Abstract
Background & Aims:
Estimates of absolute risk of colorectal cancer (CRC) are needed to facilitate communication and better inform the public about the potentials and limits of cancer prevention.
Methods:
Using data from a large population-based case-control study in Germany (DACHS study, which began in 2003) and population registry data, we calculated 30-year absolute risk estimates for development of CRC, based on a healthy lifestyle score (derived from 5 modifiable lifestyle factors: smoking, alcohol consumption, diet, physical activity, and body fatness), a polygenic risk score (based on 90 single nucleotide polymorphisms), and colonoscopy history.
Results:
We analyzed data from 4220 patients with CRC and 3338 individuals without CRC. Adherence to a healthy lifestyle and colonoscopy in the preceding 10 y were associated with a reduced relative risk of CRC in men and women. We observed a higher CRC risk in participants with high or intermediate genetic risk scores. For 50-year-old men and women without a colonoscopy, the absolute risk of CRC varied according to the polygenic risk score and the healthy lifestyle score (men, 3.5%–13.4% and women, 2.5%–10.6%). For 50-year-old men and women with a colonoscopy, the absolute risk of developing CRC was much lower but still varied according to the polygenic risk score and the healthy lifestyle score (men, 1.2%–4.8% and women, 0.9%–4.2%). Among all risk factor profiles, the 30-y absolute risk estimates consistently decreased with adherence to a healthy lifestyle.
Conclusions:
In a population-based study, we found that a colonoscopy can drastically reduce the absolute risk of CRC and that the genetically predetermined risk of CRC can be further reduced by adherence to a healthy lifestyle. Our results show the magnitude of CRC prevention possible through colonoscopy and lifestyle at a predefined genetic risk.
Keywords: colon cancer, epidemiology, food, exercise
Graphical Abstract
Introduction
Colorectal cancer (CRC) is the third most common cancer and the fourth most common cause of cancer related death worldwide1. It is a complex disease with both genetic and lifestyle factors contributing to individual risk of CRC2, 3. Including the most recent genome-wide association studies (GWAS), more than 90 independent loci have been identified that are associated with the risk of CRC4–16. Although these individual genetic variants are only weakly associated with CRC, when aggregated into a polygenic risk score they are predictive of CRC and provide a continuous and quantitative measure of genetic susceptibility of CRC15, 17. Moreover, recent studies have also shown that these genetic risk variants may provide additional information that appears largely independent of a first-degree family history of CRC15.
In addition to the genetic susceptibility of CRC, there is well established evidence that lifestyle factors such as smoking18, alcohol consumption19, poor diet20–24, physical inactivity25, and body fatness26, 27 are risk factors for CRC. Using data from a large population based case-control study, we previously found that a healthy lifestyle score including five potentially modifiable lifestyle factors (non-smoking, moderate alcohol consumption, a healthy diet, physical activity, and a healthy weight) was associated with lower risk of CRC and risk further decreased with increasing adherence to the healthy lifestyle score28. Moreover, we found that adherence to healthy lifestyle reduced the risk of CRC, similarly in participants with higher and lower polygenic risk scores.
Though these results show that adherence to a healthy lifestyle was associated with reduced risk of CRC within each category of genetic risk, the results do not show the absolute risk or probability of developing CRC given a specific set of risk and protective factors. On the other hand, substantial evidence has shown that the risk of CRC can be greatly reduced through colonoscopy, allowing for the removal of precancerous lesions29, which may attenuate the influence of lifestyle and the genetic risk profile. Estimates of absolute risk are needed to facilitate communication and to better inform the public about the potentials and limits of cancer prevention.
Therefore, the aim of this analysis was to calculate detailed absolute risk estimates of CRC based on our healthy lifestyle score, an updated polygenic risk score, and information on colonoscopy history.
Materials & Methods
Study design and study population
The DACHS study (Darmkrebs: Chancen der Verhütung durch Screening) is an ongoing population-based case-control study conducted in southwest Germany since 2003. This analysis includes patients and controls recruited until 2016. Details of the DACHS study have been reported previously30, 31. Briefly, patients with a histologically confirmed, first diagnosis of CRC (International Classification of Diseases, 10th Revision [ICD-10] codes C18-C20) are eligible to participate if they are at least 30 years of age (no upper age limit), can speak German, and are physically able to participate in an interview of about one hour. All 22 hospitals in the study area offering first line treatment to patients with CRC are involved in recruitment. Approximately 50% of all eligible patients in the study area are recruited. Incomplete recruitment of patients is largely due to lack of time among the clinicians in charge of recruiting patients and notifying the study centre in the routine setting. Community-based controls are randomly selected from population registries using frequency matching with respect to age, sex and county of residence (participation rate: 51%). The DACHS study was approved by the ethics committees of the University of Heidelberg and the state medical boards of Baden-Wuerttemberg and Rhineland-Palatinate. Written informed consent was obtained from each participant before taking part.
Data collection
Patients were informed about the study by their physicians, usually a few days after surgery. Patients participated in an interview with trained interviewers who collected information on patients’ socio-demographic, medical and lifestyle history using a standardized questionnaire. In addition, we collected hospital discharge letters and pathology reports for all cases. Patients who could not be recruited during their hospital stay were contacted by mail shortly after discharge by clinicians or clinical cancer registries. The median time between CRC diagnosis and interview was 24 days. Controls were contacted by the study centre through mail and follow-up calls, and interviews were scheduled at their homes. A minority of control participants not willing to participate in a personal interview provided some key information in a self-administered short questionnaire. However, as this questionnaire did not include a food frequency questionnaire (FFQ), these participants were excluded from this analysis.
Derivation of the healthy lifestyle score
A healthy lifestyle score was created by dichotomizing the information on five lifestyle factors (smoking, alcohol consumption, diet, physical activity and BMI) based on a priori knowledge of the risk factors for CRC18–26, 32–34. The assessment of the lifestyle factors is described in the Supplementary Methods and further details on the derivation of the healthy lifestyle score were published recently28.
Derivation of the polygenic risk score
DNA was extracted from blood samples (in 99.1% of participants) or from buccal cells (in 0.9% of participants) using conventional methods. Details about genome wide single nucleotide polymorphism (SNP) analyses and imputation of missing genotypes in the DACHS study are provided in Supplementary Table 1.
