Abstract
Objectives.
To determine whether calf muscle hemoglobin oxygen saturation (StO2) obtained during a standardized treadmill test was associated with ambulatory function and health-related quality of life (HRQoL) in patients with symptomatic peripheral artery disease (PAD). We hypothesized that a rapid decline in calf muscle StO2 during walking was associated with impaired ambulatory function and HRQoL, and that these associations were independent of ankle/brachial index (ABI).
Methods.
Calf muscle StO2, peak walking time (PWT) and claudication onset time (COT) were obtained during a treadmill test in 151 symptomatic men and women with PAD. Patients were further characterized on demographic variables, comorbid conditions, cardiovascular risk factors, ABI, 6-minute walk distance (6MWD), daily ambulatory activity, walking impairment questionnaire (WIQ), and on the Medical Outcomes Study Short-Form 36 (MOS SF-36) survey to assess HRQoL.
Results.
The median calf muscle StO2 value at rest was 52%, which declined to 22% after only one minute of walking during the treadmill test, and reached a minimum value of 9% after a median time of 87 seconds of walking. Of the various calf muscle StO2 measurements obtained during the treadmill test, the exercise time to the minimum calf muscle StO2 value (log transformed) had the strongest univariate associations with PWT (r=0.56, p<0.001), COT (r=0.49, p<0.001), 6MWD (r=0.31, p<0.001), WIQ distance score (r=0.33; p<0.001), WIQ speed score (r=0.39, p<0.001), WIQ stair climbing score (r=0.37; p<0.001), and the MOS SF-36 physical function score (r=0.32; p<0.001). In adjusted multiple regression models, these associations persisted (p<0.001) after adjusting for demographic measures, cardiovascular risk factors, comorbid conditions, and ABI.
Conclusions.
More rapid decline in oxygen saturation of the calf musculature during walking, indicative of impaired microcirculation, was predictive of impaired ambulatory function and HRQoL in patients with symptomatic PAD. Of particular importance, these associations were independent of ABI and other common health burdens, highlighting the clinical relevance that the microcirculation has on ambulatory function and HRQoL in patients with symptomatic PAD.
Table of Contents Summary
This prospective cross-sectional study of 151 patients with claudication found that the time taken for oxygen saturation of the calf muscle to decline to a minimum value during treadmill exercise was associated with peak walking time and claudication onset time. This study suggests that more rapid decline in calf oxygen saturation, indicative of impaired microcirculation, was predictive of impaired ambulatory function and quality of life.
INTRODUCTION
Peripheral artery disease (PAD) is highly prevalent, as 202 million people are affected worldwide,1 and prevalence grew by 23.5% in the first decade of this century.1 Furthermore, PAD is costly,2 leads to poor quality of life,3 and is associated with a high rate of all-cause and cardiovascular mortality.4 Between 40 and 75 percent of patients with PAD have symptomatic leg pain during ambulation that is either typical or atypical of classic claudication,5 resulting in ambulatory dysfunction,6 impaired functional status which declines over time,7,8 and low daily physical activity.9
The hallmark non-invasive clinical test to determine the presence and severity of PAD is the ankle/brachial index (ABI), which is a measure of the macrocirculation that can be obtained quickly and easily in the clinical setting.10 Additionally, the ABI is clinically relevant because it predicts cardiovascular risk associated with mortality and major coronary events beyond that explained by the Framingham Risk Score.10 However, ABI is a measure of large vessel disease obtained at rest which has relatively low correlation with claudication measurements both at baseline11–18 and following exercise intervention.19 In contrast, non-invasive vascular measures of the microcirculation of the lower extremity musculature during exercise may correlate better with claudication and ambulatory function outcomes, thereby providing more useful clinical information.
Near Infrared Spectroscopy (NIRS) is a non-invasive technique that provides real-time measurement of the microcirculation, as determined by the hemoglobin oxygen saturation (StO2) of the calf musculature.20–27 Although a number of factors may influence calf muscle StO2, such as lesions in proximal large arteries, microvascular function, and extraction and utilization of oxygen by muscle, calf muscle StO2 represents the balance between oxygen delivery and utilization to the local tissue,22 and is a marker of the blood volume in the microvascular territory of the muscle.28
NIRS technology is readily available, but to date it has been an under-utilized methodology to provide insight into calf muscle perfusion and claudication during exercise in patients with PAD. We have previously found that calf muscle StO2 during treadmill exercise is associated with initial and absolute claudication distances in a small cohort of patients with PAD,13,29 but the association between calf muscle StO2 with a common battery of clinically important exercise outcomes has not been examined. Therefore, it is not clear whether calf muscle StO2 during standardized treadmill exercise is associated with a variety of clinically relevant ambulatory function outcomes obtained from the treadmill test, 6-minute walk test, daily physical activity monitoring, and patient-based outcomes of walking and health-related quality of life (HRQoL).
The aim of this investigation was to determine whether calf muscle StO2 obtained during a standardized treadmill test was associated with ambulatory function and HRQoL in patients with symptomatic PAD. We hypothesized that a rapid decline in calf muscle StO2 during walking was associated with impaired ambulatory function and HRQoL, and that these associations were independent of ABI.
METHODS
Patients
Approval and Informed Consent.
The procedures of this study were approved by the institutional review board, and written informed consent was obtained from each patient prior to beginning the investigation.
Recruitment.
Patients who had symptomatic PAD (Fontaine Stage II and Rutherford Grade I)5 and who were not currently exercising were recruited from vascular laboratories and vascular clinics for possible enrollment into the study.
Medical Screening through History and Physical Examination
Protocol.
Patients were evaluated in the morning at the Clinical Research Center.30 Patients arrived in the morning fasted, but were allowed to take their usual medications. To begin the study visit, patients completed the consent form, and their vital signs, demographic information, height, weight, body mass index, anthropometric measurements, and waist circumference31 were recorded by research personnel. Subsequently, patients had blood samples drawn by study nurses, which were then sent to a central lab for analyses for fasting blood chemistries. Patients then underwent a medical history and physical examination by study physicians, in which claudication history, co-morbid conditions, cardiovascular risk factors and current medications were recorded. Following this assessment, patients rested supine for 10 minutes under standardized laboratory conditions. Ankle and brachial systolic blood pressures were then obtained according to standard guidelines32 by exercise physiologists for the calculation of ABI. Patients then performed an eligibility screening graded treadmill test in which the ABI was measured from the more affected leg immediately after exercise,6 for the purpose of confirming that leg pain was of vascular origin, which was one of the criteria for study inclusion. Based on this battery of baseline assessments, patients were coded on cardiovascular risk factors and co-morbid conditions according to standard definitions,33 and patients were characterized on the presence, severity, and symptoms of PAD.
