Abstract
Background:
It is unknown whether carcinogenic and endocrine disrupting polychlorinated biphenyls (PCBs) influence mortality following breast cancer. We examined plasma levels of 17 PCB congeners in association with mortality among women with breast cancer.
Methods:
Participants included 456 white and 292 black women in North Carolina who were diagnosed with primary invasive breast cancer from 1993 to 1996, and who had PCB and lipid measurements from blood samples obtained an average of 4.1 months after diagnosis. Over a median follow-up of 20.6 years, there were 392 deaths (210 from breast cancer). We used Cox regression to estimate covariate-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for all-cause and breast cancer-specific 5-year mortality, and 20-year mortality (conditional on 5-year survival) in association with tertiles and continuous ln-transformed lipid-adjusted PCB levels.
Results:
The highest (vs. lowest) tertiles of PCB74, PCB99, and PCB118 were associated with 5-year breast cancer-specific mortality HRs of 1.46 (95%CI = 0.86–2.47), 1.57 (95%CI = 0.90–2.73), and 1.86 (95%CI = 1.07–3.23), respectively. Additionally, one-ln unit increases in PCB74, PCB99, PCB118, and total PCBs were each associated with 33–40% increases in 5-year breast cancer-specific mortality rates. The PCBs were not, however, associated with longer-term breast cancer-specific mortality. For all-cause mortality, one-ln unit increases in PCB118, PCB146, PCB153, PCB182, PCB187, and total PCBs were associated with 20–37% increases in 20-year all-cause mortality rates among women who survived at least 5 years.
Conclusion:
PCBs may increase the risk of short-term breast cancer-specific mortality and long-term all-cause mortality among women with breast cancer.
Keywords: Organochlorine compounds, PCBs, Breast cancer, Survival, Mortality
1. Introduction
Polychlorinated biphenyls (PCBs) are synthetic organic chemicals manufactured in the United States (US) for half a century from 1929 to 1979 (United States Environmental Protection Agency, 2019). There are 209 PCB congeners, which are sequentially-numbered and distinguished by their degree and pattern of chlorination (Agency for Toxic Substances and Disease Registry (ATSDR) 2015). Because PCBs are very stable, non-flammable, and resistant to extreme temperatures and pressures, they were used in hundreds of industrial and commercial applications including in electrical and hydraulic equipment and in paints and plastics (United States Environmental Protection Agency, 2019). Although no longer produced in the US, environmental contamination by PCBs continues due to poorly maintained hazardous waste sites, disposal of PCB-containing products into landfills, and releases from electrical transformers (United States Environmental Protection Agency, 2019). PCBs are also environmentally persistent and can be redistributed over long distances (Sinkkonen and Paasivirta, 2000), and because they are highly lipophilic and not easily degraded by metabolism, readily enter the food chain and are biomagnified (Agency for Toxic Substances and Disease Registry (ATSDR) 2015). While occupational studies (Chen and Luo, 1982; Wolff et al., 1992) and one study of Southeastern US women (Vo et al., 2008) documented declines in PCB levels after their ban, PCB levels are hypothesized to still be of concern because of their known health effects, including cancer.
PCBs are classified by the International Association for Research on Cancer (IARC) as carcinogenic to humans due to studies documenting excess risk of melanoma in occupational cohort studies (Lauby-Secretan et al., 2013). PCBs or their metabolites have been shown to induce genotoxic effects, immune suppression, inflammatory responses, and endocrine effects via a number of mechanisms (Agency for Toxic Substances and Disease Registry (ATSDR) 2015). Because PCBs are readily absorbed and distributed in the body and accumulate in adipose tissue, PCBs are hypothesized to influence breast cancer risk. A meta-analysis of 25 studies with 12,866 participants from eight countries reported increases in breast cancer risk among women with the highest levels of group II (potentially anti-estrogenic, immunotoxic, and dioxin-like) and group III (phenobarbital, CYP1A and CYP2B inducers), but not with group I, PCBs (Zhang et al., 2015). A congener-specific meta-analysis reported an increase in the risk of breast cancer among women with higher levels of PCBs 99, 183, and 187 (Leng et al., 2016). Importantly, studies of early life exposure indicate that PCB exposures during developmentally sensitive periods may increase the risk of developing breast cancer (Cohn et al., 2012).
In contrast to studies on breast cancer risk, the influence of PCBs on mortality following breast cancer has received limited scientific attention. However, there is accumulating evidence that PCBs and other organochlorine compounds may influence the risk of metastasis (Koual et al., 2019), recurrence (Muscat et al., 2003), and mortality following breast cancer (Parada et al., 2016a, 2016b, 2019). Furthermore, to our knowledge, these associations have not been examined in minority populations including black women. This is important given that US non-Hispanic blacks have been documented to have higher body burdens of pollutants than non-Hispanic whites (Pumarega et al., 2016) and black women have higher rates of breast cancer mortality than non-Hispanic white women (National Cancer Institute, 2019).
The aims of this study were to examine the associations between plasma levels of 17 PCB congeners and all-cause and breast cancer-specific mortality among women who participated in a population-based study of breast cancer. We also examined associations stratified by race and evaluated associations among black and white women separately. We hypothesized that higher plasma PCB levels are associated with greater risk of breast cancer-specific-mortality and that higher levels in black women than in white women may contribute to the racial disparity in mortality.
2. Methods
2.1. Study population
Participants in this study were from the Carolina Breast Cancer Study Phase I (CBCSI), a population-based North Carolina study of breast cancer with follow-up for mortality among women with newly diagnosed invasive breast cancer from 1993 to 1996 (Newman et al., 1995). In the CBCSI, 861 women with breast cancer were enrolled and interviewed. Approximately 98% of participants who were interviewed in-person by nurses provided three 10 mL blood samples an average of 4.1 months after diagnosis (range = 0.8–19.2 months). Here, we included 456 white non-Hispanic and 292 black women with breast cancer who had available PCB and lipid measurements, as previously reported (Millikan et al., 2000; Parada et al., 2019). All procedures performed in the CBCS involving human participants were in accordance with the ethical standards of the Institutional Review Boards of the University of North Carolina at Chapel Hill.
2.2. Follow-up for mortality
CBCSI participant records were linked to the National Death Index (Centers for Disease Control and Prevention, 2017) to ascertain date and cause of death. International Statistical Classification of Diseases (ICD) codes ICD-9-174.9 and ICD-10-C-50.9 listed on the death certificate were used to identify breast cancer-related deaths. Follow-up for mortality occurred from date of diagnosis in 1993–1996 until December 31, 2016. For all-cause mortality, participants alive at the end of follow-up were censored. For breast cancer-specific mortality, participants who died from other causes were censored at the time of death. The median survival time for the 748 women included in this study was 20.6 years (min = 0.4, max = 23.7 years), during which 392 women died from any cause, of whom 210 died from breast cancer.
2.3. Laboratory assessment
Plasma PCBs were measured using gas chromatography (GC) with electron capture detection, as previously reported (Millikan et al., 2000). In brief, plasma samples (2.0 mL) were treated with methanol (1.0 mL), spiked with a surrogate standard of PCB 198), and then extracted with three 2.5 mL portions of hexane:diethyl ether (1:1). The extract was fractionated using Florisil (R) open-column chromatography and eluted with 35 mL of hexane. The fraction was concentrated to 0.5 mL and spiked with octachloronaphthalene as an external quantitation standard. Individual compounds were identified based on chromatographic retention times relative to the internal standards and pattern recognition in the sample extract. Calibration solutions were prepared from certified standard solutions for each of the 35 individual congeners measured. Of these, 18 PCBs were detected in < 70% of participants; proportions below the limit of detection (LOD = 0.0625 ng/mL) ranged from 37.1% for PCB105 to 99.3% for PCB185 (Supplemental eTable 1). These 18 PCBs were not considered in analyses of individual PCB congeners. Additionally, four PCB pairs were quantitated and reported as the sum of their concentrations because they co-elute during GC analysis. Thus, here we report on nine individual PCB congeners and four pairs of PCB congeners. PCB levels below the LOD were imputed as LOD/√2 (Phillips et al., 1989) (Supplemental eTable 1). Total PCBs were determined by summing the wet-weights of all 35 measured PCB congeners, as previously reported in the CBCS (Millikan et al., 2000). In sensitivity analyses, we summed moles per gram of lipid, which gave similar results as summing wet weights. Here, we present results based on wet-weights.