We considered a most recently reported set of 95 SNPs that were identified to be associated with a higher risk of CRC in the world’s largest CRC GWAS in populations of European descent2. No linkage disequilibrium criterion was employed for generating the polygenic risk score given the pre-defined SNP set, however, checks revealed no high linkage disequilibrium (D’≥0.95) between any SNPs in our dataset. Out of the reported 95 SNPs, a total of 90 SNPs could be extracted from our dataset. The polygenic risk score was calculated as the sum of risk alleles as reported by Huyghe et al2.
Information on colonoscopy
Endoscopies prior to diagnosis of CRC (excluding those leading to the current diagnosis) (cases) or before the interview (controls) were assessed in detail during the interviews. We requested endoscopy and histology reports from the respective physicians for up to three prior endoscopies. Self-reported information was corrected if reported endoscopies could not be confirmed by medical records. Although we did not validate the information among all those who reported no prior endoscopy, we previously found the information to be accurate in a validation study35. Information on endoscopies leading to the current diagnosis were also assessed in detail during interviews. We classified a history of colonoscopy within the preceding 10 years of the reference time, including screening colonoscopies which led to the CRC diagnosis, as ‘yes’, and no history of colonoscopy or no history of colonoscopy in the preceding 10 years as ‘no’, to reflect the decreased protective effect of colonoscopy beyond this time36.
Statistical analysis
The distribution of the demographic and lifestyle characteristics of the study population according to case-control status was evaluated in descriptive analyses using the Pearson chi-square test or t-test.
Multiple logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) of the association of CRC risk with the healthy lifestyle score, polygenic risk score and colonoscopy in the preceding 10 years. We stratified the model by sex (men and women) to allow for potential differential effects for men and women and included adjustment for age in all models. In these analyses, the lifestyle score was divided into three categories: favourable lifestyle (at least four of the five healthy lifestyle factors), intermediate (three healthy lifestyle factors), or unfavourable (zero, one or two healthy lifestyle factors), and the polygenic risk score was modelled as a categorical variable in tertiles (low, intermediate, and high genetic risk).
To replicate our findings published previously28, we performed analyses on the healthy lifestyle score stratified by polygenic risk score, using this expanded dataset (which included a much larger number of participants and an updated polygenic risk score) and tested for interaction by including a cross-product term along with the main effect terms in the models, adjusting for the same covariates as previously28.
Absolute risk calculations
We estimated the 30-year absolute risk and 95% CIs for developing CRC for 50 year old men and women, with specific risk profiles, based on the principles of the modelling described by Freedman et al37 and Pfeiffer and Petracci38, considering only the healthy lifestyle score, the polygenic risk score, and colonoscopy. Briefly, the estimation of the absolute risk of CRC with this method includes estimating relative risks of CRC (calculated from population-based case-control data) and attributable risk parameters39, and combining these estimates with baseline age-specific cancer hazard rates based on incidence rates and competing mortality rates from the German Centre for Cancer Registry Data, Robert Koch Institute (the German Federal Institute within the portfolio of the Federal Ministry of Health) to estimate the probability of developing CRC over a pre-specified time interval (here: 30 years) given a person’s age and risk factors (healthy lifestyle score, polygenic risk score and colonoscopy status). Exact details of the calculations are provided in the Supplementary Methods. In sensitivity analyses, we recalculated the absolute risks using different RRs for colonoscopy history: 1. the estimate reported in a meta-analysis on screening colonoscopy29, OR=0.33, for both men and women; 2. an estimate closer to findings of a large cohort study40, RR=0.50, for both men and women, in case the effect of colonoscopy was overestimated in our case-control study.
All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R software version 2.15.3 (R Foundation for Statistical Computing, Vienna, Austria). Statistical tests were two-sided, with an alpha level of 0.05.
Results
Overall, 4220 patients with CRC and 3338 control participants recruited in 2003–2016 were included in this analysis (Figure 1). The mean age of the cases and controls was 68.4 years and 61.5% of the participants were male (Table 1).
Figure 1.
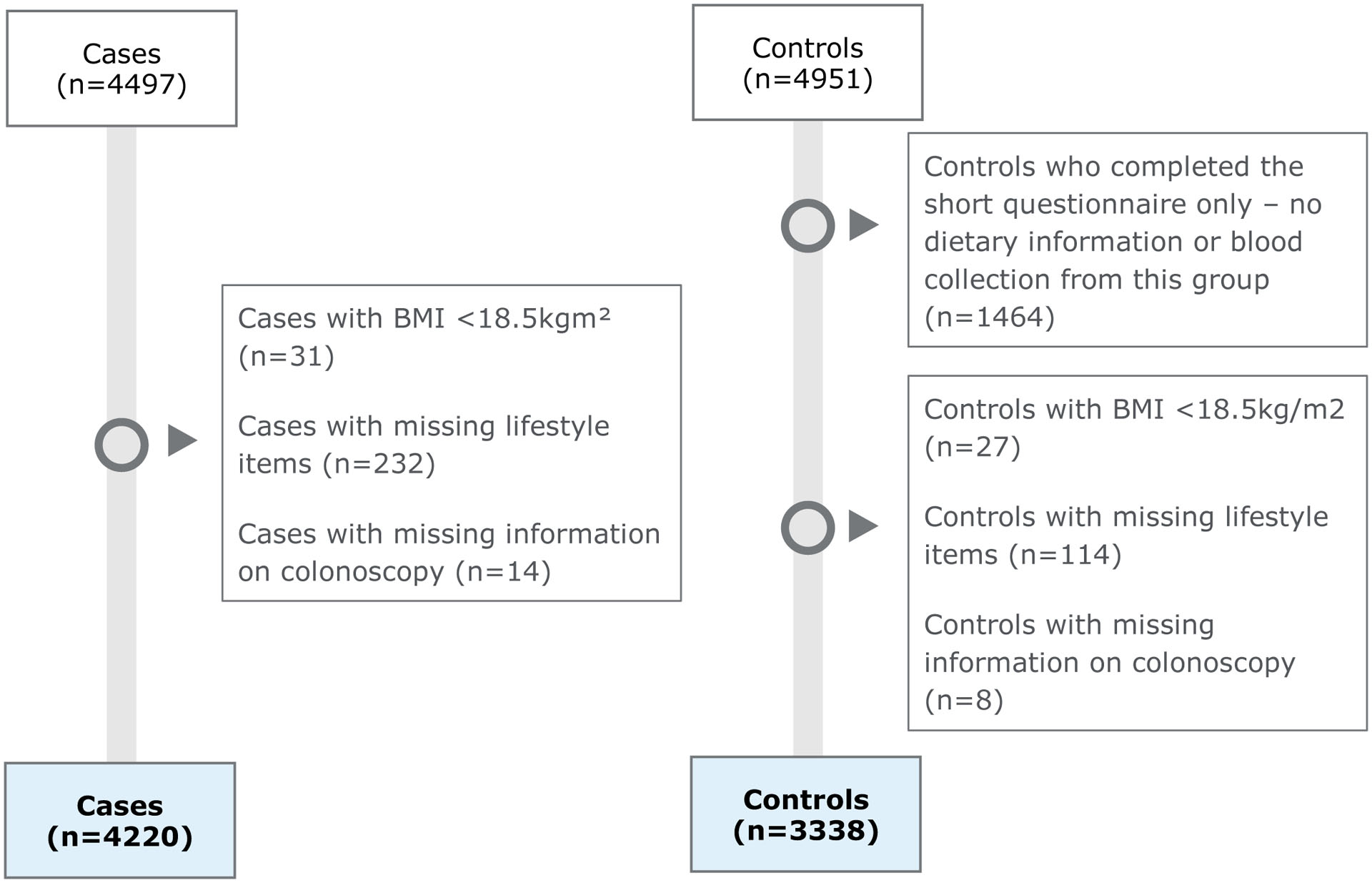
Study participants
Table 1.
