Summary
With technological advances in culture-independent molecular methods, we are uncovering a new facet of our natural history by accounting for the vast diversity of microbial life which colonizes the human body. The human microbiome contributes functional genes and metabolites which affect human physiology and are, therefore, considered an important factor for maintaining health. Much has been described in the past decade based primarily on 16S rRNA gene amplicon sequencing regarding the diversity, structure, stability and dynamics of human microbiota in their various body habitats, most notably within the gastrointestinal tract (GIT). Relatively high levels of variation have been described across different stages of life and geographical locations for the GIT microbiome. These observations may prove helpful for the future contextualization of patterns in other body habitats especially in relation to identifying generalizable trends over human lifetime. Given the large degree of complexity and variability, a key challenge will be how to define baseline healthy microbiomes and how to identify features which reflect deviations therefrom in the future. In this context, metagenomics and functional omics will likely play a central role as they will allow resolution of microbiome-conferred functionalities associated with health. Such information will be vital for formulating therapeutic interventions aimed at managing microbiota-mediated health particularly in the GIT over the course of a human lifetime.
Introduction
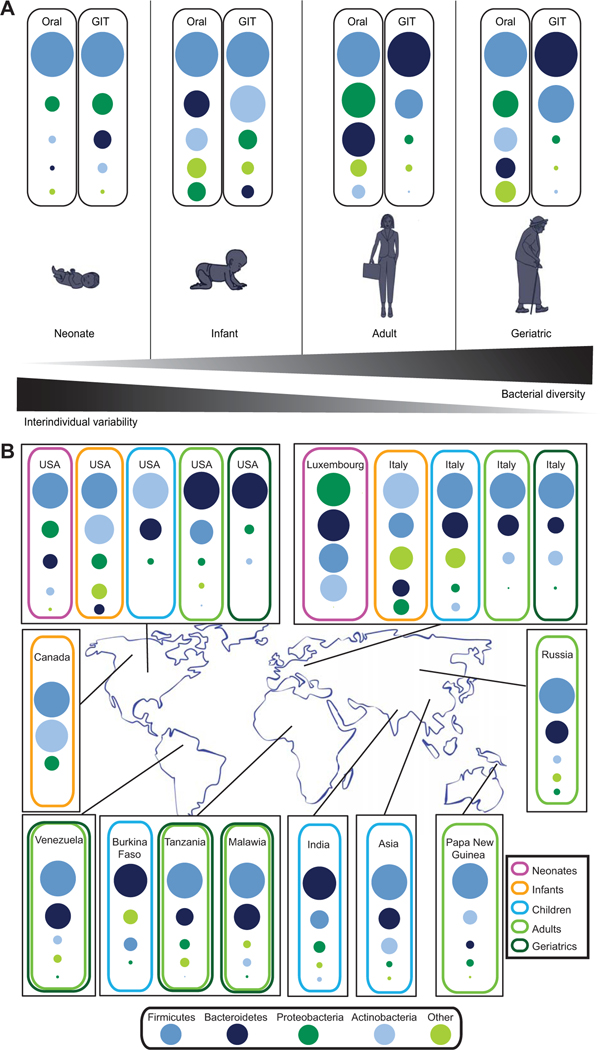
Technological advances in culture-independent molecular methods are allowing us to uncover a new facet of our natural history by accounting for the vast diversity of microbial life, which colonizes the human body. Human beings are now more than ever regarded as microbial ecosystems, comprising bacteria, archaea, eukaryotes and viruses with whom we have coevolved and which colonize different body habitats. The human microbiome contributes essential functionalities to human physiology and is considered essential for the maintenance of human health (Rooks et al., 2014). As the microbiota in certain body habitats, e.g. the gastrointestinal tract (GIT), are temporally stable over the short- and medium-term (Voigt et al., 2015), the definition of microbiome attributes associated with general host characteristics, e.g. health, should be possible. However, microbial community compositions vary over human lifetimes and geographies (Yatsunenko et al., 2012). Furthermore, relatively large intraindividual differences exist between distinct body sites and considerably less but notable interindividual variation is apparent even among healthy individuals for a given body site (Grice et al., 2009; Franzosa et al., 2015; Voigt et al., 2015). Consequently, it may be difficult to define site-specific baseline microbiomes associated with human health especially at lower taxonomic ranks (such as at the genus, species and/or strain levels). This restriction is in part due to the limited resolution afforded by 16S rRNA gene amplicon sequencing which presently is the method of choice in most studies. The structural differences which are typically resolved at higher taxonomic ranks (such as at the phylum, class and/or order levels) may not be very meaningful as many distinct taxa are typically regrouped, thereby confounding linkages between specific microbial community structures and health status. Therefore, future studies encompassing metagenomics and functional omics will allow much deeper insights into the structural and functional complements of microbiota associated with health. Nonetheless, individual body sites exhibit certain broad features associated with health, that are resolvable using 16S rRNA gene-based surveys, which include specific distributions of microbial phyla, diversity and relative stability over time (Fig. 1A).
Fig. 1.
Characteristics of the ‘healthy’ human microbiome. A. The ‘healthy’ microbiome of individual body sites throughout human lifetime. B. The ‘healthy’ gastrointestinal tract microbiome across geographies. Dots are weighted according to the overall abundance of distinct phyla. Additional information regarding the studies, based on which Fig. 1 was devised, are listed in Table S1.
The human GIT microbiome has been the major focus of studies as it contains the vast majority of microbial biomass (Eckburg et al., 2005) and can relatively easily be sampled by collection of fecal material. Additionally, it is intimately involved in digestion, metabolite production, cross-talk with the immune system (Flint et al., 2012), and has been implicated in numerous disease processes (Kinross et al., 2011). The human GIT microbiome is dominated by four main bacterial phyla including Firmicutes, Bacteroidetes, Actinobacteria and Proteobacteria (Ley et al., 2008). Given their dominance, the Bacteroidetes and Firmicutes have probably received the most attention with respect to the microbial ecology of the GIT. While the Firmicutes are primarily associated with energy harvest from food (Turnbaugh et al., 2006), the Bacteroidetes are linked with several health benefits due to their capacity to degrade complex sugars and proteins into metabolizable short chain fatty acids (SCFAs) (Ley et al., 2006).
Based on recent surveys, it is apparent that the human GIT microbiome changes significantly over human lifetime and that age-specific differences may be key to understanding microbiome-mediated effects on health. Although other body habitats are distinct in their microbiota compositions from the GIT, they remain much less explored with regards to differences across age and geography. Consequently, we use here the human GIT microbiome as a template for discussing the development and changes of the human microbiome over a lifetime while accounting for biogeography, host genetics, diet and other environmental influences. Although the microbial community compositions in other body habitats, e.g. the skin, are shaped by combinations of factors distinct from those in the GIT, observations in the GIT microbiome with respect to colonization, succession and dynamics may prove helpful for the future contextualization of patterns observed in other body habitats in the context of human health. In particular, the refinement of our understanding of normal variation within microbial communities will further our knowledge of their structure and function and allow us to identify key features which reflect deviations therefrom, thereby facilitating the design of advanced therapeutics aimed at managing microbiota-mediated health over the course of life.
Development and succession of the GIT microbiome over the human lifetime
In order to understand how the GIT microbiome is involved in human health, we need to understand how the microbiome develops and evolves with age. The GIT microbiome is dynamic over a human lifetime (Fig. 1A); colonization may begin in utero, and the undifferentiated, low diversity GIT microbiome at birth proceeds through various developmental stages with associated changes in diversity, structure and functional gene repertoires (Yatsunenko et al., 2012; Hollister et al., 2015).
First exposures: in utero colonization
Until recently, it was assumed that the fetal environment is sterile and that microbial colonization begins with birth. However, with the advent of culture-independent molecular methods, recent evidence has called this assumption into question. The placenta, amniotic fluid, fetal membranes, and cord blood from healthy, term pregnancies have been shown to harbour microorganisms, suggesting that the presence of bacteria in these tissues is not necessarily indicative of a pathogenic state (Jiménez et al., 2005; Steel et al., 2005; Rautava et al., 2012; Aagaard et al., 2014). Furthermore, the application of culture-dependent and culture-independent methods have demonstrated that meconium is not sterile but contains bacterial communities similar to those detected in amniotic fluid (Jiménez et al., 2008a; Gosalbes et al., 2013; Ardissone et al., 2014). Taken together, these results challenge the assumption of a sterile in utero environment and suggest that initial colonization of the GIT may begin before birth.
