Abstract
Background
Inhaled corticosteroids are an integral part of asthma management, and act as an anti‐inflammatory agent in the airways of the lung. These agents confer significant benefit in terms of symptom management and improvement in lung function, but may also cause harm in terms of local and systemic side‐effects. Ciclesonide is a novel steroid that has efficient distribution and release properties that mean it can be taken once daily, making it potentially useful in ongoing asthma management.
Objectives
To assess the efficacy of inhaled ciclesonide in adults and children with chronic asthma.
Search methods
We searched the Cochrane Airways Group register of trials with pre‐defined terms. Additional searches of CENTRAL and PubMed were undertaken. The literature searches for this review are current up to June 2007.
Selection criteria
Randomised parallel or crossover studies were eligible for the review. We included studies comparing ciclesonide with placebo, and we also included studies comparing ciclesonide at different doses.
Data collection and analysis
Two authors assessed studies for inclusion in the review, extracted data independently and checked each others' work. We contacted study investigators in order to obtain additional data. Extracted data were entered into RevMan 4.2 and analysed as fixed effect mean differences for continuous data, and fixed effect risk ratios for dichotomous data.
Main results
Eighteen trials (reporting 20 study comparisons) met the review entry criteria. We report findings from 18 group comparisons where data were available (6343 participants, of whom 1692 were children).
Ciclesonide versus placebo: The short duration of the included studies means that there is a lack of data with respect to the impact of ciclesonide on asthma exacerbations. At doses of 100 mcg/d or less up to 400 mcg/d in mild to moderate asthma, ciclesonide improved lung function, asthma symptoms and rescue inhaler use, compared with placebo.
Dose response outcomes: Comparisons of 100 versus 200 mcg/d, 100 versus 400 mcg/d and 400 versus 800 mcg/d did not yield significant differences in lung function outcomes. Adverse event data were not available in sufficient detail to permit assessment of the safety profile of this drug.
Authors' conclusions
Ciclesonide was more effective than placebo, in the short term, in improving lung function in patients with mild to moderate asthma previously treated with inhaled corticosteroids. There remain questions as to dose response, and the lack of data on the longer term impact on exacerbations and safety profile should be addressed in future studies.
Keywords: Adult, Child, Humans, Anti-Asthmatic Agents, Anti-Asthmatic Agents/therapeutic use, Asthma, Asthma/drug therapy, Chronic Disease, Placebo Effect, Pregnenediones, Pregnenediones/therapeutic use, Randomized Controlled Trials as Topic
Plain language summary
Ciclesonide versus placebo for chronic asthma in adults and children
In asthma, inflammation (swelling in the wall) narrows the airway and is the main factor giving rise to asthmatic symptoms of cough, wheeze, shortness of breath and chest tightness. Inhaled corticosteroids (ICS) which are given usually more than once daily are now recommended as first line therapy for most people with asthma. The currently available ICS, such as budesonide (BUD), beclomethasone (BDP) or fluticasone (FP), have been available for many years and have proven to be an important therapy for controlling inflammation and symptoms. However, these drugs can be associated with significant side‐effects, especially local effects in the upper airways such as hoarseness and oral candida (thrush infection). The main reputed advantage of ciclesonide (CIC, a new generation of ICS), is its ability not only (as with other ICS) to be delivered locally by inhalation but specifically to the lower airways of the lung in a form which potentially minimises local side‐effects. Overall this advantage of CIC could lead to a reduction of local airway side‐effects with once daily therapy and thereby improving adherence to therapy. The results from this review indicate that CIC at low to moderate doses improves lung function and reduces asthma symptoms compared to placebo, but the short duration of the studies means that there is a lack of information about the impact on asthma exacerbations. Thus the currently recommended doses of CIC of 100‐200 mcg daily would seem appropriate. However, the number of studies in the higher dose range are low and further studies are therefore required in adults and children to determine whether higher CIC doses will give significant benefit without increasing adverse events. It will also be important to determine in clinical studies how CIC compares to the other currently available ICS in terms of efficacy and safety in asthmatic adults and children in order to determine the precise role of CIC therapy in asthma. The published data are insufficient to assess the reputed safety advantage of ciclesonide, and better assessment and reporting in studies is required to address this important question.
Background
On a worldwide basis asthma is a common chronic disease in clinical practice affecting over 300 million people. It is responsible for one in 250 deaths per year and 15 million disability adjusted life years (DALYs) lost worldwide. It is a condition which can develop in early childhood and generally persists into adulthood (Gerritsen 1989; Martin 1982; Williams 1969). Asthma is a chronic inflammatory disease of the airways involving a complex interaction between airway structural cells and specific allergic inflammatory cells including mast cells, eosinophils and T‐lymphocytes, and the release of specific cytokines and mediators of inflammation. This inflammatory response is associated with airway narrowing, especially in smaller airways, which cause patients to complain of symptoms such as cough and wheeze (Tattersfield 2002; GINA 1998). The anti‐inflammatory corticosteroids have been an effective therapy for asthma for over 30 years and are now the main therapy for asthma control currently for those with persistent asthma (Adams 2000; Adams 2007; Powell 2003; BGAM 1997; BTS/SIGN 2003; Consensus 1999; Consensus 2005; GINA 1998).
Corticosteroids deal effectively with the asthma inflammatory process through interaction with the glucocorticoid receptor, thus leading to the amelioration in asthma symptoms and control of the disease. The main advantage of the inhaled route is to bring the therapy directly to the disease location and at a reduced dose and hence less systemic side‐effects compared to higher dose oral steroid therapy (Mash 2001). There are different types of inhaled corticosteroids available on the market given either by multi‐dose dry powder or aerosol inhaler devices (e.g. beclomethasone, fluticasone, budesonide, and mometasone). Inhaled corticosteroids significantly reduce the hospitalisation rate for asthma (and hence reduce cost associated with the disease) and the mortality from the condition when taken on a regular basis (Suissa 2000; Suissa 2002). Non‐compliance is a significant problem with inhaled corticosteroid therapy due to a number of factors including increased dosing frequency and local or systemic side effects (Buston 2000). However, while inhaled steroids may be effective when used morning and evening, reducing dosing to a once daily dosing regimen can give also effective control (Malo 1989; Toogood 1982). Compliance with increased dosing frequency of inhaled steroids in asthmatics, especially four times daily can be poor (Coutts 1992; Eisen 1990). The novel inhaled corticosteroid ciclesonide (CIC) has recently been approved in Europe. Research from clinical trials has shown the drug to be an effective therapy in persistent asthma in improving lung function, and in reducing asthma symptoms. This therapy has novel release and distribution properties, reported to result in better targeting of the anti‐inflammatory effects in the airways especially to the small airways. It is inhaled as a pro‐drug, which is converted to an active metabolite (des CIC) in the airways, reportedly with reduced systemic and local (e.g. oropharyngeal) side effects. In addition, ciclesonide can also be given once daily, and may lead to better compliance with inhaled corticosteroids.
Objectives
The objectives of this review were to compare the efficacy and safety of ciclesonide in adults (aged 18 years and older) and children (less than 18 years) who have persistent asthma of any severity compared with placebo therapy, and with ciclesonide at alternative doses.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCT) comparing the inhaled ciclesonide with placebo were considered for inclusion. Trials that use parallel group designs or cross‐over design with a washout period of two weeks or more were eligible. Studies published in abstract form and unpublished data were also eligible for inclusion.
Types of participants
Adults (aged 18 years and older) and children (less than 18 years) were eligible for inclusion. All study subjects had a diagnosis of chronic asthma, including those with intermittent and chronic symptoms. Studies that base the diagnosis of asthma on physician opinion or on objective criteria related to symptoms, airway reversibility to an inhaled short‐acting 2‐agonist or airway hyper‐responsiveness in keeping with international asthma guidelines such as GINA 1998 (Global Initiative On Asthma) / National Institutes of Health (NIH) or BTS/SIGN 2003) or evidenced based guidelines were included. Studies that delivered interventions to patients in the community/family practice setting or hospital‐based settings were included. Studies with subjects with pulmonary diagnosis other than asthma (e.g. COPD) were excluded.
Types of interventions
Studies that included inhaled ciclesonide at any dose versus placebo, or compared different doses of ciclesonide were considered. A second review of ciclesonide in comparison with other inhaled corticosteroids, such as budesonide, beclomethasone, fluticasone, triamcinolone, flunisolide has been undertaken separately. Therapy should be for at least 4 weeks. Concomitant therapies for asthma, such as short‐acting beta2‐agonists (rescue therapy), theophyllines, long‐acting 2‐agonists (Serevent or formoterol), inhaled anti‐cholinergics were allowed provided that patient's asthma is stable.
Types of outcome measures
The primary outcomes were: 1.
4.
Primary outcomes
Asthma exacerbations requiring use of systemic steroid
Measures of lung function, forced expired volume in one second (FEV1) and or peak expiratory flow rates (PEF
Secondary outcomes
Mild asthma exacerbations not requiring systemic steroids or severe exacerbations requiring hospital admissions.
Rescue medication use
Symptoms
Measures of adverse effects including oropharyngeal (candidiasis, sore throat, hoarseness), and systemic (osteopenia, adrenal suppression, growth rate) side‐effects and withdrawal rate due to side‐effects.
Measures of healthcare utilisation: doctor visits, emergency visits and or hospital admissions for asthma.
Measures of morbidity: days of school absences, days of restricted activities, nights disturbed by asthma symptoms, health‐related quality of life, asthma severity, asthma‐free days. Measures of compliance. As a surrogate to include study withdrawal or patient preference in crossover studies.
We have given a narrative overview of the following metrics, creating an additional table and re‐expressing their effects as standardised mean differences:
am PEF
FEV1
Symptoms
Rescue medication usage
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL, and handsearching of respiratory journals and meeting abstracts. All records in the Specialised Register coded as 'asthma' were searched using the following terms:
ciclesonide* or Alveso* or pregnenedione* or CIC
Additional searches of CENTRAL and PubMed were carried out (see Table 1 for the full CENTRAL search strategy). Abstracts from the American Thoracic Society annual conference 2006‐2007 were searched online with the keyword 'ciclesonide'. Searches are current to June 2007.
1. CENTRAL Search Strategy.
| Search strategy |
| #1 MeSH descriptor Asthma, explode all trees in MeSH products #2 asthma* #3 wheez* #4 MeSH descriptor Bronchial Spasm, explode all trees in MeSH products #5 bronch* near spas* #6 bronchospas* #7 bronchoconstrict* #8 bronch* near constrict* #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8) #10 ciclesonide* #11 alvesco #12 CIC #13 (#10 OR #11 OR #12) #15 (#9 AND #13) |
Searching other resources
Reference lists of all primary studies and review articles were reviewed for additional references. We consulted www.clinicalstudyresults.org for unpublished studies.
Data collection and analysis
Selection of studies
The title and abstract of each citation identified using the search strategy identified was screened independently by PM and TL for eligibility. Articles that appear to fulfil the inclusion criteria were retrieved in full text. From the full text of the articles, PM and TL independently established whether each study met the inclusion criteria as a RCT with the above interventions. Disagreement was resolved by consensus. Translation into English was not necessary.
Data extraction and management
Data from included trials were extracted independently and checked before TL entered the data into RevMan 4.2. We attempted to obtain additional outcome data from investigators where possible.
We extracted the following characteristics of each study: Methods Design, randomisation method, blinding, follow‐up procedures and withdrawals. Population Sample size, age, gender, inclusion and exclusion criteria (including asthma therapy), asthma diagnosis and severity, pulmonary function, other medical diagnoses and therapies. Intervention Type and dose of comparator inhaled steroid, dose of ciclesonide, timing and duration of therapy, method of delivery, co‐intervention medications. Outcomes Reported outcomes
Assessment of risk of bias in included studies
Study quality was assessed using the Cochrane approach to assessment of allocation concealment. All trials were scored and entered using the following principals.
Grade A: adequate concealment Grade B: uncertain Grade C: clearly inadequate concealment
Jadad scores were also calculated for each study (Jadad 1996).
Data synthesis
Trial data were combined using RevMan 4.2. Data were pooled using a fixed effect model.
A mean difference (MD) and 95% CI were calculated for continuous variables measured on identical metrics. SMD (standardised mean difference) was used for the same continuous variables measured with different metrics. Generic inverse variance was used to pool data derived from the same scale if they are only available as mean differences with 95% CIs or standard errors.
For dichotomous outcomes, a Risk Ratio (RR) was calculated based upon the number of participants with an event versus the number of participants without an event. Fixed Effect modelling was used to pool data for RRs unless heterogeneity was observed (I square >/=20%; Higgins 2003), in which case a sensitivity analysis with Random Effects modelling was used.
Studies were grouped according to whether they were conducted in adults or children.
Results
Description of studies
Results of the search
Literature searches current up to June 2007 identified a total of 148 citations and 18 trials representing 53 of these citations, and reporting 20 study comparisons, met the review entry criteria (see Figure 1). For details of each included study see table 'Characteristics of included studies'. Reasons for the exclusion of 16 studies are detailed in the table 'Characteristics of excluded studies'. Of the 18 trials which met the review entry criteria, two yielded additional group comparisons. Gelfand 2006a; Gelfand 2006b assessed similar doses of ciclesonide (50 and 100 mcg/d of ciclesonide), and EFC6163a; EFC6163b assessed once daily and twice daily inhalation of the same dose of ciclesonide. One of the 18 trials was a crossover study (Wilson 2006). Two studies do not contribute data to the analysis of the review as we have not been able to obtain effect estimates: Baena Cagnani 2006 (N=661); Bernstein 2004 (N=531). The following description of study characteristics pertains to the 18 parallel group study comparisons which yielded data, since these were the primary source of evidence for the review.
1.
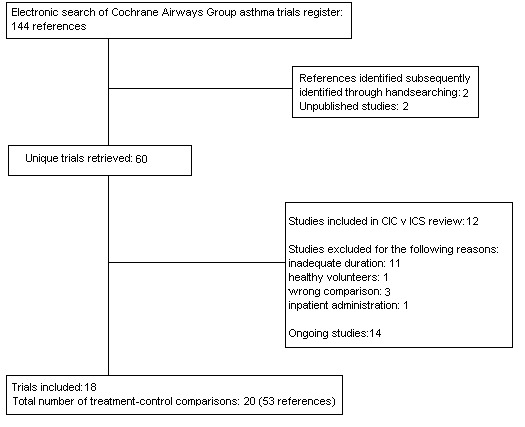
Flow diagram of studies in this review
Included studies
Study design Three studies reported integrated analyses of more than one trial (Pearlman 2005; Gelfand 2006a; Gelfand 2006b). Two dose comparison trials were open label; the remainder were double‐blind.
Participants The studies randomised 6343, of whom 1692 were children. The remainder of the study participants were either adults or their age could not be determined.
Baseline FEV1 predicted and requirement for maintenance treatment varied between the studies. One study recruited participants on oral steroid therapy (Bateman 2006), with a mean FEV1 predicted of 55%. Mean FEV1 predicted at baseline was above 80% in one study (Lipworth 2005), in five studies it was between 70 and 80% (Adachi 2007a; Chapman 2005; Hansel 2006; Langdon 2005; Magnussen 2007), and in two studies it was below 70% (Adachi 2007b; Gelfand 2006a). In the remaining studies, maximum allowable baseline FEV1 was 85% in Pearlman 2005, 80% in DFI6153, 70% in Bateman 2006a and 65% in Bernstein 2004.
Requirement for pre‐study maintenance ICS treatment featured in Adachi 2007a; Adachi 2007b; Chapman 2005; DFI6153; Langdon 2005; O'Connor 2002 and Zietkowski 2006 as a criterion of study entry, and in Gelfand 2006a and Pearlman 2005 use of ICS prior to baseline was permitted. Lipworth 2005 only included participants whose asthma was controlled with as needed short‐acting beta‐agonist. In the remaining studies no mention of inhaled steroid treatment was made in study entry or exclusion criteria.
Intervention We assessed three comparisons represented by the following studies (all doses quoted are ex‐valve): 1. Ciclesonide versus placebo (non‐OCS) analysing doses separately (100 mcg/d or less (Adachi 2007a; Gelfand 2006a; Gelfand 2006b); 200 mcg/d (Adachi 2007a; Chapman 2005; DFI6153; EFC6163a; EFC6163b; Gelfand 2006a); 400 mcg/d (Adachi 2007a; DFI6153; Pearlman 2005); 800 mcg/d (Chapman 2005; DFI6153) and greater than 800 mcg/d (DFI6153) 2. Ciclesonide versus placebo (OCS users) analysing doses separately (<800 and >800 mcg/d): Bateman 2006. 3. Ciclesonide at different doses (50 versus 100 mcg/d (Gelfand 2006a); 100 versus 200 mcg/d (Adachi 2007a; Gelfand 2006a; Magnussen 2007; Pearlman 2005; Zietkowski 2006); 100 versus 400 mcg/d (Hansel 2006; Langdon 2005) 200 versus 400 mcg/d (Bernstein 2004; DFI6153; Pearlman 2005); 200 versus 800 mcg/d (Bateman 2006a; Chapman 2005; DFI6153); 400 versus 800 mcg/d (Adachi 2007b; Lipworth 2005; DFI6153); 800 versus 1600 mcg/d (O'Connor 2002).
Delivery of drug and duration of studies Ciclesonide was delivered via metered dose inhalers in all the trials with the exception of DFI6153 where it was delivered via dry powder inhaler.
Dosing regimens varied, with ciclesonide given once daily in all studies with the exception of Bateman 2006; Bernstein 2004; DFI6153 where it was administered twice daily.
One study was 12 months (Baena Cagnani 2006) and another six weeks in duration (DFI6153). The remaining studies were 12 weeks long, but some included a run in period of up to 4 weeks
Outcomes assessed Baena Cagnani 2006 and Lipworth 2005 were the only two studies where lung function outcome data were not reported. Symptoms or rescue medication use were assessed in all studies except for Lipworth 2005.
Excluded studies
Risk of bias in included studies
Thirteen studies were described as randomised and double‐blinded. The method of blinding was known in two studies. One dose‐comparison study was open label (Adachi 2007b). Methodological quality, as assessed by the Jadad scoring system, was variable. Only one of the studies achieved a score of 5 (high quality), three studies a score of 4 (good quality), six a score of 3 (fair quality) and the remaining four studies a score of 2 (poor quality). Three of the low quality studies were published in abstract form for presentation at international respiratory or allergy conferences and we had only limited details about patient withdrawals from study, methods of randomisation and blinding. It is therefore possible that these scores represent an underestimation of the true methodological quality.
Effects of interventions
1. Ciclesonide versus placebo (non‐OCS users)
Primary outcomes
No data were found in relation to asthma exacerbations.
Change in FEV1 (Litres):
100 mcg/d or less: 0.08; 95% confidence interval 0.05 to 0.11 (five studies, N = 1677). The subgroup estimates for this outcome were significantly different from each other (children versus adults: 0.05 versus 0.13 (P = 0.019). 200 mcg/d 0.12; 95% confidence interval 0.08 to 0.15 (four studies, N = 1543). One paediatric study contributed to this outcome. 400 mcg/d: 0.17 L; 95% confidence interval 0.14 to 0.21 (six studies, N = 1717). All of these studies were conducted in adults. 800 mcg/d: One unpublished study reported a significant difference in favour of ciclesonide (DFI6153, N = 374). Chapman 2005 reported a significant difference in favour of ciclesonide for end of treatment FEV1. 1600 mcg/d: One unpublished study reported a significant difference in favour of ciclesonide (DFI6153, N = 372).
Change in FEV1 percent predicted:
100 mcg/d or less: 2.96%; 95% confidence interval 1.80 to 4.12 (four studies, N = 1432). 200 mcg/d: 3.06%; 95% confidence interval 1.85 to 4.26 (three studies, N = 1171). 400 mcg/d: 3.10%; 95% confidence interval 1.66 to 4.53 (two studies, N = 666).
Change in am PEF:
100 mcg/d or less: 14 L/min; 95% confidence interval 10 to 18 (five studies, N = 1677). 200 mcg/d: 19 L/min; 95% confidence interval 15 to 23 (five studies, N = 1768). 400 mcg/d: 18 L/min; 95% confidence interval 14 to 22 (six studies, N = 1722). 800 mcg/d: 28 L/min; 95% confidence interval 21 to 35 (two studies, N = 594). 1600 mcg/d: One unpublished study reported a significant difference in favour of ciclesonide (DFI6153, N = 372)
Change in pm PEF:
100 mcg/d or less: 12 L/min; 95% confidence interval 8 to 16 (five studies, N = 1677). 200 mcg/d: 14 L/min; 95% confidence interval 10 to 18 (three studies, N = 1166). 400 mcg/d: 15 L/min; 95% confidence interval 10 to 21 (three studies, N = 906).
Secondary outcomes
Quality of life & asthma symptoms
Change in asthma symptoms favoured ciclesonide in studies conducted in adults, across three dose ranges where data could be pooled as standardised mean differences (100 mcg/d or less: ‐0.39; 95% confidence interval ‐0.52 to ‐ 0.34; 200 mcg/d: ‐0.51; 95% confidence interval ‐0.65 to ‐0.38; 400 mcg/d: ‐0.48; 95% confidence interval ‐0.59 to ‐0.38).
Rescue medication use
There was a significant reduction in the frequency of rescue medication use in favour of low dose CIC compared with placebo (CIC 100 mcg or less: ‐0.87 puffs/d (‐1.36 to ‐0.37, three studies); 200 mcg/d: ‐1.1 puffs/d; 95% confidence interval ‐1.32 to ‐0.89; three studies). One unpublished study found a significant reduction in rescue medication use in favour of ciclesonide (DFI6153, N = 372)
Study withdrawal & adverse event data
Study withdrawal occurred less frequently in the low dose CIC groups compared with placebo in adults and children (100 mcg/d or less: RR 0.67; 95% confidence intervals 0.55 to 0.83, four studies N =1174). Withdrawals were primarily due to a loss of efficacy or adverse events. Similar results were seen with CIC 200 mcg (RR 0.55; 95% confidence intervals 0.43 to 0.7, three studies, N = 877). Specific adverse events did not differ between treatment groups (pharyngitis, nasopharyngitis, headache, URTI and rhinitis). Very little data were available to indicate the frequency of oral candidiasis (confirmed by culture) in each treatment group.
2. Ciclesonide versus Placebo (OCS users)
No data were identified for OCS‐treated exacerbations.
Lung function
A single parallel study (Bateman 2006) of good methodological quality (Jadad score 4) was conducted in 141 asthmatic patients age range 12‐75 years with severe persistent oral steroid dependant asthma. There was no significant changes from baseline in FEV1 for CIC but it was reduced in the placebo group. Improvement in morning PEF occurred in CIC versus placebo (MD 16.67; 95% confidence interval ‐1.85 to 35.59) this was only significant in the CIC1280 group.
Oral steroid reduction
In the single study (Bateman 2006) the prednisolone dose was significantly reduced in the CIC group by 58.26% compared to placebo MD (95% confidence interval ‐86.13 to ‐30.39). Discontinuation of maintenance of oral steroids was more common in CIC compared to placebo RR 2.81 (95% confidence intervals 1.11 to 7.11).
3. Ciclesonide at different doses
Results are presented in favour of the lower dose of CIC, and significant differences were not shown in the between dose comparisons.
Primary outcomes
Exacerbations requiring oral corticosteroids were only reported in one study (Magnussen 2007) and the numbers were too small to draw any conclusions (there were 2 patients with exacerbations in each arm given 100 mcg and 200 mcg of ciclesonide).
Change in FEV1 (Litres):
100 versus 200 mcg/d: 0.02; 95% confidence interval ‐0.01 to 0.06 (four studies, N = 1726) 100 versus 400 mcg/d: 0.00; 95% confidence interval ‐0.05 to 0.05 (three studies, N = 747) 200 versus 400 mcg/d: 0.03; 95% confidence interval ‐0.02 to 0.09 (two studies, N = 537) 400 versus 800 mcg/d: ‐0.06; 95% confidence interval ‐0.12 to 0.00 (two studies, N = 583)
One paediatric study compared CIC 50 and 100 mcg/d and did not detect a significant difference between treatments in change from baseline in FEV1 (0.03 L; 95% confidence interval ‐0.02 to 0.08).
Change in FEV1 (predicted):
100 versus 200 mcg/d: 0.31; 95% confidence interval ‐0.74 to 1.35 (three studies, N = 1176).
Change in am PEF L/min:
100 versus 200 mcg/d: 3.80; 95% confidence interval ‐0.78 to 8.37 (three studies, N = 1176) 100 versus 400 mcg/d: ‐0.48; 95% confidence interval ‐7.59 to 6.64 (three studies, N = 749) 200 versus 400 mcg/d: 2.17; 95% confidence interval ‐5.04 to 9.38 (two studies, N = 537) 200 versus 800 mcg/d: ‐4.78; 95% confidence interval ‐11.65 to 2.09 (two studies, N = 594)
Change in pm PEF L/min:
100 versus 200 mcg/d: 2.28; 95% confidence interval ‐2.04 to 6.60 (three studies, N = 1176) 100 versus 400 mcg/d: 1.36; 95% confidence interval ‐6.05 to 8.78 (two studies, N = 396)
Secondary outcomes
Quality of life and asthma symptoms
800 mcg and 1600 mcg/d: standardised mean difference ‐0.06; 95% confidence interval ‐0.20 to 0.08 (two studies, N = 737).
There were no significant differences in the change from baseline found in terms of quality of life (AQLQ questionnaire) between 100 and 200 mcg/d with ‐0.08 (95% confidence intervals ‐0.25 to 0.09; P=0.35) in adults (Hansel 2006).
Change in rescue medication use (puffs/d)
400 versus 800 mcg/d: 0.12; 95% confidence interval ‐0.13 to 0.37 (two studies, N = 583) 800 versus 1600 mcg/d: 0.15; 95% confidence interval ‐0.07 to 0.37 (two studies, N = 735)
Study withdrawal & adverse event data
There was no difference in the likelihood of study withdrawal due to lack of efficacy between 100 and 200 mcg and between 100 and 400 mcg/d. There was a high level of statistical heterogeneity between studies assessing 100 and 200 in adverse events. Specific adverse events from individual studies were too imprecise to derive meaningful results from our analyses.
Discussion
From the 20 study comparisons included in this review, data from 18 contribute to the analyses. The two parallel group studies which did not yield any data remain unpublished in full text form as of August 2007 (Baena Cagnani 2006; Bernstein 2004). The studies did not provide information on asthma exacerbations, but in comparison with placebo, lung function assessments favoured ciclesonide in studies of up to 12 weeks duration. The evidence available on adverse events is insufficient to draw reliable inferences regarding its safety and tolerability.