Baseline characteristics of participants by case and control status.
| Characteristics | Total | Cases | Controls | P value |
|---|---|---|---|---|
| N=7558 | N=4220 | N=3338 | ||
| Sex | ||||
| Female | 2912 (38.5) | 1636 (38.8) | 1276 (38.2) | - |
| Male | 4646 (61.5) | 2584 (61.2) | 2062 (61.7) | |
| Age | ||||
| Range | 30–102 | 30–96 | 33–102 | - |
| Mean, (SD) | 68.4 (10.6) | 68.3 (10.7) | 68.5 (10.5) | |
| Education1 | ||||
| ≤9 years | 4687 (62.1) | 2795 (66.4) | 1892 (56.8) | |
| 10–11 years | 1410 (18.7) | 728 (17.3) | 682 (20.5) | <0.0001 |
| ≥12 years | 1447 (19.2) | 689 (16.4) | 758 (22.8) | |
| Smoking status | ||||
| Current or former smokers | 1558 (20.6) | 949 (22.5) | 609 (18.2) | <0.0001 |
| Alcohol consumption, g/day, mean | ||||
| Women | 5.4 | 5.2 | 5.7 | 0.11 |
| Men | 21.2 | 22.5 | 19.5 | <0.0001 |
| Dietary quality score*, mean | 31.2 | 30.5 | 32.2 | <0.0001 |
| Leisure time physical activity, MET-h/week, mean | 42.9 | 40.3 | 46.2 | <0.0001 |
| BMI, kg/m2, mean | 26.9 | 27.3 | 26.4 | <0.0001 |
| 1st degree family history of CRC2 | ||||
| Yes | 971 (12.9) | 616 (14.6) | 355 (10.6) | <0.0001 |
| Colonoscopy in the preceding 10 years | ||||
| Yes | 2840 (37.6) | 1140 (27.0) | 1700 (50.9) | <0.0001 |
| Participation in a health check up3 | ||||
| Yes | 6624 (88.0) | 3569 (84.9) | 3055 (91.9) | <0.0001 |
| NSAIDs4 | ||||
| Yes | 2184 (29.3) | 1072 (25.8) | 1112 (33.7) | <0.0001 |
| Healthy lifestyle score | ||||
| Unfavourable lifestyle (0 or 1 or 2 factors) | 2053 (27.2) | 1321 (31.3) | 732 (21.9) | |
| Intermediate lifestyle (3 factors) | 2633 (34.8) | 1504 (35.6) | 1129 (33.8) | <0.0001 |
| Favourable lifestyle (4 or 5 factors) | 2872 (38.0) | 1395 (33.1) | 1477 (44.2) | |
| Polygenic risk score | ||||
| Low (T1) | 2015 (26.7) | 901 (21.4) | 1114 (33.4) | |
| Intermediate (T2) | 2506 (33.2) | 1368 (32.4) | 1138 (34.1) | <0.0001 |
| High (T3) | 3037 (40.2) | 1951 (46.2) | 1086 (32.5) | |
| Mean (SD) | 85.8 (5.7) | 86.7 (5.6) | 84.6 (5.6) |
Abbreviations: MET, metabolic equivalent of task; BMI, body mass index; CRC, colorectal cancer; NSAIDs, non-steroidal anti-inflammatory drug;
Data missing for 14 participants;
Data missing for 6 participants;
Data missing for 32 participants;
Data missing for 93 participants;
Diet quality score max 50 points
When comparing the baseline characteristics of the study participants, patients with CRC were more likely to have a lower level of education, smoke, have a higher BMI, were less likely to have had a colonoscopy in the preceding 10 years, and were less likely to have participated in a health check-up. Control participants were more likely to be more physically active, less likely to have a family history of CRC, and more likely to use NSAIDs. Males with CRC were more likely to have a higher alcohol consumption but no difference was seen among women. Overall, patients with CRC had a lower healthy lifestyle score compared to control participants and a higher polygenic risk score (median cases: 86.8; median controls: 84.9) (Table 1).
Association of adherence to a healthy lifestyle, polygenic risk score and colonoscopy with CRC risk in men and women
In our study population, adherence to a healthy lifestyle was associated with reduced risk of CRC among both men and women after adjustment for age, polygenic risk score and previous colonoscopy (Table 2). A higher CRC risk was observed among participants at high and intermediate genetic risk than among those at low genetic risk. A colonoscopy in the preceding 10 years was associated with a strong risk reduction of CRC, as reported previously30, 36(Table 2).
Table 2.
