Abstract
Objective
The etiology of osteoarthritis (OA) is unknown, however, there appears to be a significant contribution from genetics. We have identified recombinant-inbred strains of mice derived from LG/J and SM/J strains that vary significantly in their ability to repair articular cartilage and susceptibility to post-traumatic OA due to their genetic composition. Here, we report cartilage repair phenotypes in the same strains of mice in which OA susceptibility was analyzed previously, and determine the genetic correlations between phenotypes.
Design
We used 12 recombinant-inbred strains, including the parental strains, to test three phenotypes: ear-wound healing (n=263), articular cartilage repair (n=131), and post-traumatic OA (n=53) induced by DMM. Genetic correlations between various traits were calculated as Pearson correlation coefficients of strain means.
Results
We found a significant positive correlation between ear-wound healing and cartilage regeneration (r=0.71;P=0.005). We observed a strong inverse correlation between cartilage regeneration and susceptibility to OA based on maximum (r=−0.54;P=0.036) and summed OARSI scores (r=−0.56;P=0.028). Synovitis was not significantly correlated with cartilage regeneration but was significantly positively correlated with maximum (r=0.63;P=0.014) and summed (r=0.70;P=0.005) scores. Ectopic calcification was correlated with cartilage regeneration (r=0.59;P=0.021).
Conclusions
Using recombinant-inbred strains, our study allows, for the first time, the measurement of genetic correlations of regeneration phenotypes with degeneration phenotypes that are characteristic of post-traumatic OA (cartilage degeneration, synovitis). We demonstrate that OA is positively correlated with synovitis and inversely correlated with the ability to repair cartilage. These results suggest a shift in the risk paradigm for OA from a focus on degeneration to regeneration.
Keywords: ear-wound, cartilage injury, DMM surgery, synovitis, ectopic calcification, LG/J, SM/J
INTRODUCTION
The ability to stimulate cartilage regeneration/repair has been an elusive target leading to the common wisdom that cartilage cannot repair. While nearly impossible to study in humans, we and others have undertaken repair studies in mice[1–3]. In particular, we undertook studies to examine the genetics of cartilage repair in informative recombinant-inbred mouse lines[1]. Using a healer mouse line (LG/J) and a non-healer mouse line (SM/J), recombinant-inbred strains were developed that demonstrate phenotypic continuum in their ability to heal a 2-mm ear-wound[4]. We have previously reported differences in cartilage healing in some strains of these recombinant-inbred lines[1].
Osteoarthritis (OA) of the knee affects all tissues of the joint such as bone, cartilage, synovium, meniscus and ligaments[5, 6]. While OA is recognized as the whole joint disease, cartilage degeneration is still considered the hallmark of end-stage disease[7]. OA is a complex polygenic disease with a genetic contribution accounting for 39–65% of OA variation[8, 9]. Numerous other risk factors, such as aging, obesity, female sex and joint trauma influence OA development and progression[6, 10]. At least 12% of the overall prevalence of symptomatic OA is attributable to post-traumatic OA[11] stemming from intra-articular injuries and cartilage damage. As such OA pathology accumulates over a time-course of 10–15 years subsequent to ligament and meniscus tears[12].
Genetic variability in response to injury and post-traumatic OA has been described in a number of mouse strains: MRL/MpJ, a super-healer mouse, along with its close relative, the LG/J mouse, possesses remarkable ability to regenerate a wide range of body tissues[3, 13–15]. In contrast, the C57BL/6J and SM/J mouse strains bear much less healing potential. Unlike unrelated mouse strains, such as MRL and C57BL/6J, recombinant-inbred strains can be used to measure and map the genetic variations that confer phenotypic traits[16, 17]. The recombinant-inbred cross generated from LG/J (healer) and SM/J (non-healer) strains demonstrates variation in a number of phenotypes (ear-wound healing, cartilage healing and OA) depending on the genetic contribution derived from the parental strains[1, 16, 18, 19]. We have used the recombinant-inbred strains to measure and map the genetic variations that confer OA resistance[19, 20].
Having established that both cartilage regeneration and post-traumatic OA are heritable traits and that recombinant-inbred lines would demonstrate a spectrum of phenotypic traits, we were able to probe the relationship between cartilage healing and aspects of the OA phenotype, such as synovitis. In this study we report the results of cartilage healing in 12 recombinant-inbred mouse strains including the parental strains. We further calculate genetic correlations between cartilage healing phenotypes, ear-wound repair and OA from previously reported results in the same strains[20]. Our findings demonstrate a phenotypic distribution of cartilage repair in recombinant-inbred mouse strains and show genetic correlations between healing of cartilage and protection from OA. We have previously reported that the correlation between synovitis and OA in mice parallel those in human[20]. We predict that the genetic correlations between the ability to heal cartilage and protection from OA will be born out in humans as well.
METHODS
Mice
All procedures were approved by the Institutional Animal Care and Use Committee. We used 12 recombinant-inbred strains of mice generated from LG/J by SM/J intercross (Table-1) including the parental strains. LG/J and SM/J mice were obtained from The Jackson Laboratory (Bar Harbor ME, USA). LGXSM recombinant-inbred lines were generated from the F2 intercross of LG/J females with the SM/J males. The detailed information and history of the recombinant- inbred lines has been described before[1, 16, 20]. Male mice were housed in individually ventilated cages under pathogen-free conditions with ad libitum access to standard mouse chow and water and a 12:12h light:dark cycle. All mice were housed in groups in cages (5 mice/cage). Mice were randomized for two phenotypes namely articular cartilage repair and destabilization of medial meniscus (DMM): some animals underwent only knee cartilage injury, and others received only DMM surgery. Ear-wound phenotype was, however, performed on all animals selected for the above two phenotypes as well as on additional animals that did not undergo knee cartilage or DMM surgery.
