Abstract
Animal studies have suggested that transient receptor potential (TRP) ion channels and G protein-coupled receptors (GPCRs) play important roles in itch transmission. TRPV3 gain-of-function mutations have been identified in patients with Olmsted syndrome which is associated with severe pruritus. However, the mechanisms causing itch remain poorly understood. Here, we show that keratinocytes lacking TRPV3 impair the function of protease activated receptor 2 (PAR2), resulting in reduced neuronal activation and scratching behavior in response to PAR2 agonists. Moreover, we show that TRPV3 and PAR2 were upregulated in skin biopsies from patients and mice with atopic dermatitis (AD), whereas their inhibition attenuated scratching and inflammatory responses in mouse AD models. Taken together, these results reveal a previously unrecognized link between TRPV3 and PAR2 in keratinocytes to convey itch information and suggest that a blockade of PAR2 or TRPV3 individually or both may serve as a potential approach for antipruritic therapy in AD.
Keywords: TRPV3, PAR2, itch, keratinocytes, calcium, atopic dermatitis
INTRODUCTION
Atopic dermatitis (AD) is a common inflammatory skin disease with chronic, intractable and severe itch (Hong et al., 2011, Mack and Kim, 2018). It constitutes a major unmet problem that adversely impacts the quality of life of patients because of lack of effective treatments. Defects in the skin barrier are known to underlie the pathogenesis of AD (Fallon et al., 2009). AD is mediated by type 2 immune response that involves an enrichment of basophils, groups 2 innate lymphoid cells (ILC2s), T helper 2 (Th2) cells in response to thymic stromal lymphopoietin (TSLP)(Kim et al., 2013, Kim et al., 2014, Liu, 2006, Roediger et al., 2013), resulting in elevated production of cytokines, IL-4, IL-13 and IL-31 (Brandt and Sivaprasad, 2011, Brunner et al., 2017). Despite these studies, the molecular mechanisms linking epithelial dysfunction to itch in AD are poorly understood (Mollanazar et al., 2016, Voisin and Chiu, 2018). Transient receptor potential (TRP) cation channels and G-protein coupled receptors (GPCRs) play essential roles in inflammatory skin diseases and itch transmission (Bautista et al., 2014, Dong and Dong, 2018, Geppetti et al., 2015, Gouin et al., 2018, Gouin et al., 2015, Mollanazar et al., 2016). While there is increased recognition that skin keratinocytes could function as the first sensor for itch signaling (Mack and Kim, 2018, Mollanazar et al., 2016, Nilius and Szallasi, 2014, Veldhuis et al., 2015), the contribution of GPCR-TRP signaling pathway to itch transmission remains poorly defined.
TRPV3 is a warm temperature-sensitive Ca2+-permeable cation channel abundantly expressed in skin keratinocytes (Moqrich et al., 2005, Peier et al., 2002, Xu et al., 2002). TRPV3 can also be directly activated by several natural compounds derived from plants (e.g. carvacrol and camphor) (Nilius and Szallasi, 2014). Importantly, we previously identified gain-of-function mutations in TRPV3 from Olmsted syndrome patients, clinically characterized by diffuse palmoplantar keratoderma, alopecia, and severe pruritus (Lin et al., 2012). Activation of TRPV3 in human keratinocytes is well known to regulate inflammatory responses (Szollosi et al., 2018). In mice, a Gly573Ser substitution in TRPV3 in keratinocytes resulted in an AD-like phenotype, including severe pruritus(Asakawa et al., 2006, Yoshioka et al., 2006, Yoshioka et al., 2009). To date, TRPV3 remains the only TRP channel whose mutation was directly implicated in pruritus in a human skin disease. How TRPV3 may mediate itch in the context of inflammatory skin diseases in the absence of spontaneous mutation remains unclear. One possibility is that TRPV3 may be activated by upstream GPCR signaling such as Protease-activated receptor 2 (PAR2) (Park et al., 2017, Veldhuis et al., 2015, Xu et al., 2006).
PAR2, a GPCR that belongs to the protease-activated receptor family, can be cleaved and activated by proinflammatory factors such as proteolytic enzymes, tryptase and trypsin during skin inflammation (Dery et al., 1998, Ossovskaya and Bunnett, 2004). PAR2 is also highly expressed in keratinocytes and has been implicated in AD pruritus (Briot et al., 2009, Kempkes et al., 2014, Steinhoff et al., 1999, Steinhoff et al., 2003). Activation of PAR2 leads to the production of cytokines and chemokines like TSLP that are involved in immune responses and sustained epidermal barrier disruption (Kempkes et al., 2014, Wilson et al., 2013). We hypothesize that PAR2 may couple to TRPV3 to integrate itch signaling in keratinocytes.
Here we used pharmacological, genetic, mouse behavioral assay, single-cell Ca2+ imaging, and a mouse model of AD to explore the causal relationship between TRPV3 and PAR2 in keratinocytes. Our results suggest that the PAR2-TRPV3 interactions mediate acute and AD-associated pruritus.