Placebo comparison
Although the doses assessed indicated favourable effects of ciclesonide, the effect sizes were somewhat smaller than those established by similar reviews of beclomethasone, budesonide and fluticasone (Adams 1999; Adams 2005a; Adams 2005b). However, indirect comparison between these preparations may not be reliable, since it assumes that the populations recruited to the efficacy studies of the respective preparations were pre‐treated with similar doses of inhaled steroids. Nevertheless it is noteworthy that ciclesonide studies conducted in adults and adolescents yielded a difference in FEV1 of between 0.13 to 0.17 against placebo in the ranges from 100 to 400 mcg/d. Studies conducted in pre‐trial maintenance inhaled steroid users from meta‐analysis of fluticasone studies gave a larger effect size of 0.33 L (personal communication). The eligibility criteria of the trials stipulated a requirement for maintenance inhaled corticosteroid treatment as a maximum BDP equivalent of 800‐1000 mcg/d (Adachi 2007a; Chapman 2005; EFC6163a; EFC6163b; Langdon 2005; Pearlman 2005), and group mean airway obstruction was lower than 80% in the studies. Thus, requirement for pre‐baseline steroid treatment was indicative of a more severe patient population in the ciclesonide studies than those of fluticasone trials.
In OCS users, (i.e. subjects on lowest maintenance oral steroids for 12 months), a single study found that ciclesonide permitted a reduction in the oral maintenance steroid dosage and prevented falls in FEV1 while improving morning PEF (daily dose of 800 and 1600 mcg). This was also associated with a greater likelihood of oral candidiasis in the combined ciclesonide group, but the increase was not statistically significant. Pharyngitis was not a problem in these high doses of ciclesonide. A similar finding was reported for high dose FP 1000‐1500 in Adams 2005b, whereby FP was associated not only with a greater likelihood of oral candidiasis, but also sore throat and symptoms of hoarseness.
Adverse event data did not yield findings that provide firm guidance on the effects of ciclesonide. The studies available may not been conducted over a sufficient period of time in order to provide useful data to this end. The reputed benefits of ciclesonide in terms of fewer local side‐effects remain to be shown by additional trials of longer duration, that assess for oral candida using microbiological confirmation of infection.
Dose response analysis
The analyses of the dose ranging studies was limited, with inconclusive effects on FEV1 found in adults between 100 and 200 mcg/d, and also between doses of 100 and 400 mcg/d. In terms of am and pm PEF no significant changes were found between low and higher doses of ciclesonide. This highlights some uncertainty in the lower dose ranges of ciclesonide, and reinforces the requirement for more work in this area. Currently the effect estimates do not provide evidence that increasing the dose of ciclesonide confers significant benefit, but the confidence intervals are too wide to draw any conclusions.
Since the number of studies and participants is low, the evidence base may be underpowered to identify a dose response curve in ciclesonide. The confidence limits do not exclude the possibility of a meaningful effect in favour of higher doses. The data from placebo groups are also noteworthy with baseline details of Gelfand 2006a indicating that participants had moderate airway obstruction (around 69% predicted of FEV1), and placebo‐treated children saw FEV1 improve by 0.22 L over baseline values.
Quality of Life, exacerbations and asthma symptoms
Data from studies conducted in adults indicated that ciclesonide led to significant improvements in both quality of life (QoL) and asthma symptoms at low doses compared with placebo. Currently the effect estimates do not provide evidence that increasing the dose of ciclesonide confers significant benefit; as with the lung function endpoints in the confidence limits do not exclude the possibility of a meaningful effect in favour of higher doses.
There is a lack of evidence regarding the impact of ciclesonide on the prevention of exacerbations of asthma which prompt additional treatment with steroids or presentation in acute care settings. Studies reporting outcome data which are defined adequately in terms of the required treatment strategy would help to establish the role of ciclesonide in the management of asthma better.
Effect size overview (Table 1)
Since the number of studies in the medium and high dose ranges were low, we tabulated effect sizes (given as standard deviation units) for FEV1, am PEF, symptoms and rescue medication use for the low dose comparison with placebo. This indicated that the effect size was stronger for symptoms and rescue medication use than for lung function outcomes.
Limitations of the review
One large parallel group trial only available as a conference abstract has not contributed data to this review, which is a safety study conducted over 12 months (Baena Cagnani 2006). Given the relatively low numbers of studies contributing data to primary outcomes analysed in this review, the absence of published data could leave the effect estimates open to the threat of publication bias. We could not reliably detect this since the most number of studies available for analysis was for any one outcome was six, meaning that funnel plot asymmetry may not be a sensitive instrument for assessing this.
In summary, the evidence from this review indicates that in short term studies at low and moderate doses (50‐800 mcg/d), ciclesonide is effective in improving lung function, and reducing symptoms compared with placebo in patients previously treated with regular inhaled steroids. There is a lack of evidence regarding the impact of ciclesonide on exacerbations. Effects from dose response studies have been conflicting and do not yet show how the dose response curve of ciclesonide compares with that of other steroid preparations such as fluticasone or beclomethasone. It is feasible that differing characteristics of the participants recruited to the studies in this review may have affected the behaviour of outcomes in the dose response comparisons, since placebo controlled trials with low attrition rates likely reflect a response to inhaled therapy in people with mild asthma (Adams 2006). In view of the limited evidence available on the effects of increasing the dose of ciclesonide this review cannot make firm recommendations regarding the dose response curve of ciclesonide.
Authors' conclusions
Implications for practice.
The results of this review clearly show a short‐term benefit of ciclesonide compared to placebo, in terms of lung function, symptoms and rescue inhaler use. The results have not identified an apparent dose‐response effect of ciclesonide across a wide range of doses.
Implications for research.
The included studies are of short duration and the number of studies in the medium and high dose range are low; thus further longer term studies in adults and children at higher doses are required to determine the impact of ciclesonide on exacerbations and to assess whether increasing the doses of ciclesonide confers significant benefit and to evaluate, as yet, unidentified safety issues with this strategy. In Bateman 2006, CIC at higher doses allowed a reduction in oral steroid doses in steroid dependant asthmatics . Further studies are needed to identify the usefulness and safety of recommending this strategy on a wider basis for such patients. Finally, it will be important to evaluate and compare the efficacy and safety of ciclesonide with currently available inhaled corticosteroids.
What's new
| Date | Event | Description |
|---|---|---|
| 22 July 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 2, 2008
| Date | Event | Description |
|---|---|---|
| 11 February 2008 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We are grateful to the staff of the editorial base of the Cochrane Airways Group for providing assistance in running searches and obtaining papers. The contact author received a Cochrane Fellowship from the Health Research Board, for the island of Ireland. We thank Dr Mitsuru Adachi, Prof Eric Bateman & Prof Erwin Gelfand for corresponding with us in our attempts to obtain unpublished data from their studies (Adachi 2007a; Adachi 2007b; Bateman 2006a; Gelfand 2006a).
Data and analyses
Comparison 1. Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 | 5 | 1673 | Litres (Fixed, 95% CI) | 0.08 [0.05, 0.11] |
| 1.1 Children | 2 | 765 | Litres (Fixed, 95% CI) | 0.05 [0.00, 0.09] |
| 1.2 Adults | 3 | 908 | Litres (Fixed, 95% CI) | 0.13 [0.08, 0.18] |
| 2 Change in FEV1 predicted | 4 | 1428 | Mean Difference (IV, Fixed, 95% CI) | 2.97 [1.80, 4.13] |
| 2.1 Children | 2 | 765 | Mean Difference (IV, Fixed, 95% CI) | 2.09 [‐0.19, 4.36] |
| 2.2 Adults | 2 | 663 | Mean Difference (IV, Fixed, 95% CI) | 3.27 [1.92, 4.63] |
| 3 Change in FVC | 2 | 402 | Litres (Fixed, 95% CI) | 0.11 [0.03, 0.19] |
| 3.1 Children | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 2 | 402 | Litres (Fixed, 95% CI) | 0.11 [0.03, 0.19] |
| 4 Change in clinic PEF | 1 | L/min (Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in am PEF | 5 | 1673 | L/min (Fixed, 95% CI) | 13.94 [9.74, 18.14] |
| 5.1 Children | 2 | 765 | L/min (Fixed, 95% CI) | 9.5 [3.26, 15.74] |
| 5.2 Adults | 3 | 908 | L/min (Fixed, 95% CI) | 17.62 [11.94, 23.30] |
| 6 Change in pm PEF | 5 | 1677 | L/min (Fixed, 95% CI) | 10.70 [6.91, 14.49] |
| 6.1 Children | 2 | 769 | L/min (Fixed, 95% CI) | 8.25 [2.80, 13.71] |
| 6.2 Adults | 3 | 908 | L/min (Fixed, 95% CI) | 12.98 [7.72, 18.24] |
| 7 Change in rescue beta‐agonist use | 3 | 908 | Puffs/d (Random, 95% CI) | ‐0.87 [‐1.36, ‐0.37] |
| 7.1 Children | 0 | 0 | Puffs/d (Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Adults | 3 | 908 | Puffs/d (Random, 95% CI) | ‐0.87 [‐1.36, ‐0.37] |
| 8 Change in total symptoms | 2 | 741 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐0.53, ‐0.24] |
| 8.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Adults | 2 | 741 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐0.53, ‐0.24] |
| 9 Change in daytime symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Change in nighttime symptoms | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Change in nighttime awakenings (n/night) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Change in quality of life score (paediatric AQLQ) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13 Change from baseline in quality of life score (AQLQ) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Adverse event | 4 | 1174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.81, 0.99] |
| 14.1 Children | 2 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.80, 0.99] |
| 14.2 Adults | 2 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.70, 1.22] |
| 15 Candidiasis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.1 Children | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Pharyngitis | 2 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.53, 2.28] |
| 16.1 Children | 2 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.53, 2.28] |
| 16.2 Adults | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Nasopharyngitis | 3 | 929 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.74, 1.42] |
| 17.1 Children | 2 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.62, 1.53] |
| 17.2 Adults | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.69, 1.73] |
| 18 Headache | 4 | 1174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.58, 1.34] |
| 18.1 Children | 2 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.56, 1.39] |
| 18.2 Adults | 2 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.33, 2.44] |
| 19 Upper respiratory tract infection | 4 | 1174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.65, 1.36] |
| 19.1 Children | 2 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.44, 1.12] |
| 19.2 Adults | 2 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.83, 2.93] |
| 20 Withdrawals | 4 | 1174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.55, 0.83] |
| 20.1 Children | 2 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.56, 1.06] |
| 20.2 Adults | 2 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.46, 0.78] |
| 21 Withdrawals (lack of efficacy) | 5 | 1680 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.38, 0.60] |
| 21.1 Children | 2 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.37, 0.90] |
| 21.2 Adults | 3 | 908 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.34, 0.59] |
| 22 Withdrawals (adverse events) | 4 | 1174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.43, 0.99] |
| 22.1 Children | 2 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.39, 0.92] |
| 22.2 Adults | 2 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.32, 29.36] |
| 23 Rhinitis | 3 | 1017 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.72, 1.96] |
| 23.1 Children | 2 | 772 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.63, 2.04] |
| 23.2 Adults | 1 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.52, 3.48] |
| 24 Asthma (not otherwise specified) | 3 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.40, 0.75] |
| 24.1 Children | 2 | 768 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.46, 0.92] |
| 24.2 Adults | 1 | 245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.13, 0.62] |
1.1. Analysis.
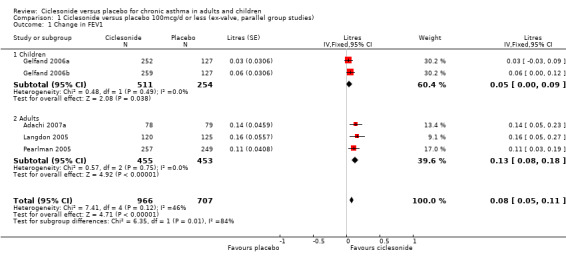
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 1 Change in FEV1.
1.2. Analysis.
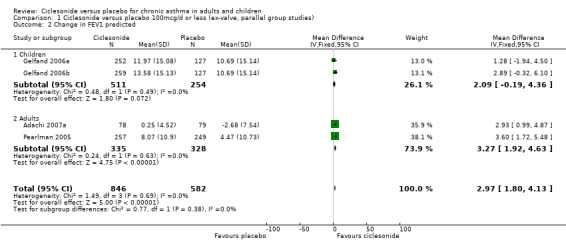
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 2 Change in FEV1 predicted.
1.3. Analysis.
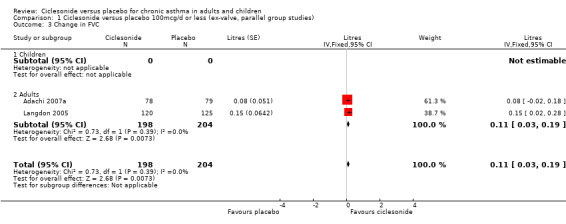
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 3 Change in FVC.
1.4. Analysis.
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 4 Change in clinic PEF.
1.5. Analysis.
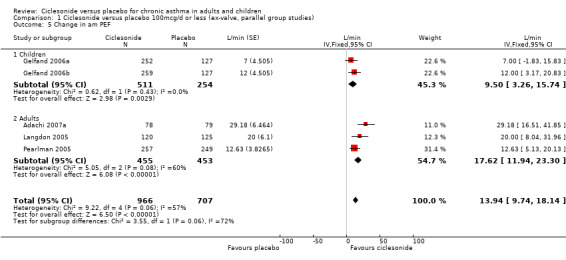
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 5 Change in am PEF.
1.6. Analysis.
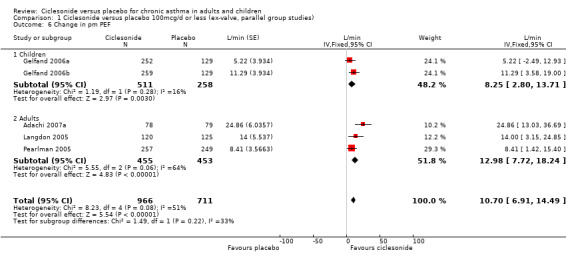
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 6 Change in pm PEF.
1.7. Analysis.
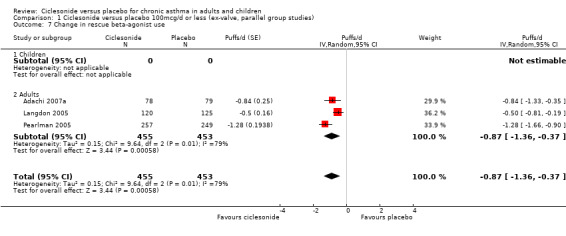
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 7 Change in rescue beta‐agonist use.
1.8. Analysis.
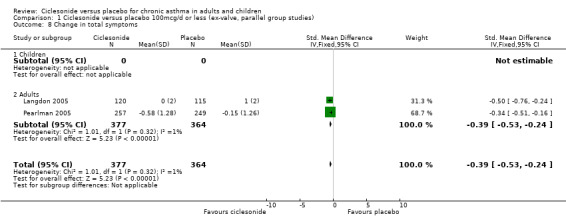
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 8 Change in total symptoms.
1.9. Analysis.
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 9 Change in daytime symptoms.
1.10. Analysis.
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 10 Change in nighttime symptoms.
1.11. Analysis.
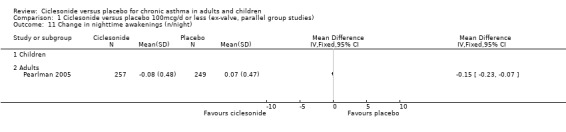
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 11 Change in nighttime awakenings (n/night).
1.12. Analysis.
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 12 Change in quality of life score (paediatric AQLQ).
1.13. Analysis.
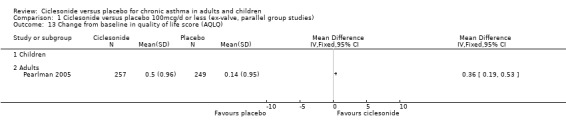
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 13 Change from baseline in quality of life score (AQLQ).
1.14. Analysis.
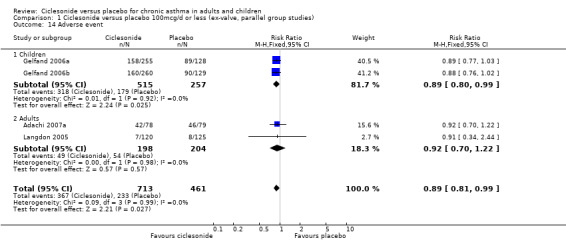
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 14 Adverse event.
1.15. Analysis.
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 15 Candidiasis.
1.16. Analysis.
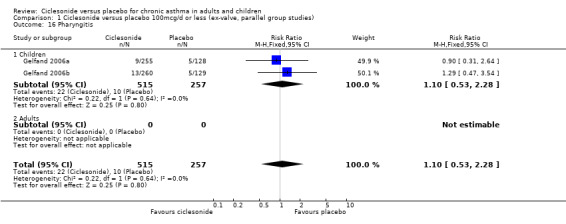
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 16 Pharyngitis.
1.17. Analysis.
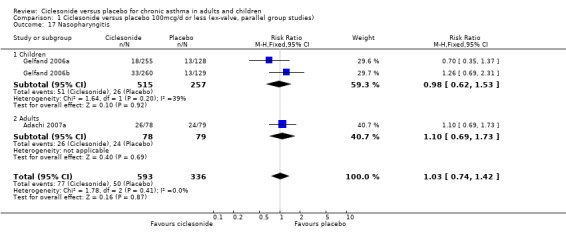
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 17 Nasopharyngitis.
1.18. Analysis.
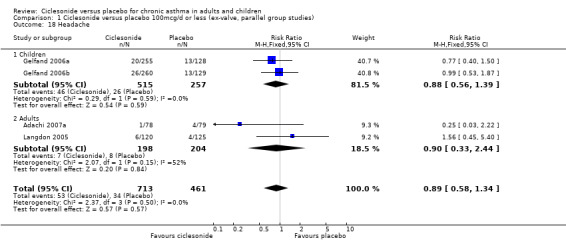
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 18 Headache.
1.19. Analysis.
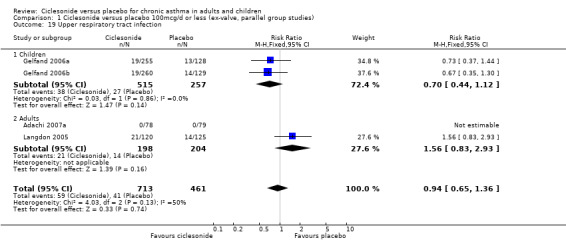
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 19 Upper respiratory tract infection.
1.20. Analysis.
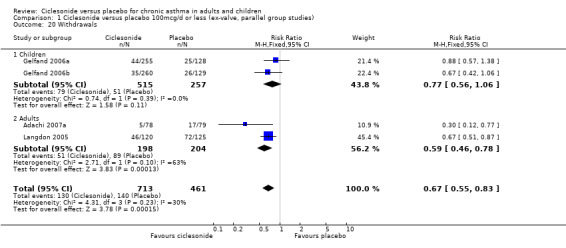
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 20 Withdrawals.
1.21. Analysis.
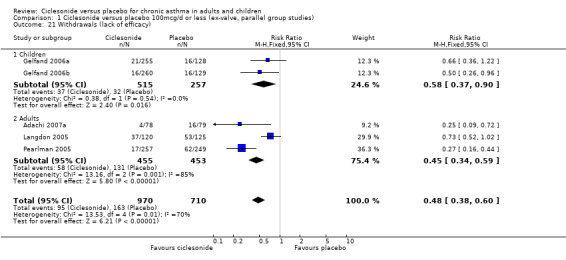
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 21 Withdrawals (lack of efficacy).
1.22. Analysis.
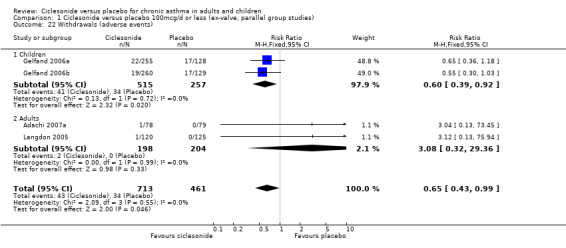
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 22 Withdrawals (adverse events).
1.23. Analysis.
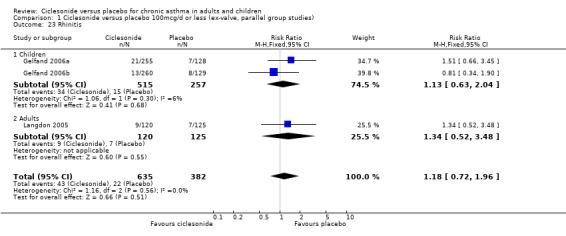
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 23 Rhinitis.
1.24. Analysis.
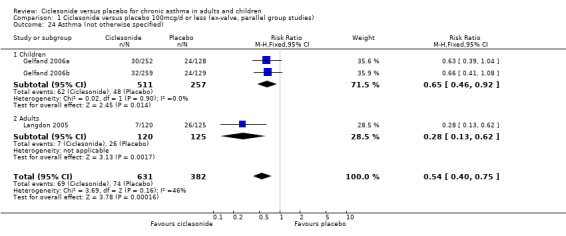
Comparison 1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies), Outcome 24 Asthma (not otherwise specified).
Comparison 2. Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 at endpoint | 1 | L (Fixed, 95% CI) | Totals not selected | |
| 1.1 Children | 0 | L (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Adults | 1 | L (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in FEV1 | 4 | 1543 | L (Fixed, 95% CI) | 0.12 [0.08, 0.15] |
| 2.1 Children | 1 | 507 | L (Fixed, 95% CI) | 0.07 [0.02, 0.12] |
| 2.2 Adults | 3 | 1036 | L (Fixed, 95% CI) | 0.17 [0.12, 0.22] |
| 3 Change in FEV1 predicted | 3 | 1171 | Mean Difference (IV, Fixed, 95% CI) | 3.06 [1.85, 4.26] |
| 3.1 Children | 1 | 507 | Mean Difference (IV, Fixed, 95% CI) | 3.48 [0.86, 6.10] |
| 3.2 Adults | 2 | 664 | Mean Difference (IV, Fixed, 95% CI) | 2.94 [1.58, 4.30] |
| 4 Change in FVC | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in clinic PEF (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Change in am PEF | 5 | 1768 | L/min (Fixed, 95% CI) | 18.66 [14.74, 22.57] |
| 6.1 Children | 1 | 507 | L/min (Fixed, 95% CI) | 7.53 [0.49, 14.57] |
| 6.2 Adults | 4 | 1261 | L/min (Fixed, 95% CI) | 23.64 [18.93, 28.35] |
| 7 Change in pm PEF (L/min) | 4 | 1383 | Mean Difference (IV, Fixed, 95% CI) | 13.93 [9.52, 18.35] |
| 7.1 Children | 1 | 507 | Mean Difference (IV, Fixed, 95% CI) | 8.7 [2.40, 15.00] |
| 7.2 Adults | 3 | 876 | Mean Difference (IV, Fixed, 95% CI) | 18.97 [12.78, 25.15] |
| 8 Change in rescue 2‐agonists use | 3 | 1034 | Puffs/d (Fixed, 95% CI) | ‐1.11 [‐1.32, ‐0.89] |
| 8.1 Children | 0 | 0 | Puffs/d (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Adults | 3 | 1034 | Puffs/d (Fixed, 95% CI) | ‐1.11 [‐1.32, ‐0.89] |
| 9 Change in asthma symptom scores | 3 | 1091 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐0.65, ‐0.38] |
| 9.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Adults | 3 | 1091 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐0.65, ‐0.38] |
| 10 Change in nighttime awakenings (SMD) | 2 | 874 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.47, ‐0.20] |
| 10.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Adults | 2 | 874 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.47, ‐0.20] |
| 11 Change in quality of life score (paediatric AQLQ) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Change from baseline in quality of life score (AQLQ) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Loss of efficacy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14 Withdrawals | 3 | 877 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.43, 0.70] |
| 14.1 Children | 1 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.41, 0.93] |
| 14.2 Adults | 2 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.38, 0.69] |
| 15 Withdrawals (lack of efficacy) | 3 | 1159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.22, 0.45] |
| 15.1 Children | 1 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.24, 0.81] |
| 15.2 Adults | 2 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.16, 0.41] |
| 16 Withdrawals (adverse events) | 3 | 1159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.27, 0.56] |
| 16.1 Children | 1 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.27, 0.84] |
| 16.2 Adults | 2 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.22, 0.55] |
| 17 Changes in cortisol levels (serum) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18 Changes in cortisol levels (urinary) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19 Asthma (not otherwise specified) | 2 | 724 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.43, 0.83] |
| 19.1 Children | 1 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.44, 1.01] |
| 19.2 Adults | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.28, 0.84] |
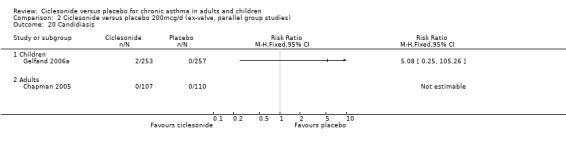
| 20 Candidiasis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
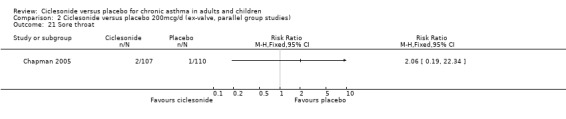
| 21 Sore throat | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
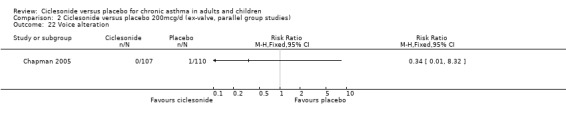
| 22 Voice alteration | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
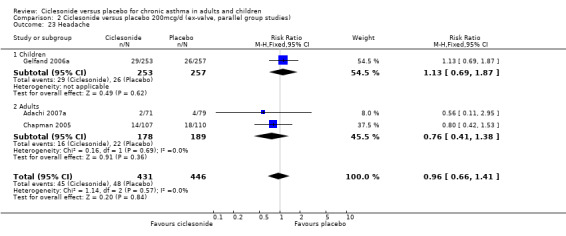
| 23 Headache | 3 | 877 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.41] |
| 23.1 Children | 1 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.69, 1.87] |
| 23.2 Adults | 2 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.41, 1.38] |
| 24 Upper respiratory tract infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 24.2 Adults | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25 Symptoms of asthma (wheeze, dsypnea or cough) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 26 Rhinitis | 2 | 727 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.33, 1.00] |
| 26.1 Children | 1 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.23, 1.26] |
| 26.2 Adults | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.29, 1.26] |
| 27 Adverse event | 2 | 670 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.84, 1.05] |
| 27.1 Children | 1 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.89, 1.11] |
| 27.2 Adults | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.54, 1.01] |
| 28 Pharyngitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 28.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 28.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 29 Nasopharyngitis | 2 | 660 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.81, 1.61] |
| 29.1 Children | 1 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.79, 2.09] |
| 29.2 Adults | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.60, 1.59] |
2.1. Analysis.
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 1 FEV1 at endpoint.