Table 1:
Study sample size, strains and time points for each phenotype
| Strain | Ear wound healing phenotype | Articular cartilage regeneration phenotype | OA phenotype (DMM model) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain | N | Age at phenotype | Analysis time point | N | Age at phenotype | Analysis time point | N | Age at phenotype | Analysis time point |
| LGXSM-5 | 14 | 6 weeks | 30 days | 12 | 8 weeks | 16 weeks | 3 | 10 weeks | 8 weeks |
| LGXSM-6 | 72 | 6 weeks | 30 days | 29 | 8 weeks | 16 weeks | 11 | 10 weeks | 8 weeks |
| LGXSM-18 | 7 | 6 weeks | 30 days | 6 | 8 weeks | 16 weeks | 3 | 10 weeks | 8 weeks |
| LGXSM-19 | 9 | 6 weeks | 30 days | 4 | 8 weeks | 16 weeks | 3 | 10 weeks | 8 weeks |
| LGXSM-33 | 37 | 6 weeks | 30 days | 20 | 8 weeks | 16 weeks | 8 | 10 weeks | 8 weeks |
| LGXSM-35 | 12 | 6 weeks | 30 days | 14 | 8 weeks | 16 weeks | 3 | 10 weeks | 8 weeks |
| LGXSM-46 | 8 | 6 weeks | 30 days | 6 | 8 weeks | 16 weeks | 3 | 10 weeks | 8 weeks |
| LGXSM-163 | 36 | 6 weeks | 30 days | 4 | 8 weeks | 16 weeks | 3 | 10 weeks | 8 weeks |
| LGXSM-120a | 22 | 6 weeks | 30 days | 7 | 8 weeks | 16 weeks | 3 | 10 weeks | 8 weeks |
| LGXSM-128a | 25 | 6 weeks | 30 days | 8 | 8 weeks | 16 weeks | 3 | 10 weeks | 8 weeks |
| LG/J | 8 | 6 weeks | 30 days | 11 | 8 weeks | 16 weeks | 5 | 10 weeks | 8 weeks |
| SM/J | 13 | 6 weeks | 30 days | 10 | 8 weeks | 16 weeks | 5 | 10 weeks | 8 weeks |
OA = osteoarthritis; DMM = destabilization of medial meniscus
Ear-wound healing phenotype
A bilateral 2 mm through-and-through hole was produced in the center of the cartilaginous part of external ear of 6-week-old mice (n=263) with the use of a metal ear punch (Thermo-Fisher-Scientific) as previously described[21]. The hole diameter was measured 30 days after wounding using a grid-etched reticle in a blinded fashion and healing diameter was calculated by subtracting the residual hole diameter from original hole size (2 mm).
Articular cartilage repair phenotype
Bilateral full-thickness knee articular cartilage lesions were created through microsurgery in 8-week-old mice (n=126) as detailed previously[3]. Briefly, while under anesthesia through intraperitoneal injection of rodent-cocktail comprised of ketamine (100-mg/ml), xylazine (20-mg/ml) and acepromazine (10-mg/ml), the joint capsule was opened and the patella luxated laterally to expose the trochlear groove articular surface. Cartilage lesion was created with a 27Gx½” needle (BD Syringe). The joint capsule was closed, subcutaneous tissue was apposed by suturing followed by application of Vetclose skin-glue (Suture-Vet-Buttler). After 16-weeks, mice were sacrificed, joints were processed for histology, paraffin-embedded, and sectioned in sagittal plane. The sections were mounted on polylysine-coated slides (Thermo-Fischer- Scientific) and were stained with toluidine-blue after de-waxing. Stained sections were imaged on a microscope (Eclipse-E800; Nikon) attached to a camera (2000R-Fast1394; Retiga) with the use of QCapturePro (Qlmaging) software. Sections were scored in a blinded fashion (by at least two expert scorers) according to an established protocol for an overall healing score ranging from 0 (no healing) to 14 (full healing).
Post-traumatic OA phenotype
DMM surgery was performed on 10-week-old mice (n=53) to induced post-traumatic OA as before[19, 22]. Briefly, under anesthesia the joint capsule immediately medial to the patellar tendon was opened and the anterior attachment of medial meniscotibial ligament was transected. The joint capsule was closed by suturing as well as skin using Vetclose skin-glue. The left knee served as sham in which the joint capsule was opened and the ligament was visualized but left intact. 8-weeks post-surgery, mice were sacrificed by CO2 asphyxiation. Knee joints were collected, fixed in 10% neutral-buffered-formalin and decalcified. Eight coronal sections (5-pm thick) were taken from each joint at eight levels separated by 80-pm intervals. From each level, we stained three sections with toluidine-blue to perform scoring for cartilage degeneration using the Osteoarthritis Research Society International (OARSI) scoring system[23–25]. Cartilage damage score was measured in all four quadrants of the joint at all sectioned levels[23]. Summed OARSI scores, representing whole joint changes and maximum OARSI scores, which represents the highest score within all sectioned levels of each knee[19, 23, 25] were recorded. Synovitis was measured from all four quadrants by examining the enlargement of the synovial lining cell layer and density of the cells using a previously established scoring system[25, 26]. All scoring was performed in a blinded manner with respect to mouse strains and type of surgery by at least two expert scorers with a high inter-rater reliability.
Prior to decalcification, joints were scanned using in-vivo micro-CT scanner (SCANCO Medical) to analyze the 3-dimensional structure of bone for the following parameters as reported previously[19, 25, 27, 28]: BV/TV, Tb.N, Tb.Th, Tb.Sp, SMI, and CDI. Subchondral-bone-plate thickness was outlined separately from trabecular bone at the tibial side using contouring methods on the cortical regions as described previously[25]. As some strains of mice developed calcified nodules in the joints after injury[29], we quantified the number of nodules after joints were scanned by micro-CT scanner[30]. Briefly, after visualization by generating 3-dimensional images in OsiriX imaging software (OsiriX), these nodules were counted by including all mineralized areas in and around the joint space except for the patella, anterior horns of the menisci, and sesamoid bones.
