 discontinued intervention (dizziness).” “Allocated to receive placebo (n=53), Analyzed (n=49). Withdrawals: 2 loss to follow‐up – perceived lack of effect, and 2 cramping and diarrhoea, nausea.” Comment: we found an unbalanced percentage of dropouts (treatment (2/53 = ˜3%) versus placebo (4/53 = ˜7%), but these numbers were small. Overall the dropout rate was 6/106 = 5.6%. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study authors did not report data on adverse effects for the control group. |
| Other bias | Unclear risk | Comment: one participant in the treatment group left the study because of dizziness, which might have been an adverse effect of the treatment. |
Doubova 2007.
| Methods | RCT | |
| Participants | Included: all subjects were aged between 17 and 25 years; had regular menstrual cycles; primary dysmenorrhoea, defined as regular pain since the first menstrual period (menarche) during at least 1 day of the menstruation, with no medical history of other gynaecological diseases; moderate to severe pain intensity, varying from 5.0 to 10.0 on the VAS; an absence of hormonal treatment, oral contraceptives or intrauterine devices. 192 participants met the inclusion criteria. Age: mean age 19 years. Source: National Autonomous University of Mexico (UNAM) Location: Mexico |
|
| Interventions | Group 1: 3 mg Psidii guajavae folium extract (1 mg of active ingredient in 300 mg extract) 1 capsule taken every 8 hours (N = 52) Group 2: 6 mg Psidii guajavae folium extract (1 mg of active ingredient in 300 mg extract) 2 capsules taken every 8 hours (N = 57) Group 3: one capsule of placebo taken every 8 hours (300 mg/day starch) (N = 42) Group 4: one capsule 400 mg ibuprofen taken every 8 hours (1200 mg/day) (N = 46) All treatments were for 5 days and started 24 hours before menstruation and for three consecutive cycles (3 cycles) |
|
| Outcomes |
|
|
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Previous informed consent subjects were randomly assigned to one of four treatment "groups". “After this first month, during the second consultation, each participant was randomly assigned to one of the treatment groups, through a table of codes that was ex profeso designed with random numbers.” |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details. Although only the investigators who manufactured the products knew the codes it is unknown if they were also the study investigators. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “The phyto‐drug, ibuprofen and placebo were manufactured, standardized and packaged at Phyto‐drugs Technology Research and Development Laboratory of the Mexican Institute of Social Security.” “All vials of medication (extract, placebo or ibuprofen) were identical and labelled with codes, which were known only to the investigators who manufactured the products.” Comment: participants were likely to be blinded as the interventions were standardised and packaged. All vials of medication were identical and labelled with codes, which were known only to the investigators who manufactured the products. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “During the study, 12 participants taking 3 mg/day extract (23.1%), 19 taking 6 mg/day extract (33.4%), 11 taking placebo (26.2%) and 12 taking ibuprofen (26%) abandoned the study. In all cases, the reason cited for abandonment was lack of time. No statistically significant differences in demographic characteristics, gynaecological history and characteristics of dysmenorrhoeal were observed between students who abandoned the study and those who completed it.” Comment: although the percentage of dropouts in the treatment (3 and 6 mg) (28.4%), placebo (26.2%), and ibuprofen groups were not small (26%), the study authors reported no difference between participants who left the study and those who remained. The overall dropout rate was 54/197 = 27.4%. |
| Selective reporting (reporting bias) | Low risk | Comment: the study reported a priori outcomes. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Ghodsi 2014.
| Methods | RCT | |
| Participants | Included: students with primary dysmenorrhoea and a pain score > 3 based on VAS, willing to take part in the study, aged between 18 to 23 years Excluded: mild dysmenorrhoea, student transferring to another university, unwilling to attend for the research, irregular uptake of capsules, any similar analgesic drug uptake, occurrence of unbearable side effects, and cases with the following diagnosis: pelvic inflammatory disease, endometriosis, and any pelvic tumour Age: mean age of fennel soft capsule group = 20.8 years, control group = 20.5 years Source: Islamic Azad University, Toyserkan Location: Iran |
|
| Interventions | Group 1: soft capsule fennel (30 mg) (every 4 hours, 3 days before menstruation until the 5th day and continued for 3 months) (N = 40) Group 2: no treatment (N = 40, 38 analysed) |
|
| Outcomes |
|
|
| Notes | We searched for more information regarding the McGill pain questionnaire. It consisted of 15 descriptors (11 sensory; 4 affective) which are rated on an intensity scale as: 0 = none, 1 = mild, 2 = moderate, or 3 = severe. (Scoring 0 to 45; 0 = no pain, 45 = severe pain) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Students with primary dysmenorrhoea and pain scoring higher than 3, based on visual analog scales (VAS) entered the study and were divided randomly into 2 equal groups of intervention (n =40) and control (n=40).” Comment: the study authors did not describe the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the study authors did not describe the method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | “The intervention group received 30 mg fennel capsules each 4 hours (8 am to 12 midnight) from about 3 days before menstruation till end of fifth day for 3 months. There was no treatment for control group and they did not receive any placebo.” Comment: it was not possible to blind the participant to treatment as the control group did not receive any treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Two participants of the control group were excluded due to unwillingness to continue participation in the study, so the control group consisted of 38.” Comment: the dropout rate was 2.5% (2/80)). |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study authors did not prespecify adverse event as an outcome. The study prespecified pain as an outcome but did not report any statistical data. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Gokhale 1996.
| Methods | RCT | |
| Participants | Included: 556 girls aged 12 to 21 years, with moderate to very severe spasmodic dysmenorrhoea Location: India |
|
| Interventions | Group 1: thiamine hydrochloride (vitamin B1) 100 mg orally daily for 90 days Group 2: placebo |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "using random tables...a randomised, double‐blind, placebo‐controlled study was carried out on 556 girls aged 12‐21 yr". |
| Allocation concealment (selection bias) | Unclear risk | "a randomised, double‐blind, placebo‐controlled study was carried out on 556 girls aged 12‐21 yr". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: participants and observers were blinded, and the placebo was identical. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: final outcomes were unobtainable for 24 girls, and the dropout rate was 4.3% (24/556). |
| Selective reporting (reporting bias) | Unclear risk | Comment: there were no comparative data on adverse events. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Heidarifar 2014.
| Methods | RCT | |
| Participants | Included: single and being educated in the Nursing and Midwifery School and Paramedical Faculty of Qom University of Medical Sciences in 2011 (Qom city is almost situated in the centre of Iran with about 150 km distance from Tehran). Excluded: participants with a history of pelvic or organic disorders; any known gastrointestinal, urogenital, hematological, or other systems disorders; irregular menstrual cycles; taking any drug; and previous sensitivity to NSAIDs or dill; or mildly dysmenorrhoeal. Age: the mean age of the dill group = 20.95 years, mefenamic acid group = 22.04 years, placebo group = 20.95 years Source: Nursing and Midwifery School and Paramedical Faculty of Qom University of Medical Sciences of Iran Location: Iran |
|
| Interventions | Group 1: dill capsules that contained 500 mg dill seed powder (treated 2 capsules orally q12h (totally 1000 mg q12h)) (N = 25, 23 analysed) Group 2: 250 mg mefenamic acid capsule (orally q12h) (N = 25, 24 analysed) Group 3: starch as placebo (500 mg q12h) (N = 25, 23 analysed) The participants were treated since 2 days before the beginning of their menstruation for 5 days. Participants were followed for two cycles (2 months) and asked to answer the questionnaires at the end of every cycle. |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “This double‐blind, randomised study was conducted among 75 female students between 18 and 28 years old.” Comment: the study authors did not describe the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | “The participants were allocated randomly into one of the following groups”. “In regarding the blinding process, the researchers and the participants were uninformed of allocating manner of each group and a third one that did not involve in analysing and interpreting, etc., allocated the participants in groups and allotted a code number to everyone.” Comment: there was insufficient detail regarding whether allocation sequence was concealed or not. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “All of the capsules were made by Boo Ali Research Center of Qom city and were completely similar in shape. In regarding the blinding process, the researchers and the participants were uninformed of allocating manner of each group and a third one that did not involve in analysing and interpreting”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Of 75 participants of this study, five of them did not continue the study due to fearing of its side‐effects; therefore we evaluated 70 students”. Comment: the dropout rate was 6.67% (5/75). |
| Selective reporting (reporting bias) | Unclear risk | “In assessing the side‐effects, in Dill group, two students reported menstrual changes as increasing in amount and duration of bleeding and one student reported gastrointestinal discomfort. In mefenamic acid group, menstrual changes and gastrointestinal discomfort were reported in one and two students, respectively. In placebo group, each of the mentioned side‐effects was only observed in one student.” Comment: the study authors did not prespecify adverse events and rate of satisfaction as the outcomes, but reported them in the text and table 3, respectively. In addition, the number of participants in each group was not entirely clear. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Hosseinlou 2014.
| Methods | RCT | |
| Participants | Included: women 13 to 18 years of age, single, suffering from dysmenorrhoea, with regular menstrual cycles, no other health problems (according to their medical history), and low dietary fish intake (not more than once per week). Excluded: no information Age: no information Source: high school female students in Urmia city Location: Iran |
|
| Interventions | Group 1: vitamin B1 tablet (100 mg/day) (N = 60) Group 2: fish oil pearl capsule (500 mg/day) (N = 60) Group 3: a mixture of both fish oil capsules and vitamin B1 (N = 60) Group 4: placebo (N = 60) Participants took treatments as a single dose starting at the beginning of the menstrual cycle and continued for 2 consecutive months |
|
| Outcomes |
The study requested participants to complete a detailed questionnaire that assessed their menstrual pain and duration 3 times: at the study start, 1 month, and 2 months after taking the drugs or placebo |
|
| Notes | We presume that all participants had mild or severe pain, as all groups had a VAS score of 7.39 to 7.59 at baseline | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The girls were randomly assigned to 1 of 4 schedules.” Comment: the study authors did not describe the methods of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the study authors did not describe the allocation concealment methods. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | “A double‐blind, randomised, placebo‐controlled study carried on 240 high schools female students with dysmenorrhea in Urmia city by dividing into four groups with 60 members.” “They received drug boxes each month, and for all of them, possible drug complications were described and asked them to mention any occurred complications.” Comment: it is unclear whether or not the appearance of the treatment to both groups was the same in all aspects (different form; tablet, capsule). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the study does not state how many participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study authors did not prespecify adverse events as an outcome. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Iravani 2009.
| Methods | Double‐blind, placebo‐controlled RCT | |
| Participants | Included: adolescents with mild, moderate or severe dysmenorrhoea (N = 36 in each group). Findings for pain reported separately for participants with moderate or severe dysmenorrhoea (N = 98) Age: aged 18 to 24 years Source: 108 adolescents with primary dysmenorrhoea Location: Iran |
|
| Interventions | Group 1: Zataria multiflora 1% (N = 32 with mild/severe pain) 25 drops q 4 hours orally when pain started Group 2: Z. multiflora 2% (N = 33 with mild/severe pain) 25 drops q 4 hours orally when pain started Group 3: placebo (N = 33 with moderate/severe pain) Paticipants were evaluated for 3 cycles. |
|
| Outcomes |
|
|
| Notes | Z. multiflora is also known as Shirazi thyme, and grows in the wild only in Iran, Pakistan, and Afghanistan. One review author, VS, translated this study from Persian to English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the study authors did not describe the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the study authors did not describe the method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: the study states double‐blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: the study reported findings for all participants. |
| Selective reporting (reporting bias) | Unclear risk | Comment: adverse effects were not reported. |
| Other bias | High risk | Comment: we read the numbers directly off the published graph. We based the extracted data on the assumption that none of the women with mild or no pain at baseline reported moderate or severe pain after intervention. |
Jenabi 2010.