RESULTS
TRPV3 mediated PAR2-dependent itch
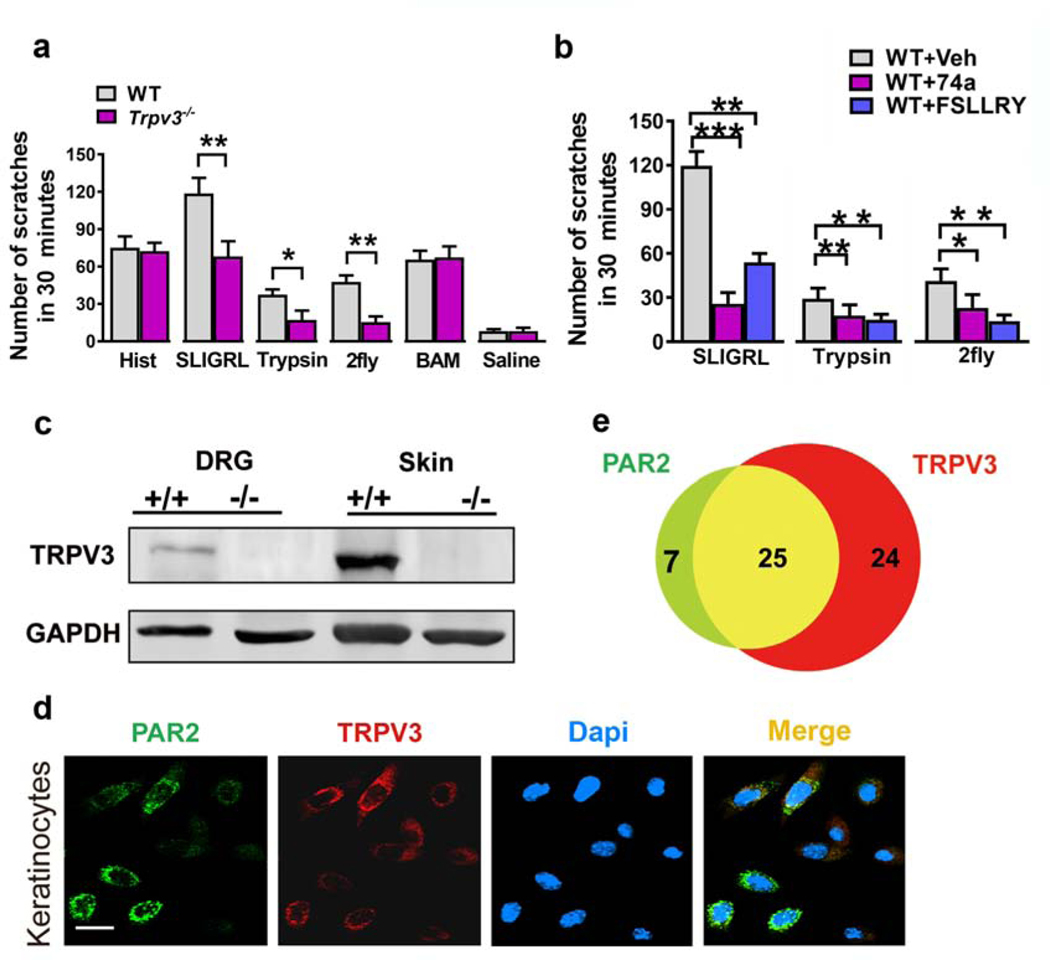
To examine whether TRPV3 is involved in acute itch transmission, we first compared the number of scratches in Trpv3−/− mice and their WT littermates after intradermal injection of a series of pruritogens (Figure 1a). Compared to WT mice, Trpv3−/− mice exhibited fewer scratches in response to SLIGRL, a PAR2 agonist, but not to histamine, indicating that TRPV3 mediates non-histaminergic itch responses. Since SLIGRL also activates MrgprC11 in DRG (Liu et al., 2011), an intradermal injection of 2fly (a PAR2 selective agonist) (McGuire et al., 2004), trypsin (an endogenous PAR2 agonist) and BAM 8–22 (a MrgprC11 agonist) were also tested. Trpv3−/− mice displayed fewer scratches in response to 2fly and trypsin but not to BAM8–22 (Figure 1a). These data strongly suggest that TRPV3 is involved in PAR2-mediated scratching response. We also pre-treated WT mice with intradermal injections of vehicle (8% DMSO), FSLLRY (a specific PAR2 antagonist) and 74a (a specific TRPV3 antagonist) (Gomtsyan et al., 2016) (Shimada et al., 2006) to confirm the effects of PAR2 and TRPV3 on scratching behavior. As expected, the number of scratches induced by SLIGRL, trypsin and 2fly decreased significantly when TRPV3 and PAR2 were pharmacologically blocked (Figure 1b).
Figure 1. TRPV3 is involved in PAR2-induced acute itch.
(a) Acute itch behaviors of Trpv3−/− mice and their WT littermates induced by intradermal injections of SLIGRL (50 μg), trypsin (100 μg), 2fly (10 μg), histamine (200 μg), and BAM 8-22 (150 μg). Unpaired t tests. n = 6–8. (b) WT mice were pre-injected with vehicle (Veh) (8% DMSO), 74a (150 μg, i.d.) or FSLLRY (100 μg, i.d.) 15 min before testing the scratching behaviors elicited by SLIGRL, trypsin, or 2fly. One-way ANOVA followed by Tukey’s post hoc. n = 6-8 mice per group. (c) TRPV3 expression in the skin and DRG of Trpv3+/+ and Trpv3−/− mice as determined by western blot. (d) Double immunofluorescence staining of PAR2 (green) and TRPV3 (red) in mouse keratinocytes. Scale bar, 20 μm. (e) Venn diagram showing the overlapping of PAR2 and TRPV3 in mouse keratinocytes. *p < 0.05, **p < 0.01, ***p < 0.001. Data are presented as mean ± s.e.m.
We next examined the co-expression of PAR2 and TRPV3 in mouse keratinocytes. Immunohistochemical (IHC) staining using antibodies against TRPV3 and PAR2 showed that 87.5% (49/56) of mouse keratinocytes expressed TRPV3 and 51% (25/49) of these cells expressed PAR2. Among PAR2 positive keratinocytes, 78.1% (25/32) of the cells expressed TRPV3 (Figure 1d and Figure 1e). The specificity of the antibodies was verified using Par2-/- and Tprv3-/- tissues (Figure 1c and Supplemental Figure S1).
TRPV3 is required for PAR2 signaling in keratinocytes
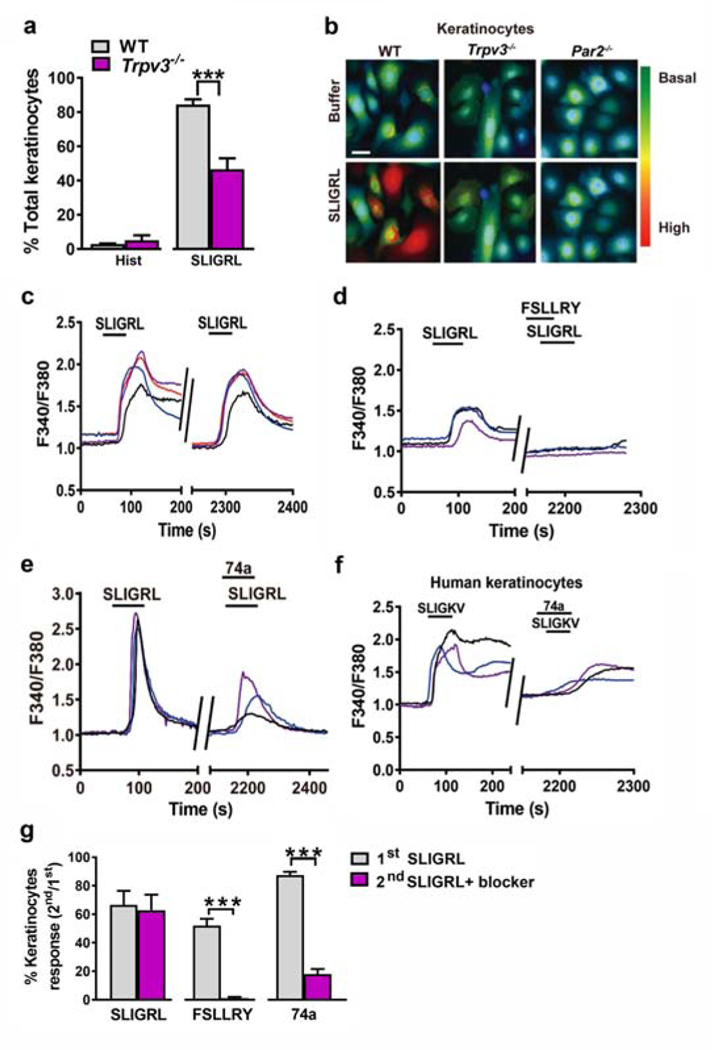
To examine the functional interactions between PAR2 and TRPV3, keratinocytes from Trpv3−/− and WT mice were treated with SLIGRL and histamine, and intracellular Ca2+ transients were quantified by calcium imaging. The proportion of SLIGRL-responsive cells was significantly reduced in Trpv3−/− keratinocytes but no significant change was observed in histamine-responsive keratinocytes (Figure 2a). Meanwhile, SLIGRL elicited stronger cytosolic Ca2+ responses in WT keratinocytes than in Trpv3−/− keratinocytes and no obvious responses in Par2−/− keratinocytes (Figure 2b). Together, these results indicate that TRPV3 is involved in PAR2-induced Ca2+ signaling in keratinocytes.