Inclusion and Exclusion Criteria.
Patients with symptomatic PAD were included in this study if they met the following criteria: (a) history of ambulatory leg pain, (b) ambulatory leg pain confirmed by treadmill exercise,6 and (c) ABI ≤ 0.90 at rest34 or ≤ 0.73 after exercise.35 Patients were excluded for the following conditions: (a) absence of PAD (ABI > 0.90 at rest and ABI > 0.73 after exercise), (b) non-compressible vessels (ABI > 1.40), (c) asymptomatic PAD (Fontaine Stage I; Rutherford Grade 0),34 (d) rest pain due to PAD (Fontaine stage III; Rutherford Grade II),34 (e) tissue loss due to PAD (Fontaine stage IV; Rutherford Grades III and IV),34 (f) use of medications indicated for the treatment of claudication (cilostazol or pentoxifylline) initiated within 3 months prior to investigation, (g) exercise limited by other diseases or conditions, (h) active cancer, (i) stage 5 chronic kidney disease (end stage), as defined by an estimated glomerular filtration rate < 15 ml/min per 1.73 m36 (j) abnormal liver function (alanine aminotransferase ≥ 56 IU/L), (k) a calf skin fold measurement > 50 mm because of the potential interference with the light path of the NIRS probe from penetrating the subcutaneous tissue, (l) pulse arterial oxygen saturation of the index finger < 95% because of potential deleterious effect on calf muscle StO2 from poor pulmonary gas exchange, and (m) failure to complete all of the tests within 3 weeks. A total of 218 patients were evaluated for eligibility in this study between January, 2009 and December, 2014. Of these patients, 151 patients were deemed eligible to participate in the study and 67 were excluded.
Tests and Measurements
Maximal Treadmill Test.
Patients performed a standardized Gardner-Skinner graded treadmill test to obtain the outcome measures of claudication onset time (COT) and peak walking time (PWT), as previously described.6 The COT, defined as the amount of time walked at which the patient first experienced pain, and the PWT, defined as the total time walked at which ambulation could not continue due to maximal pain, were both recorded to quantify the severity of claudication.
Calf Muscle Microcirculation.
Calf muscle StO2 was measured before and during the treadmill test using a continuous-wave, NIRS spectrometer (InSpectra model 325; Hutchinson Technology, Inc, Hutchinson, MN), an optical cable attached to a 25-mm probe, InSpectra software (version 2.0), and a dedicated laptop computer.13 The non-invasive technique of NIRS uses specific, calibrated wavelengths of near infrared light to quantify the percentage of hemoglobin oxygen saturation in the microvasculature of the tissue at a depth of 25 mm below the NIRS probe, as well as a small contribution of intracellular myoglobin. The degree of light absorption is dependent on the amount of oxygen attached to hemoglobin in the arterioles, venules, and capillaries. Non-absorbed light is returned as an optical signal and analyzed to produce a ratio of oxygenated hemoglobin to total hemoglobin, expressed as percentage of StO2 saturation continuously displayed on the spectrometer interfaced to a laptop computer.
The probe was attached to the skin over the medial gastrocnemius muscle of the more symptomatic leg using a double-sided adhesive light-excluding patch.21 If the severity of symptoms were similar for each leg in those with bilateral PAD, we selected the leg with the lower ABI value for placement of the probe regardless of history of prior revascularization. A baseline measure of calf muscle StO2 was obtained at rest as patients stood on the treadmill for two minutes to allow for equilibration. At the initiation of the treadmill test calf muscle StO2 was continuously recorded during exercise, and the following measurements were obtained: calf muscle StO2 at the end of the first minute of exercise, the absolute and percentage drops in calf muscle StO2 from rest to 1 minute of exercise, the minimum calf muscle StO2 value attained during exercise, the absolute and percentage drops in calf muscle StO2 from rest to the minimum exercise value, the exercise time taken to reach the minimum StO2 value, the rate of decline to the minimum StO2 value, and the calf muscle StO2 at the end of the treadmill test.
6-Minute Walk Test.
On a subsequent study visit, patients performed an over ground, 6-minute walk test in which two cones were placed 100 feet apart in a marked corridor.37 Trained exercise physiologists supervised the test and instructed the patients to walk as many laps around the cones as possible. The 6-minute walk pain-free distance (6MWPFD) as well as the total 6-minute walk distance (6MWD) were recorded in feet, and subsequently converted to meters.
Walking Impairment Questionnaire (WIQ).
Patient-based ambulatory ability was obtained using a validated questionnaire for PAD patients that assesses ability to walk at various speeds and distances, and to climb stairs.38
Medical Outcomes Study Short-Form 36 (MOS SF-36 – Rand Version 1.0).
The patient-based HRQoL was assessed from this instrument.39 We previously found that the physical function subscale was the most impaired subscale in patients with symptomatic PAD compared to national norms.40 Additionally, we successfully used the physical function subscale as the criterion variable to determine minimal clinically important differences in treadmill, 6-minute walk, and patient-based outcomes in these patients.41 Therefore, the physical function subscale was the primary outcome measure obtained from this questionnaire.
Activity Monitoring.
Daily ambulatory activity was assessed during seven consecutive days using a step activity monitor (StepWatch3™, Orthoinnovations, Inc., Oklahoma City, OK) to determine both the number of steps accumulated each day, and the number of steps taken at or above a moderate-to-vigorous pace.42
Statistical Analyses
The data were presented as means (standard deviations, SD) and quartiles (i.e., median, minimum and maximum) for continuous variables, and frequencies with percentage (%) for categorical variables (Tables I–II). The normality assumption for continuous variables was checked based on Shapiro-Wilk tests, and if necessary, log-transformation was applied to decrease the variability of data and make data conform more closely to normality. Pairwise correlation between the calf muscle StO2 measurements and the clinical exercise outcome variables were evaluated by Pearson’s correlation and the non-parametric measure via Spearman rank correlation (Table III). Thereafter, multiple regression analyses were conducted particularly for the exercise time to the minimum calf muscle StO2 value to evaluate its effect on clinical exercise outcomes adjusted for different sets of potential confounding variables including age, sex, race, body mass index, smoking, hypertension, diabetes and ABI (Table IV). The parameter estimates with 95% confidence interval (CI) as well as adjusted p-values based on Wald tests were obtained. In addition, the partial R2 values quantifying the partial correlation of determination for the variable of interest and the overall R2 values were achieved from multiple regressions. Model diagnosis was also explored via the Akaike information criterion and regression assumptions (e.g., linearity, residual normality and homogeneity) were investigated through residuals plots. All hypothesis tests were two-sided with the significance level of 0.05. Data was analyzed using SAS 9.4 Software (SAS Institute, Cary, North Carolina).