| Methods | RCT | |
| Participants | Iranian study Source: 82 university students with primary dysmenorrhoea, with a pain severity score of about 3 on a 1 to 10 scale Location: Iran |
|
| Interventions | Group 1: chamomile tea (Matricaria recutita), 2 cups per day, 1 week prior to menstruation and first 5 days of menses for 3 months (N = 40) Group 2: control (N = 40) |
|
| Outcomes |
|
|
| Notes | Rana Taghipouran (RT) translated this study from Persian to English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the study authors did not describe the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the study authors did not describe the method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: the study was not blinded, and there was no placebo intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: the study included 80 participants included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: adverse effects were not reported. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Jenabi 2012.
| Methods | RCT | |
| Participants | Included: high school students with primary dysmenorrhoea, with pain score > 3 on 1 to 10 scale Source: preuniversity centres in Hamedan Location: Iran |
|
| Interventions | Group 1: Valeriana officinalis capsules of 250 mg (N = 54) Group 2: mefenamic acid capsules of 250 mg (N = 54) Both treatment groups took the treatment every 8 hours in the first 3 days of menstruation for a 2‐month period |
|
| Outcomes |
|
|
| Notes | RT translated this study from Persian to English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the study authors did not describe the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the study authors did not describe the method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: the study gave capsules "in a similar way" to both groups. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the study analysed 99/108 participants (92%: 3 in the valerian group and 4 in the mefenamic acid group were excluded from analysis due to a lack of medicine consumption). |
| Selective reporting (reporting bias) | Unclear risk | Comment: adverse effects were not reported clearly in both groups. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Jenabi 2013.
| Methods | RCT | |
| Participants | Included: 70 single female students with primary dysmenorrhoea at Toyserkan Azad University in western Iran. The subjects underwent general physical examinations and students with pain scoring higher than 3 on the VAS (a 10 cm vertical line; 0 (no pain) to 10 (pain as bad as it could be)) were included Excluded: mild dysmenorrhoea cases Age: mean age of the intervention group = 21.3 years, control group = 21.5 years Source: Toyserkan Azad University in western Iran Location: Iran |
|
| Interventions | Group 1: ginger powder capsule (500 mg) (N = 35) Group 2: placebo (N = 34) Both treatment groups took 3 capsules daily in the first menstruation cycles for 3 days (1st cycle) |
|
| Outcomes |
|
|
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The 70 students subsequently randomised into two groups using a table of random numbers.” |
| Allocation concealment (selection bias) | Unclear risk | Comment: the study authors did not describe the method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | “The ginger and placebo capsules were prepared by a pharmacist. Briefly, fresh ginger root was chopped into small pieces, baked at 60°C for 24 hours, and then ground into powder. Ginger powder was weighed and 500 mg of it was filled in each capsule. Excess powder was wiped off the capsule surface with a clean dry cloth. Both placebo and ginger capsules were packed in an envelope containing nine capsules.” Comment: it is unclear whether or not capsule appearance in both groups was the same in all aspects (e.g. colour). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “After one (2.8%) student from the placebo group withdrew herself, the group finally consisted of 34.” Comment: risk was low despite no reason given for one withdrawal case, and an unbalanced percent of dropouts; treatment (0%) versus placebo (2.8%), but the number who dropped out was small. The overall dropout rate was 1.4% (1/69). |
| Selective reporting (reporting bias) | Unclear risk | Comment: adverse effects were not systematically reported in both groups. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Kashanian 2013.
| Methods | RCT | |
| Participants | Included: single women from 18 to 25 years of age, whose dysmenorrhoea started at menarche, without any increase over time, regular menstrual cycles, and normal pelvic examination Excluded: women that had any pelvic and abdominal surgeries, prior genital infection or pelvic pain, used other sedatives during the cycle, history of psychological problems or drug use, abnormal or heavy bleeding, smoking or alcohol consumption, digestive problems, prolonged stress in family or job, known pelvic or uterine anomaly, abnormal ultrasound of uterus and ovaries, and vitamin E allergy Age: vitamin E group = 22.8 years, placebo = 23.5 years Source: randomised 120 women aged 18 to 25 with primary dysmenorrhoea, of whom only 94 finished the study and were analysed. One woman had mild pain (VAS < 3), 44 had moderate pain (VAS 3 to 7) and 49 had severe pain (VAS 7 to 10) Location: Iran |
|
| Interventions | Group 1: vitamin E 400 IU/day for 2 days before and 3 days after menses (total 5 days), for 2 cycles (N = 60, 42 analysed) Group 2: placebo (N = 60, 52 analysed) |
|
| Outcomes |
|
|
| Notes | One woman had only mild dysmenorrhoea (and it is unclear which group she was in) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: participants chose an envelope. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the study used sealed sequentially distributed envelopes labelled A, B, C, or D. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: outcome assessors and treating physicians were blinded. Vitamin E and placebo were identical in shape, colour, taste, and smell. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: only 42/60 participants in the intervention group and 52/60 of the control group completed the study. The dropout rate was 21.7% (26/120). |
| Selective reporting (reporting bias) | Unclear risk | Comment: adverse effects were not reported. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Kashefi 2014.
| Methods | RCT | |
| Participants | Included: 15 to 18 years of age; had regular menstrual cycles; experienced dysmenorrhoea during the first 3 days of menstrual bleeding; and obtained a score > 4 on the pain VAS Excluded: secondary dysmenorrhoea due to an underlying disease or disorder, and using hormonal medications, birth control pills, or pain relief medications Age: mean age = 17 years Source: high schools in Bojnurd, Iran Location: Iran |
|
| Interventions | Group 1: ginger powder (250 mg capsule) (N = 48) Group 2: zinc sulphate (220 mg capsule) (N = 56) Group 3: placebo (lactose capsule) (N = 46) The participants were instructed to take the capsules 3 times a day for 4 days, starting from the day before menstrual bleeding to the third day of menstrual bleeding for 2 consecutive cycles |
|
| Outcomes |
Participants completed the VAS every 24 hours during the intervention days for 2 consecutive cycles, collected after each intervention cycle |
|
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Then, 150 participants were randomly assigned to three study groups by using the random table.” |
| Allocation concealment (selection bias) | Unclear risk | Comment: the study authors did not describe allocation concealment methods. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “The capsules were identical in shape, package, and colour, and were coded by the pharmacologist. Neither the researcher nor the participants were informed of the type of ingredient in each capsule.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | “A total of 150 participants received intervention. Thirteen participants failed to complete the study. Thus, data from 137 participants were analysed based on the actual treatment received and the available follow up”. Comment: based on the text, the dropout rate was 8.67% (13/150). According to information on the flowchart, the dropout rate was 6.67% (10/150). The study authors did not give any reason for participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Comment: the study authors reported a priori outcomes. Adverse events were clearly reported. |
| Other bias | Unclear risk | “The first group (n=48) received capsules containing zinc sulfate, the second group (n=56) received capsules containing ginger, and the third group (n=46) received placebo capsules.” Comment: there was inconsistency of the number of participants in each group between the study text and flowchart of participants. |
Khorshidi 2003.
| Methods | Cross‐over trial | |
| Participants | Included: 60 women, unmarried, outpatients females aged 17 to 25 years who showed the following conditions participated in the study:
Excluded: no information Age: mean = 21.05 years (range 17 to 25 years) Source: 60 people were chosen between total volunteers Location: Iran |
|
| Interventions | Group 1: fennel essential oil 1%, 0.3 to 1 mL Group 2: fennel essential oil 2%, 0.3 to 1 mL Group 3: placebo The trial medicines were administered as soon as the participants felt pain. The participants took all subsequent doses on an “as needed” basis, depending on the pain severity. These doses were not administered at intervals less than 4 hours |
|
| Outcomes |
|
|
| Notes | Rescue medication (usual therapy for the participants' symptoms) was permitted after 1 hour of administration, if the trial medication was not effective to control the symptoms ie If there was no relief of pain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “A randomised, double blind study comparing fennel essential oil (FEO) 1% and 2% with placebo, according to a 3 period crossover design was carried out on eligible patients that randomly allocated to 1 of the 6 treatment sequences according to the randomizations list”. Comment: there was insufficient information about the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | “Thirty percent of this people had grade 2 intensity of primary dysmenorrhoea and the rest had grade 3. They were allocated in one of the 6 treatment sequences randomly.” Comment: there were insufficient details on how to maintain blinding to treatment allocation. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | “A randomised, double blind study comparing FEO 1% and 2% with placebo”. Comment: there was no information on either the form of placebo, or the appearance of FEO and placebo. The study may not have accomplished blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “During period of treatment, four individuals in the placebo group, two in 1% FEO treated group and one in 2% FEO treated group were rejected from the treatment program. Six people in first two groups discontinued the protocol due to unsatisfactory therapeutic response and one person in the latter group discontinued due to poor tolerability.” Comment: the dropout rate was 23.3% (14/60). |
| Selective reporting (reporting bias) | Unclear risk | Comment: there was no indication that adverse effects were prospectively reported. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of biases. |
Modaress 2011.
| Methods | Cross‐over trial | |
| Participants | Included: primary dysmenorrhoea, 160 single students, with moderate or severe pain at least in their last 2 cycles Excluded: previous surgery in the hip/abdomen area, antibiotic intake 48 hours prior to the studied cycle and during treatment, intake of benzodiazepam (eg lorazepam, diazepam), barbiturates (phenobarbitals), narcotics, antidepressants (fluoxetine), also alcohol, aspirin, warfarin, and heparin due to pharmacological interactions. Known liver, kidney disease, or depression. Or if the participants took another drug without consultations or did not complete the questionnaire. Age: range of 20 to 30 years, average was 24.72 years (standard deviation (SD) = 2.55) Source: Tehran University students Location: Iran |
|
| Interventions | Group 1: Matricaria chamomilla 400 mg capsules same dosage. Total daily dose: mefenamic acid 1000 mg, chamomile: 1600 mg (N = 80) Group 2: mefenamic acid 250 mg capsule, 4 capsules per day for 3 days in each cycle (N = 80) |
|
| Outcomes |
|
|
| Notes | One review author, VS, translated this study from Persian to English. We used first cycle data Matricaria chamomilla is German chamomile. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: participants had to randomly select an unknown code which represented one of the drugs (A: mefenamic acid, B: chamomile). The study authors gave insufficient information about the sequence generation process. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the study authors did not describe the method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: both capsules were identical and packed in similar packages and were only identifiable by a code. Both clinicians and participants were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the state number was randomised, and the number was included in the analysis. Eighty people per group finished the study, but the study authors did not mention the initial number of participants recruited. |
| Selective reporting (reporting bias) | Unclear risk | Comment: adverse effects were not reported. |
| Other bias | Low risk | Comment: we did not identify any other likely sources of bias. |
Moslemi 2012.
| Methods | RCT | |
| Participants | Included: female medical students with regular menstrual cycles at the Sari Branch of Azad University who suffered, based on multi‐dimensional speech criteria, from mild or acute dysmenorrhoea Excluded: any known disease, signs of vaginal infection, history of pelvic inflammatory disease, myoma or tumour, drug or plant allergies, stressful incidence (parental divorce, or relative deaths) in the past 6 months or during the treatment Age: mean of fennel group = 25.05 years, vitamin E group = 23.25 years, and placebo group = 25.9 years Source: the Sari Branch of Azad University students Location: Iran |
|
| Interventions | Group 1: fennel extract (46 mg of hydro‐alcoholic fennel fruit extract mixed with starch) (N = 25, 22 analysed) Group 2: vitamin E (the 100‐unit vitamin E capsules) (N = 25, 20 analysed) Group 3: placebo (N = 25, 21 analysed) Participants took the pills every 6 hours for 3 days after their menstruation started for 2 consecutive menstrual cycles. (Total daily dose: vitamin E: 400 mg; fennel: 184 mg (each tablet contain 46 mg) |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Each student was placed randomly in one of three groups – vitamin E, fennel extract or placebo (each group consisted of 25 individuals) – by means of 1:1: 1 randomness and the table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | "The 100‐unit vitamin E capsules were purchased from local drugstores, and fennel extract capsules called Fenalgin and placebos with completely identical appearances and special coding for researchers were provided by Barij Essence Pharmaceutical Company." Comment: the study authors provided insufficient information regarding the allocation concealment method. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Drugs and placebos in exactly identical covering with codes only known to the researchers were given to the study samples." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Seventy‐five female individual were selected for this study". "At the end, individuals who failed to take the drugs regularly or lost one of the conditions needed to qualify for the research were removed from the study. Eventually, 63 individuals remained in the study (22 in the fennel extract group, 20 in the vitamin E group and 21 in the placebo group)." Comment: the dropout rate was 16% (12/75). |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study authors did not prespecify adverse events as an outcome. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Nasehi 2013.