Figure 2. TRPV3 is involved in PAR2/Ca2+ signaling in keratinocytes.
(a) The percentages of responding keratinocytes from WT and Trpv3−/− mice stimulated with histamine (100 μM) and SLIGRL (50 μM). ***p < 0.001, unpaired t tests. n = 3 mice per group. (b) Representative fluorescence images of Fura2 (2 μM)-loaded WT, Trpv3−/− and Par2−/− keratinocytes stimulated with SLIGRL (50 μM). Scale bar, 20 μm. (c) Representative traces showing intracellular Ca2+ responses elicited by SLIGRL (50 μM) in WT mouse keratinocytes. (d, e) The effect of FSLLRY (100 μM) (d) and 74a (100 μM) (e) on SLIGRL-induced Ca2+ responses in WT mouse keratinocytes. (f) The effect of 74a (100 μM) on SLIGKV-induced (100 μM) Ca2+responses in human keratinocytes. (g) Quantified data showing the percentages of SLIGRL-responsive keratinocytes before and after the incubation of FSLLRY or 74a. ***p < 0.001, unpaired t test, n = 3 mice per group. Data are presented as mean ± s.e.m.
Furthermore, pre-incubation of FSLLRY, a PAR2 antagonist, completely abrogated SLIGRL-induced Ca2+ responses (Figure 2c, 2d, and 2g), while pre-incubation of 74a, the TRPV3 antagonist, largely attenuated SLIGRL or SLIGKV-induced Ca2+ responses in mouse and human keratinocytes, respectively (Figure 2e, 2f and 2g). These findings indicate that PAR2 is indispensable for SLIGRL- induced intracellular Ca2+ transients and TRPV3 is necessary to achieve adequate Ca2+ responses during PAR2 activation by SLIGRL in keratinocytes.
PAR2 activation induced Ca2+ mobilization via phospholipase C (PLC) and TSLP release from keratinocytes
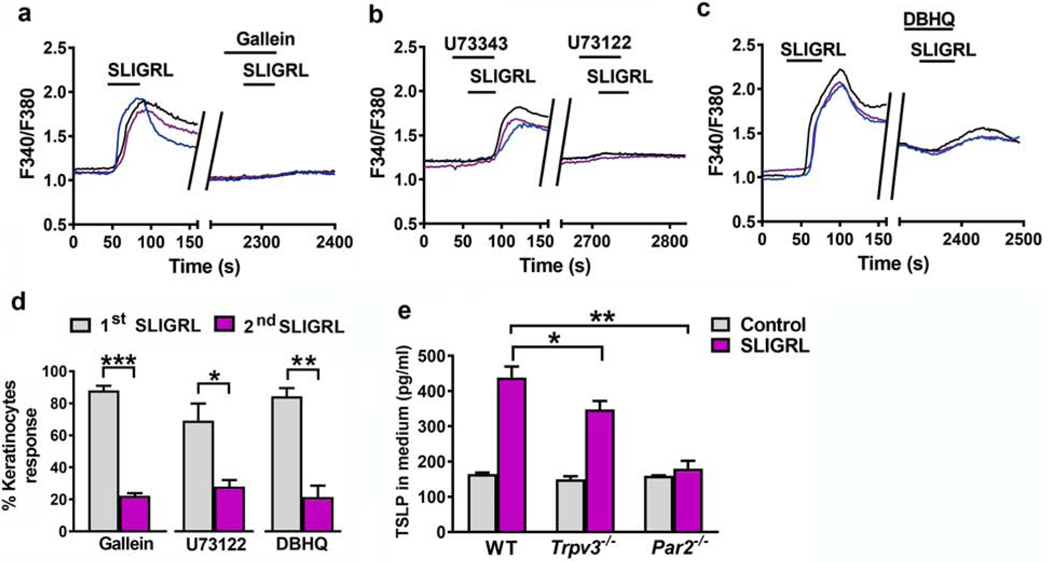
We next used a series of inhibitors to examine SLIGRL-induced signaling process in keratinocytes. The Gβγ signaling inhibitor gallein and PLC inhibitor U73122 almost completely abolished the intracellular Ca2+ response evoked by SLIGRL (Figure 3a, 3b, and 3d), consistent with a previous study (Macfarlane et al., 2005). However, after the application of endoplasmic reticulum ATPase inhibitor DBHQ, keratinocytes still showed slight Ca2+ responses (Figure 3c, and 3d), suggesting that SLIGRL activates PAR2 to elicit intracellular Ca2+ responses through not only IP3 mediated Ca+2 stores but also additional pathways probably via TRPV3.
Figure 3. The G-protein/PLC/Ca2+ pathway mediated PAR2 activation in keratinocytes, and PAR2 and TRPV3 were necessary for adequate TSLP release from keratinocytes.
(a-c) Representative traces showing that Ca2+ transients elicited by SLIGRL (50 μM) in WT mouse keratinocytes were completely inhibited by co-incubation with 100 μM gallein, a Gβγ-protein inhibitor (a) and 10 μM U73122, a PLC inhibitor (b), but were partially inhibited by co-incubation with 10 μM DBHQ, an ATPase inhibitor (c). (d) Quantified data showing the percentages of SLIGRL-responsive keratinocytes before and after the incubation of gallein, U73122 or DBHQ. *p < 0.05, **p < 0.01, ***p < 0.001, unpaired t test, n = 3 mice per group. (e) TSLP in cultured media after SLIGRL stimulation was assayed by ELISA. Keratinocytes from Trpv3−/− and Par2−/− mice released less TSLP in response to SLIGRL stimulation. n = 3 mice per group. *p < 0.05, **p < 0.01, two-way ANOVA with Bonferroni post hoc test.
PAR2 activation triggers robust TSLP release in keratinocytes (Siracusa et al., 2011), which activates sensory neurons to transmit itch and stimulates immune cells to promote TH2 cell differentiation and inflammation (Wilson et al., 2013). If PAR2 functions upstream of TRPV3 in a signaling cascade, we predicted that TSLP release would be similarly affected without either PAR2 or TRPV3 in keratinocytes. Indeed, TSLP released from Trpv3−/− and Par2−/− keratinocytes after SLIGRL stimulation was significantly less than WT (Figure 3e).