Plasma lipid profiles were measured using automated enzymatic assays, as previously reported (Millikan et al., 2000). Lipids were used to adjust PCB levels to account for non-fasting variation (Phillips et al., 1989) and to better approximate adipose tissue levels (López-Carrillo et al., 1999).
2.4. Interview and medical records data
As part of the in-person interview, participants reported on demographic and behavioral characteristics including self-reported race, education, smoking status, and parity and lactation history. At the time of the interview, nurses also collected anthropometric measurements in duplicate including height and weight, which were used to compute body mass index (BMI in kg/m2). Breast cancer stage at diagnosis, grade, tumor size, nodal status, and estrogen receptor (ER) status were obtained from medical records. Potential confounders were selected based on their known associations with PCB levels (Wolff et al., 2005) or breast cancer mortality (Soerjomataram et al., 2008).
2.5. Statistical analysis
Plasma PCB levels were first lipid-adjusted by dividing their concentrations by total lipids and then ln-transformed. We examined Spearman rank-order correlations between the ln-transformed lipid-adjusted PCBs among all women. We categorized levels of lipid-adjusted PCB congeners into tertiles using the 33rd and 66th percentiles as cut-points (Supplemental eTable 1). We used Kaplan-Meier survival curves to examine all-cause and breast cancer-specific survival in association with each PCB/PCB pair. In general, the Kaplan-Meier curves revealed a divergence in survival curves by PCB tertiles five years following breast cancer diagnosis, violating the proportional hazards assumption. Thus, in multivariable models, we examined associations within five years of diagnosis, as well as 20 years after diagnosis conditional on 5-year survival. We used Cox regression with a heaviside function (Kleinbaum and Klein, 2012) (due to the violations in the proportional hazards assumption) to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations between tertiles of lipid-adjusted PCB levels and all-cause and breast cancer-specific mortality. We first included adjustment for age and race, then added smoking status, education, BMI, parity/lactation history, stage, and ER status. Tests for log-linear trend (i.e., PTrend) used continuous ln-transformed lipid-adjusted PCBs in full covariate-adjusted regression models. For PCBs that were statistically significantly associated with breast cancer-specific mortality, we examined effect measure modification by race by conducting stratified Cox models. Multiplicative interactions were evaluated by comparing regression models with continuous PCB-by-race interaction terms to models without the interaction terms (i.e., PInteraction).
All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC).
3. Results
As shown in Table 1, 39.0% of the participants included in this study were black, 44.1% had some college education or greater, and 58.6% were diagnosed with breast cancer before the age of 50. The majority of women (88.2%) were diagnosed with stage I or stage II breast cancer and 58.6% had ER+ tumors. Total PCB levels increased with increasing age and with decreasing education. PCB levels were generally similar across other demographic and clinical characteristics. By race, black women had higher mean levels of total PCBs as compared to white women. Furthermore, as compared to white women, black women had more aggressive disease (stage III/IV, 14.8% vs. 9.9%; grade III, 48.6% vs. 38.2%; tumor size >2.0 cm, 51.1% vs. 39.0%, node positive status, 43.5% vs. 24.0%; and ER+, 52.1% vs. 62.9%) and lower median overall survival (14.7 years vs. 21.0 years). Of the 292 black women, 180 (61.6%) died over the follow-up period and of the 456 white women, 212 (46.5%) died over the follow-up period.
Table 1.
Characteristics of CBCSI women with invasive breast cancer and with available plasma PCB and lipid data, overall and by total PCB tertiles (n = 748).
| n (%) | Total PCBs (μg/g lipid) | Total PCBs Tertiles (μg/g lipid)a | |||
|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | |||
| Mean (SD) | n (%) | n (%) | n (%) | ||
| Race | |||||
| White | 456 (61.0) | 0.38 (0.21) | 180 (72.3) | 169 (67.6) | 107 (43.0) |
| Black | 292 (39.0) | 0.56 (0.39) | 69 (27.7) | 81 (32.4) | 142 (57.0) |
| Age at diagnosis (years) | |||||
| < 50 | 438 (58.6) | 0.34 (0.22) | 209 (83.9) | 158 (63.2) | 71 (28.5) |
| ≥50 | 310 (41.4) | 0.60 (0.35) | 40 (16.1) | 92 (36.8) | 178 (71.5) |
| Education | |||||
| Less than high school | 137 (18.3) | 0.59 (0.37) | 28 (11.2) | 34 (13.6) | 75 (30.1) |
| High school | 281 (37.6) | 0.45 (0.28) | 82 (32.9) | 101 (40.4) | 98 (39.4) |
| Some college or greater | 330 (44.1) | 0.39 (0.29) | 139 (55.8) | 115 (46.0) | 76 (30.5) |
| Body mass index (kg/m2) | |||||
| < 25.0 | 221 (30.1) | 0.43 (0.29) | 76 (31.3) | 73 (30.0) | 72 (29.5) |
| 25.0–29.9 | 229 (31.2) | 0.50 (0.36) | 66 (27.2) | 69 (27.9) | 94 (38.5) |
| ≥30.0 | 284 (38.7) | 0.42 (0.27) | 101 (41.6) | 105 (42.5) | 78 (32.0) |
| Missing | 14 | 6 | 3 | 5 | |
| Smoking status | |||||
| Never | 392 (52.4) | 0.46 (0.35) | 138 (55.4) | 111 (44.4) | 143 (57.4) |
| Former | 183 (24.5) | 0.44 (0.27) | 54 (21.7) | 67 (26.8) | 62 (24.9) |
| Current | 173 (23.1) | 0.42 (0.27) | 57 (22.9) | 72 (28.8) | 44 (17.7) |
| Parity/Lactation history | |||||
| Nulliparous | 112 (15.0) | 0.47 (0.33) | 39 (15.7) | 37 (14.8) | 36 (14.5) |
| Parous/never lactated | 387 (51.7) | 0.42 (0.28) | 120 (48.2) | 150 (60.0) | 117 (47.0) |
| Parous/ever lactated | 249 (33.3) | 0.47 (0.34) | 90 (36.1) | 63 (25.2) | 96 (38.5) |
| Stage | |||||
| I/II | 613 (88.2) | 0.45 (0.31) | 204 (88.7) | 211 (89.0) | 198 (86.8) |
| III/IV | 82 (11.8) | 0.45 (0.30) | 26 (11.3) | 26 (11.0) | 30 (13.2) |
| Missing | 53 | 19 | 13 | 21 | |
| Grade | |||||
| I/II | 376 (57.7) | 0.48 (0.31) | 100 (47.6) | 138 (61.6) | 138 (63.3) |
| III | 276 (42.3) | 0.41 (0.32) | 110 (52.4) | 86 (38.4) | 80 (36.7) |
| Missing | 96 | 39 | 26 | 31 | |
| Tumor size (cm) | |||||
| ≤2.0 | 396 (56.3) | 0.47 (0.31) | 120 (51.3) | 135 (46.5) | 141 (61.0) |
| > 2.0 | 308 (43.8) | 0.42 (0.31) | 114 (48.7) | 104 (43.5) | 90 (39.0) |
| Missing | 44 | 15 | 11 | 18 | |
| Node status | |||||
| Negative | 442 (62.3) | 0.45 (0.29) | 136 (58.1) | 151 (63.2) | 155 (64.4) |
| Positive | 268 (37.7) | 0.44 (0.34) | 98 (41.9) | 88 (36.8) | 82 (34.6) |
| Missing | 38 | 15 | 11 | 12 | |
| Estrogen receptor (ER) status | |||||
| ER+ | 404 (58.7) | 0.47 (0.31) | 127 (56.0) | 134 (57.5) | 143 (62.7) |
| ER− | 284 (41.3) | 0.42 (0.29) | 100 (44.0) | 99 (42.5) | 85 (37.3) |
| Missing | 60 | 22 | 17 | 21 | |
| Deaths from any cause | |||||
| Deaths | 392 (52.4) | 0.50 (0.32) | 101 (40.6) | 122 (48.8) | 169 (67.9) |
| Censored | 356 (47.6) | 0.39 (0.29) | 148 (59.4) | 128 (51.2) | 80 (32.1) |
| Deaths from breast cancer | |||||
| Deaths | 210 (28.1) | 0.43 (0.29) | 78 (31.3) | 64 (25.6) | 68 (27.3) |
| Censored | 538 (71.9) | 0.45 (0.32) | 171 (68.7) | 186 (74.4) | 181 (72.7) |
Carolina Breast Cancer Study (CBCS) participants were diagnosed with invasive breast cancer from 1993 to 1996 (Phase I) and followed-up for vital status through December 31, 2016.