| Methods | Cross‐over trial (no wash‐out period) | |
| Participants | Included: participants with a history of primary dysmenorrhoeal, with regular menses in the last 3 months prior to the first visit, no previous history of gynaecologic disease or of allergy to NSAIDs, or suspected contraindication to herbal remedies, no history of pelvic major surgery, seizure, stressor factor in the last 6 months, severe gastrointestinal disorders, or any diseases that might have interfered with the study conduct or the interpretation of results. Excluded: participants with pain for the menstruation period duration or before the onset of menstrual bleeding, use of oral hormonal contraception during treatment, urinary tract infection during the study, simultaneous participation in another clinical trial or participation in another clinical trial prior to study entry that might have had an impact on the study objectives at the discretion of the investigator. Age: mean = 21.8 years, range 18 to 30 years Source: students at Tabriz University and Tabriz University of Medical Science, who were living in the dormitory Location: Iran |
|
| Interventions | Group 1: combination of fennel extract/vitamin E capsule (60 mg fennel extract/150 UI vitamin E, QID (4 times a day) first 2 days of menstruation) (N = 34) Group 2: ibuprofen capsule (400 mg, qid (4 times a day) for the first 2 days of menstruation) (N = 34) |
|
| Outcomes |
The participants were requested to record the intensity of menstrual pain by VAS at 1, 2, 3, 6, 12, 24, and 48 hours after the onset of bleeding. |
|
| Notes | We assumed all participants had mild or severe pain, as all groups had a mean VAS score of 6.5 at baseline (range: 3.5 to 9). We extracted data from the trial publication and the trial registry entry at http://www.irct.ir/searchresult.php?id=5664&number=1. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “68 students from Tabriz University and Tabriz University of Medical Science, who were living in the dormitory, suffering from primary dysmenorrhea, and were eligible for the study, were randomly divided into two groups of 34 students each (the two groups used combination of fennel extract/vitamin E and ibuprofen cross‐over form in the 2 months).” “After that, the eligible students were determined; every student was randomly (Systematic Random Sampling) assigned to fennel/vitamin E group or ibuprofen group (34 participants in each group).” Comment: a systematic sample might lead to bias, and the study authors did not report a selected unit (selected every kth unit). |
| Allocation concealment (selection bias) | Unclear risk | “For both groups, questionnaires 1 and 2 and drug packages with randomization codes of A and B were dispensed.” Comment: the study authors provided insufficient details on how to maintain blinding to treatment allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “The shape, size, and colour of the capsule of drugs were similar.” “The study was double‐blinded, i.e. the researcher and students did not know which medication was administered.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the study authors did not state how many participants they included in the analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study authors did not prespecify adverse events as an outcome. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Nayeban 2014.
| Methods | RCT | |
| Participants | 90 participants randomised Included: participants within the age range of 18 to 26 years, single, with regular menstruation, no urogenital and coagulation disorders, and no previous history of abdominal or pelvic surgery Excluded: participants whose mean score of pain severity was < 40 (based on VAS) Age: mean = 29.7 years Source: students in the dormitories of Ferdowsi University of Mashhad Location: Iran |
|
| Interventions | Group 1: vitamin B1 100 mg/day (since the 15th day of the menstrual cycle until the beginning of the next cycle) Group 2: vitamin E 400 units/day (5 days in a month, from 2 days before the menstruation until the first 3 days) The treatment course continued for 3 menstrual cycles, and then the study authors collected all forms |
|
| Outcomes |
The study authors asked participants to record their most severe pain and its duration in the first 3 days, based on VAS and CMSS, respectively. They also asked participants if they had taken any analgesics for their pain; if so, the name and dosage of the medication was recorded in the treatment form. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The participants whose mean score of pain severity was less than 40 (based on VAS), were excluded from the study, and the rest were randomly assigned to two groups.” Comment: the study authors did not describe the methods of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | “Each medication package was placed on a separate box, coded by letters A and B, and was given to each participant.” Comment: the study authors did not describe the method of allocation concealment in sufficient detail. |
| Blinding (performance bias and detection bias) All outcomes | High risk | “Each medication package was placed on a separate box, coded by letters A and B, and was given to each participant.” Comment: it is unclear whether or not the medication appearance in both groups was the same in all aspects, and also whether there was a difference of administrator. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the study authors did not state how many participants they included in the analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study authors did not prespecify adverse events as an outcome. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Nazarpour 2007.
| Methods | RCT, parallel‐group | |
| Participants | Included: students with moderate to severe primary dysmenorrhoea Excluded: underlying disease (which leads to dysmenorrhoea Secondary cause, such as endometriosis, myoma, ovarian tumours). And contraindications to the use of drugs. Age: mean 20.7 years Source: student population Location: Iran |
|
| Interventions | Group 1: fennel (N = 36) Group 2: mefenamic acid (N = 36) Group 3: placebo (N = 32) Dosing regimen: 2 cycles Total daily dose: fennel 20 to 30 drops every 4 to 8 hours (according to need) Mefenamic acid: 250 mg every 6 hours (100 mg/day) |
|
| Outcomes |
|
|
| Notes | Translated from Persian | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the study authors did not report the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the study authors did not report the method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: both the clinician and participants were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the study authors included 104/120 participants (86%) in the analyses. |
| Selective reporting (reporting bias) | Unclear risk | Comment: does not systematically report adverse events. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Rahnama 2010.
| Methods | RCT | |
| Participants | Included: subjects with primary dysmenorrhoea who had moderate or severe pain. Source: 78 students in Shahed University Location: Iran |
|
| Interventions | Group 1: ginger (Zingiber officinale R.) 500 mg tds for 3 days from start of menstruation (N = 37) Group 2: placebo tds for 3 days from start of menstruation (N = 41) |
|
| Outcomes |
|
|
| Notes | We used data from the English abstract. Apparently this is not the same study as Rahnama 2012; Rahnama 2012 references it as a separate study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: the study authors did not report the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the study authors did not report the method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: the study authors did not mention blinding but used a placebo for the comparator. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: the study authors did not report the number of participants analysed or any statistical data apart from P values. |
| Selective reporting (reporting bias) | Unclear risk | Comment: adverse effects were not reported. |
| Other bias | Unclear risk | Comment: we were unable to access the full‐text article, and few details regarding methodology or study conduct were available. |
Rahnama 2012.
| Methods | RCT | |
| Participants | Included: 118 female students aged 18 and over, single, with a menstrual cycle that lasted from 21 to 35 days with 2 to 6 days of flow and average blood loss of 20 to 60 mL, with moderate to severe primary dysmenorrhoea (determined by a verbal multi‐dimensional scoring system) Excluded: women with diagnoses of a disease, a history of pregnancy or taking oral contraceptives, body mass index (BMI) < 19 kg/m2 or > 25 kg/m2, and mild dysmenorrhoea Age: the mean age of the intervention group = 21.4 years, control group = 21.3 years Source: students of the dormitories of Shahed University Location: Iran |
|
| Interventions | Group 1: ginger powder (Zingiber officinale R. rhizomes) 500 mg per capsule (N = 59) Group 2: placebo (toast powder) 500 mg per capsule (N = 46) Both treatment groups took treatment 3 times a day in 2 different treatment protocols Protocol 1: ginger and placebo were given 2 days before the onset of the menstrual period and continued through the first 3 days of the menstrual period (1st cycle) Protocol 2: ginger and placebo were given only for the first 3 days of the menstrual period (2nd cycle) |
|
| Outcomes |
|
|
| Notes | Apparently this is not the same study as Rahnama 2010, as Rahnama 2012 references it as a separate study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “A random numbers table was used for assigning participants in a 1:1 ratio to receive placebo and ginger using a block of two. An odd number was assigned to one patient and an even number to the other patient in each block. For each individual student recruited in the trial, a coded package was used” Comment: using a block of 2 it is possible to predict future assignments (block size is fixed and small). |
| Allocation concealment (selection bias) | Low risk | “The randomization code was available only to the midwife who had not participated in the process of patient recruitment. The code was disclosed to the researchers when the statistical analysis had been completed by researchers.” |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “The placebo capsules contained toast powder. The capsules were similar in shape, taste and colour but one set contained 500 mg ginger powder per capsule and the others were placebo capsules. The ginger capsules did not have distinguishable smell. The capsules were prepared in the Institute of Medicinal Plants and put into coded packages. Capsules and their packages were identical in appearance.” “This was a double‐ blind trial. Both the students and midwife providing care were blind to the treatment allocation. For this purpose, coded packages containing ginger and placebo capsules were used. The ginger and placebo capsules were identical in appearance, colour and taste.” “Students were asked to indicate a perception of pain intensity (most commonly) along a 10 cm horizontal line. Duration of pain was determined by asking each student to indicate the number of hours she had experienced pain during the first three days of the menstrual period.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “Thirteen students who had received placebo discontinued the trial before completing the evaluation due to the fact that they indicated did not like to be involved in this research project any longer. More information on reasons for leaving was not captured. However, there were no significant differences between characteristics of 13 patients who left the placebo group and those who remained in the study (Table 1).” Comment: we found an imbalance regarding participants lost to follow‐up found; treatment (0%) versus placebo (13/46 = 28.3%). The overall dropout rate was 12.4% (13/105). |
| Selective reporting (reporting bias) | Unclear risk | Comment: the denominator for reporting of adverse events was unclear. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Rehman 2015.
| Methods | RCT | |
| Participants | Included: 45 unmarried participants (primary dysmenorrhoea) between the ages of 15 to 25 years with regular menstrual cycles that lasted from 21 to 35 days with 2 to 6 days of flow, with moderate to severe dysmenorrhoea Excluded: women with pelvic pathology, irregular cycles, systemic illness, married women, allergic to NSAIDs and mild dysmenorrhoea, with a thyroid profile (to exclude systemic illnesses), taking any drug that could influence the study outcomes (such as NSAIDs and oral contraceptive pills) Age: mean rhubarb group = 18.87 years, mefenamic acid group = 17.73 years Source: women with primary dysmenorrhoea Location: India |
|
| Interventions | Group 1: rhubarb was finely powdered and filled in 500 mg capsules (N = 30); each capsule contained ˜420 mg of the powdered drug. Participants took 3 capsules of rhubarb twice a day, began treatment 2 days before the menstruation, and continued until the first 3 days of menstruation for 3 consecutive cycles Group 2: mefenamic acid (250 mg) (N = 15) was powdered and filled in capsules similar to the test drug Control group patients took capsules of mefenamic acid 3 times a day after meals for the same protocol |
|
| Outcomes |
Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomised into 2:1 ration by simple randomizations (lottery method) to receive either rhubarb (i.e. experimental group, n=30) or mefenamic acid (control group, n=15).” |
| Allocation concealment (selection bias) | High risk | “Investigator assigned the participants to interventions but the participants were not aware about the treatment.” “the participants were blinded to the treatment allocation.” Comment: the investigator knew in advance what intervention a particular participant would have. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “This was a single‐blind trial. The appearances of the experimental and control drug capsules were identical, and no aroma was detected from either”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “45 were randomised into experimental and control groups. Three patients in the control group did not come for last follow up; information regarding lost to follow up was not obtained. The final analysis was conducted on 45 patients” Comment: an intention to treat analysis was applied |
| Selective reporting (reporting bias) | Unclear risk | “In the rhubarb group, six (20%) patients were given the history of side effects out of which two patients complained bloating and four patients diarrhoea for the last two days of last cycle. As diarrhoea and bloating were mild and were not so much troubling, so the trial continued. No other side effects such as heart burn, abdominal pain and vomiting were reported by patients.” Comment: it is unclear whether or not data on adverse effects was collected systematically in both groups. |
| Other bias | Low risk | Comment: we did not identify any other potential sources of bias. |
Schwertner 2013.