Potential sources of these pioneering microbes include the vaginal microbiome (Aagaard et al., 2012; Romero et al., 2014), resident bacteria in the uterus (Hemsell et al., 1989; Cowling et al., 1992; Møller et al., 1995), and the maternal digestive track including the oral cavity (Aagaard et al., 2014). The vagina harbours an abundant, low diversity microbiome dominated by Lactobacillus spp. and its proximity to the fetus makes it a possible candidate for fetal colonization (Aagaard et al., 2012). However, bacterial taxa identified in the fetal environment are diverse and include taxa associated with the oral and GIT microbiomes such as Fusobacterium spp. and Bacteroides spp., suggesting the vagina may not be the primary source of inoculum (Rautava et al., 2012; Aagaard et al., 2014). Recent studies have demonstrated that placentas from healthy, term pregnancies harbour a low abundance but diverse microbiome most akin to the maternal oral microbiome, suggesting that bacteria translocated from the oral cavity may colonize the placenta and serve as an initial source for fetal exposure (Aagaard et al. 2014; Zheng et al., 2015). Oral bacteria may enter the blood stream via minor abrasions in the oral mucosa (Lockhart et al., 2008), and preterm birth is associated with both periodontal disease and intrauterine infection, suggesting hematogenous dissemination of bacteria from the oral cavity may be involved in both physiological and pathological uterine bacterial colonization (Goepfert et al., 2004; Klein and Gibbs, 2005; Schwendicke et al., 2015). However, these routes of colonization remain largely speculative, and further detailed studies are needed to characterize the impact of the prenatal maternal microbiome on offspring colonization.
Taking into account that development of the GIT microbiome may begin before birth, we must explore the factors and exposures that influence this process. It is well established that diet modulates the GIT microbiome in adults (David et al., 2014), and recent studies have revealed that maternal diet likely influences the offspring’s microbiome as well. In a nonhuman primate study, a high fat maternal diet (36% fat) during gestation and lactation was associated with a persistent microbial dysbiosis in offspring even after they were weaned onto an isocaloric control diet (13% fat) (Ma et al., 2014). While this study failed to separate the effects of maternal diet during gestation and lactation, another study demonstrated that excess gestational weight gain, but not obesity, is associated with changes in the placental microbiome, suggesting maternal diet during gestation may modulate colonization in utero (Antony et al., 2015). In addition to maternal diet, antenatal infection (Aagaard et al., 2014) and maternal stress during pregnancy (Bailey et al., 2004) are associated with changes in the placental microbiome. Moreover, pregestational diabetes (Hu et al., 2013) and prenatal probiotic supplementation (Lahtinen et al., 2009) correlate with significant changes in the offspring’s microbiome. Together, this evidence suggests that the prenatal period plays a significant role in shaping the offspring microbiome.
The microbiome at birth: perinatal influences
Compared to the adult microbiome, the GIT microbiome at birth has significantly lower diversity and higher variability among individuals (Fig. 1A) (Dominguez-Bello et al., 2010; Yatsunenko et al., 2012). The dominant phyla in the neonatal GIT include Firmicutes, Proteobacteria and Actinobacteria with lower levels of Bacteroidetes, a dominant phylum in the adult GIT microbiome (Fig. 1A) (Gosalbes et al., 2013; Ardissone et al., 2014; Del Chierico et al., 2015). Culture-based studies have reported a predominance of Bifidobacterium spp. (phylum Actinobacteria) in the neonatal microbiome (Penders et al., 2006). These taxa are known to elicit beneficial effects in their host by breaking down dietary carbohydrates and interacting directly with host metabolism (Davis et al., 2011). However, many culture-independent studies have reported relatively low abundances of Actinobacteria in the neonatal microbiome (Fig. 1A). This inconsistency may be explained by poor PCR amplification of Bifidobacteria spp. due to variation in the conserved regions of the 16S rRNA gene. In general, this circumstance highlights the need to consider extraction and PCR bias when evaluating results from studies employing molecular methods (Milani et al., 2014).
Gestational age at delivery as well as mode of delivery influence the neonatal microbiome at birth (Dominguez-Bello et al., 2010; Ardissone et al., 2014). Compared to infants delivered at term, the neonatal microbiome of preterm infants exhibits overall lower diversity and lower abundance of Lactobacillus spp., Bacteroides spp. and Bifidobacterium spp., with some differences persisting until 90 days postpartum (Arboleya et al., 2011, 2012, 2015). A study of a large cohort of preterm infants demonstrated that gestational age at birth appears to influence the pace, but not necessarily the progression of bacterial colonization, and that gestational age has a larger influence on the progression of colonization compared to other exogenous factors including antibiotic use, diet, and mode of delivery (La Rosa et al., 2014). This association may be explained by a microbiota-driven process involved in the pathogenesis of preterm birth, an altered neonatal environment of the preterm infant or possibly an interruption in fetal colonization in utero. While much has yet to be learnt about the importance of these factors, recent evidence suggests that the GIT microbiome of preterm infants is largely shaped by microbiota derived from feeding and intubation as well as the immediate incubator and room environment within neonatal intensive care units rather than by individuals who come into contact with the infants (parents and healthcare providers) (Brooks et al., 2014). Regardless of the main factors at play, the microbial perturbations associated with preterm birth are considered to be problematic for the infant, as necrotizing enterocolitis, a severe inflammatory disease of the GIT, is strongly associated with both preterm birth and microbial dysbiosis (Wang et al., 2009; Mai et al., 2011).
In addition to gestational age, recent studies suggest that mode of delivery may influence the immediate neonatal microbiome at birth, although the longer-term impact remains unclear. In the immediate neonatal period, Cesarean delivery is associated with an overall lower bacterial diversity, a lower abundance of Bacteroides spp., Bifidobacterium spp. and Lactobacillus spp. and a bacterial composition akin to maternal skin, while the meconium microbiome of vaginally delivered infants is more similar to the mother’s vaginal microbiome (Grönlund et al., 1999; Penders et al., 2006; Biasucci et al., 2008, 2010; Dominguez-Bello et al., 2010; Jakobsson et al., 2014). However, many of these studies were limited in sample size and failed to stratify subjects based on indication for Cesarean delivery (e.g. unlabored repeat Cesarean versus labored with fetal macrosomia). Thus, differences attributed to mode of delivery may be due to the underlying pathology or fetal physiology that necessitates initial Cesarean delivery. A recent study has found that, in an attempt to recapitulate inoculation during passage through the birth canal, exposure of Cesarean-delivered infants to maternal vaginal fluids at birth results in a relatively minimal effect on the bacterial community profile of anal swab samples, reflecting the partial restoration of a few bacterial taxa (Lactobacillus spp. and Bacteroides spp.) when compared to levels found in samples from vaginally delivered infants (Dominguez-Bello et al., 2016). At present, it is difficult to definitively determine the significance of these findings with respect to the GIT microbiome, as for example the presence of these taxa may be due to direct inoculation of vaginal bacteria to the perianal region during the inoculation procedure rather than actual changes in the GIT microbiome. Overall, further studies employing larger sample sizes, longer sampling duration and stratification by delivery indication are necessary to determine if failure to pass through the birth canal has a significant, lasting impact on infant microbial development.
The infant microbiome: development during the first year of life
The first year of life represents a significant period of fluctuation and maturation of the GIT microbiome (Fig. 1A). Taxonomic diversity is relatively low at birth but increases over time as the infant is colonized with bacteria acquired from breast milk and the environment (Fig. 1A) (Schanche et al., 2015; Thompson et al., 2015).