Table I.
Clinical characteristics of symptomatic patients with peripheral artery disease (n = 151).
| Variables | Values |
|---|---|
| Mean (SD) [Min, Max] | |
| Age (years) | 65 (10) [39, 89] |
| Weight (kg) | 82.5 (18.5) [47.2, 140.6] |
| Height (cm) | 168.7 (9.5) [147.0, 193.0] |
| Body Mass Index (kg/m2) | 29.0 (6.1) [18.0, 48.4] |
| Ankle/Brachial Index - Rest | 0.70 (0.27) [0.32, 1.28] |
| Claudication Onset Time (sec) | 193 (162) [21, 782] |
| Peak Walking Time (sec) | 398 (262) [36, 1200] |
| 6-Minute Walk Pain-Free Distance (m) | 184 (117) [15.2, 516.0] |
| 6-Minute Walk Distance (m) | 342 (96) [121.9, 520.0] |
| Total Daily Steps (steps/day) | 6438 (3442) [56, 20,112] |
| Moderate-to-Vigorous Daily Steps (steps/day) | 1416 (1364) [0, 8,020] |
| WIQ Distance Score (%) | 35 (32) [1, 100] |
| WIQ Speed Score (%) | 33 (23) [0, 100] |
| WIQ Stair Climbing Score (%) | 39 (29) [0, 100] |
| MOS SF-36 Physical Function Score (%) | 43 (20) [5,90] |
| Number (Percentage %) with Characteristics Present | |
| Sex (% Men) | 81 (54) |
| Race (% Caucasian) | 79 (52) |
| Current Smoking (% yes) | 62 (41) |
| Hypertension (% yes) | 132 (87) |
| Dyslipidemia (% yes) | 136 (90) |
| Diabetes (% yes) | 69 (46) |
| Obesity (% yes) | 63 (42) |
| Lower Extremity Revascularization (% yes) | 57 (38) |
| Bilateral Leg Pain (% yes) | 96 (64) |
| Coronary Artery Disease (% yes) | 47 (31) |
| Chronic Kidney Disease (% yes) | 38 (25) |
| Chronic Obstructive Pulmonary Disease (% yes) | 42 (28) |
MOS SF-36 = Medical Outcomes Study Short-Form 36 survey, WIQ = walking impairment questionnaire
Table II.
Calf muscle hemoglobin oxygen saturation (StO2) variables in symptomatic patients with peripheral artery disease.
| Variables | Mean (SD) | Median (Min, Max) |
|---|---|---|
| StO2 at rest (% saturation) | 53 (19) | 54 (3, 96) |
| StO2 at 1 minute of exercise (% saturation) | 26 (23) | 22 (1, 86) |
| Absolute drop in StO2 from rest to 1 minute of exercise (% saturation) | 27 (19) | 24 (0, 82) |
| Percentage drop in StO2 from rest to 1 minute of exercise (%) | 56 (35) | 58 (0, 98) |
| Minimum exercise StO2 (% saturation) | 16 (19) | 9 (1, 79) |
| Absolute drop in StO2 from rest to the minimum exercise value (% saturation) | 36 (19) | 34 (1, 85) |
| Percentage drop in StO2 from rest to the minimum exercise value (%) | 71 (28) | 81 (2, 99) |
| Exercise time to minimum StO2 (sec) | 203 (250) | 87 (4, 1092) |
| StO2 rate of decline to exercise minimum (% saturation/sec) | 0.57 (0.75) | 0.35 (0.02, 7.14) |
| StO2 at peak walking time (% saturation) | 22 (21) | 15 (1, 94) |
Table III.
Univariate regression models between calf muscle hemoglobin oxygen saturation (StO2) variables and clinical exercise outcome variables in 151 symptomatic patients with peripheral artery disease. Values are Pearson correlation coefficients.
| Calf Muscle StO2 Variables | COT | PWT | 6MWPFD § | 6MWD | Total Steps | MV Steps | WIQ Dist | WIQ Speed | WIQ Stairs | PF Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Absolute drop in StO2 from rest to 1 minute of exercise | −0.25 † | −0.17 | 0.02 | 0.14 | 0.18 * | 0.15 | −0.06 | −0.06 | −0.09 | −0.05 |
| Percentage drop in StO2 from rest to 1 minute of exercise | −0.30 ‡ | −0.26 † | −0.08 | −0.03 | 0.09 | 0.07 | −0.11 | −0.15 | −0.19 * | −0.13 |
| Absolute drop in StO2 from rest to the minimum exercise value | −0.08 | 0.02 | 0.11 | 0.22 * | 0.25 † | 0.25 † | −0.00 | 0.03 | 0.06 | 0.03 |
| Percentage drop in StO2 from rest to the minimum exercise value | −0.14 | −0.08 | −0.02 | 0.04 | 0.13 | 0.17 | −0.07 | −0.06 | −0.06 | −0.05 |
| Exercise time to minimum StO2 § | 0.49 ‡ | 0.56 ‡ | 0.19 * | 0.31 ‡ | 0.14 | 0.20 * | 0.33 ‡ | 0.39 ‡ | 0.37 ‡ | 0.32 ‡ |
| StO2 rate of decline to exercise minimum § | −0.48 ‡ | −0.49 ‡ | −0.11 | −0.19 * | −0.00 | −0.06 | −0.30 ‡ | −0.33 ‡ | −0.31 ‡ | −0.28 ‡ |
(p < 0.05),
(p < 0.01),
(p < 0.001).
Log transformed data.
COT = claudication onset time
PWT = peak walking time
6MWPFD = 6-minute walk pain-free distance
6MWD = 6-minute walk distance
MV = moderate-to-vigorous walking
WIQ = walking impairment questionnaire
Dist = distance
PF = physical function from the Medical Outcomes Study Short-Form 36 survey
Table IV.