Statistical analysis
Parts of published and new unpublished data were used from 12 recombinant-inbred strains including the parental LG/J and SM/J strains (Table-2). We have previously published ear-wound healing and knee cartilage repair data from both male and female mice[1]; however, here, we limited our analysis to only male mice since DMM was performed only in males. For ear- wound, we have historical data from 9 strains and new data from 3 strains (indicated by $ in Table-2). Likewise, knee cartilage repair data were published only from 6 strains and new data come from 6 new strains (indicated by $ in Table-2). To match strains (not individual mice) for each phenotype, DMM data was chosen from 12 out of 14 strains published previously[19, 20]. Ear-wound healing and articular cartilage regeneration data from right and left sides were averaged for each phenotype. For OA data, sham knees were subtracted from DMM knees.
Table 2:
Data used for calculation of correlation coefficients
| Strain | Ear wound | Articular cartilage | Max. OARSI | Sum. OARSI | Synovitis | BV/TV | CDI | SMI | Tb.N | Tb.Th | Tb.Sp | SCB | Ectopic calcification |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LGXSM-5 | 1.15 | 3.93 | 3.83 | 20.33 | 1.38 | −0.07 | −37.55 | 0.50 | −0.56 | 0.00 | 0.03 | −0.70 | 5.33 |
| LGXSM-6 | 1.19 | 5.45 | 2.00 | 6.57 | 0.48 | −0.10 | −23.06 | 1.67 | −1.12 | 0.00 | 0.04 | −26.80 | 8.00 |
| LGXSM-18$ | 0.70 | 3.22 | 2.00 | 5.50 | 0.33 | −0.03 | −32.89 | −0.01 | −0.15 | 0.00 | 0.00 | 21.34 | 3.00 |
| LGXSM-19$ | 1.16 | 4.17 | 2.33 | 11.67 | 0.88 | −0.10 | −56.46 | 0.98 | −0.90 | 0.00 | 0.05 | −42.56 | 4.67 |
| LGXSM-33 | 0.69 | 2.36 | 4.08 | 23.92 | 0.85 | −0.03 | −5.12 | 0.48 | −0.55 | 0.00 | 0.03 | 56.86 | 0.67 |
| LGXSM-35 | 1.15 | 5.30 | 1.00 | 9.83 | 0.57 | 0.00 | −5.31 | −0.10 | −0.15 | 0.00 | 0.01 | 4.77 | 2.33 |
| LGXSM-46$ | 1.31 | 2.76 | 3.00 | 20.17 | 1.40 | 0.00 | −14.67 | −0.09 | −0.01 | 0.00 | 0.00 | −1.69 | 5.00 |
| LGXSM-163$^ | 1.02 | 4.83 | 0.33 | 0.67 | 0.12 | −0.02 | −6.93 | 0.10 | −0.25 | 0.00 | 0.01 | −7.47 | 1.60 |
| LGXSM-120a$^ | 1.16 | 4.17 | 2.00 | 8.83 | 0.34 | −0.01 | −6.51 | 0.02 | −0.08 | 0.00 | 0.00 | 9.13 | 2.75 |
| LGXSM-128a$^ | 1.14 | 4.21 | 1.17 | 4.50 | 1.05 | −0.12 | −58.89 | 1.15 | −0.83 | −0.01 | 0.04 | −10.79 | 2.33 |
| LG/J | 1.44 | 8.45 | 1.50 | 5.50 | 0.61 | −0.02 | −12.40 | 0.26 | −0.30 | 0.01 | 0.01 | 5.92 | 7.00 |
| SM/J | 0.50 | 2.08 | 2.50 | 14.00 | 0.66 | 0.01 | −33.35 | 0.25 | −0.10 | 0.01 | 0.00 | 4.08 | 1.00 |
Max. OARSI = maximum Osteoarthritis Research Society International (score); Sum. OARSI = summed OARSI (score); BV/TV = bone volume/total volume; CDI = connectivity density index; SMI = structure model index; Tb.N = trabecular number; Tb.Th = trabecular thickness; Tb.Sp = trabecular spacing; SCB = subchondral bone plate thickness
new data for articular cartilage regeneration phenotype
new data for ear-wound healing phenotype
There are different numbers of animals in each strain for each trait so the number of cases per strain needs adjustment for each trait[31]. Sokal and Rohlf (1981) provide an equation (eq 9.1) calculating the appropriate pooled within-group sample size to use when considering variation in sample sizes across strains[31]. For this study, the number of cases per strain varies from 20.54 for ear-wound healing, 10.51 for articular cartilage repair, and 4.29 for OA. The general group size averaged over all the traits is 10 individuals per strain. Note that the corrected number of cases per strain is always lower than the raw average of cases per strain. Our units of analysis are strains because individual animals do not correspond across the traits.
The data for each trait was collected from different individuals with different sample sizes depending on trait and strain. OARSI score, synovitis score and bone-parameters were examined only in mice that underwent DMM while overall knee cartilage healing was assessed in mice subjected to knee cartilage injury. Ear-wound diameter was measured in mice undergoing ear- wounding, however these mice also had undergone either DMM or cartilage surgery or none of the two. We cannot use a MANOVA here for analysis because while measures are obtained from the same strains, there are different individuals for each trait. Therefore, we correlated strain means. Correlation coefficients were calculated in GraphPad Prims (GraphPad Software Inc.) and values are shown as 95% confidence interval unless stated otherwise.
By using the means to represent the units of analysis rather than individuals, both variances and covariances among traits are inflated[32] by adding one-tenth (n=10 animals per strain averaged over the experiment) of the within-group variance or covariance to each between-group estimate. These additions are small and made to both the numerator and denominator of the correlation estimates. A more serious problem might occur when the within- and between-strain correlations are in opposite directions. This is rarely the case for morphological traits[33].
We tested the hypotheses about correlations using a one-tailed p-value based on justifications from the literature and considerations of biological processes. Several of the traits are anabolic formed by adding or regenerating damaged cartilage[34], including ear cartilage repair, regenerated articular cartilage, and ectopic-calcifications. Other traits measure catabolic or degenerative processes[35], such as the OARSI measures after DMM, and synovitis. We expect positive correlations within these groups and negative correlations between them. Correlations of opposite sign from our expectation cannot be interpreted in this study but will be noted for future reference.
