Abstract
Dysfunction in neurophysiological systems that regulate food intake and metabolism are at least partly responsible for obesity and related comorbidities. An important component of this process is the hypothalamic melanocortin system, where an imbalance can result in severe obesity and deficits in glucose metabolism. Exercise offers many health benefits related to cardiovascular improvements, hunger control, and blood glucose homeostasis. However, the molecular mechanism underlying the exercise-induced improvements to the melanocortin system remain undefined. Here, we review the role of the melanocortin system to sense hormonal, nutrient, and neuronal signals of energy status. This information is then relayed onto secondary neurons in order to regulate physiological parameters, which promote proper energy and glucose balance. We also provide an overview on the effects of physical exercise to induce biophysical changes in the melanocortin circuit which may regulate food intake, glucose metabolism and improve overall metabolic health.
1. Introduction
The concept that the brain influences energy balance arose more than century ago. Joseph P. Babinski and Alfred Fröhlich reported development of obesity in a patient with a pituitary tumor [10, 76]. In 1904 pathologist Jakob Erdheim suggested that a similar type of obesity was observed independent of damage to the pituitary gland opposing the idea of pituitary involvement in the development of obesity in these patients [68]. His-observation was later supported by several groups that demonstrated ablation of the hypothalamic region (tuber cinereum/infundibulum of the hypothalamus) independent of injury to the pituitary induced adiposity and weight gain [[1]. Hetherington, 1940; [114, 167]]. This led to the concept that the hypothalamus was an important region of the brain necessary for proper control of energy balance however, the hypothalamic regions involved in regulating food intake and body weight, remained unclear at that time.
Several approaches were implemented to determine the regions of hypothalamus that affect food intake and body weight. Jean Camus and Gustave Roussy performed a series of surgical procedures on the hypothalamus leaving the pituitary intact and successfully reproduced the increased adiposity and obesity first observed by Babinski and Fröhlich [[38] and Roussy, 1913a, [39–41]]. Hetherington and Ranson observed adiposity and obesity after inducing hypothalamic injury limited to the ventromedial hypothalamic nucleus at the level of the median eminence [105–107]. Physiologists John R Brobeck and Bal K. Anand further showed that small localized bilateral electrolytic lesions in the extreme lateral part of the hypothalamus results in complete inhibition of food intake even in hyperphagic and obese animals [28, 29]. However, large lesions of the ventromedial nucleus with unilateral lesions in lateral hypothalamus failed to alter food intake [28, 29]. This pioneering work in the early twentieth century introduced the hypothalamus and associated nuclei as an important endocrine structure, which contributes to feeding behavior and body weight.
2. The arcuate nucleus: POMC and NPY/AGRP neurons
Over the past 20–30 years, considerable progress has been made in the characterization of brain circuits in murine models leading to the identification of signaling pathways that regulate metabolism. This has been accelerated with the use of mouse genetics and the identification of cell populations and receptors systems, including the melanocortin and leptin systems [89, 90, 146, 147, 162, 212]. Due to the shared physiology of these systems across species, both leptin and melanocortins have aided in the description of neural pathways involved in the regulation of energy balance and glucose homeostasis [70–72]. The hypothalamic arcuate nucleus (ARH) is a crucial brain region that receives input from several peripheral hormones that reflect energy stores and nutrient availability (e.g. leptin, insulin, ghrelin, etc.) and relays this information to both intra- and extra-hypothalamic brain regions (Fig. 1) [8, 195]. Two neuronal cell groups which comprise the melanocortin system, the anorexigenic pro-opiomelanocortin (POMC) neurons and orexigenic neuropeptide Y (NPY)/agouti-related peptide (AgRP) neurons of hypothalamic ARH, are regarded as critical nodes in this process [162]. These two neuronal populations form neural circuits with various hypothalamic target sites such as the lateral (LHA), and paraventricular (PVH) nucleus as well as with extrahypothalamic nuclei including the bed nucleus of the stria terminalis (BNST), amygdala (AMY), lateral parabrachial nucleus (LPB), periaqueductal grey (PAG), the nucleus of the solitary tract (NTS) and the intermediolateral cell column of the spinal cord (IML) (Fig. 1) [8, 15, 19, 30, 65, 66, 93, 123, 124, 128, 170, 173, 182, 192].These neuronal circuit connections are crucial for the proper regulation of feeding behavior, energy homeostasis, and glucose metabolism [9, 19, 67, 84, 115, 129, 178, 187, 195].
Fig. 1. Melanocortin reciprocal projections.
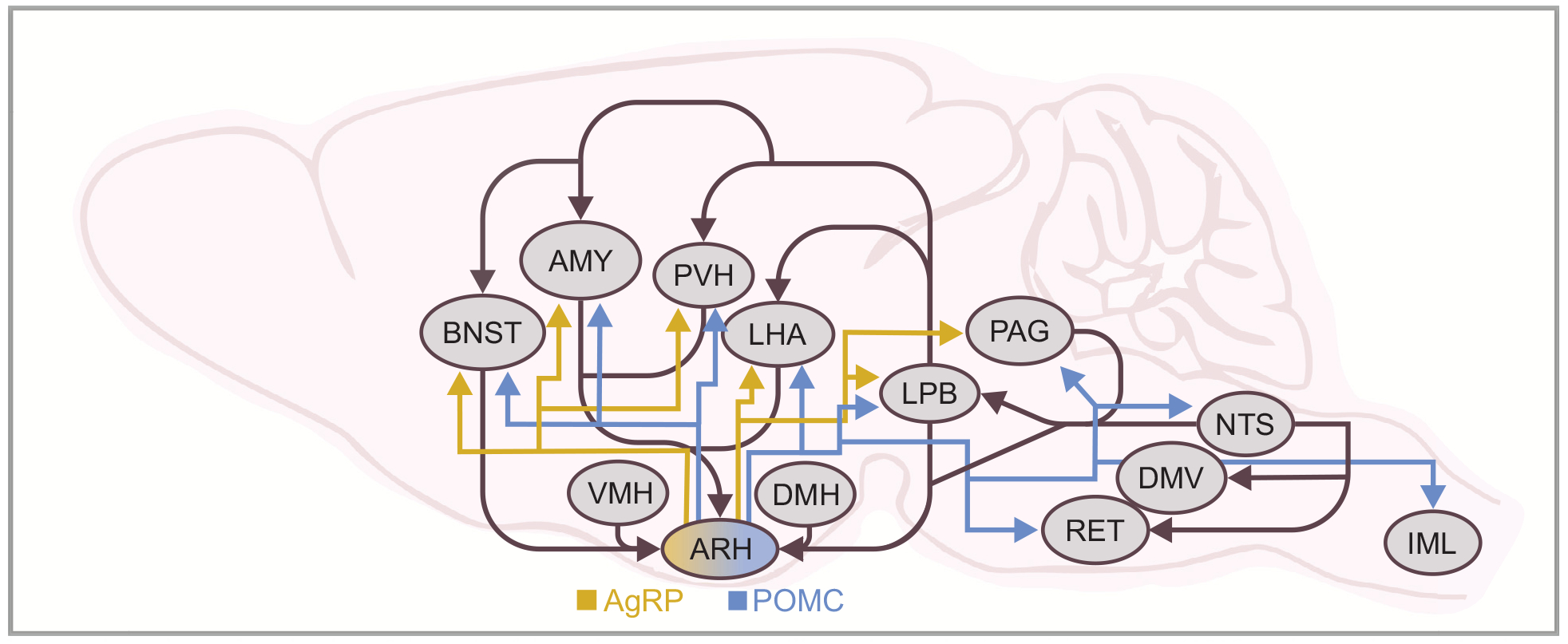
Schematic of the melanocortin system illustrating projections of the arcuate (ARH) nucleus and respective downstream targets. The blue arrows signify POMC projections and the yellow arrows signify NPY/AgRP projections. AMY, central amygdala; ARH, arcuate nucleus; BNST, bed nucleus of the stria terminalis; DMV, dorsal motor nucleus of the vagus; LHA, lateral hypothalamic area; LPB, lateral parabrachial nucleus; NTS, nucleus tractus solitarius; PAG, periaqueductal grey; PVH, paraventricular nucleus of the hypothalamus; RET, reticular formation; VMH, ventromedial hypothalamus; DMH, dorsomedial hypothalamus; IML, intermediolateral cell column of the spinal cord.