PAR2 signaling is independent of TRPV4 in keratinocytes and TRPV3 in DRG
PAR2 can sensitize TRPV4 to mediate inflammatory pain in DRGs (Grant et al., 2007, Zhao et al., 2014), the latter of which has also been implicated in itch signaling (Akiyama et al., 2016, Kim et al., 2016, Luo et al., 2018). TRPV4 in keratinocytes also mediates histaminergic itch (Chen et al., 2016). To examine whether PAR2 may also activate TRPV4 in itch signaling, we compared scratching behavior of Trpv4−/− mice and their WT littermates after intradermal injection of SLIGRL and found no significant difference between the two groups (Supplementary Figure S2a). Moreover, Trpv4−/− and WT keratinocytes showed comparable Ca2+ responses to SLIGRL stimulation (Supplementary Figure S2c–S2e). Together, we conclude that TRPV4 is not involved in PAR2-induced itch, consistent with a previous study (Akiyama et al., 2016).
Next, we examined whether TRPV3, which is also expressed in DRG (Smith et al., 2002, Xu et al., 2002), may function downstream of PAR2 in DRGs. We found that TRPV3 expression levels in DRG were much lower than that in the skin (Figure 1c). Furthermore, Ca2+ responses to SLIGRL and histamine in DRG were similar between Trpv3−/− and WT mice (Supplementary Figure S2b). These data suggest that PAR2 signaling is likely independent of TRPV3 in the DRG.
PAR2/TRPV3 signaling cascade plays a critical role in AD pruritus
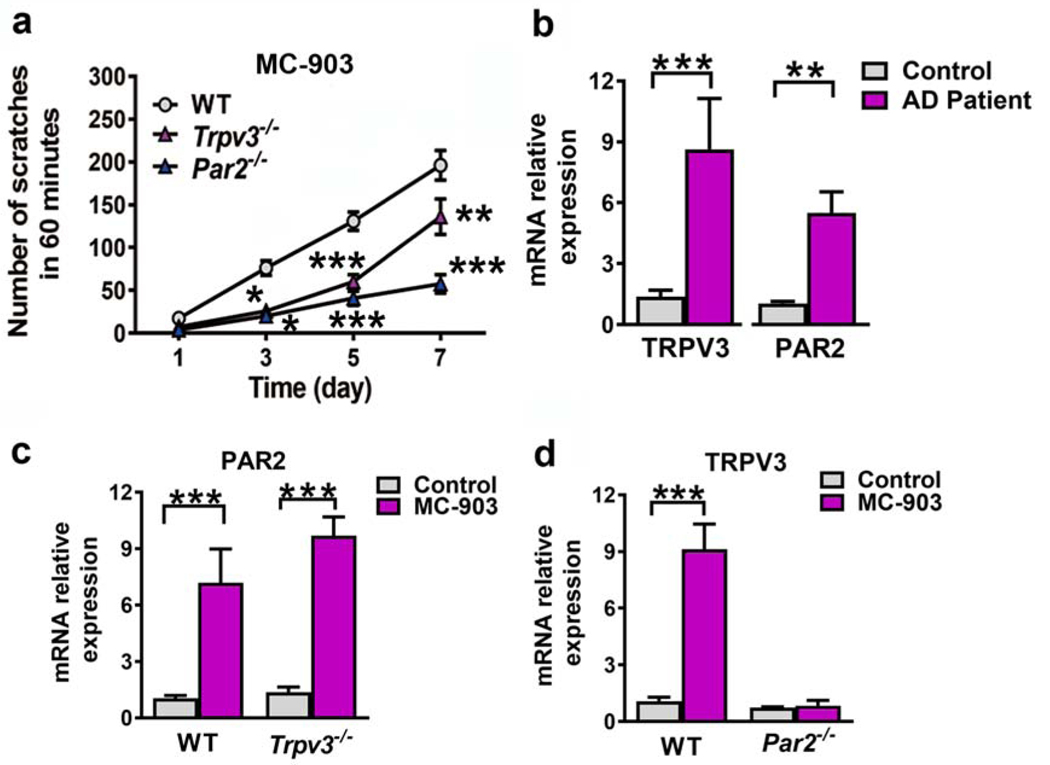
Next, we examined the expression of PAR2 and TRPV3 by collecting skin samples from 12 control individuals and 12 AD patients. PAR2 and TRPV3 mRNA levels were significantly increased in pruritic areas of AD patients compared to the control group (Figure 4b). To investigate the role of PAR2/TRPV3 signaling pathway in the pathogenesis of chronic itch in AD, topical application of calcipotriol (MC-903), a vitamin D3 analogue, was employed to induce AD-like disease characterized by erythema, edema, dry skin and excoriation accompanied by spontaneous scratching (Li et al., 2006). Scratching bouts were significantly reduced in Trpv3−/− and Par2−/− mice in the context of AD-like disease compared to control WT mice (Figure 4a). In histological studies, the ear skin of Trpv3−/− and Par2−/− mice appeared less hyperkeratotic and with less spongiosis compared with WT controls (Supplementary Figure S3a–f). Moreover, ear thickness was reduced in knockout (KO) mice treated with MC903 compared to WT controls (Supplementary Figure S3g). Importantly, PAR2 mRNA levels were increased significantly in the skin of both WT and Trpv3−/− mice (Figure 4c). In contrast, TRPV3 mRNA levels were increased only in WT mice but not in Par2−/− mice with AD-like disease (Figure 4d). These findings suggest that PAR2 expression functions upstream of TRPV3 to regulate its expression.
Figure 4. PAR2 and TRPV3 were involved in the pathogenesis of chronic itch.
(a) WT, Trpv3−/− and Par2−/− mice were treated daily with a topical application of MC-903. Scratching numbers were recorded on day 1, 3, 5, and 7. Two-way ANOVA followed by Bonferroni post hoc. n = 6–8 mice per group. (b) Real time RT-PCR showing the relative levels of TRPV3 and PAR2 mRNA in pruritic skin lesions of AD patients and normal control. Unpaired t test. n = 12. (c, d) Relative levels of PAR2 mRNA (c) and TRPV3 mRNA (e) in the ears of MC-903-treated mice on day 5. Two-way ANOVA with Bonferroni post hoc. n = 4–5. Data are presented as mean ± s.e.m. *p < 0.05, **p < 0.01, ***p < 0.001.
We also compared mRNA levels of cytokines in lesioned AD-like skin of WT, Par2−/− and Trpv3-/- mice and found that the mRNA levels of IL-6, IL-17A and IgE receptor were significantly decreased in Trpv3−/− and Par2−/− mice relative to the control (Supplementary Figure S4). These data suggest that the PAR2/TRPV3 signaling pathway modulates the production of cytokines associated with AD-like symptoms in mice.