| Methods | RCT | |
| Participants | Included: 40 participants that complained of pelvic pain, between 18 and 45 years old, from the gynaecology outpatient clinic at the Hospital de Clínicas de Porto Alegre and by newspaper publicity. Chronic pelvic pain or dyspareunia, or both, defined as a moderate‐to‐severe pain intensity lasting for > 6 months, that elicited pain scores on a categorical scale (0 to 10) ≥ 4 and required regular analgesic use. All participants had an endometriosis diagnosis confirmed by laparoscopic surgery in a pelvic pain investigation by the same investigator Excluded: women with non‐gynaecological causes of pelvic pain based on medical history, physical examination, and laboratory examinations when appropriate; with diagnosed malignancies, uterine myomas, ovarian cysts, inflammatory pelvic disease, and pregnancy; history of neurological or oncological disease, ischaemic heart disease, kidney or hepatic insufficiency, or a regular intake of antidepressants or anticonvulsants that could not be discontinued at least 15 days before the study start; history of alcohol or substance abuse in the past 6 months or were undergoing hormonal therapy or had irregular menstrual cycles Age: mean age of intervention group = 36.8 years, control group = 37.6 years Source: gynaecology outpatient clinic at the Hospital de Clínicas de Porto Alegre and by newspaper publicity Location: Brazil |
|
| Interventions | Group 1: melatonin capsules (10 mg) (N = 20) Group 2: placebo capsules (N = 20) Both treatment groups began the treatment at the onset of the menstrual cycle for 8 weeks (2 cycles) |
|
| Outcomes |
Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “We used a fixed block size of 4 to ensure that equal numbers of participants were randomised into the 2 groups.” |
| Allocation concealment (selection bias) | Low risk | “Before the recruitment phase, envelopes containing the protocol materials were prepared. Each envelope was sealed and numbered sequentially and contained an allocated treatment. After the participant agreed to participate in the trial, the envelope in the sequence was opened by the nurse who administered the medications.” “Twenty patients were allocated to the placebo group, and 20 were allocated to the melatonin group.” |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “10‐mg melatonin tablets (Sigma Chemical, Munich, Germany, provided batch‐by‐batch certificates of analysis authenticating the purity of each batch) or a placebo with identical characteristics. The capsules were manufactured in such a way that the placebo and active treatment appeared to be identical.” “Other individuals who were involved in patient care were unaware of the treatment group to which the patients belonged.” “Two independent medical examiners who were blind to the group assignments were trained to administer the pain scales and to conduct the psychological tests.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | “Twenty patients were allocated to the placebo group, and 20 were allocated to the melatonin group. Thirty‐six patients completed the study; 3 patients in the melatonin group (1=depression, 2= no reason) and one in the placebo group withdrew due to treatment inefficacy (1=no reason).” Comment: the groups were unbalanced regarding numbers of dropouts across groups; treatment 15% (3/20), placebo 5% (1/20). The overall dropout rate was 10% (4/40). |
| Selective reporting (reporting bias) | Unclear risk | Comment: adverse effects were not reported as an outcome. |
| Other bias | High risk | Comment: the baseline characteristics seemed to differ between the 2 groups (N = 20 in each group):
|
Abbreviations:
RCT: randomised controlled trial.
q12h: every 12 hours
q 4 hours: every 4 hours
qid: 4 times a day
tds: 3 times a day
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amoyi Rokn‐Abaad 2012 | This study was cited in Mirabi 2014, but library interloan services were unable to retrieve it as the reference appears to be wrong. Our attempts to contact the study authors were unsuccessful. |
| Atallahi 2014 | The study included women with mild dysmenorrhoea, and did not present results separately. |
| Butler 1955 | Not a RCT; "the girls reported to the warden of the hostel ten days before the period was due and were given either vitamin E or the placebo in strict rotation". |
| Delaram 2001 | This study was cited in Mirabi 2014, but library interloan services were unable to retrieve it as the reference appears to be wrong. Our attempts to contact the study authors were unsuccessful. |
| Deutch 1995 | An epidemiological study, not a RCT. |
| Deutch 2000 | The study include women using oral contraceptives. |
| Direkvand‐Moghadam 2012 | The study included women who suffered from menstrual pain, and possibly included women with mild dysmenorrhoea. |
| Fontana 1990 | This trial allocated women to each treatment group as they came to the gynaecological clinic. |
| Geng 2010 | Chinese traditional medicine ‐ this is the subject of a separate Cochrane review (Zhu 2008). |
| Harel 1996 | This study included women who reported dysmenorrhoea and no other health problems, and possibly included women with mild dysmenorrhoea. |
| Jang 2009 | Peer review suggested that the ingredients were commonly used in Chinese herbal medicine and the structure of the formula was based on a couple of classic Chinese herbal formulae for treating menstrual pain and the name is pronounced similar to Chinese. Therefore we excluded this trial and it will be included in Zhu 2008. |
| Karimian 2013 | This study had no inclusion criteria that related to the severity of dysmenorrhoea. The full text is in Persian and one review author, VS, checked its inclusion criteria. |
| Khodayari 2004 | This was available only as an abstract, which gave no indication of pain severity. |
| Kooshki 2013 | There were no separate results for women with moderate or severe dysmenorrhoea. |
| Kotani 1997 | This is not a RCT, but a controlled clinical trial. |
| Modaress 2006 | There were no inclusion criteria related to the severity of dysmenorrhoea. The full text is in Persian and one review author, VS, checked the study's inclusion criteria. |
| Moghadamnia 2010 | This study included women who suffered from primary dysmenorrhoea, and possibly included women with mild dysmenorrhoea. |
| Nahid 2009 | This study included students who complained of primary dysmenorrhoea, and included women with mild dysmenorrhoea. It did not give separate results for women with moderate/severe pain. |
| Navamar Jahromi 2003 | Allocation was not randomised. |
| Nazar 2006 | The study included women who suffered from primary dysmenorrhoea, and it is possible it recruited women with mild dysmenorrhoea. |
| Olfati 2010 | Trial emerged as a single trial arm only. |
| Ozgoli 2009 | Not a RCT; "alternately divided into three equal groups." |
| Praseetha 2012 | Progressive muscle relaxation versus ginger; relaxation is not in the scope of this Cochrane review. |
| Rahbar 2012 | The study included women who exhibited primary dysmenorrhoea, and possibly included women with mild dysmenorrhoea. |
| Salazar de Roldan 1993 | This is an abstract (which is in Spanish) and did not mention randomisation. |
| Salmalian 2014 | This study was apparently restricted to women with mild or moderate dysmenorrhoea. |
| Sampalis 2003 | The inclusion criteria were premenstrual syndrome. |
| Santanam 2013 | This study included women with secondary dysmenorrhoea, but did not state which level of pain (mild, moderate, severe). |
| Satarzadeh 2009 | This study reported duration of symptoms (pain and bleeding), rather than pain intensity. |
| Seifert 1989 | This is not a RCT but a controlled clinical trial. |
| Sekhavat 2010 | The primary outcome was gastrointestinal symptoms rather than pain. |
| Sesti 2007 | This study included women with secondary dysmenorrhoea, but did not state which level of pain (mild, moderate, severe). |
| Shahhosseini 2006 | Allocation was not randomised, and the study used alternate allocation. |
| Sriyakul 2012 | This study included women who suffered from primary dysmenorrhoea and required analgesic drug for relieving pain, and it is possible it recruited women with mild dysmenorrhoea. |
| Suzuki 2008 | A RCT that compared pycnogenol plus NSAIDs versus NSAIDs alone. It was designed to see whether use of pycnogeneol would reduce use of NSAIDs. The study determined pain severity by need for NSAIDs. |
| Tavasoli 2001 | This study was cited in Mirabi 2014 as a RCT of mefenamic acid and cumin, but the reference appears to be wrong. Our attempts to contact the study authors were unsuccessful. |
| Torkzahrani 2007 | Allocation was not randomised, and used alternate allocation. |
| Tseng 2005 | A cluster‐RCT. |
| Zamani 2001 | This study did not mention pain severity in the abstract, and we were unable to obtain the full‐text article. |
| Zangene 2014 | This study was quasi‐randomised. Allocation was based on the day of the week (review author VS translated the study from Persian to English). |
| Zeraati 2014 | Mean pain severity was low at baseline, and the study apparently included women with mild dysmenorrhoea. |
| Ziaei 2001 | The study included women who suffered from primary dysmenorrhoea, and possibly women with mild dysmenorrhoea. |
| Ziaei 2005 | The study included women who suffered from primary dysmenorrhoea, and possibly women with mild dysmenorrhoea. |
Abbreviations:
RCT: randomised controlled trial.
NSAIDs: non‐steroidal anti‐inflammatory drugs
Characteristics of studies awaiting assessment [ordered by study ID]
Davis 1988.
| Methods | Design: repeated measures parallel trial Method of allocation and concealment: random but method unclear Blinding: double blind Number of subjects at outset: unclear (see notes) Withdrawals: 2 (reasons unclear) |
| Participants | Inclusion: primary dysmenorrhoea with moderate to severe cramping, regular menstruation Exclusion: over the counter medication, vitamins or minerals, use of OCP or IUD, secondary dysmenorrhoea, health or reproductive problems Age: 18 to 35 years Location: college campus in Texas, USA |
| Interventions | Group 1: vitamin B6 200 mg daily (N = 10) Group 2: magnesium 500 mg daily (N = 13) Group 3: vitamin B6 200 mg plus magnesium 500 mg daily (N = 12 or 11) Group 4: pressed lactose placebo (N = 11) Duration: 5 consecutive cycles (1 pretreatment and 4 with treatment) |
| Outcomes |
|
| Notes | Dissertation
On page 85 the study author states "only women with menstrual cramping {n=46} included", but on page 38 they state 47 women We are awaiting the full‐text article. We had difficulties with the interlibrary loan (November 2015) |
Characteristics of ongoing studies [ordered by study ID]
IRCT201203045975N3.
| Trial name or title | The effect of Melissa Officinalis extract on the severity of primary dysmenorrhoea and related symptoms |
| Methods | RCT |
| Participants | Inclusion criteria: single; 18 to 26 years old; with moderate and severe dysmenorrhoea; no known chronic disease, such as fibromyalgia; no vaginal discharge, dysuria, or itching; regular menstruation; no history of pelvic inflammatory disease, Fibrom; not taking medication; no stress condition, such as parental separation or death of a family member (within 2 months). Exclusion criteria: drug intolerance; lack of proper medication or stopped taking the capsules; taking any herbal medicine in the 3 months prior and during the intervention (except melissa). Location: Iran |
| Interventions | Group 1: In the melissa group, students were given capsules of the herb (330 mg) 3 times a day (morning, noon, night) over 3 days at the onset of haemorrhage for two consecutive menstrual cycles Group 2: in placebo group, students were given (330 mg) capsules that contained starch 3 times a day (morning, noon, night) over 3 days at the onset of haemorrhage for 2 consecutive menstrual cycles |
| Outcomes |
Primary
Secondary
|
| Starting date | 04/05/2012 |
| Contact information | Parvaneh Mirabi (parvaneh_mirabi@yahoo.com) |
| Notes |
IRCT201205198348N3.