It should be noted that POMC neurons are also expressed in the hindbrain nucleus tractus solitarius (NTS) [50, 213]. NTS POMC neurons project to and receive inputs from brain regions which both overlap and are distinct from connections of ARH POMC neurons [50, 192]. This likely contributes to observations that ARH and NTS POMC neurons have distinct roles in the regulation of metabolism and the source of POMC neurons must be considered with various genetic manipulations when describing metabolic phenotypes [32, 55, 209, 211, 213].
2.1. Heterogeneity of arcuate POMC and NPY/AGRP neurons
NPY/AgRP and POMC neuronal projections are distributed topographically in the ARH according to their axon projection targets (Fig. 1) [19, 65, 66]. In particular, AgRP neurons projecting to anterior brain regions are enriched in the anterior portion of the ARH while those projecting to the hindbrain are most prevalent towards the posterior ARH [19]. Neurons of the retrochiasmatic area (RCA) and ARH, which express POMC, project to the paraventricular hypothalamus (PVH) and lateral hypothalamic area (LHA) [65, 66]. Additionally, POMC neurons of the RCA and lateral arcuate project caudally to autonomic areas [e.g. intermediolateral cell column (IML) and dorsal vagal complex (DVC)] [8, 65, 66]. Therefore, divergent axon projections originate from heterogeneous ARH NPY/AgRP and POMC neuronal populations.
Various metabolic signals modulate POMC and NPY/AgRP neuronal activity. In particular, leptin, serotonin (via serotonin 2c receptors – 5-HT2cR), adiponectin, and glucagon-like peptide 1 (GLP-1) serve to activate, while insulin inhibits POMC neurons via their cognate receptors [5, 48, 52, 82, 98, 99, 102, 103, 108, 109, 161, 163, 174, 180, 181]. Conversely, leptin, serotonin (via serotonin 1b receptors - 5-HT1bR), adiponectin, insulin, and GLP-1 inhibit NPY/AgRP cells [5, 13, 48, 98, 99, 103, 111, 127, 163, 190]. While leptin, serotonin, adiponectin, and insulin directly inhibit NPY/AgRP neurons, GLP-1 indirectly inhibits NPY/AgRP neurons via GABAergic synaptic connectivity [98, 163]. Similarly, ghrelin directly excites NPY/AgRP neurons while indirectly inhibiting POMC neurons [44, 53, 111, 126, 190]. Together, these findings contribute to the model by which POMC and NPY/AgRP neurons survey hormonal changes and adjust their activity in response to nutrient state. It’s important to note that recent imaging techniques have suggested that some of these metabolic signals may not directly alter the activities of these cells in vivo [4, 46, 138, 179]. The disparity between in vivo imaging and ex vivo observations warrants further investigation.
2.2. Functional heterogeneity and relationship to metabolism
While anatomical studies are necessary to map circuits, the functional assessment of neuronal circuits is not fully realized through analysis of neuroanatomy alone. Thus, there is a need to perform functional experiments to determine the structure-function relationships that influence behavior. Initial utilization of optogenetic and chemogenetic strategies demonstrated that the acute activity of arcuate NPY/AgRP and POMC neurons modifies feeding behavior (Fig. 2) [9, 129, 211]. While both populations of neurons regulate feeding, the AgRP population drives rapid feeding responses (within minutes to hours) while the arcuate POMC population alters chronic feeding behavior (within hours to days) [9, 129, 211]. Interestingly, the ARH POMC neuron induced reduction of food intake and body weight requires melanocortin receptor signaling [9] while the AgRP neuron induced hyperphagia is independent of melanocortins [9, 45, 130]. As AgRP is an inverse agonist of melanocortin receptors, these studies highlight the role of NPY and GABA, independent of melanocortin signaling, for the acute ability of AgRP neurons to regulate feeding [45, 58, 67, 199–202].
Fig. 2. Metabolic signal dependent regulation of POMC and NPY/AgRP neurons and effects on metabolism.