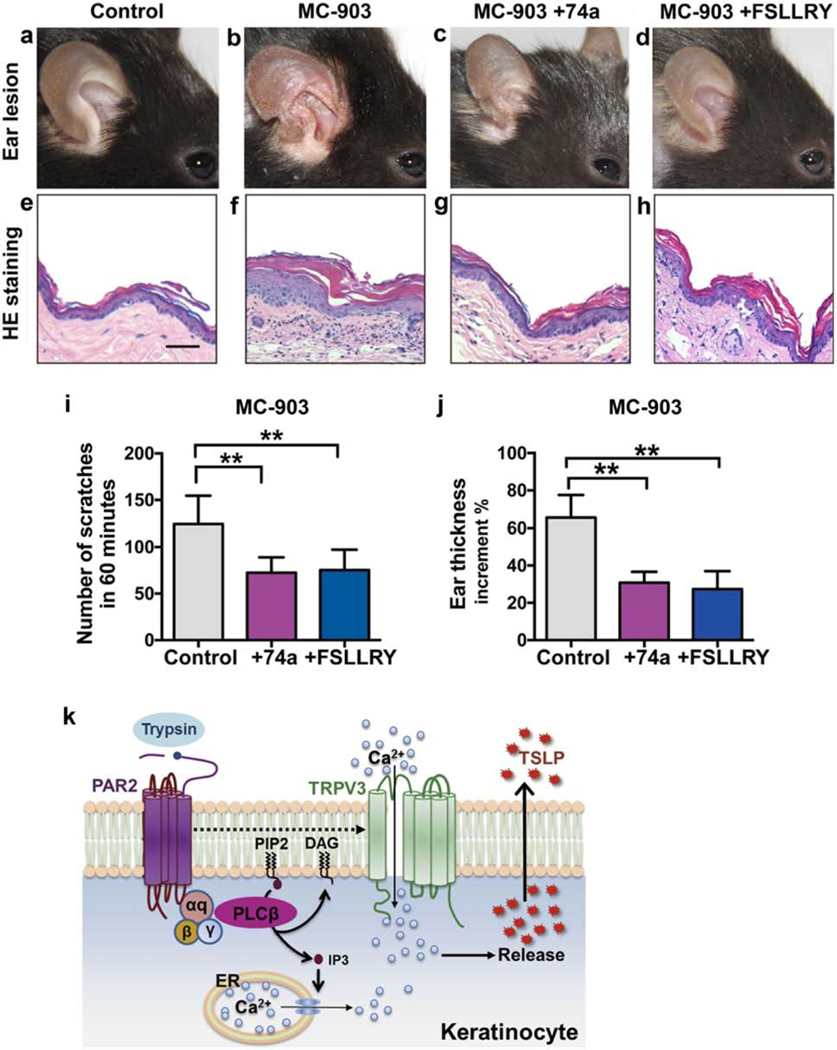
To test whether AD-like pathology could be ameliorated by a blockade of the PAR/TRPV3 signaling, topical treatment of the PAR2 antagonist FSLLRY or the TRPV3 antagonist 74a was applied to AD mice. Compared to the control, the 74a and FSLLRY-treated AD mice showed milder hyperkeratosis and acanthosis in the epidermis, less leukocyte infiltration and angiogenesis in dermis (Figure 5a–5h), reduced number of scratches (Figure 5i), and improved ear swelling (Figure 5j).
Figure 5. Topical blockade of PAR2 and TRPV3 attenuated the manifestations of AD mouse model.
(a-d) Ear appearance of WT mice topically applied with vehicle as a control (a), MC-903 (b), MC-903+74a (c), and MC-903+FSLLRY (d) for 7 days. (e-h) Hematoxylin-eosin staining showing changes in hyperkeratosis and inflammatory infiltration in the ears of corresponding mice in panels a-d, respectively. Scale = 50 μm. (i. j) The scratching numbers (i) and ear thickness increment (j) of mice treated with MC-903, MC-903+74a and MC-903+FSLLRY for 7 days. One-way ANOVA followed by Tukey’s post hoc. n = 6–8 mice per group. (k) Diagram illustrating PAR2-TRPV3 signaling pathway in itch.
DISCUSSION
Using an interdisciplinary approach, our study shows that PAR2 functions upstream of TRPV3 in a signaling cascade for itch in keratinocytes. To the best of our knowledge, the present study provides the first direct behavioral evidence supporting the hypothesis that PAR2 mediates itch in a TRPV3-dependent manner. This conclusion is further supported by the evidence showing that PAR2-induced TSLP release requires TRPV3 in keratinocytes.
Our studies clarify the role of SLIGRL in itch, with respect to two distinct receptors: PAR2 and MrgprC11. It has been shown that SLIGRL mediates itch via MrgprC11 in DRGs (Liu et al., 2011), giving rise to the notion that SLIGRL is not an agonist for PAR2. The present study shows that not only SLIGRL, but also 2fly, a PAR2 selective agonist, activates PAR2 in keratinocytes.
Thus, SLIGRL-induced itch requires PAR2 in the skin which do not express Mrgprs (Liu et al., 2011), and MrgprC11 in DRGs, respectively. These data reconcile previous conflicting results concerning whether SLIGRL is a PAR2 agonist (Kempkes et al., 2014), suggesting tissue and cell-type specific roles of SLIGRL.
The finding that PAR2 acts upstream of TRPV3 is consistent with a recent in vitro study showing that attenuated TRPV3 function compromised the response of PAR2 to an agonist in keratinocytes (Park et al., 2017). Together with previous studies supporting the roles of PAR2 and TRPV3, independently of one another, in AD (Barr et al., 2019, Yoshioka et al., 2006, Yoshioka et al., 2009), the present study provides a causal link between PAR2 and TRPV3 in acute itch as well as chronic pruritus associated with AD. The observation that TRPV3 antagonist 74a attenuated PAR2-induced intracellular Ca2+ responses in mouse and human keratinocytes implies that the function of TRPV3 signaling in itch transmission relies at least in part on the presence of PAR2 in keratinocytes. Importantly, we found that the PAR2 response was impaired in mice lacking TRPV3. Because PAR2 agonists do not act on PAR2 in DRGs, this suggests that the impaired function of PAR2 originates in TRPV3 deficiency in the skin. Given that the PAR2-TRPV3 signaling cascade has also been implicated in post-burn pruritus, with the former activating the latter through protein kinase A and protein kinase C-dependent pathways in keratinocytes (Park et al., 2017), we propose that regardless of etiology of skin diseases, the PAR2-TRPV3 pathway may be a conserved mechanism for itch transmission.
How does PAR2 cross-activate TRPV3 in itch transmission in keratinocytes? One possibility is that PAR2 sensitizes TRPV3 through intracellular PLC-Ca2+ signaling, which directly opens a TRPV3 channel (Figure 5k), reminiscent of Gq/11-coupled receptor-dependent PI(4,5)P2 hydrolysis (Doerner et al., 2011). Whether there is direct physical interaction between PAR2 and TRPV3 remains to be determined. It should be noted that impaired itch behavior of PAR2 and Trpv3-/- and Par2-/- mice may also be attributed to their expression in other tissues such as immune cells and sensory neurons (Shpacovitch et al., 2008, Smith et al., 2002, Xu et al., 2002). In the context of AD, however, activation or upregulation of PAR2/TRPV3 should be sufficient to trigger spontaneous scratching behavior, as suggested by gain-of-function of TRPV3 mutations. Accompanied by scratching, the PAR2-TRPV3 pathway in the skin would further trigger the release of TSLP, a proinflammatory cytokine (Figure 5k), resulting in heightened inflammation and exacerbation of the vicious itch-scratch cycle. On the other hand, TSLP release and induction of other type 2 cytokines in turn could activate their respective receptors and TRPs in sensory neurons, further contributing to itch responses.