| Trial name or title | The effect of honey on severity of primary dysmenorrhoea |
| Methods | RCT |
| Participants | Inclusion criteria: age 18 to 35, regular menstrual cycles of 28 days, moderate to severe menstrual pain each menstrual cycle, lack of sensitivity to the honey, absence of genital disease, failure to menstruate during the night. Exclusion criteria: use of anti‐inflammatory drugs oral contraceptives and nonsteroidal pain or bleeding at all during the study. Location: Iran |
| Interventions | Group 1: the intervention group, after the onset of menstruation, consumed 5 teaspoons of honey a day (40 g) in the morning until the next menstrual cycle Group 2: the control group, after the onset of menstruation, consumed 5 teaspoons of impure honey a day (40 g) in the morning until the next menstrual cycle |
| Outcomes |
Primary
Secondary
|
| Starting date | 19 April 2012 |
| Contact information | Neda Mirbagher (salam_20012003@yahoo.co.uk) |
| Notes |
IRCT2012070410160N2.
| Trial name or title | Comparing the effect of Camomilla and mefenamic acid on dysmenorrhoea |
| Methods | RCT |
| Participants | Inclusion criteria: moderate or severe dysmenorrhoea; not having history of drug taking; doesn't have certain medical conditions; regular cycles. Exclusion criteria: married; mild dysmenorrhoea; history of drug taking or diseases; history of taking oral contraceptive pills (OCP). Location: Iran |
| Interventions | Group 1: 250 mg of camomilla. Treatment was started 48 hours before the beginning of menstruation and continued until the 24 hours of bleeding for 2 cycles Group 2: received 250 mg of mefenamic acid. Treatment was started 48 hours before the beginning of menstruation and continued until the 24 hours of bleeding for 2 cycles |
| Outcomes |
Primary
Secondary
|
| Starting date | 02 June 2011 |
| Contact information | Karimian Zahra (karimian62@yahoo.com) |
| Notes |
IRCT2012080610517N1.
| Trial name or title | Oral zinc sulfate for dysmenorrhoea in adolescent Iranian girls: a randomised double‐blind placebo‐controlled clinical trial. |
| Methods | RCT |
| Participants | Inclusion: history of regular menstrual cycles of 21 to 35 days with actual menstruation period of 3 to 7 days; if the participants experienced at least 4 consecutive painful menstruation periods in the past 6 months; the pain started 1 day before or at the starting day of menstruation and if dysmenorrhoea disturbed daily activity of the patients for at least 1 day per month. After describing the study protocol, an informed consent was taken from the participants' parents. Enrolled participants underwent pelvic ultrasonography examination by a gynaecologist. Exclusion: a clinically significant medical history or active disease; pelvic pathology diagnosed by the gynaecologist; used other medications or supplements; and uninterested in using the drug and filling the pain charts of the study. |
| Interventions | Group 1: intervention: 50 mg capsule of zinc sulphate, QD, for 3 months Group 2: placebo, using the same method as the intervention group. QD 1 capsule for 3 months |
| Outcomes |
|
| Starting date | 02 September 2012 |
| Contact information | Seyed Omid Reza Zekavat (ozekavat@gmail.com) |
| Notes |
IRCT2012100110980N1.
| Trial name or title | Effect of vitamin D on menstrual pain in women of childbearing age in comparison with placebo; double‐blind randomised controlled clinical trial |
| Methods | RCT |
| Participants | Inclusion criteria: women aged 18 to 30 years; with normal menstrual cycles (cycles lasted 21 to 35, with menstrual lasting 3 to 7 days); at least 4 consecutive painful periods in the past 6 months with a diagnosis of primary dysmenorrhoea; Serum 25‐hydroxyvitamin D level ≤ 30 ng/dL Exclusion criteria: secondary menstrual pain diagnosed by ultrasound examination; Previous and current use of intrauterine contraceptive devices within the 6 months prior; previous and current use of drugs including calcium or vitamin D within the 6 months prior; recent use of oral contraception drugs during the past 2 months; smoker; BMI ≥ 30; history of kidney stones, granulomatous disease, hyperparathyroidism, and any malignant disease. Location: Iran |
| Interventions | Group 1: Cholecalciferol 50,000 IU oral tablets, once a week for 8 weeks Group 2: Placebo, oral tablet, once a week for 8 weeks. |
| Outcomes |
|
| Starting date | 19/02/2013 |
| Contact information | Akbar Fotouhi (tumspre.news@sina.tums.ac.ir) |
| Notes |
IRCT201311216807N10.
| Trial name or title | Evaluating the effect of Rosaceous Sinensis on the severity of dysmenorrhoea in subjects with primary dysmenorrhoea |
| Methods | RCT |
| Participants | Inclusion criteria: 18 to 24 years of age; regular menstrual periods with 21 to 35 days cycles and 3 to 10 days bleeding period; no identified physical and pelvic diseases, primary dysmenorrhoea. Exclusion criteria: taking 20 drop of Rosaceous or less on 2 consecutive days; allergy to Rosaceous during intervention. Location: Iran |
| Interventions | Group 1: Rosaceous sinensis, 10 drops, twice a day for the first 3 days of period Group 2: water and sugar, 10 drops, twice a day for the first 3 days of period |
| Outcomes |
|
| Starting date | 16 June 2014 |
| Contact information | Seddighe Amir Ali Akbari (asa_akbari@yahoo.com) |
| Notes |
IRCT2013122114668N2.
| Trial name or title | Comparison Effect of Cinnamon Zeylanicum and Ibubrofen for Treatment of Primary Dysmenorrhea ‐ Comparison Effect of Cinnamon Zeylanicum and Ibubrofen for Treatment of Primary Dysmenorrhea |
| Methods | RCT |
| Participants | Inclusion criteria: age range "18 to 130 years"; presence of menstrual pains among recent 6 months; regular menstrual cycles Exclusion criteria: history of chronic diseases; history of abdominal/pelvic surgery; usage of OCPs; herbal or drug allergy Location: Iran |
| Interventions | Group 1: in the experimental group, 2 oral capsules of powdered cinnamon bark extract (420 mg capsule), 3 times a day during in the first 3 days of menstrual cycle for 3 consecutive months Group 2: in the Ibuprofen group, 2 oral capsules 200 mg of Ibuprofen 3 times a day during the first 3 days of each cycle for 3 consecutive months Group 3: in the placebo group, 2 oral capsules containing 420 mg starch 3 times a day in the first 3 days of each cycle for 3 consecutive months |
| Outcomes |
Primary
Seconday
|
| Starting date | 12 December 2012 |
| Contact information | Molouk Jaafarpour (jaafarpour‐@medilam.ac.ir jaafarpourm@gmail.com) |
| Notes |
IRCT2014040117111N1.
| Trial name or title | Comparison of the influence Nigella sativa with mefenamic acid on primary dysmenorrhoea |
| Methods | RCT |
| Participants | Inclusion criteria: age range between 18 to 30 years, single, female students staying in Iran Mashhad University dormitories, with regular menstrual periods of moderate to severe primary dysmenorrhoea grade based on a visual scale Exclusion criteria: history abdominal or pelvic surgery, history of sexual problems or mishaps during study, coagulation disorders or abnormal uterine pelvic ultrasound, consumption of hormonal drugs (e.g. contraception), NSAIDs, opioids, or any sensitivity to drugs, obesity (BMI > 30) Location: Iran |
| Interventions | Group 1: oral Nigella sativa 3 g daily (two capsules of 500 mg every 8 hours) during the first 3 days of menstruation Group 2: mefenamic acid 250 mg (two 500 mg capsules with 125 mg of active ingredient mefenamic acid every 8 hours during the first 3 days of menstruation |
| Outcomes |
|
| Starting date | 01 November 2014 |
| Contact information | Farzaneh Jafaarnejad (jaafarnejadf@mums.ac.ir) |
| Notes |
IRCT2014102519669N1.
| Trial name or title | Effect of Omega 3 fatty acids and vitamin E supplementation on symptoms of primary dysmenorrhoea in resident students at Ahvaz Jundishapur University of Medical Sciences student accommodation: A randomised, single‐blind, placebo‐controlled clinical trial |
| Methods | RCT |
| Participants | Inclusion criteria: women with primary dysmenorrhoea and "use irregular NSAIDS and OCP" Exclusion criteria: women with secondary dysmenorrhoea (endometriosis, adenomyosis, leiomyoma, uterine anomaly, endometrial polyps, ovarian cyst) Location: Iran |
| Interventions | Group 1: vitamin E 200 units + omega 3 300 mg (180 docosahexaenoic acid mg + 120 mg eicosapentaenoic acid), manufacturing in the Zahravi pharmaceutical company, taken daily for 2 days before and during first 3 days of menstruation, for 2 menstrual periods Group 2: omega 3 300 mg (180 docosahexaenoic acid mg + 120 mg eicosapentaenoic acid), manufactured in the Zahravi pharmaceutical company, taken daily for 2 days before and during first 3 days of menstruation for 2 menstrual periods Group 3: vitamin E 200 units (manufactured in Zahravi pharmaceutical company) taken daily, 2 days before and during first 3 days of menstruation, for 2 menstrual periods. Group 4: placebo |
| Outcomes |
|
| Starting date | 22 December 2013 |
| Contact information | Hossein Khadem Haghighian (khadem.h@ajums.ac.ir) |
| Notes |
IRCT2014120917501N1.
| Trial name or title | Comparison the effect of mefenamic acid and Teucrium Polium on intensity of dysmenorrhoea |
| Methods | RCT |
| Participants | Inclusion criteria: single; aged between 20 to 30 years; girls with primary dysmenorrhoea and pain intensity of moderate to severe based on the McGill ruler; not suffering from chronic diseases (diabetes, hypertension, cardiac diseases, infectious diseases, liver, kidney); regular menstruation (period between 21 to 35 days); no symptoms of itching, abnormal discharge during study; not using any herbal remedies within last 3 months; no deplorable event, such as near relatives’ death or surgical operation, in last 6 months. Exclusion criteria: lack of individual's consent to continue participation in the study; improper use of capsules; use of any herbal drug during the study Location: Iran |
| Interventions | Group 1: Teucrium polium capsule, 250 mg, taken orally daily (first 3 days of menstruation) for 2 cycles. Group 2: mefenamic acid capsule, 250 mg, taken orally daily (first 3 days of menstruation) for 2 cycles. Group 3: placebo, contains 250 mg starch powder, oral daily (first 3 days of menstruation) during 2 cycles. For each group, pain intensity with associated systemic characteristics will be recorded using the McGill and verbal multi‐dimensional scoring system |
| Outcomes |
|
| Starting date | 22 October 2014 |
| Contact information | Masume Simbar (msimbar@yahoo.com www.sbmu.ac.ir) |
| Notes |
NCT01598012.
| Trial name or title | The Efficacy of Ayurved Siriraj Prasaplai for Treatment Primary Dysmenorrhea |
| Methods | Block randomisation and double blinded |
| Participants | Inclusion: women diagnosed with primary dysmenorrhoea, with regular menstruation; has a numeric rating score for pain during menstruation (dysmenorrhoea) > 5 and continuous for 3 menstrual cycles, and want to participate in this study. Exclusion: women with allergy history to herbal or other components in Ayurved Siriraj Prasaplai drug; using hormonal contraception; has other diseases which cause abdominal pain; breast feeding Location: Thailand |
| Interventions | Group 1: Ayurved Siriraj Prasaplai with or without mefenamic acid. Prasaplai in capsule, 2 capsules 3 times a day for 3 days. Mefenamic acid 500 mg prn for severe pain (as rescue medication). Other name: Ponstan Group 2: Placebo with or without mefenamic acid. Placebo in a capsule (physically identical appearance to Prasaplai) 2 capsules 3 times a day for 3 days. Mefenamic acid (Ponstan) 500 mg prn for severe pain, every 6 hours (rescue medication) |
| Outcomes |
Primary
Secondary
|
| Starting date | December 2011 |
| Contact information | Thanyarat Wongwananuruk, Gynecologic Endocrinology Unit, Department of Obstetric and Gynecology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, 10700 |
| Notes | Data collection has finished. As of November 2014 the study authors are writing up their results. Not published at February 2016. |
prn: as needed
qd: every day / daily
OCP: oral contraceptive pills
Differences between protocol and review
We changed the review title from 'Herbal and dietary therapies for dysmenorrhoea' to 'Dietary supplements for dysmenorrhoea' as herbal supplements are covered by the Cochrane review 'Chinese herbal medicine for primary dysmenorrhoea' (Zhu 2008).