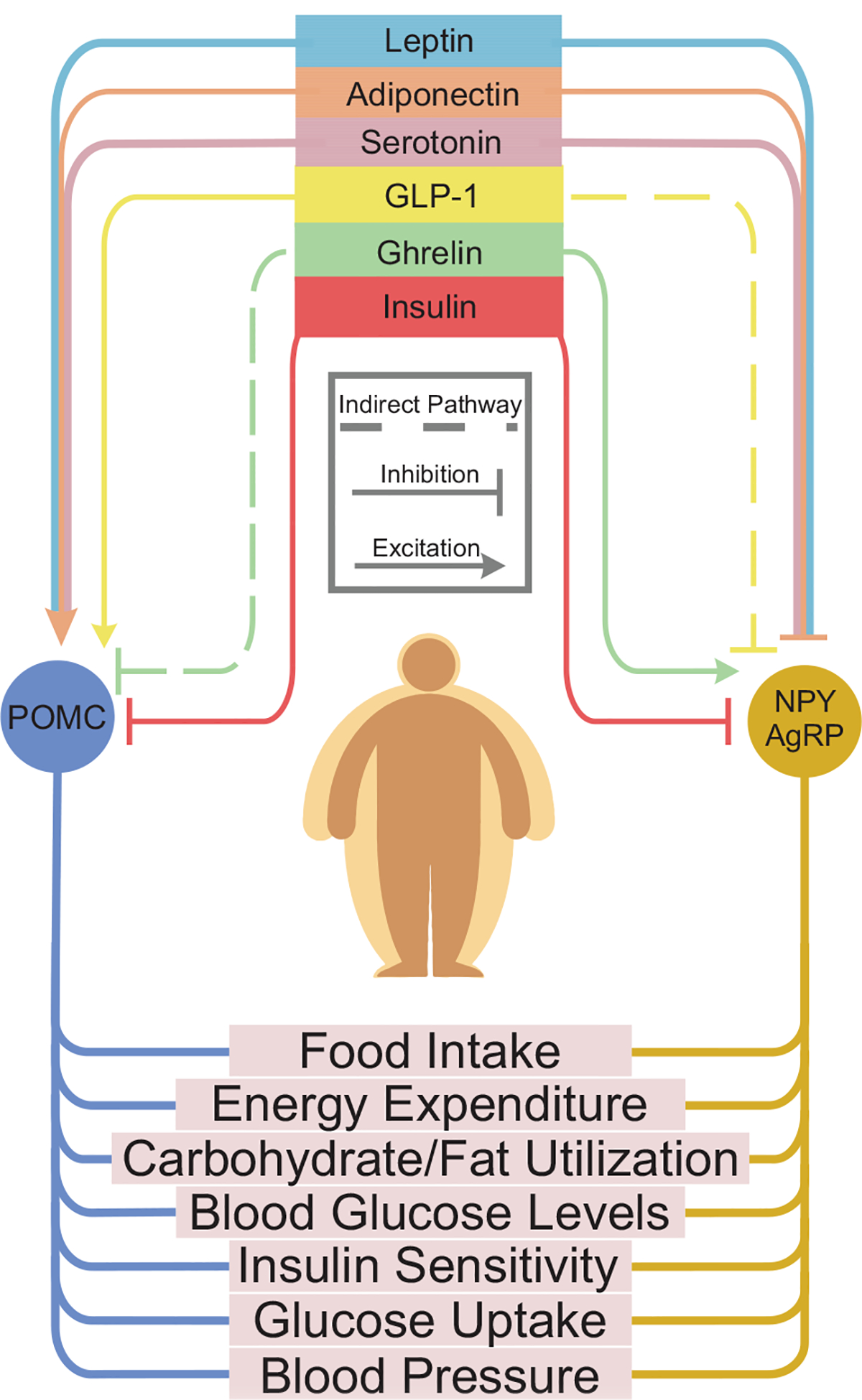
Flow chart of key peripheral factors (shown at the top) and their effect on POMC and/or NPY/AgRP neurons. In the top portion: Each color represents a different metabolic signal: Leptin, adiponectin, serotonin, GLP-1, Ghrelin, and Insulin. Each metabolic signal either excites or inhibits (represented by line with arrow or line with butt end, respectively), depending on the target cell. The bottom half of this figure illustrates several metabolic outcomes that can be modified by NPY/AgRP and/or POMC neuronal activity. In particular, the activity of NPY/AgRP and POMC neurons has been directly linked to feeding behavior. There is increasing evidence that the activity of these neurons also play a role in various metabolic outcomes, including: energy expenditure, carbohydrate/fat utilization, blood glucose levels, insulin sensitivity, glucose uptake and blood pressure. A more complete understanding of how NPY/AgRP and POMC neurons regulate these physiological parameters will require further investigation.
To better understand the functional organization of AgRP circuits, a systematic assessment of individual projection subpopulations that project to intra- and extra-hypothalamic sites was performed [19]. Optogenetic stimulation of AgRP neurons that project to the aBNST, PVH, and LHA were sufficient to evoke voracious food consumption [19]. Thus, AgRP neuron subpopulations form parallel and redundant circuits which project to distinct regions and are sufficient to independently evoke food intake. Importantly, several other AgRP projection subpopulations did not initiate food intake [19], suggesting these populations may play a role in regulating other behaviors that compete with food intake .
Notably, ablation of NPY/AgRP neurons that co-express GABA in the ARH of adult mice leads to starvation. These studies demonstrated that GABAergic signaling from AgRP neurons in the parabrachial nucleus (PBN) is essential for food intake behavior [136, 199]. These findings may seem surprising given that optogenetic activation of AgRP terminals within the PBN is not sufficient to evoke feeding behaviors [19]. However, this GABAergic projection is necessary for food intake, as it suppresses the visceral malaise associated with overconsumption [37]. Interestingly, NPY released from AgRP terminals into the PBN can also suppress inflammatory pain during hunger [7], suggesting that different neurotransmitters released from the same AgRP projection subpopulation serve diverse functions that enable food seeking. As such, NPY/AgRP neurons sit at the intersection of survival needs that influence behavior, which can include overcoming aversive stimuli in order to attain nutrients.
2.2.1. Substrate metabolism
Substrate utilization, the selection of either carbohydrate or fat during metabolic reactions [168], is an underappreciated component of energy balance regulation. Different energy substrates result in disparate amounts of free energy, and importantly, the inability to shift between substrates is associated with obesity [86]. Activation of AgRP neurons results in swift modifications of whole-body substrate utilization in which carbohydrate usage is increased simultaneously with a decrease in fat usage, independent of food intake (Fig. 2) [43]. Therefore, activity in AgRP neurons can spur a metabolic shift towards lipid storage and contribute to peripheral substrate utilization and lipogenesis.