Odds ratios of risk factors associated with colorectal cancer risk stratified by sex
| Cases n(%)/Controls n(%) | OR (95% CI) | |||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Healthy lifestyle score | ||||
| Unfavourable lifestyle | 1013(39)/590(29) | 308(19)/142(11) | 1.00 (Ref.) | 1.00 (Ref.) |
| Intermediate lifestyle | 931(36)/746(36) | 573(35)/383(30) | 0.78 (0.67–0.90) | 0.72 (0.57–0.93) |
| Favourable lifestyle | 640(25)/726(35) | 755(46)/751(59) | 0.55 (0.47–0.64) | 0.50 (0.40–0.63) |
| Polygenic risk score | ||||
| Low genetic risk | 555(21)/699(34) | 346(21)/415(33) | 1.00 (Ref.) | 1.00 (Ref.) |
| Intermediate genetic risk | 851(33)/699(34) | 517(32)/439(34) | 1.54 (1.32–1.80) | 1.45 (1.19–1.77) |
| High genetic risk | 1178(46)/664(32) | 773(47)/422(33) | 2.24 (1.93–2.62) | 2.23 (1.84–2.70) |
| Colonoscopy in the preceding 10 years | ||||
| No | 1888(73)/989(48) | 1192(73)/649(51) | 1.00 (Ref.) | 1.00 (Ref.) |
| Yes | 696(27)71073(52) | 444(27)7627(49) | 0.34 (0.30–0.39) | 0.38 (0.32–0.44) |
Abbreviations: OR, odds ratio; CI, confidence interval;
The logistic regression models included age, healthy lifestyle score, polygenic risk score and colonoscopy in the preceding 10 years
Association of adherence to a healthy lifestyle and risk of CRC according to polygenic risk score
Among both men and women, multivariable analyses revealed that within each tertile of the polygenic risk score, participants with more favourable lifestyle had a lower risk of CRC (Supplementary Table 2). In an additional analysis, we assessed in this larger number of participants and using an updated polygenic risk score, the association of the healthy lifestyle score and CRC risk according to two groups of the polygenic risk score as published previously28 and found similar results (Supplementary Table 3). Similar results were also seen when we stratified by tertiles of polygenic risk score (Supplementary Table 4).
Absolute risk estimates for CRC based on adherence to a healthy lifestyle score, polygenic risk score and previous colonoscopy
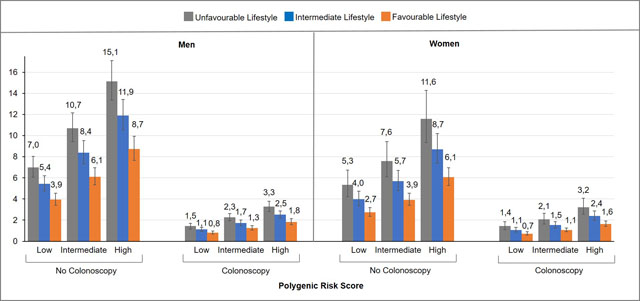
Table 3 presents estimates of the 30-year projected absolute risks of developing CRC for men and women separately, aged 50 years, combining information on polygenic risk score, adherence to a healthy lifestyle and colonoscopy status, accounting for competing causes of death. The 30-year absolute risk of CRC was largely determined by colonoscopy status. Without a colonoscopy, the 30-year absolute risk of developing CRC varied substantially depending on the individual risk profile, but across all risk factor profiles, the 30-year absolute risk estimates consistently decreased with higher adherence to a healthy lifestyle within each category of polygenic risk score, regardless of the colonoscopy status (Figure 2).
Table 3.
30-year absolute risk estimates of colorectal cancer for 50-year-old men and women
| Subgroup | Cases n(%)/Controls n(%) | 30 Year Risk, % (95% CI) | ||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| No colonoscopy | ||||
| Low genetic risk | ||||
| Unfavourable lifestyle | 164(40)/88(26) | 40(15)/26(12) | 6.2 (5.4–7.1) | 4.9 (3.9–6.2) |
| Intermediate lifestyle | 157(38)/123(36) | 101 (38)/53(24) | 4.9 (4.3–5.6) | 3.6 (3.0–4.3) |
| Favourable lifestyle | 87(21)/134(39) | 123(47)/143(64) | 3.5 (3.0–4.0) | 2.5 (2.1–2.9) |
| Intermediate genetic risk | ||||
| Unfavourable lifestyle | 260(41)/97(30) | 76(21)/20(9) | 9.4 (8.3–10.7) | 7.1 (5.7–8.8) |
| Intermediate lifestyle | 216(34)/120(37) | 131(36)/78(35) | 7.4 (6.5–8.3) | 5.2 (4.4–6.1) |
| Favourable lifestyle | 153(24)/108(33) | 156(43)/126(56) | 5.3 (4.7–6.1) | 3.6 (3.1–4.2) |
| High genetic risk | ||||
| Unfavourable lifestyle | 341(40)/115(36) | 118(21)/27(13) | 13.4 (11.8–15.1) | 10.6 (8.6–13.1) |
| Intermediate lifestyle | 302(35)/105(33) | 199(35)/57(28) | 10.6 (9.4–11.9) | 7.8 (6.7–9.1) |
| Favourable lifestyle | 208(24)/99(31) | 248(44)/119(59) | 7.6 (6.7–8.7) | 5.5 (4.8–6.3) |
| Colonoscopy | ||||
| Low genetic risk | ||||
| Unfavourable lifestyle | 53(36)/80(23) | 7(9)/21(11) | 2.1 (1.9–2.6) | 1.9 (1.5–2.4) |
| Intermediate lifestyle | 59(40)/145(41) | 33(40)/65(34) | 1.7 (1.5–2.0) | 1.4 (1.1–1.7) |
| Favourable lifestyle | 35(24)/129(36) | 42(51)/107(55) | 1.2 (1.0–1.4) | 0.9 (0.8–1.1) |
| Intermediate genetic risk | ||||
| Unfavourable lifestyle | 75(34)/110(29) | 23(15)/26(12) | 3.3 (2.9–3.1) | 2.7 (2.2–3.4) |
| Intermediate lifestyle | 80(36)/126(34) | 60(28)/60(28) | 2.6 (2.3–3.0) | 1.9 (1.7–2.4) |
| Favourable lifestyle | 67(30)/138(37) | 88(57)/129(60) | 1.9 (1.6–2.1) | 1.4 (1.2–1.6) |
| High genetic risk | ||||
| Unfavourable lifestyle | 120(38)/100(29) | 44(21)/22(10) | 4.8 (4.2–5.5) | 4.2 (3.3–5.2) |
| Intermediate lifestyle | 117(36)/127(37) | 66(32)/70(32) | 3.8 (3.3–4.3) | 3.0 (2.6–3.6) |
| Favourable lifestyle | 90(28)7118(34) | 98(47)7127(58) | 2.6 (2.3–3.1) | 2.1 (1.8–2.4) |
Abbreviations: CI, confidence interval
Figure 2.