RESULTS
Genetic correlation between articular cartilage regeneration and various characteristics of post-traumatic OA
A significant inverse correlation was observed between articular cartilage regeneration and susceptibility to cartilage degeneration based on maximum OARSI (r=−0.54; [−0.85 to 0.05]; P=0.036) (Fig.-1A) as well as summed OARSI scores (r=−0.56; [−0.86 to 0.02]; P=0.028) (Fig.-1B). With regards to susceptibility to synovitis, cartilage regeneration was weakly negatively correlated (r=0.29; [−0.74 to 0.34] P=0.182) (Fig.-1C). Mice showing greater cartilage regeneration exhibited increased ectopic-calcification as is evident by their positive significant linear correlation coefficient (r=0.59; [0.03 to 0.87]; P=0.021) (Fig.-1D).
Figure 1: Genetic correlation between articular cartilage regeneration and various characteristics of post-traumatic OA.
Correlation between knee cartilage regeneration and (A) maximum OARSI score, (B) summed OARSI score, (C) synovitis, and (D) ectopic calcification. Note: for maximum and summed OARSI scores as well as synovitis score and ectopic calcification, sham knees were subtracted from DMM knees. Results are shown as Pearson correlations for best-fitted curve (solid line) with 95% confidence interval (dotted lines) from the curve. Each dot represents genotypic mean value for a given phenotype.
Genetic correlation of various phenotypes with ear-wound healing
Data obtained from ear-wound healing and knee cartilage regeneration revealed a significant positive correlation between the two phenotypes (r=0.71; [0.22 to 0.91]; P=0.005) (Fig.-2A). However, despite strong significant correlation between these (anabolic) phenotypes, there was only a weak correlation between ear-wound healing and various features of OA. For instance, both maximum OARSI score (r=−0.25; [−0.72 to 0.37]; P=0.212) (Fig.-2B) and summed OARSI score (r=−0.19; [−0.69 to 0.42]; P=0.269) (Fig.-2C) were only weakly correlated with ear-wound healing and nature of correlation was negative meaning that better ear-wound healing leads to less susceptibility to cartilage degeneration. In contrast, synovitis was weakly correlated with ear-wound healing (r=0.24; [−0.39 to 0.72] P=0.224) in a direction opposite to our prediction (Fig.-2D). Interestingly, ectopic bone-formation was significantly positively correlated with ear- wound healing (r=0.70; [0.21 to 0.91]; P=0.003) (Fig.-2E).
Figure 2: Genetic correlation of various phenotypes with ear-wound healing.
Correlation between ear-wound healing and (A) knee cartilage repair, (B) maximum OARSI score, (C) summed OARSI score, (D) synovitis and (E) ectopic calcification. Note: for maximum and summed OARSI scores as well as synovitis score and ectopic calcification, sham knees were subtracted from DMM knees Results are shown as Pearson correlations for best-fitted curve (solid line) with 95% confidence interval (dotted lines) from the curve. Each dot represents genotypic mean value for a given phenotype.
Genetic correlations within post-traumatic OA phenotypes
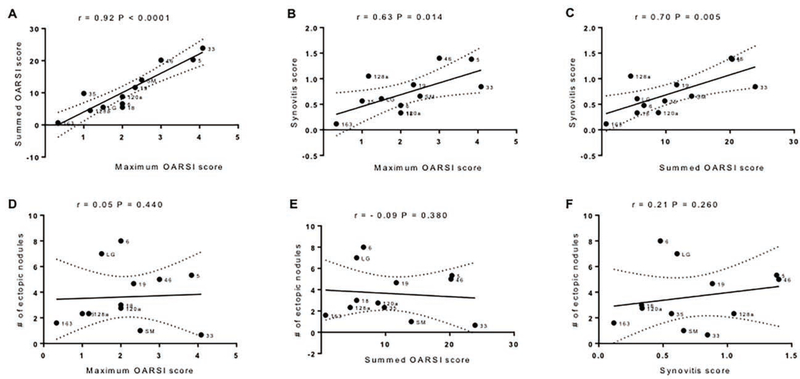
To examine the interrelationship between various phenotypes characteristic of post-traumatic OA, correlation coefficients were computed for maximum and summed OARSI scores, synovitis as well as ectopic-calcification. We observed that maximum and summed OARSI scores were highly significantly associated with each other (r=0.92; [0.73 to 0.97]; P<0.0001) (Fig.-3A) so that they should behave similarly in correlations with other variables. While synovitis was not significantly correlated with ear and knee cartilage healing, it has significant positive correlations with maximum (r=0.63; [0.09 to 0.88]; P=0.014) (Fig.-3C) and summed OARSI scores (r=0.70; [0.22 to 0.91]; P=0.005) (Fig.-3C). Neither maximum (r=0.05; [−0.54 to 0.61]; P=0.440) (Fig.-3D), nor summed OARSI scores (r=−0.10; [−0.64 to 0.50]; P=0.380) (Fig.-3E) showed any correlation with the ectopic-calcification. Finally, synovitis and ectopic-calcification were not significantly correlated (r=0.21; [−0.42 to 0.70]; P=0.260) (Fig.-3F) and the sign goes against expectation.
Figure 3: Genetic correlations within post-traumatic OA phenotypes.
A. Correlation between maximum and summed OARSI scores. B. Correlation between synovitis and maximum OARSI score. C. Correlation between synovitis and summed OARSI score. D. Correlation between maximum OARSI score and ectopic calcification. E. Correlation between summed OARSI score and ectopic calcification. F. Correlation between synovitis and ectopic calcification Note: for maximum and summed OARSI scores, as well as synovitis score and ectopic calcification, sham knees were subtracted from DMM knees. Results are shown as Pearson correlations for best-fitted curve (solid line) with 95% confidence interval (dotted lines) from the curve. Each dot represents genotypic mean value for a given phenotype.