Diet is a significant driver of the developing infant microbiome as it adapts to the changing availability of nutrients (Thompson et al., 2015). Early in infancy, the GIT microbiome is enriched in genes involved in digestion of oligosaccharides found in breast milk, while later in infancy, due to the introduction of solid foods, the metagenome is enriched in genes involved in the digestion of polysaccharides (contributed for example to the microbiome by Bacteroides spp. (Ravcheev et al., 2013)) and vitamin biosynthesis (Koenig et al., 2011; Bäckhed et al., 2015). Furthermore, the mode of feeding significantly influences microbial composition in the infant GIT (Thompson et al., 2015). Breast-fed infants show an increase in the relative abundance of Actinobacteria and a decrease in Firmicutes and Proteobacteria, while formula-fed infants exhibit enrichments in putative pathogens, including Escherichia coli and Clostridium difficile (Penders et al., 2006; Fallani et al., 2010; Azad et al., 2013; Lee et al., 2015). Breast milk contains multiple components that have the potential to impact the composition of the infant GIT microbiome, including immunoglobulins (Rogier et al., 2014), prebiotic oligosaccharides (which favor the growth of Bifidobacterium spp.) (Hardy et al., 2013), and diverse breast milk microbiota that continually seed the infant GIT (Hunt et al., 2011). Intriguingly, the breast milk microbiome includes bacteria possibly derived from the maternal GIT via enteromammary trafficking, a proposed pathway by which enteric bacteria are engulfed by leukocytes and delivered to the mammary glands via systemic circulation (Stagg et al., 2003; Perez et al., 2007; LaTuga et al., 2014). In support of this potential pathway are results showing that oral probiotics given to lactating mothers are detectable in breast milk and identical bacterial strains are shared between the maternal GIT, breast milk and the infant GIT (Jiménez et al., 2008a,b; Jost et al., 2014). Thus, enteromammary trafficking may represent a mechanism for vertical transmission of the GIT microbiome. Given that diet significantly affects the adult GIT microbiome (David et al., 2014), the trafficking from maternal to infant GIT may explain the observation that high fat maternal diet is associated with persistent microbial dysbiosis in nursing offspring (Ma et al., 2014). However, further studies are clearly needed to elucidate the impact of the maternal GIT microbiota on the developing microbiome of nursing infants.
In addition to infant diet, environmental and pharmacological exposures are also associated with differences in the developing infant microbiome. These factors include antibiotic exposure (Penders et al., 2006; Mangin et al., 2010), number of siblings (Penders et al., 2006), exposure to pets (Nermes et al., 2015), daycare attendance (Thompson et al., 2015) and geography (Fallani et al., 2010). Finally, the interindividual variation within the GIT microbiome is much larger in infants compared to adults (Yatsunenko et al., 2012) suggesting that early successional patterns are not uniform, but nevertheless reach a relatively similar endpoint in the adult microbiome (Yatsunenko et al., 2012).
The GIT microbiome in childhood
Based on initial culture-based surveys, it was thought that the human GIT microbiome reaches a mature state of colonization between the ages of 1–4 years (Ellis-Pegler et al., 1975). However, more recent molecular studies have uncovered key differences in the GIT microbiome of children and adults (Hopkins et al., 2001; Cheng et al., 2015; Hollister et al., 2015). A study in the United States revealed that compared to the adult GIT microbiome, there is an overall clear enrichment in Firmicutes (Cheng et al., 2015; Hollister et al., 2015), Proteobacteria (Saulnier et al., 2011) and Actinobacteria (Cheng et al., 2015; Hollister et al., 2015) and a decrease in Bacteroidetes (Saulnier et al., 2011) in the GIT microbiome of American children. Furthermore, Roseburia spp., Faecalibacterium spp., Ruminococcus spp., Alistipes spp., Bacteroides vulgatus and Bacteroides xylanisolvens were all found to be enriched in preadolescent American children when compared to adult counterparts (Hollister et al., 2015). Roseburia spp., Faecalibacterium spp. and Ruminococcus spp. are butyrate-producing bacteria which are associated with a healthy GIT microbiome (Khan et al., 2012; Neyrink et al., 2012; Zhang et al., 2015). On a functional level, the GIT microbiomes of children in the United States are enriched in functions which may support ongoing development, e.g. vitamin B12 (Hollister et al., 2015) when compared to American adults. The functional repertoires appear to shift over time and culminate in the adult GIT microbiome. Moreover, as we age the GIT microbiome is enriched in more traits associated with inflammation and metabolic dysfunction (Hollister et al., 2015).
As with other stages of life, there also appear to be clear geographical differences with respect to the childhood microbiome. In particular, the GIT microbiome of Italian children is mainly comprised of Firmicutes, Bacteroidetes and a smaller proportion of Actinobacteria (Fig. 1B). In contrast, children residing in rural villages in Burkina Faso exhibit a significant enrichment of Bacteroidetes and depletion of Firmicutes and an enrichment in bacterial genes capable of degrading cellulose and xylan (De Filippo et al., 2010). To date, the functional characteristics of the microbiome of children in all other geographical regions remain to be explored in detail. Overall, the GIT microbiome in childhood, while more established than the infant microbiome, is not completely mature and, therefore, represents a dynamic community which likely influences health outcomes later on in life.
The GIT microbiome in adolescence
SimiIar to the child microbiome, it was assumed that the microbiomes of adolescents are no different than those of adults. However, a single molecular study has identified differences in the adolescent GIT microbiome, including a higher abundance of Clostridium spp. and Bifidobacteria spp. compared to the adult microbiome (Hopkins et al., 2001). Research on the healthy GIT microbiome in adolescence barely exists and future studies are clearly needed in this area. In particular, as fluctuating hormone levels are a hallmark of adolescence, changes in the GIT microbiome likely also occur during this important transitional period similar to other life events associated with major hormonal changes, e.g. pregnancy (Koren et al., 2012).
The GIT microbiome in adulthood
The majority of microbiome studies to date have focused on the adult GIT microbiome, which comprises mainly Firmicutes, Bacteroidetes and Proteobacteria (Fig. 1B) (Huttenhower et al., 2012). The proportion of each phylum varies according to geographical location (Schnorr et al., 2014). For example, a few studies have shown that Firmicutes are enriched in adults in nonindustrialized countries (Schnorr et al., 2014; Martinez et al., 2015), whereas adults in westernized societies appear to exhibit a higher Bacteroidetes to Firmicutes ratio (Huttenhower et al., 2012; Zhu et al., 2015). However, this trend is not reflected in two separate studies in which enrichments in Firmicutes were observed for GIT microbiomes from European countries (Mueller et al., 2006; Arumugam et al., 2011). A higher ratio of Firmicutes to Bacteroidetes has been mostly attributed to energy harvest and body weight gain (Turnbaugh et al., 2006). Additional future work is required to clarify the relative levels of Firmicutes and Bacteroidetes and their associated functionalities in healthy human populations across different geographies and their potential impact on health and obesity.
Overall the adult GIT microbiome remains relatively stable through adulthood, except following perturbations such as infections, antibiotic treatment or drastic dietary interventions (David et al., 2014). Even though the GIT microbiome recovers to its initial state relatively quickly (Wu et al., 2011; David et al., 2014), these perturbations subtly alter the GIT microbiome composition over time and are likely strong drivers behind the extensive strain-level interindividual differences which are apparent in healthy adults (Zhu et al., 2015). Taken together, this suggests that the human hologenome [the collective of human and microbial genomes (Moran and Sloan, 2015)] is very much individual-specific.
The GIT microbiome in old age
Studies from Europe suggest that the GIT microbiome in the elderly is distinct from adults (Fig. 1B) (Mariat et al., 2009). The GIT microbiome is thought to influence the overall health of the elderly, as changes in its composition have been associated with declines in health (Claesson et al., 2012). Overall, the GIT microbiome of the elderly exhibits a higher Firmicutes to Bacteroidetes ratio when compared to adults (Mariat et al., 2009) with a concomitant reduction in protective commensal bacteria such as Bifidobacteria and Bacteroides (Mariat et al., 2009). A reduction in Bacteroides spp., Prevotella spp. and Faecalibacterium prausnitzii (Mariat et al., 2009; Cho and Blaser, 2012), and an increase in Enterobacteriaceae, has been associated with on overall decrease in the quality of life in old age (Van Tongeren et al., 2005). Factors which play a greater role in old age and which might lead to the observed changes in GIT microbiota include an overall increased use of medication, dietary deficiency, as well as changing hormonal levels (Voreades et al., 2014). In order to study the dynamics of the healthy microbiome as we age, further in-depth studies, including a focus on the identification of specific microbiome-conferred functionalities in different geographical regions are necessary. Furthermore, as impaired dentition accompanies old age, it may be worth-while to explicitly investigate the oral microbiome as this is likely a contributor to changes in GIT microbiota (Claesson et al., 2012) and consequently health status.