Concentrations of lipid-adjusted total PCBs were divided into tertiles using the following cut points: tertile 1, ≤0.291 μg/g lipid; tertile 2, > 0.291 to ≤0.459 μg/g lipid; and tertile 3, > 0.459 μg/g lipid.
3.1. 5-Year mortality
From the Kaplan-Meier curves, most PCBs were not associated with 5-year all-cause or breast cancer-specific mortality when comparing the highest to the lowest PCB tertiles (Fig. 1). After covariate adjustment, however, 5-year breast cancer-specific mortality HRs were 1.46 (95%CI = 0.86–2.47) for PCB74, 1.53 (95%CI = 0.89–2.64) for PCB138, 1.57 (95%CI = 0.90–2.73) for PCB99, and 1.86 (95%CI = 1.07–3.23) for PCB118, comparing the highest to the lowest tertiles (Table 2). Furthermore, one-ln unit increases in PCB74, PCB99, PCB118, and total PCBs were associated with 5-year breast cancer-specific mortality HRs of 1.33 (95%CI = 1.02–1.74), 1.35 (95%CI = 1.03–1.78), 1.40 (95%CI = 1.02–1.92), and 1.35 (95%CI = 0.89–2.03), respectively. Breast cancer-specific mortality rates were elevated among women with the highest versus lowest tertiles of PCBs 138, 146, and 153, but confidence intervals were wide and included the null. The remaining PCBs were not associated with 5-year mortality.
Fig. 1.
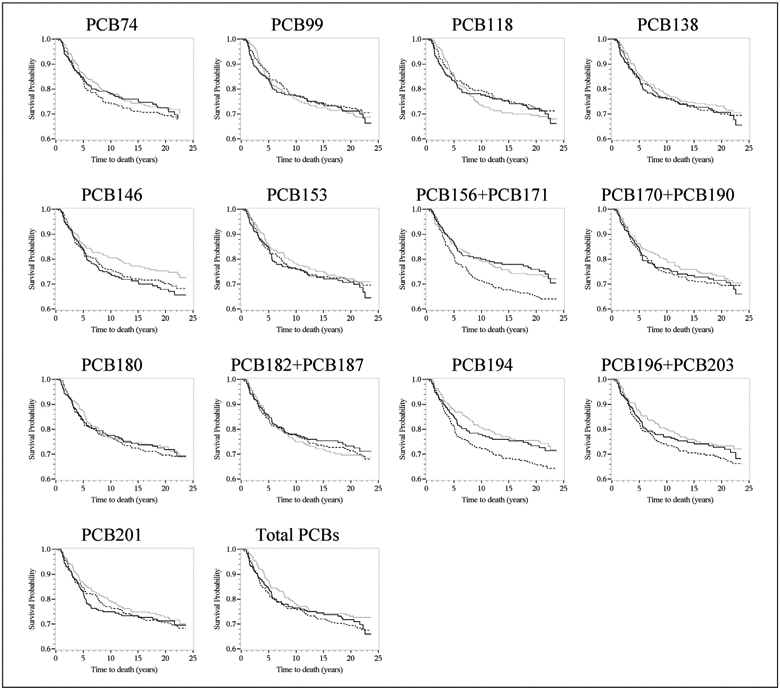
Kaplan–Meier survival curves for breast cancer-specific mortality and tertiles (Tertile 3, solid line vs. Tertile 1, dashed line) of lipid-adjusted plasma polychlorinated biphenyls among CBCSI women diagnosed with breast cancer in 1993–1996 (n = 748). The x-axis shows times to death in years; the y-axis shows proportion of participants alive.
Table 2.
Cox regression hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations between plasma levels of lipid-adjusted polychlorinated biphenyls and 5-year mortality in the CBCSI women diagnosed with invasive breast cancer in 1993–1996 (n = 748).
| PCBs (μ/g lipid) | 5-Year All-Cause Mortality | 5-Year Breast Cancer Specific-Mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| Deaths (n = 133) | Censored (n = 615) | Model 1a | Model 2b | Deaths (n = 111) | Censored (n = 637) | Model 1a | Model 2b | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||||
| PCB74 | ||||||||
| Tertile 1 | 45 | 204 | 1.00 (Reference) | 1.00 (Reference) | 41 | 208 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 37 | 213 | 0.69 (0.45–1.08) | 0.74 (0.45–1.21) | 34 | 216 | 0.89 (0.56–1.41) | 0.89 (0.53–1.49) |
| Tertile 3 | 51 | 198 | 0.78 (0.50–1.21) | 1.00 (0.63–1.59) | 36 | 213 | 1.25 (0.76–2.05) | 1.46 (0.86–2.47) |
| Ln(PCB74) | 0.87 (0.71–1.06) | 1.08 (0.86–1.35) | 1.08 (0.85–1.36) | 1.33 (1.02–1.74) | ||||
| PCB99 | ||||||||
| Tertile 1 | 36 | 211 | 1.00 (Reference) | 1.00 (Reference) | 33 | 214 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 43 | 204 | 1.01 (0.65–1.58) | 1.06 (0.64–1.76) | 39 | 208 | 1.17 (0.73–1.86) | 1.28 (0.75–2.18) |
| Tertile 3 | 53 | 104 | 0.97 (0.62–1.51) | 1.12 (0.68–1.83) | 38 | 209 | 1.38 (0.84–2.28) | 1.57 (0.90–2.73) |
| Ln(PCB99) | 0.97 (0.79–1.21) | 1.11 (0.88–1.41) | 1.17 (0.92–1.50) | 1.35 (1.03–1.78) | ||||
| PCB118 | ||||||||
| Tertile 1 | 40 | 207 | 1.00 (Reference) | 1.00 (Reference) | 36 | 211 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 38 | 209 | 0.79 (0.51–1.24) | 0.85 (0.52–1.39) | 35 | 212 | 1.02 (0.64–1.63) | 1.15 (0.68–1.94) |
| Tertile 3 | 54 | 193 | 0.85 (0.55–1.32) | 1.14 (0.70–1.85) | 39 | 208 | 1.37 (0.83–2.25) | 1.86 (1.07–3.23) |
| Ln(PCB118) | 0.88 (0.70–1.10) | 1.12 (0.86–1.46) | 1.08 (0.83–1.41) | 1.40 (1.02–1.92) | ||||
| PCB138 | ||||||||
| Tertile 1 | 43 | 204 | 1.00 (Reference) | 1.00 (Reference) | 39 | 208 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 39 | 208 | 0.75 (0.48–1.16) | 0.89 (0.54–1.45) | 34 | 213 | 0.96 (0.60–1.53) | 1.22 (0.72–2.07) |
| Tertile 3 | 50 | 197 | 0.72 (0.47–1.11) | 0.95 (0.59–1.54) | 37 | 210 | 1.17 (0.72–1.90) | 1.53 (0.89–2.64) |
| Ln(PCB138) | 0.77 (0.60–1.00) | 0.93 (0.69–1.24) | 1.01 (0.75–1.35) | 1.21 (0.87–1.69) | ||||
| PCB146 | ||||||||
| Tertile 1 | 43 | 204 | 1.00 (Reference) | 1.00 (Reference) | 40 | 207 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 40 | 207 | 0.77 (0.50–1.19) | 0.86 (0.53–1.39) | 34 | 213 | 0.94 (0.59–1.50) | 1.06 (0.63–1.79) |
| Tertile 3 | 50 | 197 | 0.73 (0.47–1.15) | 0.83 (0.51–1.35) | 37 | 210 | 1.16 (0.71–1.91) | 1.23 (0.71–2.14) |
| Ln(PCB146) | 0.87 (0.71–1.07) | 0.94 (0.74–1.17) | 1.04 (0.82–1.31) | 1.09 (0.84–1.41) | ||||
| PCB153 | ||||||||
| Tertile 1 | 43 | 204 | 1.00 (Reference) | 1.00 (Reference) | 40 | 207 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 40 | 207 | 0.79 (0.52–1.23) | 0.86 (0.53–1.39) | 34 | 213 | 0.95 (0.60–1.51) | 1.06 (0.63–1.79) |
| Tertile 3 | 49 | 198 | 0.72 (0.46–1.11) | 0.89 (0.55–1.45) | 36 | 211 | 1.14 (0.70–1.86) | 1.36 (0.78–2.35) |
| Ln(PCB153) | 0.80 (0.63–1.03) | 0.94 (0.69–1.28) | 1.02 (0.75–1.37) | 1.26 (0.87–1.81) | ||||
| PCB156 + PCB171c | ||||||||