Genetic correlations with bone-parameters
Our previous data have shown that various bone-parameters are inter-correlated[20]. To get some insights as to how these bone-parameters associated with DMM-surgery (i.e. posttraumatic OA) are correlated with ear-wound healing and cartilage regeneration, correlation coefficients were computed for a number of bone-parameters (Table-3). None of the individual bone-parameters summarized by OARSI were significantly correlated with ear-wound healing or articular cartilage regeneration.
Table 3:
Correlations coefficients for various bone parameters
| Phenotypes | BV/TV* | CDI* | SMI* | Tb.N* | Tb.Th* | Tb.Sp* | SCB* | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | r | P | r | P | |
| Ear wound healing | −0.26 [−0.72 to 0.37] | 0.212 | 0.06 [−0.53 to 0.61] | 0.422 | 0.15 [−0.46 to 0.66] | 0.322 | −0.19 [−0.69 to 0.43] | 0.279 | −0.07 [−0.62 to 0.52] | 0.410 | 0.16 [−0.45 to 0.67] | 0.307 | −0.48 [−0.83 to 0.13] | 0.057 |
| Articular cartilage | −0.14 [−0.66 to 0.47] | 0.331 | 0.18 [−0.44 to 0.68] | 0.287 | 0.14 [−0.47 to 0.66] | 0.330 | −0.17 [−0.68 to 0.44] | 0.294 | 0.20 [−0.42 to 0.69] | 0.266 | 0.09 [−0.51 to 0.63] | 0.390 | −0.30 [−0.75 to 0.33] | 0.169 |
= values are difference between DMM minus sham; BV/TV = bone volume over total volume; CDI = connectivity density index; SMI = structural model index; Tb.N = trabecular number; Tb.Th = trabecular thickness; Tb.Sp = trabecular thickness; SCB = subchondral bone plate thickness; Pearson correlation coefficients are shown with 95% confidence interval.
DISCUSSION
Studies in the field of cartilage regeneration/repair are primarily concerned with the anabolic stimulation of cartilage chondrocytes in-vitro or in-vivo or construction of tissue engineered cartilage from chondrocytes or stem cells. Our approach has been to study native cartilage repair and to explore the potential of population genetics to uncover genetic differences within the population. We initially demonstrated that cartilage repair is heritable and correlated with the ability to heal ear-wounds[1]. Using new data on cartilage repair in additional strains and recently published data on post-traumatic OA in the same strains, the current study shows for the first time, the measurement of genetic correlations of the regeneration phenotype (anabolic) with degeneration phenotypes (catabolic) characteristic of post-traumatic OA. Utilizing recombinant-inbred mouse strains developed from “healer” and “non-healer” parental strains (defined by healing of ear-wounds), we demonstrate a linear relationship between the ability to heal an articular cartilage defect and resistance to cartilage degeneration after DMM surgery. Thus, for the first time, studies can be undertaken on the genes and gene variants that protect from cartilage degeneration as well as the genes the confer susceptibility. We discuss how these results can be translated to humans.
Unrelated vs Related Mouse Strains
A number of studies using unrelated inbred strains indicated that there would be genetic differences in the ability to heal cartilage and the susceptibility to cartilage degeneration. Glasson and colleagues recognized that different strains of mice reacted differently to DMM and thus developed different degrees of cartilage degeneration[22]. On the repair side, studies were largely informed by the healing ability of the MRL mouse. Fitzgerald et al.[3] were first to demonstrate differences in cartilage healing between the MRL/MpJ mouse and the C57BL/6 mouse. The MRL strain was generated from a cross involving several strains but 75% of its genes come from the LG/J strain used here. Dell’Accio and colleagues also demonstrated differences between the C57BL/6 and two strains of DBA mice[2] in their ability to heal articular cartilage and their susceptibility to cartilage degeneration. Finally, Olson and colleagues[36] demonstrated differences between the MRL mouse and the C57BL/6 in their response to articular fracture and subsequent development of cartilage degeneration. For heritability studies, the use of recombinant-inbred strains is preferred because any quantitative genetic variation detected is due to different combinations of the two parental genomes without the confounding influence of different genetic backgrounds that random inbred strains would have[16].
Correlation of Phenotypes
All correlations within the anabolic and catabolic trait sets are strong, statistically significant and in the predicted directions. None of the correlations between anabolic and catabolic traits are statistically significant, except that between cartilage regeneration and cartilage degeneration (r=−0.59) which is in the predicted direction. None of the 5 other correlations across anabolic and catabolic trait sets are significant, with three in the predicted direction and two not. This leads to the generalization that we have strong, positive genetic correlations within trait sets and no correlation between the trait sets, with the exception noted above.
There was a significant positive correlation between summed and maximum OARSI scores. As mentioned before, summed OARSI score represents changes in all four quadrants of the knee joint while maximum OARSI score represents the highest score within all sectioned levels of the knee. This finding supports the initial notion for OARSI scoring system that was designed to express OA severity either combining maximum and summed OARSI scores or use them in isolation[23].
We originally reported the correlation between cartilage regeneration and ear-wound healing in six inbred strains and four LGXSM recombinant-inbred strains[1]. We demonstrated that articular cartilage regeneration in mice is heritable and there is a strong genetic correlation between ear-wound healing and cartilage regeneration indicating that they plausibly share a common genetic basis. In the current study, we extended this correlation to include a total of 12 recombinant-inbred lines including the parental strains and calculate a correlation of r=0.71 (P=0.005). We also observed a strong inverse correlation between cartilage regeneration and susceptibility to cartilage degeneration suggesting that cartilage regeneration is positively correlated with resistance to cartilage degeneration. Synovitis was not significantly correlated with knee articular cartilage healing but was significantly positively correlated with maximum and summed OARSI scores. In the current study, we observed weak correlations between OARSI scores and ectopic-calcification and between synovitis and ectopic-calcification. This is in contrast to a prior more powered study in which we showed that ectopic-calcification had a moderate to severe inverse correlation with these catabolic traits: OARSI score, synovitis and subchondral-bone-plate-thickness[20]. However, it was strongly, positively associated with ear- wound healing and cartilage regeneration. Interestingly, despite weak correlation between severity of cartilage degeneration and ectopic-calcification, we have previously shown that mice exhibiting cartilage repair potential and protection from cartilage degeneration developed more calcifications in the joints than non-healer mice and mice susceptible to cartilage degeneration[20, 29]. In addition, in the current study, we have observed a positive correlation between cartilage regeneration and ectopic-calcification and an inverse correlation between cartilage regeneration and susceptibility to cartilage degeneration. The three anabolic phenotypes are correlated: ear-wound healing, cartilage healing and ectopic bone-formation.
