Abstract
Background:
Serum immunoglobulin G (IgG) concentrations are integral to the workup of immune deficiencies and IgG4-related disease (IgG4-RD). Demographic differences in IgG concentrations are poorly described but could influence test interpretation, contribute to racial disparities in primary immunodeficiency diagnosis, and explain demographic differences in IgG concentrations in IgG4-RD.
Objective:
To assess differences in IgG and IgG subclass concentrations according to sex and race.
Methods:
We identified patients with IgG and IgG subclass concentrations measured in a large healthcare system. Multivariate-adjusted differences in IgG and IgG subclass concentrations and the proportion of subjects with results outside of reference ranges according to sex and race were estimated.
Results:
Of the 12,851 patients, the mean age was 54.7 years and 7,917 (62%) were female. 11,673 (91%), 611 (5%), and 302 (2%) were White, Black, and Asian, respectively. Compared to White patients, Asian and Black patients had higher mean concentrations of IgG (1340.0 and 1504.4 vs. 988.1 mg/dL; p<0.001), IgG1 (782.0 and 938.4 vs. 592.4 mg/dL, p<0.001), IgG2 (493.5 and 384.2 vs. 305 mg/dL; p<0.001), IgG3 (76.6 and 91.9 vs. 55.9 mg/dL; p<0.001), and IgG4 (140.4 and 53.6 vs. 41.6 mg/dL; p<0.001). IgG4 concentrations were higher in males than females (56.3 vs. 37.4 mg/dL; p < 0.001). Similar observations were made when comparing the proportions of patients with results outside of reference ranges and after stratifying by diagnosis.
Conclusion:
IgG and IgG subclass concentrations differ according to sex and race. These findings may have implications for the interpretation of these test results but require confirmation in diverse, healthy populations.
INTRODUCTION
Serum immunoglobulin G (IgG) and IgG subclass concentrations are used in the evaluation of patients for immune deficiencies (e.g., primary immunodeficiency (1, 2); common variable immunodeficiency (3, 4)) and some autoimmune diseases (5, 6) (e.g., IgG4-related disease (IgG4-RD) (7–10) and Sjögren’s syndrome (11)). While the interpretations of some laboratory tests are made in the context of patient demographics, such as the glomerular filtration rate and creatinine kinase (12–14), sex and race are not considered when interpreting IgG and IgG subclass concentration results.
Although some data have suggested IgG and IgG subclass concentrations may vary by demographics, studies were small and/or restricted to particular conditions (15–19). For instance, Black patients with periodontal disease have higher IgG subclass concentrations than non-Black patients (15–17) and Asian and male patients with IgG4-RD have higher IgG4 concentrations than non-Asian and female patients, respectively (18, 19). Small studies have also found differences in other immunoglobulin concentrations (i.e., IgA, IgE, and IgM) concentrations according to sex and race (20–24). In addition, studies have described unexplained racial disparities in the diagnosis of immunodeficiency (25, 26).
Comprehensively measuring and characterizing these differences may shed light on the observed demographic differences in the manifestation and prevalence of certain conditions and would impact the interpretation of immunoglobulin testing across clinical settings. To address these knowledge gaps, we investigated demographic differences in IgG and IgG subclass concentrations in a large, racially diverse cohort of patients who underwent testing for a variety of indications.
METHODS
Cohort Description:
The Partners Research Patient Data Registry (RPDR) was queried to identify patients who had at least one IgG subclass concentrations measured between January 1, 1989 and March 15, 2018 within the Partners HealthCare Network. RPDR is a centralized clinical data warehouse that contains electronic health information (e.g., demographics, diagnoses) for more than 6.5 million patients. Approximately half of the population in the greater Boston, Massachusetts area receives care within the Partners HealthCare Network. Because of known variations in IgG concentrations in the pediatric population, only patients over the age of 18 years were included (27, 28). This study was approved by the institutional review board of Partners HealthCare.
Immunoglobulin Concentrations:
All IgG, IgG subclass, IgA, IgE, and IgM laboratory results, their associated reference ranges, and the date of each test were extracted from RPDR.
Covariates:
Date of birth, sex, self-reported race, home address, and diagnostic (ICD-9 or ICD-10) billing codes were extracted from RPDR. Patients with unknown race were excluded from the analysis after confirming that their inclusions did not impact our findings. Median household income was assigned according to the subject’s billing address ZIP code and was used to estimate socioeconomic status (29). The medical condition for which the test was ordered was determined using the primary diagnostic billing code closest in time to the date of the test result. Clinical Classifications Software (CCS) grouped diagnostic codes into mutually exclusive categories, including immune deficiencies, autoimmune diseases, infectious conditions, gastrointestinal disorders, and pulmonary diseases (Appendix Material) (30).