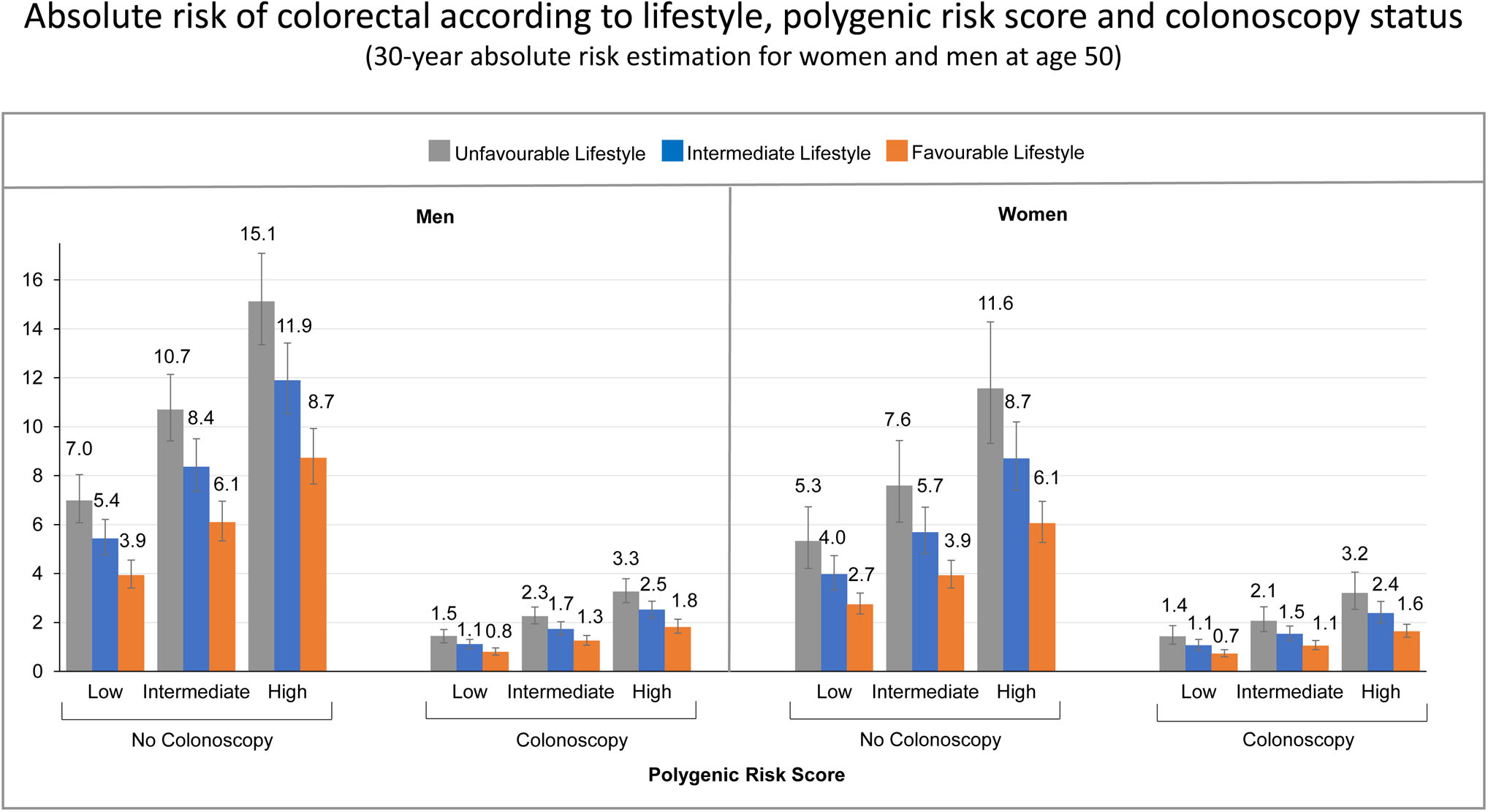
30-year absolute risk estimates of colorectal cancer for 50 year old men and women, according to lifestyle, polygenic risk score and colonoscopy status.
To illustrate, a 50-year-old male, with a high polygenic risk score, an unfavourable lifestyle and without colonoscopy, the estimated 30-year absolute risk of developing CRC was 13.4% (95% CI, 11.8%-15.1%). In contrast, for a 50-year-old male with the same risk profile, but adhering to a healthy lifestyle, the estimated 30-year absolute risk of CRC was 7.6% (95% CI, 6.7%-8.7%). Furthermore, a 50-year-old male with a favourable lifestyle who had a colonoscopy, had an estimated 30-year absolute risk of CRC of only 2.6% (95% CI, 2.3%-3.1%).
For a 50 year old female, with the highest risk profile (high genetic risk, unfavourable lifestyle and without colonoscopy), the estimated 30-year absolute risk of CRC was 10.6% (95% CI, 8.6%-13.1%). With adherence to a healthy lifestyle, the 30-year absolute risk was much lower (5.5%, 95% CI 4.8%-6.3%), and with colonoscopy, the 30 year absolute risk of CRC was estimated to be 2.1% only (95% CI, 1.8%-2.4%).
The estimated 30-year absolute risk of developing CRC for men with the lowest risk profile (50 year old male, with a low genetic risk, favourable lifestyle, who had a colonoscopy) was 1.2% (95% CI, 1.0%-1.4%), and similarly, the estimated 30-year absolute risk of developing CRC for women with the lowest risk profile (50 year old female, with a low genetic risk, favourable lifestyle, who had a colonoscopy) was 0.9% (95% CI, 0.8%-1.1%).
