Abstract
Background
Upper gastrointestinal bleeding is one of the most frequent causes of morbidity and mortality in the course of liver cirrhosis. Several treatments are used for upper gastrointestinal bleeding in people with liver diseases. One of them is vitamin K administration, but it is not known whether it benefits or harms people with acute or chronic liver disease and upper gastrointestinal bleeding. This is an update of this Cochrane review.
Objectives
To assess the beneficial and harmful effects of vitamin K for people with acute or chronic liver disease and upper gastrointestinal bleeding.
Search methods
We searched The Cochrane Hepato‐Biliary Controlled Trials Register (February 2015), the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 2 of 12, 2015), MEDLINE (Ovid SP) (1946 to February 2015), EMBASE (Ovid SP) (1974 to February 2015), Science Citation Index EXPANDED (1900 to February 2015), and LILACS (1982 to 25 February 2015). We sought additional randomised trials from two registries of clinical trials: the World Health Organization Clinical Trials Search Portal and the metaRegister of Controlled Trials. We looked through the reference lists of the retrieved publications and review articles.
Selection criteria
Randomised clinical trials irrespective of blinding, language, or publication status for assessment of benefits and harms. We considered observational studies for assessment of harms only.
Data collection and analysis
\We aimed to summarise data from randomised clinical trials using Standard Cochrane methodology and assess them according to the GRADE approach.
Main results
We found no randomised trials on vitamin K for upper gastrointestinal bleeding in people with liver diseases assessing benefits and harms of the intervention. We identified no quasi‐randomised studies, historically controlled studies, or observational studies assessing harms.
Authors' conclusions
This updated review found no randomised clinical trials of vitamin K for upper gastrointestinal bleeding in people with liver diseases. The benefits and harms of vitamin K need to be tested in randomised clinical trials. Until randomised clinical trials are conducted to assess the trade‐off between benefits and harms, we cannot recommend or refute the use of vitamin K for upper gastrointestinal bleeding in people with liver diseases.
Keywords: Humans, Acute Disease, Antifibrinolytic Agents, Antifibrinolytic Agents/therapeutic use, Chronic Disease, Gastrointestinal Hemorrhage, Gastrointestinal Hemorrhage/drug therapy, Gastrointestinal Hemorrhage/etiology, Liver Diseases, Liver Diseases/complications, Vitamin K, Vitamin K/therapeutic use
Plain language summary
Vitamin K for upper gastrointestinal bleeding in people with acute or chronic liver diseases
Review question We reviewed vitamin K for upper gastrointestinal bleeding in people with acute liver disease (that is, loss of normal liver functions which occurs in days or weeks; most often, people do not have a pre‐existing liver disease) or chronic liver disease (that is, progressive destruction of normal liver functions, usually associated with fibrotic regeneration of the liver tissue).
Background Upper gastrointestinal bleeding (vomiting of blood) is one of the most frequent causes of morbidity and mortality in the course of liver disease. Vitamin K administration is used as a supplementary intervention, but it is unknown whether it benefits or harms people with acute or chronic liver disease and upper gastrointestinal bleeding.
Study characteristics We searched scientific databases for studies assessing the benefits and harms of vitamin K in people of any age with liver disease. This updated review found no randomised clinical trials (clinical studies where people are randomly put into one of two or more treatment groups) on the benefits or harms of vitamin K for upper gastrointestinal bleeding in people with acute or chronic liver diseases. The evidence is current to February 2015.
Key results There is no evidence to recommend or refute the use of vitamin K for upper gastrointestinal bleeding in people with liver diseases.
Quality of evidence We found no randomised clinical trials.
Background
Description of the condition
Upper gastrointestinal bleeding is the loss of blood caused by illnesses that affect the alimentary canal from the mouth to the duodenum. According to the volume and speed of blood loss, upper gastrointestinal bleeding can lead to chronic anaemia or acute, life‐threatening symptoms. Haematemesis (vomiting of blood) and melena (dark coloured, tarry stools containing digested blood) are the most common symptoms and signs of upper gastrointestinal bleeding (Green 2003). The annual incidence of upper gastrointestinal bleeding in France was 143 people per 100,000 (Czernichow 2000). The annual incidence of acute upper gastrointestinal bleeding in the UK was between 47 and 116 people per 100,000 (Dallal 2001). In the United States, from 1998 to 2006, the number of hospitalisations for upper gastrointestinal bleeding per 100,000 people decreased by 14% (Zhao 2008). Epidemiology and demographics of upper gastrointestinal bleeding (i.e., prevalence, incidence, and mortality) have been reviewed (Sostres 2011).