Statistical Analysis:
Continuous variables are presented as mean (standard deviation, SD) or median (interquartile range, IQR), as appropriate. Categorical variables are presented as N (%). In the primary analysis, we analyzed each subject’s first test result. IgG and IgG subclass concentrations were compared across sex and race categories using t-tests and ANOVA tests, respectively. The proportion of patients in each demographic group with a result above the upper limit of normal (ULN) and below the lower limit of normal (LLN) was determined and differences in the distribution of these proportions across demographic groups were compared using Chi-square tests. Multivariate linear regression was used to estimate differences in IgG and IgG subclass concentrations across sex and race subgroups after adjustment for age, sex, and/or race. We used logistic regression to compare the proportion of patients across demographic groups with results above or below the assay’s reference range after adjustment for age, sex, and/or race.
We performed multiple sensitivity analyses. First, we compared sex and race differences in of IgG and IgG subclass concentrations among patients with specific conditions using linear regression. Second, we repeated the primary analysis (i.e., univariate and multivariate linear regression) using each subject’s highest test result as well as the first test result standardized to the associated reference range ULN. Third, we used generalized estimation equation linear regression models to compare IgG and IgG subclass concentration measurements using all available results for each subject. Fourth, we repeated the primary analysis after excluding patients with an associated diagnosis code commonly used for immunodeficiency (ICD9: 279.x or ICD10: D80, D81, D82, D83, D84) and after excluding patients with an IgG or IgG subclass concentration below the lower limit of normal.
To evaluate potential explanations for observed sex and race in IgG and IgG subclass concentrations, we performed two secondary analyses. First, we assessed whether similar differences were observed when comparing IgA, IgE, and IgM concentrations across demographic groups using the same methods as in the primary analysis. Second, we evaluated whether our observed associations regarding sex and race differences in IgG and IgG subclass concentrations persisted after adjustment for household income using multivariate linear regression.
All p values were 2-sided with a significance threshold <0.05. Statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC) and R, version 3.5.0.
RESULTS
Cohort Description:
Of the 12,851 cases, 4,934 (38.4%) were male and the mean (SD) age was 54.7 (±18.3) years. 11,673 (90.8%) were White, 611 (4.8%) were Black, and 302 (2.4%) were Asian (Table 1). The median (IQR) number of IgG subclass measurements per subject during the study period was 2 (1, 3). The mean (SD) serum IgG, IgG1, IgG2, IgG3, and IgG4 concentrations were 1,024.6 (±500.2) mg/dL, 616.7 (±347.4) mg/dL, 314.6 (±184.6) mg/dL, 58.4 (±77.5) mg/dL, and 44.6 (±109.7) mg/dL, respectively. The IgG, IgG1, IgG2, IgG3, and IgG4 concentrations were above the ULN in 9.6%, 8.8%, 3.7%, 5.4%, and 7.8% of patients, respectively, and below the LLN in 15.5%, 19.0%, 23.8%, 12.5%, and 9.1%, respectively. (Table 2).