2.2.2. Energy expenditure and glucose metabolism
Acute activity of arcuate POMC and NPY/AgRP neurons has been implicated in the regulation of energy expenditure and glucose metabolism [43, 129, 178, 187]. For example, activation of arcuate AgRP neurons decreases energy expenditure [129]. Moreover, acute activation of AgRP neurons but not POMC neurons impairs systemic insulin sensitivity as insulin stimulated glucose uptake was inhibited in brown adipose tissue (BAT), culminating in insulin resistance [178]. Neuronal activation of AgRP also coordinates sympathetic nerve activity to BAT and modifies gene expression in BAT toward a myogenic signature, increasing myostatin levels [178]. Ablation of arcuate POMC neurons reduces energy expenditure contributing to increased adiposity and metabolic dysfunction [211]. Moreover, inhibition of POMC neurons stimulates feeding while decreasing glucose levels in normoglycaemic mice [187]. Together, POMC and NPY/AgRP neurons modify energy balance and glucose homeostasis, however the influence of melanocortin signaling on metabolism might depend on both metabolic state and the activity level in melanocortin circuits.
3. Metabolic signals and cell-type specificity of hypothalamic neurons
There is a distribution of multiple receptors for metabolic signals in POMC and NPY/AgRP neurons, suggesting heterogeneity in these defined cell types. Our lab and others, have demonstrated that the activity of various metabolic signals either synergize or segregate into subpopulations of POMC and NPY/AgRP neurons(Fig. 3) [51, 52, 82, 99, 101, 108, 109, 111, 127, 160, 163, 173, 174, 180, 195, 197, 198]. For example, acute leptin receptor activity in ARH POMC neurons colocalizes with adiponectin and GLP-1 receptor (GLP-1r) activity [98, 180]. Additional work suggests that the activity of neurotensin synergizes with GLP-1r activity in POMC neurons [160]. Thus, the acute activity of neurotensin may also colocalize with leptin and adiponectin receptor activity (Fig. 3). While several metabolic signals overlap in ARH POMC neurons, both insulin and serotonin (via 5-HT2cR) are largely segregated into distinct POMC populations [173, 174, 195, 197]. Initial findings suggest that NPY/AgRP neurons are more homogenous in acute responses to leptin, insulin, adiponectin, and ghrelin (Fig. 3) [111, 180]. This is supported, at least in part, by recent evidence suggesting that distinct AgRP projection-populations have a similar neural activity response to energy state and metabolic hormones. In particular, food deprivation or ghrelin activated all AgRP neuron projection subpopulations, regardless of their role in evoking food intake [19]. However, some evidence for heterogeneity in AgRP neurons exists, as direct leptin responsiveness may be limited by the postnatal expression of leptin receptors to extra-hypothalamic AgRP neuron projection subpopulations [19]. Importantly, the receptor activities in arcuate POMC and NPY/AgRP neurons likely contribute to the effects of these receptor systems on metabolism (Fig. 2 and Fig. 3) [12, 16, 17, 34, 82, 98, 108, 111, 127, 160, 163, 180, 189, 203–205].
Fig. 3. Segregation of acute activity and cell transcriptional gene expression in POMC and NPY/AgRP cells.
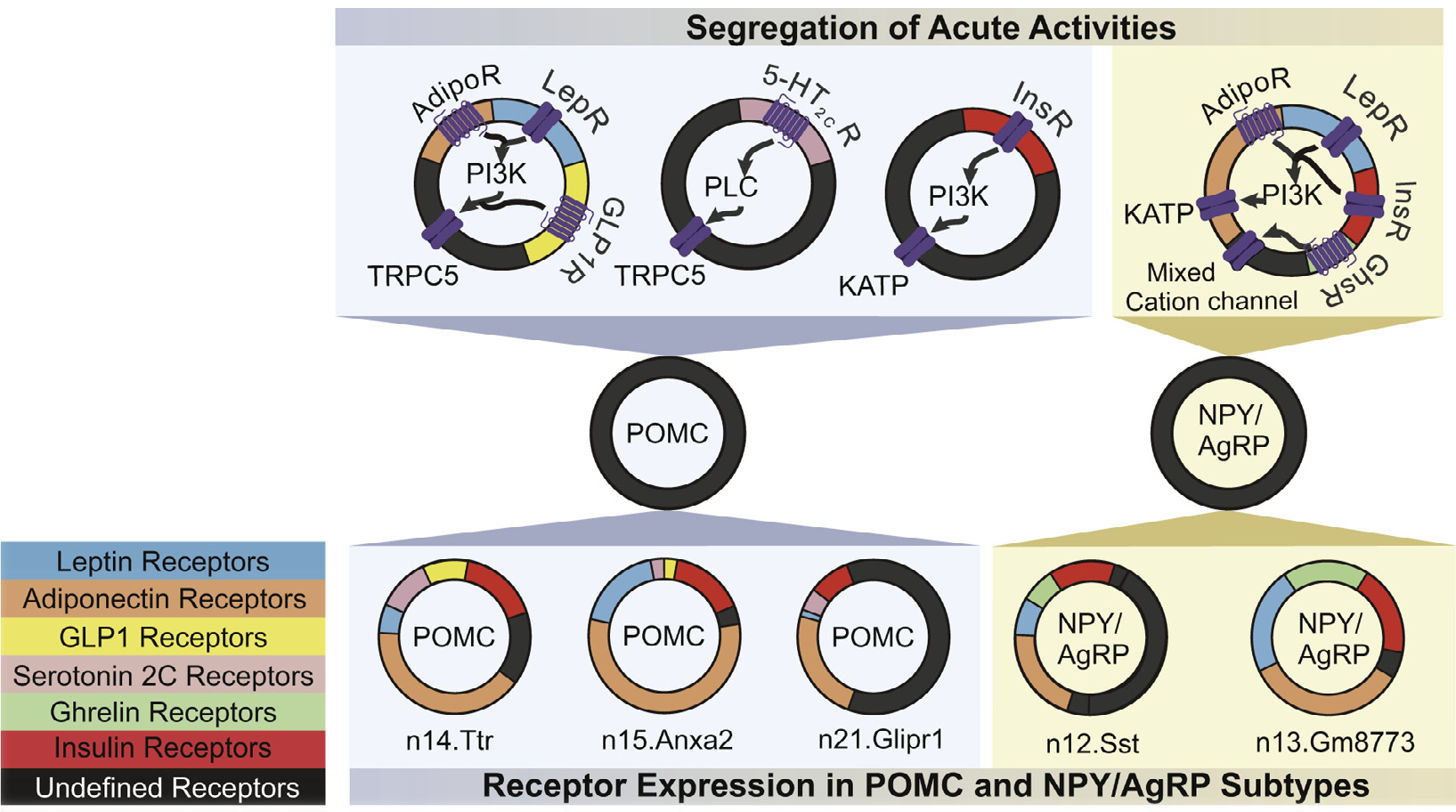
The top portion represents the segregation of acute activity in POMC and NPY/AgRP cells. Each receptor type is dedicated by a color to represent receptor expression and is shown on the bottom left hand side of the figure. The signaling pathway of each receptor type is then shown by arrow movement. In POMC neurons, Leptin and Adiponectin act through Phosphoinositide 3-kinase signaling pathway leading to activation of a putative transient receptor protein 5 (TRPC5) channel. Serotonin acts via Phospholipase C and TRPC5. Insulin acts through PI3K as well but ultimately targets an ATP-sensitive potassium channels (KATP). In AgRP cells, Adiponectin, Leptin, and Insulin act through a PI3K-KATP dependent mechanism. Ghrelin acts through a mixed cation channel. Adiponectin Receptors (AdipoR); Ghrelin Receptors (GhsR); Glucagon-like peptide Receptors (GLP-1r); Insulin Receptors (InsR); Leptin Receptors (LepR); Serotonin 2C Receptors (5-HT2cR). The bottom portion of the figure shows cell subtypes based on transcriptional profiling data in Campbell et al. [36]. Neuron clusters are named after the specified gene. POMC has three subtypes: n14.Ttr, n15.Anxa2, and n21.Glipr1; AgRP has 2 subtypes: n12.Sst and n13.Gm8773. The size of each partition, although not to scale, is meant to represent the expression value of each specified receptor in relation to the other subtypes of the cell (POMC or NPY/AgRP).