In a sensitivity analysis where we used an estimate of CRC risk reduction closer to findings of a large cohort study (RR=0.50), the absolute risk estimates were overall only slightly lower than in the main analyses, however, the same pattern was observed. Similar to the main analyses, the 30-year absolute risk estimates consistently decreased with adherence to a healthy lifestyle within each category of genetic risk, regardless of colonoscopy status (Supplementary Table 5, Supplementary Figure 1).
Discussion
Using data from a large epidemiological study and population registry data, we present 30 year absolute risk estimates for developing CRC combining information on adherence to a healthy lifestyle, polygenic risk score, and colonoscopy history. Of the three factors, colonoscopy status was the strongest preventive factor. If a colonoscopy was performed, absolute risks of CRC were overall much lower and the range of absolute risks determined by lifestyle and polygenic risk score was narrower. However, adherence to a healthy lifestyle and genetic risk still played an important role. Within any polygenic risk category, increased adherence to a healthy lifestyle resulted in lower 30 year absolute risk estimates of CRC, suggesting that the genetically predetermined increased risk of CRC can be offset at least to some extent by healthy lifestyle. Healthy lifestyle and genetic risk played a much stronger role if no colonoscopy was performed.
The reduction of CRC risk associated with a healthy lifestyle has been well reported28, 41–46, but we present for the first time absolute risk estimates of developing CRC based on genetic information, adherence to a healthy lifestyle and history of colonoscopy. The absolute risk results together with the sensitivity analysis results support our previous findings that lifestyle factors may powerfully modify risk of CRC regardless of the person’s genetic profile28. Although individuals may perceive that having an increased genetic risk means that they are powerless against their genetic predisposition, our results show that a healthy lifestyle can still reduce CRC risk. Moreover, while the 30-year absolute risks associated with adherence to a healthy lifestyle were greatest in the group at high genetic risk and for those with no previous colonoscopy, these results still emphasize the benefit of everyone adhering to a healthy lifestyle.
Of the three factors included in our absolute risk calculations, history of colonoscopy was the strongest preventive factor. For a 50-year-old man or woman with a history of colonoscopy, absolute risks of CRC were much lower and variation of risk according to lifestyle and polygenic risk score was less pronounced. This is consistent with the well-established evidence that gastrointestinal endoscopy (in particular polypectomy during sigmoidoscopy and colonoscopy) has a major protective effect against CRC29. Since most sporadic CRCs develop slowly over many years, the precursor lesions, adenomas and serrated polyps, can be detected and removed by colonoscopy47. Based on the current available evidence, most national and international screening guidelines therefore recommend beginning CRC screening at age 50 in average risk adults48, 49. In this large study, we only considered history of colonoscopy although stool based tests for blood (the guaiac based faecal occult blood test [gFOBT] and the faecal immunochemical test [FIT]) were also used for CRC screening. In some countries however, stool-based tests are used as the primary screening tests (for example in the UK and the Netherlands)50. Still, as we did not differentiate by indication for colonoscopy in this study, our results refer to colonoscopies for any reason, including those to follow up positive stool tests. Also, although the effect of colonoscopy might be overestimated in our case-control study, the sensitivity analyses using an effect estimate closer to those reported in a large cohort study from the US40, confirmed that the strongest risk reduction was still determined by colonoscopy, and that with adherence to a healthy lifestyle the 30-year absolute risk estimates consistently decreased within each category of genetic risk regardless of colonoscopy status. However, the sensitivity analyses also showed that with less pronounced risk reduction of colonoscopy the difference in the absolute risks between unfavourable and favourable lifestyle increased.
Strengths and limitations of this study
The major strengths of the current study include the large sample size, which enabled the combination of genetic risk, lifestyle and colonoscopy information in detail. Furthermore, we used an updated polygenic risk score for CRC using the most recently reported set of 95 SNPs that were identified to be associated with a higher risk of CRC in the world’s largest CRC GWAS in populations of European descent. Our model estimates the probability of developing CRC over a 30-year time interval using data from a large German population-based case-control study, incidence data from the German Centre for Cancer Registry Data, and data from national mortality rates. Thus, it is expected that our risk prediction models are mostly representative of the general German population. Moreover, this model includes information on lifestyle that can be easily ascertained in a clinical setting. Although genetic information is not available from the patients yet, it is increasingly being incorporated in electronic health records particularly in the US51. Also, our absolute risk estimates may facilitate communication about the risk of CRC, thereby allowing physicians to improve their patient education leading to better lifestyle management in higher risk patients (even without knowledge of the genetic risk).
Our study also has some limitations. Firstly, since we only had information collected at the reference time, the lifestyle factors were treated as fixed variables that did not change. However, diet and lifestyle behaviour may change over a person’s lifetime. Therefore, we cannot conclude how an individual’s absolute risk may change if they make healthier lifestyle choices. Secondly, in this model we estimated the relative risks and attributable risks from a case-control study. Although case-control data has previously been used for the development of risk prediction models for CRC37 or breast cancer52, 53 our estimates could be subject to recall bias. The ascertainment of lifestyle was based on self-reported information; therefore, the effects may be underestimated. In addition, we cannot rule out the possibility of selection bias, particularly in the recruitment of controls. Control participants may have been more health conscious and may have reported overall healthier lifestyles compared to the entire underlying control population. For example, control participants who only provided a self-administered questionnaire were excluded from the analysis due to lack of information on diet and genetic risk score. These participants were slightly older (70.7 years vs 68.5 years) and reported less often participation in health check-ups (74.2% vs 91.9%), which would result in some overestimation of the healthy lifestyle effect. However, on the other hand, it is possible that due to the dichotomization of risk factors in our healthy lifestyle score, the importance of healthy lifestyle is underestimated in this study. It is likely that with a more refined lifestyle score, relative risk and absolute risk estimates may be much more pronounced. In this study, we classified a small percentage of participants who had a colonoscopy more than 10 years ago together with participants who never underwent lower endoscopy, which may have led to an underestimation of the effects of colonoscopy. In addition, in rare cases, participants in our study may have had sigmoidoscopy or rectoscopy rather than colonoscopy35, but since these are rarely performed anymore in Germany, the results are likely to be unchanged. Finally, the population included in the present analyses were primarily people of European descent. Therefore, these results may not be generalizable to populations that are more diverse.