Translation to Humans
These results are predicted to translate to humans because the known correlations between phenotypes in humans match those found in our mouse model. First, the correlation of synovitis and cartilage degeneration reflect the estimates of synovitis in human OA. On arthroscopy, synovitis is seen in approximately 50% of the knees of patients with painful OA and in an even higher percentage using MRI[37, 38]. Secondly, there is a lack of correlation between ectopic bone-formation and cartilage degeneration in mice, which also is true for humans[39]. In addition, the relationship between synovitis and chondrocalcinosis in humans has recently been examined showing no differences in synovitis scores at baseline and at 4-years between the chondrocalcinosis and the control groups[40], suggesting their independence. Therefore, the extension to the correlation between cartilage healing and resistance to cartilage degeneration can be predicted to be true for humans as well as mice. Very little is known in humans regarding the differential ability to heal cartilage, however, Chu and colleagues[41] suggested that the variation in the ability of human stem cells to undergo chondrogenesis reflects variability in the ability to heal cartilage. There have been no attempts to study cartilage regeneration in humans, only the degenerative phenotype, primarily due to the inability to identify people that are not susceptible to cartilage degeneration. We suggest that the ability to heal cartilage may reflect a “healer” phenotype that confers resistance to degeneration. Interestingly, in a set of experiments undertaken in collaboration with Dr. Robert Brophy (personal communication), we have been able to identify a set of genes that are expressed in people who do not progress to OA while those who progress actually have little expression of these genes, thus potentially identifying a “healing” phenotype for these individuals. Another set of potential “healing” biomarkers was demonstrated in a clinical study by Garnero et al.,[42] where they predict that the loss of cartilage arises from an imbalance between cartilage synthesis and cartilage degradation. In the Garnero study, high levels of the anabolic chondrocyte marker, PIIANP, coupled with low levels of the degradation marker, CTX-II, predicted which patients would not progress in OA, while low PIIANP and high CTX-II predicted the progressors. Lastly, the concept of “responders” and “non-responders” has been discussed in terms of the response to cell-based therapies such as stem cells[43]. Taken together, these studies point to the possibility of a “healer/regenerative/anabolic” or “responder” phenotype in humans similar to that in mice.
Since we have shown that articular cartilage regeneration is positively correlated with ear-wound healing, screening for ear-wound healing could be sentinel for articular cartilage healing indicating that the gene or pathway is also likely to be involved in susceptibility to cartilage degeneration. This finding suggests that if we wish to examine articular cartilage regeneration in mice with single gene defects, we can use ear-wound healing to first screen mice as this does not require sacrificing of mice or extensive histological analyses. Further knee cartilage analyses could be performed once a mouse strain with a potential for ear-wound healing is identified, as it is a good candidate to heal articular cartilage and be less susceptible to cartilage degeneration. Some laboratories have shown that inactivation of the p21[44] and TGFP receptor I genes[45] improved ear-wound healing. We have shown that similar sets of genes and pathways such as DNA repair and WNT signaling are correlated with ear-wound healing and knee cartilage regeneration[46]. Therefore, the ear-punch results could help identify specific genes’ involvement in OA.
A limitation of the study was that we did not include female mice in our analysis. As some sexual dimorphism has been reported for these strains[18], and as is customary for OA model due to the more consistent response in males[22], we limited our analyses at this time only to male mice. Further studies should consider sexual dimorphism and determine if the same correlations hold in females. Another potential limitation could be the fact that for DMM experiment, we subtracted contralateral sham score from DMM score and one can assume that this approach may produce false-positive or false-negative results as the sham operated knees are shown to undergo bone remodeling and that this can potentially affect ipsilateral DMM-operated knees. However, this effect, if present in our experiment, would be consistent across groups and would not result in a systematic bias. Lastly, sample size for OA phenotype was low for some strains. With this sample size, we can detect effects of 0.979 within-group standard deviations at a power of 0.80 with a=0.05 and most of our tests were significant implying that this sample size was sufficient. Furthermore, all mice in a recombinant-inbred line are genetically identical (i.e. isogenic) and homozygous at all genetic loci due to many generations of inbreeding and therefore provide a very robust and reproducible phenotype.
In the current study, we chose data from only one time-point for each phenotype. We have, however, reported time-course studies for each phenotype in two informative mouse strains, LGXSM-6 (healer) and LGXSM-33 (non-healer). We studied ear-wound healing on 2-week and 4-week time-points finding that LGXSM-6 strain showed a 45% reduction in residual ear hole diameter by 2-weeks post-surgery and a 60% reduction by 4-week time-point pointing to an increased ear-wound healing[19]. Moreover, our unpublished data (personal communication) on ear-wound healing for additional time-points (0, 5, 9, 14, 28 and 56 days) revealed that significant differences in ear-wound healing between LGXSM-6 and LGXSM-33 existed on or after day 9 and the magnitude of differences also increased over time. Time-course experiment of knee cartilage regeneration in LGXSM-6 and LGXSM-33 yielded that at early time-points (4, 8, and 12 weeks) there were no significant differences between the two mouse strains in terms of cartilage repair. In contrast, 16-week data showed that LGXSM-6 exhibited significantly higher cartilage repair potential than LGXSM-33[1]. For OA phenotype, we studied cartilage and bone changes at 2, 4 and 8 week time-points[19]. Histological assessment of cartilage showed increased degeneration in LGXSM-33 mice than LGXSM-6 mice at all time-points. Specifically, LGXSM-33 displayed proteoglycan loss with some cartilage fibrillation at 2-weeks, and significant loss of hyaline cartilage accompanied by extended lesion to calcified cartilage at 4- and 8-weeks. No such changes were evident in LGXSM-6 mice except for some focal loss of proteoglycan staining. In addition, we observed time-dependent changes in subchondral-bone between the two mouse strains. While LGXSM-33 strain exhibited subchondral-bone-plate thinning at early time-points (2- and 4-weeks) and thickening between 4 and 8-weeks, LGXSM-6 strain showed thinning at all time-points. Taken together, these data suggest natural course of tissue regeneration and degeneration over time.