Given the functional activity of metabolic related receptors in AgRP neurons, it is surprising that functionally identified and discrete subpopulations of these neuron types have not been identified. Past studies employing traditional immunohistochemistry (IHC) or in situ hybridization techniques were limited by their scope of transcripts or proteins assessed and have a bias towards known markers. Similarly, population based analyses of gene expression can often mask distinct cellular properties [118]. Recent advances in transcriptomics have now made it possible to directly quantify gene expression of hundreds of individual cells in a population at a time, with the potential to elucidate pathways that counteract weight-loss and discover gene expression profiles of various neurons. Ultimately, linking these transcriptional profiles to function will enable a better understanding of the metabolic signals that control energy balance.
3.1. Population-based transcriptional profiling
Cell type-specific transcriptomics reveal that the expression of hundreds of genes in AgRP and POMC neurons are modified by energy state [104]. AgRP neurons are more sensitive to changes in food intake than POMC neurons, as food deprivation has a greater effect on gene expression in AgRP neurons [104]. The changes in transcriptional profiles of AgRP neurons observed may also help explain alterations in cellular activity in response to food deprivation. For example, the SK3 channel transcript, Kcnn3, an important contributor to AGRP neuron excitability and burst firing, is elevated in fed mice and reduced during food deprivation [96, 104]. Analogous to NPY/AgRP neuronal activity after an overnight fast [96, 135, 183], blockage or deficiency of SK3 channels in AgRP neurons promotes increased action potential firing [96, 104]. In addition to contributing to NPY/AgRP neuronal activity during energy deficit, this channel has been further linked in NPY/AgRP neurons to regulate long-term energy balance [96]. Additional changes in genes associated with cellular excitability were also observed and will be discussed in section 4.2 – energy state dependent plasticity.
3.2. Single-cell transcriptional profiles
While understanding global patterns of gene expression in pooled AgRP and POMC neurons is important, analysis at the single cell level enables a better understanding of the heterogeneity within anatomically localized populations of neurons [36, 132]. Unbiased clustering analysis revealed multiple classes of ARH POMC neurons (n14.Ttr, n15.Anxa2 and N21.Glipr) and two classes of ARH AgRP neurons (n12.SST and n13.Gm8773), each with distinct cell surface receptor gene expression profiles (Fig. 3) [36]. Leptin, insulin, adiponectin, and ghrelin receptors are expressed in both AgRP subtypes (Fig. 3) (n12.SST and n13.Gm8773) [36]. However, all transcripts are enriched to varying degrees in the n13.Gm8773 AgRP subtype compared to n12.SST (Fig. 3) [36]. Leptin receptors (LepR) are more highly expressed in ARH AgRP neurons compared to POMC neurons while insulin receptors (IR) are expressed at similar frequency across POMC and AgRP expressing neurons (Fig. 3) [36, 132]. Notably, among POMC subtypes, the LepR transcript is largely restricted to a distinct neuronal subtype (n15.Anxa2; Fig. 3), whereas serotonin 2c receptors (Htr2c) are predominantly expressed by other POMC subtypes (n14.Ttr and n21.Glipr; Fig. 3) [36]. As little as 10% of ARH POMC neurons expressed GLP-1r [132]. GLP-1r and Htr2c were both found in n14.Ttr POMC neurons to a higher degree than n15.Anxa2 POMC neurons (Fig. 3) [36]. However, of the POMC neurons expressing either Htr2c or GLP-1r, even fewer express both receptors [132]. GLP-1r and adiponectin receptors (AdipoR1/AdipoR2) are largely restricted to n.14.Ttr and n15.Anxa2 POMC neuron subtypes that also express LepRs [36]. Insulin receptors were found to be expressed in all three POMC subtypes (Fig. 3) [36].
The diverse genetic expression of POMC and AgRP subtypes is largely in agreement with the leptin receptor-, serotonin/Htr2c-, AdipoR1/R2-, insulinR-, and GLP-1r-sensing subtypes reported by previous studies (Fig. 3) [82, 98, 111, 173, 174, 180, 197]. However, segregation or overlap of these various receptors is not absolute with respect to the transcriptional profiles of these neuronal subtypes (Fig. 3) [36, 132]. This may provide important additional avenues for therapeutic intervention in obesity and diabetes. For example, the 5-HT2cR agonist, Lorcaserin [169], and various GLP-1 analogues [61] are targeted therapies that have been marketed for blood glucose control and chronic weight management. Both of these compounds mediate their effects on energy balance and glucose metabolism, at least in part via ARH POMC neurons expressing 5-HT2cR [33, [82], 102] or GLP-1r [80, 98, 163], respectively. As 5-HT2cRs and GLP-1rs are not typically expressed on the same POMC neurons [132], it is likely that distinct subpopulations contribute to the pharmacological effects of each compound, advancing the idea of a potential combinatorial therapy to achieve greater weight loss and/or blood glucose control.