While PAR2 can cross activate TRPs, the mode of action of PAR2-TRPV3 in the skin may differ from PAR2-TRPs in the sensory neurons, where PAR2 could sensitize TRPV1/A1, resulting in neuropeptide release and cutaneous neurogenic inflammation (Gouin et al., 2018, Gouin et al., 2017, Gouin et al., 2015). In sensory neurons, TRPV4 may be involved in PAR2-mediated scratching and inflammatory responses in the skin (Kittaka and Tominaga, 2017, Luo et al., 2018) (Kim et al., 2016). On the other hand, given that TRPV1 is also expressed in the skin (Oh et al., 2013), whether it is involved in PAR2-mediated response also remains to be determined. Nevertheless, accumulating evidence points to an important role for PAR2-TRPV3 pathway in the development of skin inflammatory response and pruritus, especially associated with AD.
TRPV3 activation or sensitization can contribute to pruritus via PAR2-independent and – dependent mechanisms. The gain-of-function mutations of TRPV3 evidently occurs independent of prior PAR2 activation, as shown in Olmsted Syndrome patients (Lin et al., 2012) and mice (Asakawa et al., 2006, Yoshioka et al., 2006, Yoshioka et al., 2009). In the context of AD or other chronic itch, TRPV3 can also be potentiated by PAR2 whose activation results from inflammatory response, manifested by TSLP release to activate its receptors in DRGs (Wilson et al., 2013). Taken together, our data suggest that the canonical Gq/11-protein coupled PLC-Ca2+ signaling pathway is engaged in SLIGRL-induced PAR2 activation in mouse keratinocytes, followed by Ca2+ mobilization from endoplasmic reticulum to activate TRPV3, followed by TSLP release in inflammatory skin disease conditions (Figure 5k). Therapeutically, TRPV3 or PAR2 can be either individually or concurrently inhibited to attenuate the allergic inflammation and AD-associated pruritus together (Barr et al., 2019, Nakajima et al., 2014), and such an inhibition could target skin exclusively or dampen neuroinflammation such as decreasing leukocyte recruitment via their targets in other tissues. The PAR2-TRV3 signaling interface may also be an AD-associated itch-specific axis for future exploration of therapeutic strategies for treating AD.
MATERIALS & METHODS
Animals
Adult male (2–3-month-old) Trpv3-/-, Par2−/− and Trpv4-/- mice and their wild-type (WT) littermates were used for the study (Trpv3-/-, Par2−/− mice were obtained from Jackson Laboratory and Trpv4-/- mice were purchased from Riken). Mice were housed in a controlled environment at a constant temperature of 23°C, a light/dark cycle of 12/12 h and humidity of 50±10% with food and water available ad libitum. All experimental procedures were performed in accordance with the National Institutes of Health guide for the care and use of laboratory animals and were approved by the Animal Studies Committee at Peking University First Hospital and Washington University School of Medicine.
Itch Behavior
Drugs were dissolved in 0.9% saline and injected intradermally at the nape of the neck: SLIGRL (50 μg, GenScript, Piscataway, NJ), trypsin (100 μg, Gibco), histamine (200 μg, Sigma-Aldrich), BAM8–22 (150 μg, GenScript, Piscataway, NJ), 2-Furoyl-LIGRLO-amide (2fly, 10 μg, GenScript, Piscataway, NJ) or FSLLRY (100 μg, Tocris Bioscience, Minneapolis, MN). TRPV3 antagonist 74a (100 μM, AbbVie) was first dissolved in DMSO and then diluted with saline to a final DMSO concentration of 8% for intradermal injections. Saline with 8% DMSO was used as vehicle control for 74a and FSLLRY. Scratching behavior was quantified by recording the number of scratching bouts in a period of 30 min. In the chronic itch model of AD, mouse ears were topically treated once a day with calcipotriol (MC-903) (Tocris Bioscience, 2 nmol/20 μl) dissolved in ethanol. Scratching behavior in chronic itch models was recorded for one hour as described (Zhao et al., 2013).
Culture of mouse and human epidermal keratinocytes
The primary keratinocyte culture was prepared as previously described with minor modifications (Luo et al., 2012). Newborn mouse pups (P0–P3) or normal human skin specimens from plastic surgery or skin benign tumor resection were soaked in 10% povidone-iodine for 5 min. After rinsing in 70% ethanol several times, the skin was placed in a Petri dish containing phosphate-buffered saline (PBS) solution with 2.5% Dispase II (Roche) and incubated at 4°C overnight. The epidermis was then separated from dermis. Keratinocytes were dissected by gentle scraping and flushing with CnT-07 medium (Advanced Cell Systems). Harvested cells were plated on coverslips covered with coating matrix (Life Technologies) and cultured in CnT-07 medium. Mouse cells were used after 72 hours and human cells were used after 7 days.
Culture of mouse DRG
DRGs were prepared from 3–4 week old mice (Kim et al., 2016). Mice were sacrificed, DRG were dissected out and incubated in Neurobasal-A medium (Gibco) containing 30 μl papain (Worthington) at 37°C for 20 min, and an additional 20 min digestion at 37°C with collagenase type 2 (Worthington). After washing, gentle trituration was performed using a glass pipette and cells were filtered through a 40 μm nylon cell strainer (BD Falcon). The homogenate was centrifuged at 500 xg for 5 min. Cell pellets were resuspended in culture medium composed of Neurobasal medium (Gibco, 92% vol/vol), fetal bovine serum (Invitrogen, 2% vol/vol), horse serum (Invitrogen, 2% vol/vol), GlutaMax (2 mM, Invitrogen, 1% vol/vol) and B27 (Invitrogen, 2% vol/vol), and then plated onto coverslips coated with laminin and poly-ornithine. Calcium imaging was performed after culturing the cells overnight.
Calcium imaging
Calcium imaging was performed on a Nikon Eclipse Ti microscope using Fura-2 AM (Invitrogen) as described(Kim et al., 2016). Drugs were diluted to the required concentrations in artificial cerebrospinal fluid buffer: 140 mM NaCl, 2.4 mM CaCl2, 1.3 mM MgCl2, 4 mM KCl, 10 mM HEPES and 5 mM glucose. Results are presented as ratios of F340/F380.
Immunofluorescence
Primary antibodies used for immunofluorescence of cultured primary keratinocytes were: anti-TRPV3 antibody (Alomone Labs, 1:300) and anti-PAR2 antibody (Santa Cruz, 1:100). This was followed by secondary antibodies conjugated to FITC (Jackson ImmunoResearch, 1:1000) or Cy3 (Jackson ImmunoResearch, 1:500). Samples were examined with a Nikon A1 confocal laser microscope.