As a consequence of these findings, it is apparent that the LG/J by SM/J intercross segregates for multiple genetic factors affecting articular cartilage regeneration and degeneration and that the genetic basis for this difference can be genetically mapped in the intercross population. In addition to the recombinant-inbred lines used here, there is also an advanced intercross line[47] in which fine-mapping studies can be performed. The previously mapped quantitative trait loci (QTLs) effecting ear-wound healing are also likely to affect cartilage regeneration in the knee, so that fine-mapped ear-hole healing loci are strong candidates for loci also affecting articular cartilage regeneration. In a recent study, we have been able to measure the genetic susceptibility to ectopic-calcification in the LG/J and SM/J advanced intercross mice identifying 20 QTLs associated with this phenotype[29].
Conclusions from these studies that have implications for future regeneration-gene hunting efforts are that the articular cartilage regeneration trait is heritable, and that ear tissue regeneration, articular cartilage healing and susceptibility to cartilage degeneration following trauma are affected by a common set of genes. These finds suggest that the responses to these pathologies are likely to have the same underlying genetic basis and are not tissue or location specific. With these new lines, we can now support our hypothesis that OA is indeed inversely proportionate to cartilage repair due to genetic variants: that is, born out in a statistical number of recombinant-inbred lines. This is particularly important because cartilage repair or regeneration cannot be studied in humans, and the mouse data provides the first data to demonstrate a relationship between degeneration and regeneration of cartilage, and that relationship has a significant genetic contribution. These mice are a valuable experimental resource for the next phase of research into mammalian epimorphic regeneration: gene identification.
Acknowledgements
We acknowledge with thanks the important technical support by Crystal Idleburg and John Freeman. We also thank Drs. Shingo Hashimoto, Ken Takebe, and Nobuaki Chinzei for their help with mouse surgeries and blinded evaluation of histology and micro-CT data.
Role of funding source
This study was supported by R01 AR063757 (Sandell, Cheverud) and by P30 AR074992 (Musculoskeletal Research Center) from the National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH). Dr. Rai was supported through NIH Pathway to Independence Award (R00 AR064837) from NIAMS. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIAMS or the NIH.
Footnotes
Conflict of interest
The authors have no competing interest to declare. All authors declare that no financial conflict of interest exists with regard to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rai MF, Hashimoto S, Johnson EE, Janiszak KL, Fitzgerald J, Heber-Katz E, et al. Heritability of articular cartilage regeneration and its association with ear wound healing in mice. Arthritis Rheum 2012; 64: 2300–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eltawil NM, De Bari C, Achan P, Pitzalis C, Dell’accio F. A novel in vivo murine model of cartilage regeneration. Age and strain-dependent outcome after joint surface injury. Osteoarthritis Cartilage 2009; 17: 695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzgerald J, Rich C, Burkhardt D, Allen J, Herzka AS, Little CB. Evidence for articular cartilage regeneration in MRL/MpJ mice. Osteoarthritis Cartilage 2008; 16: 1319–1326. [DOI] [PubMed] [Google Scholar]
- 4.Cheverud JM, Lawson HA, Bouckaert K, Kossenkov AV, Showe LC, Cort L, et al. Fine- mapping quantitative trait loci affecting murine external ear tissue regeneration in the LG/J by SM/J advanced intercross line. Heredity (Edinb) 2014; 112: 508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poole AR. Osteoarthritis as a whole joint disease. HSS J 2012; 8: 4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 2012; 64: 1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poulet B Models to define the stages of articular cartilage degradation in osteoarthritis development. Int J Exp Pathol 2017; 98: 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandell LJ. Etiology of osteoarthritis: genetics and synovial joint development. Nat Rev Rheumatol 2012; 8: 77–89. [DOI] [PubMed] [Google Scholar]
- 9.Spector TD, Cicuttini F, Baker J, Loughlin J, Hart D. Genetic influences on osteoarthritis in women: a twin study. BMJ 1996; 312: 940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rai MF, Brophy RH, Sandell LJ. Osteoarthritis following meniscus and ligament injury: insights from translational studies and animal models. Curr Opin Rheumatol 2019; 31: 70–79. [DOI] [PubMed] [Google Scholar]
- 11.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma 2006; 20: 739–744. [DOI] [PubMed] [Google Scholar]
- 12.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage 1995; 3: 261–267. [DOI] [PubMed] [Google Scholar]
- 13.Heydemann A The super super-healing MRL mouse strain. Front Biol (Beijing) 2012; 7: 522–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leferovich JM, Bedelbaeva K, Samulewicz S, Zhang XM, Zwas D, Lankford EB, et al. Heart regeneration in adult MRL mice. Proc Natl Acad Sci U S A 2001; 98: 9830–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rai MF, Sandell LJ. Regeneration of articular cartilage in healer and non-healer mice. Matrix Biol 2014; 39: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hrbek T, de Brito RA, Wang B, Pletscher LS, Cheverud JM. Genetic characterization of a new set of recombinant inbred lines (LGXSM) formed from the inter-cross of SM/J and LG/J inbred mouse strains. Mamm Genome 2006; 17: 417–429. [DOI] [PubMed] [Google Scholar]