2.2. Analysis.
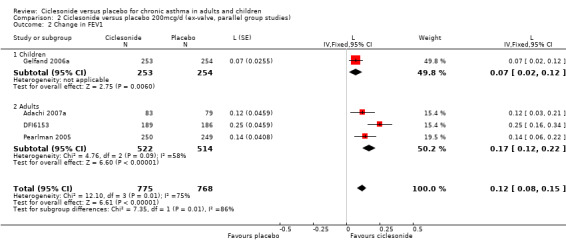
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 2 Change in FEV1.
2.3. Analysis.
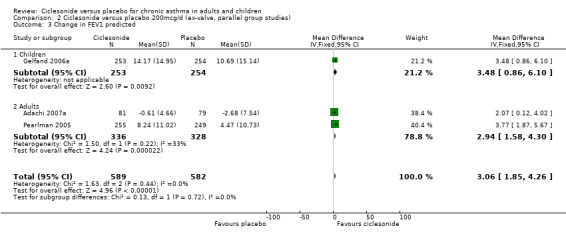
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 3 Change in FEV1 predicted.
2.4. Analysis.
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 4 Change in FVC.
2.5. Analysis.
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 5 Change in clinic PEF (L/min).
2.6. Analysis.
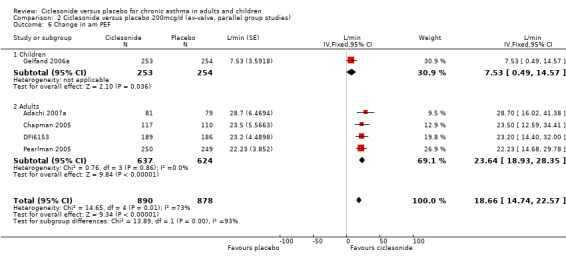
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 6 Change in am PEF.
2.7. Analysis.
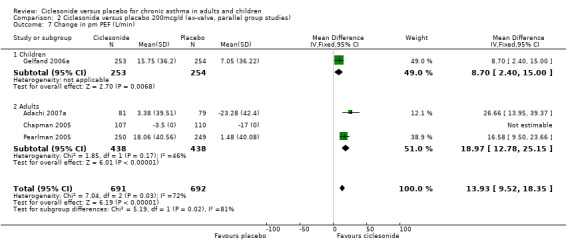
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 7 Change in pm PEF (L/min).
2.8. Analysis.
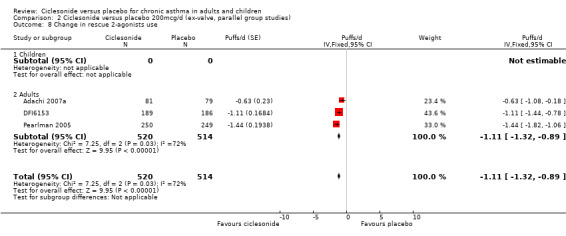
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 8 Change in rescue 2‐agonists use.
2.9. Analysis.
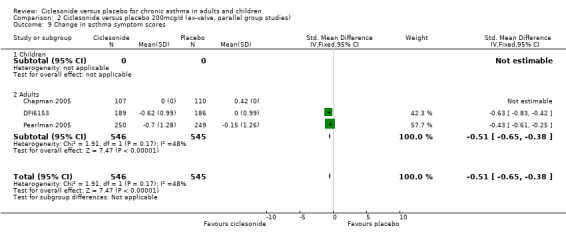
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 9 Change in asthma symptom scores.
2.10. Analysis.
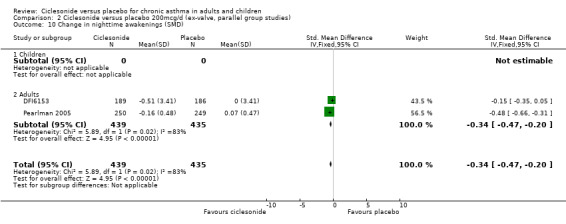
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 10 Change in nighttime awakenings (SMD).
2.11. Analysis.
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 11 Change in quality of life score (paediatric AQLQ).
2.12. Analysis.
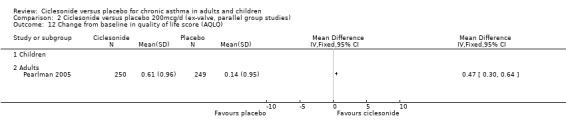
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 12 Change from baseline in quality of life score (AQLQ).
2.13. Analysis.
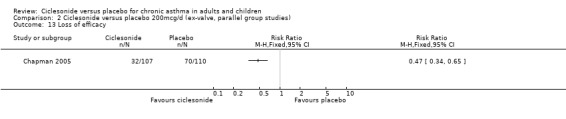
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 13 Loss of efficacy.
2.14. Analysis.
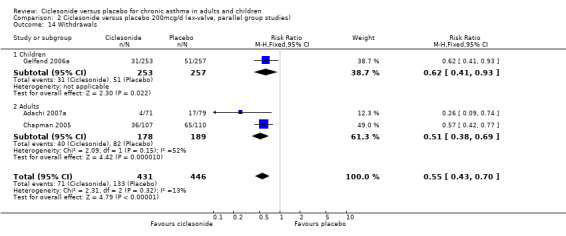
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 14 Withdrawals.
2.15. Analysis.
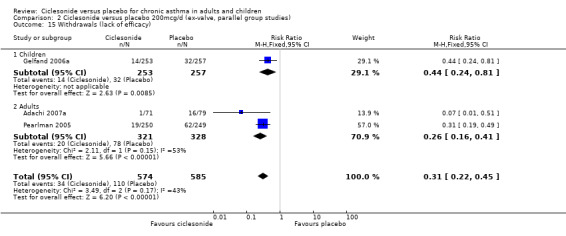
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 15 Withdrawals (lack of efficacy).
2.16. Analysis.
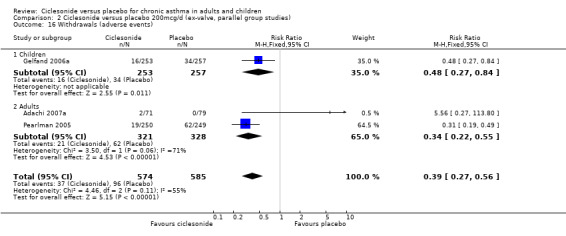
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 16 Withdrawals (adverse events).
2.17. Analysis.
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 17 Changes in cortisol levels (serum).
2.18. Analysis.
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 18 Changes in cortisol levels (urinary).
2.19. Analysis.
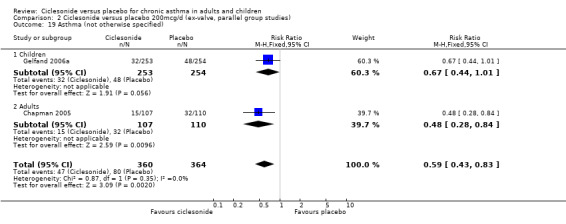
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 19 Asthma (not otherwise specified).
2.20. Analysis.
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 20 Candidiasis.
2.21. Analysis.
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 21 Sore throat.
2.22. Analysis.
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 22 Voice alteration.
2.23. Analysis.
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 23 Headache.
2.24. Analysis.
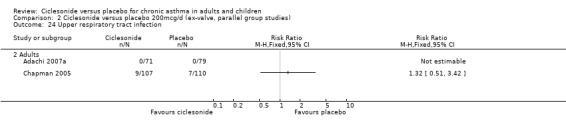
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 24 Upper respiratory tract infection.
2.25. Analysis.
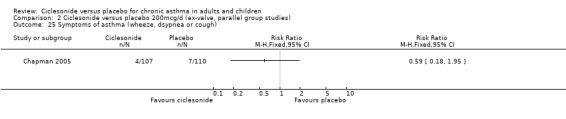
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 25 Symptoms of asthma (wheeze, dsypnea or cough).
2.26. Analysis.
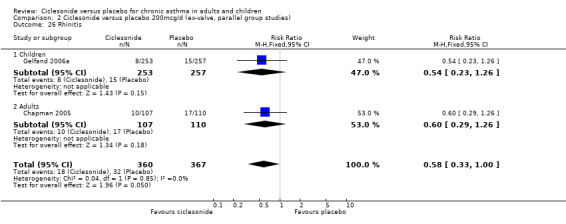
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 26 Rhinitis.
2.27. Analysis.
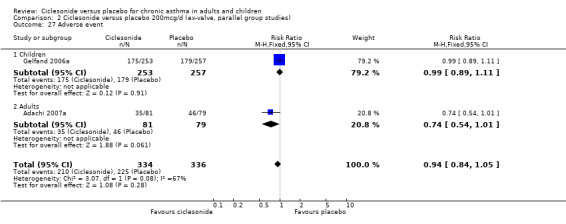
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 27 Adverse event.
2.28. Analysis.
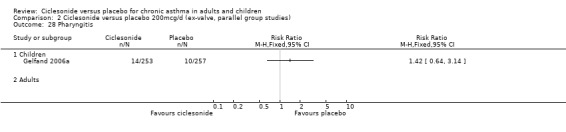
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 28 Pharyngitis.
2.29. Analysis.
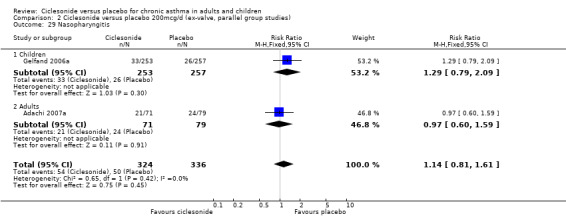
Comparison 2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies), Outcome 29 Nasopharyngitis.
Comparison 3. Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 | 6 | 1717 | Litres (Fixed, 95% CI) | 0.17 [0.14, 0.21] |
| 1.1 Children | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 6 | 1717 | Litres (Fixed, 95% CI) | 0.17 [0.14, 0.21] |
| 2 Change from baseline in FEV1% predicted | 2 | 666 | Mean Difference (IV, Fixed, 95% CI) | 3.10 [1.66, 4.53] |
| 2.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 2 | 666 | Mean Difference (IV, Fixed, 95% CI) | 3.10 [1.66, 4.53] |
| 3 Change in FVC | 2 | 402 | Litres (Fixed, 95% CI) | 0.12 [0.03, 0.20] |
| 3.1 Children | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 2 | 402 | Litres (Fixed, 95% CI) | 0.12 [0.03, 0.20] |
| 4 Change in am PEF | 6 | 1722 | L/min (Fixed, 95% CI) | 18.19 [14.31, 22.07] |
| 4.1 Children | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Adults | 6 | 1722 | L/min (Fixed, 95% CI) | 18.19 [14.31, 22.07] |
| 5 Change in pm PEF | 3 | 906 | L/min (Fixed, 95% CI) | 15.30 [10.04, 20.55] |
| 5.1 Children | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Adults | 3 | 906 | L/min (Fixed, 95% CI) | 15.30 [10.04, 20.55] |
| 6 Change in asthma symptom scores | 5 | 1560 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.59, ‐0.38] |
| 6.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Adults | 5 | 1560 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.59, ‐0.38] |
| 7 Change in rescue 2‐agonists use | 6 | 1722 | Puffs/d (Fixed, 95% CI) | ‐0.78 [‐0.94, ‐0.62] |
| 7.1 Children | 0 | 0 | Puffs/d (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Adults | 6 | 1722 | Puffs/d (Fixed, 95% CI) | ‐0.78 [‐0.94, ‐0.62] |
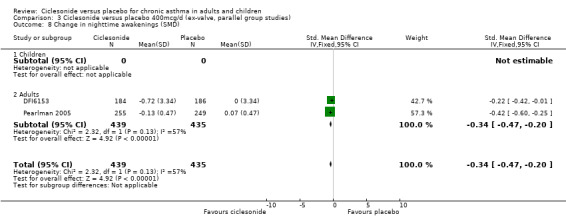
| 8 Change in nighttime awakenings (SMD) | 2 | 874 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.47, ‐0.20] |
| 8.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Adults | 2 | 874 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.34 [‐0.47, ‐0.20] |
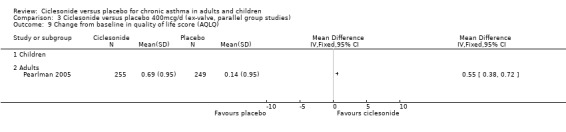
| 9 Change from baseline in quality of life score (AQLQ) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
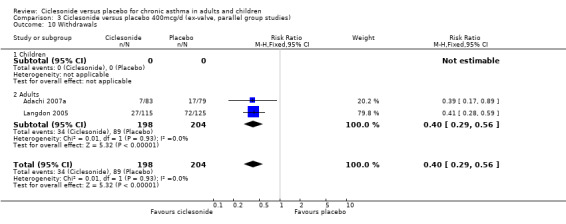
| 10 Withdrawals | 2 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.29, 0.56] |
| 10.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Adults | 2 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.29, 0.56] |
| 11 Withdrawals (lack of efficacy) | 3 | 906 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.23, 0.44] |
| 11.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 Adults | 3 | 906 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.23, 0.44] |
| 12 Adverse events | 4 | 858 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.85, 1.13] |
| 12.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Adults | 4 | 858 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.85, 1.13] |
| 13 Upper respiratory tract infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Adults | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Headache | 2 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.65, 3.68] |
| 14.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Adults | 2 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.65, 3.68] |
| 15 Rhinitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Nasopharyngitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17 Change in high dose peak serum cortisol levels | 2 | 157 | mcg/dL (Fixed, 95% CI) | ‐0.75 [‐2.24, 0.75] |
| 17.1 Children | 0 | 0 | mcg/dL (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 Adults | 2 | 157 | mcg/dL (Fixed, 95% CI) | ‐0.75 [‐2.24, 0.75] |
3.1. Analysis.
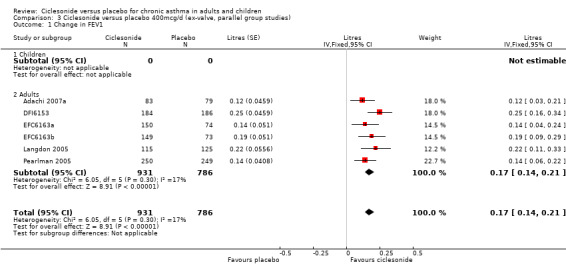
Comparison 3 Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies), Outcome 1 Change in FEV1.
3.2. Analysis.
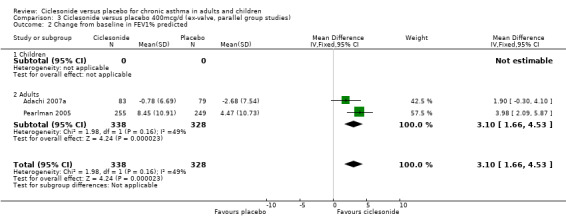
Comparison 3 Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies), Outcome 2 Change from baseline in FEV1% predicted.
3.3. Analysis.
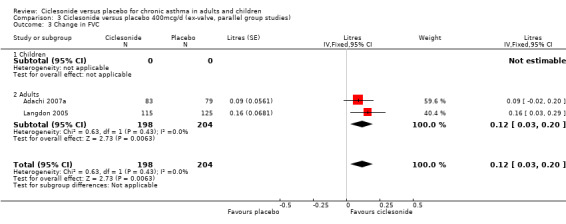
Comparison 3 Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies), Outcome 3 Change in FVC.
3.4. Analysis.
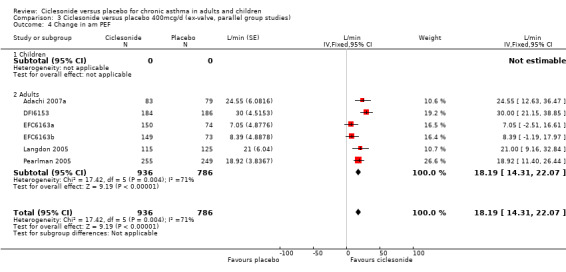
Comparison 3 Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies), Outcome 4 Change in am PEF.
3.5. Analysis.
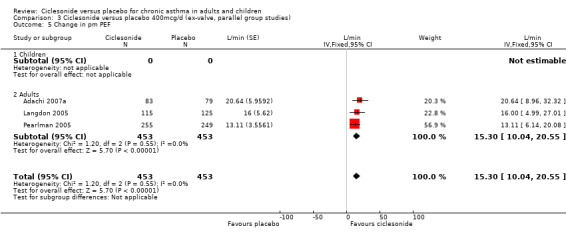
Comparison 3 Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies), Outcome 5 Change in pm PEF.
3.6. Analysis.
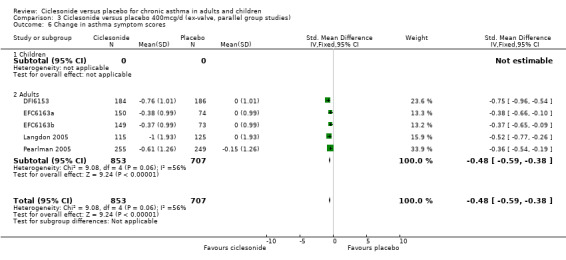
Comparison 3 Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies), Outcome 6 Change in asthma symptom scores.
3.7. Analysis.
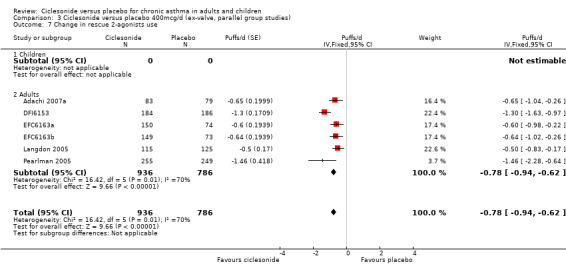
Comparison 3 Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies), Outcome 7 Change in rescue 2‐agonists use.
3.8. Analysis.
Comparison 3 Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies), Outcome 8 Change in nighttime awakenings (SMD).
3.9. Analysis.
Comparison 3 Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies), Outcome 9 Change from baseline in quality of life score (AQLQ).
3.10. Analysis.
Comparison 3 Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies), Outcome 10 Withdrawals.
3.11. Analysis.
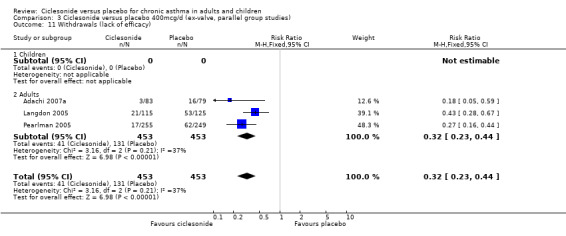
Comparison 3 Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies), Outcome 11 Withdrawals (lack of efficacy).
3.12. Analysis.
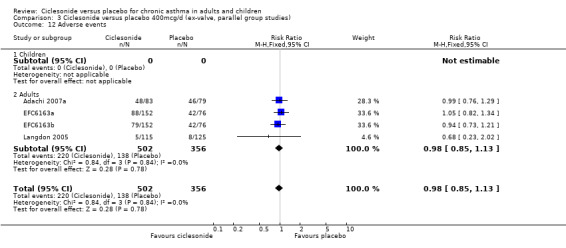
Comparison 3 Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies), Outcome 12 Adverse events.
3.13. Analysis.
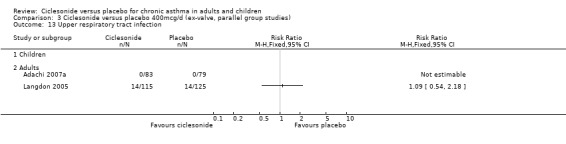
Comparison 3 Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies), Outcome 13 Upper respiratory tract infection.
3.14. Analysis.
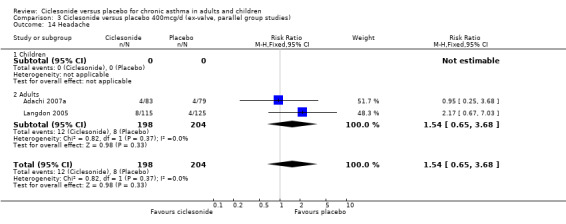
Comparison 3 Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies), Outcome 14 Headache.
3.15. Analysis.
Comparison 3 Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies), Outcome 15 Rhinitis.
3.16. Analysis.
Comparison 3 Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies), Outcome 16 Nasopharyngitis.
3.17. Analysis.
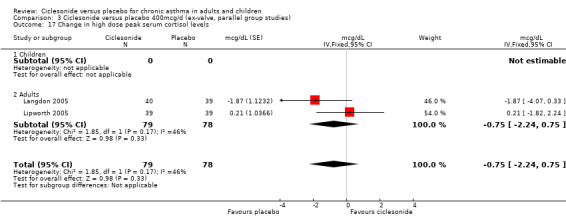
Comparison 3 Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies), Outcome 17 Change in high dose peak serum cortisol levels.
Comparison 4. Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 | 1 | Litres (Fixed, 95% CI) | Totals not selected | |
| 1.1 Children | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Adults | 1 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FEV1 at endpoint | 1 | L (Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 0 | L (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 1 | L (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in FEV1 predicted | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in FVC | 0 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in am PEF | 2 | 594 | L/min (Fixed, 95% CI) | 28.09 [21.24, 34.94] |
| 5.1 Children | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Adults | 2 | 594 | L/min (Fixed, 95% CI) | 28.09 [21.24, 34.94] |
| 6 Change in pm PEF (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Change in clinic PEF (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Change in asthma symptom scores | 2 | 594 | Mean Difference (IV, Fixed, 95% CI) | ‐0.87 [‐1.08, ‐0.66] |
| 8.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Adults | 2 | 594 | Mean Difference (IV, Fixed, 95% CI) | ‐0.87 [‐1.08, ‐0.66] |
| 9 Change in rescue beta2‐agonists use | 2 | 594 | Puffs/d (Fixed, 95% CI) | ‐1.19 [‐1.52, ‐0.86] |
| 9.1 Children | 0 | 0 | Puffs/d (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Adults | 2 | 594 | Puffs/d (Fixed, 95% CI) | ‐1.19 [‐1.52, ‐0.86] |
| 10 Change in high dose peak serum cortisol levels | 1 | mcg/dL (Fixed, 95% CI) | Totals not selected | |
| 10.1 Children | 0 | mcg/dL (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Adults | 1 | mcg/dL (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Changes in cortisol levels (urinary) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Worsening asthma | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13 Loss of efficacy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14 Withdrawals | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15 Candidiasis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16 Sore throat | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 17 Voice alteration | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 18 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19 Upper respiratory tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 20 Symptoms of asthma (wheeze, dsypnea or cough) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 21 Rhinitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 22 Adverse events | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 22.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
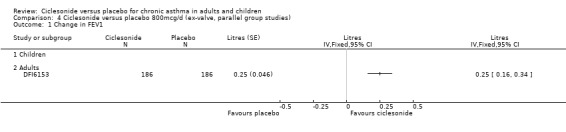
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 1 Change in FEV1.
4.2. Analysis.
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 2 FEV1 at endpoint.
4.5. Analysis.
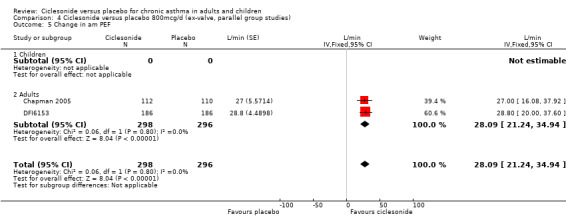
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 5 Change in am PEF.
4.6. Analysis.
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 6 Change in pm PEF (L/min).
4.7. Analysis.
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 7 Change in clinic PEF (L/min).
4.8. Analysis.
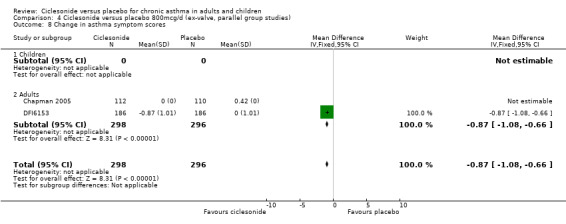
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 8 Change in asthma symptom scores.
4.9. Analysis.
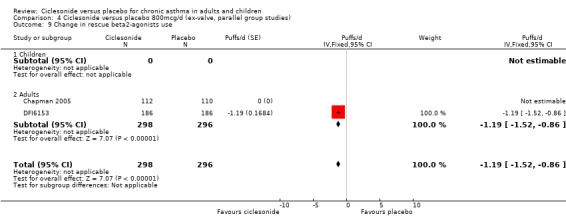
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 9 Change in rescue beta2‐agonists use.
4.10. Analysis.
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 10 Change in high dose peak serum cortisol levels.
4.11. Analysis.
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 11 Changes in cortisol levels (urinary).
4.12. Analysis.
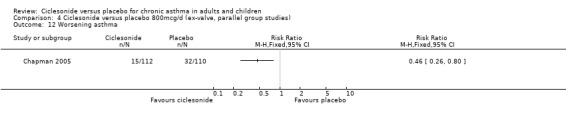
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 12 Worsening asthma.
4.13. Analysis.
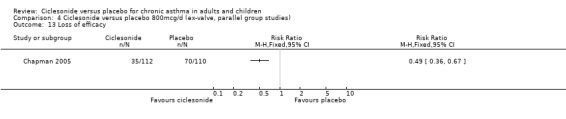
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 13 Loss of efficacy.
4.14. Analysis.
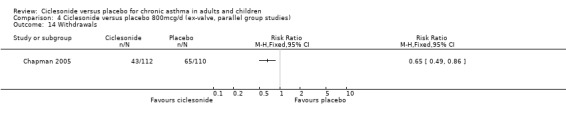
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 14 Withdrawals.
4.15. Analysis.
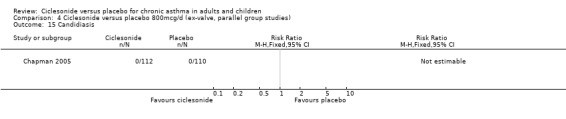
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 15 Candidiasis.