Western blot
Frozen skin and DRG from WT and Trpv3-/- mice were ground in liquid nitrogen and then lysed in RIPA buffer (Keygen Biotech). Protein samples were resolved on a 10% SDS-PAGE gel and transferred onto a nitrocellulose membrane. The primary antibodies used were goat anti-TRPV3 (Alomone Labs, 1:100) and goat anti-GAPDH (Leagene, 1:1000) antibodies. Blots were incubated in anti-goat horseradish peroxidase-conjugated secondary antibody and visualized by enhanced chemiluminescence (Millipore).
AD patients and sample collection
Human skin biopsy specimens were collected from patients diagnosed with AD based on typical manifestations of pruritus, eczema and chronic dermatitis. All patients agreed to enroll in the study. The experiments on human samples were conducted after obtaining written informed consent and approval from the Clinical Research Ethics Committee of the Peking University First Hospital.
Real time RT-PCR
Total RNA was extracted from human or mouse skin tissues using TRIzol Reagent (Invitrogen). cDNA was synthesized using the high capacity cDNA reverse transcription kit (Applied Biosystems). cDNA was quantified with the SYBR Green Master Mix (Roche) using the Step OnePlus real-time PCR system (Applied Biosystems). The generated cycle threshold (Ct) value was normalized to the Ct value of GAPDH. Primers used are listed in Table S1.
ELISA Assay
The mouse TSLP ELISA kit was obtained from Abcam (ab155461). The cultured medium of mouse keratinocytes stimulated with SLIGRL for 48 hours was used for TSLP assay following the manufacturer’s instruction. OD450 values were measured on a microplate reader (Bio-Rad).
Statistics
Statistical comparisons were performed using Graphpad Prism (version 7.0, GraphPad), with student’s t test or one or two-way analysis of variance (ANOVA) as indicated (*P < 0.05; **P < 0.01; ***P < 0.001;). Numerical results were presented as mean ± standard error of the mean (s.e.m.).
Data availability
Datasets related to this article are available from the corresponding author upon reasonable request.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (grant no. 81271744 to YY), China National Funds for Distinguished Young Scientists (grant no. 81425020 to YY) CAMS Innovation Fund for Medical Sciences (CIFMS, 2018-I2M-3-006) and the NIH grants (1R01AR056318-05, R21 NS088861-01A1, R01NS094344, R01 DA037261-01A1 and R56 AR064294-01A1 to ZFC). We thank all the patients for participating in this study. We thank Arthur R Gomtsyan and Phil Kym (Abbvie) for providing TRPV3 antagonist 74a. We thank J. Yin for mouse genotyping. We thank Dr. Y Li Lab for calcium imaging support at Peking University.
CONFLICT OF INTEREST
BSK: Consulting and advisory board payments received from Regeneron-Sanofi, AbbVie, Pfizer, Cara Therapeutics, Menlo Therapeutics, Concert Pharmaceuticals, Boehringer Ingelheim, Theravance Pharmaceuticals, Celgene, LEO Pharma; grants received from Cara Therapeutics, Celgene, and LEO Pharma. All other authors state no conflict of interest.
Abbreviations:
- AD
atopic dermatitis
- BAM 8–22
bovine adrenal medulla peptide 8–22
- DRG
dorsal root ganglia
- GPCR
G protein coupled receptor
- Mrgpr
Mas-related G-protein coupled receptor
- PAR2
protease-activated receptor 2
- TRPV3
transient receptor potential cation channel V3
- TSLP
thymic stromal lymphopoietin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akiyama T, Ivanov M, Nagamine M, Davoodi A, Carstens MI, Ikoma A, et al. Involvement of TRPV4 in Serotonin-Evoked Scratching. J Invest Dermatol 2016;136(1):154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa M, Yoshioka T, Matsutani T, Hikita I, Suzuki M, Oshima I, et al. Association of a mutation in TRPV3 with defective hair growth in rodents. J Invest Dermatol 2006;126(12):2664–72. [DOI] [PubMed] [Google Scholar]
- Barr TP, Garzia C, Guha S, Fletcher EK, Nguyen N, Wieschhaus AJ, et al. PAR2 Pepducin-Based Suppression of Inflammation and Itch in Atopic Dermatitis Models. J Invest Dermatol 2019;139(2):412–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Wilson SR, Hoon MA. Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci 2014;17(2):175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt EB, Sivaprasad U. Th2 Cytokines and Atopic Dermatitis. J Clin Cell Immunol 2011;2(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briot A, Deraison C, Lacroix M, Bonnart C, Robin A, Besson C, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med 2009;206(5):1135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol 2017;139(4S):S65–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Fang Q, Wang Z, Zhang JY, MacLeod AS, Hall RP, et al. Transient Receptor Potential Vanilloid 4 Ion Channel Functions as a Pruriceptor in Epidermal Keratinocytes to Evoke Histaminergic Itch. J Biol Chem 2016;291(19):10252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dery O, Corvera CU, Steinhoff M, Bunnett NW. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol 1998;274(6):C1429–52. [DOI] [PubMed] [Google Scholar]
- Doerner JF, Hatt H, Ramsey IS. Voltage- and temperature-dependent activation of TRPV3 channels is potentiated by receptor-mediated PI(4,5)P-2 hydrolysis. J Gen Physiol 2011;137(3):271–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XT, Dong XZ. Peripheral and Central Mechanisms of Itch. Neuron 2018;98(3):482–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet 2009;41(5):602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppetti P, Veldhuis NA, Lieu T, Bunnett NW. G Protein-Coupled Receptors: Dynamic Machines for Signaling Pain and Itch. Neuron 2015;88(4):635–49. [DOI] [PubMed] [Google Scholar]
- Gomtsyan A, Schmidt RG, Bayburt EK, Gfesser GA, Voight EA, Daanen JF, et al. Synthesis and Pharmacology of (Pyridin-2-yl)methanol Derivatives as Novel and Selective Transient Receptor Potential Vanilloid 3 Antagonists. Journal of medicinal chemistry 2016;59(10):4926–47. [DOI] [PubMed] [Google Scholar]
- Gouin O, ĽHerondelle K, Buscaglia P, Le Gall-Ianotto C, Philippe R, Legoux N, et al. Major Role for TRPV1 and InsP3R in PAR2-Elicited Inflammatory Mediator Production in Differentiated Human Keratinocytes. J Invest Dermatol 2018;138(7):1564–72. [DOI] [PubMed] [Google Scholar]
- Gouin O, ĽHerondelle K, Lebonvallet N, Le Gall-Ianotto C, Sakka M, Buhe V, et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017;8(9):644–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin O, Lebonvallet N, ĽHerondelle K, Le Gall-Ianotto C, Buhe V, Plee-Gautier E, et al. Self-maintenance of neurogenic inflammation contributes to a vicious cycle in skin. Exp Dermatol 2015;24(10):723–6. [DOI] [PubMed] [Google Scholar]
- Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol 2007;578(Pt 3):715–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Buddenkotte J, Berger TG, Steinhoff M. Management of itch in atopic dermatitis. Semin Cutan Med Surg 2011;30(2):71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempkes C, Buddenkotte J, Cevikbas F, Buhl T, Steinhoff M. Role of PAR-2 in Neuroimmune Communication and Itch In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Frontiers in Neuroscience; Boca Raton (FL); 2014. [PubMed] [Google Scholar]
- Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med 2013;5(170):170ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Wang K, Siracusa MC, Saenz SA, Brestoff JR, Monticelli LA, et al. Basophils promote innate lymphoid cell responses in inflamed skin. J Immunol 2014;193(7):3717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Barry DM, Liu XY, Yin S, Munanairi A, Meng QT, et al. Facilitation of TRPV4 by TRPV1 is required for itch transmission in some sensory neuron populations. Sci Signal 2016;9(437):ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittaka H, Tominaga M. The molecular and cellular mechanisms of itch and the involvement of TRP channels in the peripheral sensory nervous system and skin. Allergol Int 2017;66(1):22–30. [DOI] [PubMed] [Google Scholar]
- Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A 2006;103(31):11736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Chen Q, Lee M, Cao X, Zhang J, Ma D, et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am J Hum Genet 2012;90(3):558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Weng HJ, Patel KN, Tang ZX, Bai HH, Steinhoff M, et al. The Distinct Roles of Two GPCRs, MrgprC11 and PAR2, in Itch and Hyperalgesia. Science Signaling 2011;4(181). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med 2006;203(2):269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Feng J, Yu G, Yang P, Mack MR, Du J, et al. Transient receptor potential vanilloid 4-expressing macrophages and keratinocytes contribute differentially to allergic and nonallergic chronic itch. J Allergy Clin Immunol 2018;141(2):608–19 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Stewart R, Berdeaux R, Hu H. Tonic inhibition of TRPV3 by Mg2+ in mouse epidermal keratinocytes. J Invest Dermatol 2012;132(9):2158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane SR, Sloss CM, Cameron P, Kanke T, McKenzie RC, Plevin R. The role of intracellular Ca2+ in the regulation of proteinase-activated receptor-2 mediated nuclear factor kappa B signalling in keratinocytes. Br J Pharmacol 2005;145(4):535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack MR, Kim BS. The Itch-Scratch Cycle: A Neuroimmune Perspective. Trends Immunol 2018;39(12):980–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JJ, Saifeddine M, Triggle CR, Sun K, Hollenberg MD. 2-furoyl-LIGRLO-amide: a potent and selective proteinase-activated receptor 2 agonist. J Pharmacol Exp Ther 2004;309(3):1124–31. [DOI] [PubMed] [Google Scholar]
- Mollanazar NK, Smith PK, Yosipovitch G. Mediators of Chronic Pruritus in Atopic Dermatitis: Getting the Itch Out? Clin Rev Allergy Immunol 2016;51(3):263–92. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005;307(5714):1468–72. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Kitoh A, Egawa G, Natsuaki Y, Nakamizo S, Moniaga CS, et al. IL-17A as an inducer for Th2 immune responses in murine atopic dermatitis models. J Invest Dermatol 2014;134(8):2122–30. [DOI] [PubMed] [Google Scholar]
- Nilius B, Szallasi A. Transient Receptor Potential Channels as Drug Targets: From the Science of Basic Research to the Art of Medicine. Pharmacol Rev 2014;66(3):676–814. [DOI] [PubMed] [Google Scholar]
- Oh MH, Oh SY, Lu J, Lou H, Myers AC, Zhu Z, et al. TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. J Immunol 2013;191(11):5371–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev 2004;84(2):579–621. [DOI] [PubMed] [Google Scholar]
- Park CW, Kim HJ, Choi YW, Chung BY, Woo SY, Song DK, et al. TRPV3 Channel in Keratinocytes in Scars with Post-Burn Pruritus. Int J Mol Sci 2017;18(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, et al. A heat-sensitive TRP channel expressed in keratinocytes. Science 2002;296(5575):2046–9. [DOI] [PubMed] [Google Scholar]
- Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol 2013;14(6):564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada SG, Shimada KA, Collins JG. Scratching behavior in mice induced by the proteinase-activated receptor-2 agonist, SLIGRL-NH2. Eur J Pharmacol 2006;530(3):281–3. [DOI] [PubMed] [Google Scholar]
- Shpacovitch V, Feld M, Hollenberg MD, Luger TA, Steinhoff M. Role of protease-activated receptors in inflammatory responses, innate and adaptive immunity. J Leukoc Biol 2008;83(6):1309–22. [DOI] [PubMed] [Google Scholar]
- Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature 2011;477(7363):229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 2002;418(6894):186–90. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Corvera CU, Thoma MS, Kong W, McAlpine BE, Caughey GH, et al. Proteinase-activated receptor-2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Exp Dermatol 1999;8(4):282–94. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci 2003;23(15):6176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szollosi AG, Vasas N, Angyal A, Kistamas K, Nanasi PP, Mihaly J, et al. Activation of TRPV3 Regulates Inflammatory Actions of Human Epidermal Keratinocytes. J Invest Dermatol 2018;138(2):365–74. [DOI] [PubMed] [Google Scholar]
- Veldhuis NA, Poole DP, Grace M, McIntyre P, Bunnett NW. The G Protein-Coupled Receptor-Transient Receptor Potential Channel Axis: Molecular Insights for Targeting Disorders of Sensation and Inflammation. Pharmacol Rev 2015;67(1):36–73. [DOI] [PubMed] [Google Scholar]
- Voisin T, Chiu IM. Molecular link between itch and atopic dermatitis. P Natl Acad Sci USA 2018;115(51):12851–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013;155(2):285–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci 2006;9(5):628–35. [DOI] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002;418(6894):181–6. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Hikita I, Asakawa M, Hirasawa T, Deguchi M, Matsutani T, et al. Spontaneous scratching behaviour in DS-Nh mice as a possible model for pruritus in atopic dermatitis. Immunology 2006;118(3):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka T, Imura K, Asakawa M, Suzuki M, Oshima I, Hirasawa T, et al. Impact of the Gly573Ser substitution in TRPV3 on the development of allergic and pruritic dermatitis in mice. J Invest Dermatol 2009;129(3):714–22. [DOI] [PubMed] [Google Scholar]
- Zhao P, Lieu T, Barlow N, Metcalf M, Veldhuis NA, Jensen DD, et al. Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J Biol Chem 2014;289(39):27215–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZQ, Huo FQ, Jeffry J, Hampton L, Demehri S, Kim S, et al. Chronic itch development in sensory neurons requires BRAF signaling pathways. J Clin Invest 2013;123(11):4769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets related to this article are available from the corresponding author upon reasonable request.