Upper gastrointestinal bleeding is one of the most frequent causes of morbidity and mortality in the course of liver cirrhosis (Protell 1981; Longstreth 1998; del Olmo 2000; Zaltman 2002). In people with advanced cirrhosis, acute upper gastrointestinal bleeding is the most serious and life‐threatening complication (Afessa 2000; van Leerdam 2008). The causes of acute upper gastrointestinal bleeding in people with cirrhosis are: 1. rupture of varices in the oesophagus, stomach, or duodenum; 2. reflux oesophagitis; 3. acute lesions in the mucosal lining of the stomach membrane lesion; 4. peptic ulceration; and 5. reduction in blood coagulation factors (Amitrano 2002; Green 2003).
Description of the intervention
Several primary treatments are used for upper gastrointestinal bleeding in people with liver diseases. These are directed against the source of bleeding, for example, vasoactive treatment or endoscopic therapy for bleeding varices (Ioannou 1999; D'Amico 2002). Supplementary interventions are also often used including administration of vitamin K, which participates in the control of the formation of coagulation factors II, VII, IX, and X and anticoagulant proteins C and S by the liver. In liver disease, there is impaired synthesis of these clotting factors, resulting in a prolonged prothrombin time (Blonski 2007; Pluta 2010; Kavanagh 2011; Tripodi 2011; Wicklund 2011). This lack of production of clotting factors is more likely to be associated with impaired hepatocyte function than with a deficiency in vitamin K. One study, designed to research thrombin generation for assessment of the function of blood coagulation in people with cirrhosis, showed that although the prothrombin time is prolonged in people with cirrhosis, this may not correspond to an actual coagulation impairment (Tripodi 2005). The proposed explanation is that, in liver disease, the reduction of procoagulant factors in people with cirrhosis is compensated for by the reduction of anticoagulant factors, thus leaving the coagulation balance unaltered (Tripodi 2005). Therefore, administration of vitamin K to people with liver disease may have only partial or no effect in improving prolonged prothrombin time.
Why it is important to do this review
While vitamin K is used for treating coagulopathies in people with upper gastrointestinal bleeding, there appears to be no specific studies supporting its use (Lucena 2002). Furthermore, vitamin K is associated with anaphylactoid reactions (Pereira 1998; Fiore 2001).
We identified no systematic reviews or meta‐analyses on vitamin K administration to people with upper gastrointestinal bleeding, apart from our own Cochrane Hepato‐Biliary systematic reviews (Martí‐Carvajal 2005; Marti‐Carvajal 2008; Martí‐Carvajal 2012). The present review presents an update of the most recent review published in 2012 (Martí‐Carvajal 2012).
Objectives
To assess the beneficial and harmful effects of vitamin K for people with acute or chronic liver disease and upper gastrointestinal bleeding.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised clinical trials (parallel group or cross‐over trials) irrespective of publication status (trials could be unpublished or published as an article, an abstract, or a letter), language, and blinding. For cross‐over trials, we planned to include only data from the first intervention period.
We intended to include quasi‐randomised studies, prospective observational studies, and observational studies with a historical control group in order to assess data on harms.
Types of participants
People with liver disease and upper gastrointestinal bleeding, irrespective of aetiology.
Types of interventions
Vitamin K at any dose, route of administration, and duration of treatment versus placebo or no intervention. Since upper gastrointestinal bleeding in people with liver disease requires different medical and endoscopic treatments (i.e., primary intervention), vitamin K is considered a supplementary intervention. Thus, for the purpose of the review, eligible randomised clinical trials had to compare the same primary interventions with and without vitamin K supplementation.
Types of outcome measures
Primary outcomes
Mortality.
Early re‐bleeding.
Serious adverse events. We used the International Conference on Harmonisation (ICH) Guidelines for Good Clinical Practice's definition of a serious adverse event (ICH‐GCP 1997), that is, any untoward medical occurrence that resulted in death, was life threatening, required hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability or incapacity, or was a congenital anomaly or birth defect. We considered all other adverse events as non‐serious.
Secondary outcomes
Quality of life.
Non‐serious adverse events.
Number of participants transfused.
Number of blood transfusions.
Coagulation factors II, VII, and X.
Length of hospital stay.
Length of intensive care unit stay.
We planned to report the first seven of the above primary and secondary outcomes in the 'Summary of findings' table.
Search methods for identification of studies
We searched The Cochrane Hepato‐Biliary Controlled Trials Register (Gluud 2015), the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Ovid SP), EMBASE (Ovid SP), Science Citation Index EXPANDED, and LILACS (Royle 2003) to February 2015. Appendix 1 shows the search strategies with the time spans of the searches.
We looked through the reference lists of the retrieved publications and review articles.
We also searched the following trial databases for ongoing and unpublished trials:
World Health Organization Clinical Trials Search Portal (apps.who.int/trialsearch/);
metaRegister of controlled trials (www.controlled‐trials.com/mrct/search.html).
In future updates, we will also search for study reports at regulatory agencies (US Food and Drug Administration and European Medicines Agency) and trials at www.clinicaltrials.gov.