Table 1:
Cohort Characteristics
| Sex | Race | |||||
|---|---|---|---|---|---|---|
| Female (N=7,917) | Male (N=4,934) | Asian (N = 302) | Black (N=611) | White (N= 11,673) | Overall (N = 12,851) | |
| Male (N, %) | 4,934 (100%) | 143 (47.4%) | 222 (36.3%) | 4,470 (38.3%) | 4,934 (38.4%) | |
| Race (N, %) | ||||||
| White | 7,203 (91.0%) | 4,470 (90.6%) | -- | -- | 11673 | 11,673 (90.8%) |
| Black | 389 (4.9%) | 222 (4.5%) | -- | 611 | -- | 611 (4.8%) |
| Asian | 159 (2.0%) | 143 (2.9%) | 302 | -- | -- | 302 (2.4%) |
| Other | 166 (2.1%) | 99 (2.0%) | -- | -- | -- | 265 (2.1%) |
| Age (years) at first test (mean, SD) | 54.9 (17.9) | 54.3 (18.9) | 51.6 (18.2) | 52.8 (16.5) | 55.0 (18.3) | 54.7 (18.2) |
| No of tests per subject (median, IQR) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) |
Table 2:
Distribution of Patients (N, %) with IgG or IgG Subclass Concentrations Above or Below Reference Ranges According to Sex and Race
| Sex | Race | |||||
|---|---|---|---|---|---|---|
| IgG Subclass | Female (N=7,917) | Male (N=4,934) | Asian (N = 302) | Black (N=611) | White (N= 11,673) | Overall (N = 12,851) |
| IgG | ||||||
| > ULN (N, %) | 747 (9.4%) | 490 (9.9%) | 68 (22.5%) | 209 (34.2%) | 907 (7.8%)* | 1237 (9.6%) |
| < LLN (N, %) | 1142 (14.4%) | 847 (17.2%)* | 16 (5.3%) | 25 (4.1%) | 1922 (16.5%)* | 1989 (15.5%) |
| IgG1 | ||||||
| > ULN (N, %) | 673 (8.5%) | 463 (9.4%) | 61 (20.2%) | 198 (32.4%) | 822 (7.0%)* | 1136 (8.8%) |
| < LLN (N, %) | 1428 (18.0%) | 1012 (20.5%)* | 24 (8.0%) | 31 (5.1%) | 2356 (20.2%)* | 2440 (19.0%) |
| IgG2 | ||||||
| > ULN (N, %) | 271 (3.4%) | 200 (4.1%) | 52 (17.2%) | 48 (7.9%) | 355 (3.0%)* | 471 (3.7%) |
| < LLN (N, %) | 1883 (23.8%) | 1181 (23.9%) | 24 (8.0%) | 104 (17.0%) | 2891 (24.8%)* | 3064 (23.8%) |
| IgG3 | ||||||
| > ULN (N, %) | 394 (5.0%) | 297 (6.0%)* | 34 (11.3%) | 84 (13.8%) | 551 (4.7%)* | 691 (5.4%) |
| < LLN (N, %) | 949 (12.0%) | 653 (13.2%)* | 30 (10.0%) | 28 (4.6%) | 1520 (13.0%)* | 1602 (12.5%) |
| IgG4 | ||||||
| > ULN (N, %) | 483 (6.1%) | 517 (10.5%)* | 75 (24.8%) | 73 (12.0%) | 819 (7.0%)* | 1000 (7.8%) |
| < LLN (N, %) | 744 (9.4%) | 425 (8.6%) | 10 (3.3%) | 37 (6.1%) | 1106 (9.5%)* | 1169 (9.9%) |
p < 0.05
Differences According to Sex:
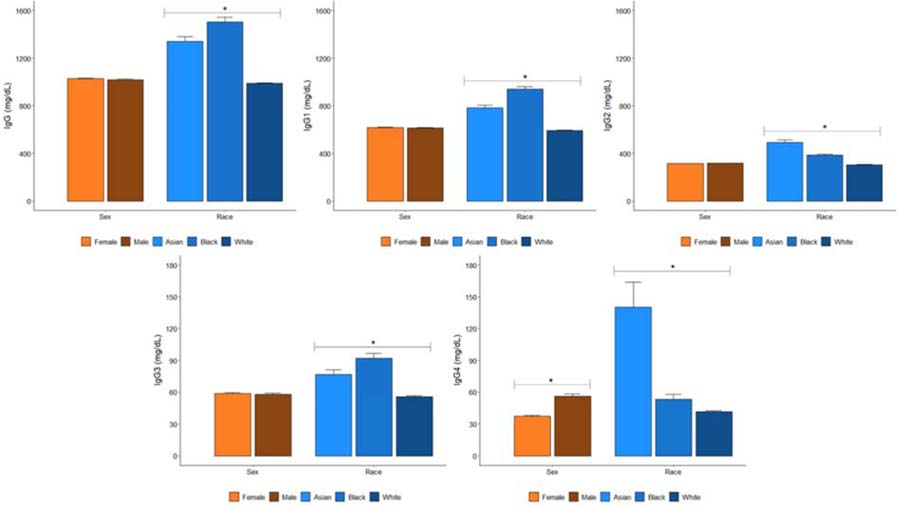
Female patients had lower IgG4 concentrations (37.4 [±75.2] mg/dL vs 56.3 [±148.5] mg/dL, p < 0.001) and less often had IgG3 (5.0% vs 6.0%, p = 0.01) and IgG4 (6.1% vs 10.5%, p < 0.001) concentrations above the ULN compared with male patients in crude analyses (Figure 1, Table 2). In adjusted analyses (Table 3), only differences in IgG4 concentrations persisted, such that female patients had lower serum IgG4 concentrations (β = −18.2 mg/dL [95% CI: −22.0, −14.3], p < 0.001) and female patients were less likely to have an IgG4 concentration above the ULN (adjusted OR 0.56 [95% CI: 0.49–0.64]).