Age-independent influences on the GIT microbiome and its modulation
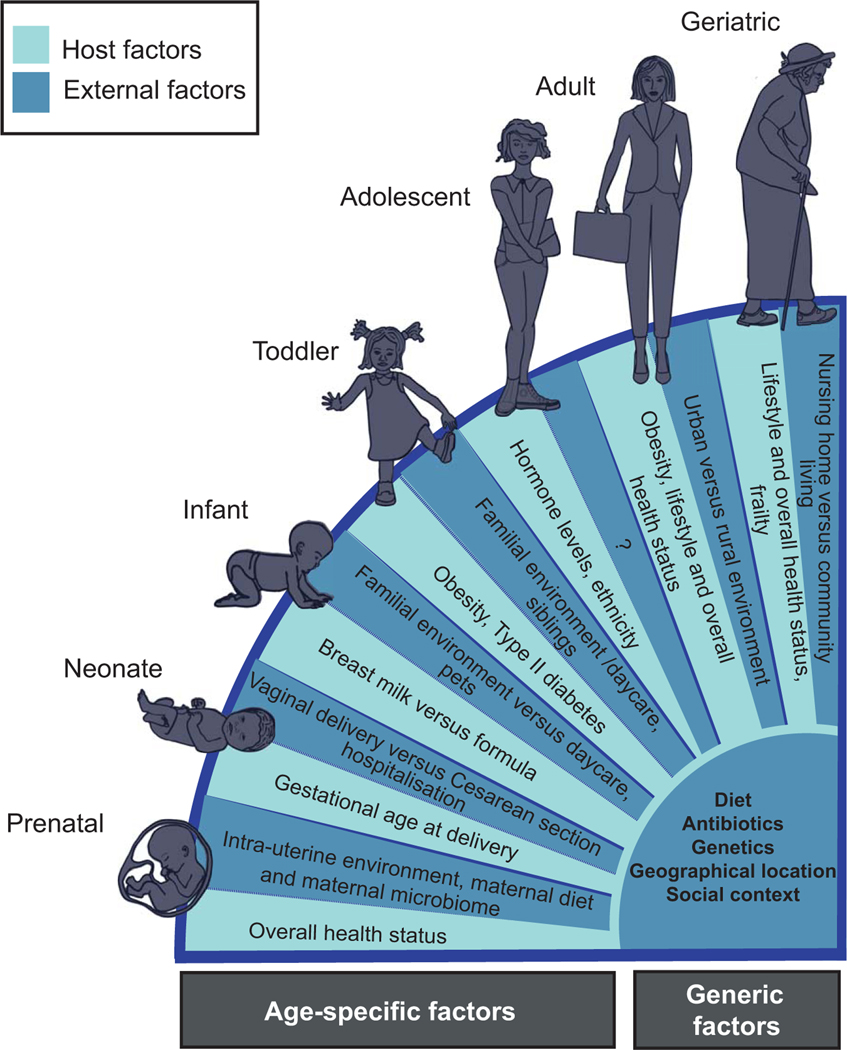
Apart from age-associated changes in the GIT microbiota of healthy individuals, other factors including geographical location, social context, gender, host genetics, diet and antibiotic use may also greatly impact the composition of the GIT microbiome (Fig. 2) (Dethlefsen et al., 2008; Walker et al., 2011). In particular, apparent geographical differences, for example between rural and urban areas (Tyakht et al., 2013), may be attributable to variations in dietary habits or other environmental influences (Fig. 2) (De Filippo et al., 2010; Rampelli et al., 2015; Nakayama et al., 2015). Apart from these factors, the differences observed between school children attending private schools compared to children living in impoverished conditions may be the result of several social factors which are presently difficult to deconvolute (De Mello et al., 2009). These factors likely also contribute to the described association between taxonomic structures and level of education (Ding and Schloss, 2014). Furthermore, elderly individuals that live in a community setting have higher proportions of Firmicutes to Bacteroidetes in comparison to long-stay residential individuals (Fig. 2) (Claesson et al., 2012). Data from the Home Microbiome Project has found that the human microbiome fingerprint is very similar between individuals that share the same home (Lax et al., 2014) which in turn suggests an overall high degree of nestedness for the GIT microbiome which likely further reinforces social effects. These results as well as those from other studies (Yatsunenko et al., 2012) suggest that the overall environmental setting is a strong factor in shaping the human GIT microbiome and, in more general terms, the microbiomes of other body habitats.
Fig. 2.
Factors which influence the gastrointestinal tract microbiome according to different life stages.
Apart from environmental factors, host genetics have also been found to shape GIT microbiome composition (Goodrich et al., 2014; Blekhman et al., 2015). More specifically, many microbial taxa whose abundances appear to be affected by host genetics have been identified (Goodrich et al., 2014). Interestingly, the most heritable taxon identified, the family Christensenellaceae, was found to be enriched in individuals with low body mass index. When transplanted into germ-free mice, a Christensenellaceae amendment reduced weight gain significantly and overall altered the microbiome of recipient mice (Goodrich et al., 2014). Strikingly, the heritability of Christensenellaceae did not appear to be driven by diet and was, therefore, mainly due to host genetic factors (Goodrich et al., 2014). Furthermore, gender-specific differences in GIT microbiome composition have been observed, which may be explained by combinations of host genetics and social context (Markle et al., 2013; Ding and Schloss, 2014).
Although diet appears not to be the main driver influencing microbiome composition, certain major taxa, e.g. the Bacteroidetes, have been found to respond very rapidly to dietary changes (David et al., 2014). In particular, Western dietary habits have been found to significantly affect the GIT microbiome which in turn has been linked to metabolic diseases (Fukuda and Ohno, 2014; Hur and Lee, 2015). The Western diet consists largely of processed foods and is high in simple carbohydrates, animal protein, fat and low in dietary fibre (De Filippo et al., 2010). In contrast, high fibre diets are typically composed of fresh vegetables, fruits and whole grains (Fung et al., 2001). Fibre-rich diets can have short term-impacts on the GIT microbiome (David et al., 2014), but more importantly long-term fibre intake has been linked to microbiome-mediated beneficial, systemic effects (Kuo, 2013). Interesting in this context are Prevotella spp. and Xylanibacter spp., two genera capable of degrading nondigestible fibres, which are enriched in children from Burkina Faso and completely absent in European children (De Filippo et al., 2010). Fibre-degrading bacteria ferment dietary fibre to SCFAs (Flint et al., 2012), which provide an important source of energy for host cells (Bäckhed et al., 2004; Turnbaugh et al., 2006) as well as being involved in the maintenance of the epithelial barrier (Kelly et al., 2015) and modulation of the immune system (Furusawa et al., 2013). The two latter processes are particularly pertinent in the context of allergic diseases, which show an increasing incidence world-wide, particularly in Western countries (Azad et al., 2013). More specifically, increased circulating levels of fibre-derived SCFAs have been found to protect against inflammation in the lung of a mouse model, whereas a low-fibre diet decreased levels of SCFAs and increased allergic airway disease (Trompette et al., 2014). Additionally, even though the results are not conclusive, it seems that there is potential that the co-administration of nondigestible fibre during antibiotic treatment has promising effects on the GIT microbiome such as prevention or alleviation of antibiotic-associated diarrhea (Moore et al., 2015).
Epidemiological data suggests that the increasing prevalence of antibiotic usage and general cleanliness in Western countries (‘the hygiene hypothesis’) negatively affects the GIT microbiome and that these contribute to the increasing incidence in allergic diseases in these countries (Azad et al., 2013). The administration of antibiotics directly perturbs the GIT microbiome leading to decreased bacterial diversity and richness (Dethlefsen et al., 2008; Claesson et al., 2011). Even though the GIT microbiome is restored after approximately one month (Claesson et al., 2011), some taxa are permanently lost (Dethlefsen et al., 2008). However, these and other studies (Penders et al., 2006; Mangin et al., 2010) have only investigated microbial diversity before and after interventions and have not examined transient and subsequent microbial metabolic profiles nor the molecular repercussions over longer durations. Even though antibiotic exposure might only have relatively short-term effects on the microbiota, it might activate long-term changes in gene expression that could affect the immune system or metabolism which in turn might culminate in chronic diseases (Nobel et al., 2015). Furthermore, facultative pathogens may occupy larger niches postadministration which may lead to sustained proinflammatory states (Gagliani et al., 2014). Clearly, detailed longitudinal studies aimed at understanding the long-term impact of antibiotic treatment on microbiome-mediated health are needed to fully understand the resilience of the GIT microbiome in relation to antibiotic exposure and to identify possible supportive treatment strategies.