Multiple regression models between the log transformed exercise time to the minimum calf muscle StO2 (predictor) and clinical exercise outcome variables in 151 symptomatic patients with peripheral artery disease.
| Clinical Exercise Outcome Variables | Model | Estimate (95% CI) | Partial R2 | P Value | AIC | Overall R2 |
|---|---|---|---|---|---|---|
| Claudication Onset Time | 1 | 67.50 (48.32, 86.67) | 0.24 | < 0.001 | 1927.3 | 0.24 |
| 2 | 64.81 (45.63, 83.98) | 0.22 | < 0.001 | 1929.5 | 0.26 | |
| 3 | 67.81 (48.64, 86.98) | 0.23 | < 0.001 | 1920.7 | 0.29 | |
| 4 | 65.56 (46.39, 84.73) | 0.22 | < 0.001 | 1920.4 | 0.31 | |
| Peak Walking Time | 1 | 125.26 (95.68, 154.84) | 0.32 | < 0.001 | 2058.2 | 0.32 |
| 2 | 120.17 (90.59, 149.75) | 0.29 | < 0.001 | 2053.0 | 0.37 | |
| 3 | 125.00 (95.42, 154.58) | 0.32 | < 0.001 | 2040.9 | 0.40 | |
| 4 | 120.33 (90.76, 149.91) | 0.31 | < 0.001 | 2032.4 | 0.44 | |
| 6-Minute Walk Pain-Free Distance * | 1 | 0.11 (0.02, 0.20) | 0.04 | 0.018 | 296.7 | 0.04 |
| 2 | 0.09 (−0.01, 0.18) | 0.02 | 0.073 | 296.0 | 0.08 | |
| 3 | 0.10 (0.01, 0.19) | 0.03 | 0.045 | 296.9 | 0.12 | |
| 4 | 0.08 (−0.01, 0.18) | 0.02 | 0.081 | 296.7 | 0.14 | |
| 6-Minute Walk Distance | 1 | 25.36 (12.54, 38.18) | 0.09 | < 0.001 | 1749.8 | 0.09 |
| 2 | 22.93 (10.11, 35.76) | 0.08 | < 0.001 | 1738.2 | 0.20 | |
| 3 | 26.14 (13.32, 38.96) | 0.11 | < 0.001 | 1721.3 | 0.27 | |
| 4 | 23.26 (10.44, 36.08) | 0.10 | < 0.001 | 1708.4 | 0.35 | |
| Total Daily Steps | 1 | 210.40 (−22.29, 443.10) | 0.02 | 0.078 | 2662.7 | 0.02 |
| 2 | 155.27 (−77.42, 387.96) | 0.01 | 0.212 | 2665.1 | 0.04 | |
| 3 | 201.11 (−31.58, 433.80) | 0.02 | 0.100 | 2641.7 | 0.13 | |
| 4 | 163.33 (−69.37, 396.02) | 0.01 | 0.180 | 2639.8 | 0.16 | |
| Moderate-to-Vigorous Daily Steps | 1 | 113.99 (22.68, 205.30) | 0.04 | 0.016 | 2382.1 | 0.04 |
| 2 | 81.08 (−10.23, 172.39) | 0.02 | 0.095 | 2382.2 | 0.08 | |
| 3 | 95.84 (4.53, 187.15) | 0.03 | 0.046 | 2363.4 | 0.14 | |
| 4 | 86.90 (−4.41, 178.21) | 0.02 | 0.072 | 2363.5 | 0.16 | |
| WIQ Distance Score | 1 | 9.03 (4.93, 13.12) | 0.11 | < 0.001 | 1461.3 | 0.11 |
| 2 | 8.28 (4.18, 12.38) | 0.09 | < 0.001 | 1460.2 | 0.15 | |
| 3 | 8.64 (4.55, 12.74) | 0.10 | < 0.001 | 1455.9 | 0.17 | |
| 4 | 7.96 (3.86, 12.06) | 0.09 | < 0.001 | 1447.6 | 0.23 | |
| WIQ Speed Score | 1 | 7.65 (4.71, 10.60) | 0.15 | < 0.001 | 1361.3 | 0.15 |
| 2 | 7.18 (4.23, 10.12) | 0.13 | < 0.001 | 1360.6 | 0.19 | |
| 3 | 7.63 (4.69, 10.58) | 0.14 | <0.001 | 1355.2 | 0.21 | |
| 4 | 7.27 (4.33, 10.21) | 0.14 | < 0.001 | 1349.6 | 0.26 | |
| WIQ Stair Climbing Score | 1 | 9.30 (5.59, 13.00) | 0.14 | <0.001 | 1421.6 | 0.14 |
| 2 | 7.89 (4.18, 11.59) | 0.11 | < 0.001 | 1403.3 | 0.27 | |
| 3 | 8.15 (4.44, 11.86) | 0.12 | < 0.001 | 1396.1 | 0.30 | |
| 4 | 8.11 (4.40, 11.81) | 0.12 | < 0.001 | 1398.7 | 0.30 | |
| MOS SF-36 Physical Function Score | 1 | 5.40 (2.80, 8.00) | 0.10 | < 0.001 | 1323.7 | 0.10 |
| 2 | 4.54 (1.95, 7.14) | 0.07 | < 0.001 | 1313.0 | 0.19 | |
| 3 | 4.65 (2.05, 7.25) | 0.08 | < 0.001 | 1307.3 | 0.22 | |
| 4 | 4.33 (1.74, 6.93) | 0.07 | 0.002 | 1306.7 | 0.24 |
Log transformed data, AIC = akaike information criterion, MOS SF-36 = Medical Outcomes Study Short-Form 36 survey, StO2 = hemoglobin oxygen saturation ,WIQ = walking impairment questionnaire.
Model 1: unadjusted. Model 2: adjusted for age, sex, race. Model 3: adjusted for Model 2 plus body mass index, smoking, hypertension, diabetes. Model 4: adjusted for Model 3 plus ankle-brachial index.