Conclusion
In conclusion, after quantifying absolute risk estimates for CRC based on three major determinants of CRC risk - adherence to a healthy lifestyle, polygenic risk score and history of colonoscopy, colonoscopy was the strongest preventive factor. We still found that better adherence to a healthy lifestyle was associated with much lower absolute risks of CRC within each category of genetic risk. These findings highlight the strong protective effect of colonoscopy and the potential of lifestyle interventions to reduce the risk of CRC across the population, even among those at high genetic risk of CRC and still among those who have had a colonoscopy. Our absolute risk estimates can be useful to facilitate communication and to better inform the public about the magnitude, potentials and limits of CRC prevention.
Supplementary Material
What you need to know:
BACKGROUND AND CONTEXT: Estimates of absolute risk of colorectal cancer (CRC) are needed to educate the public about the potentials and limits of cancer prevention.
NEW FINDINGS: A population-based study showed that a colonoscopy greatly reduces the absolute risk of CRC. The genetically predetermined risk of CRC can be reduced by adherence to a healthy lifestyle.
LIMITATIONS: The lifestyle factors in this study were treated as fixed variables that did not change, therefore, the authors cannot conclude how an individual’s absolute risk may change if they make healthier lifestyle choices.
IMPACT: Risk of CRC can be greatly reduced with colonoscopy screening and lifestyle modification for persons with all levels of genetic risk.
LAY SUMMARY: In an analysis of a large population in Europe, the authors found that colonoscopy screening and healthy lifestyles greatly reduce risk of colorectal cancer, even in persons with genetic risk factors.
Acknowledgements
The authors thank Ute Handte-Daub, Ansgar Brandhorst and Petra Bächer for their excellent technical assistance. The authors thank the study participants and the interviewers who collected the data. The authors also thank the following hospitals and cooperating institutions that recruited patients for this study: Chirurgische Universitätsklinik Heidelberg, Klinik am Gesundbrunnen Heilbronn, St. Vincentiuskrankenhaus Speyer, St. Josefskrankenhaus Heidelberg, Chirurgische Universitätsklinik Mannheim, Diakonissenkrankenhaus Speyer, Krankenhaus Salem Heidelberg, Kreiskrankenhaus Schwetzingen, St. Marienkrankenhaus Ludwigshafen, Klinikum Ludwigshafen, Stadtklinik Frankenthal, Diakoniekrankenhaus Mannheim, Kreiskrankenhaus Sinsheim, Klinikum am Plattenwald Bad Friedrichshall, Kreiskrankenhaus Weinheim, Kreiskrankenhaus Eberbach, Kreiskrankenhaus Buchen, Kreiskrankenhaus Mosbach, Enddarmzentrum Mannheim, Kreiskrankenhaus Brackenheim, and Cancer Registry of Rhineland-Palatinate, Mainz.
Grant support:
This work was supported by the German Research Council (BR 1704/6-1, BR 1704/6-3, BR 1704/6-4, BR 1704/6-6, CH 117/1-1, BR 1704/17-1, HO 5117/2-1), the German Federal Ministry of Education and Research (01KH0404, 01ER0814, 01ER0815, 01GL1712), the Interdisciplinary Research Program of the National Center for Tumor Diseases (NCT), Germany, and the National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services (NIH R01 CA195789; U01 CA137088; and R01 CA059045). The funders played no role in the design of the study, the collection, analysis and interpretation of data; and in the decision to approve publication of the finished manuscript. The authors assume full responsibility for analyses and interpretation of these data.
Abbreviations:
- BMI
body mass index
- CI
confidence interval
- CRC
colorectal cancer
- DACHS
Darmkrebs: Chancen der Verhütung durch Screening
- FFQ
food frequency questionnaire
- GWAS
genome-wide association studies
- ICD-10
International Classification of Diseases, 10th Revision
- NSAIDs
nonsteroidal anti-inflammatory drugs
- OR
odds ratio
- SNPs
single nucleotide polymorphisms
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors declare that they have no conflict of interest.
Registration: This observational study has been registered in the German Clinical Trials Register (DRKS00011793), which is a primary registry in the WHO Registry Network.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018. [DOI] [PubMed] [Google Scholar]
- 2.Huyghe JR, Bien SA, Harrison TA, et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat Genet 2019;51:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson CM, Wei C, Ensor JE, et al. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control 2013;24:1207–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berndt SI, Potter JD, Hazra A, et al. Pooled analysis of genetic variation at chromosome 8q24 and colorectal neoplasia risk. Hum Mol Genet 2008;17:2665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broderick P, Carvajal-Carmona L, Pittman AM, et al. A genome-wide association study shows that common alleles of SMAD7 influence colorectal cancer risk. Nat Genet 2007;39:1315–7. [DOI] [PubMed] [Google Scholar]
- 6.Houlston RS, Webb E, Broderick P, et al. Meta-analysis of genome-wide association data identifies four new susceptibility loci for colorectal cancer. Nat Genet 2008;40:1426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaeger E, Webb E, Howarth K, et al. Common genetic variants at the CRAC1 (HMPS) locus on chromosome 15q13.3 influence colorectal cancer risk. Nat Genet 2008;40:26–8. [DOI] [PubMed] [Google Scholar]
- 8.Tenesa A, Farrington SM, Prendergast JG, et al. Genome-wide association scan identifies a colorectal cancer susceptibility locus on 11q23 and replicates risk loci at 8q24 and 18q21. Nat Genet 2008;40:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomlinson I, Webb E, Carvajal-Carmona L, et al. A genome-wide association scan of tag SNPs identifies a susceptibility variant for colorectal cancer at 8q24.21. Nat Genet 2007;39:984–8. [DOI] [PubMed] [Google Scholar]
- 10.Tomlinson IP, Carvajal-Carmona LG, Dobbins SE, et al. Multiple common susceptibility variants near BMP pathway loci GREM1, BMP4, and BMP2 explain part of the missing heritability of colorectal cancer. PLoS Genet 2011;7:e1002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomlinson IP, Webb E, Carvajal-Carmona L, et al. A genome-wide association study identifies colorectal cancer susceptibility loci on chromosomes 10p14 and 8q23.3. Nat Genet 2008;40:623–30. [DOI] [PubMed] [Google Scholar]
- 12.Whiffin N, Hosking FJ, Farrington SM, et al. Identification of susceptibility loci for colorectal cancer in a genome-wide meta-analysis. Hum Mol Genet 2014;23:4729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanke BW, Greenwood CM, Rangrej J, et al. Genome-wide association scan identifies a colorectal cancer susceptibility locus on chromosome 8q24. Nat Genet 2007;39:989–94. [DOI] [PubMed] [Google Scholar]