A well-established instance where persistent antibiotic use leads to a major disruption of the GIT microbiota balance with potentially life-threatening consequences is that of recurrent Clostridium difficile infection, which is particularly prevalent in elderly individuals. In terms of treatment, fecal microbiota transplantation (FMT) has recently proven to be very efficacious as it results in the reestablishment of a more diverse, ‘healthy’ microbiome (Kelly et al., 2014; Dutta et al., 2014; Cohen et al., 2015). Apart from FMT, other more classical means for modulating the GIT microbiome include the administration of defined pre-, pro- or synbiotic formulations (Hardy et al., 2013). Prebiotics comprise nondigestible food components that promote the growth of certain beneficial bacteria, e.g. SCFA-producing bacteria, in the GIT. Examples include dietary supplementation with nondigestible fibre which promotes the growth of Actinobacteria (Gerritsen et al., 2011; Johnson and Versalovic, 2012; Simeoni et al., 2015). Actinobacteria have been shown to be associated with health benefits and to protect against pathogens by modulating the host’s immune responses (Ventura et al., 2007). Probiotics are living bacteria that can provide health benefits to the host when administered in adequate amounts (Rijkers et al., 2011) as they may potentially restore a disturbed GIT microbiome (Sullivan and Nord, 2002) and activate anti-inflammatory pathways (Eloe-Fadrosh et al., 2015). Certain probiotic species produce SCFAs and may secrete other molecules, which, inter alia, may stimulate the growth of other beneficial bacteria (Eloe-Fadrosh et al., 2015) and thereby prevent the expansion of pathogenic bacteria (Collado et al., 2007). Synbiotics, which involve a combination of probiotics and prebiotics administered together, have been shown to favourably modulate interactions between the GIT microbiome and host, and thereby confer overall health benefits (Druart et al., 2014). More specifically, modulation of the microbiome by synbiotic regimes has been demonstrated to be efficacious for the protection against sensitization to food allergens (Stefka et al., 2014), the reduction of inflammation in the GIT (Furrie et al., 2005) and a corresponding reduction in colorectal cancer risk (Ohashi et al., 2002; Rafter et al., 2007; Liu et al., 2011). Beyond the use of undefined modulatory regimes, i.e. FMT, targeted age-group specific synbiotic regimes may be further developed (Ley et al., 2006; De Filippo et al., 2010) to prevent or/and treat imbalanced GIT microbiota over the course of human life.
Attributes of baseline ‘healthy’ microbiomes
Even though the GIT microbiome in healthy individuals is considered to be relatively stable (Manichanh et al., 2008; Ding and Schloss, 2014), the identification of features characteristic of a ‘healthy’ microbiome is a challenging task. Indeed, desirable microbial community structures associated with human health may be different for different individuals according to their age, environment, genetics, diet, etc., and may be more dependent on microbial gene carriage patterns than taxonomic composition (Huttenhower et al., 2012; Zhu et al., 2015). In contrast, given the relative stability of functions encoded in GIT metagenomes (Huttenhower et al., 2012), it is likely that a core of microbiome-conferred functionalities exists. The characteristics of such a ‘functional core’ should be explored and its specific attributes in relation to human health should be investigated.
Many studies have identified microbial community structures associated with specific diseases and correspondingly, it is tempting to associate ‘health’ with the features identified in the healthy control cohorts of these studies. However, assigning causality to features associated with health and disease is a difficult problem and often requires use of model systems, which at present do not accurately reflect human physiology (Fritz et al., 2013). Additionally, careful consideration of exclusion criteria should be taken before these features are considered to be reflective of a ‘healthy’ microbiome, as many studies may identify ‘healthy’ features which may be associated with other factors not under investigation. For example, many studies have identified a relatively low Firmicutes to Bacteroidetes ratio to be associated with health, as elevated ratios have been linked to metabolic diseases (Ley et al., 2006; Mariat et al., 2009). However, due to considerable variation in the observed Bacteroidetes to Firmicutes ratios in healthy subjects, which may in turn be due to several factors (Ley et al., 2006; Mariat et al., 2009), this ratio is likely not be a generalizable indicator of human health (Hollister et al., 2015).
Large-scale studies of healthy individuals screened with extensive exclusion criteria such as the Human Microbiome Project and MetaHIT, have allowed us to begin to define what is characteristic of healthy individuals, and this should form the framework for defining features of ‘healthy’ microbiomes in the future. In addition to identifying microbial community structures associated with human health, studies should also focus on identifying specific functionalities and the corresponding bacterial taxa that confer these functions to the individual. For example, some Bacteroides spp. are known to produce specific beneficial products such as polysaccharide A (Johnson et al., 2015) and SCFAs (Walker et al., 2005; Ley et al., 2006). Although 16S rRNA gene-based analyses have a role to play for the ab initio characterization of community structures, future studies should involve the systematic generation of metagenomic and functional omic [metatranscriptomic, metaproteomic, (meta-)metabolomics] data for resolving the functional complements of microbiomes. Ultimately, the functionalities encoded and conferred by the microbiota are key to ensuring health. Finally, the establishment of a repertoire of beneficial bacterial strains is necessary as their administration as probiotics potentially in conjunction with prebiotic formulations represents one of the most promising avenues of microbiome-related therapeutics.
Characterization efforts aimed at defining ‘healthy’ microbiota compositions must be expanded beyond the lower GIT microbiome, and links between different body sites must be systematically investigated. For example, the oral cavity is a main gateway for microorganisms to enter and interact with the human body and the community types of the oral and lower GIT microbiomes are predictive of each other (Ding and Schloss, 2014). Additionally, the oral microbiome has been associated with oral infectious diseases as well as systemic diseases (Ley et al., 2006; Seymour et al., 2007). Consequently, the oral microbiome might be a key factor for understanding human health over a human life-time. Furthermore, it is also likely that alterations to the microbiota in other body habitat, e.g. the skin (Weyrich et al., 2015), may trigger systemic effects not least via immune system responses. Clearly, microbial communities at different body sites are involved in different aspects of human health, and the systematic characterization of many body sites is therefore imperative for our overall understanding of microbiota-mediated health in the future.
Concluding remarks and perspectives
Given the complexity and diversity of microbial community structures in healthy individuals (Huttenhower et al., 2012), it may be at present difficult to define attributes characteristic of baseline ‘healthy’ microbiomes. In spite of the relatively conserved, characteristic features of body site-specific microbiota (Kurokawa et al., 2007; Huttenhower et al., 2012; Hollister et al., 2015), it remains unclear which factors influence microbial community assembly and their relative importance. Key factors which likely influence microbiota compositions include age (Palmer et al., 2007; Mariat et al., 2009; Koenig et al., 2011), geographical location (Yatsunenko et al., 2012), host genetics (Gagliani et al., 2014; Blekhman et al., 2015), social context (De Mello et al., 2009; Koenig et al., 2011; Nakayama et al., 2015), and diet (David et al., 2014) (Fig. 2). Therefore, in order to understand how the microbiome plays a role in maintaining human health, studies aimed at identifying measures that sustain beneficial microbiome compositions over the course of a human lifetime along with high-resolution longitudinal data in different geographic locales is essential. Such data would need to include information on highlighted factors influencing microbiome composition as well as provide a sampling from different body sites of individuals of different ages, ethnicities, socio-economic backgrounds and geographies. Furthermore, such efforts should go beyond mere 16S rRNA gene-based taxonomic surveys and include systematically generated metagenomic and functional omic data. Given the microbiome’s contribution of key functionalities to host physiology, the inclusion of functional information will likely greatly enhance the linking of microbial contributions to overall health status. Additionally, it is essential that all data is stored in a web-accessible comprehensive database to allow comparisons of body-site specific microbiome characteristics across studies. Therefore, in our opinion, a standardized, concerted international effort is needed to resolve and define the healthy human microbiome.
Supplementary Material
Relative abundance of bacterial phyla in stool samples obtained in different geographical locations world-wide (average %).
Acknowledgements
This work was funded by an ATTRACT program grant (ATTRACT/A09/03) and a European Union Joint Programming in Neurodegenerative Diseases grant (INTER/JPND/12/01) awarded to P.W. as well as a PhD grant Aide à la Formation Recherche (AFR) to K.M.G. (PHD/9964547), all funded by the Luxembourg National Research Fund (FNR). The authors have no further conflicts of interest relevant to the content of this review.