RESULTS
The clinical characteristics of 151 patients are shown in Table I. The group had a similar distribution of men and women. The men and women were older patients who were, on average, in the overweight classification. The mean ABI of 0.70 is indicative of moderately severe PAD, and all patients were symptomatic during the treadmill test with mean COT and PWT occurring at 193 and 398 seconds, respectively. Cardiovascular risk factors and comorbid conditions were highly prevalent, particularly for dyslipidemia (90%), hypertension (87%), diabetes (46%), obesity (42%), and current smoking (41%). Most patients were managed with medications, as 92.4% of the hypertensive patients were treated with anti-hypertensive medications, 78.7% of those with dyslipidemia were treated with statin therapy, and 87.0% of patients with diabetes were on medication therapy.
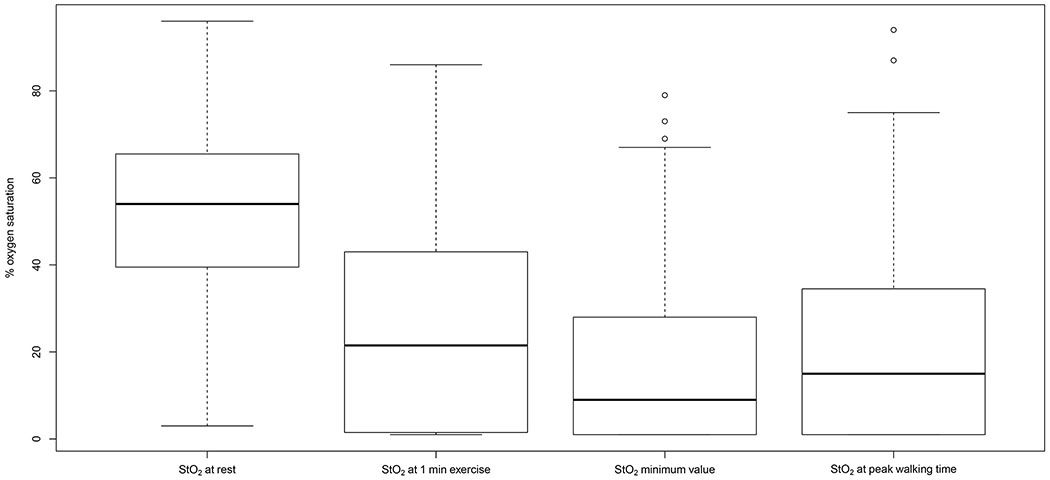
The calf muscle StO2 measurements in the patients at rest and during treadmill exercise are displayed in Table II and Figure 1. The median calf muscle StO2 at rest was 52% saturation, which declined to a median of 22% saturation after only one minute of walking during the treadmill test, representing an absolute drop from rest of 24% saturation and a relative percentage drop of 58%. As exercise continued, the median calf muscle StO2 declined further and reached a minimum value of 9% saturation after a median time of 87 seconds of exercise, representing an absolute drop from rest of 34% saturation and a relative percentage drop of 81%. The median rate of decline of calf muscle StO2 from the value at rest to the minimum value during exercise was 0.35% saturation/sec. After reaching the minimum value of 9% saturation at 87 seconds of exercise, calf muscle StO2 gradually increased to a median of 15% saturation by the end of the test.
Figure 1.
Box plot of calf muscle hemoglobin oxygen saturation (StO2) at rest, at 1 min of exercise, at the minimum value during exercise, and at peak walking time (PWT) in symptomatic patients with peripheral arterial disease.
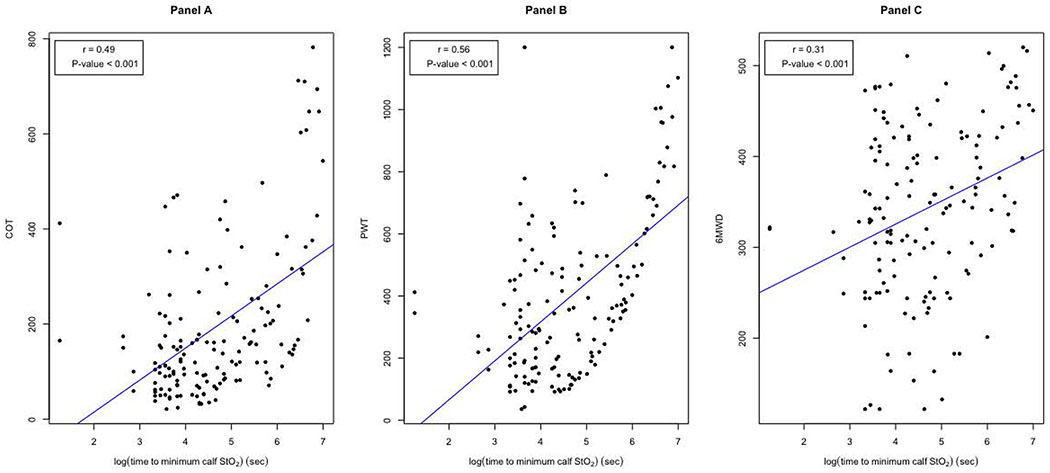
Univariate regression models between calf muscle StO2 variables and clinical exercise outcome variables are shown in Table III. Both Pearson and Spearman correlation coefficients were performed and were found to be similar, therefore only the Pearson correlation coefficients are presented in Table III. Of the calf muscle StO2 variables, the log transformed exercise time to the minimum calf muscle StO2 value had the strongest associations. Of particular interest, the associations between log transformed exercise time to the minimum calf muscle StO2 value and PWT (r=0.56, p<0.001), COT (r=0.49, p<0.001), and 6MWD (r=0.31, p<0.001) are shown in Figure 2.
Figure 2.
Scatter plots between the log-transformed exercise time to the minimum calf muscle hemoglobin oxygen saturation (StO2) and claudication onset time (COT) (Panel A), peak walking time (PWT) (Panel B), and 6-minute walk distance (6MWD) (Panel C).
Multiple regression models between log transformed exercise time to the minimum calf muscle StO2 value and clinical exercise outcome variables are shown in Table IV. Four models are shown for each clinical exercise outcome variable. Model 1 examines the association between the exercise time to the minimum calf muscle StO2 value and each exercise outcome variable without adjustment. Model 2 adjusts for demographic measures of age, sex, and race (coded as Caucasian or non-Caucasian). Model 3 adjusts for model 2 variables plus cardiovascular risk factors and comorbid conditions consisting of body mass index, current smoking, hypertension, and diabetes. Model 4 adjusts for model 3 variables plus ABI. All four regression models for COT, PWT, 6MWD, WIQ distance score, WIQ speed score, WIQ stair climbing score, and MOS SF-36 physical function score were highly associated (p < 0.001) with the log transformed exercise time to the minimum calf muscle StO2 value. Models 1 and 3 were significant for the log transformed 6MWPFD, and for the number of daily steps accumulated during a moderate-to-vigorous walking pace. None of the regression models were significant for the total number of daily steps accumulated during walking.