We updated the methods in line with current Cochrane methodological standards.
Contributions of authors
For this review update, Porjai Pattanittum (PP) led the review process. PP, Naowarat Kunyanone (NK), Julie Brown (JB), and Jane Marjoribanks (JM) screened articles identified by searches against inclusion criteria, extracted data from eligible studies, and assessed risk of bias of the included studies.
Vahid Seyfoddin (VS) extracted data from the included Persian studies, and assessed risk of bias in the Persian studies.
Ussanee Sangkomkamhang (US) acted as a third review author for conflicts in applying eligibility criteria.
PP and JM entered the extracted data into RevMan (RevMan 2014), performed and interpreted the analyses, and were responsible for drafting the review.
Jo Barnes (JoB) critically reviewed the final review draft, and provided supervisory assistance and content expert advice.
All review authors read and checked the review before submission.
Sources of support
Internal sources
-
Khon Kaen University, Thailand.
Porjai Pattanittum
External sources
-
Thailand Research Fund (Distinguished Research Professors Award), Thailand.
Grant for honorarium to first review author Porjai Pattanittum
-
Cochrane Thailand, Thailand.
Grant for infrastructural support of this Cochrane review
Declarations of interest
Porjai Pattanittum, Naowarat Kunyanone, Julie Brown, Ussanee S Sangkomkamhang, Joanne Barnes, Vahid Seyfoddin and Jane Marjoribanks have no known conflicts of interest.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abdelmaeboud 2014 {published and unpublished data}
- Abd‐El‐Maeboud KHI, Kortam MAMF, Ali MS, Ibrahim MI, Mohamed RMMZ. A preliminary pilot randomized crossover study of uzara (Xysmalobium undulatum) versus ibuprofen in the treatment of primary dysmenorrhea. PLoS One 2014;9(8):e104473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelmaeboud KH, Kortam MAMF, Ali MS, Ibrahim MI, Mohamed RMMZ. Comparative study of uzara and ibuprofen in the treatment of Egyptian students with primary dysmenorrhea: a randomized, crossover study. International Journal of Gynecology & Obstetrics 2012;119:S426‐7. [DOI: 10.1016/S0020-7292(12)60896-0] [DOI] [Google Scholar]
Akbari 2012 {published and unpublished data}
- Akbari SAA, Younesy S, Nouraei S, Esmaeili S, Majd HA. Effects of fenugreek seed on the severity and systemic manifestations of dysmenorrhea. International Journal of Obstetrics & Gynaecology 2012;119(S1):140. [Google Scholar]
- Younesy S, Amiraliakbari S, Esmaeili S, Alavimajd H, Nouraei S. Effects of fenugreek seed on the severity and systemic symptoms of dysmenorrhea. Journal of Reproduction & Infertility 2014;15(1):41‐8. [PUBMED: 24695380] [PMC free article] [PubMed] [Google Scholar]
Akhavan Amjadi 2009 {published data only}
- Akhavan Amjadi M, Mojab F, Shahbazzadegan S. Effects of Cinnamomum zeylanicum on the severity and systemic manifestations of dysmenorrhea. Journal of Ardabil University of Medical Sciences 2009;9(3):204‐9. [Google Scholar]
Bani 2014 {published data only}
- Bani S, Hasanpour S, Mousavi Z, Mostafa Garehbaghi P, Gojazadeh M. The effect of rosa damascena extract on primary dysmenorrhea: a double‐blind cross‐over clinical trial. Iranian Red Crescent Medical Journal 2014;16(1):e14643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bokaie 2013 {published data only}
- Bokaie M, Farajkhoda T, Enjezab B, Khoshbin A, Karimi‐Zarchi M. Oral fennel (Foeniculum vulgare) drop effect on primary dysmenorrhea: Effectiveness of herbal drug. Iranian Journal of Nursing and Midwifery Research 2013;18(2):128‐32. [PMC free article] [PubMed] [Google Scholar]
- Bokaie M, Farajkhoda T, Enjezab B, Khoshbin A, Karimi‐Zarchi M. Oral fennel (Foeniculum vulgare) drop effect on primary dysmenorrhea: Effectiveness of herbal drug. Iranian Journal of Nursing and Midwifery Research 2014;19(2):216. [PMC free article] [PubMed] [Google Scholar]
Dolation 2010 {published data only}
- Mirabe P, Dolation M, Mojab F, Majd AH. Effects of Valerian officinalis on the severity of dysmenorrheal symptoms. Journal of Reproduction & Infertility 2010;10(4):253‐259. [Google Scholar]
- Mirabi P, Dolatian M, Mojab F, Majd HA. Effects of valerian on the severity and systemic manifestations of dysmenorrhea. International Journal of Gynaecology & Obstetrics 2011;115(3):285‐8. [PUBMED: 21959068] [DOI] [PubMed] [Google Scholar]
Doubova 2007 {published data only}
- Doubova SV, Morales HR, Hernández SF, Carmen Martinez‐Garcia M, González de Cossío Ortiz M, Chávez Soto MA, et al. Effect of a Psidii guavae folium extract in the treatment of primary dysmenorrhoea: A randomized clinical trial. Journal of Ethnopharmacology 2007;110(2):305‐10. [DOI] [PubMed] [Google Scholar]
Ghodsi 2014 {published data only}
- Ghodsi Z, Asltoghiri M. The effect of fennel on pain quality, symptoms, and menstrual duration in primary dysmenorrhea. Journal of Pediatric and Adolescent Gynecology 2014;27(5):283‐6. [DOI] [PubMed] [Google Scholar]
Gokhale 1996 {published data only}
- Gokhale LB. Curative treatment of primary (spasmodic) dysmenorrhoea. Indian Journal of Medical Research 1996;103:227‐31. [PubMed] [Google Scholar]
Heidarifar 2014 {published data only}
- Heidarifar R, Mehran N, Heidari A, Ahmari Tehran H, Koohbor M, Mansourabad MK. Effect of Dill (Anethum graveolens) on the severity of primary dysmenorrhea in compared with mefenamic acid: A randomized, double‐blind trial. Journal of Research in Medical Sciences 2014;19(4):326‐30. [PMC free article] [PubMed] [Google Scholar]
Hosseinlou 2014 {published data only}
- Hosseinlou A, Alinejad V, Alinejad M, Aghakani N. The effects of fish oil capsules and vitamin B1 tablets on duration and severity of dysmenorrhea in students of high school in Urmia‐Iran. Global Journal of Health Science 2014;6(7):124‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iravani 2009 {published data only}
- Iravani M. Clinical effects of Zataria multiflora essential oil on primary dysmenorrhoea. Journal of Medicinal Plants 2009;8(30):54‐60. [Google Scholar]
Jenabi 2010 {published data only}
- Jenabi E, Ebrahimzadeh S. Chamomile tea for relief of dysmenorrhea. Iranian Journal of Obstetrics, Gynaecology and Infertility 2010;13(1):39‐42. [Google Scholar]
Jenabi 2012 {published data only}
- Jenabi E, Toghiri MA, Parisa Hejrati. The comparison of the effects of antiplain of valeriana officinalis risom and mefenamic acid in relief of primary dismenorrhea. Iranian Journal of Obstetrics, Gynecology and Infertility 2012;15(2):44‐8. [Google Scholar]
Jenabi 2013 {published data only}
- Jenabi E. The effect of ginger for relieving of primary dysmenorrhoea. Journal of the Pakistan Medical Association 2013;63(1):8‐10. [PUBMED: 23865123] [PubMed] [Google Scholar]
Kashanian 2013 {published data only}
- Kashanian M, Lakeh MM, Ghasemi A, Noori S. Evaluation of the effect of vitamin E for pelvic pain reduction in primary dysmenorrhea [Persian]. Iranian Journal of Obstetrics, Gynecology and Infertility 2012;14(8):34‐8. [Google Scholar]
- Kashanian M, Lakeh MM, Ghasemi A, Noori S. Evaluation of the effect of vitamin E on pelvic pain reduction in women suffering from primary dysmenorrhea. Journal of Reproductive Medicine 2013;58(1‐2):34‐8. [PubMed] [Google Scholar]
Kashefi 2014 {published data only}
- Kashefi F, Khajehei M, Tabatabaeichehr M, Alavinia M, Asili J. Comparison of the effect of ginger and zinc sulfate on primary dysmenorrhea: a placebo‐controlled randomized trial. Pain Management Nursing 2014;15(4):826‐33. [DOI] [PubMed] [Google Scholar]
Khorshidi 2003 {published data only}
- Khorshidi N, Ostad SN, Mosaddegh M, Soodi M. Clinical effects of fennel essential oil on primary dysmenorrhea. Iranian Journal of Pharmaceutical Research 2003;2:89‐93. [Google Scholar]
Modaress 2011 {published data only}
- Modaress M, Mirmohhamad M, Oshireh Z, Mehran A. Comparison of the effect of mefenamic acid and Matricaria chamomilla capsules on primary dysmenorrhea. Journal of Babol University of Medical Sciences 2011;13:50‐8. [Google Scholar]
Moslemi 2012 {published data only}
- IRCT201101225664N1. Comparison effect of Fennel/Vitamin E and Ibuprofen on intensity of primary dysmenorrhea at students of Tabriz, 2007‐2009. http://www.irct.ir/searchresult.php?id=5664&number=1 (accessed 24 May 2015).