Figure 1:
IgG and IgG Subclass Concentrations (mean and standard error) According to Sex and Race (*indicates P<0.05 for comparison across groups)
Table 3:
Multivariate-Adjusted IgG Concentration Differences (mg/dL) According to Sex and Race (β, 95% CI)
| Sex | Race | ||||
|---|---|---|---|---|---|
| IgG Subclass (mg/dL) | Female | Male | Asian | Black | White |
| IgG | +10.7 (−7.4, +29.9) | 0.0 (Reference) | +356.3 (+295.9, +416.7)* | +517.6 (+475.3, +559.9)* | 0.0 (Reference) |
| IgG1 | +5.8 (−6.3, +17.9) | 0.0 (Reference) | +189.7 (+150.7, +228.7)* | +345.6 (+317.6, +373.6)* | 0.0 (Reference) |
| IgG2 | −1.7 (−8.2, +4.8) | 0.0 (Reference) | +189.7 (+168.8, +210.7)* | +80.1 (+65.1, +95.2)* | 0.0 (Reference) |
| IgG3 | +0.7 (−2.1, +3.4) | 0.0 (Reference) | +21.1 (+12.2, +29.9)* | +36.1 (+29.5, +42.5)* | 0.0 (Reference) |
| IgG4 | −18.2 (−22.0, −14.3)* | 0.0 (Reference) | +97.9 (+85.5, +110.2)* | +12.8 (+3.9, +21.6)* | 0.0 (Reference) |
p < 0.05
Adjusted for age but data not presented
Differences According to Race:
In unadjusted analyses, Asian and Black patients had higher IgG (1340.0 and 1504.4 vs. 988.1 mg/dL; p < 0.001), IgG1 (782.0 and 938.4 vs. 592.4 mg/dL; p < 0.001), IgG2 (493.5 and 384.1 vs. 305.0 mg/dL; p < 0.001), IgG3 (76.6 and 91.9 vs. 55.9 mg/dL; p < 0.001), and IgG4 (140.4 and 53.6 vs. 41.6 mg/dL; p < 0.001) subclass concentrations than White patients (Figure 1). As a result of these differences, Asian and Black patients more often had IgG (22.5% and 34.2% vs. 7.8%; p < 0.001), IgG1 (20.2% and 32.4% vs. 7.0%; p < 0.001), IgG2 (17.2% and 7.9% vs. 3.0%; p < 0.001), IgG3 (11.3% and 13.8% vs. 4.7%; p < 0.001), and IgG4 (24.8% and 12.0% vs. 7.0%; p < 0.001) concentrations above the ULN. In contrast, White patients more often had IgG (16.5% vs. 5.3% and 4.1%; p < 0.001), IgG1 (20.2% vs. 8.0% and 5.1%; p < 0.001), IgG2 (24.8% vs. 8.0% and 17.0%; p < 0.001), IgG3 (13.0% vs. 10.0% and 4.6%; p < 0.001), and IgG4 (9.5% vs. 3.3% and 6.1%; p < 0.001) concentrations below the LLN compared with Asian and Black patients, respectively (Table 2).
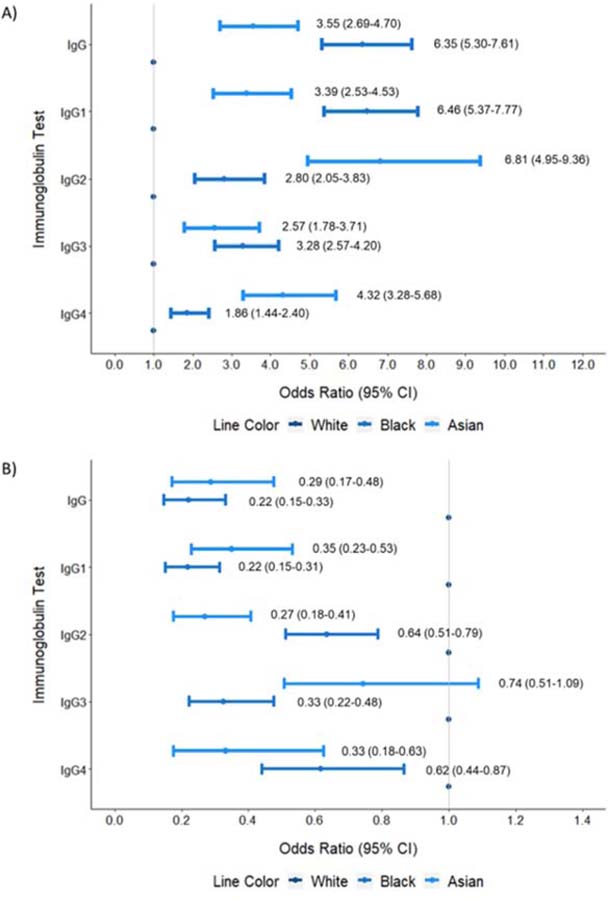
These differences persisted in adjusted analyses (Table 3, Figures 2A–B). For example, compared with White patients, Black patients were less likely to have an IgG concentration below the LLN (adjusted OR: 0.22 [95% CI: 0.15–0.33]) and Asian patients were more likely to have an IgG4 concentration greater than the ULN (adjusted OR: 4.32 [95% CI: 3.28–5.68]).