While the focus of this review is on gene changes related to cellular activity and distribution of various metabolic cue/signal related receptors, transcriptional profiling of NPY/AgRP and POMC neurons also revealed extensive gene expression changes during energy deficit associated with increased protein translation and folding, circadian gene expression, and alterations in secreted proteins [36, 104]. Additionally, energy deficit activated pathways within NPY/AgRP neurons that protect from cellular damage and death were also described [36, 104]. These changes in gene profiles are also not limited to neurons of origin as the activity of POMC neurons non-autonomously regulates hepatic transcriptional profiles in a melanocortin dependent manner [27, 196]. Thus, the widespread changes in transcriptional regulation observed in NPY/AgRP and POMC neurons in these studies may have profound implications for the transcriptional profiles of downstream targets, which ultimately alter physiology.
It is important to consider that a cell-type census is only the beginning of unraveling the complex architecture of the brain, and thus may present an incomplete picture. In particular, differential expression of genes does not equate to differential function as there may be redundancy built within the brain as a compensatory mechanism. Cell transcriptomics only assesses dynamic transcriptional responses comprehensively across cell types, affirming the close relationship between a cell’s transcriptional and functional identities. Further research is needed to establish associative rationale and connect transcriptional changes with functional output. However, these resources, combined with methods to validate and manipulate these pathways, provide an exciting framework for extensive metabolic circuit analysis. The cell type-specific circuit nodes in the brain provide molecular insights towards unraveling the neuronal regulation of metabolism and will aid in the development of future therapeutic strategies.
4. Cellular plasticity of POMC and NPY/AGRP neurons in response to metabolic challenges
One of the most remarkable features of the brain is its ability to undergo functional and structural changes referred to as plasticity [73, 110]. The plasticity of the brain has probably been most appreciated with regard to long-term potentiation associated with learning and memory [14, 20, 100, 117, 137]. However, there is increasing evidence that the brain responds transiently and/or chronically in response to metabolic state and/or signals [57, 162]. The plasticity of neurons in response to peripheral metabolic cues/signals, e.g. hormones, glucose and fatty acid levels, may be beneficial in maintaining a regulatory balance with regard to feeding, energy expenditure, and glucose metabolism [57, 108, 195, 210].
Leptin has been implicated in the plasticity of metabolically relevant circuits during development and metabolic disease states. In particular, leptin participates in neurite outgrowth and synaptic rewiring of ARH neurons during development ([23], b; [25]). This defines leptin as an important trophic factor responsible for the proper formation of hypothalamic circuits during development with ramifications for metabolic disorders. Moreover, Pinto and colleagues examined the synaptic structure and electrophysiological properties of POMC and NPY/AgRP neurons in the absence or presence of leptin [159]. Specifically, they utilized a genetic model of obesity - mice that are deficient for leptin (ob/ob mice). NPY neurons from ob/ob mice received a larger amount of excitatory input than compared to their wildtype counterparts. POMC neurons from ob/ob mice received less excitatory tone than their wildtype counterparts. The synaptic structure/activity of NPY and POMC neurons from ob/ob mice corresponds with the phenotype of the mice, as they were obese and diabetic [113, 162, 212]. Interestingly, treating ob/ob mice with leptin as a monotherapy restored the synaptic organization of NPY and POMC neurons to their wildtype state [159]. Remarkably, this reorganization occurred within 6 h of leptin treatment. These data highlight the rapid plasticity of melanocortin circuits during development and in mature circuits ([23], b; [25, 159]).
4.1. Energy state dependent plasticity
The energy state of an organism can elicit synaptic reorganization of NPY/AgRP and POMC neurons [206]. In particular, fasting activates NPY/AgRP neurons, leading to an increase in action potential firing as well as an increase in the frequency of excitatory postsynaptic currents to NPY/AgRP neurons [96, 104, 135, 183]. The neurotransmitter glutamate is released from presynaptic terminals into the synaptic cleft and post-synaptically binds to both N-methyl-D-aspartate receptors (NMDARs) and α-amino-3‑hydroxy‑5-methyl-4-isoxazolepropionic acid receptors (AMPARs), allowing for the influx of Na+ and Ca2+ which in turn influence signal activity. Calcium then triggers a series of intracellular signaling cascades, which lead to additional AMPAR recruitment [119]. The increase in postsynaptic ion channels strengthen synaptic signals and may lead to dendritic spinogenesis [119]. Importantly, fasting results in significant changes in genes associated with glutamate signaling in AgRP neurons, including increased expression of AMPA and kainate glutamate receptors [104]. Elevated levels of genes associated with spine morphology and excitatory synaptic input were also observed in AgRP neurons from food-deprived animals [104]. Functionally, the increased excitatory synaptic input has been suggested to be dependent upon NMDARs, which trigger the fasting-induced activation and dendritic spinogenesis [135, 206]. Additional work suggests postsynaptic NMDARs have the potential to regulate energy balance independent of energy state [135]. It is currently unclear whether NMDAR activity is required for the aforementioned fasting-induced SK3 channel activity [96, 104] or if these are functionally redundant mechanisms to stimulate NPY/AgRP neurons during energy deficit. While a direct trigger in the fasting induced plasticity of the melanocortin circuit has yet to be identified, it should be noted that leptin levels fall during a fast [3, 42]. Leptin or leptin receptor deficiency alone also drives the activity of NPY neurons [183] and mice that are deficient for leptin or leptin receptors exhibit a blunting of the fasting induced activation of NPY neurons [183]. Thus, leptin appears to be a prime candidate to contribute to the energy sensing plasticity of the melanocortin circuit.
Similar to fasting, energy excess (as occurs with HFD exposure) has been linked to both transient and chronic changes in the activity of NPY/AgRP and POMC neurons [13, 149, 194]. In particular, mice on HFD exhibit an increase in NPY/AgRP activity [13, 194]. This effect may occur in just a few days and during chronic exposure of HFD this activity may persist [13]. Furthermore, this effect is mediated by macronutrient content of the diet, as the increases in NPY/AgRP activity are independent of caloric intake and weight gain. Age is also a factor in synaptic architecture of the hypothalamus as AgRP innervation to POMC neurons increases with aging [149]. This increased AgRP dependent GABAergic inhibition of arcuate POMC neurons is associated with decreased POMC neuronal activity with age [149]. Various hormones have been linked to this age-dependent plasticity of the melanocortin circuit. In particular, mice deficient for leptin or leptin signaling are protected from age-dependent inhibition of POMC neurons by AgRP projections, and HFD accelerates the AgRP dependent increase in inhibitory tone to POMC neurons [149]. There also appears to be projection specific effects as HFD disrupts AgRP projections to the PVH and leptin deficiency mimics this activity [22, 24, 194]. Similarly, ghrelin has been implicated in increasing AgRP activity while suppressing POMC projections to the PVH [177]. Importantly, this may be specific to development as ghrelin signaling failed to alter these projections in adult mice [177]. Together, peripheral hormonal signals -such as leptin, ghrelin, and environmental cues - can greatly influence excitatory/inhibitory tone in ARH POMC and NPY/AgRP neurons as well as innervation to downstream target nuclei. This plasticity facilitates adaptations to various metabolic challenges.