4.16. Analysis.
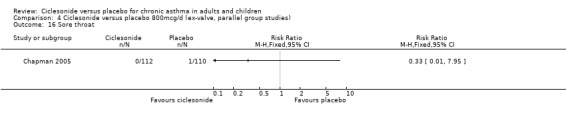
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 16 Sore throat.
4.17. Analysis.
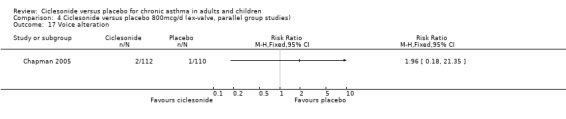
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 17 Voice alteration.
4.18. Analysis.
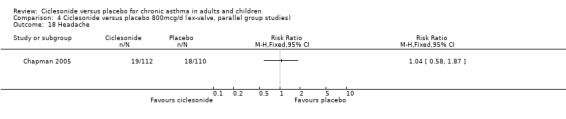
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 18 Headache.
4.19. Analysis.
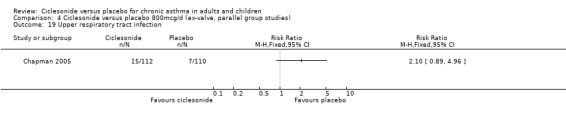
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 19 Upper respiratory tract infection.
4.20. Analysis.
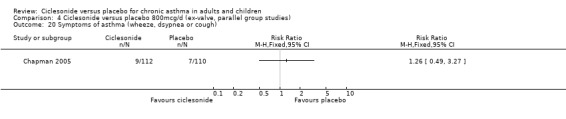
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 20 Symptoms of asthma (wheeze, dsypnea or cough).
4.21. Analysis.
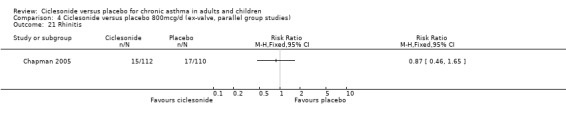
Comparison 4 Ciclesonide versus placebo 800mcg/d (ex‐valve, parallel group studies), Outcome 21 Rhinitis.
Comparison 5. Ciclesonide versus placebo 1600mcg/d (ex‐valve, parallel group studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 | 1 | Litres (Fixed, 95% CI) | Totals not selected | |
| 1.1 Children | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Adults | 1 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in am PEF | 1 | L/min (Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 1 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in rescue beta2‐agonists use | 1 | Puffs/d (Fixed, 95% CI) | Totals not selected | |
| 3.1 Children | 0 | Puffs/d (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adults | 1 | Puffs/d (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in asthma symptom scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.
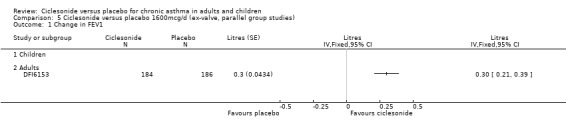
Comparison 5 Ciclesonide versus placebo 1600mcg/d (ex‐valve, parallel group studies), Outcome 1 Change in FEV1.
5.2. Analysis.
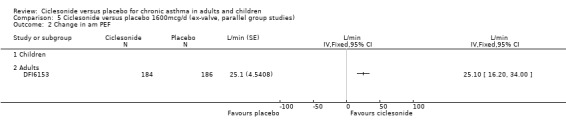
Comparison 5 Ciclesonide versus placebo 1600mcg/d (ex‐valve, parallel group studies), Outcome 2 Change in am PEF.
5.3. Analysis.
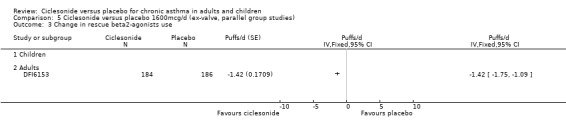
Comparison 5 Ciclesonide versus placebo 1600mcg/d (ex‐valve, parallel group studies), Outcome 3 Change in rescue beta2‐agonists use.
5.4. Analysis.
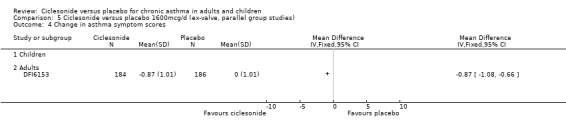
Comparison 5 Ciclesonide versus placebo 1600mcg/d (ex‐valve, parallel group studies), Outcome 4 Change in asthma symptom scores.
Comparison 6. Ciclesonide versus placebo plus OCS 800mcg/d (ex‐valve, parallel group studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in maintenance oral steroid dose (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Discontinuation of maintenance oral steroid | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Change in FEV1 | 1 | L (Fixed, 95% CI) | Totals not selected | |
| 4 Change in rescue medication use (puffs/d) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Change in 24hr symptom scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Change in am PEF (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Withdrawals (total) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Withdrawals (lack of efficacy) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Candidiasis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
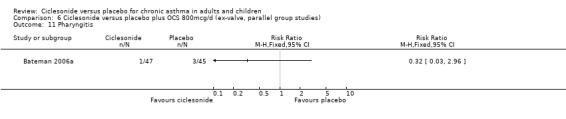
| 11 Pharyngitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Hoarseness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13 Suppression of HPA axis function (end point) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14 Withdrawals (adverse events) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
6.1. Analysis.
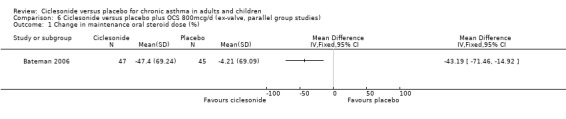
Comparison 6 Ciclesonide versus placebo plus OCS 800mcg/d (ex‐valve, parallel group studies), Outcome 1 Change in maintenance oral steroid dose (%).
6.2. Analysis.
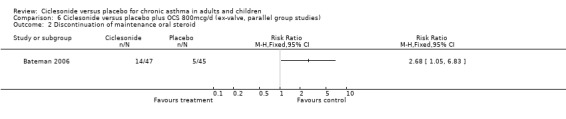
Comparison 6 Ciclesonide versus placebo plus OCS 800mcg/d (ex‐valve, parallel group studies), Outcome 2 Discontinuation of maintenance oral steroid.
6.3. Analysis.
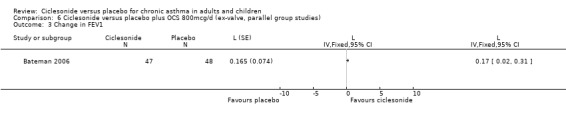
Comparison 6 Ciclesonide versus placebo plus OCS 800mcg/d (ex‐valve, parallel group studies), Outcome 3 Change in FEV1.
6.4. Analysis.
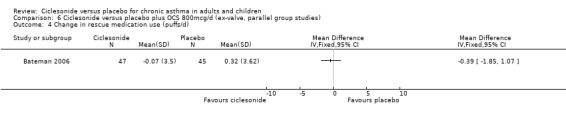
Comparison 6 Ciclesonide versus placebo plus OCS 800mcg/d (ex‐valve, parallel group studies), Outcome 4 Change in rescue medication use (puffs/d).
6.5. Analysis.
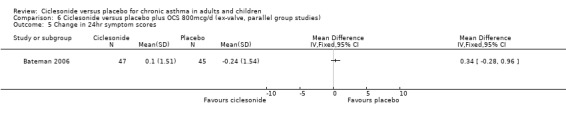
Comparison 6 Ciclesonide versus placebo plus OCS 800mcg/d (ex‐valve, parallel group studies), Outcome 5 Change in 24hr symptom scores.
6.6. Analysis.
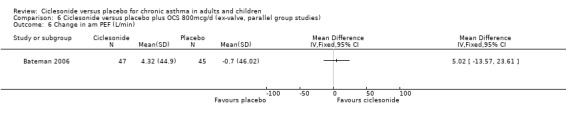
Comparison 6 Ciclesonide versus placebo plus OCS 800mcg/d (ex‐valve, parallel group studies), Outcome 6 Change in am PEF (L/min).
6.7. Analysis.
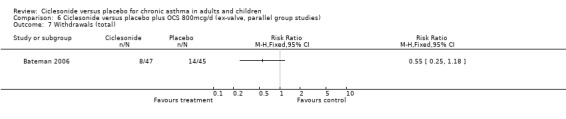
Comparison 6 Ciclesonide versus placebo plus OCS 800mcg/d (ex‐valve, parallel group studies), Outcome 7 Withdrawals (total).
6.8. Analysis.
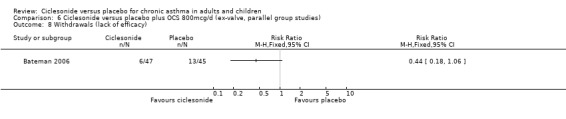
Comparison 6 Ciclesonide versus placebo plus OCS 800mcg/d (ex‐valve, parallel group studies), Outcome 8 Withdrawals (lack of efficacy).
6.9. Analysis.
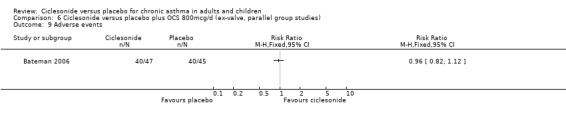
Comparison 6 Ciclesonide versus placebo plus OCS 800mcg/d (ex‐valve, parallel group studies), Outcome 9 Adverse events.
6.10. Analysis.
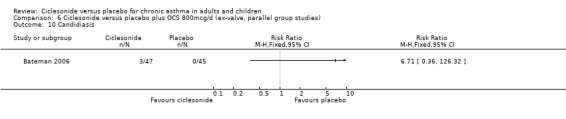
Comparison 6 Ciclesonide versus placebo plus OCS 800mcg/d (ex‐valve, parallel group studies), Outcome 10 Candidiasis.
6.11. Analysis.
Comparison 6 Ciclesonide versus placebo plus OCS 800mcg/d (ex‐valve, parallel group studies), Outcome 11 Pharyngitis.
6.12. Analysis.
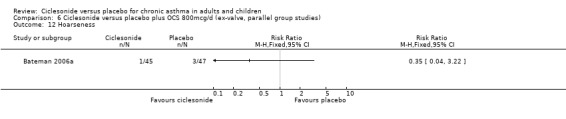
Comparison 6 Ciclesonide versus placebo plus OCS 800mcg/d (ex‐valve, parallel group studies), Outcome 12 Hoarseness.
6.13. Analysis.
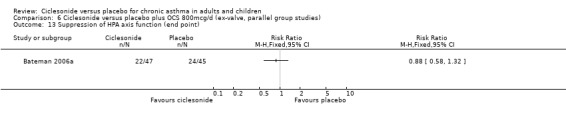
Comparison 6 Ciclesonide versus placebo plus OCS 800mcg/d (ex‐valve, parallel group studies), Outcome 13 Suppression of HPA axis function (end point).
6.14. Analysis.
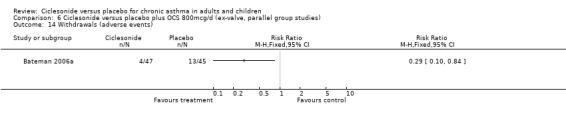
Comparison 6 Ciclesonide versus placebo plus OCS 800mcg/d (ex‐valve, parallel group studies), Outcome 14 Withdrawals (adverse events).
Comparison 7. Ciclesonide versus placebo plus OCS greater than 800mcg/d (ex‐valve, parallel group studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in maintenance oral steroid dose (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Discontinuation of maintenance oral steroid | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Change in FEV1 | 1 | L (Fixed, 95% CI) | Totals not selected | |
| 4 Change in rescue medication use (puffs/d) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Change in 24hr symptom scores | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Change in am PEF (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Withdrawals (total) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Withdrawals (lack of efficacy) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Candidiasis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Pharyngitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Hoarseness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13 Suppression of HPA axis function (end point) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14 Withdrawals (adverse events) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
7.1. Analysis.
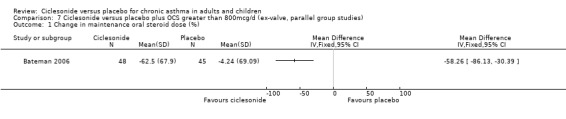
Comparison 7 Ciclesonide versus placebo plus OCS greater than 800mcg/d (ex‐valve, parallel group studies), Outcome 1 Change in maintenance oral steroid dose (%).
7.2. Analysis.
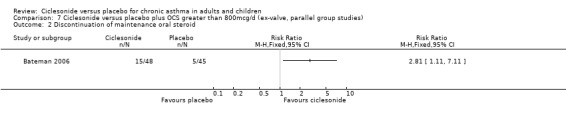
Comparison 7 Ciclesonide versus placebo plus OCS greater than 800mcg/d (ex‐valve, parallel group studies), Outcome 2 Discontinuation of maintenance oral steroid.
7.3. Analysis.

Comparison 7 Ciclesonide versus placebo plus OCS greater than 800mcg/d (ex‐valve, parallel group studies), Outcome 3 Change in FEV1.
7.4. Analysis.
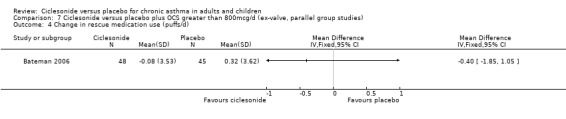
Comparison 7 Ciclesonide versus placebo plus OCS greater than 800mcg/d (ex‐valve, parallel group studies), Outcome 4 Change in rescue medication use (puffs/d).
7.5. Analysis.
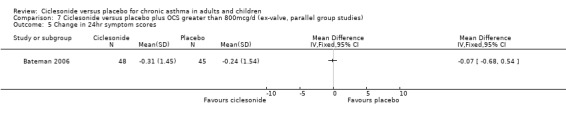
Comparison 7 Ciclesonide versus placebo plus OCS greater than 800mcg/d (ex‐valve, parallel group studies), Outcome 5 Change in 24hr symptom scores.
7.6. Analysis.
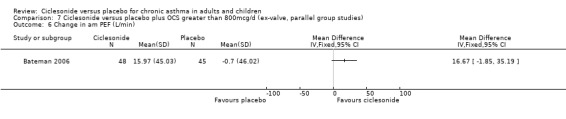
Comparison 7 Ciclesonide versus placebo plus OCS greater than 800mcg/d (ex‐valve, parallel group studies), Outcome 6 Change in am PEF (L/min).
7.7. Analysis.
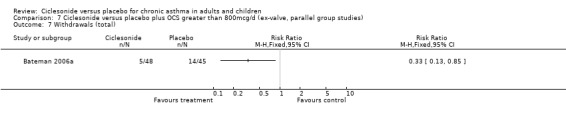
Comparison 7 Ciclesonide versus placebo plus OCS greater than 800mcg/d (ex‐valve, parallel group studies), Outcome 7 Withdrawals (total).
7.8. Analysis.
Comparison 7 Ciclesonide versus placebo plus OCS greater than 800mcg/d (ex‐valve, parallel group studies), Outcome 8 Withdrawals (lack of efficacy).
7.9. Analysis.
Comparison 7 Ciclesonide versus placebo plus OCS greater than 800mcg/d (ex‐valve, parallel group studies), Outcome 9 Adverse events.
7.10. Analysis.
Comparison 7 Ciclesonide versus placebo plus OCS greater than 800mcg/d (ex‐valve, parallel group studies), Outcome 10 Candidiasis.
7.11. Analysis.
Comparison 7 Ciclesonide versus placebo plus OCS greater than 800mcg/d (ex‐valve, parallel group studies), Outcome 11 Pharyngitis.
7.12. Analysis.
Comparison 7 Ciclesonide versus placebo plus OCS greater than 800mcg/d (ex‐valve, parallel group studies), Outcome 12 Hoarseness.
7.13. Analysis.
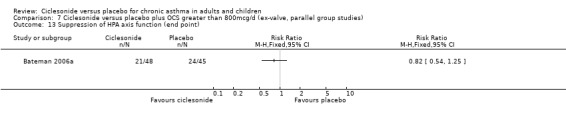
Comparison 7 Ciclesonide versus placebo plus OCS greater than 800mcg/d (ex‐valve, parallel group studies), Outcome 13 Suppression of HPA axis function (end point).
7.14. Analysis.
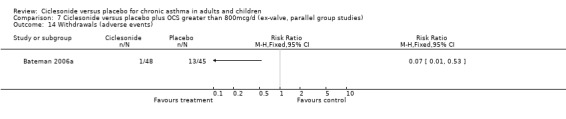
Comparison 7 Ciclesonide versus placebo plus OCS greater than 800mcg/d (ex‐valve, parallel group studies), Outcome 14 Withdrawals (adverse events).
Comparison 8. Ciclesonide 50 versus 100mcg/d (ex‐valve, parallel group studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 | 1 | Litres (Fixed, 95% CI) | Totals not selected | |
| 1.1 Children | 1 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Adults | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in FEV1 predicted (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in am PEF (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in pm PEF (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in quality of life score (paediatric AQLQ) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Candidiasis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Pharyngitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Nasopharyngitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Asthma (not otherwise specified) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Upper respiratory tract infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Rhinitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Withdrawals | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 Withdrawals (lack of efficacy) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Withdrawals (adverse events) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.
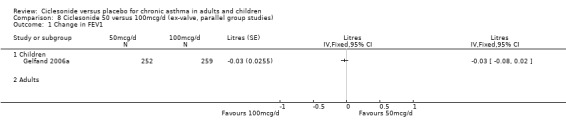
Comparison 8 Ciclesonide 50 versus 100mcg/d (ex‐valve, parallel group studies), Outcome 1 Change in FEV1.
8.2. Analysis.
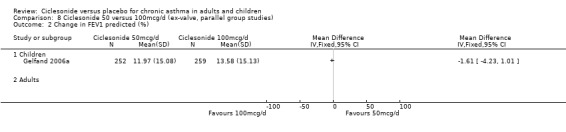
Comparison 8 Ciclesonide 50 versus 100mcg/d (ex‐valve, parallel group studies), Outcome 2 Change in FEV1 predicted (%).
8.3. Analysis.
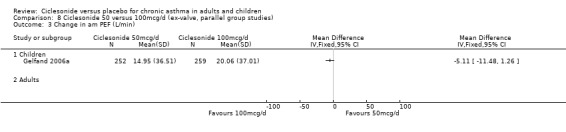
Comparison 8 Ciclesonide 50 versus 100mcg/d (ex‐valve, parallel group studies), Outcome 3 Change in am PEF (L/min).
8.4. Analysis.
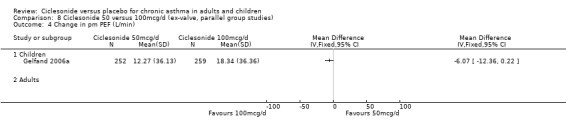
Comparison 8 Ciclesonide 50 versus 100mcg/d (ex‐valve, parallel group studies), Outcome 4 Change in pm PEF (L/min).
8.5. Analysis.
Comparison 8 Ciclesonide 50 versus 100mcg/d (ex‐valve, parallel group studies), Outcome 5 Change in quality of life score (paediatric AQLQ).
8.6. Analysis.
Comparison 8 Ciclesonide 50 versus 100mcg/d (ex‐valve, parallel group studies), Outcome 6 Adverse event.
8.7. Analysis.
Comparison 8 Ciclesonide 50 versus 100mcg/d (ex‐valve, parallel group studies), Outcome 7 Candidiasis.
8.8. Analysis.
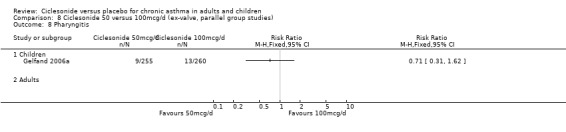
Comparison 8 Ciclesonide 50 versus 100mcg/d (ex‐valve, parallel group studies), Outcome 8 Pharyngitis.
8.9. Analysis.
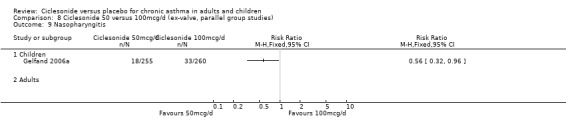
Comparison 8 Ciclesonide 50 versus 100mcg/d (ex‐valve, parallel group studies), Outcome 9 Nasopharyngitis.
8.10. Analysis.
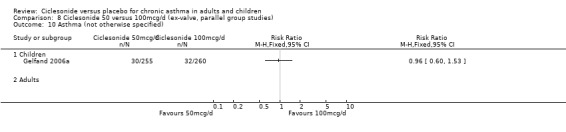
Comparison 8 Ciclesonide 50 versus 100mcg/d (ex‐valve, parallel group studies), Outcome 10 Asthma (not otherwise specified).
8.11. Analysis.
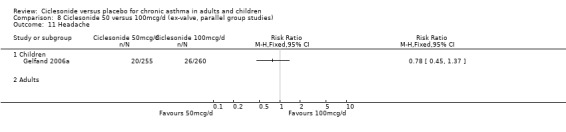
Comparison 8 Ciclesonide 50 versus 100mcg/d (ex‐valve, parallel group studies), Outcome 11 Headache.
8.12. Analysis.
Comparison 8 Ciclesonide 50 versus 100mcg/d (ex‐valve, parallel group studies), Outcome 12 Upper respiratory tract infection.
8.13. Analysis.
Comparison 8 Ciclesonide 50 versus 100mcg/d (ex‐valve, parallel group studies), Outcome 13 Rhinitis.
8.14. Analysis.
Comparison 8 Ciclesonide 50 versus 100mcg/d (ex‐valve, parallel group studies), Outcome 14 Withdrawals.
8.15. Analysis.
Comparison 8 Ciclesonide 50 versus 100mcg/d (ex‐valve, parallel group studies), Outcome 15 Withdrawals (lack of efficacy).
8.16. Analysis.
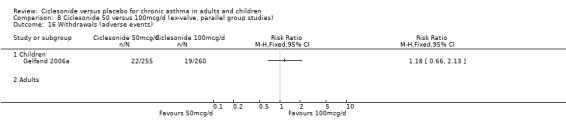
Comparison 8 Ciclesonide 50 versus 100mcg/d (ex‐valve, parallel group studies), Outcome 16 Withdrawals (adverse events).
Comparison 9. Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 | 4 | 1726 | Litres (Fixed, 95% CI) | ‐0.01 [‐0.04, 0.03] |
| 1.1 Children | 1 | 512 | Litres (Fixed, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 1.2 Adults | 3 | 1214 | Litres (Fixed, 95% CI) | ‐0.00 [‐0.05, 0.04] |
| 2 Change in FEV1 predicted (%) | 3 | 1176 | Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐0.74, 1.35] |
| 2.1 Children | 1 | 512 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐3.20, 2.02] |
| 2.2 Adults | 2 | 664 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [‐0.67, 1.62] |
| 3 Change in am PEF (L/min) | 3 | 1176 | Mean Difference (IV, Fixed, 95% CI) | 4.04 [‐0.53, 8.61] |
| 3.1 Children | 1 | 512 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐1.37, 11.37] |
| 3.2 Adults | 2 | 664 | Mean Difference (IV, Fixed, 95% CI) | 3.02 [‐3.55, 9.59] |
| 4 Change in pm PEF (L/min) | 3 | 1176 | Mean Difference (IV, Fixed, 95% CI) | 2.26 [‐2.05, 6.58] |
| 4.1 Children | 1 | 512 | Mean Difference (IV, Fixed, 95% CI) | 2.56 [‐3.73, 8.85] |
| 4.2 Adults | 2 | 664 | Mean Difference (IV, Fixed, 95% CI) | 2.00 [‐3.94, 7.94] |
| 5 Change in quality of life score (paediatric AQLQ) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Adverse event | 2 | 672 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.07] |
| 6.1 Children | 1 | 513 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.01] |
| 6.2 Adults | 1 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.90, 1.72] |
| 7 Candidiasis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Pharyngitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Nasopharyngitis | 2 | 672 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.75, 1.47] |
| 9.1 Children | 1 | 513 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.62, 1.53] |
| 9.2 Adults | 1 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.72, 1.95] |
| 10 Asthma (not otherwise specified) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Headache | 2 | 672 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.52, 1.39] |
| 11.1 Children | 1 | 513 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.53, 1.44] |
| 11.2 Adults | 1 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.05, 5.61] |
| 12 Upper respiratory tract infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Rhinitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13.1 Children | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Withdrawals | 2 | 672 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.73, 1.72] |
| 14.1 Children | 1 | 513 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.70, 1.73] |
| 14.2 Adults | 1 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.36, 4.66] |
| 15 Withdrawals (lack of efficacy) | 3 | 1177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.78, 1.92] |
| 15.1 Children | 1 | 513 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.55, 2.23] |
| 15.2 Adults | 2 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.72, 2.37] |
| 16 Withdrawals (adverse events) | 2 | 672 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.59, 2.01] |
| 16.1 Children | 1 | 513 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.61, 2.20] |
| 16.2 Adults | 1 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.05, 5.61] |
| 17 Change in symptoms | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 17.1 Children | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 Adults | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 Change in rescue medication usage (puffs/d) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 18.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19 Change from baseline in quality of life score (AQLQ) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 19.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 19.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Change from baseline in nocturnal awakenings (n/night) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 20.1 Children | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 Adults | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21 FEV1 (L) (end of treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 21.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 21.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22 FEV1 predicted (%) (end of treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 22.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 22.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23 Day symptoms (end of treatment) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 23.1 Children | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 23.2 Adults | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 24 Night symptoms (end of treatment) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 24.1 Children | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 24.2 Adults | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25 Rescue medication usage (puffs/d) (end of treatment) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 25.1 Children | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 25.2 Adults | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
9.1. Analysis.
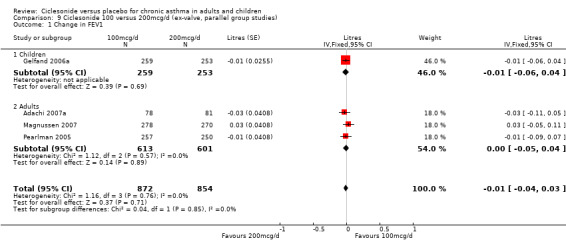
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 1 Change in FEV1.
9.2. Analysis.
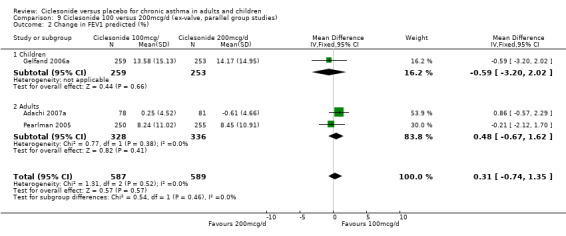
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 2 Change in FEV1 predicted (%).