Figure 2:
Multivariate-Adjusted Odds Ratio (95% Confidence Interval) of Having an IgG or IgG Subclass Concentration Above (A) or Below (B) the Reference Range According to Race
Sensitivity Analyses:
We found similar differences in IgG and IgG subclass concentrations across demographic groups when adjusted analyses were restricted to patients with similar diagnoses (Table 4), when concentrations were standardized to each assay reference range’s ULN (eTable 1), when the highest concentrations for each subject were used (eTable 2), when all available IgG and IgG subclass concentration measurements for each subject were used (eTable 3), and when all subjects with a diagnosis of immune deficiency were excluded (eTable 4). Additionally, we performed an analysis in which we excluded subjects with an IgG or IgG subclass concentration below the lower limit of normal and our results remained unchanged.
Table 4:
Multivariate-Adjusted IgG-Concentration Differences (mg/dL) According to Sex and Race Stratified by Selected Indication for Testing (Asian vs White Patients and Black vs White Patients) (β, 95% CI)
| Allergy/Immunology | Autoimmune Disease | |||||
|---|---|---|---|---|---|---|
| Asian | Black | White | Asian | Black | White | |
| No. of Patients (N, %) | 35 (2.6%) | 18 (1.3%) | 1317 (96.1%) | 20 (4.0%) | 36 (7.3%) | 440 (88.7%) |
| IgG | +375.1 (+207.3, +543.0)* | +191.8 (−32.3, +415.9) | Ref | +508.3 (+206.0, +810.7)* | +342.8 (+128.0, +557.6)* | Ref |
| IgG1 | +162.2 (+61.1, +263.3)* | +163.3 (+25.5, +301.2)* | Ref | +324.9 (+121.3, +528.4)* | +215.8 (+64.4, +367.1)* | Ref |
| IgG2 | +233.7 (+175.4, +292)* | +57.2 (−19.2, +133.6) | Ref | +323.0 (+224.0, +422.0)* | +55.0 (−13.1, +123) | Ref |
| IgG3 | +10.5 (−2.4, +23.5) | +20.8 (+3.0, +38.6)* | Ref | +36.5 (+3.5, +69.5)* | +25.2 (+0.5, +50.0)* | Ref |
| IgG4 | +201.7 (+155.0, +248.4)* | +2.5 (−49.3, +54.2) | Ref | +280.4 (+202.4, +358.3)* | +4.1 (−37.7, +46.0) | Ref |
| GI Disorder | Infection | |||||
| Asian | Black | White | Asian | Black | White | |
| No. of Patients (N, %) | 29 (3.7%) | 60 (7.7%) | 694 (88.6%) | 36 (2.3%) | 80 (5.1%) | 1449 (92.6%) |
| IgG | +136.8 (−49.6, +323.3) | +445.2 (+313.4, +577.0)* | Ref | +222.0 (+62.2, +381.7)* | +598.1 (+491.6, +704.6)* | Ref |
| IgG1 | +108.6 (−25.6, +242.8) | +322.8 (+223.8, +421.9)* | Ref | +124.4 (+16.4, +232.4)* | +475.9 (+394.1, +557.7)* | Ref |
| IgG2 | +130.8 (+67, +194.6)* | +101.8 (+58.1, +145.5)* | Ref | +130.9 (+78.4, +183.5)* | +65.0 (+28.6, +101.3)* | Ref |
| IgG3 | +15.2 (−13.2, +43.7) | +16.5 (−3.3, +36.4) | Ref | +16.7 (−3.3, +36.7) | +69.8 (+49.6, +90.0)* | Ref |
| IgG4 | +19.6 (+1.9, +37.3)* | +14.9 (+2.1, +27.7)* | Ref | +10.8 (−4.2, +25.8) | +15.4 (+5.0, +25.7)* | Ref |
p < 0.05
Adjusted for age and sex but data not presented
Secondary Analyses:
IgA and IgE concentrations also differed according to sex and race. Females had lower IgA (β = −18.3mg/dL [95% CI: −25.3, −11.3], p < 0.001) and IgE (β = −122.0mg/dL [95% CI: −178.4, −65.6], p < 0.001) and higher IgM (β = +19.3mg/dL [95% CI: +11.6, +27.0], p < 0.001) than male patients (eTable 5). Compared with White patients, Asian and Black patients had higher IgA (β = +62.1mg/dL [95% CI: +40.3, +83.9], p < 0.001 and β = +103.4mg/dL [95% CI: +86.9, +119.8], p < 0.001, respectively) and IgE (β = +172.8mg/dL [95% CI: −11.0, +356.6], p = 0.07 and β = +488.9mg/dL [95% CI: +338.6, +639.3], p < 0.001, respectively) concentrations (eTable 5). IgM concentrations did not differ across race subgroups.