4.2. Sexual dimorphism in the responses of pomc and npy/agrp neurons
There is a sexual dimorphism in the number of cells within the ARH that contain NPY/AgRP, and POMC [153]. Compared to females, male mice have fewer POMC neuronal fibers and increased NPY gene expression in the ARH [153, 188]. The associative decrease in POMC gene and protein expression is concomitant with an increased energy intake [153]. Accordingly, female rats are more sensitive to the anorectic action of centrally injected leptin than males [49]. Conversely, males are more sensitive to the hyperphagic effects of centrally administered AgRP [85], an effect which might also be influenced by estrous cycle in females [155]. Sex differences have been further highlighted by work using mouse genetics [17, 33, 165, 166]. These patterns are not limited to rodents as sex differences in energy expenditure regulation are conserved across species, including humans [49, 153, 164]. Better understanding sex-based differences in the plasticity of metabolically relevant circuits is a pressing need.
5. Exercise training exerts complex adaptive responses in the arcuate
Regular exercise contributes to improvements in metabolism via activity within the CNS and can decrease the risk of obesity and diabetes [64, 78, 79, 81, 94, 140, 184]. The CNS, including the hypothalamus, is essential for many of the beneficial effects of exercise [78, 79, 140]. As previously discussed, melanocortin circuits regulate energy/ glucose homeostasis and are highly sensitive to various metabolic challenges. Exercise itself is a metabolic challenge, which raises the question if exercise might improve metabolism at least in part via plasticity of melanocortin circuits.
5.1. Cellular responses of arcuate POMC and NPY/AGRP neurons during and after exercise
Recent work suggests that exercise (both single bout and chronic training) activates ARH POMC neurons while simultaneously inhibiting NPY/AgRP neurons (Fig. 4) [97]. In particular, arcuate POMC neurons from exercised mice are depolarized and receive an increased excitatory synaptic tone after exercise (Fig. 4). Complementary studies also demonstrated that acute exercise enhances POMC transcripts in wistar rats [77]. Opposite to the effects on POMC neurons, NPY/AgRP neurons from exercised mice are hyperpolarized and receive an increased inhibitory synaptic tone after exercise (Fig. 4). Importantly, these effects were observed ex vivo and in vivo, utilizing both patch-clamp and in-vivo fiber photometry methods.
Fig. 4.
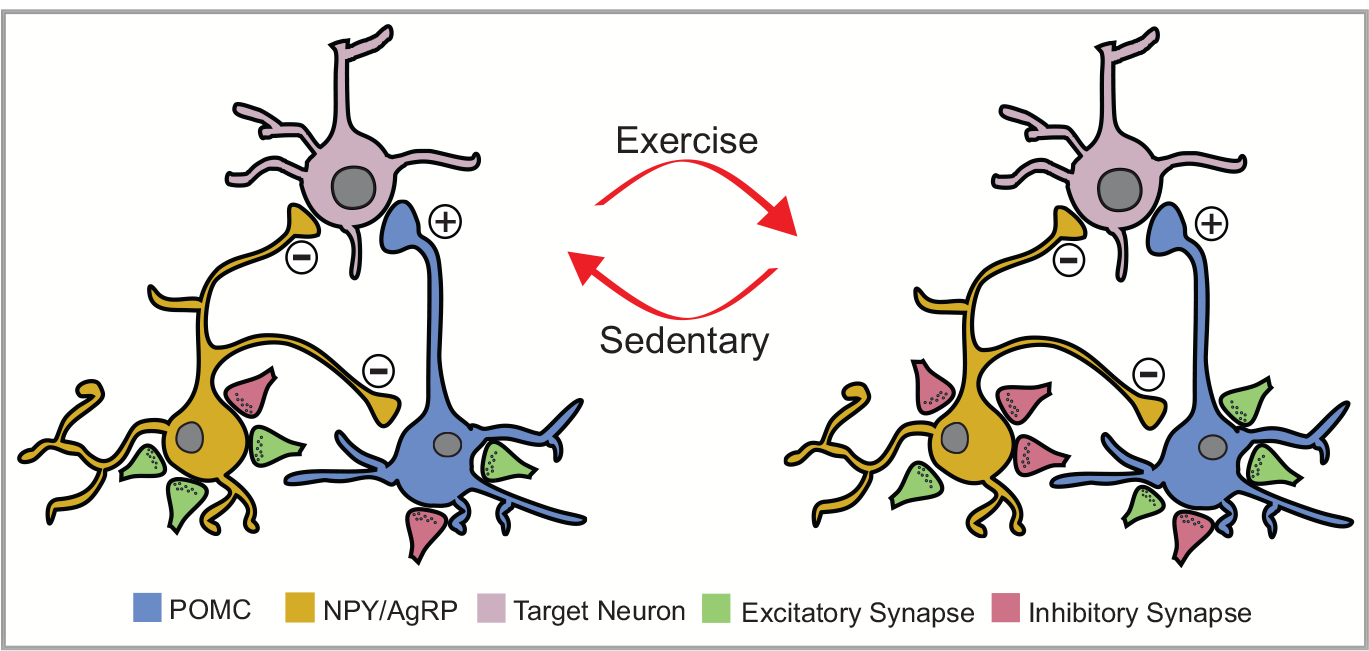
Plasticity of POMC and NPY/AgRP neurons after exercise The plasticity of POMC (depicted in blue) and NPY/AgRP (depicted in yellow) neurons in response to exercise. The arrows demonstrate the changes in the circuit depending on the state of the animal - sedentary or exercise. The pink cell represents downstream targets of these cell populations. The green synaptic terminals (boutons) are meant to represent excitatory synaptic projections or inputs, while the red terminals are inhibitory inputs. POMC neurons are activated while NPY/ AgRP neurons are inhibited after exercise.
While these data support changes in arcuate POMC and NPY/AgRP neuronal activity after exercise [97], the effects on these neurons during exercise have remained undefined. In order to monitor changes in neural activity during exercise, we measured whole-cell calcium dynamics of arcuate NPY/AgRP and POMC neurons during a single bout of HIIE using in-vivo fiber photometry [97, 179]. Within minutes of starting a single bout of HIIE, calcium levels were increased in ARH NPY/AgRP neurons and decreased in ARH POMC neurons of ad libitum fed mice (Fig. 5A), demonstrating the rapid ability of hypothalamic neurons to detect and respond to physical activity. In contrast, whole cell calcium levels were decreased in both ARH NPY/AgRP and POMC neurons of food restricted mice (Fig. 5B). Interestingly, exercise intensity impacts the magnitude but not direction of calcium dynamics as the neural activity changes were greater with increasing running speeds (Fig. 5).