- Moslemi L, Aghamohammadi A, Bekhradi R, Zafari M. The comparison effect of vitamin E and Fennel extract on intensity of primary dysmenorrhea. Journal of Mazandaran University of Medical Sciences 2012;22(88):103‐7. [Google Scholar]
Nasehi 2013 {published data only}
- Nasehi M, Sehhatie F, Zamanzadeh V, Delazar A, Javadzadeh Y, Chongheralu BM. Comparison of the effectiveness of combination of fennel extract/vitamin E with ibuprofen on the pain intensity in students with primary dysmenorrhea. Iranian Journal of Nursing and Midwifery Research 2013;18(5):355‐9. [PMC free article] [PubMed] [Google Scholar]
Nayeban 2014 {published data only}
- Nayeban S, Jafarnejad F, Nayeban S, Sefidgaran A. A comparison of the effects of vitamin E and vitamin B1 on the severity and duration of pain in primary dysmenorrhea. Journal of Midwifery & Reproductive Health 2014;2(2):143‐6. [Google Scholar]
Nazarpour 2007 {published data only}
- Nazarpour S, Azimi H. Comparison of therapeutic effects of fennelin and mefenamic acid on primary dysmenorrhea. Journal of Mazandaran University of Medical Sciences 2007;17(61):54‐61. [Google Scholar]
Rahnama 2010 {published data only}
- Rahnama P, Fallah Huseini H, Mohammadi K, Modares M, Khajavi Shojaei K, Askari M, et al. The effects of Zingiber officinal R. on primary dysmenorrhoea. Journal of Medicinal Plants 2010;9(36):81‐6. [Google Scholar]
Rahnama 2012 {published data only}
- Rahnama P, Montazeri A, Huseini HF, Kianbakht S, Naseri M. Effect of Zingiber officinale R. rhizomes (ginger) on pain relief in primary dysmenorrhea: a placebo randomized trial. BMC Complementary and Alternative Medicine 2012;12:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rehman 2015 {published data only}
- Rehman H, Begum W, Anjum F, Tabasum H, Zahid S. Effect of rhubarb (Rheum emodi) in primary dysmenorrhoea: a single‐blind randomized controlled trial. Journal of Complementary and Integrative Medicine 2015;12(1):61‐9. [DOI] [PubMed] [Google Scholar]
Schwertner 2013 {published data only}
- Schwertner A, Conceição Dos Santos CC, Costa GD, Deitos A, Souza A, Souza IC, et al. Efficacy of melatonin in the treatment of endometriosis: a phase II, randomized, double‐blind, placebo‐controlled trial. Pain 2013;154(6):874‐81. [PUBMED: 23602498] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amoyi Rokn‐Abaad 2012 {published data only}
- Amoyi Rokn‐Abaad M, Sarafraz N. Comparison effect of supermint and ibuprofen on dysmenorrhea. Journal of Qom University of Medical Sciences 2012;5:37‐41. [Google Scholar]
Atallahi 2014 {published data only}
- Atallahi M, Akbari SAA, Mojab F, Majd HA. Effects of wheat germ extract on the severity and systemic symptoms of primary dysmenorrhea: a randomized controlled clinical trial. Iranian Red Crescent Medical Journal 2014;16(8):e19503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Butler 1955 {published data only}
- Butler EB, McKnight E. Vitamin E in the treatment of primary dysmenorrhoea. Lancet 1955;268(6869):844‐7. [DOI] [PubMed] [Google Scholar]
Delaram 2001 {published data only}
- Delaram M, Forozandeh N. Effects of Foeniculum vulgare extract on primary dysmenorrhea. Journal of Medical Sciences 2001;10:81‐8. [Google Scholar]
Deutch 1995 {published data only}
- Deutch B. Menstrual pain in Danish women correlated with low n‐3 polyunsaturated fatty acid intake. European Journal of Clinical Nutrition 1995;49(7):508‐16. [PubMed] [Google Scholar]
Deutch 2000 {published data only}
- Deutch B, Jørgensen EB, Hansen JC. Menstrual discomfort in Danish women reduced by dietary supplements of omega‐3 PUFA and B12 (fish oil or seal oil capsules). Nutrition Research 2000;20(5):621‐31. [Google Scholar]
Direkvand‐Moghadam 2012 {published data only}
- Direkvand‐Moghadam A, Khosravi A. Evaluating Shirazi (Thymus vulgaris) on menstrual pain using verbal multidimensional scoring system (VMS). African Journal of Pharmacy and Pharmacology 2012;6(39):2761‐6. [DOI: 10.5897/AJPP11.818] [DOI] [Google Scholar]
- Direkvand‐Moghadam A, Khosravi A. The impact of a novel herbal Shirazi Thymus Vulgaris on primary dysmenorrhea in comparison to the classical chemical Ibuprofen. Journal of Research in Medical Sciences 2012;17(7):668‐70. [PUBMED: 23798928] [PMC free article] [PubMed] [Google Scholar]
Fontana 1990 {published data only}
- Fontana‐Klaiber H, Hogg B. Therapeutic effects of magnesium in dysmenorrhea [German]. Schweizerische Rundschau für Medizin Praxis 1990;79(16):491‐4. [PubMed] [Google Scholar]
Geng 2010 {published data only}
- Geng SS, Li HZ, Wu XK, Dang JM, Tong H, Zhao CY, et al. Effect of Wujijing oral liquid on menstrual disturbance of women. Journal of Ethnopharmacology 2010;128(3):649‐53. [DOI: 10.1016/j.jep2009.12.041] [DOI] [PubMed] [Google Scholar]
Harel 1996 {published data only}
- Harel Z, Biro FM, Kottenhahn RK, Rosenthal SL. Supplementation with omega‐3 polyunsaturated fatty acids in the management of dysmenorrhea in adolescents. American Journal of Obstetrics and Gynecology 1996;174(4):1335‐8. [DOI] [PubMed] [Google Scholar]
Jang 2009 {published data only}
- Jang J‐B, Yoon Y‐J, Park J‐H, Jeong H‐G, Cho J‐H, Ko S‐G, et al. Therapeutic effects of Chiljehyangbuhwan on primary dysmenorrhoea: a randomized double blind, placebo controlled study. Complementary Therapies in Medicine 2009;17(3):123‐30. [DOI] [PubMed] [Google Scholar]
Karimian 2013 {published data only}
- Karimian Z, Sadat Z, Abedzadeh M, Sarafraz N, Kafaei Atrian M, Bahrami N. Comparison the effect of mefenamic acid and Matricaria chamomilla on primary dysmenorrhea in Kashan Medical University Students. Journal of Ardabil University of Medical Sciences 2013;13(4):413‐20. [Google Scholar]
Khodayari 2004 {published data only}
- Khodayari N, Moatar F. Efficacy of traditional medicine for the treatment of primary dysmenorrhea. Iranian Journal of Pharmaceutical Research 2004;3(2):37. [Google Scholar]
Kooshki 2013 {published data only}
- Kooshki A, Tofighiyan T, Rakhshani MH. Comparison of the effects of marine omega‐3 fatty acids and ibuprofen on primary dysmenorrehea. Life Science Journal 2013;10(4s):1‐3. [Google Scholar]
Kotani 1997 {published data only}
- Kotani N, Oyama T, Sakai I, Hashimoto H, Muraoka M, Ogawa Y, et al. Analgesic effect of a herbal medicine for treatment of primary dysmenorrhea‐‐a double‐blind study. American Journal of Chinese Medicine 1997;25(2):205‐12. [DOI] [PubMed] [Google Scholar]
Modaress 2006 {published data only}
- Modaress Nejad V, Asadipour M. Comparison of the effectiveness of fennel and mefenamic acid on pain intensity in dysmenorrhoea. Eastern Mediterranean Health Journal 2006;12(3‐4):423‐7. [PUBMED: 17037712] [PubMed] [Google Scholar]
- Modaress Nejad V, Motamedi B, Asadi‐pour M. Comparison between the pain‐relief effect of fennel and mefenamic acid on primary dysmenorrhea. Journal of Rafsanjan University of Medical Sciences 2006;5(1):1‐6. [Google Scholar]
Moghadamnia 2010 {published data only}
- Moghadamnia AA, Mirhosseini N, Abadi MH, Omranirad A, Omidvar S. Effect of Clupeonella grimmi (anchovy/kilka) fish oil on dysmenorrhoea. Eastern Mediterranean Health Journal 2010;16(4):408‐13. [PubMed] [Google Scholar]
Nahid 2009 {published data only}
- Nahid K, Fariborz M, Ataolah G, Solokian S. The effect of an Iranian herbal drug on primary dysmenorrhea: a clinical controlled trial. Journal of Midwifery & Women's Health 2009;54(5):401‐4. [DOI] [PubMed] [Google Scholar]
Navamar Jahromi 2003 {published data only}
- Namavar Jahromi B, Tartifizadeh A, Khabnadideh S. Comparison of fennel and mefenamic acid for the treatment of primary dysmenorrhea. International Journal of Gynecology and Obstetrics 2003;80(2):153‐7. [DOI] [PubMed] [Google Scholar]
Nazar 2006 {published data only}
- Nazar H, Usmanghani K. Clinical evaluation to assess the safety and efficacy of coded herbal medicine "Dysmo‐off" versus allopathic medicine "Diclofenac sodium" for the treatment of primary dysmenorrhea. Journal of Herbal Pharmacotherapy 2006;6(1):21‐39. [PUBMED: 17135158] [PubMed] [Google Scholar]
Olfati 2010 {published data only}
- Olfati F, Azarbaijani S, Hadizadeh M, Sadeghi T, Hajseiedjavadi E. Effect of powder of Stachys Iavandulifolia flowers on primary dysmenorrhoea. Journal of Medicinal Plants 2010;34:84‐9. [Google Scholar]
Ozgoli 2009 {published data only}
- Ozgoli G, Goli M, Moattar F. Comparison of effects of ginger, mefenamic acid, and ibuprofen on pain in women with primary dysmenorrhoea. Journal of Alternative and Complementary Medicine 2009;15(2):129‐32. [DOI] [PubMed] [Google Scholar]
Praseetha 2012 {published data only}
- Praseetha KV. Effectiveness of progressive muscle relaxation versus ginger powder on dysmenorrhea among students in selected nursing colleges of Mangalore [Thesis of Master Degree in Nursing]. Ullal, Mangalore: K. Pandyarajah Ballal College of Nursing, 2012. [Google Scholar]
Rahbar 2012 {published data only}
- Rahbar N, Asgharzadeh N, Ghorbani R. Effect of omega‐3 fatty acids on intensity of primary dysmenorrhea. International Journal of Gynaecology and Obstetrics 2012;117(1):45‐7. [PUBMED: 22261128] [DOI] [PubMed] [Google Scholar]
Salazar de Roldan 1993 {published data only}
- Salazar de Roldan M, Castro S. Treatment of primary dysmenorrhoea with ibuprofen or vitamin E [Tratamiento de la dismenorrea primaria con ibufofeno y vitamina E]. Revista de Obstetricia y Ginecología de Venezuela 1993;53(1):35‐7. [Google Scholar]
Salmalian 2014 {published data only}
- Salmalian H, Saghebi R, Moghadamnia AA, Bijani A, Faramarzi M, Nasiri Amiri F, et al. Comparative effect of thymus vulgaris and ibuprofen on primary dysmenorrhea: a triple‐blind clinical study. Caspian Journal of Internal Medicine 2014;5(2):82‐8. [PMC free article] [PubMed] [Google Scholar]
Sampalis 2003 {published data only}
- Sampalis F, Bunea R, Pelland M, Kowalski O, Duget N, Dupuis S. Evaluation of the effects of neptune krill oilTM on the management of premenstrual syndrome and dysmenorrhoea. Alternative Medicine Review 2003;8(2):171‐9. [PubMed] [Google Scholar]
Santanam 2013 {published data only}
- Santanam N, Kavtaradze N, Murphy A, Dominguez C, Parthasarathy S. Antioxidant supplementation reduces endometriosis‐related pelvic pain in humans. Translational Research, The Journal of Laboratory and Clinical Medicine 2013;161(3):189‐95. [PUBMED: 22728166] [DOI] [PMC free article] [PubMed] [Google Scholar]
Satarzadeh 2009 {published data only}
- Satarzadeh N, Nazemie H, Maleki N, Hashemi M. Effect of achillea willhemsii on menstrual bleeding and dysmenorrhea. Nursing and Midwifery Journal of Tabriz University of Medical Sciences & Health Services 2009;12:4‐10. [Google Scholar]
Seifert 1989 {published data only}
- Seifert B, Wagler P, Dartsch S, Schmidt U, Nieder J. Magnesium‐‐a new therapeutic alternative in primary dysmenorrhea [German]. Zentralblatt für Gynäkologie 1989;111(11):755‐60. [PubMed] [Google Scholar]
Sekhavat 2010 {published data only}
- Sekhavat L, Jouzshari MN. Therapeutic effect of vitamin B6 on gastrointestinal symptoms of primary dysmenorrhoea in young women in Yazd City (2006‐2009). Iranian Journal of Obstetrics, Gynaecology and Infertility 2010;13(5):25‐9. [Google Scholar]
Sesti 2007 {published data only}