| Tertile 1 | 50 | 199 | 1.00 (Reference) | 1.00 (Reference) | 45 | 204 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 41 | 209 | 0.72 (0.48–1.09) | 0.73 (0.46–1.17) | 34 | 216 | 0.82 (0.52–1.28) | 0.90 (0.54–1.49) |
| Tertile 3 | 42 | 207 | 0.50 (0.32–0.77) | 0.67 (0.42–1.07) | 32 | 217 | 0.81 (0.50–1.32) | 1.15 (0.67–0.96) |
| Ln(PCB156 + PCB171) | 0.79 (0.67–0.94) | 0.90 (0.74–1.09) | 0.92 (0.75–1.11) | 1.08 (0.86–1.34) | ||||
| PCB170 + PCB190c | ||||||||
| Tertile 1 | 43 | 197 | 1.00 (Reference) | 1.00 (Reference) | 39 | 201 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 38 | 203 | 0.73 (0.47–1.13) | 0.73 (0.45–1.18) | 32 | 209 | 0.98 (0.61–1.57) | 0.88 (0.52–1.48) |
| Tertile 3 | 47 | 193 | 0.71 (0.46–1.10) | 0.75 (0.47–1.21) | 36 | 204 | 1.16 (0.71–1.87) | 1.02 (0.60–1.75) |
| Ln(PCB170 + PCB 190) | 0.85 (0.71–1.01) | 0.92 (0.76–1.12) | 1.02 (0.83–1.25) | 1.07 (0.85–1.35) | ||||
| PCB180 | ||||||||
| Tertile 1 | 45 | 201 | 1.00 (Reference) | 1.00 (Reference) | 40 | 206 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 34 | 213 | 0.57 (0.36–0.89) | 0.55 (0.33–0.92) | 30 | 217 | 0.83 (0.52–1.35) | 0.74 (0.43–1.28) |
| Tertile 3 | 51 | 195 | 0.70 (0.46–1.07) | 0.86 (0.54–1.36) | 39 | 207 | 1.14 (0.71–1.82) | 1.33 (0.79–2.23) |
| Ln(PCB180) | 0.79 (0.60–1.04) | 0.92 (0.67–1.27) | 1.06 (0.80–1.41) | 1.19 (0.86–1.66) | ||||
| PCB182 + PCB187c | ||||||||
| Tertile 1 | 41 | 208 | 1.00 (Reference) | 1.00 (Reference) | 39 | 210 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 44 | 206 | 0.83 (0.54–1.28) | 0.95 (0.58–1.51) | 38 | 212 | 1.06 (0.67–1.68) | 1.15 (0.69–1.93) |
| Tertile 3 | 48 | 201 | 0.66 (0.41–1.05) | 0.86 (0.52–1.42) | 34 | 215 | 0.99 (0.58–1.68) | 1.14 (0.64–2.03) |
| Ln(PCB182 + PCB187) | 0.87 (0.73–1.05) | 0.95 (0.78–1.16) | 1.06 (0.86–1.30) | 1.10 (0.88–1.38) | ||||
| PCB194 | ||||||||
| Tertile 1 | 47 | 202 | 1.00 (Reference) | 1.00 (Reference) | 46 | 203 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 41 | 209 | 0.66 (0.43–1.02) | 0.66 (0.41–1.05) | 30 | 220 | 0.74 (0.46–1.19) | 0.67 (0.39–1.14) |
| Tertile 3 | 45 | 204 | 0.53 (0.34–0.83) | 0.69 (0.43–1.12) | 35 | 214 | 0.95 (0.58–1.55) | 1.08 (0.64–1.85) |
| Ln(PCB194) | 0.76 (0.61–0.94) | 0.87 (0.68–1.11) | 1.00 (0.78–1.27) | 1.10 (0.83–1.44) | ||||
| PCB196 + PCB203c | ||||||||
| Tertile 1 | 44 | 205 | 1.00 (Reference) | 1.00 (Reference) | 43 | 206 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 39 | 211 | 0.66 (0.43–1.03) | 0.71 (0.44–1.16) | 31 | 219 | 0.81 (0.50–1.30) | 0.85 (0.50–1.45) |
| Tertile 3 | 50 | 199 | 0.63 (0.40–1.00) | 0.73 (0.44–1.21) | 37 | 212 | 1.16 (0.69–1.93) | 1.14 (0.65–2.01) |
| Ln(PCB196 + PCB203) | 0.78 (0.62–0.98) | 0.86 (0.66–1.11) | 1.01 (0.78–1.31) | 1.03 (0.76–1.38) | ||||
| PCB201 | ||||||||
| Tertile 1 | 39 | 210 | 1.00 (Reference) | 1.00 (Reference) | 38 | 211 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 41 | 209 | 0.83 (0.53–1.30) | 0.85 (0.52–1.39) | 33 | 217 | 1.00 (0.62–1.61) | 1.02 (0.60–1.73) |
| Tertile 3 | 53 | 196 | 0.84 (0.53–1.32) | 0.84 (0.51–1.39) | 40 | 209 | 1.51 (0.92–2.48) | 1.29 (0.75–2.23) |
| Ln(PCB201) | 0.91 (0.75–1.10) | 0.92 (0.74–1.15) | 1.18 (0.95–1.47) | 1.13 (0.88–1.45) | ||||
| Total PCBs | ||||||||
| Tertile 1 | 45 | 204 | 1.00 (Reference) | 1.00 (Reference) | 43 | 206 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 37 | 213 | 0.67 (0.43–1.04) | 0.70 (0.44–1.14) | 31 | 219 | 0.77 (0.48–1.23) | 0.81 (0.48–1.36) |
| Tertile 3 | 51 | 198 | 0.68 (0.44–1.05) | 0.78 (0.48–1.26) | 37 | 212 | 1.10 (0.68–1.80) | 1.18 (0.68–2.05) |
| Ln(Total PCBs) | 0.75 (0.55–1.03) | 0.95 (0.66–1.35) | 1.08 (0.75–1.54) | 1.35 (0.89–2.03) | ||||
Carolina Breast Cancer Study (CBCS) participants were diagnosed with invasive breast cancer from 1993 to 1996 (Phase I) and followed-up for vital status through December 31, 2016.
Adjusted for age at diagnosis (continuous in years) and race (black vs. white).
Adjusted for age at diagnosis (continuous in years), race (black vs. white), smoking status (never, former, and current smoker), education (less than high school, high school, and college or greater), BMI (< 25.0, 25.0–29.9, ≥30.0 kg/m2), parity/lactation history (nulliparous, parous/never lactated, parous/ever lactated), stage (III/IV vs. I/II), and ER status (ER+ vs. ER−).
These analytes are quantitated and reported as the sum of their concentrations because they co-elute during GC analysis.
Because PCBs 74, 99, 118, and the total PCBs were significantly associated with 5-year breast cancer-specific mortality, we examined effect measure modification by race for these three PCBs and for total PCBs. As shown in Table 3, a one-ln unit increase in PCB74 was associated with a 47% (HR = 1.47; 95%CI = 1.01–2.14) increase in breast cancer-specific mortality risk among black women, and a 19% (HR = 1.19; 95%CI = 0.79–1.77) increase in breast cancer-specific mortality risk among white women (PInteraction = 0.05).
Table 3.