DISCUSSION
This is the first study to report how the microcirculation response during a standardized treadmill test was associated with a battery of commonly measured and clinically relevant exercise outcomes related to ambulatory function and HRQoL in patients with symptomatic PAD. In support of our hypothesis, the primary novel finding was that shorter exercise time to the minimum calf muscle StO2 value during the treadmill test was highly associated with worse ambulatory function and HRQoL outcomes. More importantly, these associations persisted even after adjusting for demographic measures, cardiovascular risk factors, comorbid conditions, and ABI. The clinical relevance is that exercise, dietary, and pharmacological therapies that may improve the microcirculation of the calf muscle may be especially beneficial in improving ambulatory function and HRQoL in patients with symptomatic PAD.
Change in Calf Muscle StO2 During Exercise.
During the treadmill test, calf muscle StO2 declined rapidly within the first minute of walking, indicating that the oxygen delivery was far inadequate to match the increased oxygen utilization of the calf muscle in patients with symptomatic PAD. This rapid decline in calf muscle StO2 is characteristic of patients with PAD during exercise, as previous studies have found a greater decrease in calf muscle StO2 during exercise in patients than in controls.24,26,28,43 It is important to note that our study is the first to report that the calf muscle StO2 data during exercise was not normally distributed, and thus we provided the median responses in which the minimum StO2 value reached a median of 9% saturation at a median time of 87 seconds. We have previously found that the most important characteristic of calf muscle StO2 during treadmill exercise in patients with symptomatic PAD is the time to reach the minimum StO2 value, as this measure was positively associated with COT and PWT in smaller cohorts,13,29 and this association persists even after adjusting for ABI.29 As such, we primarily focused on this variable to determine the association with key ambulatory function and HRQoL outcome measures. A final interesting observation is that once calf muscle StO2 reached the minimum value during exercise, there was a slight increase in it beyond that point to the end of exercise (ie, PWT). Since calf muscle StO2 is a balance between oxygen delivery to the local tissue and oxygen utilization by the tissue, the slight increase after the nadir indicates either a slight increase in oxygen delivery to the calf musculature, or a decrease in utilization of oxygen by the calf muscle, or a combination of both. The potential mechanisms associated with these changes are beyond the scope of this investigation.
Associations of Calf Muscle StO2 with Ambulatory function and HRQoL Outcomes
In the current study, we found that in unadjusted analyses, a shorter exercise time to the minimum calf muscle StO2 value was associated with worse ambulatory function and HRQoL. The exercise time to minimum calf muscle StO2 accounted for 26% of the variance in PWT, 22% of the variance in COT, 8% of the variance in 6MWD, between 10 and 15% of the variance in perceived walking ability and physical function related to HRQoL, and 4% of the variance in the 6-minute walk pain-free distance and in number of steps taken each day at a moderate-to-vigorous pace in patients with symptomatic PAD. Although the exercise time to minimum calf muscle StO2 accounted for significant variance in these clinical exercise outcomes, the moderate-to-low values suggest that other unexplained factors may also exist that are associated with these exercise outcomes. The current findings with PWT and COT support our previous observations that a rapid decline in calf muscle StO2 was associated with low COT and PWT,13,29 and indicates that poor microcirculation during the initial phase of the treadmill test hastens the development of claudication. This is the first study to extend these findings by showing that rapid decline in calf muscle StO2 is associated with impaired 6-minute walk performance, lower WIQ scores, a worse physical function subscale score of HRQoL, and lower levels of daily activity of at least moderate intensity.
In contrast to the association with daily activity of moderate-to-vigorous intensity, it is interesting to note that this is not true for total daily steps, as the exercise time to the minimum calf muscle StO2 value was not related to the accumulated number of steps taken each day in univariate or multiple regression analyses. The reason for this lack of association is not clear, but we speculate that physical activity performed at light intensity, which is the primary activity level completed by patients with PAD,42 is of insufficient intensity to induce changes in microcirculation of the calf musculature. We have previously found that both supervised and home-based exercise training programs lengthen the exercise time to the minimum calf muscle StO2 value, indicative of improved microcirculation.30 The current observation that exercise time to minimum calf muscle StO2 is associated with the amount of daily ambulation performed at moderate-to-vigorous intensity, but not with total activity that includes light intensity, may support our speculation that daily activity needs to be of sufficient intensity to impact the calf microcirculation.
Another unique aspect to this study was that we examined the association between the exercise time to minimum calf muscle StO2 value and ambulatory function and HRQoL outcomes after adjusting for covariates, including ABI. We have previously shown that sex,29,44 smoking,21 and dyslipidemia45 are related to the exercise time to minimum calf muscle StO2 value. Thus, we used a multiple regression approach to determine if the microcirculation of the lower extremity independently explains variation in the ambulatory function and HRQoL outcomes of patients with symptomatic PAD. For most of the variables, adjusting for age, sex and race in model 2 did not change the association with exercise time to minimum calf StO2. These findings suggest that age, sex and race have minimal impact on the association between microcirculation and exercise outcomes. The exceptions were for the 6-minute walk pain-free distance and the number of daily steps accumulated during moderate-to-vigorous activity, in which the association with exercise time to minimal calf StO2 was no longer significant after the adjustment. We have previously found that women with PAD have lower daily activity levels than men,44 which supports the current observation that sex partially explains the relationship between microcirculation and daily activity. After further adjustment for body mass index, current smoking, hypertension, and diabetes in model 3, and adjustment for ABI in model 4, the association between the exercise time to minimal calf StO2 with each clinical exercise outcome remained significant, indicating that they did not partially explain the association between microcirculation and exercise outcomes. The observation with ABI is of particular importance, as the interpretation is that key ambulatory function outcomes such as claudication values during treadmill exercise, 6MWD, daily activity, and perceived walking ability and physical function are strongly associated with the microcirculation of the lower extremities independent of the impairment in the macrocirculation. It is not clear what factors may contribute to impairment in microcirculation in patients with PAD and claudication, but we speculate that some factors may include endothelial dysfunction, microvascular damage, and lower capillary density.