9.3. Analysis.
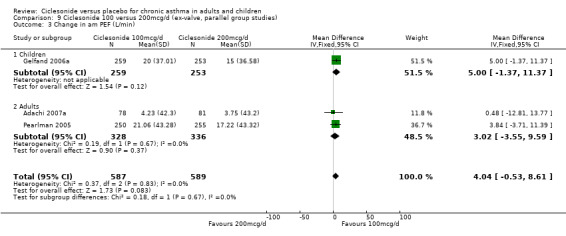
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 3 Change in am PEF (L/min).
9.4. Analysis.
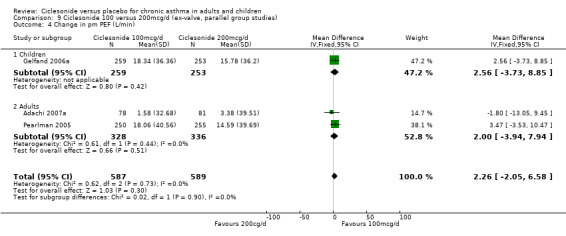
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 4 Change in pm PEF (L/min).
9.5. Analysis.
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 5 Change in quality of life score (paediatric AQLQ).
9.6. Analysis.
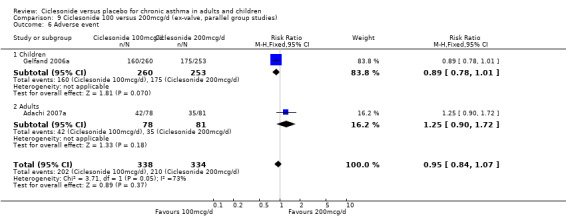
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 6 Adverse event.
9.7. Analysis.
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 7 Candidiasis.
9.8. Analysis.
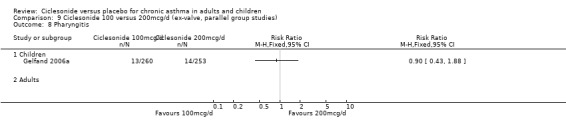
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 8 Pharyngitis.
9.9. Analysis.
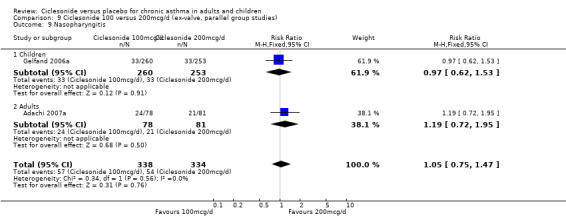
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 9 Nasopharyngitis.
9.10. Analysis.
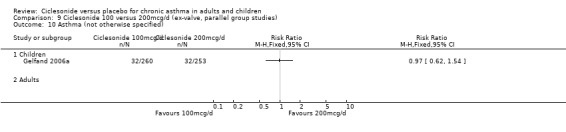
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 10 Asthma (not otherwise specified).
9.11. Analysis.
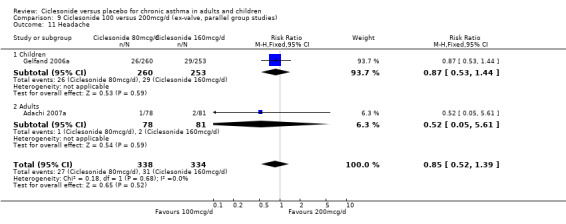
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 11 Headache.
9.12. Analysis.
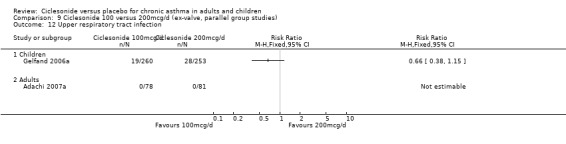
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 12 Upper respiratory tract infection.
9.13. Analysis.
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 13 Rhinitis.
9.14. Analysis.
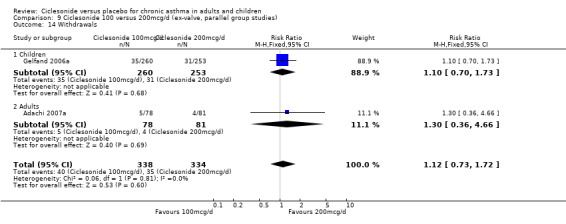
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 14 Withdrawals.
9.15. Analysis.
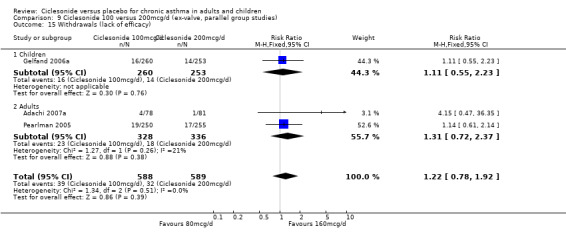
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 15 Withdrawals (lack of efficacy).
9.16. Analysis.
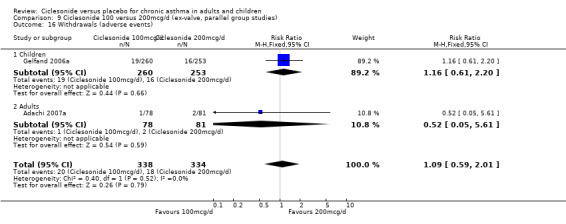
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 16 Withdrawals (adverse events).
9.17. Analysis.
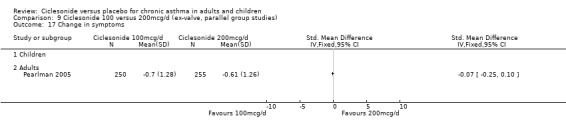
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 17 Change in symptoms.
9.18. Analysis.
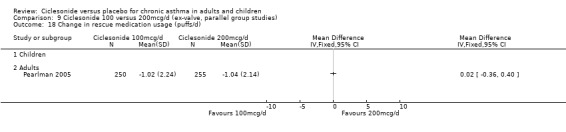
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 18 Change in rescue medication usage (puffs/d).
9.19. Analysis.
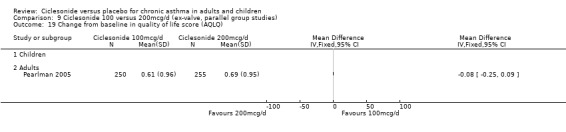
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 19 Change from baseline in quality of life score (AQLQ).
9.20. Analysis.
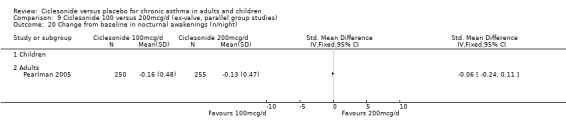
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 20 Change from baseline in nocturnal awakenings (n/night).
9.21. Analysis.
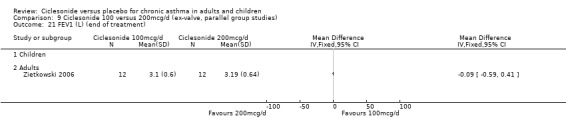
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 21 FEV1 (L) (end of treatment).
9.22. Analysis.
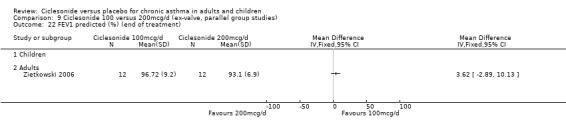
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 22 FEV1 predicted (%) (end of treatment).
9.23. Analysis.
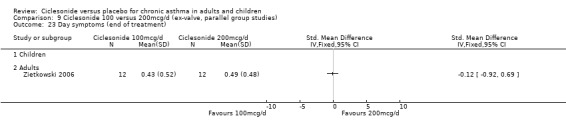
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 23 Day symptoms (end of treatment).
9.24. Analysis.
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 24 Night symptoms (end of treatment).
9.25. Analysis.
Comparison 9 Ciclesonide 100 versus 200mcg/d (ex‐valve, parallel group studies), Outcome 25 Rescue medication usage (puffs/d) (end of treatment).
Comparison 10. Ciclesonide 100 versus 400mcg/d (ex‐valve, parallel group studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 | 3 | 747 | Litres (Fixed, 95% CI) | ‐0.00 [‐0.05, 0.05] |
| 1.1 Children | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 3 | 747 | Litres (Fixed, 95% CI) | ‐0.00 [‐0.05, 0.05] |
| 2 Change in FEV1 predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in am PEF | 3 | 749 | L/min (Fixed, 95% CI) | ‐0.48 [‐7.59, 6.64] |
| 3.1 Children | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 3 | 749 | L/min (Fixed, 95% CI) | ‐0.48 [‐7.59, 6.64] |
| 4 Change in pm PEF | 2 | 396 | L/min (Fixed, 95% CI) | 1.36 [‐6.05, 8.78] |
| 4.1 Children | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Adults | 2 | 396 | L/min (Fixed, 95% CI) | 1.36 [‐6.05, 8.78] |
| 5 Change clinic PEF | 1 | L/min (Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 1 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Change in FVC | 2 | 396 | Litres (Fixed, 95% CI) | ‐0.01 [‐0.08, 0.06] |
| 6.1 Children | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Adults | 2 | 396 | Litres (Fixed, 95% CI) | ‐0.01 [‐0.08, 0.06] |
| 7 Change from baseline in quality of life score (AQLQ) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Adverse event | 2 | 539 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.76, 1.10] |
| 8.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Adults | 2 | 539 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.76, 1.10] |
| 9 Headache | 2 | 539 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.32, 2.21] |
| 9.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Adults | 2 | 539 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.32, 2.21] |
| 10 Upper respiratory tract infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Adults | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Rhinitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Withdrawals | 2 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.87, 2.40] |
| 12.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Adults | 2 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.87, 2.40] |
| 13 Withdrawals (lack of efficacy) | 2 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.50, 4.06] |
| 13.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Adults | 2 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.50, 4.06] |
| 14 Withdrawals (adverse events) | 2 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.58, 4.45] |
| 14.1 Children | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Adults | 2 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.58, 4.45] |
| 15 Increased cough | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
10.1. Analysis.
Comparison 10 Ciclesonide 100 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 1 Change in FEV1.
10.2. Analysis.
Comparison 10 Ciclesonide 100 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 2 Change in FEV1 predicted.
10.3. Analysis.
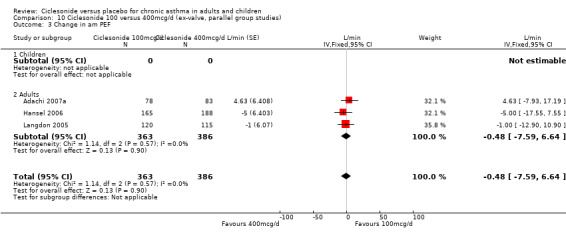
Comparison 10 Ciclesonide 100 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 3 Change in am PEF.
10.4. Analysis.
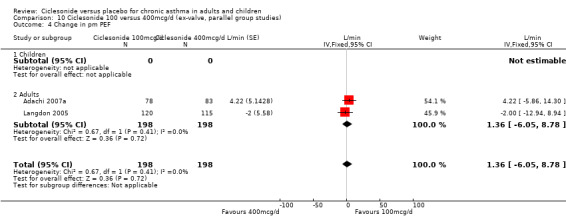
Comparison 10 Ciclesonide 100 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 4 Change in pm PEF.
10.5. Analysis.
Comparison 10 Ciclesonide 100 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 5 Change clinic PEF.
10.6. Analysis.
Comparison 10 Ciclesonide 100 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 6 Change in FVC.
10.7. Analysis.
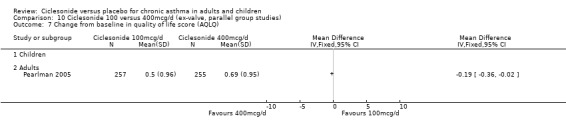
Comparison 10 Ciclesonide 100 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 7 Change from baseline in quality of life score (AQLQ).
10.8. Analysis.
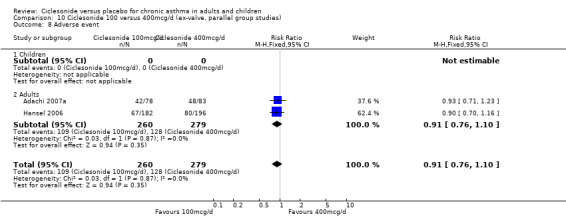
Comparison 10 Ciclesonide 100 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 8 Adverse event.
10.9. Analysis.
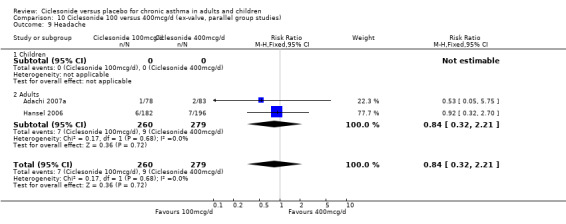
Comparison 10 Ciclesonide 100 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 9 Headache.
10.10. Analysis.
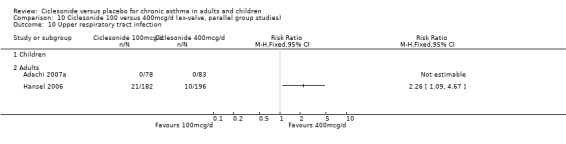
Comparison 10 Ciclesonide 100 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 10 Upper respiratory tract infection.
10.11. Analysis.
Comparison 10 Ciclesonide 100 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 11 Rhinitis.
10.12. Analysis.
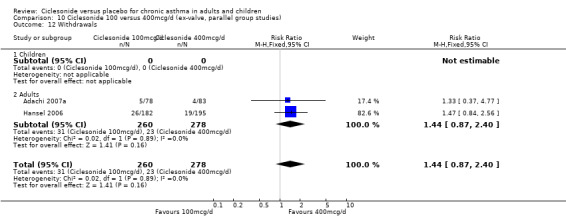
Comparison 10 Ciclesonide 100 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 12 Withdrawals.
10.13. Analysis.
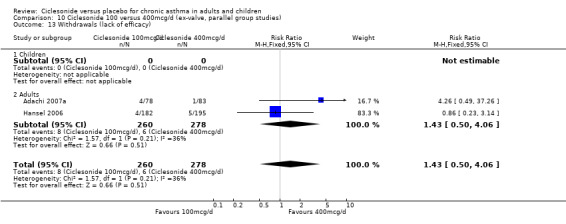
Comparison 10 Ciclesonide 100 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 13 Withdrawals (lack of efficacy).
10.14. Analysis.
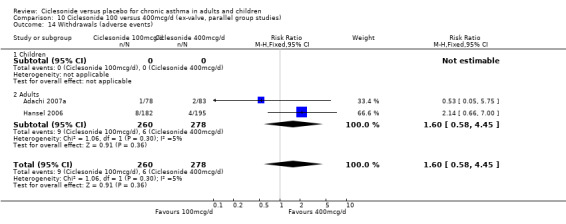
Comparison 10 Ciclesonide 100 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 14 Withdrawals (adverse events).
10.15. Analysis.
Comparison 10 Ciclesonide 100 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 15 Increased cough.
Comparison 11. Ciclesonide 200 versus 400mcg/d (ex‐valve, parallel group studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 | 2 | 537 | Litres (Fixed, 95% CI) | 0.03 [‐0.02, 0.09] |
| 1.1 Children | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 2 | 537 | Litres (Fixed, 95% CI) | 0.03 [‐0.02, 0.09] |
| 2 Change in FEV1 predicted (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in am PEF (L/min) | 2 | 537 | Litres (Fixed, 95% CI) | 2.17 [‐5.04, 9.38] |
| 3.1 Children | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 2 | 537 | Litres (Fixed, 95% CI) | 2.17 [‐5.04, 9.38] |
| 4 Change in FVC (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
11.1. Analysis.
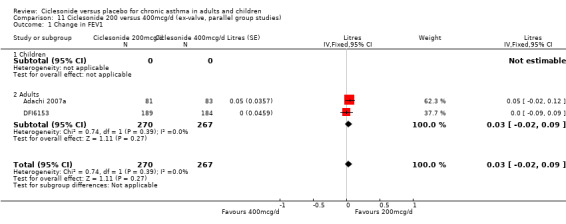
Comparison 11 Ciclesonide 200 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 1 Change in FEV1.
11.2. Analysis.
Comparison 11 Ciclesonide 200 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 2 Change in FEV1 predicted (%).
11.3. Analysis.
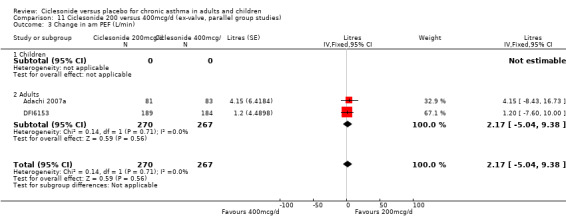
Comparison 11 Ciclesonide 200 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 3 Change in am PEF (L/min).
11.4. Analysis.
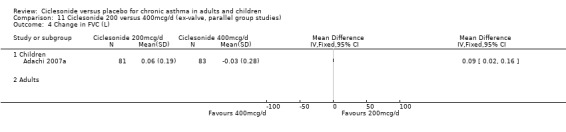
Comparison 11 Ciclesonide 200 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 4 Change in FVC (L).
11.5. Analysis.
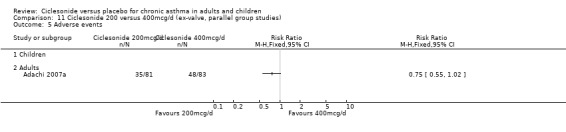
Comparison 11 Ciclesonide 200 versus 400mcg/d (ex‐valve, parallel group studies), Outcome 5 Adverse events.
Comparison 12. Ciclesonide 200 versus 800mcg/d (ex‐valve, parallel group studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 | 1 | Litres (Fixed, 95% CI) | Totals not selected | |
| 1.1 Children | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Adults | 1 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in am PEF | 2 | 594 | L/min (Fixed, 95% CI) | ‐4.78 [‐11.65, 2.09] |
| 2.1 Children | 0 | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Adults | 2 | 594 | L/min (Fixed, 95% CI) | ‐4.78 [‐11.65, 2.09] |
| 3 Change in pm PEF | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in clinic PEF | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in rescue medication usage (puffs/d) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Change in symptoms | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Children | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Adults | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Adverse event | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Candidiasis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Withdrawals | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Sore throat | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
12.1. Analysis.
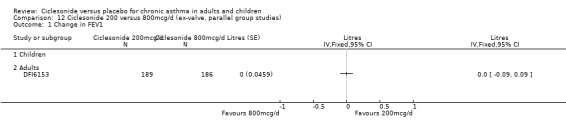
Comparison 12 Ciclesonide 200 versus 800mcg/d (ex‐valve, parallel group studies), Outcome 1 Change in FEV1.
12.2. Analysis.
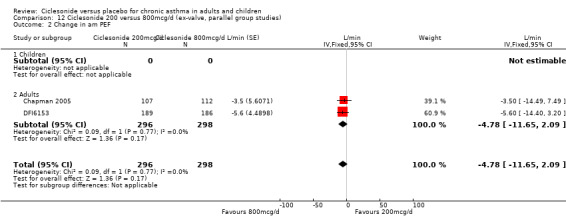
Comparison 12 Ciclesonide 200 versus 800mcg/d (ex‐valve, parallel group studies), Outcome 2 Change in am PEF.
12.3. Analysis.
Comparison 12 Ciclesonide 200 versus 800mcg/d (ex‐valve, parallel group studies), Outcome 3 Change in pm PEF.
12.4. Analysis.
Comparison 12 Ciclesonide 200 versus 800mcg/d (ex‐valve, parallel group studies), Outcome 4 Change in clinic PEF.
12.5. Analysis.
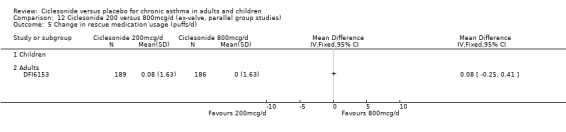
Comparison 12 Ciclesonide 200 versus 800mcg/d (ex‐valve, parallel group studies), Outcome 5 Change in rescue medication usage (puffs/d).
12.6. Analysis.
Comparison 12 Ciclesonide 200 versus 800mcg/d (ex‐valve, parallel group studies), Outcome 6 Change in symptoms.
12.7. Analysis.
Comparison 12 Ciclesonide 200 versus 800mcg/d (ex‐valve, parallel group studies), Outcome 7 Adverse event.
12.8. Analysis.
Comparison 12 Ciclesonide 200 versus 800mcg/d (ex‐valve, parallel group studies), Outcome 8 Candidiasis.
12.9. Analysis.
Comparison 12 Ciclesonide 200 versus 800mcg/d (ex‐valve, parallel group studies), Outcome 9 Withdrawals.
12.10. Analysis.
Comparison 12 Ciclesonide 200 versus 800mcg/d (ex‐valve, parallel group studies), Outcome 10 Sore throat.
Comparison 13. Ciclesonide 200 versus 1600mcg/d (ex‐valve, parallel group studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 | 1 | Litres (Fixed, 95% CI) | Totals not selected | |
| 1.1 Children | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Adults | 1 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in am PEF | 1 | L/min (Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 0 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 1 | L/min (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in rescue medication usage (puffs/d) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in symptoms | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse event | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Candidiasis | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Withdrawals | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Sore throat | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Adults | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
13.1. Analysis.
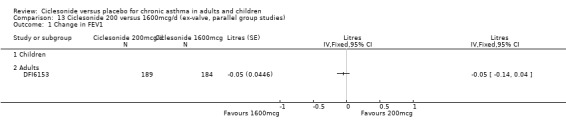
Comparison 13 Ciclesonide 200 versus 1600mcg/d (ex‐valve, parallel group studies), Outcome 1 Change in FEV1.
13.2. Analysis.
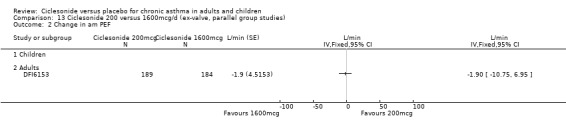
Comparison 13 Ciclesonide 200 versus 1600mcg/d (ex‐valve, parallel group studies), Outcome 2 Change in am PEF.
13.3. Analysis.
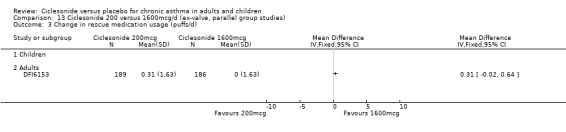
Comparison 13 Ciclesonide 200 versus 1600mcg/d (ex‐valve, parallel group studies), Outcome 3 Change in rescue medication usage (puffs/d).
13.4. Analysis.
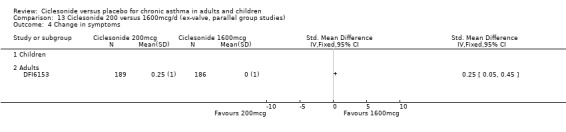
Comparison 13 Ciclesonide 200 versus 1600mcg/d (ex‐valve, parallel group studies), Outcome 4 Change in symptoms.
Comparison 14. Ciclesonide 400 versus 800mcg/d (ex‐valve, parallel group studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 | 2 | 583 | Litres (Fixed, 95% CI) | ‐0.06 [‐0.12, 0.00] |
| 1.1 Children | 0 | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Adults | 2 | 583 | Litres (Fixed, 95% CI) | ‐0.06 [‐0.12, 0.00] |
| 2 Change in FEV1 predicted (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in FVC (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in am PEF | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in pm PEF | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Change in rescue beta2‐agonists use | 2 | 583 | Puffs/d (Fixed, 95% CI) | 0.12 [‐0.13, 0.37] |
| 6.1 Children | 0 | 0 | Puffs/d (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Adults | 2 | 583 | Puffs/d (Fixed, 95% CI) | 0.12 [‐0.13, 0.37] |
| 7 Change in high dose peak serum cortisol levels | 0 | mcg/dL (Fixed, 95% CI) | Totals not selected | |
| 7.1 Children | 0 | mcg/dL (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Adults | 0 | mcg/dL (Fixed, 95% CI) | 0.0 [0.0, 0.0] |
14.1. Analysis.
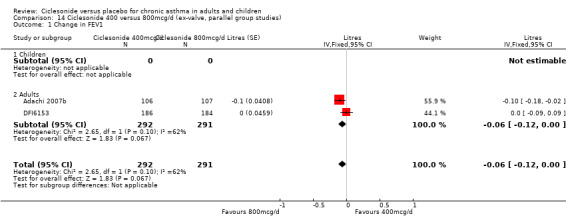
Comparison 14 Ciclesonide 400 versus 800mcg/d (ex‐valve, parallel group studies), Outcome 1 Change in FEV1.
14.2. Analysis.
Comparison 14 Ciclesonide 400 versus 800mcg/d (ex‐valve, parallel group studies), Outcome 2 Change in FEV1 predicted (%).
14.3. Analysis.
Comparison 14 Ciclesonide 400 versus 800mcg/d (ex‐valve, parallel group studies), Outcome 3 Change in FVC (L).
14.4. Analysis.
Comparison 14 Ciclesonide 400 versus 800mcg/d (ex‐valve, parallel group studies), Outcome 4 Change in am PEF.
14.5. Analysis.
Comparison 14 Ciclesonide 400 versus 800mcg/d (ex‐valve, parallel group studies), Outcome 5 Change in pm PEF.
14.6. Analysis.
Comparison 14 Ciclesonide 400 versus 800mcg/d (ex‐valve, parallel group studies), Outcome 6 Change in rescue beta2‐agonists use.
Comparison 15. Ciclesonide 800 versus 1600mcg/d (ex‐valve, parallel group studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 | 1 | Litres (Fixed, 95% CI) | Totals not selected | |
| 1.1 Children | 0 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Adults | 1 | Litres (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in clinic PEF (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Children | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Adults | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in symptoms | 2 | 737 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.20, 0.08] |
| 3.1 Children | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Adults | 2 | 737 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.20, 0.08] |
| 4 Change in rescue medication usage (puffs/d) | 2 | 735 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.07, 0.37] |
| 4.1 Children | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Adults | 2 | 735 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.07, 0.37] |
| 5 Withdrawals (lack of efficacy) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Children | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Adults | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
15.1. Analysis.
Comparison 15 Ciclesonide 800 versus 1600mcg/d (ex‐valve, parallel group studies), Outcome 1 Change in FEV1.
15.2. Analysis.
Comparison 15 Ciclesonide 800 versus 1600mcg/d (ex‐valve, parallel group studies), Outcome 2 Change in clinic PEF (%).