- Sesti F, Pietropolli A, Capozzolo T, Broccoli P, Pierangeli S, Bolea M, et al. Hormonal suppression treatment or dietary therapy versus placebo in the control of painful symptoms after conservative surgery for endometriosis stage III‐IV. A randomized comparative trial. Fertility and Sterility 2007;88(6):1541‐7. [DOI] [PubMed] [Google Scholar]
Shahhosseini 2006 {published data only}
- Shahhosseini Z, Amin GH, Salehi Sormaghi MH, Danesh M, Abedian K. The effect of vitex drop on dysmenorrhea. Journal of Mazandaran University of Medical Sciences 2006;15:15‐21. [Google Scholar]
Sriyakul 2012 {published data only}
- Sriyakul K, Kietinun S, Pattaraarchachai J, Ruangrungsi N. A comparative double‐blinded randomized study: the efficacy of prasaplai herbal extract versus mefenamic acid in relieving pain among primary dysmenorrhea patients. The Open Complementary Medicine Journal 2012;4:16‐21. [Google Scholar]
Suzuki 2008 {published data only}
- Suzuki N, Uebaba K, Kohama T, Moniwa N, Kanayama N, Koike K. French maritime pine bark extract significantly lowers the requirement for analgesic medication in dysmenorrhea: a multicenter, randomized, double‐blind, placebo‐controlled study. The Journal of Reproductive Medicine 2008;53(5):338‐46. [PUBMED: 18567279] [PubMed] [Google Scholar]
Tavasoli 2001 {published data only}
- Tavasoli F, Sharifian J, Mazlom SR. Comparison of the effect of mefenamic acid and carumcarvi on the severity of primary dysmenorrhea in Mashhad high school students (1999‐2000). Journal of Sabzevar University of Medical Sciences 2001;8(1(19)):4‐9. [Google Scholar]
- Tavasouli F, Sharifian AJ, Mazlom SR. Comparison of the effect of mefenamic acid and carumcarvi on the severity of primary dysmenorrhea in Mashhad high school students. Ofogh‐e‐Danesh 2001;6(2):1‐6. [Google Scholar]
Torkzahrani 2007 {published data only}
- Torkzahrani SH, Akhavan Amjadi M, Mojab F, Alavi‐Majd H. Clinical effects of Foeniculum vulgare extract on primary dysmenorrhea. Journal of Reproduction and Infertility 2007;8(1):45‐51. [Google Scholar]
Tseng 2005 {published data only}
- Tseng YF, Chen CH, Yang YH. Rose tea for relief of primary dysmenorrhea in adolescents: a randomized controlled trial in Taiwan. Journal of Midwifery & Women's Health 2005;50(5):e51‐7. [PUBMED: 16154059] [DOI] [PubMed] [Google Scholar]
Zamani 2001 {published data only}
- Zamani M, Soltan BF. Evaluation of the treatment effect of vitamin B1 in primary dysmenorrhoea. Arak Medical University Journal 2001;4(3(16)):7‐15. [Google Scholar]
Zangene 2014 {published data only}
- Zangene M, Veisi F, Nankali A, Rezaei M, Ataee M. Evaluation of the effects of oral vitamin‐D for pelvic pain reduction in primary dysmenorrheal. Iranian Journal of Obstetrics, Gynecology and Infertility 2014;16(88):14‐20. [Google Scholar]
Zeraati 2014 {published data only}
- Zeraati F, Shobeiri F, Nazari M, Araghchian M, Bekhradi R. Comparative evaluation of the efficacy of herbal drugs (fennelin and vitagnus) and mefenamic acid in the treatment of primary dysmenorrhea. Iranian Journal of Nursing and Midwifery Research 2014;19(6):581‐4. [PMC free article] [PubMed] [Google Scholar]
Ziaei 2001 {published data only}
- Ziaei S, Faghihzadeh S, Sohrabvand F, Lamyian M, Emamgholy T. A randomised placebo‐controlled trial to determine the effect of vitamin E in treatment of primary dysmenorrhoea. British Journal of Obstetrics and Gynaecology 2001;108(11):1181‐3. [DOI] [PubMed] [Google Scholar]
Ziaei 2005 {published data only}
- Ziaei S, Zakeri M, Kazemnejad A. A randomised controlled trial of vitamin E in the treatment of primary dysmenorrhoea. British Journal of Obstetrics and Gynaecology 2005;112(4):466‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Davis 1988 {published data only}
- Davis LS. Stress, vitamin B6 and magnesium in women with and without dysmenorrhea: a comparison and intervention study [Dissertation]. Austin (TX): The University of Texas at Austin, 1988. [Google Scholar]
References to ongoing studies
IRCT201203045975N3 {unpublished data only}
- IRCT201203045975N3. The effect of Melissa Officinalis extract on the severity of primary dysmenorrhea and related symptoms of Islamic Azad University students in Zanjan. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201203045975N3 (accessed 24 May 2015).
IRCT201205198348N3 {unpublished data only}
- IRCT201205198348N3. The effect of honey on severity of primary dysmenorrhea. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201205198348N3 (accessed 24 May 2015).
IRCT2012070410160N2 {unpublished data only}
- IRCT2012070410160N2. Comparing the effect of camomilla and mefenamic acid on dysmenorrhea. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2012070410160N2 (accessed 24 May 2015).
IRCT2012080610517N1 {unpublished data only}
- IRCT2012080610517N1. Oral zinc sulfate for dysmenorrhea in adolescent Iranian girls: a randomized double‐blind placebo‐controlled clinical trial. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2012080610517N1 (accessed 24 May 2015).
IRCT2012100110980N1 {unpublished data only}
- IRCT2012100110980N1. Effect of vitamin D on menstrual pain in women of childbearing age in comparison with placebo; double‐blind randomized controlled clinical trial. http://www.irct.ir/searchresult.php?id=10980&number=1 (accessed 24 May 2015).
IRCT201311216807N10 {unpublished data only}
- IRCT201311216807N10. Evaluating the effect of Rosaceous Sinensis on the severity of dysmenorrhea in subjects with primary dysmenorrhea. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201311216807N10 (accessed 24 May 2015).
IRCT2013122114668N2 {unpublished data only}
- IRCT2013122114668N2. Comparison Effect of Cinnamon Zeylanicum and Ibubrofen for Treatment of Primary Dysmenorrhea ‐ Comparison Effect of Cinnamon Zeylanicum and Ibubrofen for Treatment of Primary Dysmenorrhea. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2013122114668N2 (accessed 24 May 2015).
IRCT2014040117111N1 {unpublished data only}
- IRCT2014040117111N1. Comparison of the influence nigella sativa with mefenamic acid on primary dysmenorrhea. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2014040117111N1 (accessed 24 May 2015).
IRCT2014102519669N1 {unpublished data only}
- IRCT2014102519669N1. Effect of Omega 3 fatty acids and vitamin E supplementation on symptoms of primary dysmenorrhea in resident students at Ahvaz Jundishapur University of Medical Sciences student accommodation: A randomized, single‐blind, placebo‐controlled clinical trial. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2014102519669N1 (accessed 24 May 2015).
IRCT2014120917501N1 {unpublished data only}
- IRCT2014120917501N1. Comparison the effect of mefenamic acid and Teucrium polium on intensity of dysmenorrhea. http://www.irct.ir/searchresult.php?id=17501&number=1 (accessed 24 May 2015).
NCT01598012 {unpublished data only}
- NCT01598012. The efficacy of ayurved siriraj prasaplai for treatment primary dysmenorrhea. https://clinicaltrials.gov/ct2/show/NCT01598012?term=dysmenorrhea&rank=21 (accessed 24 May 2015).
Additional references
Barnes 2008
- Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. National Health Statistics Reports 2008;12:1‐23. [PubMed] [Google Scholar]
Benassi 1992
- Benassi L, Barlette FP, Baroncini L, Bertani D, Filippini F, Beski L, et al. Effectiveness of magnesium pidolate in the prophylactic treatment of primary dysmenorrhoea. Clinical and Experimental Obstetrics & Gynecology 1992;19(3):176‐9. [PubMed] [Google Scholar]
Cupp 1999
- Cupp MJ. Herbal remedies: adverse effects and drug interactions. American Family Physician 1999;59(5):1239‐45. [PubMed] [Google Scholar]
French 2005
- French L. Dysmenorrhea. American Family Physician 2005;71(2):285‐91. [PUBMED: 15686299] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool. Available from www.gradepro.org: McMaster University (developed by Evidence Prime, Inc.), 2015.
Harel 2006
- Harel Z. Dysmenorrhea in adolescents and young adults: etiology and management. Journal of Paediatric and Adolescent Gynaecology 2006;19(6):363‐71. [PUBMED: 17174824] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Ju 2014
- Ju H, Jones M, Mishra G. The prevalence and risk factors of dysmenorrhoea. Epidemiologic Reviews 2014;36:104‐13. [PUBMED: 24284871] [DOI] [PubMed] [Google Scholar]
Lichten 1987
- Lichten EM, Bombard J. Surgical treatment of primary dysmenorrhea with laparoscopic uterine nerve ablation. Journal of Reproductive Medicine 1987;32:37‐41. [PubMed] [Google Scholar]
Marjoribanks 2010
- Marjoribanks J, Proctor M, Farquhar C, Derks Roos S. Nonsteroidal anti‐inflammatory drugs for dysmenorrhoea. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD001751.pub2] [DOI] [PubMed] [Google Scholar]
Mirabi 2014
- Mirabi P, Alamolhoda SH, Esmaeilzadeh S, Mojab F. Effect of medicinal herbs on primary dysmenorrhoea ‐ a systematic review. Iranian Journal of Pharmaceutical Research 2014;13(3):757‐67. [PMC free article] [PubMed] [Google Scholar]
Nahin 2009
- Nahin RL, Barnes PM, Stussman BJ, Bloom B. Costs of complementary and alternative medicine (CAM) and frequency of visits to CAM practitioners: United States, 2007. National Health Statistics Reports 2009;18:1‐14. [PubMed] [Google Scholar]
Osayande 2014
- Osayande AS, Mehulic S. Diagnosis and initial management of dysmenorrhea. American Family Physician 2014;89(5):341‐6. [PubMed] [Google Scholar]
Parker 2010
- Parker MA, Sneddon AE, Arbon P. The menstrual disorder of teenagers (MDOT) study: determining typical menstrual patterns and menstrual disturbance in a large population‐based study of Australian teenagers. BJOG: An International Journal of Obstetrics and Gynaecology 2010;117(2):185‐92. [DOI] [PubMed] [Google Scholar]
Penland 1993
- Penland JG, Johnson PE. Dietary calcium and manganese effects on menstrual cycle symptoms. American Journal of Obstetrics and Gynecology 1993;168(5):1417‐23. [DOI] [PubMed] [Google Scholar]
Proctor 2006
- Proctor M, Farquhar C. Diagnosis and management of dysmenorrhoea. BMJ (Clinical research ed.) 2006;332(7550):1134‐8. [PUBMED: 16690671] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Terry 2011
- Terry R, Posadzki P, Watson LK, Ernst E. The use of ginger (Zingiber officinale) for the treatment of pain: a systematic review of clinical trials. Pain Medicine 2011;12(12):1808‐18. [PUBMED: 22054010] [DOI] [PubMed] [Google Scholar]
US FDA 2014
- U.S. Food, Drug Administration. United States Department of Health and Human Services. What is a dietary supplement?. http://www.fda.gov/aboutfda/transparency/basics/ucm195635.htm (accessed 4 April 2014).
Wallace 2010
- Wallace S, Keightley A, Gie C. Dysmenorrhoea. The Obstetrician & Gynaecologist 2010;12(3):149–54. [DOI: 10.1576/toag.12.3.149.27596] [DOI] [Google Scholar]
Winslow 1998
- Winslow LC, Kroll DJ. Herbs as medicine. Archives of Internal Medicine 1998;158(20):2192‐9. [DOI] [PubMed] [Google Scholar]
Zhu 2008
- Zhu X, Proctor M, Bensoussan A, Wu E, Smith CA. Chinese herbal medicine for primary dysmenorrhoea. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD005288.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Proctor 2001
- Proctor ML, Murphy PA. Herbal and dietary therapies for primary and secondary dysmenorrhoea. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD002124; PUBMED: 11687013] [DOI] [PubMed] [Google Scholar]