Data collection and analysis
We planned to summarise data using standard Cochrane methodology (Higgins 2011a).
Selection of studies
AMC and IS screened the search results for potentially relevant trials and independently assessed them for inclusion or exclusion using a pre‐designed eligibility form based on the inclusion criteria. We resolved disagreements through discussion until consensus was reached.
Data extraction and management
Due to the lack of randomised clinical trials, we could not follow what is described below. However, if we identify trials in the future, we will follow our protocol as described (Marti‐Carvajal 2004). We will use a form to extract data from each relevant trial (Zavala 2006). Both review authors will independently extract data from the papers and will contact the authors if data are missing. AMC will enter the data into Review Manager 5 (RevMan 2014) and IS will check the data. We will extract information on study design and participant characteristics (age, sex, and disease severity as measured by the Child‐Pugh score).
Assessment of risk of bias in included studies
If we identify randomised clinical trials fulfilling the inclusion criteria of our review, we will independently assess the risk of bias of each included trial according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), The Cochrane Hepato‐Biliary Module (Gluud 2015), and methodological studies (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Lundh 2012; Savović 2012a; Savović 2012b). We will use the following definitions in the assessment of risk of bias.
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice were adequate if performed by an independent adjudicator.
Unclear risk of bias: the trial was described as randomised, but the method of sequence generation was not specified.
High risk of bias: the sequence generation method was not, or may not have been, random. Quasi‐randomised studies, those using dates, names, or admittance numbers in order to allocate participants, were inadequate and were excluded for the assessment of benefits but included for assessing harms.
Allocation concealment
Low risk of bias: allocation was controlled by a central and independent randomisation unit, sequentially numbered, opaque and sealed envelopes or similar, so that intervention allocations could not have been foreseen in advance of, or during, enrolment.
Unclear risk of bias: the trial was described as randomised, but the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: the allocation sequence was known to the investigators who assigned participants, or the study was quasi‐randomised.
Blinding of participants and personnel
Low risk of bias: it was mentioned that both participants and personnel providing the interventions were blinded, and the method of blinding was described, so that knowledge of allocation was prevented during the trial.
Unclear risk of bias: it was not mentioned if the trial was blinded, or the trial was described as blinded, but the method or extent of blinding was not described, so that knowledge of allocation was possible during the trial.
High risk of bias: the trial was not blinded, so that the allocation was known during the trial.
Blinded outcome assessment
Low risk of bias: outcome assessment was carried out blinded for all relevant outcomes, and the method of blinding was described, so that knowledge of allocation was prevented.
Unclear risk of bias: blinding of outcome assessment was not described, or the outcome assessment was described as blinded, but the method of blinding was not described, so that knowledge of allocation was possible.
High risk of bias: outcome assessment was not blinded, so that the allocation was known to outcome assessors.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, were employed to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: the trial reported the following pre‐defined outcomes: mortality, early re‐bleeding, and serious adverse events. If the original trial protocol was available, the outcomes should be those called for in that protocol. If the trial protocol was obtained from a trial registry (e.g., www.clinicaltrials.gov), the outcomes sought should be those enumerated in the original protocol if the trial protocol was registered before or at the time that the trial was begun. If the trial protocol was registered after the trial was begun, those outcomes were not considered to be reliable.
Unclear risk of bias: not all pre‐defined outcomes were reported fully, or it was unclear whether data on these outcomes were recorded or not.
High risk of bias: one or more pre‐defined outcomes were not reported.
For‐profit bias
Low risk of bias: the trial appeared to be free of industry sponsorship or other type of for‐profit support that may manipulate the trial design, conductance, or results of the trial.
Unclear risk of bias: the trial may or may not be free of for‐profit bias as no information on clinical trial support or sponsorship was provided.
High risk of bias: the trial was sponsored by industry or received other type of for‐profit support.
Other bias
Low risk of bias: the trial appeared to be free of other bias domains that could put it at risk of bias.
Unclear risk of bias: the trial may or may not have been free of other domains that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias.
We will judge trials at low risk of bias if assessed with low risk of bias in all above domains. We will judge trials at high risk of bias if assessed with unclear risk of bias or high risk of bias in one or more of the above domains.
We will generate a 'Risk of bias' graph and a 'Risk of bias' summary to show a summary of this assessment.
Measures of treatment effect
For binary outcomes, such as mortality, early re‐bleeding and safety, we planned to calculate the risk ratio (RR) with 95% confidence interval (CI). For continuous outcomes, such as number of people transfused and number of blood transfusions, we planned to calculate the mean difference (MD) with 95% CI.
Dealing with missing data
In the case of missing data on participants or missing statistics (such as standard deviations), we intended to contact the trial authors. If unsuccessful, we planned to base our main analysis on participants who had completed the trial, but we planned to perform sensitivity analysis for worse‐case and best‐case scenarios according to the Cochrane Handbook for Systematic Reviews of Interventions Section 16.1 (Higgins 2011b).