Limitations
Several limitations exist for this study. As with many clinical studies, there may have been a self-selection bias for participating. Patients who agreed to participate were volunteers and may have represented those with the highest interest in clinical research, the best access to transportation, and the best health compared with non-volunteers. Another study limitation is related to the cross-sectional design. As such, significant associations between the calf muscle StO2 variables and the exercise variables does not indicate causality. Additionally, information on the specific locations of arterial lesions in the lower extremities was not available on all patients and was not considered in the analyses. Fourteen percent (n = 22) of the patients had an ABI at rest in the borderline to normal range, and they qualified for the study based on the drop in ABI below 0.73 after exercise. Although this group had mild PAD, inclusion of this group had minimal impact on the results because controlling for ABI in the multiple regressions (model 4) had little impact on the findings that log transformed exercise time to the minimum calf muscle StO2 was significantly associated with the clinical exercise outcome variables. Another limitation is that our findings are applicable to symptomatic PAD patients, and may not generalize to patients with different stages of PAD, such as asymptomatic PAD or critical limb ischemia. However, our results are representative of patients with claudication because of the typically high prevalence of comorbid conditions.
There are limitations to this study that are associated with the measurement of calf muscle StO2. Although the calf muscle StO2 primarily reflects the relative balance between oxygen delivery and oxygen utilization of the local tissue, the contribution of myoglobin to the StO2 measurement cannot be excluded, especially during the onset on exercise.17 However, any contribution that local myoglobin may have on the calf muscle StO2 should be minimal beyond the initial few seconds of exercise. Another limitation with the StO2 measure is that the redistribution of oxyhemoglobin and de-oxyhemoglobin in the local tissue represents a mixture of capillary and venular blood that have different oxygen saturations.17 The subcutaneous fat thickness also may interfere with the calf muscle StO2 measurement. However, it is unlikely that this affected the results because there is no relationship between calf skin fold and calf muscle StO2 in PAD patients,16 and because only patients having calf skin folds <50 mm participated. Since subcutaneous tissue is only half the thickness of the skin fold measurement, the penetration of the light path of the NIRS probe to a depth of 25 mm should go beyond the subcutaneous tissue into the calf musculature. Finally, we only had one probe to measure calf muscle StO2, and therefore we selected the more symptomatic leg to study at the exclusion of the contralateral leg.
Conclusion and Clinical Significance
In conclusion, more rapid decline in oxygen saturation of the calf musculature during walking, indicative of impaired microcirculation, was predictive of impaired ambulatory function and HRQoL in patients with symptomatic PAD. Of particular importance, these associations were independent of ABI and other common health burdens, highlighting the clinical relevance that the microcirculation has on ambulatory function and HRQoL in patients with symptomatic PAD. The clinical significance is that behavioral and medical therapies that may improve the microcirculation of the calf muscle may be especially beneficial in improving ambulatory function and HRQoL in patients with symptomatic PAD.
ARTICLE HIGHLIGHTS.
Type of Research:
Single-center, prospective, cross-sectional study.
Key Findings:
Calf muscle oxygen saturation was measured during a standardized treadmill test, and the time taken to reach the minimum value (log transformed) was predictive of peak walking time (r=0.56, p<0.001) and claudication onset time (r=0.49, p<0.001). These associations persisted (p<0.001) in adjusted multiple regression models.
Take home Message:
More rapid decline in oxygen saturation of the calf muscle during walking, indicative of impaired microcirculation, was predictive of impaired ambulatory function and quality of life in patients with claudication.
Acknowledgments
Supported by grants from the National Institute on Aging (R01-AG-24296) and General Clinical Research Center (M01-RR-14467) sponsored by the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–1340. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch AT, Hartman L, Town RJ, Virnig BA. National health care costs of peripheral arterial disease in the Medicare population. Vasc Med. 2008;13(3):209–215. [DOI] [PubMed] [Google Scholar]
- 3.Regensteiner JG, Hiatt WR, Coll JR, et al. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med. 2008;13(1):15–24. [DOI] [PubMed] [Google Scholar]
- 4.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326(6):381–386. [DOI] [PubMed] [Google Scholar]
- 5.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45 Suppl S:S5–67. [DOI] [PubMed] [Google Scholar]
- 6.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single-stage treadmill tests for evaluation of claudication. Med Sci Sports Exerc. 1991;23(4):402–408. [PubMed] [Google Scholar]
- 7.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286(13):1599–1606. [DOI] [PubMed] [Google Scholar]
- 8.McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292(4):453–461. [DOI] [PubMed] [Google Scholar]
- 9.Sieminski DJ, Gardner AW. The relationship between free-living daily physical activity and the severity of peripheral arterial occlusive disease. Vasc Med. 1997;2(4):286–291. [DOI] [PubMed] [Google Scholar]
- 10.Fowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300(2):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Prediction of claudication pain from clinical measurements obtained at rest. Med Sci Sports Exerc. 1992;24(2):163–170. [PubMed] [Google Scholar]
- 12.Gardner AW, Ricci MA, Case TD, Pilcher DB. Practical equations to predict claudication pain distances from a graded treadmill test. Vasc Med. 1996;1(2):91–96. [DOI] [PubMed] [Google Scholar]
- 13.Gardner AW, Parker DE, Webb N, Montgomery PS, Scott KJ, Blevins SM. Calf muscle hemoglobin oxygen saturation characteristics and exercise performance in patients with intermittent claudication. J Vasc Surg. 2008;48(3):644–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiatt WR, Nawaz D, Regensteiner JG, Hossack KF. The evaluation of exercise performance in patients with peripheral vascular disease. J Cardiopulmonary Rehabil. 1988;12:525–532. [Google Scholar]