15.3. Analysis.
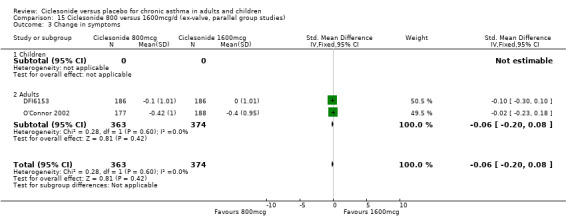
Comparison 15 Ciclesonide 800 versus 1600mcg/d (ex‐valve, parallel group studies), Outcome 3 Change in symptoms.
15.4. Analysis.
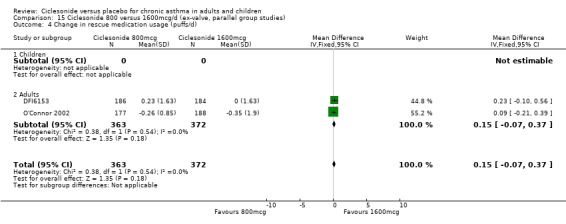
Comparison 15 Ciclesonide 800 versus 1600mcg/d (ex‐valve, parallel group studies), Outcome 4 Change in rescue medication usage (puffs/d).
15.5. Analysis.
Comparison 15 Ciclesonide 800 versus 1600mcg/d (ex‐valve, parallel group studies), Outcome 5 Withdrawals (lack of efficacy).
Comparison 16. WMD archive.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 | 9 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies) | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group trials) | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Ciclesonide 400mcg (ex‐valve, parallel group trials) | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Ciclesonide 50 versus 100mcg/d | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Ciclesonide 100 versus 200mcg/d | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Ciclesonide 100 versus 400mcg/d | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Ciclesonide 200 versus 400mcg/d | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Ciclesonide 400 versus 800mcg/d (ex‐valve, parallel group studies) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in am PEF (L/min) | 9 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies) | 5 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group trials) | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Ciclesonide 400mcg (ex‐valve, parallel group trials) | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Ciclesonide 800mcg (ex‐valve, parallel group trials) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Ciclesonide 100 versus 400mcg/d | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Ciclesonide 200 versus 400mcg/d | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Ciclesonide 200 versus 800mcg/d (ex‐valve, parallel group studies) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in pm PEF (L/min) | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies) | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Ciclesonide 400mcg (ex‐valve, parallel group trials) | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Ciclesonide 100 versus 400mcg/d | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Changes in cortisol levels (serum) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Ciclesonide versus placebo greater than 600mcg/d (parallel group studies) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Change in FVC | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve, parallel group studies) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Ciclesonide versus placebo 400mcg/d (ex‐valve, parallel group studies) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Ciclesonide 100 versus 400mcg/d | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Change in rescue 2‐agonists use | 5 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Ciclesonide versus placebo 100mcg/d or less (ex‐valve parallel group studies) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group trials) | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Ciclesonide 400mcg (ex‐valve, parallel group trials) | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 Ciclesonide 800mcg (ex‐valve, parallel group trials) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 Ciclesonide 400 versus 800mcg/d (ex‐valve, parallel group studies) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Change in asthma symptom scores | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Ciclesonide versus placebo 200mcg/d (ex‐valve, parallel group studies) | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
16.1. Analysis.
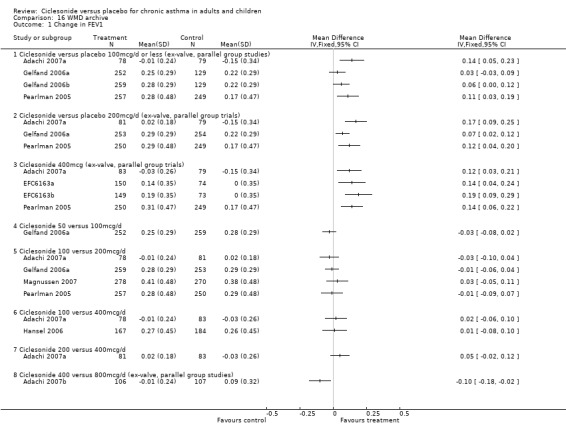
Comparison 16 WMD archive, Outcome 1 Change in FEV1.
16.2. Analysis.
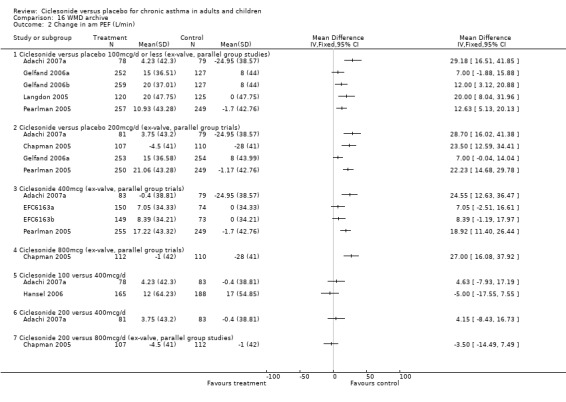
Comparison 16 WMD archive, Outcome 2 Change in am PEF (L/min).
16.3. Analysis.
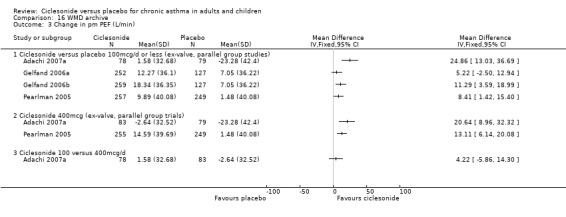
Comparison 16 WMD archive, Outcome 3 Change in pm PEF (L/min).
16.4. Analysis.
Comparison 16 WMD archive, Outcome 4 Changes in cortisol levels (serum).
16.5. Analysis.
Comparison 16 WMD archive, Outcome 5 Change in FVC.
16.6. Analysis.
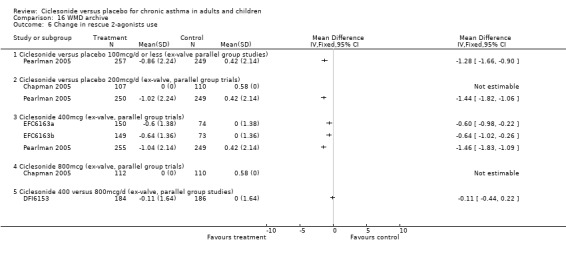
Comparison 16 WMD archive, Outcome 6 Change in rescue 2‐agonists use.
16.7. Analysis.
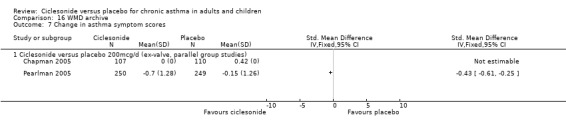
Comparison 16 WMD archive, Outcome 7 Change in asthma symptom scores.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adachi 2007a.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: 50 centres in Japan DURATION OF STUDY: 8 weeks (4 week run‐in period reported with BDP 400mcg/d) CONCEALMENT OF ALLOCATION: Unclear COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Not reported DESCRIPTION OF WITHDRAWALS/DROPOUTS: Not stated JADAD SCORE (5‐1): 2 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): Available case (assumed) COMPLIANCE: Not reported CONFOUNDERS: Slight imbalance between groups in terms of PEF% predicted at baseline | |
| Participants | N SCREENED: 435 N RANDOMISED: 311 N COMPLETED: 311 (assumed) M= 171, F= 140 MEAN AGE: 51 years BASELINE DETAILS: FEV1 % predicted: 71% INCLUSION CRITERIA: 16‐75 years; mild to moderate asthma according to the Japanese guidelines; treatment with 400‐800 mcg/day BDP/200‐400 mcg/d FP >4 weeks; mean morning PEF during the last week of run‐in 60% to 90% predicted PEF. EXCLUSION: Significant coexisting respiratory disease; hospitalisation, emergency room care for asthma or treatment with systemic steroids <4 weeks before run‐in. | |
| Interventions | 1. Ciclesonide 100mcg OD
2. Ciclesonide 200mcg OD
3. Ciclesonide 400mcg OD
4. Placebo DELIVERY: MDI TREATMENT PERIOD: 8 weeks RESCUE: SABA CO‐INTERVENTIONS PERMITTED: Not stated CO‐INTERVENTIONS: Not reported % on ICS baseline: 100 |
|
| Outcomes | am PEF; pm PEF; FEV1; FVC; symptoms; use of rescue medication; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Adachi 2007b.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: 59 centres in Japan DURATION OF STUDY: 8 weeks (4 week run‐in period CFC‐BDP 800 mcg/day). CONCEALMENT OF ALLOCATION: Unclear COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: No METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not stated METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Open label DESCRIPTION OF WITHDRAWALS/DROPOUTS: Not stated JADAD SCORE (5‐1): 1 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): Not clear COMPLIANCE: Not reported CONFOUNDERS: Balanced groups at baseline | |
| Participants | N SCREENED: 478 N RANDOMISED: 316 (213 to groups of interest to this review) N COMPLETED: Not clear M= 105, F= 108 MEAN AGE: 52.3 years BASELINE DETAILS: FEV1 69% predicted; FVC: 2.76 L INCLUSION CRITERIA: 16‐75 years; moderate to severe asthma according to the Japanese Guidelines; treated with >800 mg/day CFC‐BDP or >400 mg/day of FP for more than four weeks; mean morning PEF during last week of run‐in of <80% predicted PEF; reversibility of airflow limitation of >15%. EXCLUSION: Significant coexisting respiratory disease; hospitalisation, emergency room care for asthma or treatment with systemic steroids <4 weeks before run‐in. | |
| Interventions | 1. Ciclesonide 200mcg BID (400mcg/d)
2. Ciclesonide 400mcg BID (800mcg/d)
3. Beclomethasone 400mcg BID (800mcg/d) DELIVERY: CIC: HFA‐MDI; BDP: CFC‐MDI + spacer TREATMENT PERIOD: 8 weeks RESCUE: SABA CO‐INTERVENTIONS PERMITTED: Not stated % on ICS baseline: 100 |
|
| Outcomes | am PEF; pm PEF; FEV1; FVC; symptoms; use of rescue medication | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Baena Cagnani 2006.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: Multicentre, USA, South America DURATION OF STUDY: 12 months (6 month run‐in period reported, unclear treatment regime) CONCEALMENT OF ALLOCATION: Not reported COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Not reported DESCRIPTION OF WITHDRAWALS/DROPOUTS: Not reported JADAD SCORE (5‐1): 2 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT (presumed as most likely) COMPLIANCE: Not reported CONFOUNDERS: Not reported | |
| Participants | N SCREENED: Unknown N RANDOMISED: 661 (eligible patients) N COMPLETED: Unknown M= unknown F= unknown MEDIAN AGE: Range ‐ males 5‐8.5 years; females 5‐7.5 years BASELINE DETAILS: Unknown INCLUSION CRITERIA: Mild persistent asthma; children EXCLUSION: Not reported | |
| Interventions | 1. Ciclesonide 50mcg OD
2. Ciclesonide 100cg OD
3. Placebo DELIVERY: MDI TREATMENT PERIOD: 12 months RESCUE: Not reported CO‐INTERVENTIONS PERMITTED: Not reported CO‐INTERVENTIONS: Not reported % on ICS baseline: Not reported |
|
| Outcomes | Growth velocity; adverse events; withdrawals | |
| Notes | Unpublished conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bateman 2006.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: USA and South Africa, 60 centres. DURATION OF STUDY: 12 weeks (lowest effective oral steroid dose achieved for each patient during screening period) CONCEALMENT OF ALLOCATION: Not reported COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Not reported DESCRIPTION OF WITHDRAWALS/DROPOUTS: YesJADAD SCORE (5‐1): 4TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITTCOMPLIANCE: yesCONFOUNDERS: Baseline values comparable " | |
| Participants | N SCREENED: 241 N RANDOMISED: 141 N COMPLETED: 114 (PP) and 140 (ITT analysis ‐ 1 patient excluded CIC1280 group because no post baseline measurements) M= 44F= 96 MEDIAN AGE (range):CIC640 48.3 years (13‐74); CIC1280 48.2 (17‐70); Placebo 48.3 (12‐73) BASELINE DETAILS: FEV1 (% Pred): CIC640 1.583 (52.07%); CIC1280 1.713 (57.21%); Placebo 1.621 (56.36%) INCLUSION CRITERIA: Male and females; Aged >= 12 years; oral corticosteroid‐dependant (OCS) asthma for 12 months GINA definition; oral steroids daily or alternate days 5 of 6 months and ICS daily for 6 months, using inhaled beta2 agonists for rescue for 2 weeks; lowest effective OCS according to criteria at randomisation; FEV1 40‐80% (pred) on withholding beta2‐agnoists for 6 hours, and >= 12% reversibility following inhaled medication with an absolute increase >=200mls within the 12 months. EXCLUSION: Smokers or ex‐smokers with smoking history >= 10 pack year of cigs and who quit less than 6 months. | |
| Interventions | 1. Ciclesonide 400mcg BD
2. Ciclesonide 800mcg BD
3. Placebo DELIVERY: HFA‐MDI TREATMENT PERIOD: 12 weeks RESCUE: Albuterol HFA‐MDI CO‐INTERVENTIONS PERMITTED: To continue on intranasal steroids for rhinitis, cromolyn, antihistamines, hydrocortisone creams or ointments, < 1% at stable dose, montelukast, anti‐cholinergics, oral beta2‐agonists, LABAs, SABA or nebulised SABA CO‐INTERVENTIONS: reduction in OCS% on ICS baseline: all patients |
|
| Outcomes | Am PEF; rescue medication use; reduction in maintenance OCS use; asthma symptoms; HPA function; adverse events; withdrawals | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bateman 2006a.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: Multicentre DURATION OF STUDY: 12 weeks (2‐4 weeks on FP250mcg/d) CONCEALMENT OF ALLOCATION: Not reported COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Not reported DESCRIPTION OF WITHDRAWALS/DROPOUTS: Not reported JADAD SCORE (5‐1): 2 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT( presumed as most likely) COMPLIANCE: Not reported CONFOUNDERS: Not reported | |
| Participants | N SCREENED: Unknown N RANDOMISED: 680 (eligible patients) N COMPLETED: Not reported M= unknown F= unknown MEDIAN AGE Not reported BASELINE DETAILS: Not reported INCLUSION CRITERIA: Severe asthma; FEV1 < 70% predicted age 12‐75 years EXCLUSION: Not reported | |
| Interventions | 1. Ciclesonide 200mcg OD
2. Ciclesonide 400mcg BD DELIVERY: HFA‐MDI TREATMENT PERIOD: 12 weeks RESCUE: Not reported CO‐INTERVENTIONS PERMITTED: Not reported CO‐INTERVENTIONS: Not reported % on ICS baseline: 100 (FP 250mcg BD) |
|
| Outcomes | FEV1, am PEF; symptoms; asthma exacerbations | |
| Notes | Unpublished conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Bernstein 2004.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: Multicentre STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: Multicentre DURATION OF STUDY: 12 weeks (run‐in unclear) CONCEALMENT OF ALLOCATION: Not reported COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Not reported DESCRIPTION OF WITHDRAWALS/DROPOUTS: Not reported JADAD SCORE (5‐1): 2 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT(presumed) COMPLIANCE: Not reported CONFOUNDERS: Not reported. | |
| Participants | N SCREENED: Not reported N RANDOMISED: 531 N COMPLETED: Not reported M= unknown; F= unknown MEDIAN AGE: Not reported BASELINE DETAILS: Not reported INCLUSION CRITERIA: Moderate‐severe asthma for 6 months or more; FEV1 of 40‐65%;age >= 12 years. EXCLUSION: unknown | |
| Interventions | 1. Ciclesonide 200mcg BD
2. Ciclesonide 400mcg BD
3. Fluticasone 1000mcg BD
4. Placebo DELIVERY: CIC: MDI; FP CFC‐MDI TREATMENT PERIOD: 12 weeks RESCUE: unknown CO‐INTERVENTIONS PERMITTED: Not reported CO‐INTERVENTIONS: Not reported % on ICS baseline: Not reported |
|
| Outcomes | FEV1; am PEF; AQLQ symptom score; adverse events | |
| Notes | Unpublished conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chapman 2005.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: Canada, 25 centres. DURATION OF STUDY: 12 weeks (2 week run‐in on current ICS) CONCEALMENT OF ALLOCATION: Unclear COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Identical devices DESCRIPTION OF WITHDRAWALS/DROPOUTS: Yes JADAD SCORE (5‐1): 4 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT COMPLIANCE: Not reported CONFOUNDERS: Baseline values comparable | |
| Participants | N SCREENED: 440 N RANDOMISED: 329 N COMPLETED: 185 M=166; F= 163 MEDIAN AGE (range):CIC160 41 years (18‐68); CIC640 39 (18‐69); Placebo 41 (19‐69) BASELINE DETAILS: FEV1 (% Pred): CIC160 2.70 (78%); CIC640 2.66 78; 2.61 (77%) INCLUSION CRITERIA: Aged 18‐70 years; either sex; persistent asthma ATS definition; previous use of ICS for 4 weeks (400‐800 ug/day BDP, Tri, BUD or Fluisolide or 200‐500 ug/day Fluticasone; at randomisation FEV1 (% pred) between >= 60% ‐ <=90% pred bronchodilator and have 1 of the following ; 12% variability in FEV1 or >= 200 mls after two‐4 puffs salbutamol at randomisation or methacholine tests 8 mg/ml or less, or diurnal variation in PEF >= 15% at least 3 of 7 days before randomisation. EXCLUSION: asthma exacerbation of asthma or RTI within 6 weeks of the study, hospitalisation for asthma within 6 months, COPD or current or former smokers > 10 pack years, pregnant or lactating females or premenopausal women not using effective contraception, Lack of efficacy (LOC) criteria before randomisation, use of oral steroids, or more than 8 puffs of salbutamol on two successive days during baseline. | |
| Interventions | 1. Ciclesonide 200mcg OD
2. Ciclesonide 800mcg OD
3. Placebo DELIVERY: CIC: HFA MDI without a spacer, Placebo MDI without spacer TREATMENT PERIOD: 12 weeks RESCUE: Salbutamol MDI CO‐INTERVENTIONS PERMITTED: Theophylline. % on ICS baseline: 100 |
|
| Outcomes | Am PEF; rescue medication use; cortisol levels; adverse events; withdrawals | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
DFI6153.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: USA, 66 centres DURATION OF STUDY: 6 weeks (2 week run‐in on FP 100mcg) CONCEALMENT OF ALLOCATION: Unclear COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Not clear DESCRIPTION OF WITHDRAWALS/DROPOUTS: Not clear JADAD SCORE (5‐1): 2 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT COMPLIANCE: Not reported CONFOUNDERS: Not clear | |
| Participants | N SCREENED: Not clear N RANDOMISED: 1145 N COMPLETED: Not clear M=not clear ; F= not clear MEDIAN AGE (range): Not clear BASELINE DETAILS: Not available INCLUSION CRITERIA: >12 years; history of persistent asthma (>6 months before screening); treatment with ICS for >1 month before screening; FEV1 >40% and <80% predicted. EXCLUSION: Not reported. | |
| Interventions | 1. Ciclesonide 100mcg BD
2. Ciclesonide 200mcg BD
3. Ciclesonide 400mcg BD
4. CIcliesonide 400mcg BD
5. Ciclesonide 800mcg BD
6. Placebo DELIVERY: CIC: DPI (CIC group 3 given via MDI) TREATMENT PERIOD: 6 weeks RESCUE: Salbutamol MDI CO‐INTERVENTIONS PERMITTED: % on ICS baseline: 100 |
|
| Outcomes | FEV1; am PEF; rescue medication use; symptoms; nocturnal awakenings; safety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
EFC6163a.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: USA, 38 centres DURATION OF STUDY: 6 weeks (1 week run‐in on FP <440mcg) CONCEALMENT OF ALLOCATION: Unclear COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: identical devices DESCRIPTION OF WITHDRAWALS/DROPOUTS: Not clear JADAD SCORE (5‐1): 3 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT COMPLIANCE: Not reported CONFOUNDERS: Not clear | |
| Participants | N SCREENED: Not clear N RANDOMISED: 456 N COMPLETED: Not clear M=not clear ; F= not clear MEDIAN AGE (range): Not clear BASELINE DETAILS: Not available INCLUSION CRITERIA: >12 years; history of persistent asthma (6 months); ICS monotherapy for >1 month or use of ICS/LABA combination therapy for >1 month; FEV1 60‐90% predicted (ICS monotherapy)/FEV1 70‐95% (ICS/LABA combination therapy); </=440mcg/d FP or equivalent or </=200/100 FP/SAL equivalent. EXCLUSION: Not reported. | |
| Interventions | 1. Ciclesonide 100mcg BD
2. Ciclesonide 200mcg OD
3. Placebo DELIVERY: CIC: MDI TREATMENT PERIOD: weeks RESCUE: Not reported CO‐INTERVENTIONS PERMITTED: % on ICS baseline: 100 |
|
| Outcomes | FEV1; am PEF; rescue medication use; symptoms; nocturnal awakenings; safety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
EFC6163b.
| Methods | See above | |
| Participants | See above | |
| Interventions | See above | |
| Outcomes | See above | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gelfand 2006a.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: USA (100 ctrs), Mexico (21), Poland (10); 131 centres. DURATION OF STUDY: 12 weeks (4 week run‐in period, stable maintenance medications) CONCEALMENT OF ALLOCATION: Unclear COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Not reported DESCRIPTION OF WITHDRAWALS/DROPOUTS: Yes JADAD SCORE (5‐1): 3 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT COMPLIANCE: Not reported CONFOUNDERS: Baseline values comparable | |
| Participants | N SCREENED: Not stated N RANDOMISED: 1031 (ITT population 1018) N COMPLETED: 870 M= 652; F= 366 MEDIAN AGE (range): CIC40 8.14 years; CIC80 8.20; CIC160 8.33; Placebo 8.2 (all ranges 4‐11 years) BASELINE DETAILS: FEV1 (% Pred): CIC40 1.29 (68.5); CIC80 1.29 (68.3); CIC160 1.30 (68.2); Placebo 1.32 (68.5%) INCLUSION CRITERIA: 4‐11 years; persistent asthma (all severities ‐ 1997 NIH criteria); FEV1 (% predicted between 40% and 90%; 12% variability in FEV1 after two puffs albuterol at randomisation or within 12 months of screening; if on controller therapy must have had a 10% drop in FEV1 (between screening and randomisation after maintenance treatment stopped); patients using only beta2agonist at screening had to have at least 1 of 3: 24 hour asthma symptom score >= 3, PEF variability > 20%, albuterol use >= 2 puffs daily; effective use of MDI inhaler and ability to perform spirometry EXCLUSION: Oral or systemic steroid use within 4 weeks of screening; life‐threatening asthma, two or more hospitalizations for asthma exacerbation with 1 year before study, urinary cortisol level < 10 ug/dL at screening | |
| Interventions | 1. Ciclesonide 50mcg OD
2. Ciclesonide 100mcg OD
3. Ciclesonide 200mcg OD
4. Placebo DELIVERY: HFA MDI without a spacer TREATMENT PERIOD: 12 weeks RESCUE: Albuterol HRA‐MDI CO‐INTERVENTIONS PERMITTED: steroid creams (<1%), nasal and eye cromoglycate, anti‐histamines, decongestants and regular immunotherapy % on ICS baseline: 0 |
|
| Outcomes | FEV1; pm PEF; Quality of life (AQLQ); adverse events; withdrawals | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gelfand 2006b.