Race-stratified Cox regression hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations between lipid-adjusted plasma levels of select polychlorinated biphenyls and 5-year breast cancer-specific mortality in the CBCSI women diagnosed with invasive breast cancer in 1993–1996 (n = 748).
| PCBs (μ/g lipid) | White (n = 456) | Black (n = 292) | PInteraction | ||||
|---|---|---|---|---|---|---|---|
| Deaths (n = 54) | Censored (n = 402) | HR (95% CI)a | Deaths (n = 57) | Censored (n = 235) | HR (95% CI)a | ||
| PCB74 | 0.05 | ||||||
| Tertile 1 | 27 | 150 | 1.00 (Reference) | 14 | 58 | 1.00 (Reference) | |
| Tertile 2 | 14 | 136 | 0.84 (0.40–1.77) | 20 | 80 | 0.98 (0.45–2.10) | |
| Tertile 3 | 13 | 116 | 1.37 (0.63–2.96) | 23 | 97 | 1.67 (0.78–3.56) | |
| Ln(PCB74) | 1.19 (0.79–1.77) | 1.47 (1.01–2.14) | |||||
| PCB99 | 0.77 | ||||||
| Tertile 1 | 23 | 168 | 1.00 (Reference) | 10 | 46 | 1.00 (Reference) | |
| Tertile 2 | 19 | 134 | 1.34 (0.66–2.72) | 20 | 74 | 1.10 (0.46–2.60) | |
| Tertile 3 | 12 | 95 | 2.04 (0.94–4.43) | 26 | 114 | 1.28 (0.54–3.02) | |
| Ln(PCB99) | 1.46 (0.97–2.19) | 1.33 (0.88–2.00) | |||||
| PCB118 | 0.31 | ||||||
| Tertile 1 | 21 | 160 | 1.00 (Reference) | 15 | 51 | 1.00 (Reference) | |
| Tertile 2 | 19 | 142 | 1.44 (0.70–2.95) | 16 | 70 | 0.86 (0.39–1.88) | |
| Tertile 3 | 14 | 95 | 3.00 (1.36–6.62) | 25 | 113 | 1.29 (0.59–2.84) | |
| Ln(PCB118) | 1.66 (1.05–2.64) | 1.25 (0.79–1.98) | |||||
| Total PCBs | 0.63 | ||||||
| Tertile 1 | 29 | 151 | 1.00 (Reference) | 14 | 55 | 1.00 (Reference) | |
| Tertile 2 | 14 | 155 | 0.75 (0.38–1.49) | 17 | 64 | 0.90 (0.39–2.03) | |
| Tertile 3 | 11 | 96 | 1.29 (0.57–2.93) | 26 | 116 | 1.35 (0.59–3.09) | |
| Ln(Total PCBs) | 1.50 (0.75–2.99) | 1.34 (0.78–2.29) | |||||
Carolina Breast Cancer Study (CBCS) participants were diagnosed with invasive breast cancer from 1993 to 1996 (Phase I) and followed-up for vital status through December 31, 2016.
Adjusted for age at diagnosis (continuous in years), race, smoking status (never, former, and current smoker), education (less than high school, high school, and college or greater), BMI (< 25.0, 25.0–29.9, ≥ 30.0 kg/m2), parity/lactation history (nulliparous, parous/never lactated, parous/ever lactated), stage (III/IV vs. I/II), and ER status (ER+ vs. ER−), as appropriate.
3.2. 20-Year conditional mortality
From the Kaplan-Meier curves, most PCBs were associated with 20-year all-cause mortality when comparing the highest to the lowest PCB tertiles (Supplemental eFigure 1). Furthermore, the breast cancer-specific mortality survival curves tended to cross roughly 5-years after diagnosis for PCB156+PCB171 and PCB194. We therefore performed long-term survival analysis conditioning upon survival to five years. Among those who survived at least five years, PCBs were not associated with 20-year breast cancer-specific mortality (Table 4). Additionally, while PCBs 74, 99, and 118 were associated with 5-year breast cancer-specific mortality, associations were attenuated at 20-years. For 20-year conditional all-cause mortality, most PCBs were associated with an increased risk of mortality, though not all associations were statistically significant. HRs comparing the highest to the lowest tertiles ranged from 1.33 (95%CI = 0.92–1.93) for PCB156+PCB171 to 1.96 (95%CI = 1.33–2.89) for PCB153. Furthermore, there was an apparent dose-response association for most PCBs as shown by the log-linear trend analyses (Table 4).
Table 4.
Cox regression hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations between lipid-adjusted plasma levels of polychlorinated biphenyls and 20-year conditional mortality in the CBCSI women diagnosed with invasive breast cancer in 1993–1996 (n = 748).
| 20-year Conditional All-Cause Mortality | 20-year Conditional Breast Cancer-Specific Mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| PCBs (μ/g lipid) | Deaths (n = 259) | Censored (n = 356) | Model 1a | Model 2b | Deaths (n = 97) | Censored (n = 518) | Model 1a | Model 2b |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||||
| PCB74 | ||||||||
| Tertile 1 | 60 | 146 | 1.00 (Reference) | 1.00 (Reference) | 36 | 170 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 80 | 133 | 1.12 (0.79–1.57) | 0.91 (0.63–1.33) | 34 | 179 | 1.02 (0.63–1.64) | 0.84 (0.50–1.42) |
| Tertile 3 | 119 | 77 | 1.61 (1.14–2.28) | 1.54 (1.06–2.24) | 27 | 169 | 1.14 (0.66–1.96) | 1.23 (0.70–2.18) |
| Ln(PCB74) | 1.19 (1.00–1.40) | 1.17 (0.98–1.41) | 1.04 (0.81–1.34) | 1.11 (0.85–1.46) | ||||
| PCB99 | ||||||||
| Tertile 1 | 65 | 146 | 1.00 (Reference) | 1.00 (Reference) | 36 | 175 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 80 | 124 | 1.15 (0.82–1.60) | 1.33 (0.92–1.92) | 34 | 170 | 0.99 (0.61–1.58) | 1.15 (0.68–1.92) |
| Tertile 3 | 112 | 82 | 1.49 (1.07–2.08) | 1.57 (1.08–2.29) | 27 | 167 | 0.97 (0.57–1.65) | 1.11 (0.63–1.96) |
| Ln(PCB99) | 1.16 (0.99–1.36) | 1.19 (0.99–1.43) | 0.99 (0.77–1.28) | 1.10 (0.83–1.45) | ||||
| PCB118 | ||||||||
| Tertile 1 | 63 | 144 | 1.00 (Reference) | 1.00 (Reference) | 32 | 175 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 81 | 128 | 1.23 (0.88–1.71) | 1.46 (1.01–2.10) | 39 | 170 | 1.38 (0.86–2.21) | 1.79 (1.07–2.98) |
| Tertile 3 | 113 | 80 | 1.52 (1.08–2.13) | 1.74 (1.19–2.56) | 26 | 167 | 1.15 (0.66–2.00) | 1.57 (0.86–2.86) |
| Ln(PCB118) | 1.24 (1.05–1.47) | 1.37 (1.12–1.68) | 1.06 (0.81–1.39) | 1.23 (0.90–1.69) | ||||
| PCB138 | ||||||||
| Tertile 1 | 59 | 145 | 1.00 (Reference) | 1.00 (Reference) | 34 | 170 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 81 | 127 | 1.23 (0.88–1.73) | 1.24 (0.85–1.80) | 33 | 175 | 1.12 (0.69–1.82) | 1.24 (0.73–2.10) |
| Tertile 3 | 117 | 80 | 1.64 (1.16–2.31) | 1.72 (1.18–2.50) | 30 | 167 | 1.19 (0.71–2.01) | 1.40 (0.80–2.46) |
| Ln(PCB138) | 1.21 (0.99–1.48) | 1.20 (0.97–1.50) | 1.01 (0.75–1.37) | 1.08 (0.78–1.50) | ||||
| PCB146 | ||||||||
| Tertile 1 | 53 | 151 | 1.00 (Reference) | 1.00 (Reference) | 35 | 169 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 76 | 131 | 1.24 (0.87–1.77) | 1.11 (0.75–1.63) | 27 | 180 | 0.84 (0.51–1.40) | 0.78 (0.45–1.36) |