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
References
- Aagaard K, Riehle K, Ma J, Segata N, Mistretta T-A, Coarfa C, et al. (2012) A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One 7: e36466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, and Versalovic J (2014) The placenta harbors a unique microbiome. Science Transl Med 6: 237ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony KM, Ma J, Mitchell KB, Racusin DA, Versalovic J, and Aagaard K (2015) The preterm placental microbiome varies in association with excess maternal gestational weight gain. Am J Obstet Gynecol 212: e1–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arboleya S, Binetti A, Salazar N, Ferná Ndez N, Solís G, Herná Ndez-Barranco, et al. (2011) Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol Ecol 79: 763–772. [DOI] [PubMed] [Google Scholar]
- Arboleya S, Ang L, Margolles A, Yiyuan L, Dongya Z, Liang X, et al. (2012) Deep 16S rRNA metagenomics and quantitative PCR analyses of the premature infant fecal microbiota. Anaerobe 18: 378–380. [DOI] [PubMed] [Google Scholar]
- Arboleya S, Sánchez B, Milani C, Duranti S, Solís G, Fernández N, et al. (2015) Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J Pediatr 166: 538–544. [DOI] [PubMed] [Google Scholar]
- Ardissone AN, de la Cruz DM, Davis-Richardson AG, Rechcigl KT, Li N, Drew JC, et al. (2014) Meconium microbiome analysis identifies bacteria correlated with premature birth. PLoS One 9: e90784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, et al. (2011) Enterotypes of the human gut microbiome. Nature 473: 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, et al. (2013) Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ 185: 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. (2004) The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U. S. A. 101: 15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. (2015) Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17: 690–703. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Lubach GR, and Coe CL (2004) Prenatal stress alters bacterial colonization of the gut in infant monkeys. J Pediatr Gastroenterol Nutr 38: 414–421. [DOI] [PubMed] [Google Scholar]
- Biasucci G, Benenati B, Morelli L, Bessi E, and Boehm G (2008) Cesarean delivery may affect the early biodiversity of intestinal bacteria. J Nutr 138: 1796S–1800S. [DOI] [PubMed] [Google Scholar]
- Biasucci G, Rubini M, Riboni S, Morelli L, Bessi E, and Retetangos C (2010) Mode of delivery affects the bacterial community in the newborn gut. Early Hum Dev 86: 13–15. [DOI] [PubMed] [Google Scholar]
- Blekhman R, Goodrich JK, Huang K, Sun Q, Bukowski R, Bell JT, et al. (2015) Host genetic variation impacts microbiome composition across human body sites. Genom Biol 16: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks B, Firek BA, Miller CS, Sharon I, Thomas BC, Baker R, et al. (2014) Microbes in the neonatal intensive care unit resemble those found in the gut of premature infants. Microbiome 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Ringel-Kulka T, Heikamp-de Jong I, Ringel Y, Carroll I, de Vos WM, et al. (2015) Discordant temporal development of bacterial phyla and the emergence of core in the fecal microbiota of young children. ISME J 4: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I and Blaser MJ (2012) The Human Microbiome: at the interface of health and disease. Nat Rev Genet 13: 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, et al. (2011) Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA 108(Suppl.): 4586–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Jeffery IB, Conde S, Power SE, O ‘Connor EM, Cusack S, et al. (2012) Gut microbiota composition correlates with diet and health in the elderly. Nature 488: 178–184. [DOI] [PubMed] [Google Scholar]
- Cohen NA, Ben Ami R, Guzner-Gur H, Santo ME, Halpern Z, and Maharshak N (2015) Fecal microbiota transplantation for Clostridium difficile-Associated Diarrhea. Isr Med Assoc J 17: 510–514. [PubMed] [Google Scholar]
- Collado MC, Meriluoto J, and Salminen S (2007) Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Lett Appl Microbiol 45: 454–460. [DOI] [PubMed] [Google Scholar]
- Cowling P, McCoy DR, Marshall RJ, Padfield CJ, and Reeves DS (1992) Bacterial colonization of the non-pregnant uterus: a study of pre-menopausal abdominal hysterectomy specimens. Eur J Clin Microbiol Infect Dis 11: 204–205. [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LMG, Martínez I, Walter J, Goin C, and Hutkins RW (2011). Barcoded Pyrosequencing Reveals That Consumption of Galactooligosaccharides Results in a Highly Specific Bifidogenic Response in Humans. PLoS One 6: e25200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. (2010) Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 107: 14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Chierico F, Vernocchi P, Petrucca A, Paci P, Fuentes S, Praticò G, et al. (2015) Phylogenetic and metabolic tracking of gut microbiota during perinatal development. PLoS One 10: e0137347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L, Huse S, Sogin ML, and Relman DA (2008) The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA Sequencing. PLoS One 6: e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druart C, Alligier M, Salazar N, Neyrinck AM, and Delzenne NM (2014) Modulation of the gut microbiota by nutrients with prebiotic and probiotic properties. Adv Nutr 5: 624S–633S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding T, and Schloss PD (2014) Dynamics and associations of microbial community types across the human body. Nature 509: 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta SK, Girotra M, Garg S, Dutta A, von Rosenvinge EC,Maddox C, et al. (2014) Efficacy of combined jejunal and colonic fecal microbiota transplantation for recurrent Clostridium difficile Infection. Clin Gastroenterol Hepatol 12: 1572–1576. [DOI] [PubMed] [Google Scholar]
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, and Knight R (2010) Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA 107: 11971–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Bello MG, De Jesus-Laboy KM, Shen N, Cox LM, Amir A, Gonzalez A, Bokulich NA, Song SJ, et al. (2016) Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med 22: 250–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent, et al. (2005) Diversity of the human intestinal microbial flora. Science 308: 1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis-Pegler RB, Crabtree C, and Lambert HP (1975) The faecal flora of children in the United Kingdom. J Hyg Camb 75: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eloe-Fadrosh EA, Brady A, Crabtree J, Drabek EF, Ma B, Mahurkar A, et al. (2015) Functional dynamics of the gut microbiome in elderly people during probiotic consumption. MBio 6: e00231–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallani M, Young D, Scott J, Norin E, Amarri S, Adam R, et al. (2010) Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J Pediatr Gastroenterol Nutr 51: 77–84. [DOI] [PubMed] [Google Scholar]
- Flint HJ, Scott KP, Louis P, Duncan SH, Flint HJ, Scott KP, et al. (2012) The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol 9: 577–589. [DOI] [PubMed] [Google Scholar]
- Franzosa EA, Huang K, Meadow JF, Gevers D, Lemon KP, Bohannen BJ and Huttenhower C et al. (2015) Identifying personal microbiomes using metagenomic codes. Proc Natl Acad Sci USA 112: E2930–E2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz JV, Desai MS, Shah P, Schneider JG, and Wilmes P (2013) From meta-omics to causality: experimental models for human microbiome research. Microbiome 1: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda S, and Ohno H (2014) Gut microbiome and metabolic diseases. Semin Immunopathol 36: 103–114. [DOI] [PubMed] [Google Scholar]
- Fung TT, Rimm EB, Spiegelman D, Rifai N, Tofler GH, Willett WC, and Hu FB (2001) Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr 73: 61–67. [DOI] [PubMed] [Google Scholar]
- Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O’neil DA, and Macfarlane GT (2005) Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut 54: 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504: 446–450. [DOI] [PubMed] [Google Scholar]
- Gagliani N, Hu B, Huber S, Elinav E, and Flavell RA (2014) The fire within: microbes inflame tumors. Cell 157: 776–783. [DOI] [PubMed] [Google Scholar]
- Gerritsen J, Smidt H, Rijkers GT, and De Vos WM (2011) Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr 6: 209–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goepfert AR, Jeffcoat MK, Andrews WW, Faye-Petersen O, Cliver SP, Goldenberg, et al. (2004) Periodontal disease and upper genital tract inflammation in early spontaneous preterm birth. Obstet Gynecol 104: 777–783. [DOI] [PubMed] [Google Scholar]
- Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, et al. (2014) Human genetics shape the gut microbiome. Cell 159: 789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosalbes MJ, Llop S, Vallès Y, Moya A, Ballester F, and Francino MP (2013) Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clin Exp Allergy 43: 198–211. [DOI] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. (2009) Topographical and temporal diversity of the human skin. Science 324: 1190–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönlund MM, Lehtonen OP, Eerola E, and Kero P (1999) Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr 28: 19–25. [DOI] [PubMed] [Google Scholar]