| Methods | As above | |
| Participants | As above. Second study ID created for estimates involving lower dose range of study (i.e. 40 & 80mcg/d) |
|
| Interventions | As above | |
| Outcomes | FEV1; pm PEF; Quality of life (AQLQ); adverse events; withdrawals | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hansel 2006.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: Europe, 62 centres. DURATION OF STUDY: 12 weeks (1‐4 week run‐in on prn SABA) CONCEALMENT OF ALLOCATION: Not reported COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes to ciclesonide dose but open label for BUD (no BUD placebo available) METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Yes (computer generated randomisation list) METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Appropiate for CIC arms DESCRIPTION OF WITHDRAWALS/DROPOUTS: yes JADAD SCORE (5‐1): 5 (CIC v CIC); 3 (CIC v BUD) TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT COMPLIANCE: Not reported CONFOUNDERS: Baseline values comparable | |
| Participants | N SCREENED: 684 N RANDOMISED: 554 N COMPLETED: 490 (64 withdrawn). 49 excluded from PP analysis (protocol violations) M= 301F= 253 MEDIAN AGE (range): CIC80 38 years (12‐73); CIC320 41 (14‐74); BUD 45 (13‐73) BASELINE DETAILS: FEV1 % predicted 72% INCLUSION CRITERIA: Aged 12‐75 years; mild to moderate persistent asthma of over 6 months duration according to ATS criteria including asthma symptoms and spontaneous fluctuations in obstruction. EXCLUSION: Oral or systemic steroid use within 4 weeks of screening or more than 3 times during preceding 6 months; inhaled daily dose of BDP > 500 ug or equivalent steroids within 4 weeks of screening, contraindication to inhaled corticosteroids use, hypersensitivity to study meds, asthma exacerbation or LRTI within 4 weeks of screening, COPD or other relevant respiratory disease, pregnancy, breast feeding, lack of contraceptive in women of child bearing potential, inability to follow study procedures, with clinically relevant lab values suggestive of disease. | |
| Interventions | 1. Ciclesonide 100mcg OD
2. Ciclesonide 400mcg OD
3. BUD 200 mcg BD (open labelled) DELIVERY: HFA‐MDI (CIC) and Turbohaler (BUD) TREATMENT PERIOD: 12 weeks RESCUE: SABA salbutamol or terbutaline CO‐INTERVENTIONS PERMITTED: rescue only (withdrawal if asthma exacerbation needing oral or systemic steroids, other ICS) % on ICS (pre‐run in): 0 |
|
| Outcomes | FEV1; am PEF; asthma symptom score, rescue medication use; adverse events; 24‐hour urinary cortisols (HPA‐axis) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Langdon 2005.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: UK and Canada, 51 centres. DURATION OF STUDY: 12 weeks (2 week run‐in on maintenance ICS) CONCEALMENT OF ALLOCATION: Unclear COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Identical devices DESCRIPTION OF WITHDRAWALS/DROPOUTS: Reported JADAD SCORE (5‐1): 4 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT COMPLIANCE: Not assessed CONFOUNDERS: Baseline values comparable. | |
| Participants | N SCREENED: Unclear N RANDOMISED: 360 (488 entered run‐in phase) N COMPLETED: 215 M= 161 F= 299 MEAN AGE: 40.56 years BASELINE DETAILS: FEV1: 2.54L; FEV1 % predicted: 78; am PEF: 412L/min; PEF variability: 7.2L INCLUSION CRITERIA: 18‐70 years old; history of asthma; FEV1 60‐95% predicted; inhaled corticosteroid maintenance therapy (BDP equivalent 4‐800mcg/d; stable dosage regimen for four weeks); post‐run in FEV1 60‐90% predicted; FEV1 reversibility >/=15% EXCLUSION: COPD; exacerbation of asthma within 6 weeks of study entry; >10 cigarettes/day; nasal/topical corticosteroids and LABA therapy before 4 weeks of study entry | |
| Interventions | 1. Ciclesonide 100mcg OD
2. Ciclesonide 400mcg OD
3. Placebo DELIVERY: HFA‐metered dose inhaler TREATMENT PERIOD: 12 weeks RESCUE: salbutamol CO‐INTERVENTIONS PERMITTED: Medications not listed under exclusion criteria % on ICS (pre‐run in): 100 |
|
| Outcomes | FEV1; FVC; Clinic PEF; am PEF; pm PEF; Symptoms (total, day, night, rescue medication use); adverse events; withdrawals | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lipworth 2005.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: USA, 20 centres. DURATION OF STUDY: 12 weeks (no run‐in described) CONCEALMENT OF ALLOCATION: Unclear COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Identical inhaler devices DESCRIPTION OF WITHDRAWALS/DROPOUTS: Reported JADAD SCORE (5‐1): 3 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT COMPLIANCE: Similar across treatment groups (assessed by canister weight) CONFOUNDERS: Baseline characteristics comparable. | |
| Participants | N SCREENED: Not reported. N RANDOMISED: 164 N COMPLETED: 148 M= 79F= 85 MEAN AGE: 37 BASELINE DETAILS: FEV1: 3L; FEV1 predicted: 81% INCLUSION CRITERIA: >/‐18 years; mild to moderate persistent asthma; acceptable inhaler technique; SABA only for 6 months (at least 2 x daily); FEV1 >/=70% predicted. Females taking oral contraceptives and HRT were required to have an increase in serum cortisol levels of 7mcg/dL or greater from basal to peak levels. EXCLUSION: Systemic steroid use within 6 months of screening; inhaled steroids within 2 months. | |
| Interventions | 1. Ciclesonide 400mcg OD
2. Ciclesonide 400mcg BD
3. Fluticasone 500mcg BD
4. Placebo DELIVERY: CIC: HFA MDI without a spacer; FP: CFC MDI without a spacer. TREATMENT PERIOD: 12 weeks RESCUE: Not reported CO‐INTERVENTIONS PERMITTED: None permitted % on ICS baseline: 0 |
|
| Outcomes | Hypothalmic pituitary axis function; serum cortisol; safety | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Magnussen 2007.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: Europe, number of centres not reported DURATION OF STUDY: 12 weeks (1‐4 weeks prn SABA) CONCEALMENT OF ALLOCATION: Unclear COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Both treatments given via MDIs DESCRIPTION OF WITHDRAWALS/DROPOUTS: Stated JADAD SCORE (5‐1): 4 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT and PP COMPLIANCE: Not reported CONFOUNDERS: Balanced groups at baseline | |
| Participants | N SCREENED: Not reported N RANDOMISED: 808 N COMPLETED: 764 M = 409; F = 398 MEDIAN AGE: 29‐33 BASELINE DETAILS: FEV1 predicted: 79%; reversibility: 25% INCLUSION CRITERIA: ATS defined asthma; 12‐75 years; 61‐90% predicted (if treated with ICS), or 61‐105% predicted if not treated with ICS; maximum daily dose was FP 250 mcg; post‐run in participants had to demonstrate FEV1 between 60‐90% predicted. EXCLUSION: Concomitant severe disease; smoking history of >10 pack years; LABA or OCS treatment in previous 4 weeks. | |
| Interventions | 1. Ciclesonide 100 mcg OD
2. Ciclesonide 200 mcg OD
3. Fluticasone 100 mcg BID DELIVERY: MDI TREATMENT PERIOD: 12 weeks RESCUE: Salbutamol CO‐INTERVENTIONS PERMITTED: Not reported CO‐INTERVENTIONS: Not reported % on ICS: Not reported |
|
| Outcomes | FEV1; peak flow; asthma symptoms; asthma exacerbations requiring oral steroids | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
O'Connor 2002.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: DURATION OF STUDY: 12 weeks (2 week run‐in on 1600mcg/d BDP) CONCEALMENT OF ALLOCATION: Not reported COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Not reported DESCRIPTION OF WITHDRAWALS/DROPOUTS: Not reported JADAD SCORE (5‐1): 2 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): Not clear COMPLIANCE: Not reported CONFOUNDERS: Not reported. | |
| Participants | N SCREENED: Not reported N RANDOMISED: 365 N COMPLETED: 275 M= Not reported; F= Not reported MEAN AGE: Not reported BASELINE DETAILS: Not reported INCLUSION CRITERIA: Moderate to severe asthma; treatment with BDP equivalent 800‐2000mcg/d; symptom score >4 over last week of run in; sum of rescue medication >14 puffs over run in. EXCLUSION: Not reported. | |
| Interventions | 1. Ciclesonide 400mcg BD
2. Ciclesonide 800mcg BD DELIVERY: MDI TREATMENT PERIOD: 12 weeks (2 week run in on high dose BDP) RESCUE: Not reported CO‐INTERVENTIONS PERMITTED: Not reported % on ICS: 100 |
|
| Outcomes | FEV1; rescue medication usage; symptoms; withdrawal due to lack of efficacy. | |
| Notes | Reported as unpublished conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pearlman 2005.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: United States, number of centres: not clear. DURATION OF STUDY: 12 weeks (1‐4 weeks on single‐blind placebo) CONCEALMENT OF ALLOCATION: Not clear COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not described METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Not described DESCRIPTION OF WITHDRAWALS/DROPOUTS: Not reported JADAD SCORE (5‐1): 2 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): ITT COMPLIANCE: Not assessed CONFOUNDERS: Similar characteristics at baseline. | |
| Participants | N SCREENED: 2512 N RANDOMISED: 1015 (study i: 526; study ii: 489) N COMPLETED: Not reported (only withdrawals due to lack of efficacy reported) M= 413F= 598 MEAN AGE: 36.6 BASELINE DETAILS: FEV1: 2.44L; am PEF: 364L/min; pm PEF: 380L/min; symptom score: 2.63; saba use: 3.16 puffs/d; AQLQ: 4.6 INCLUSION CRITERIA: >12 years; mild‐moderate asthma; not using more than 400mcg/d FP, 250/50 FP/SAL combination, 1000mcg/d BUD or equivalent, leukotrienes, SABA or LABA for ess than 30 days before study entry; FEV1 60‐85% predicted; greater than 12% reversibility to SABA; symptomatic over last 7 days prior to randomisation (score >3; PEF variability >20%; SABA usage 2 or more puffs/d over 3 of last 7 days). EXCLUSION: Continual asthma; frequent night symptoms; history of life threatening asthma; two or more hospitalisations with asthma in past year; COPD; systemic steroids in 3 months prior to randomisation. | |
| Interventions | 1. Ciclesonide 100mcg OD
2. Ciclesonide 200mcg OD
3. Ciclesonide 400mcg OD
4. Placebo DELIVERY: HFA‐MDI TREATMENT PERIOD: 12 weeks RESCUE: Salbutamol CO‐INTERVENTIONS PERMITTED: None CO‐INTERVENTIONS: Not reported % on ICS: Not reported. |
|
| Outcomes | FEV1; FEV1 predicted; am PEF L/min; pm PEF L/min; symptoms; rescue medication use; quality of life; night awakenings; serum cortisol; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wilson 2006.
| Methods | STUDY DESIGN: Crossover LOCATION, NUMBER OF CENTRES: Single centre in Canada. DURATION OF STUDY: 2 x 4 week treatment periods (1 week run‐in and 2 week washout) CONCEALMENT OF ALLOCATION: Performed by a pharmacist and coded in a sealed envelope COCHRANE QUALITY SCORE: A DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not clear METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Not clear. DESCRIPTION OF WITHDRAWALS/DROPOUTS: 3/20 JADAD SCORE (5‐1): 3 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): Available case. COMPLIANCE: Participants asked to fill in a chart when medication was taken. CONFOUNDERS: NA | |
| Participants | N SCREENED: Not reported. N RANDOMISED: 20 N COMPLETED: 17 M= 8 F= 9 MEAN AGE: 30 years BASELINE DETAILS: FEV1: 3.35 INCLUSION CRITERIA: History of mild‐moderate asthma; 18‐71 years of age; atopic; non‐smokers; stable for 4 weeks prior to study entry; bronchial hyperresponsiveness to methacholine challenge; prn SABA only. EXCLUSION: Cardiac or pulmonary disease beside asthma. | |
| Interventions | 1. Ciclesonide 200mcg QD
2. Placebo DELIVERY: HFA‐MDI TREATMENT PERIOD: 4 weeks RESCUE: Salbutamol. CO‐INTERVENTIONS PERMITTED: SABA CO‐INTERVENTIONS: SABA only. % on ICS baseline: 0 |
|
| Outcomes | Methacholine challenge; exhaled nitric oxide; FEV1; PEF; symptoms; adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zietkowski 2006.
| Methods | STUDY DESIGN: Parallel group LOCATION, NUMBER OF CENTRES: One centre in Poland. DURATION OF STUDY: 12 weeks (1‐4 week run in on prn SABA) CONCEALMENT OF ALLOCATION: Unclear COCHRANE QUALITY SCORE: B DESCRIBED AS RANDOMISED: Yes DESCRIBED AS DOUBLE BLIND: Yes METHOD OF RANDOMISATION WELL DESCRIBED/APPROPRIATE: Not reported METHOD OF BLINDING WELL DESCRIBED/APPROPRIATE: Double‐dummy DESCRIPTION OF WITHDRAWALS/DROPOUTS: Not reportedJADAD SCORE (5‐1): 3 TYPE OF ANALYSIS (AVAILABLE CASE/TREATMENT RECEIVED/ ITT): Assumed available case. COMPLIANCE: Not reported CONFOUNDERS: Baseline values comparable. | |
| Participants | N SCREENED: Not clear N RANDOMISED: 35 N COMPLETED: 35 M= 19; F= 16 MEAN AGE: 45 years BASELINE DETAILS: Duration of symptoms: 15 years; allergic rhinitis: 23/35 participants INCLUSION CRITERIA: Mild allergic asthma (according to GINA guidelines); free from exacerbations in previous four weeks; non‐smokers; treatment with FP equivalent 250mcg/d. EXCLUSION: Not reported. | |
| Interventions | 1. Ciclesonide OD 100mcg
2. Ciclesonide OD 200mcg
3. Fluticasone BD 100mcg DELIVERY: unclear TREATMENT PERIOD: 12 weeks RESCUE: Salbutamol CO‐INTERVENTIONS PERMITTED: Not reported. CO‐INTERVENTIONS: Not listed. % on ICS pre‐baseline: 100. |
|
| Outcomes | FEV1 L; FEV1 predicted; symptoms; rescue medication use | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
BD: twice daily; BDP: beclomethasone dipropionate; DPI: dry powder inhaler; FP: fluticasone propionate; ICS: inhaled corticosteroid; MDI: metered dose inhaler; OD: once daily; SABA: short‐acting beta‐agonist
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agertoft 2005 | Treatment <4 weeks |
| Bethke 2002 | Healthy volunteers |
| Dahl 1998 | Treatment <4weeks |
| Derom 2005 | Treatment <4 weeks |
| Drollman 2004 | Treatment <4 weeks |
| Erin 2005 | Treatment <4 weeks |
| Gauvreau 2005 | Treatment <4 weeks |
| Kanniess 2001 | Treatment <4 weeks |
| Larsen 2003 | Treatment <4 weeks |
| Lee 2004 | Wrong comparison |
| Lee 2005 | Wrong comparison |
| Postma 2001 | Morning versus evening administration |
| Richter 2005 | Treatment <4 weeks |
| Subbarao 2006 | Treatment <4 weeks |
| Szefler 2005 | Inpatient administration |
| Taylor 1999 | Treatment <4 weeks |
Characteristics of ongoing studies [ordered by study ID]
Arshad.
| Trial name or title | An assessment of safety and efficacy in treating moderate to severe asthmatics with inhaled Ciclesonide vs Fluticasone |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Beck.
| Trial name or title | Efficacy and safety of ciclesonide administered with or without different spacers in patients with asthma (12 to 75 y). Clinicaltrials.Gov. 2005 |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Beckman.
| Trial name or title | Efficacy of ciclesonide inhaled once daily versus other corticosteroids used for treatment of mild asthma in children (4‐11yrs). Clinicaltrials.Gov. 2005 |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Colatruglio.
| Trial name or title | Effect of ciclesonide on quality of life in patients with moderate persistent asthma (21 to 65 y). Clinicaltrials.Gov. 2005 |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Dahl.
| Trial name or title | Efficacy of ciclesonide versus fluticasone propionate in patients with mild to moderate asthma (12 to 75 y). Clinicaltrials.Gov. 2005 |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Derom.
| Trial name or title | Effect of inhaled ciclesonide versus fluticasone propionate in patients with mild to moderate asthma (18 to 65 y). Clinicaltrials.Gov. 2005 |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Dusser.
| Trial name or title | Efficacy of ciclesonide and fluticasone propionate in adult patients with moderate and severe persistent asthma (18 to 75 y). Clinicaltrials.Gov. 2005 |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Engelstätter.
| Trial name or title | Efficacy of ciclesonide inhaled once daily versus fluticasone propionate inhaled twice daily in children with asthma (4 to 15 y). Clinicaltrials.Gov. 2005 |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Giwa.
| Trial name or title | Comparison of inhaled ciclesonide and fluticasone proprionate in moderate to severe asthma patients, well controlled under high doses of inhaled corticosteroids |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Hansel.
| Trial name or title | A 3‐period double‐blind, cross‐over study on the onset of action of inhaled ciclesonide on airway responsiveness to adenosine monophosphate, sputum eosinophils and exhaled breath NO in patients with asthma |
| Methods | |
| Participants | Outpatients of either sex who are between 18‐45 years with a history of atopic disease, who have a history of perennial bronchial asthma for at least 6 months as defined by ATS criteria (increased responsiveness to a variety of stimuli; symptoms like dyspnoe, wheezing and cough of varying degree; spontaneous fluctuations in the severity of obstruction with substantial improvements following bronchodilators or corticosteroids (American Thoracic Society, 1987) |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
O'Byrne.
| Trial name or title | Efficacy of ciclesonide vs fixed combination of fluticasone propionate/salmeterol vs placebo in patients with mild persistent asthma (12 to 75 y). Clinicaltrials.Gov. 2005. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Park.
| Trial name or title | Effectiveness of ciclesonide versus budesonide in patients with asthma (18 to 75 y). Clinicaltrials.Gov. 2005 |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Sanofi Aventis.
| Trial name or title | Effects of ciclesonide and beclomethasone on lens opacification in adult subjects with moderate to severe persistent asthma. Clinicaltrials.Gov . 2005; Efficacy of ciclesonide vs. placebo administered as once daily or twice daily in patients not treated with inhaled corticosteroid. Clinicaltrials.Gov. 2005; Effects of ciclesonide MDI 50mg/day and 200mg/day (ex‐value) once‐daily on growth in children with mild persistent asthma. Clinicaltrials.Gov. 2005; Sanofi‐Aventis. Dose response study of inhaled ciclesonide (glucocorticosteroid) to patients with persistent asthma. Clinicaltrials.Gov. 2005. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Stenton.
| Trial name or title | A double‐blind randomised parallel group study comparing the efficacy and safety of 800 and 1000mcg CIC/day in patients with asthma followed by an open long‐term study to assess the safety of CIC in patients with asthma |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Contributions of authors
PM: Protocol initiation & development; study assessment & data extraction; write‐up. PG: Protocol development; review development; contact editor TL: Protocol development; study assessment & data extraction; data entry & analysis; write‐up
Sources of support
Internal sources
No sources of support supplied
External sources
Cochrane Fellowship from the Health Research Board, Ireland.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Adachi 2007a {published data only}
- Adachi M, Ishihara K, Inoue H, Kudo K, Takahashi K, Morita Y, et al. Efficacy and safety of once‐daily inhaled ciclesonide in adults with mild to moderate asthma: A double‐blind, placebo‐controlled study. Respirology 2007;12(4):566‐72. [DOI] [PubMed] [Google Scholar]
Adachi 2007b {published data only}
- Adachi M, Ishihara K, Inoue H, Kudo K, Takahashi K, Morita Y, et al. Efficacy and safety of inhaled ciclesonide compared with chlorofluorocarbon beclomethasone dipropionate in adults with moderate to severe persistent asthma. Respirology 2007;12(4):573‐80. [DOI] [PubMed] [Google Scholar]
Baena Cagnani 2006 {unpublished data only}
- Baena‐Cagnani CE, Mansfeild L, Zhang P, Lloyd M, Banjeri D. Once daily ciclesonide has no effect on growth in children with mild persistent asthma a subgroup analysis of north and South American populations. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):S191. [Google Scholar]
- Bernstein D, Caballero‐Fonseca F, Zhang P, Lloyd M, Banjeri D. Once daily ciclesonide has no effect on hypothalmic pituitary adrenal axis function in prepubertal children with mild persistent asthma. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):S184. [Google Scholar]
- Maspero J, Maika S, Kundu S, Lloyd M, Banjerji D. Ciclesonide once daily is well tolerated in children with mild persistent asthma a 1 year double blind placebo controlled study. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):S94. [Google Scholar]
- Nayak A, Herrera O, Kudu S, Lloyd M, Banjeri D. Ciclesonide has no effect on growth in prepubertal children with mild persistent asthma irrespective of age and gender. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):S191. [Google Scholar]
- Neffen H, Ruff M, Zhang P, Lloyd M, Banjeri D. Ciclesonide administered once daily has no effect on skeletal maturity in prepubertal children with mild persistent asthma. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):S184. [Google Scholar]
- Skoner D, Maspero J, Kundu S, Lloyd M, Banerji D. Ciclesonide administered once daily has no effect on growth velocity in prepubertal children with mild persistent asthma. Journal of Allergy and Clinical Immunology 2006;117(2 Suppl 1):S11. [Google Scholar]
Bateman 2006 {published data only}
- Bateman E, Karpel J, Casale T, Craig T, Wenzel S, Fish J, et al. Ciclesonide reduces oral corticosteroid use in adults with severe persistent asthma while maintaining asthma control. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B35; Poster: G16.
- Bateman E, Karpel J, Casale T, Wenzel S, Banerji D. Ciclesonide reduces the need for oral steroid use in adult patients with severe, persistent asthma. Chest 2006;129(5):1176‐87. [DOI] [PubMed] [Google Scholar]
Bateman 2006a {published data only}
- Bateman ED, Velazquez‐Samano G, Záková L, Chuchalin A, Göhring UM, Engelstätter R. Comparison of ciclesonide 160 versus 640 mcg per day in patients with severe asthma. European Respiratory Journal 2006;28(50):206s. [Google Scholar]
Bernstein 2004 {unpublished data only}
- Bernstein D, Berger W, Welch M, Kundu S, Banerji D. Once‐daily ciclesonide significantly improves pulmonary function in adults/adolescents with mild‐moderate asthma. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B35; poster: G27.
- Bernstein D, Nathan R, Ledford D, Ledoux E, Pedinoff A, Crivera C, et al. Ciclesonide, a new inhaled corticosteroid, significantly improves asthma‐related quality of life in patients with severe, persistent asthma. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S210. [Google Scholar]
- Bernstein JA, Noonan MJ, Rim C, Fish J, Kundu S, Williams J, et al. Ciclesonide has minimal oropharyngeal side effects in the treatment of patients with moderate‐to‐severe asthma. Journal of Allergy and Clinical Immunology 2004;113(2 Suppl):S113. [Google Scholar]
- Busse W, Kaliner M, Bernstein D, Nayak A, Kundu S, Williams J. The novel inhaled corticosteroid ciclesonide is efficacious and has a favourable safety profile in adults and adolescents with severe persistent asthma. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S213. [Google Scholar]
- Skoner D, Baena‐Cagnani CE, Maspero J, Neffen H, Banerji D. Once‐daily ciclesonide has no effect on growth velocity, as assessed by stadiometer height, in children with mild, persistent asthma. American Thoracic Society International Conference, San Diego, California, May 19‐24, 2006. 2006:A73 [Poster G20].
- Weinstein S, Friedman B, Kundu S, Banerji D. Ciclesonide is effective and well tolerated in adults/adolescents with severe persistent asthma. American Thoracic Society 2005 International Conference; May 20‐25 San Diego, California. 2005:[B35] [Poster: G26].
Chapman 2005 {published data only}
- Chapman KR, Buhl R, Engelstätter R. Once daily ciclesonide is effective in the treatment of asthma. Thorax 2004;59(Suppl 2):ii71. [Google Scholar]
- Chapman KR, D'Urzo AD, Oedekoven C, Steinijans VW, Wurst W. Effects of ciclesonide versus placebo on lung function after 12 weeks of treatment in patients with asthma. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A767. [Google Scholar]
- Chapman KR, Patel P, Boulet LP, D'Urzo AD, Alexander M, Mehra S, et al. Efficacy and long‐term safety of ciclesonide in asthmatic patients as demonstrated in a 52 week long study [abstract]. European Respiratory Society Annual Congress. 2002:Abstract nr: 2328.
- Chapman KR, Patel P, D'Urzo AD, Alexander M, Mehra S, Oedekoven C, et al. Maintenance of asthma control by once‐daily inhaled ciclesonide in adults with persistent asthma. Allergy 2005;60(3):330‐7. [DOI] [PubMed] [Google Scholar]
DFI6153 {unpublished data only}
- Sanofi‐Aventis. A placebo‐ and active‐controlled (ciclesonide metered‐dose inhaler), randomized, parallel‐group, dose‐range finding study of ciclesonide administered by dry powder inhaler (Ultrahaler®) in adult and adolescent patients with persistent asthma. http://www.clinicalstudyresults.org [Accessed June 2007] 2006.
EFC6163a {published data only}
- Sanofi‐Aventis. A multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study to assess the efficacy of ciclesonide metered‐dose inhaler at a daily dose of 160 mcg administered for 12 weeks either in a once‐daily regimen in the morning (160 mcg qd AM) or in a twice‐daily regimen (80 mcg bid) in adults and adolescents with mild to moderate persistent asthma treated previously with inhaled corticosteroids. http://www.clinicalstudyresults.org [Accessed June 2007] 2006.
EFC6163b {unpublished data only}
- Sanofi‐Aventis. A multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study to assess the efficacy of ciclesonide metered‐dose inhaler at a daily dose of 160 mcg administered for 12 weeks either in a once‐daily regimen in the morning (160 mcg qd AM) or in a twice‐daily regimen (80 mcg bid) in adults and adolescents with mild to moderate persistent asthma treated previously with inhaled corticosteroids. http://www.clinicalstudyresults.org [Accessed June 2007] 2006. [Google Scholar]
Gelfand 2006a {published data only}
- Gelfand E, Boguniweicz M, Weinstein S, Bernstein C, Lloyd M, Zhang P, et al. Ciclesonide, administered once daily, has a low incidence of oropharyngeal adverse events in pediatric asthma patients. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S213. [Google Scholar]
- Gelfand EW, Georgitis JW, Noonan M, Ruff ME. Once‐daily ciclesonide in children: efficacy and safety in asthma. Journal of Pediatrics 2006;148(3):377‐83. [DOI] [PubMed] [Google Scholar]
- Kerwin E, Meltzer E, Kundo S, Banjeri D. Ciclesonide an effective once‐daily inhaled corticosteroid for children with persistent asthma. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:C47; Poster: A11.
- Miller D, Ratner P, Condemi J, Lawrence M, Crivera C, Lloyd M, et al. Once‐daily ciclesonide improves quality of life in pediatric patients with asthma. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S211. [Google Scholar]
Gelfand 2006b {published data only}
- Gelfand E, Boguniweicz M, Weinstein S, Bernstein C, Lloyd M, Zhang P, et al. Ciclesonide, administered once daily, has a low incidence of oropharyngeal adverse events in pediatric asthma patients. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S213. [Google Scholar]
- Gelfand EW, Georgitis JW, Noonan M, Ruff ME. Once‐daily ciclesonide in children: efficacy and safety in asthma. Journal of Pediatrics 2006;148(3):377‐83. [DOI] [PubMed] [Google Scholar]
- Kerwin E, Meltzer E, Kundo S, Banjeri D. Ciclesonide an effective once‐daily inhaled corticosteroid for children with persistent asthma. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:C47; Poster: A11.
- Miller D, Ratner P, Condemi J, Lawrence M, Crivera C, Lloyd M, et al. Once‐daily ciclesonide improves quality of life in pediatric patients with asthma. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S211. [Google Scholar]
Hansel 2006 {published data only}
- Engelstätter R, Benezet O, Kafe H, Ponitz HH, Hansel T, Cheung D, et al. Comparative study in asthma patients treated with inhaled ciclesonide (80µg or 320µg once daily) or budesonide (200µg twice daily) for 12 weeks. American Thoracic Society 99th International Conference. 2003; Vol. C105:D64.