Assessment of heterogeneity
We planned to quantify the impact of statistical heterogeneity using the I2 statistic, which describes the percentage of total variation across trials that is due to heterogeneity rather than sampling error (Higgins 2003). If the identified trials were comparable enough, we intended to summarise their findings using a random‐effects model. In the case of substantial heterogeneity (I2 > 50%), we planned to do further research to identify possible causes of heterogeneity by exploring the impact of participants' characteristics.
Assessment of reporting biases
We intended to assess whether the review was subjected to publication bias by using a funnel plot that is usually used to illustrate variability between trials in a graphical way. We needed at least 10 trials in order to be able to make judgements about asymmetry, and if asymmetry was present, we planned to attempt to explore other causes for it (Sterne 2011).
Data synthesis
We intended to carry out statistical analysis using Review Manager 5 software (RevMan 2014). If the eligible trials were sufficiently comparable in their clinical characteristics, we planned to summarise their findings using the fixed‐effect model and the random‐effects model according to the Cochrane Handbook for Systematic Reviews of Interventions Section 9.4 (Deeks 2011). In the case of statistically significant differences between the two models, we intended to report both. Otherwise, we planned to report the results of the model having the most conservative findings (Jakobsen 2014).
Trial sequential analysis
A meta‐analysis of cumulative data may run the risk of random errors ('play of chance') due to sparse data and repetitive analyses of the same data (Brok 2008; Wetterslev 2008; Brok 2009; Wetterslev 2009; Thorlund 2010; Thorlund 2011). In order to assess the risks of random errors in cumulative meta‐analyses, we planned to conduct diversity‐adjusted trial sequential analyses (Thorlund 2011; TSA 2011) based on a required information size calculated from the proportion of people with the outcome in the control group; an a priori set relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and the diversity in the meta‐analysis (Thorlund 2009; Thorlund 2011; TSA 2011). We planned to conduct sensitivity analyses of the trial sequential analysis to estimate the potential need for further trials.
Subgroup analysis and investigation of heterogeneity
We anticipated clinical heterogeneity in the effects of the intervention, and we planned to conduct the following subgroup analyses when data become available:
trials with low risk of bias (or with lower risk of bias) compared to trials with high risk of bias;
participants with cirrhosis compared to participants without cirrhosis;
Child‐Pugh classification A compared to classification B and compared to classification C.
Sensitivity analysis
We planned to conduct sensitivity analyses including only randomised trials with low risk of bias, and perform best‐case and worst‐case scenario analyses if there are missing participants.
We intended to seek data on the number of participants randomised to the intervention and control groups, irrespective of compliance and whether or not the participant was later thought to be ineligible or was otherwise excluded from the treatment group or follow‐up. If we were unable to do so, we planned to record whether the results of each trial were based on an intention‐to‐treat analysis or on available participant analysis.
'Summary of findings' tables
We intended to use the principles of the GRADE system (Guyatt 2011a) in order to assess the quality of the body of evidence associated with specific outcomes (mortality, failure to control bleeding, number of participants receiving a blood transfusion, number of blood transfusions, and adverse events) in our review and construct a 'Summary of findings' table using the GRADEPro software (ims.cochrane.org/revman/other‐resources/gradepro). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias, the indirectness of the evidence, heterogeneity of the data, imprecision of effect estimates, and risk of publication bias (Balshem 2011; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2011h; Guyatt 2011i; Guyatt 2013a; Guyatt 2013b). We planned to assess the imprecision according to Jakobsen 2014.
Results
Description of studies
The initial bibliographic search identified 462 studies. After manually checking the titles and abstracts, we identified one study to be evaluated further. We excluded the study by Pereira 2005 as it assessed the pharmacokinetics of vitamin K in people with liver diseases. We determined that none of the studies attempted to answer the research questions of this systematic review. See Figure 1.
1.
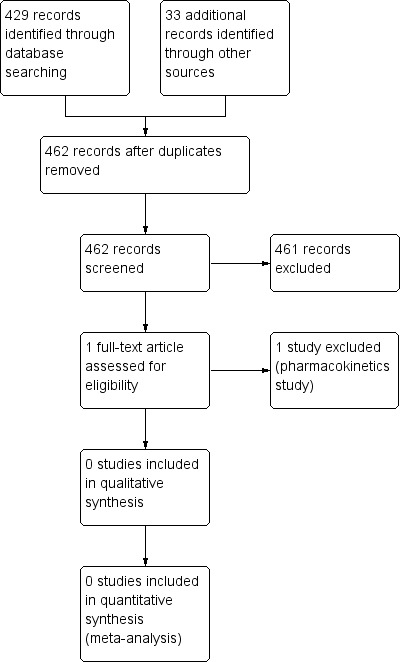
Study flow diagram (25 February 2015).
Risk of bias in included studies
We included no studies.