In analyses adjusted for household income, differences in IgG and IgG subclass concentrations among Asian, Black, and White patients were attenuated but persisted (eTable 6).
DISCUSSION
We found significant differences in IgG and IgG subclass concentrations across demographic groups. Our observations persisted regardless of the clinical indication for testing. In particular, Asian and Black patients have significantly higher IgG and IgG subclass concentrations than White patients and male patients have higher IgG4 concentrations than female patients. These variations in IgG and IgG subclass concentration measurements led to significant demographic differences in the proportion of patients with results outside of normal reference ranges. These provocative observations require confirmation in an external data source that includes a diverse, healthy population.
Our findings suggest that demographic differences in IgG and IgG subclass concentrations may lead to certain demographic groups being more or less likely to be classified as having abnormal test results and affect the likelihood that certain diagnoses are made. For instance, Asian patients are more likely to have elevated serum IgG4 concentrations and may therefore be more likely to be labeled as having IgG4-RD than a White subject with a similar clinical history. Similarly, because of differences in the proportion of patients with IgG and IgG subclass concentrations results below the lower limit of normal among White patients when compared to Black or Asian patients, the likelihood of a diagnosis of immunodeficiency may be more often rendered in White patients. The use of race in the interpretation of test results has been controversial (31), but, in contrast to other tests, not accounting for racial differences in immunoglobulin concentrations may contribute to disparities in the diagnosis of certain conditions, such as immunodeficiencies, among minority groups (25, 26). Population-based studies in diverse cohorts are necessary to determine whether the prevalence of immunodeficiencies differ across racial subgroups when conventional reference ranges are used.
To our knowledge, this is the first study to comprehensively assess differences in IgG and IgG subclass concentrations between adult Asian, Black, and White patients, as well as male and female patients, in a large cohort with diverse indications for testing. We expanded upon prior observations regarding differences in IgA and IgE concentrations across racial groups by including Asian patients. Black patients with asthma have been previously found to have higher IgE concentrations than White patients and healthy Black patients have been found to have higher IgA concentrations than White patients (20, 21, 24, 32).
Previous studies evaluated the impact of socioeconomic status and smoking on these observations but no associations were observed (21, 33). In contrast, we found some attenuation of the associations between racial groups and differences in immunoglobulin concentrations after adjusting for socioeconomic status (SES). These conflicting findings may have to do with the manner in which SES was estimated and require further study. Due to a lack of data on smoking status, we were unable to assess its impact on our observations. Given that Black and Asian patients had higher levels of all immunoglobulins, our observations may reflect generalized, nonspecific activation of immunoglobulin-producing B cells because of environmental factors, genetic predisposition, and/or chronic infection. For instance, mechanistic and clinical evidence suggest an association between higher IgE concentrations and chronic viral infections (34, 35).
Our study has certain limitations. First, although our cohort was large, the majority of patients were White. Despite this, we had sufficient power to detect significant differences across racial groups. We relied on self-reported race which is a complex concept but one that is commonly used in epidemiologic analyses. Second, the number of patients in each diagnostic condition group was relatively small which limited our ability to detect significant differences in some groups. However, we were able to detect similar trends across subgroups with these conditions as in our primary analysis. Third, we conducted this study using patients for whom testing was performed because of a clinical indication; future studies are necessary to confirm these observations in healthy patients. However, our findings are similar to those made in other studies which used healthy patients from the general population (20–22). Additionally, our observations persisted after excluding patients in whom testing was performed for immunodeficiency as the indication. Fourth, though we were unable to determine the method used for tests (e.g., nephelometry), we confirmed our observations after standardizing measurements to the upper limit of normal for each assay. Moreover, it is unlikely that the method of immunoglobulin assessment would confound observations regarding comparisons across demographic groups (i.e., any differences would not be differential by sex or race).