- 14.Peters U, Jiao S, Schumacher FR, et al. Identification of Genetic Susceptibility Loci for Colorectal Tumors in a Genome-Wide Meta-analysis. Gastroenterology 2013;144:799–807.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weigl K, Chang-Claude J, Knebel P, et al. Strongly enhanced colorectal cancer risk stratification by combining family history and genetic risk score. Clin Epidemiol 2018;10:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Tassan NA, Whiffin N, Hosking FJ, et al. A new GWAS and meta-analysis with 1000Genomes imputation identifies novel risk variants for colorectal cancer. Sci Rep 2015;5:10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weigl K, Thomsen H, Balavarca Y, et al. Genetic Risk Score Is Associated With Prevalence of Advanced Neoplasms in a Colorectal Cancer Screening Population. Gastroenterology 2018;155:88–98.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer 2009;124:2406–15. [DOI] [PubMed] [Google Scholar]
- 19.Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer 2015;112:580–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aune D, Lau R, Chan DS, et al. Dairy products and colorectal cancer risk: a systematic review and meta-analysis of cohort studies. Ann Oncol 2012;23:37–45. [DOI] [PubMed] [Google Scholar]
- 21.Aune D, Lau R, Chan DS, et al. Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies. Gastroenterology 2011;141:106–18. [DOI] [PubMed] [Google Scholar]
- 22.Aune D, Chan DS, Lau R, et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. Bmj 2011;343:d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan DS, Lau R, Aune D, et al. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One 2011;6:e20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carr PR, Walter V, Brenner H, et al. Meat subtypes and their association with colorectal cancer: Systematic review and meta-analysis. Int J Cancer 2016;138:293–302. [DOI] [PubMed] [Google Scholar]
- 25.Boyle T, Keegel T, Bull F, et al. Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. J Natl Cancer Inst 2012;104:1548–61. [DOI] [PubMed] [Google Scholar]
- 26.Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One 2013;8:e53916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut 2013;62:933–47. [DOI] [PubMed] [Google Scholar]
- 28.Carr PR, Weigl K, Jansen L, et al. Healthy Lifestyle Factors Associated With Lower Risk of Colorectal Cancer Irrespective of Genetic Risk. Gastroenterology 2018;155:1805–1815 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. Bmj 2014;348:g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med 2011;154:22–30. [DOI] [PubMed] [Google Scholar]
- 31.Brenner H, Chang-Claude J, Rickert A, et al. Risk of colorectal cancer after detection and removal of adenomas at colonoscopy: population-based case-control study. J Clin Oncol 2012;30:2969–76. [DOI] [PubMed] [Google Scholar]
- 32.Tsoi KK, Pau CY, Wu WK, et al. Cigarette smoking and the risk of colorectal cancer: a meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol 2009;7:682–688.e1–5. [DOI] [PubMed] [Google Scholar]
- 33.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC, 2007. [Google Scholar]
- 34.World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer, 2011.
- 35.Hoffmeister M, Chang-Claude J, Brenner H. Validity of self-reported endoscopies of the large bowel and implications for estimates of colorectal cancer risk. Am J Epidemiol 2007;166:130–6. [DOI] [PubMed] [Google Scholar]
- 36.Brenner H, Chang-Claude J, Jansen L, et al. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology 2014;146:709–17. [DOI] [PubMed] [Google Scholar]
- 37.Freedman AN, Slattery ML, Ballard-Barbash R, et al. Colorectal cancer risk prediction tool for white men and women without known susceptibility. J Clin Oncol 2009;27:686–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfeiffer RM, Petracci E. Variance computations for functional of absolute risk estimates. Stat Probab Lett 2011;81:807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graubard BI, Fears TR. Standard errors for attributable risk for simple and complex sample designs. Biometrics 2005;61:847–55. [DOI] [PubMed] [Google Scholar]
- 40.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013;369:1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aleksandrova K, Pischon T, Jenab M, et al. Combined impact of healthy lifestyle factors on colorectal cancer: a large European cohort study. BMC Med 2014;12:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirkegaard H, Johnsen NF, Christensen J, et al. Association of adherence to lifestyle recommendations and risk of colorectal cancer: a prospective Danish cohort study. Bmj 2010;341:c5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hang J, Cai B, Xue P, et al. The Joint Effects of Lifestyle Factors and Comorbidities on the Risk of Colorectal Cancer: A Large Chinese Retrospective Case-Control Study. PLoS One 2015;10:e0143696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Platz EA, Willett WC, Colditz GA, et al. Proportion of colon cancer risk that might be preventable in a cohort of middle-aged US men. Cancer Causes Control 2000;11:579–88. [DOI] [PubMed] [Google Scholar]
- 45.Wei EK, Colditz GA, Giovannucci EL, et al. Cumulative risk of colon cancer up to age 70 years by risk factor status using data from the Nurses’ Health Study. Am J Epidemiol 2009;170:863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Odegaard AO, Koh WP, Yuan JM. Combined lifestyle factors and risk of incident colorectal cancer in a Chinese population. Cancer Prev Res (Phila) 2013;6:360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383:1490–502. [DOI] [PubMed] [Google Scholar]
- 48.von Karsa L, Patnick J, Segnan N, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy 2013;45:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA : The Journal of the American Medical Association 2016;315:2564–75. [DOI] [PubMed] [Google Scholar]
- 50.Ponti A, Anttila A, Ronco G, et al. Against Cancer Cancer screening in the European Union. Report on the implementation of the Council Recommendation on cancer screening. Brussels, Belgium: European Commission, 2017. [Google Scholar]
- 51.Shirts BH, Salama JS, Aronson SJ, et al. CSER and eMERGE: current and potential state of the display of genetic information in the electronic health record. J Am Med Inform Assoc 2015;22:1231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maas P, Barrdahl M, Joshi AD, et al. Breast Cancer Risk From Modifiable and Nonmodifiable Risk Factors Among White Women in the United States. JAMA Oncol 2016;2:1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989;81:1879–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