Effects of interventions
The searches did not identify any randomised clinical trials eligible for inclusion in this systematic review. We identified no ongoing trials.
We found no quasi‐randomised studies, historically controlled studies, or cohort studies that we could use to assess harms from vitamin K.
Discussion
Summary of main results
We were unable to identify evidence from randomised clinical trials supporting or refuting the use of vitamin K for upper gastrointestinal bleeding in people with acute or chronic liver disease associated with acquired coagulation disorders.
Overall completeness and applicability of evidence
In one multicentre hospital study on prescribing patterns for prophylaxis and treatment of complications on cirrhosis, Lucena et al. showed that at discharge, vitamin K was utilised in 24% (range 4% to 53%) of the trial participants (Lucena 2002). Lucena et al. also observed that there was a wide variability in prescribing practices across centres, and that the use of non‐evidence based treatments such as vitamin K was higher than expected (Lucena 2002). In contrast to these observed practices, other studies have shown that there is a risk of anaphylactoid reactions associated with the use of vitamin K (Pereira 1998; ADRAC 2000; Fiore 2001).
It is widely known that clinicians make practical decisions, often on the basis of inadequate information. Decisions on treatment should be taken based on the results of randomised clinical trials (Alderson 2004; Chalmers 2004).
The basis for the rational use of vitamin K in people with liver disease with upper gastrointestinal bleeding remains unknown. Randomised clinical trials to answer the research question of this systematic review have not yet been carried out. It is possible that some studies have been carried out, but due to their negative results, they remained unpublished (Easterbrook 1991; Gluud 1998). This causes publication bias, reducing the possibilities to develop valid systematic reviews in certain areas. The existence of high‐quality data sufficient to make strong recommendations for an intervention varies considerably across specialities (Diringer 2003). This is influenced by a number of factors, from the prevalence of the conditions to the resources available to do research. Keeping clinical uncertainty does not benefit people and may increase health‐service costs (Alderson 2000). However, admitting clinical uncertainty helps clarify treatment options and encourages further research (Alderson 2000; Diringer 2003). The paucity of data on vitamin K for people with upper gastrointestinal bleeding in liver disease should stimulate the development of well‐planned randomised clinical trials to try to give a response to this widespread practice for upper gastrointestinal bleeding in people with liver disease.
Quality of the evidence
We were unable to identify evidence from randomised clinical trials supporting the use of vitamin K for upper gastrointestinal bleeding in people with acute or chronic liver disease associated with acquired coagulation disorders.
Potential biases in the review process
In the process of performing this systematic review, we also planned to look for 'significance‐chasing biases' (Ioannidis 2010), which include, among others, publication bias, selective outcome reporting bias, selective analysis reporting bias, and fabrication bias. Publication bias represents a major threat to the validity of systematic reviews, particularly in reviews that include small trials. However, this Cochrane review contains no trials and as such, we did not evaluate risk of bias. Trials with 'negative' results may have remained unpublished as suppression of information on specific outcomes may have occurred (Ioannidis 2010). We were unable to identify evidence from randomised clinical trials supporting the use of vitamin K for upper gastrointestinal bleeding in people with acute or chronic liver disease associated with acquired coagulation disorders.
Agreements and disagreements with other studies or reviews
We were unable to identify evidence from randomised clinical trials supporting or refuting the use of vitamin K for upper gastrointestinal bleeding in people with acute or chronic liver disease associated with acquired coagulation disorders.
Authors' conclusions
Implications for practice.
We found no randomised clinical trials of vitamin K for upper gastrointestinal bleeding for inclusion in this review. Therefore, it was not possible to determine whether vitamin K is beneficial or harmful for upper gastrointestinal bleeding in people with liver diseases. Accordingly, we cannot recommend or refute vitamin K for upper gastrointestinal bleeding in people with acute and chronic liver disease.
Implications for research.
This systematic review has identified the need for well‐designed, adequately powered randomised clinical trials to assess the benefits and harms of vitamin K as a way of improving the survival and decreasing mortality from upper gastrointestinal bleeding in people with liver diseases. Potential trials should be planned according to SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) statement (Chan 2013a; Chan 2013b). The trials should be reported according to the CONSORT (CONsolidated Standards Of Reporting Trials) statement (Moher 2010), which helps in improving the quality of reporting of benefits and harms in clinical research (Ioannidis 2004; Moher 2010). Trials should include participant‐centred outcomes such as mortality, re‐bleeding, and serious and non‐serious adverse events as recommended by the Patient‐Centered Outcomes Research Institute (P‐CORI) statement (Selby 2013; Frank 2014; Selby 2014).
What's new
| Date | Event | Description |
|---|---|---|
| 17 March 2015 | New citation required but conclusions have not changed | No change in conclusion. Randomised clinical trials are still lacking. |
| 25 February 2015 | New search has been performed | Searches performed in February 2015; however, there were still no trials on the subject of our review. |
Notes
This review will not be updated until relevant for the review randomised clinical trials are published.