We found that IgG and IgG subclass concentrations vary according to sex and race. These provocative observations have the potential to affect the interpretation of these diagnostic tests in the clinical setting and in research. The observed variations may contribute to differences in the manifestation (e.g., IgG4 concentration elevations in IgG4-RD) and prevalence of various diagnoses (e.g., disparities in immunodeficiency diagnoses) across racial groups. Before implementing any changes in practice, additional studies are necessary to confirm these observations in diverse, healthy populations, further examine explanatory factors, and assess the impact of these differences on the likelihood of being diagnosed with certain conditions.
Supplementary Material
Funding:
Dr. Wallace received funding from the Rheumatology Research Foundation (Scientist Development Award) and the NIH/NIAMS (K23-AR073334 and L30-AR070520). Dr. Stone received funding from NIH/NIAID (UM1-AI144295).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
References:
- 1.Parker A, Skold M, Ramsden DB, Ocejo-Vinyals JG, Lopez-Hoyos M, Harding SJ. The Clinical Utility of Measuring IgG Subclass Immunoglobulins During Immunological Investigation for Suspected Primary Antibody Deficiencies. Lab Med 2017;48(4):314–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonilla FA, Khan DA, Ballas ZK, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol 2015;136:1186–205. [DOI] [PubMed] [Google Scholar]
- 3.Oksenhendler E, Gerard L, Fieschi C, et al. Infections in 252 Patients with Common Variable Immunodeficiency. Clin Infect Dis 2008;46(10):1547–54. [DOI] [PubMed] [Google Scholar]
- 4.Filion CA, Taylor-Black S, Maglione PJ, Radigan L, Cunningham-Rundles C. Differentiation of Common Variable Immunodeficiency From IgG Deficiency. J Allergy Clin Immunol Pract 2019:7(4):1277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poplawska-Kita A, Kosciuszko-Zdrodowska M, Siewko K, et al. High Serum IgG4 Concentrations in Patients with Hashimoto’s Thyroiditis. Int J Endocrinol 2015;2015:706843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carruthers MN, Khosroshahi A, Augustin T, Deshpande V, Stone JH. The diagnostic utility of serum IgG4 concentrations in IgG4-related disease. Ann Rheum Dis 2015;74(1):14–8. [DOI] [PubMed] [Google Scholar]
- 7.Xia CS, Fan CH, Liu YY. Diagnostic performances of serum IgG4 concentration and IgG4/IgG ratio in IgG4-related disease. Clin Rheumatol 2017;36(12):2769–74. [DOI] [PubMed] [Google Scholar]
- 8.Culver EL, Sadler R, Simpson D, et al. Elevated Serum IgG4 Levels in Diagnosis, Treatment Response, Organ Involvement, and Relapse in a Prospective IgG4-Related Disease UK Cohort. Am J Gastroenterol 2016;111(5):733–43. [DOI] [PubMed] [Google Scholar]
- 9.Yamada K, Yamamoto M, Saeki T, et al. New clues to the nature of immunoglobulin G4-related disease: a retrospective Japanese multicenter study of baseline clinical features of 334 cases. Arthritis research & therapy 2017;19(1):262-017-1467-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawa S, Skold M, Ramsden DB, Parker A, Harding SJ. Serum IgG4 Concentration in IgG4-Related Disease. Clin Lab 2017;63(9):1323–37. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Li J. Preferentially Immunoglobulin (IgG) subclasses production in primary Sjogren’s syndrome patients. Clin Chem Lab Med 2012;50(2):345–9. [DOI] [PubMed] [Google Scholar]
- 12.Pottel H, Hoste L, Delanaye P, Cavalier E, Martens F. Demystifying ethnic/sex differences in kidney function: is the difference in (estimating) glomerular filtration rate or in serum creatinine concentration? Clin Chim Acta 2012;413(19–20):1612–7. [DOI] [PubMed] [Google Scholar]
- 13.Pottel H, Hoste L, Dubourg L, et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant 2016;31(5):798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grams ME, Matsushita K, Sang Y, et al. Explaining the Racial Difference in AKI Incidence. J Am Soc Nephrol 2014;25(8):1834–41.24722442 [Google Scholar]
- 15.Haralambieva IH, Salk HM, Lambert ND, et al. Associations between race, sex and immune response variations to rubella vaccination in two independent cohorts. Vaccine 2014;32(17):1946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunsolley JC, Pandey JP, Quinn SM, Tew JG, Schenkein HA. The effect of race, smoking and immunoglobulin allotypes on IgG subclass concentrations. J Periodontal Res 1997;32(4):381–7. [DOI] [PubMed] [Google Scholar]