- Hansel T, Biberger C, Engelstätter R. Ciclesonide 80 µg or 320 µg once daily achieves lung function improvement comparable with budesonide 200 µg twice daily in patients with persistent asthma. Thorax 2004;59(Suppl II):ii71. [Google Scholar]
- Hansel T, Engelstatter R, Benezet O, Kafe H, Ponitz HH, Cheung D, et al. Once daily ciclesonide (80ug or 320ug) is equally effective as budesonide 200ug given twice daily: a 12 week study in asthma patients. European Respiratory Journal 2003;22(Suppl 45):Abstract No: P2639. [Google Scholar]
- Hansel TT, Benezet O, Kafe H, Ponitz HH, Cheung D, Engelstatter R, Barnes PJ. A multinational, 12‐week, randomized study comparing the efficacy and tolerability of ciclesonide and budesonide in patients with asthma. Clinical therapeutics 2006;28(6):906‐20. [DOI] [PubMed] [Google Scholar]
Langdon 2005 {published data only}
- Drollmann A, Langdon C, Engelstätter R, Rathgeb F, Steinijans VW, Wurst W. Ciclesonide is effective in the treatment of bronchial asthma. European Respiratory Journal 2001;18(Suppl 33):95s. [Google Scholar]
- Engelstätter R, Langdon C, Bethke T, Rathgeb F, Steinijans VW, Wurst W. Efficacy of ciclesonide after 12 week treatment of bronchial asthma. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A766. [Google Scholar]
- Langdon CG, Adler M, Mehra S, Alexander M, Drollmann A. Once‐daily ciclesonide 80 or 320mug for 12 weeks is safe and effective in patients with persistent asthma. Respiratory Medicine 2005;99(10):1275‐85. [DOI] [PubMed] [Google Scholar]
Lipworth 2005 {published data only}
- LaForce CF, Baker JW, Amin D, Rohatagi S, Mendes P, Williams J, et al. Ciclesonide a novel inhaled steroid has no effect on hypothalamic pituitary adrenal (HPA) ‐ axis function in mild to moderate asthmatics. Journal of Allergy and Clinical Immunology 2003;111(2 Suppl):S218. [Google Scholar]
- Lipworth BJ, Kaliner MA, LaForce CF, Baker JW, Kaiser HB, Amin D, et al. Effect of ciclesonide and fluticasone on hypothalamic‐pituitary‐adrenal axis function in adults with mild‐to‐moderate persistent asthma. Annals of Allergy, Asthma and Immunology 2005;94(4):465‐72. [DOI] [PubMed] [Google Scholar]
Magnussen 2007 {published data only}
- Magnussen H, Hellwig M, Hellbradt S, Josten JP, Engelstätter R. Ciclesonide 80ug or 160ug once‐daily and fluticasone propionate 88ug twice‐daily similarly reduce asthma symptoms and rescue medication use in patients with mild‐to‐moderate asthma. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B35; Poster: G20.
- Magnussen H, Hofman J, Novakova B, Kaczmarek B, Hellwig M, Engelstätter R. Ciclesonide 80 µg or 160 µg once daily is comparable to fluticasone propionate 88 µg twice daily in the treatment of asthma patients. European Respiratory Journal 2005;26(Suppl 49):255s. [Google Scholar]
- Magnussen H, Hofman J, Novakova B, Kaczmarek B, Hellwig M, Engelstätter R. Ciclesonide decreases exhaled breath nitric oxide and rapidly reduces airway responsiveness to adenosine monophosphate in asthma patients. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1723. [Google Scholar]
- Magnussen H, Hofman J, Novakova B, Kaczmarek‐Czeczothka B, Koci T, Staneta P, et al. Ciclesonide 80 µg or 160 µg once‐daily is as effective as fluticasone propionate 88 µg twice‐daily in the treatment of persistent asthma. Journal of Allergy and Clinical Immunology 2005;115(2 Suppl):S4. [Google Scholar]
- Magnussen H, Hofman J, Novokova B, Kaczmarek B, Schutze J, Hellwig M. Once daily ciclesonide 80 µg or 160 µg is comparable to twice daily fluticasone propionate 88 µg in the treatment of persistent asthma. XIX World Allergy Organization Congress; Munich, Germany. 2005:Abstract 297.
- Magnussen H, Hofman J, Staneta P, Lawo JP, Hellwig M, Engelstatter R. Similar efficacy of ciclesonide once daily versus fluticasone propionate twice daily in patients with persistent asthma. Journal of Asthma 2007;44(7):555‐63. [DOI] [PubMed] [Google Scholar]
O'Connor 2002 {published data only}
- O'Connor B, Sips P, Engelstätter R, Steinijans VW, Wurst W. Management of moderate to severe bronchial asthma by ciclesonide: A 12‐week trial. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A767. [Google Scholar]
- O'Connor BJ, Kilfeather S, Cheung D, Sips P, Blagden MD, Kafe H, et al. Treatment of moderate to severe asthma with ciclesonide: a long‐term investigation over 52 weeks. European Respiratory Society Annual Congress 2002. 2002:Abstract nr: 2579.
Pearlman 2005 {published data only}
- Berger WE. Ciclesonide is well tolerated and has minimal oropharyngeal side effects at once‐daily doses of 80µg, 160µg and 320µg in the treatment of patients with mild to moderate asthma. Journal of Allergy and Clinical Immunology 2004;113(2 Suppl):S38. [Google Scholar]
- Bernstein D, Berger W, Welch M, Kundu S, Banerji D. Once‐daily ciclesonide significantly improves pulmonary function in adults/adolescents with mild‐moderate asthma. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B35; poster: G27.
- Kerwin E, Chervinsky P, Fish J, Kundu S, Williams J, Banerji D, et al. Ciclesonide has no effect on hypothalamic‐pituitary‐adrenal (HPA) axis at once‐daily doses of 80 mcg, 160 mcg, or 320 mcg in the treatment of patients with mild‐to‐moderate asthma. American Thoracic Society 100th International Conference, May 21‐26, 2004, Orlando. 2004:A 37; Poster J104.
- Nayak A, Charous BL, Finn A, Lumry W, Crivera C, Williams J, et al. A novel inhaled corticosteroid ciclesonide significantly improves quality of life in patients with mild‐to‐moderate asthma. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S210. [Google Scholar]
- Pearlman D, Creticos P, Lampl K, Gower R, Kundu S, Fish J, et al. Once‐daily ciclesonide is effective and well‐tolerated in adult and adolescent patients with mild‐to‐moderate asthma. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S212. [DOI] [PubMed] [Google Scholar]
- Pearlman DS, Berger WE, Kerwin E, LaForce C, Kundu S, Banerji D. Once‐daily ciclesonide improves lung function and is well tolerated by patients with mild‐to‐moderate persistent asthma. Journal of Allergy & Clinical Immunology 2005;116(6):1206‐12. [DOI] [PubMed] [Google Scholar]
Wilson 2006 {published data only}
- Wilson AM, Duong M, Pratt B, Dolovich M, O'Byrne PM. An assessment of the anti‐inflammatory properties of low dose inhaled ciclesonide in asthmatic patients. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California;. 2005:C33; Poster: F64.
- Wilson AM, Duong M, Pratt B, Dolovich M, O'Byrne PM. Anti‐inflammatory effects of once daily low dose inhaled ciclesonide in mild to moderate asthmatic patients. Allergy 2006;61(5):537‐42. [DOI] [PubMed] [Google Scholar]
Zietkowski 2006 {published data only}
- Zietkowski Z, Bodzenta‐Lukaszyk A, Tomasiak MM, Szymanski W, Skiepko R. Effect of ciclesonide and fluticasone on exhaled nitric oxide in patients with mild allergic asthma. Respiratory Medicine 2006;100:1651‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agertoft 2005 {published data only}
- Agertoft L, Pedersen S. Inhaled ciclesonide does not effect lower leg growth rate or HPA‐axis function in children with mild asthma. European Respiratory Journal 2004;24(Suppl 48):377s. [Google Scholar]
- Agertoft L, Pedersen S. Short‐term lower‐leg growth rate and urine cortisol excretion in children treated with ciclesonide. Journal Allergy & Clinical Immunology 2005;115(5):940‐5. [DOI] [PubMed] [Google Scholar]
- Agertorft L, Pedersen S. Lower‐leg growth rate and APA‐axis function in children with asthma during treatment with inhaled ciclesonide. Journal of Allergy and Clinical Immunology 2004;113(2 Suppl):S119. [Google Scholar]
Bethke 2002 {published data only}
- Bethke TD, Drollman A, Hauns B, Nave R, Zech K, Seinijans VW, et al. Bioequivalence of two ciclesonide dosing regimen (4 puffs of 200µg versus 16 puffs of 50µg using a MDI). European Respiratory Journal 2002;20(Suppl 38):109s. [Google Scholar]
- Drollmann A, Bethke TD, Nave R, Zech K, Hauns B, Steinjans VW, et al. Bioequivalence of two ciclesonide dosing regimen (4 puffs of 200µg vs. 8 puffs of 100µg using MDI). European Respiratory Journal 2002;20(Suppl 38):304s. [Google Scholar]
Dahl 1998 {published data only}
- Dahl R, Nielsen LP, Christensen MB, Engelstätter R. Ciclesonide ‐ an inhaled corticosteroid prodrug ‐ inhibits allergen induced early and late phase reactions. European Respiratory Journal 1998;12(Suppl 28):62S. [Google Scholar]
Derom 2005 {published data only}
- Derom E, Velde V, Marissens S, Engelstätter R, Vincken W, Pauwels R. Effects of inhaled ciclesonide and fluticasone propionate on cortisol secretion and airway responsiveness to adenosine 5'monophosphate in asthmatic patients. Pulmonary Pharmacology & Therapeutics 2005;18(5):328‐36. [DOI] [PubMed] [Google Scholar]
- Derom E, Velde V, Marissens S, Vincken W, Pauwels RA. Efficacy and systemic effects of ciclesonide and fluticasone in asthma patients. European Respiratory Journal 2001;18(Suppl 33):147s. [Google Scholar]
- Pauwels RA, Derom E, Velde V, Marissens S, Vincken W. Effects of inhaled ciclesonide and fluticasone propionate on cortisol secretion and PC²° for adenosine in asthma patients. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A768. [Google Scholar]
Drollman 2004 {published data only}
- Drollman A, Nave R, Steinijans VW, Bethke TD, Baumgartner E. Equivalent pharmacokinetics of the active metabolite of ciclesonide with and without use of a spacer for inhalation. Journal of Allergy and Clinical Immunology 2004;113(2 Suppl):S120. [DOI] [PubMed] [Google Scholar]
- Nave R, Baumgartner E, Bethkle D, Steinijans W, Drollman A. Equivalent pharmacokinetics of the active metabolite of ciclesonide when using the ciclesonide MDI without a spacer. European Respiratory Journal 2004;24(Suppl 48):583s. [Google Scholar]
Erin 2005 {published data only}
- Erin EM, Engelstätter R, Hellwig M, Hansel TT, Barnes PJ. Ciclesonide decreases exhaled breath nitric oxide and rapidly reduces airway responsiveness to adenosine monophosphate in asthma patients. European Respiratory Journal 2005;26(Suppl 49):1723. [Google Scholar]
- Erin EM, Englestätter R, Hellwig M, Hansel TT, Barnes PJ. Ciclesonide rapidly reduces airway responsiveness to adenosine monophosphate and deceases exhaled breath nitric oxide in patients with asthma. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:D99; Poster: 916.
- Erin EM, Hansel TT, Barnes PJ, Schutze J, Hellwig M, Engelstätter R. Ciclesonide rapidly decreases airway responsiveness to adenosine monophosphate, and reduces exhaled nitric oxide in patients with asthma. XIX World Allergy Organization Congress; Munich, Germany. 2005:Abstract 295.
Gauvreau 2005 {published data only}
- Gauvreau GM, Boulet LP, Postma DS, Kawayama T, Watson RM, Duong M, et al. Effect of low‐dose ciclesonide on allergen‐induced responses in subjects with mild allergic asthma. Journal of Allergy & Clinical Immunology 2005;116(2):285‐91. [DOI] [PubMed] [Google Scholar]
- Gauvreau GM, Watson RM, Postma DS, Monchy GR, Deschesnes F, Boulet L. Effect of ciclesonide 40 µg and 80 µg on early and late asthmatic reactions, and sputum eosinophils after allergen challenge in patients with mild asthma. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S210. [Google Scholar]
- Kawayama T, Gauveau GM, Watson RM, Killian KJ, Duong M. Effects of inhaled ciclesonide on circulating Th1/Th2 cells in atopic asthmatics after allergen challenge. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B35; Poster: G1.
- O'Byrne PM, Postma DS, Smau L, Widmann A, Gauvreau GM, Monchy J, et al. Once daily ciclesonide reduces allergen‐induced early and late asthmatic reaction, and sputum eosinophils in patients with mild asthma. XIX World Allergy Organization Congress; Munich, Germany 2005:Abstract No. 296. [Google Scholar]
Kanniess 2001 {published data only}
- Kanniess F, Richter K, Bohme S, Jorres RA, Magnussen H. Effect of inhaled ciclesonide on airway responsiveness to inhaled AMP, the composition of induced sputum and exhaled nitric oxide in patients with mild asthma. Pulmonary Pharmacology & Therapeutics 2001;14(2):141‐7. [DOI] [PubMed] [Google Scholar]
Larsen 2003 {published data only}
- Larsen BB, Nielsen LP, Engelstatter R, Steinijans V, Dahl R. Effect of ciclesonide on allergen challenge in subjects with bronchial asthma. Allergy 2003;58(3):207‐12. [DOI] [PubMed] [Google Scholar]
Lee 2004 {published data only}
- Fardon TC, Lee DK, Gray RD, Currie GP, Haggart K, Bates CE, Lipworth BJ. Effects of hydrofluoroalkane formulations of ciclesonide 320µg once daily versus fluticasone propionate 220µg twice daily on methacholine hyperresponsiveness in mild‐to‐moderate persistent asthma. Journal of Allergy and Clinical Immunology 2004;113(2 Suppl):S119. [Google Scholar]
- Lee DK, Haggart K, Currie GP, Bates CE, Lipworth BJ. Effects of hydrofluoroalkane formulations of ciclesonide 400 microg once daily versus fluticasone 250 microg twice daily on methacholine hyper‐responsiveness in mild‐to‐moderate persistent asthma. British Journal of Clinical Pharmacology 2004;58(1):26‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2005 {published data only}
- Lee DK, Fardon TC, Bates CE, Haggart K, McFarlane LC, Lipworth BJ. Airway and systemic effects of hydrofluoroalkane formulations of high‐dose ciclesonide and fluticasone in moderate persistent asthma. Chest 2005;127(3):851‐60. [DOI] [PubMed] [Google Scholar]
- Lee DKC, Lipworth BJ. Relative airway and systemic effects of high dose ciclesonide and fluticasone propionate in asthma. Thorax 2004;59(Suppl II):ii11. [Google Scholar]
Postma 2001 {published data only}
- Postma DS, Schlosser N, Sevette C, Martinat Y, Aumann J, Kafe H. Morning and evening administration of inhaled ciclesonide is equally effective in treatment of asthma. European Respiratory Society Annual Congress. 2002:P1913.
- Postma DS, Sevette C, Martinat Y, Schlosser N, Aumann J, Kafe H. Treatment of asthma by the inhaled corticosteroid ciclesonide given either in the morning or evening. European Respiratory Journal 2001;17(6):1083‐8. [DOI] [PubMed] [Google Scholar]
Richter 2005 {published data only}
- Richter K, Kanniess F, Biberger C, Nave R, Magnussen H. Comparison of the oropharyngeal deposition of inhaled ciclesonide and fluticasone propionate in patients with asthma. Journal of Clinical Pharmacology 2005;45(2):146‐52. [DOI] [PubMed] [Google Scholar]
Subbarao 2006 {published data only}
- Duong M, Subbarao P, Otis J, Adelroth E, Inman M, Pedersen S, et al. Relationship between exercise‐induced bronchoconstriction and sputum eosinophillia during ciclesonide treatment in asthmatics. American Thoracic Society 2005 International Conference; May 20‐25; San Diego, California. 2005:B35; Poster: G11.
- Subbarao P, Duong M, Adelroth E, Otis J, Obminski G, Inman M, et al. Effect of ciclesonide dose and duration of therapy on exercise‐induced bronchoconstriction in patients with asthma. Journal of Allercy & Clinical Immunology 2006;117(5):1008‐13. [DOI] [PubMed] [Google Scholar]
- Subbarao P, Duong M, Otis J, Obminski G, Inman M, Alderoith E, et al. Ciclesonide attenuates exercise‐induced bronchoconstriction over time in asthmatic subjects. American Thoracic Society 2005 International conference; May 20‐25; San Diego, California. 2005:B35; Poster: G10.
- Subbarao P, Duong M, Pedersen S, Eckert J, Hellbardt S, Engelstatter R, et al. Once daily ciclesonide protects against exercise‐induced bronchoconstriction. XIX World Allergy Organization Congress; Munich, Germany. 2005:Abstract 298.
- Subbarao PJ, Duong M, Otis J, Obminski G, Adelroth E, Pedersen S, et al. Ciclesonide is effective in protecting against exercise‐induced bronchoconstriction. Journal of Allergy & Clinical Immunology 2005;115(2 Suppl):S1. [DOI] [PubMed] [Google Scholar]
Szefler 2005 {published data only}
- Szefler S, Rohatagi S, Williams J, Lloyd M, Kundu S, et al. Ciclesonide, a novel inhaled steroid, does not affect hypothalamic‐pituitary‐adrenal axis function in patients with moderate‐to‐severe persistent asthma. Chest 2005;128(3):1104‐14. [DOI] [PubMed] [Google Scholar]
- Szefler SJ, Herron J, Lloyd M, Rohatagi S, Williams JE, Kundu S, et al. High doses of the novel inhaled steroid ciclesonide have no effect on HPA‐axis function in patients with moderate to severe persistent asthma. Journal of Allergy and Clinical Immunology 2003;111(2 Suppl):S216. [Google Scholar]
Taylor 1999 {published data only}
- Jensen MW, Taylor DA, Englestätter R, Kanabar V, O'Connor BJ. A novel inhaled steroid, ciclesonide, reduces airway responsiveness to AMP in a dose‐related manner. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A406. [DOI] [PubMed] [Google Scholar]
- Taylor DA, Jensen MW, Kanabar V, Engelstätter R, Steinijans VW, Barnes PJ, et al. A dose‐dependent effect of the novel inhaled corticosteroid ciclesonide on airway responsiveness to adenosine‐5'‐monophosphate in asthmatic patients. American Journal of Respiratory & Critical Care Medicine 1999;160(1):237‐43. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Arshad {published data only}
- An assessment of safety and efficacy in treating moderate to severe asthmatics with inhaled Ciclesonide vs Fluticasone. Ongoing study Starting date of trial not provided. Contact author for more information.
Beck {published data only}
- Efficacy and safety of ciclesonide administered with or without different spacers in patients with asthma (12 to 75 y). Clinicaltrials.Gov. 2005. Ongoing study Starting date of trial not provided. Contact author for more information.
Beckman {published data only}
- Efficacy of ciclesonide inhaled once daily versus other corticosteroids used for treatment of mild asthma in children (4‐11yrs). Clinicaltrials.Gov. 2005. Ongoing study Starting date of trial not provided. Contact author for more information.
Colatruglio {published data only}
- Effect of ciclesonide on quality of life in patients with moderate persistent asthma (21 to 65 y). Clinicaltrials.Gov. 2005. Ongoing study Starting date of trial not provided. Contact author for more information.
Dahl {published data only}
- Efficacy of ciclesonide versus fluticasone propionate in patients with mild to moderate asthma (12 to 75 y). Clinicaltrials.Gov. 2005. Ongoing study Starting date of trial not provided. Contact author for more information.
Derom {published data only}
- Effect of inhaled ciclesonide versus fluticasone propionate in patients with mild to moderate asthma (18 to 65 y). Clinicaltrials.Gov. 2005. Ongoing study Starting date of trial not provided. Contact author for more information.
Dusser {published data only}
- Efficacy of ciclesonide and fluticasone propionate in adult patients with moderate and severe persistent asthma (18 to 75 y). Clinicaltrials.Gov. 2005. Ongoing study Starting date of trial not provided. Contact author for more information.
Engelstätter {published data only}
- Efficacy of ciclesonide inhaled once daily versus fluticasone propionate inhaled twice daily in children with asthma (4 to 15 y). Clinicaltrials.Gov. 2005. Ongoing study Starting date of trial not provided. Contact author for more information.
Giwa {published data only}
- Comparison of inhaled ciclesonide and fluticasone proprionate in moderate to severe asthma patients, well controlled under high doses of inhaled corticosteroids. Ongoing study Starting date of trial not provided. Contact author for more information.
Hansel {published data only}
- A 3‐period double‐blind, cross‐over study on the onset of action of inhaled ciclesonide on airway responsiveness to adenosine monophosphate, sputum eosinophils and exhaled breath NO in patients with asthma. Ongoing study Starting date of trial not provided. Contact author for more information.
O'Byrne {published data only}
- Efficacy of ciclesonide vs fixed combination of fluticasone propionate/salmeterol vs placebo in patients with mild persistent asthma (12 to 75 y). Clinicaltrials.Gov. 2005.. Ongoing study Starting date of trial not provided. Contact author for more information.
Park {published data only}
- Effectiveness of ciclesonide versus budesonide in patients with asthma (18 to 75 y). Clinicaltrials.Gov. 2005. Ongoing study Starting date of trial not provided. Contact author for more information.
Sanofi Aventis {published data only}
- Effects of ciclesonide and beclomethasone on lens opacification in adult subjects with moderate to severe persistent asthma. Clinicaltrials.Gov . 2005; Efficacy of ciclesonide vs. placebo administered as once daily or twice daily in patients not treated with inhaled corticosteroid. Clinicaltrials.Gov. 2005; Effects of ciclesonide MDI 50mg/day and 200mg/day (ex‐value) once‐daily on growth in children with mild persistent asthma. Clinicaltrials.Gov. 2005; Sanofi‐Aventis. Dose response study of inhaled ciclesonide (glucocorticosteroid) to patients with persistent asthma. Clinicaltrials.Gov. 2005.. Ongoing study Starting date of trial not provided. Contact author for more information.
Stenton {published data only}
- A double‐blind randomised parallel group study comparing the efficacy and safety of 800 and 1000mcg CIC/day in patients with asthma followed by an open long‐term study to assess the safety of CIC in patients with asthma. Ongoing study Starting date of trial not provided. Contact author for more information.
Additional references
Adams 1999
- Adams N, Bestall J, Jones PW. Budesonide versus placebo for chronic asthma in children and adults. Cochrane Database of Systematic Reviews 1999, Issue 4. [Art. No.: CD003274] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 2000
- Adams N, Bestall J, Jones P. Budesonide at different doses for chronic asthma (Cochrane review). Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 2005a
- Adams NP, Bestall JB, Malouf R, Lasserson TJ, Jones PW. Beclomethasone versus placebo for chronic asthma. Cochrane Database of Systematic Reviews 2005, Issue 1. [Art. No.: CD002738. DOI: 10.1002/14651858] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 2005b
- Adams NP, Bestall JC, Lasserson TJ, Jones PW, Cates CJ. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2005, Issue 4. [Art. No.: CD003135. DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
Adams 2006
- Adams NP, Jones PW. The dose‐response characteristics of inhaled corticosteroids when used to treat asthma: An overview of Cochrane systematic reviews. Respiratory Medicine 2006;100(8):1297‐306. [DOI] [PubMed] [Google Scholar]
Adams 2007
- Adams N, Lasserson TJ, Cates CJ, Jones PW. Fluticasone versus beclomethasone or budesonide for chronic asthma in adults and children (Cochrane review). Cochrane Database of Systematic Reviews 2007, Issue 4. [Art No: CD002310] [DOI] [PMC free article] [PubMed] [Google Scholar]
BGAM 1997
- British Thoracic Society, National Asthma Campaign, Royal College of Physicians of London, et al. The British guidelines on asthma management: 1995 review and position statement. Thorax 1997;52(Suppl 1):S1‐21. [Google Scholar]
BTS/SIGN 2003
- British Thoracic Society. British Guideline on the Management of Asthma. Thorax 2003;58:Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buston 2000
- Buston KM, Wood SF. Non‐compliance amongst adolescents with asthma: listening to what they tell us about self‐management. Family Practice 2000;17:134‐8. [DOI] [PubMed] [Google Scholar]
Consensus 1999
- Canadian Asthma Concensus Group. Canadian asthma consensus report. CMAJ 1999;161(11 Suppl). [PMC free article] [PubMed] [Google Scholar]
Consensus 2005
- Summary of recommendations from the Canadian Asthma Consensus Guidelines, 2003 and Canadian Pediatric Asthma Consensus Guidelines, 2003 (updated to December 2004). Canadian Medical Association Journal 2005;173(Suppl):s1‐s156. [PMC free article] [PubMed] [Google Scholar]
Coutts 1992
- Coutts JA, Gibson NA, Paton JY. Measuring compliance with inhaled medication in asthma. Archives of Disease of Childhood 1992;67:332‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisen 1990
- Eisen SA, Miller DK, Woodward RS, Spitznagel E, Przybeck TR. The effect of prescribed daily dose frequency on patient medication compliance. Archives of Internal Medicine 1990;150:1881‐4. [PubMed] [Google Scholar]
Gerritsen 1989
- Gerritsen J, Koeter GH, Postma DS, Schouten JP, Krol K. Prognosis of asthma from childhood to adulthood. Amercian Review of Respiratory Disease 1989;140:1325‐30. [DOI] [PubMed] [Google Scholar]
GINA 1998
- National Institutes of Health (NIH), National Heart, Lung and Blood Institute. Global Strategy for Asthma Management and Prevention. NIH publication No. 96‐36598 (document available at: www.ginasthma.com) 1998.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Malo 1989
- Malo JL, Cartier A, Merland N, Ghezzo H, Burek A, Morris J, et al. Four‐times‐a‐day dosing frequency is better than twice‐a‐day regimen in subjects requiring a high‐dose inhaled steroid, budesonide, to control moderate to severe asthma. American Review of Respiratory Disease 1989;140:624‐8. [DOI] [PubMed] [Google Scholar]
Martin 1982
- Martin AJ, Landau LJ, Phelan PD. Asthma from childhood at age 21: the patient and his disease. British Medical Journal 1982;284:380‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mash 2001
- Mash B, Bheekie A, Jones PW. Inhaled versus oral steroids for adults with chronic asthma (Cochrane review). Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI] [PubMed] [Google Scholar]
Powell 2003
- Powell H, Gibson PG. High dose versus low dose inhaled corticosteroid as initial starting dose for asthma in adults and children (Cochrane review). Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suissa 2000
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low‐dose inhaled corticosteroids and the prevention of death from asthma. New England Journal of Medicine 2000;343:332‐6. [DOI] [PubMed] [Google Scholar]
Suissa 2002
- Suissa S, Ernst P, Kezoul A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation for asthma. Thorax 2002;57:880‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tattersfield 2002
- Tattersfield AE, Knox AS, Britton JR, Hall IP. Asthma. Lancet 2002;360:1313‐20. [DOI] [PubMed] [Google Scholar]
Toogood 1982
- Toogood JH, Baskerville JC, Jennings B, Lefcoe NM, Johannsson SA. Influence of dosing frequency and schedule on the response of chronic asthmatics to the aerosol steroid, budesonide. Journal Allergy and Clinical Immunology 1982;70:288‐98. [DOI] [PubMed] [Google Scholar]
Williams 1969
- Williams H, McNicol KN. Prevalence, natural history, and relationship of wheezy bronchitis and asthma in children. An epidemiological study. British Medical Journal 1969;4:321‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]