Acknowledgements
We wish to thank Sarah Louise Klingenberg of The Cochrane Hepato‐Biliary for conducting the searches and The Cochrane Hepato‐Biliary Editorial Team staff for guidance. Contact editors: Brian Davidson, UK; Christian Gluud, Denmark.
Appendices
Appendix 1. Search strategies
| Name of database | Time span of search | Search strategy |
| The Cochrane Hepato‐Biliary Controlled Trials Register | February 2015. | (vitamin k OR phylloquinon* OR menaquinon* OR menadion*) AND liver disease* |
| Cochrane Central Register of Controlled Trials (CENTRAL) | Issue 2 of 12, 2015. | #1 MeSH descriptor: [Vitamin K] explode all trees #2 (vitamin* next K) or phylloquinon* or menaquinon* or menadion* #3 #1 or #2 #4 MeSH descriptor: [Gastrointestinal Hemorrhage] explode all trees #5 bleed* or h*emorrhage* #6 #4 or #5 #7 MeSH descriptor: [Liver Diseases] explode all trees #8 liver disease* #9 #7 or #8 #10 #3 and #6 and #9 |
| MEDLINE (Ovid SP) | 1946 to February 2015. | 1. exp Vitamin K/ 2. ('vitamin K' or phylloquinon* or menaquinon* or menadion*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 3. 1 or 2 4. exp Gastrointestinal Hemorrhage/ 5. (bleed* or h*emorrhage*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 6. 4 or 5 7. exp Liver Diseases/ 8. liver disease*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 9. 7 or 8 10. 3 and 6 and 9 11. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 12. 10 and 11 |
| EMBASE (Ovid SP) | 1974 to February 2015. | 1. exp vitamin K group/ 2. ('vitamin K' or phylloquinon* or menaquinon* or menadion*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 3. 1 or 2 4. exp gastrointestinal hemorrhage/ 5. (bleed* or h*emorrhage*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 6. 4 or 5 7. exp liver disease/ 8. liver disease*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 9. 7 or 8 10. 3 and 6 and 9 11. (random* or blind* or placebo* or meta‐analys*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 12. 10 and 11 |
| Science Citation Index EXPANDED | 1900 to February 2015. | #6 #5 AND #4 #5 TS=(random* or blind* or placebo* or meta‐analys*) #4 #3 AND #2 AND #1 #3 TS="liver disease*" #2 TS=(bleed* or h*emorrhage*) #1 TS=("vitamin K" or phylloquinon* or menaquinon* or menadion*) |
| LILACS | 1982 to February 2015. | (1) Vitamin K (2) liver diseases (3) 1+2 (4) ((Pt RANDOMIZED CONTROLLED TRIAL OR Pt CONTROLLED CLINICAL TRIAL OR Mh RANDOMIZED CONTROLLED TRIALS OR Mh RANDOM ALLOCATION OR Mh DOUBLE‐BLIND METHOD OR Mh SINGLE‐BLIND METHOD OR Pt MULTICENTER STUDY) OR ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT ((CT ANIMALS OR MH ANIMALS OR CT RABBITS OR CT MICE OR MH RATS OR MH PRIMATES OR MH DOGS OR MH RABBITS OR MH SWINE) AND NOT (CT HUMAN AND CT ANIMALS)) (5) 3 + 4 |
| World Health Organization Clinical Trials Search Portal | 25 February 2015. | Upper gastrointestinal bleeding AND liver disease AND vitamin K |
| metaRegister of Controlled Trials (mRCT) | 25 February 2015. | liver disease AND vitamin K |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Pereira 2005 | A pharmacokinetics randomised trial on vitamin K in people with liver diseases. |
Differences between protocol and review
We re‐arranged the outcomes in terms of what the most relevant outcomes are for the people with liver disease.
We planned 'Summary of findings' tables and the outcomes reported within them and will apply these when we identify trials for inclusion.
We planned trial sequential analysis and will apply this when we identify trials for inclusion.
Contributions of authors
AMC took the lead on writing up the protocol based on the draft versions, with comments from IS (Marti‐Carvajal 2004).
Further contribution to the review:
searching: The Cochrane Hepato‐Biliary Controlled Trials Register;
selection of trials for inclusion: AMC, IS;
methodological assessment and extraction of data: AMC, IS;
data entry and management: AMC;
data analysis: AMC and IS;
preparation of the review: AMC, IS.
AMC is guarantor for the review. Both authors approved the final review manuscript for publication.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Centro Cochrane Iberoamericano, Spain.
Academic.
-
Cochrane Hepato Biliary, Denmark.