- 17.Quinn SM, Zhang JB, Gunsolley JC, Schenkein JG, Schenkein HA, Tew JG. Influence of smoking and race on immunoglobulin G subclass concentrations in early-onset periodontitis patients. Infect Immun 1996;64(7):2500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi R, Chen LYC, Park S, et al. Utility of Serum IgG4 Levels in a Multiethnic Population. Am J Med Sci 2018;355(1):61–6. [DOI] [PubMed] [Google Scholar]
- 19.Wallace ZS, Zhang Y, Perugino CA, et al. Clinical phenotypes of IgG4-related disease: an analysis of two international cross-sectional cohorts. Ann Rheum Dis 2019;78(3):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maddison SE, Stewart CC, Farshy CE, Reimer CB. The relationship of race, sex, and age to concentrations of serum immunoglobulins expressed in international units in healthy adults in the USA. Bull World Health Organ 1975;52(2):179–85. [PMC free article] [PubMed] [Google Scholar]
- 21.Tollerud DJ, Brown LM, Blattner WA, et al. Racial differences in serum immunoglobulin levels: relationship to cigarette smoking, T-cell subsets, and soluble interleukin-2 receptors. J Clin Lab Anal 1995;9(1):37–41. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Quintela A, Alende R, Gude F, et al. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol 2008;151(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gergen PJ, Arbes SJ, Calatroni A, Mitchell HE, Zeldin DC. Total IgE and Asthma Prevalence in the U.S. Population: Results from the National Health and Nutrition Examination Survey (NHANES) 2005–2006. J Allergy Clin Immunol 2009;124(3):447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joseph CLM, Ownby DR, Peterson EL, Johnson CC. Racial Differences in Physiologic Parameters Related to Asthma Among Middle-class Children. Chest 2000;117:1336–44. [DOI] [PubMed] [Google Scholar]
- 25.Kobrynski LJ, Powell RW, Bowen S. Prevalence and morbidity of primary immunodeficiency diseases, United States 2001–2007. J Clin Immunol 2014;34(8):954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunningham-Rundles C, Sidi P, Estrella L, Doucette J. Identifying undiagnosed primary immunodeficiency diseases in minority patients by using computer sorting of diagnosis codes. J Allergy Clin Immunol 2004;113(4):747–55. [DOI] [PubMed] [Google Scholar]
- 27.Herrod HG. Clinical significance of IgG subclasses. Curr Opin Pediatr 1993;5(6):696–9. [DOI] [PubMed] [Google Scholar]
- 28.Hanson LA, Soderstrom R, Avanzini A, Bengtsson U, Bjorkander J, Soderstrom T. Immunoglobulin subclass deficiency. Pediatr Infect Dis J 1988;7:S17–S21. [PubMed] [Google Scholar]
- 29.Berkowitz SA, Traore CY, Singer DE, Atlas SJ. Evaluating Area-Based Socioeconomic Status Indicators for Monitoring Disparities within Health Care Systems: Results from a Primary Care Network. Health Serv Res 2015;50(2):398–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.HCUP CCS Fact Sheet. Healthcare Cost and Utilization Project (HCUP) January 2012. Agency for Healthcare Research and Quality, Rockville, MD: www.hcup-us.ahrq.gov/toolssoftware/ccs/ccsfactsheet.jsp. Accessed on 7 Oct 2019. [Google Scholar]
- 31.Eneanya ND, Yang W, Reese PP. Reconsidering the Consequences of Using Race to Estimate Kidney Function. JAMA 2019;322(2):113–114. [DOI] [PubMed] [Google Scholar]
- 32.Nyenhuis SM, Krishnan JA, Berry A, et al. Race is associated with differences in airway inflammation in patients with asthma. J Allergy Clin Immunol 2017;140(1):257–65.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMillan SA, Douglas JP, Archbold GPR, McCrum EE, Evans AE. Effect of low to moderate levels of smoking and alcohol consumption on serum immunoglobulin concentrations. J Clin Pathol 1997;50:819–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welliver RC, Ogra PL. The role of IgE in pathogenesis of mucosal viral infections. Ann N Y Acad Sci 1983;409:321–32. [DOI] [PubMed] [Google Scholar]
- 35.Zambrano JC, Carper HT, Rakes GP, et al. Experimental rhinovirus challenges in adults with mild asthma: response to infection in relation to IgE. J Allergy Clin Immunol 2003;111(5):1008–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.