- 17.Broman KW. The genomes of recombinant inbred lines. Genetics 2005; 169: 1133–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blankenhorn EP, Bryan G, Kossenkov AV, Clark LD, Zhang XM, Chang C, et al. Genetic loci that regulate healing and regeneration in LG/J and SM/J mice. Mamm Genome 2009; 20: 720–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto S, Rai MF, Janiszak KL, Cheverud JM, Sandell LJ. Cartilage and bone changes during development of post-traumatic osteoarthritis in selected LGXSM recombinant inbred mice. Osteoarthritis Cartilage 2012; 20: 562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chinzei N, Rai MF, Hashimoto S, Schmidt EJ, Takebe K, Cheverud JM, et al. Evidence for Genetic Contribution to Variation in Posttraumatic Osteoarthritis in Mice. Arthritis Rheumatol 2019; 71: 370–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McBrearty BA, Clark LD, Zhang XM, Blankenhorn EP, Heber-Katz E. Genetic analysis of a mammalian wound-healing trait. Proc Natl Acad Sci U S A 1998; 95: 11792–11797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage 2007; 15: 1061–1069. [DOI] [PubMed] [Google Scholar]
- 23.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage 2010; 18 Suppl 3: S17–23. [DOI] [PubMed] [Google Scholar]
- 24.Lewis JS, Hembree WC, Furman BD, Tippets L, Cattel D, Huebner JL, et al. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthritis Cartilage 2011; 19: 864–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takebe K, Rai MF, Schmidt EJ, Sandell LJ. The chemokine receptor CCR5 plays a role in post-traumatic cartilage loss in mice, but does not affect synovium and bone. Osteoarthritis Cartilage 2015; 23: 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duan X, Rai MF, Holguin N, Silva MJ, Patra D, Liao W, et al. Early changes in the knee of healer and non-healer mice following non-invasive mechanical injury. J Orthop Res 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 2010; 25: 1468–1486. [DOI] [PubMed] [Google Scholar]
- 28.Odgaard A, Gundersen HJ. Quantification of connectivity in cancellous bone, with special emphasis on 3-D reconstructions. Bone 1993; 14: 173–182. [DOI] [PubMed] [Google Scholar]
- 29.Rai MF, Schmidt EJ, Hashimoto S, Cheverud JM, Sandell LJ. Genetic loci that regulate ectopic calcification in response to knee trauma in LG/J by SM/J advanced intercross mice. J Orthop Res 2015; 33: 1412–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rai MF, Duan X, Quirk JD, Holguin N, Schmidt EJ, Chinzei N, et al. Post-Traumatic Osteoarthritis in Mice Following Mechanical Injury to the Synovial Joint. Sci Rep 2017; 7: 45223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sokal RR, Rohlf FJ. Biometry. W. H. Freeman and Company, San Francisco, CA: 1981. [Google Scholar]
- 32.Arnold SJ. Behavioral variation in natural populations. I. Phenotypic, genetic and environmental correlations between chemoreceptive responses to prey in the garter snake, Thamnophis elegans. Evolution 1980; 35: 489–509. [DOI] [PubMed] [Google Scholar]
- 33.Cheverud JM. A comparison of genetic and phenotypic correlations. Evolution 1988; 42: 958–968. [DOI] [PubMed] [Google Scholar]
- 34.Davies OG, Liu Y, Player DJ, Martin NRW, Grover LM, Lewis MP. Defining the Balance between Regeneration and Pathological Ossification in Skeletal Muscle Following Traumatic Injury. Front Physiol 2017; 8: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res 2001; 3: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward BD, Furman BD, Huebner JL, Kraus VB, Guilak F, Olson SA. Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse. Arthritis Rheum 2008; 58: 744–753. [DOI] [PubMed] [Google Scholar]
- 37.Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis -- results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage 2005; 13: 361–367. [DOI] [PubMed] [Google Scholar]
- 38.Roemer FW, Guermazi A, Hunter DJ, Niu J, Zhang Y, Englund M, et al. The association of meniscal damage with joint effusion in persons without radiographic osteoarthritis: the Framingham and MOST osteoarthritis studies. Osteoarthritis Cartilage 2009; 17: 748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abhishek A, Doherty S, Maciewicz RA, Muir K, Zhang W, Doherty M. Does Chondrocalcinosis Associate With a Distinct Radiographic Phenotype of Osteoarthritis in Knees and Hips? A Case-Control Study. Arthritis Care Res (Hoboken) 2016; 68: 211–216. [DOI] [PubMed] [Google Scholar]
- 40.Han BK, Kim W, Niu J, Basnyat S, Barshay V, Gaughan JP, et al. Association of Chondrocalcinosis in Knee Joints With Pain and Synovitis: Data From the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2017; 69: 1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Payne KA, Didiano DM, Chu CR. Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthritis Cartilage 2010; 18: 705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garnero P, Ayral X, Rousseau J, Christgau S, Sandell L, Dougados M, et al. Uncoupling of type II collagen synthesis and degradation predicts progression of joint damage in patients with knee osteoarthritis. Arthritis Rheum 2002; 46: 2613–2624. [DOI] [PubMed] [Google Scholar]
- 43.Caplan AI. Cell-Based Therapies: The Nonresponder. Stem Cells Transl Med 2018; 7: 762–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bedelbaeva K, Snyder A, Gourevitch D, Clark L, Zhang XM, Leferovich J, et al. Lack of p21 expression links cell cycle control and appendage regeneration in mice. Proc Natl Acad Sci U S A 2010; 107: 5845–5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu J, Johnson K, Li J, Piamonte V, Steffy BM, Hsieh MH, et al. Regenerative phenotype in mice with a point mutation in transforming growth factor beta type I receptor (TGFBR1). Proc Natl Acad Sci U S A 2011; 108: 14560–14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rai MF, Schmidt EJ, McAlinden A, Cheverud JM, Sandell LJ. Molecular insight into the association between cartilage regeneration and ear wound healing in genetic mouse models: targeting new genes in regeneration. G3 (Bethesda) 2013; 3: 1881–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norgard EA, Jarvis JP, Roseman CC, Maxwell TJ, Kenney-Hunt JP, Samocha KE, et al. Replication of long-bone length QTL in the F9-F10 LG,SM advanced intercross. Mamm Genome 2009; 20: 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]