Academic.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies excluded from this review
Pereira 2005 {published data only}
Additional references
ADRAC 2000
- Adverse Drug Reactions Advisory Committee (ADRAC). Take care with vitamin K. Australian Adverse Drug Reactions Bulletin 2000;19(1):2. [Google Scholar]
Afessa 2000
- Afessa B, Kubilis PS. Upper gastrointestinal bleeding in patients with hepatic cirrhosis: clinical course and mortality prediction. American Journal of Gastroenterology 2000;95:484‐9. [DOI] [PubMed] [Google Scholar]
Alderson 2000
- Alderson P, Roberts I. Should journals publish systematic reviews that find no evidence to guide practice? Examples from injury research. BMJ (Clinical Research Ed.) 2000;320:376‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alderson 2004
- Alderson P. Absence of evidence is not evidence of absence. BMJ (Clinical Research Ed.) 2004;328:476‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amitrano 2002
- Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Seminars in Liver Disease 2002;22:83‐96. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Blonski 2007
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Chalmers 2004
- Chalmers I. Well informed uncertainties about the effects of treatments. How clinicians and patients respond?. BMJ (Clinical Research Ed.) 2004;328:475‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2013a
- Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ (Clinical Research Ed.) 2013;346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2013b
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza‐Jeric K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158(3):200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Czernichow 2000
- Czernichow P, Hochain P, Nousbaum JB, Raymond JM, Rudelli A, Dupas JL, et al. Epidemiology and course of acute upper gastro‐intestinal haemorrhage in four French geographical areas. European Journal of Gastroenterology & Hepatology 2000;12:175‐81. [DOI] [PubMed] [Google Scholar]
D'Amico 2002
- D'Amico G, Pagliaro LLP, Pietrosi GGPI, Tarantino IITA. Emergency sclerotherapy versus medical interventions for bleeding oesophageal varices in cirrhotic patients. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD002233] [DOI] [PubMed] [Google Scholar]
Dallal 2001
- Dallal HJ, Palmer KR. ABC of the upper gastrointestinal tract: upper gastrointestinal haemorrhage. BMJ (Clinical Research Ed.) 2001;323:1115‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
del Olmo 2000
- Olmo JA, Pena A, Serra MA, Wassel AH, Benages A, Rodrigo JM. Predictors of morbidity and mortality after the first episode of upper gastrointestinal bleeding in liver cirrhosis. Journal of Hepatology 2000;32:19‐24. [DOI] [PubMed] [Google Scholar]
Diringer 2003
- Diringer MN. Evidence‐based medicine: what do you do when there's no evidence?. Critical Care Medicine 2003;31(2):659‐60. [DOI] [PubMed] [Google Scholar]
Easterbrook 1991
- Easterbrook PJ, Berlin J, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet 1991;337:867‐72. [DOI] [PubMed] [Google Scholar]
Fiore 2001
- Fiore LD, Scola MA, Cantillon CE, Brophy MT. Anaphylactoid reactions to vitamin K. Journal of Thrombosis and Thrombolysis 2001;11:175‐83. [DOI] [PubMed] [Google Scholar]
Frank 2014
- Frank L, Basch E, Selby JV. The PCORI perspective on patient‐centered outcomes research. JAMA 2014;312(15):1513‐4. [DOI] [PubMed] [Google Scholar]
Gluud 1998
- Gluud C. "Negative trials" are positive!. Journal of Hepatology 1998;28(4):731‐3. [DOI] [PubMed] [Google Scholar]
Gluud 2015
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2015, Issue 1. Art. No.: LIVER.
Green 2003
- Green BT, Rockey DC. Acute gastrointestinal bleeding. Seminars in Gastrointestinal Disease 2003;14:44‐65. [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380‐2. [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines: 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [DOI] [PubMed] [Google Scholar]
Guyatt 2011h
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [DOI] [PubMed] [Google Scholar]
Guyatt 2011i
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
Guyatt 2013b
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ (Clinical Research Ed.) 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Pennsylvania: Barnett International/PAREXEL, 1997. [Google Scholar]
Ioannidis 2004
- Ioannidis JP, Evans SJ, Gotzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. [DOI] [PubMed] [Google Scholar]
Ioannidis 2010
- Ioannidis JP. The art of getting it wrong. Research Synthesis Methods 2010;1(3‐4):169‐84. [DOI] [PubMed] [Google Scholar]
Ioannou 1999
- Ioannou G, Doust J, Rockey DC. Terlipressin for acute esophageal variceal hemorrhage. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002147] [DOI] [PubMed] [Google Scholar]
Jakobsen 2014
- Jakobsen JC, Gluud C, Winkel P, Lange T, Wetterslev J. The thresholds for statistical and clinical significance ‐ a five‐step procedure for evaluation of intervention effects in randomised clinical trials. BMC Medical Research Methodology 2014;14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2011