- Hardy H, Harris J, Lyon E, Beal J, and Foey AD (2013) Probiotics, prebiotics and immunomodulation of gut mucosal defences: Homeostasis and immunopathology. Nutrients 5: 1869–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsell DL, Obregon VL, Heard MC, and Nobles BJ (1989) Endometrial bacteria in asymptomatic, nonpregnant women. J Reprod Med 34: 872–874. [PubMed] [Google Scholar]
- Hollister EB, Riehle K, Luna RA, Weidler EM, Rubio-Gonzales M, Mistretta, et al. (2015) Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome 3: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins MJ, Sharp R, and Macfarlane GT (2001) Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut 48: 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Nomura Y, Bashir A, Fernandez-Hernandez H, Itzkowitz S, Pei, et al. (2013) Diversified microbiota of meconium is affected by maternal diabetes status. PLoS One 8: e78257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt KM, Foster JA, Forney LJ, Schütte UME, Beck DL, Abdo Z, et al. (2011) Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS One 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur KY, and Lee M-S (2015) Gut microbiota and metabolic disorders. Diabetes Metab J 39: 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla, et al. (2012) Structure, function and diversity of the healthy human microbiome. Nature 486: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. (2014) Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by Caesarean section. Gut 63: 559–566. [DOI] [PubMed] [Google Scholar]
- Jiménez E, Fernández L, Marín ML, Martín R, Odriozola JM, Nueno-Palop C, et al. (2005) Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol 51: 270–274. [DOI] [PubMed] [Google Scholar]
- Jiménez E, Marín ML, Martín R, Odriozola JM, Olivares M, Xaus J, et al. (2008a) Is meconium from healthy newborns actually sterile? Res Microbiol 159: 187–193. [DOI] [PubMed] [Google Scholar]
- Jiménez E, Fernández L, Maldonado A, Martín R, Olivares M, Xaus J, and Rodríguez JM (2008b) Oral administration of Lactobacillus strains isolated from breast milk as an alternative for the treatment of infectious mastitis during lactation. Appl Environ Microbiol 74: 4650–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL, and Versalovic J (2012) The human microbiome and its potential importance to pediatrics. Pediatrics 129: 950–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Jones MB and Cobb BA (2015) Polysaccharide A from the capsule of Bacteroides fragilis induces clonal CD41 T cell expansion. J Biol Chem 190: 5007–5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost T, Lacroix C, Braegger CP, Rochat F, and Chassard C (2014) Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environ Microbiol 16: 2891–2904. [DOI] [PubMed] [Google Scholar]
- Kelly CR, Ihunnah C, Fischer M, Khoruts A, Surawicz C, Afzali A, et al. (2014) Fecal microbiota transplant for treatment of clostridium difficile infection in immunocompromised patients. Am J Gastroenterol 109: 1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, et al. (2015) Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17: 662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MT, Duncan SH, Stams AJM, van Dijl JM, Flint HJ, and Harmsen HJM (2012) The gut anaerobe Faecalibacterium prausnitzii uses an extracellular electron shuttle to grow at oxic–anoxic interphases. ISME J 6: 1578–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinross JM, Darzi AW, and Nicholson JK (2011) Gut microbiome-host interactions in health and disease. Genome Med 3: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein LL, and Gibbs RS (2005) Infection and preterm birth. Obstet Gynecol Clin North Am 32: 397–410. [DOI] [PubMed] [Google Scholar]
- Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, et al. (2011) Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci USA 108: 4578–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, et al. (2012) Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150: 470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S (2013) The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv Nutr 4: 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K, Itoh T, Kuwahara T, Oshima K, Toh H, Toyoda A, et al. (2007) Comparative metagenomics revealed commonly enriched gene sets in human gut microbiomes. DNA Res 14: 169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahtinen SJ, Boyle RJ, Kivivuori S, Oppedisano F, Smith KR, Robins-Browne R, et al. (2009) Prenatal probiotic administration can influence Bifidobacterium microbiota development in infants at high risk of allergy. J Allergy Clin Immunol 123: 499–501. [DOI] [PubMed] [Google Scholar]
- La Rosa PS, Warner BB, Zhou Y, Weinstock GM, Sodergren E, Hall-Moore CM, et al. (2014) Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci USA 111: 12522–12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaTuga MS, Stuebe A, and Seed PC (2014) A review of the source and function of microbiota in breast milk. Semin Reprod Med 32: 68–73. [DOI] [PubMed] [Google Scholar]
- Lax S, Smith DP, Hampton-Marcell J, Owens SM, Handley KM, Scott NM, et al. (2014) Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 345: 1048–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Lim JY, Kim B-S, Cho SJ, Kim NY, Kim O, et al. (2015) Comparison of the gut microbiota profile in breast-fed and formula-fed Korean infants using pyrosequencing. Nutr Res Pract 9: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RL, Peterson DA, and Gordon JI (2006) Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124: 837–848. [DOI] [PubMed] [Google Scholar]
- Ley RL, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, et al. (2008) Evolution of mammals and their gut microbes. Science 320: 1647–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Qin H, Yang Z, Xia Y, Liu W, Yang J, et al. (2011) Randomised clinical trial: the effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery – a double-blind study. Aliment Pharmacol Ther 33: 50–63. [DOI] [PubMed] [Google Scholar]
- Lockhart PB, Brennan MT, Sasser HC, Fox PC, Paster BJ, and Bahrani-Mougeot FK (2008) Bacteremia associated with toothbrushing and dental extraction. Circulation 117: 3118–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Prince AL, Bader D, Hu M, Ganu R, Baquero K, et al. (2014) High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun 5: 3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, et al. (2011) Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One 6: e20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin I, Suau A, Gotteland M, Brunser O, and Pochart P (2010) Amoxicillin treatment modifies the composition of Bifidobacterium species in infant intestinal microbiota. Anaerobe 16: 433–438. [DOI] [PubMed] [Google Scholar]
- Manichanh C, Varela E, Martinez C, Antolin M, Llopis M, Doré J, et al. (2008) The gut microbiota predispose to the pathophysiology of acute postradiotherapy diarrhea. Am J Gastroenterol 103: 1754–1761. [DOI] [PubMed] [Google Scholar]
- Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré, et al. (2009) The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol 9: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markle JGM, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. (2013) Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339: 1084–1088. [DOI] [PubMed] [Google Scholar]
- Martinez I, Stegen JC, Maldonado-Gomez MX, Eren AM, Siba PM, Greenhill AR, and Walter J (2015) The gut microbiota of rural Papua New Guineans: composition, diversity patterns, and ecological processes. Cell Rep 11: 527–538. [DOI] [PubMed] [Google Scholar]
- Mello RM, Morais MB, Tahan S, Melli LC, Rodrigues MS, Mello CS, and Scaletsky IC (2009) Lactobacilli and bifidobacteria in the feces of schoolchildren of two different socioeconomic groups: children from a favela and children from a private school. J Pediatr (Rio J) 85: 307–314. [DOI] [PubMed] [Google Scholar]
- Milani C, Lugli GA, Turroni F, Mancabelli L, Duranti S, Viappiani A, et al. (2014) Evaluation of bifidobacterial community composition in the human gut by means of a targeted amplicon sequencing (ITS) protocol. FEMS Microbiol Ecol 90: 493–503. [DOI] [PubMed] [Google Scholar]
- Møller BR, Kristiansen FV, Thorsen P, Frost L, and Mogensen SC (1995) Sterility of the uterine cavity. Acta Obstet Gynecol Scand 74: 216–219. [DOI] [PubMed] [Google Scholar]