| Tertile 3 | 126 | 71 | 2.05 (1.42–2.95) | 1.88 (1.26–2.80) | 34 | 163 | 1.38 (0.82–2.33) | 1.35 (0.77–2.36) |
| Ln(PCB146) | 1.35 (1.15–1.59) | 1.30 (1.09–1.55) | 1.19 (0.93–1.53) | 1.19 (0.91–1.55) | ||||
| PCB153 | ||||||||
| Tertile 1 | 56 | 148 | 1.00 (Reference) | 1.00 (Reference) | 32 | 172 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 81 | 126 | 1.30 (0.92–1.84) | 1.39 (0.95–2.02) | 33 | 174 | 1.17 (0.72–1.91) | 1.33 (0.78–2.25) |
| Tertile 3 | 120 | 78 | 1.80 (1.27–2.56) | 1.96 (1.33–2.89) | 32 | 166 | 1.42 (0.84–2.40) | 1.60 (0.90–2.86) |
| Ln(PCB153) | 1.27 (1.03–1.58) | 1.34 (1.05–1.70) | 1.07 (0.77–1.47) | 1.27 (0.89–1.82) | ||||
| PCB156 + PCB171c | ||||||||
| Tertile 1 | 64 | 135 | 1.00 (Reference) | 1.00 (Reference) | 41 | 158 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 81 | 128 | 1.12 (0.81–1.56) | 1.15 (0.80–1.65) | 30 | 179 | 0.79 (0.49–1.27) | 0.91 (0.55–1.52) |
| Tertile 3 | 114 | 93 | 1.27 (0.91–1.77) | 1.33 (0.92–1.93) | 26 | 181 | 0.77 (0.46–1.31) | 0.97 (0.55–1.70) |
| Ln(PCB156 + PCB171) | 1.11 (0.97–1.27) | 1.12 (0.96–1.30) | 0.90 (0.73–1.11) | 0.96 (0.76–1.20) | ||||
| PCB170 + PCB190c | ||||||||
| Tertile 1 | 58 | 139 | 1.00 (Reference) | 1.00 (Reference) | 32 | 165 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 86 | 117 | 1.30 (0.93–1.82) | 1.16 (0.81–1.68) | 32 | 171 | 1.18 (0.72–1.95) | 1.07 (0.63–1.82) |
| Tertile 3 | 109 | 84 | 1.56 (1.11–2.19) | 1.44 (0.99–2.11) | 30 | 163 | 1.30 (0.77–2.19) | 1.16 (0.66–2.06) |
| Ln(PCB170 + PCB190) | 1.21 (1.04–1.41) | 1.18 (1.00–1.39) | 1.14 (0.91–1.44) | 1.14 (0.89–1.46) | ||||
| PCB180 | ||||||||
| Tertile 1 | 64 | 137 | 1.00 (Reference) | 1.00 (Reference) | 33 | 168 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 95 | 118 | 1.21 (0.87–1.67) | 1.15 (0.81–1.64) | 38 | 175 | 1.30 (0.81–2.09) | 1.20 (0.72–2.00) |
| Tertile 3 | 98 | 97 | 1.20 (0.85–1.67) | 1.23 (0.85–1.78) | 25 | 170 | 0.94 (0.55–1.62) | 1.00 (0.57–1.78) |
| Ln(PCB180) | 1.07 (0.88–1.30) | 1.12 (0.88–1.41) | 1.05 (0.78–1.41) | 1.09 (0.79–1.52) | ||||
| PCB182 + PCB187c | ||||||||
| Tertile 1 | 54 | 154 | 1.00 (Reference) | 1.00 (Reference) | 36 | 172 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 84 | 122 | 1.39 (0.98–1.98) | 1.39 (0.95–2.04) | 35 | 171 | 1.15 (0.58–1.68) | 1.18 (0.70–1.98) |
| Tertile 3 | 121 | 80 | 1.70 (1.17–2.47) | 1.82 (1.21–2.74) | 26 | 175 | 0.91 (0.52–1.61) | 0.95 (0.52–1.74) |
| Ln(PCB182 + PCB187) | 1.23 (1.06–1.42) | 1.20 (1.03–1.41) | 1.00 (0.81–1.24) | 0.99 (0.79–1.24) | ||||
| PCB194 | ||||||||
| Tertile 1 | 60 | 142 | 1.00 (Reference) | 1.00 (Reference) | 40 | 162 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 84 | 125 | 1.12 (0.80–1.57) | 1.00 (0.69–1.45) | 31 | 178 | 0.86 (0.53–1.38) | 0.74 (0.44–1.24) |
| Tertile 3 | 115 | 89 | 1.31 (0.92–1.85) | 1.34 (0.91–1.98) | 26 | 178 | 0.83 (0.49–1.43) | 0.84 (0.47–1.51) |
| Ln(PCB194) | 1.09 (0.92–1.29) | 1.07 (0.88–1.29) | 0.88 (0.68–1.13) | 0.87 (0.66–1.14) | ||||
| PCB196 + PCB203c | ||||||||
| Tertile 1 | 53 | 152 | 1.00 (Reference) | 1.00 (Reference) | 39 | 166 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 89 | 122 | 1.39 (0.98–1.98) | 1.28 (0.87–1.89) | 32 | 179 | 0.95 (0.58–1.53) | 0.81 (0.48–1.38) |
| Tertile 3 | 117 | 82 | 1.64 (1.12–2.39) | 1.45 (0.95–2.20) | 26 | 173 | 1.00 (0.57–1.74) | 0.80 (0.44–1.45) |
| Ln(PCB196 + PCB203) | 1.23 (1.01–1.48) | 1.13 (0.92–1.39) | 26 | 173 | 0.97 (0.74–1.28) | 0.87 (0.65–1.17) | ||
| PCB201 | ||||||||
| Tertile 1 | 60 | 150 | 1.00 (Reference) | 1.00 (Reference) | 38 | 172 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 85 | 124 | 1.30 (0.93–1.82) | 1.18 (0.82–1.71) | 34 | 175 | 1.08 (0.67–1.73) | 0.97 (0.59–1.61) |
| Tertile 3 | 114 | 82 | 1.68 (1.18–2.40) | 1.38 (0.94–2.04) | 25 | 171 | 1.15 (0.66–2.00) | 0.85 (0.47–1.53) |
| Ln(PCB201) | 1.21 (1.04–1.41) | 1.11 (0.94–1.32) | 1.01 (0.81–1.26) | 0.89 (0.70–1.13) | ||||
| Total PCBs | ||||||||
| Tertile 1 | 56 | 148 | 1.00 (Reference) | 1.00 (Reference) | 35 | 169 | 1.00 (Reference) | 1.00 (Reference) |
| Tertile 2 | 85 | 128 | 1.42 (1.01–1.99) | 1.49 (1.03–2.16) | 33 | 180 | 1.08 (0.67–1.75) | 1.19 (0.71–1.98) |
| Tertile 3 | 118 | 80 | 1.71 (1.21–2.43) | 1.73 (1.17–2.56) | 29 | 169 | 1.17 (0.69–1.99) | 1.26 (0.70–2.27) |
| Ln(Total PCBs) | 1.35 (1.07–1.70) | 1.36 (1.04–1.77) | 1.13 (0.78–1.64) | 1.20 (0.80–1.81) | ||||
Carolina Breast Cancer Study (CBCS) participants were diagnosed with invasive breast cancer from 1993 to 1996 (Phase I) and followed-up for vital status through December 31, 2016. Conditional analyses are among women who survived > 5 years.
Adjusted for age at diagnosis (continuous in years) and race (black vs. white).
Adjusted for age at diagnosis (continuous in years), race (black vs. white), smoking status (never, former, and current smoker), education (less than high school, high school, and college or greater), BMI (< 25.0, 25.0–29.9, ≥30.0 kg/m2), parity/lactation history (nulliparous, parous/never lactated, parous/ever lactated), stage (III/IV vs. I/II), and ER status (ER+ vs. ER−).
These analytes are quantitated and reported as the sum of their concentrations because they co-elute during GC analysis.
4. Discussion
We examined the associations between 17 PCB congeners and the sum of 35 measured PCB congeners and mortality following breast cancer among black and white women who participated in a population-based study of breast cancer. PCBs 74, 99, and 118 and total PCBs were associated with 33–40% increase in 5-year mortality. The effect of PCBs on breast cancer outcomes may vary over time in survivorship with the strongest effects in the early years following diagnosis. Among patients who survived at least five years, PCBs had no effect on breast cancer-specific mortality. Furthermore, PCB74 was associated with a larger elevated risk of 5-year breast cancer-specific mortality among black women than among white women. Most PCBs were associated with increases in all-cause mortality, but only among women who survived at least 5 years.