- 15.Leicht AS, Crowther RG, Muller R, Golledge J. The effects of including quality of life responses in models to predict walking performance of patients with intermittent claudication. Eur J Vasc Endovasc Surg. 2011;41(4):511–517. [DOI] [PubMed] [Google Scholar]
- 16.Ouriel K, McDonnell AE, Metz CE, Zarins CK. Critical evaluation of stress testing in the diagnosis of peripheral vascular disease. Surgery. 1982;91(6):686–693. [PubMed] [Google Scholar]
- 17.Wilkinson D, Vowden P, Parkin A, Wiggins PA, Robinson PJ, Kester RC. A reliable and readily available method of measuring limb blood flow in intermittent claudication. BrJSurg. 1987;74(6):516–519. [DOI] [PubMed] [Google Scholar]
- 18.Yao ST, Needham TN, Gourmoos C, Irvine WT. A comparative study of strain-gauge plethysmography and Doppler ultrasound in the assessment of occlusive arterial disease of the lower extremities. Surgery. 1972;71(1):4–9. [PubMed] [Google Scholar]
- 19.Parmenter BJ, Raymond J, Fiatarone Singh MA. The effect of exercise on haemodynamics in intermittent claudication: a systematic review of randomized controlled trials. Sports Med. 2010;40(5):433–447. [DOI] [PubMed] [Google Scholar]
- 20.Komiyama T, Shigematsu H, Yasuhara H, Muto T. Near-infrared spectroscopy grades the severity of intermittent claudication in diabetics more accurately than ankle pressure measurement. BrJSurg. 2000;87(4):459–466. [DOI] [PubMed] [Google Scholar]
- 21.Afaq A, Montgomery PS, Scott KJ, Blevins SM, Whitsett TL, Gardner AW. The effect of current cigarette smoking on calf muscle hemoglobin oxygen saturation in patients with intermittent claudication. VascMed. 2007;12(3 ):167–173. [DOI] [PubMed] [Google Scholar]
- 22.Bauer TA, Brass EP, Hiatt WR. Impaired muscle oxygen use at onset of exercise in peripheral arterial disease. J Vasc Surg. 2004;40(3):488–493. [DOI] [PubMed] [Google Scholar]
- 23.Bauer TA, Brass EP, Barstow TJ, Hiatt WR. Skeletal muscle StO2 kinetics are slowed during low work rate calf exercise in peripheral arterial disease. Eur J Appl Physiol. 2007;100(2):143–151. [DOI] [PubMed] [Google Scholar]
- 24.Comerota AJ, Throm RC, Kelly P, Jaff M. Tissue (muscle) oxygen saturation (StO2): a new measure of symptomatic lower-extremity arterial disease. J Vasc Surg. 2003;38(4):724–729. [DOI] [PubMed] [Google Scholar]
- 25.Komiyama T, Shigematsu H, Yasuhara H, Muto T. An objective assessment of intermittent claudication by near-infrared spectroscopy. EurJVascSurg. 1994;8(3):294–296. [DOI] [PubMed] [Google Scholar]
- 26.McCully KK, Halber C, Posner JD. Exercise-induced changes in oxygen saturation in the calf muscles of elderly subjects with peripheral vascular disease. JGerontol. 1994;49(3):B128–B134. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe T, Matsushita M, Nishikimi N, Sakurai T, Komori K, Nimura Y. Near-infrared spectroscopy with treadmill exercise to assess lower limb ischemia in patients with atherosclerotic occlusive disease. SurgToday. 2004;34(10):849–854. [DOI] [PubMed] [Google Scholar]
- 28.Mohler ER III, Lech G, Supple GE, Wang H, Chance B. Impaired exercise-induced blood volume in type 2 diabetes with or without peripheral arterial disease measured by continuous-wave near-infrared spectroscopy. Diabetes Care. 2006;29(8):1856–1859. [DOI] [PubMed] [Google Scholar]
- 29.Gardner AW, Parker DE, Montgomery PS, Blevins SM, Nael R, Afaq A. Sex differences in calf muscle hemoglobin oxygen saturation in patients with intermittent claudication. J Vasc Surg. 2009;50(1):77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner AW, Parker DE, Montgomery PS, Blevins SM. Step-monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: a randomized controlled trial. J Am Heart Assoc. 2014;3(5):e001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lohman TC, Roche AF, Marubini E. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books; 1988. p. 39–70. [Google Scholar]
- 32.Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–2909. [DOI] [PubMed] [Google Scholar]
- 33.Gardner AW, Montgomery PS, Casanegra AI, Silva-Palacios F, Ungvari Z, Csiszar A. Association between gait characteristics and endothelial oxidative stress and inflammation in patients with symptomatic peripheral artery disease. Age. 2016;38(3):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–e654. [DOI] [PubMed] [Google Scholar]
- 35.Hiatt WR, Marshall JA, Baxter J, et al. Diagnostic methods for peripheral arterial disease in the San Luis Valley Diabetes Study. J Clin Epidemiol. 1990;43(6):597–606. [DOI] [PubMed] [Google Scholar]
- 36.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 37.Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc. 1998;46(6):706–711. [DOI] [PubMed] [Google Scholar]
- 38.Regensteiner JG, Steiner JF, Panzer RL, Hiatt WR. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol. 1990;2:142–152. [Google Scholar]
- 39.Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 40.Izquierdo-Porrera AM, Gardner AW, Bradham DD, et al. Relationship between objective measures of peripheral arterial disease severity to self-reported quality of life in older adults with intermittent claudication. JVascSurg. 2005;41(4):625–630. [DOI] [PubMed] [Google Scholar]
- 41.Gardner AW, Montgomery PS, Wang M. Minimal clinically important differences in treadmill, 6-minute walk, and patient-based outcomes following supervised and home-based exercise in peripheral artery disease. Vasc Med. 2018;23(4):349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardner AW, Montgomery PS, Scott KJ, Afaq A, Blevins SM. Patterns of ambulatory activity in subjects with and without intermittent claudication. J VascSurg. 2007;46(6):1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kemp GJ, Roberts N, Bimson WE, et al. Mitochondrial function and oxygen supply in normal and in chronically ischemic muscle: a combined 31P magnetic resonance spectroscopy and near infrared spectroscopy study in vivo. JVascSurg. 2001;34(6):1103–1110. [DOI] [PubMed] [Google Scholar]
- 44.Gardner AW, Parker DE, Montgomery PS, Khurana A, Ritti-Dias RM, Blevins SM. Gender differences in daily ambulatory activity patterns in patients with intermittent claudication. J Vasc Surg. 2010;52(5):1204–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Afaq A, Montgomery PS, Scott KJ, Blevins SM, Whitsett TL, Gardner AW. The effect of hypercholestrolemia on calf muscle hemoglobin oxygen saturation in patients with intermittent claudication. Angiology. 2008;59(5):534–541. [DOI] [PubMed] [Google Scholar]