- Moore JH, Pinheiro CCD, Zaenker EI, Bolick DT, Kolling GL, van Opstal E, et al. (2015) Defined nutrient diets alter susceptibility to clostridium difficile associated disease in a murine model. PLoS One 10: e0131829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N, and Sloan D (2015) The hologenome concept: helpful or hollow? PLoS Biol 13: e1002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, et al. (2006) Differences in fecal microbiota in different european study populations in relation to age, gender, and country: a cross-sectional study. Env Microbiol 72: 1027–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama J, Watanabe K, Jiang J, Matsuda K, Chao S-H, Haryono P, et al. (2015) Diversity in gut bacterial community of school-age children in Asia. Sci Rep 5: 8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermes M, Endo A, Aarnio J, Salminen S, and Isolauri E (2015) Furry pets modulate gut micrbiota composition in infants at risk for allergic disease. J Allergy Clin Immunol 136: 1688–1690. [DOI] [PubMed] [Google Scholar]
- Neyrink AM, Possemiers S, Verstraete W, De Backer F, Cani PD, and Delzenne NM (2012) Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin–glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J Nutr Biochem 23: 51–59. [DOI] [PubMed] [Google Scholar]
- Nobel YR, Cox LM, Kirigin FF, Bokulich NA, Yamanishi S, Teitler I, et al. (2015) Metabolic and metagenomic outcomes from early-life pulsed antibiotic treatment. Nat Commun 6: 7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi Y, Nakai S, Tsukamoto T, Masumori N, Akaza H, Miyanaga N, et al. (2002) Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol Int 68: 273–280. [DOI] [PubMed] [Google Scholar]
- Palmer C, Bik EM, DiGiulio DB, Relman DA, and Brown PO (2007) Development of the human infant intestinal microbiota. PloS Biol 5: e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, et al. (2006) Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118: 511–521. [DOI] [PubMed] [Google Scholar]
- Perez PF, Doré J, Leclerc M, Levenez F, Benyacoub J, Serrant P, et al. (2007) Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics 119: e724–e732. [DOI] [PubMed] [Google Scholar]
- Rafter J, Bennett M, Caderni G, Clune Y, Hughes R, Karlsson PC, et al. (2007) Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr 85: 488–496. [DOI] [PubMed] [Google Scholar]
- Rampelli S, Schnorr SL, Consolandi C, Turroni S, Severgnini M, Peano, et al. (2015) Metagenome sequencing of the Hadza Hunter-Gatherer Gut Microbiota. Curr Biol 25: 1682–1693. [DOI] [PubMed] [Google Scholar]
- Rautava S, Collado MC, Salminen S, and Isolauri E (2012) Probiotics modulate host-microbe interaction in the placenta and fetal gut: a randomized, double-blind, placebo-controlled trial. Neonatology 102: 178–184. [DOI] [PubMed] [Google Scholar]
- Ravcheev DA, Godzik A, Osterman AL, and Rodionov DA (2013) Polysaccharides utilization in human gut bacterium Bacteroides thetaiotaomicron: comparative genomics reconstruction of metabolic and regulatory networks. BMC Genomics 1: 873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijkers GT, De Vos WM, Brummer R, Morelli L, Corthier G, and Marteau P (2011) Health benefits and health claims of probiotics: bridging science and marketing. Br J Nutr 106: 1–6. [DOI] [PubMed] [Google Scholar]
- Rogier EW, Frantz AL, Bruno MEC, Wedlund L, Cohen DA, Stromberg AJ, and Kaetzel CS (2014) Secretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expression. Proc Natl Acad Sci USA 111: 3074–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Bieda J, et al. (2014) The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome 2: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks MG, Veiga P, Wardwell-Scott LH, Tickle T, Segata N, Michaud M, et al. (2014) Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J 8: 1403–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulnier DM, Riehle K, Mistretta T-A, Diaz M-A, Mandal D, Raza S, et al. (2011) Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroentero 141: 1782–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanche M, Avershina E, Dotterud C, Øien T, Storrø O, Johnsen R, and Rudi K (2015) High-resolution analyses of overlap in the microbiota between mothers and their children. Curr Microbiol 71: 283–290. [DOI] [PubMed] [Google Scholar]
- Schnorr SL, Candela M, Rampelli S, Centanni M, Consolandi C, Basaglia G, et al. (2014) Gut microbiome of the Hadza hunter-gatherers. Nat Commun 5: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendicke F, Karimbux N, Allareddy V, and Gluud C (2015) Periodontal treatment for preventing adverse pregnancy outcomes: a meta-and trial sequential analysis. PLoS One 10: e0129060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour GJ, Ford PJ, Cullinan MP, Leishman S, and Yamazaki K (2007) Relationship between infections and systemic disease. Clin Microbiol Infect 13: 3–10. [DOI] [PubMed] [Google Scholar]
- Simeoni U, Berger B, Junick J, Blaut M, Pecquet S, Rezzonico E et al. (2015) Gut microbiota analysis reveals a marked shift to bifidobacteria by a starter infant formula containing a synbiotic of bovine milk-derived oligosaccharides and Bifidobacterium animalis subsp. lactis CNCM I-3446. Environ Microbiol (in press). doi: 10.1111/1462-2920.13144. [DOI] [PubMed] [Google Scholar]
- Stagg AJ, Hart AL, Knight SC, and Kamm MA (2003) The dendritic cell: its role in intestinal inflammation and relationship with gut bacteria. Gut 52: 1522–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel JH, Malatos S, Kennea N, Edwards AD, Miles L, Duggan P, et al. (2005) Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr Res 57: 404–411. [DOI] [PubMed] [Google Scholar]
- Stefka AT, Feehley T, Tripathi P, Qiu J, McCoy K, Mazmanian SK, et al. (2014) Commensal bacteria protect against food allergen sensitization. Proc Natl Acad Sci USA 111: 13145–13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan A, and Nord CE (2002) Probiotics in human infections. J Antimicrob Chemother 50: 625–627. [DOI] [PubMed] [Google Scholar]
- Thompson AL, Monteagudo-Mera A, Cadenas MB, Lampl ML, Azcarate-Peril MA, Mcgavin MJ, and Eckstein T (2015) Milk-and solid-feeding practices and daycare attendance are associated with differences in bacterial diversity, predominant communities, and metabolic and immune function of the infant gut microbiome. Front Cell Infect Microbiol 5: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, et al. (2014) Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20: 159–166. [DOI] [PubMed] [Google Scholar]
- Turnbaugh P, Ley R, Mahoeald MA, Magrini ER, and Gordon JI (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031. [DOI] [PubMed] [Google Scholar]
- Tyakht AV, Kostryukova ES, Popenko AS, Belenikin MS, Pavlenko AV, Larin AK, et al. (2013) Human gut microbiota community structures in urban and rural populations in Russia. Nat Commun 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tongeren SP, Slaets JPJ, Welling GW, and Harmsen HJM (2005) Fecal microbiota composition and frailty fecal microbiota composition and frailty. Appl Environ Microbiol 71: 6438–6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura M, Canchaya C, Tauch A, Chandra G, Fitzgerald GF, Chater KF, and van Sinderen D (2007) Genomics of Actinobacteria: tracing the evolutionary history of an ancient phylum. Microbiol Mol Biol Rev 71: 495–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt AY, Costea PI, Kultima JR, Li SS, Zeller G, Sunagawa S, and Bork P (2015) Temporal and technical variability of human gut metagenomes. Genome Biol 16: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voreades N, Kozil A, and Weir TL (2014) Diet and the development of the human intestinal microbiome. Front Microbiol 5: 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AW, Duncan SH, Leitch ECM, Child MW, and Flint HJ (2005) pH and peptide supply can radically alter bacterial populations and short-chain fatty acid ratios within microbial communities from the human colon. Appl Environ Microbiol 71: 3692–3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, et al. (2011) Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J 5118: 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, et al. (2009) 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J 3: 944–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyrich LS, Dixit S, Farrer AG, Cooper AJ, and Cooper AJ (2015) The skin microbiome: associations between altered microbial communities and disease. Australas J Dermatol 56: 268–274. [DOI] [PubMed] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y, Keilbaugh SA, et al. (2011) Linking long-term dietary patterns with gut microbial enterotypes. Science 334: 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. (2012) Human gut microbiome viewed across age and geography. Nature 486: 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Xiao X, Zhang Q, Mao L, Yu M, and Xu J (2015) The placental microbiome varies in association with low birth weight in full-term neonates. Nutrients 7: 6924–6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li S, Gan R, Zhou T, Xu D, and Li H (2015) Impacts of Gut Bacteria on Human Health and Diseases. In J Mol Sci 16: 7493–7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A, Sunagawa S, Mende DR, and Bork P (2015) Inter-individual differences in the gene content of human gut bacterial species. Genome Biol 16: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relative abundance of bacterial phyla in stool samples obtained in different geographical locations world-wide (average %).