Only three studies have examined PCBs in association with mortality following breast cancer (Høyer et al., 2000; Parada et al., 2016a; Roswall et al., 2018), with most emphasizing all-cause mortality. The first study was conducted among 195 Danish women who provided blood samples in 1976–1978 and again in 1981–1983 and who were followed for vital status until 1996 (Høyer et al., 2000). In their study, the highest versus the lowest quartile of the sum of 27 PCBs was associated with a 44% increase in the risk of overall mortality. In contrast, a more recent Danish study of 399 postmenopausal women reported an inverse association between the sum of 18 PCBs measured in buttock tissue biopsies and overall mortality following breast cancer over a median follow-up of 16.1 years (Roswall et al., 2018). In the first US-based study, in a sample of 627 primarily non-Hispanic white women, the highest versus lowest tertile of PCB174 was associated with a twofold increase in all-cause mortality. This was the only one of the three studies that examined breast-cancer specific mortality, and found a three-fold increase in breast cancer-specific mortality within five years of diagnosis. In that study, PCB174 remained associated with breast cancer-specific mortality 15 years post-diagnosis (Parada et al., 2016a). Thus, the tendency for PCBs to be associated with mortality outcomes is consistent with our findings.
Some differences between these studies and our findings are noteworthy. In the CBCSI, PCB174 and PCB177 levels were below the LOD for 98% and 67.2% of women, respectively. We were therefore unable to evaluate these congeners. Differences in exposure patterns may help explain some discrepancies with previous studies. In particular, we found no effects on breast cancer-specific mortality after conditioning on 5 years survival (in contrast to Parada et al. 2016a,b (Parada et al., 2016a)). First, the differences in the congeners and levels detected in this study are likely attributed to different sources of exposure including geographic and cultural differences, though the time-periods during which the biological samples were collected were similar. Different PCB profiles may contribute to mortality risk differently. Second, the racial distribution of the women in this study differed from previous studies. To our knowledge, no previous study has examined PCB-mortality associations among black women with breast cancer. Racial differences in polymorphisms of metabolism genes have been reported (Dreisbach et al., 2005; Kidd et al., 2001; Tabrizi et al., 2002; Weiserbs et al., 2003). Thus, biological differences in the efficiency with which PCBs are metabolized, stored in adipose tissue, and eliminated may influence mortality, although we only found evidence of differences by race for one of the PCBs examined here. Lastly, US black women are more likely to be diagnosed with more aggressive breast cancer subtypes (O'Brien et al., 2010). It is possible that the associations between PCBs and mortality may vary by breast cancer subtypes, which we were unable to evaluate here.
PCBs are known to accumulate in human breast tissue (Ellsworth et al., 2015) where they are hypothesized to directly and indirectly modulate the microenvironment to influence cancer initiation, progression, and therapeutic response (Casey et al., 2015). Mechanisms of carcinogenicity by PCBs and PCB metabolites have been investigated using a variety of study designs. A number of mechanisms have been proposed including the formation of reactive oxygen species and highly reactive electrophilic quinones, resulting in genotoxic effects, immune suppression, inflammatory responses, and endocrine effects (Agency for Toxic Substances and Disease Registry (ATSDR) 2015). The mechanisms by which PCBs may influence overall and breast cancer-specific mortality are unknown. Here, PCBs 74, 99, and 118 were associated with 5-year breast cancer-specific mortality. PCBs 74 and 118 are potentially anti-estrogenic, immunotoxic, and dioxin-like, while PCB99 is a phenobarbital- and CYP1A/2B-inducer (Wolff et al., 1997). Dioxinlike PCBs are able to bind the aryl hydrocarbon receptor (AhR) resulting in sustained upregulation or downregulation of numerous genes controlling cell proliferation, inhibition of apoptosis, suppression of cellcell communication and adhesion, and increased cell invasiveness (Lauby-secretan et al., 2016). In addition, the AhR is also implicated as a regulator of energy metabolism (Casals-Casas and Desvergne, 2011).
This study had several strengths including the inclusion of a more racially diverse sample of women than previously considered, the use of a biomarker to estimate body burdens of PCBs, and the long follow-up, which allowed us to examine associations with mortality for over 20 years following breast cancer diagnosis. However, these results should be interpreted in light of the limitations. First, too few congeners were detected from each of the a priori PCB groupings, so we were not able to evaluate meaningful functional groupings relevant to breast cancer progression. Furthermore, the PCBs that were detected with high frequency were highly correlated (Supplemental eTable 2). Therefore, as with many studies of complex mixtures, it is unclear whether the associations observed here are confounded by other measured and unmeasured PCB congeners or other pollutants (Braun et al., 2016). Second, we only had one baseline measurement of PCBs and so we did not consider changes in PCB levels over time. However, PCBs have long biological half-lives (Agency for Toxic Substances and Disease Registry (ATSDR) 2015). Therefore, baseline measurements may still be relevant to long-term mortality. Third, although we examined associations by race, it is unclear whether racial differences are due to greater exposure in black women or due to biological differences. Here, we were underpowered to examine associations by other important indicators such as ER status and stage or by age and body mass index. Fourth, although lipid standardization is useful for comparing exposure concentrations across specimens or across study populations, several alternatives for modeling the relationships between PCBs, lipids, and health outcomes have been proposed, which may yield varying results depending on the true underlying causal structure (Schisterman et al., 2005). Fifth, blood samples drawn after diagnosis may be impacted by breast cancer treatment. However, in the CBCS case-control design, estimates were not different when stratified by weight loss or gain, as well as stage of disease, suggesting that disease or treatment-related effects may have minimal impact on PCB levels (Millikan et al., 2000). Finally, we were unable to determine the sources of PCB exposure and thus cannot make recommendations on specific ways that women may be able to reduce exposure to PCBs following breast cancer aside from avoiding contact with known contaminated soils and reducing the consumption of animal fats.
The aim of this study was to examine the associations between 17 PCB congeners measured in blood samples from women who were diagnosed with breast cancer and mortality following breast cancer. In this study, PCB74, PCB99, and PCB118 were associated with 5-year, but not longer-term, breast cancer-specific mortality. By race, PCB74 was associated with a greater increase in breast cancer-specific mortality among black women than among white women. Additional studies aimed at understanding the underlying biological mechanisms, and more broadly, evaluating how environmental exposures may affect breast cancer survivorship may be warranted. Furthermore, as PCB levels have declined since their ban, studies are needed that examine contemporary levels in association with mortality following breast cancer. These are important considerations given that an estimated 276,000 women are diagnosed with breast cancer annually (Siegel et al., 2020).
Supplementary Material
Acknowledgments
Funding
The Carolina Breast Cancer Study was funded by the University Cancer Research Fund of North Carolina, the National Cancer Institute Specialized Program of Research Excellence (SPORE) in Breast Cancer (NIH/NCI P50-CA 58223), and Susan G. Komen for the Cure (Komen OGUNC1202). H. Parada Jr was supported by the National Cancer Institute (NCI K01 CA234317, NCI U54 CA132384, and NCI U54 CA132379), and the National Institute on Aging (NIA P30 AG059299).
Footnotes
Compliance with Ethical Standards.
Ethical approval
Informed consent was obtained from all individual participants included in the study.
Ethical standards
All procedures performed in the Carolina Breast Cancer Study involving human participants were in accordance with the ethical standards of the Institutional Review Boards of the University of North Carolina at Chapel Hill and were in compliance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Declaration of competing interest
The authors declare they have no actual or potential competing financial interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijheh.2020.113522.
References
- Agency for Toxic Substances and Disease Registry (ATSDR), 2015. Toxicological profile for polychlorinated biphenyls (PCBs). Available. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=142&tid=26, Accessed date: 29 August 2019. [PubMed]
- Braun JM, Gennings C, Hauser R, Webster TF, 2016. What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ. Health Perspect 124, A6–A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals-Casas C, Desvergne B, 2011. Endocrine disruptors: from endocrine to metabolic disruption. Annu. Rev. Physiol 73, 135–162. [DOI] [PubMed] [Google Scholar]
- Casey SC, Vaccari M, Al-Mulla F, Al-Temaimi R, Amedei A, Barcellos-Hoff MH, et al. , 2015. The effect of environmental chemicals on the tumor microenvironment. Carcinogenesis 36, S160–S183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2017. National death index. Available. http://www.cdc.gov/nchs/ndi.htm, Accessed date: 26 June 2018.