- Kavanagh C, Shaw S, Webster CR. Coagulation in hepatobiliary disease. Journal of Veterinary Emergency and Critical Care 2011;21(6):589‐604. [PUBMED: 22316251] [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Longstreth 1998
- Longstreth GF, Feitelberg SP. Successful outpatient management of acute upper gastrointestinal hemorrhage: use of practice guidelines in a large patient series. Gastrointestinal Endoscopy 1998;47:219‐22. [DOI] [PubMed] [Google Scholar]
Lucena 2002
- Lucena MI, Andrade RJ, Tognoni G, Hidalgo R, Sánchez de la Cuesta F, the Spanish Collaborative Study Group on Therapeutic Management in Liver Disease. Multicenter hospital study on prescribing patterns for prophylaxis and treatment of complications of cirrhosis. European Journal of Clinical Pharmacology 2002;58:435‐40. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Moher 2010
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ (Clinical Research Ed.) 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pereira 1998
- Pereira SP, Williams R. Adverse events associated with vitamin K1: results of a worldwide postmarketing surveillance programme. Pharmacoepidemiology and Drug Safety 1998;7(3):173‐82. [DOI] [PubMed] [Google Scholar]
Pluta 2010
- Pluta A, Gutkowski K, Hartleb M. Coagulopathy in liver diseases. Advances in Medical Sciences 2010;55(1):16‐21. [PUBMED: 20513645] [DOI] [PubMed] [Google Scholar]
Protell 1981
- Protell RL, Silverstein FE, Gilbert DA, Feld AD. Severe upper gastrointestinal bleeding. Part I: causes, pathogenesis and methods of diagnosis. Clinics in Gastroenterology 1981;10:17‐26. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Selby 2013
- Selby JV. The patient‐centered outcomes research institute: a 2013 agenda for "research done differently". Population Health Management 2013;16(2):69‐70. [DOI] [PubMed] [Google Scholar]
Selby 2014
Sostres 2011
- Sostres C, Lanas A. Epidemiology and demographics of upper gastrointestinal bleeding: prevalence, incidence, and mortality. Gastrointestinal Endoscopy Clinics of North America 2011;21(4):567‐81. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ (Clinical Research Ed.) 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for trial sequential analysis (TSA), 2011. ctu.dk/tsa/files/tsa_manual.pdf (accessed 30 April 2012).
Tripodi 2005
- Tripodi A, Salerno F, Chantarangkul V, Clerici M, Cazzaniga M, Primignani M, et al. Evidence of normal thrombin generation in cirrhosis despite abnormal conventional coagulation tests. Hepatology 2005;41:553‐8. [DOI] [PubMed] [Google Scholar]
Tripodi 2011
- Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. New England Journal of Medicine 2011;365(2):147‐56. [DOI] [PubMed] [Google Scholar]
TSA 2011 [Computer program]
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
van Leerdam 2008
- Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Practice & Research. Clinical Gastroenterology 2008;22(2):209‐24. [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [PUBMED: 20042080] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wicklund 2011
- Wicklund BM. Bleeding and clotting disorders in pediatric liver disease. Hematology 2011;2011:170‐7. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaltman 2002
- Zaltman C, Souza HS, Castro ME, Sobral Mde F, Dias PC, Lemos V Jr. Upper gastrointestinal bleeding in a Brazilian hospital: a retrospective study of endoscopic records. Arquivos de Gastroenterologia 2002;39:74‐80. [DOI] [PubMed] [Google Scholar]
Zavala 2006 [Computer program]
- Venezuela: Universidad de Carabobo. Zavala D, Martí A, Peña‐Martí G, Comunián G. Sheet to enter data for performing a Cochrane review. Valencia: Venezuela: Universidad de Carabobo, 2006.
Zhao 2008
- Zhao Y, Encinosa W. Hospitalizations for gastrointestinal bleeding in 1998 and 2006 statistical brief #65, 2008. www.ncbi.nlm.nih.gov/books/NBK54562/ (accessed 26 March 2015).
References to other published versions of this review
Marti‐Carvajal 2004
- Martí‐Carvajal AJ, Martí‐Peña A. Vitamin K for upper gastrointestinal bleeding in patients with liver diseases. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD004792] [DOI] [PubMed] [Google Scholar]
Marti‐Carvajal 2008
- Marti‐Carvajal AJ, Cortes‐Jofre M, Marti‐Pena AJ. Vitamin K for upper gastrointestinal bleeding in patients with liver diseases. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD004792.pub3] [DOI] [PubMed] [Google Scholar]
Martí‐Carvajal 2005
- Martí‐Carvajal AJ, Martí‐Peña AJ. Vitamin K for upper gastrointestinal bleeding in patients with liver diseases. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD004792.pub2] [DOI] [PubMed] [Google Scholar]
Martí‐Carvajal 2012
- Martí‐Carvajal AJ, Solà I. Vitamin K for upper gastrointestinal bleeding in patients with acute or chronic liver diseases. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD004792.pub4] [DOI] [PubMed] [Google Scholar]