- Chen PH, Luo ML, 1982. Comparative rates of elimination of some individual polychlorinated biphenyls from the blood of PCB-poisoned patients in Taiwan. Food Chem. Toxicol 20, 417–425. [DOI] [PubMed] [Google Scholar]
- Cohn BA, Terry MB, Plumb M, Cirillo PM, 2012. Exposure to polychlorinated biphenyl (PCB) congeners measured shortly after giving birth and subsequent risk of maternal breast cancer before age 50. Breast Canc. Res. Treat 136, 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreisbach AW, Japa S, Sigel A, Parenti MB, Hess AE, Srinouanprachanh SL, et al. , 2005. The prevalence of CYP2C8, 2C9, 2J2, and soluble epoxide hydrolase polymorphisms in African Americans with hypertension. Am. J. Hypertens 18, 1276–1281. [DOI] [PubMed] [Google Scholar]
- Ellsworth D, Gillard D, Love B, Ellsworth R, Deyarmin B, Hooke J, et al. , 2015. Abundance and distribution of polychlorinated biphenyls (PCBs) in breast tissue. Canc. Res 291–297. [DOI] [PubMed] [Google Scholar]
- Høyer AP, Jørgensen T, Brock JW, Grandjean P, 2000. Organochlorine exposure and breast cancer survival. J. Clin. Epidemiol 53, 323–330. [DOI] [PubMed] [Google Scholar]
- Kidd RS, Curry TB, Gallagher S, Edeki T, Blaisdell J, Goldstein JA, 2001. Identification of a null allele of CYP2C9 in an African-American exhibiting toxicity to phenytoin. Pharmacogenetics 11, 803–808. [DOI] [PubMed] [Google Scholar]
- Kleinbaum DG, Klein M, 2012. Evaluating the proportional hazards assumption In: third ed. Survival Analysis: A Self-Learning Text, vol. 184 Springer, New York, NY. [Google Scholar]
- Koual M, Cano-Sancho G, Bats A-S, Tomkiewicz C, Kaddouch-Amar Y, Douay-Hauser N, et al. , 2019. Associations between persistent organic pollutants and risk of breast cancer metastasis. Environ. Int 132, 105028. [DOI] [PubMed] [Google Scholar]
- Lauby-secretan B, Loomis D, Baan R, El, Ghissassi F, Bouvard V, Benbrahim-tallaa L, et al. , 2016. Use of mechanistic data in the IARC evaluations of the carcinogenicity of polychlorinated biphenyls and related compounds. Environ. Sci. Pollut. Res 23, 2220–2229. [DOI] [PubMed] [Google Scholar]
- Lauby-Secretan B, Loomis D, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, et al. , 2013. Carcinogenicity of polychlorinated biphenyls and poly-brominated biphenyls. Lancet Oncol. 14, 287–288. [DOI] [PubMed] [Google Scholar]
- Leng L, Li J, mei Luo X., young Kim J., meng Li Y., mei Guo X., et al. , 2016. Polychlorinated biphenyls and breast cancer: a congener-specific meta-analysis. Environ. Int 88, 133–141. [DOI] [PubMed] [Google Scholar]
- López-Carrillo L, Torres-Sánchez L, López-Cervantes M, Blair A, Cebrián ME, Uribe M, 1999. The adipose tissue to serum dichlorodiphenyldichloroethane (DDE) ratio: some methodological considerations. Environ. Res 81, 142–145. [DOI] [PubMed] [Google Scholar]
- Millikan R, DeVoto E, Duell EJ, Tse CK, Savitz D a, Beach J, et al. , 2000. Dichlorodiphenyldichloroethene, polychlorinated biphenyls, and breast cancer among African-American and white women in North Carolina. Canc. Epidemiol. Biomarkers Prev. 9, 1233–1240. [PubMed] [Google Scholar]
- Muscat JE, Britton JA, Djordjevic MV, Citron ML, Kemeny M, Busch-Devereaux E, et al. , 2003. Adipose concentrations of organochlorine compounds and breast cancer recurrence in long Island, New York. Canc. Epidemiol. Biomarkers Prev 12, 1474–1478. [PubMed] [Google Scholar]
- National Cancer Institute, 2019. SEER stat fact sheets: breast cancer. Available. http://seer.cancer.gov/statfacts/html/breast.html, Accessed date: 1 August 2018.
- Newman B, Moorman PG, Millikan R, Qaqish BF, Geradts J, Aldrich TE, et al. , 1995. The Carolina Breast Cancer Study: integrating population-based epidemiology and molecular biology. Breast Canc. Res. Treat 35, 51–60. [DOI] [PubMed] [Google Scholar]
- O'Brien KM, Cole SR, Tse C-K, Perou CM, Carey LA, Foulkes WD, et al. , 2010. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin. Canc. Res 16, 6100–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada H, Sun X, Tse CK, Engel LS, Olshan AF, Troester MA, 2019. Plasma levels of dichlorodiphenyldichloroethene (DDE) and dichlorodiphenyltrichloroethane (DDT) and survival following breast cancer in the Carolina Breast Cancer Study. Environ. Int 125, 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada H, Wolff MS, Engel LS, Eng SM, Khankari NK, Neugut AI, et al. , 2016a. Polychlorinated biphenyls and their association with survival following breast cancer. Eur. J. Canc 56, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada H, Wolff MS, Engel LS, White AJ, Eng SM, Cleveland RJ, et al. , 2016b. Organochlorine insecticides DDT and chlordane in relation to survival following breast cancer. Int. J. Canc 138, 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT, Henderson LO, Needham LL, 1989. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch. Environ. Contam. Toxicol 18, 495–500. [DOI] [PubMed] [Google Scholar]
- Pumarega J, Gasull M, Lee DH, López T, Porta M, 2016. Number of persistent organic pollutants detected at high concentrations in blood samples of the United States population. PLoS One 11, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roswall N, Sørensen M, Tjønneland A, Raaschou-Nielsen O, 2018. Organochlorine concentrations in adipose tissue and survival in postmenopausal, Danish breast cancer patients. Environ. Res 163, 237–248. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Whitcomb BW, Buck Louis GM, Louis TA, 2005. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ. Health Perspect 113, 853–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A, 2020. Cancer statistics, 2020. CA A Cancer J. Clin 70. [DOI] [PubMed] [Google Scholar]
- Sinkkonen S, Paasivirta J, 2000. Degradation half-life times of PCDDs, PCDFs and PCBs for environmental fate modeling. Chemosphere 40, 943–949. [DOI] [PubMed] [Google Scholar]
- Soerjomataram I, Louwman MWJ, Ribot JG, Roukema JA, Coebergh JWW, 2008. An overview of prognostic factors for long-term survivors of breast cancer. Breast Canc. Res. Treat 107, 309–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi AR, Zehnbauer BA, Borecki IB, McGrath SD, Buchman TG, Freeman BD, 2002. The frequency and effects of cytochrome P450 (CYP) 2C9 polymorphisms in patients receiving warfarin. J. Am. Coll. Surg 194, 267–273. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency, 2019. Polychlorinated biphenyls (PCBs). Available. https://www.epa.gov/pcbs/learn-about-polychlorinated-biphenyls-pcbs, Accessed date: 29 August 2019.
- Vo TT, Gladen BC, Cooper GS, Baird DD, Daniels JL, Gammon MD, et al. , 2008. Dichlorodiphenyldichloroethane and polychlorinated biphenyls: intraindividual changes, correlations, and predictors in healthy women from the southeastern United States. Canc. Epidemiol. Biomarkers Prev 17, 2729–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiserbs KF, Jacobson JS, Begg MD, Wang LW, Wang Q, Agrawal M, et al. , 2003. A cross-sectional study of polycyclic aromatic hydrocarbon-DNA adducts and polymorphism of glutathione S-transferases among heavy smokers by race/ethnicity. Biomarkers 8, 142–155. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Britton JA, Teitelbaum SL, Eng S, Deych E, Ireland K, et al. , 2005. Improving organochlorine biomarker models for cancer research. Canc. Epidemiol. Biomarkers Prev 14, 2224–2236. [DOI] [PubMed] [Google Scholar]
- Wolff MS, Camann D, Gammon M, Stellman SD, 1997. Proposed PCB congener groupings for epidemiological studies. Environ. Health Perspect 105, 13–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Fischbein A, Selikoff IJ, 1992. Changes in PCB serum concentrations among capacitor manufacturing workers. Environ. Res 59, 202–216. [DOI] [PubMed] [Google Scholar]
- Zhang J, Huang Y, Wang X, Lin K, Wu K, 2015. Environmental polychlorinated biphenyl exposure and breast cancer risk: a meta-analysis of observational studies. Lehmler H-J (Ed.), PLoS One 10, e0142513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