Fig. 5. Neural activity changes in NPY/ AgRP and POMC neurons during exercise.
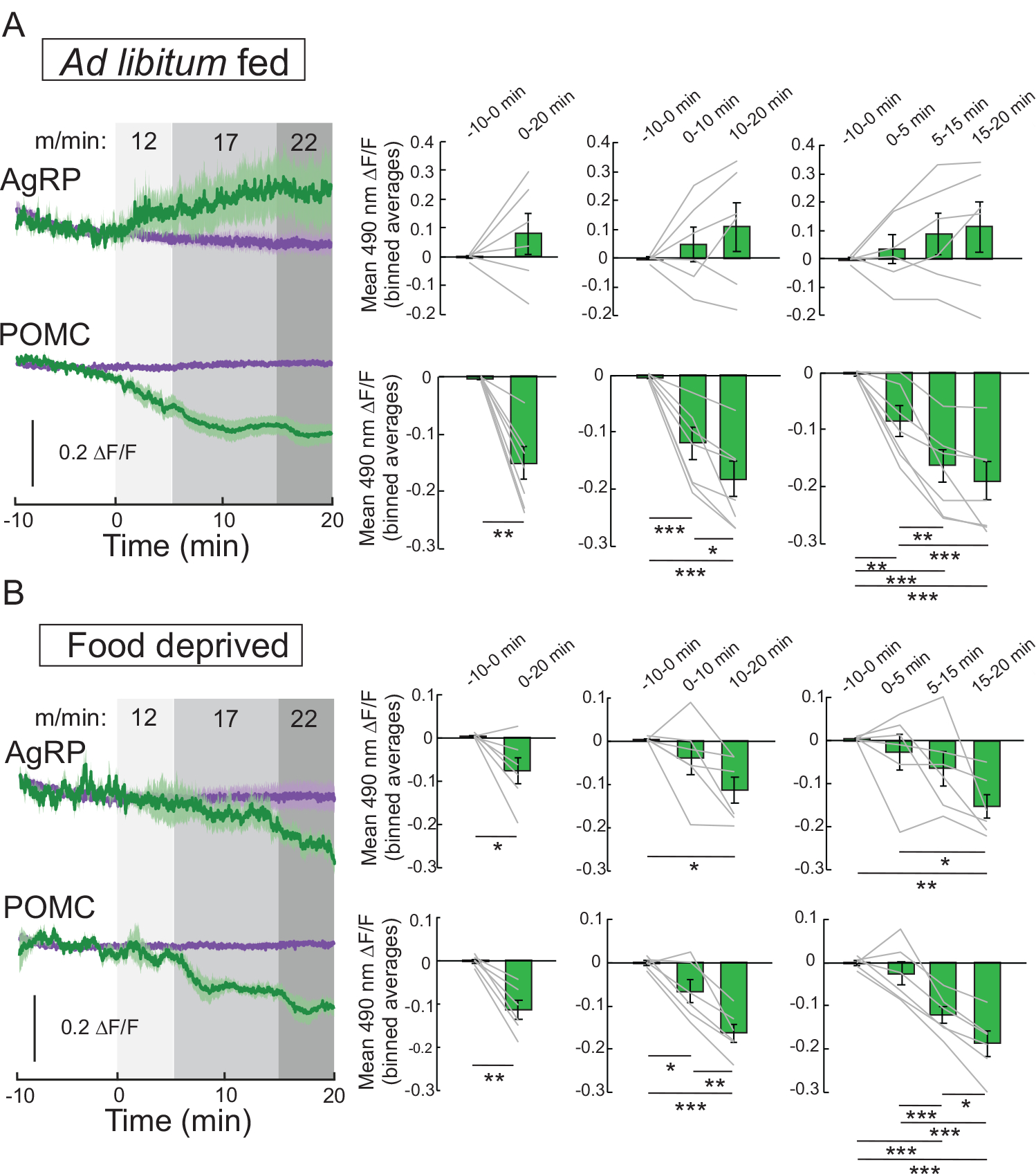
Mice were subjected to a single ramp of high intensity interval exercise (HIIE) to assess exercise-induced changes in calcium dynamics of NPY/AgRP and POMC neurons. Mice were rested on the treadmill for 10 min prior to performing 20 min of exercise at ascending speed: 5 min at 12 m/min, 10 min at 17 m/min, and 5 min at 22 m/min. Mice were tested in the ad libitum (A, n = 6 NPY/AgRP and n = 7 POMC) or food deprived (B, n = 6 NPY/ AgRP and n = 6 POMC) state. Data are expressed as mean ± SEM, t-tests and post-hoc comparisons: *p<0.05, **p<0.01, ***p<0.001.
5.2. Implications for the changes in cellular activity observed during and after exercise
The changes in calcium dynamics during exercise are different than the effects observed after exercise, raising several important questions. First, are the calcium dynamics observed after exercise a reversal of the effects observed during exercise? Our calcium imaging data combined with ex vivo electrophysiological results suggest that the changes post-exercise are not simply a reversal of exercise effects, especially because we see activity changes sometimes for several hours or days post-exercise [97]. However, it is difficult to make this assessment in vivo without measuring the firing rate of individual neurons, as photo-bleaching of fluorescent reporters can occur over extended sampling periods. One potential way to assess this question would be using in vivo extracellular neural activity recordings in NPY/AgRP and POMC neurons [138].
Second, are the changes in calcium dynamics observed in melanocortin neurons during exercise occurring in the same population of melanocortin neurons that are altered after exercise? Melanocortin neurons are highly heterogeneous, and the activity of distinct subpopulations of melanocortin neurons respond differently to numerous metabolic signals. This heterogeneity may in part explain why melanocortin populations respond differentially during and after exercise. It is also interesting to note that the variability in the calcium signal increases over time. This could reflect the stress of running and/or variability in cellular responses, possibly indicative of heterogeneous neural activity to exercise. It will therefore be important for future work to consider changes in neural activity that occur during and after exercise in individual neuronal populations.
Third, what is the physiological significance of the changes in activity of arcuate POMC and NPY/AgRP neurons during exercise versus after exercise? There are many counter-regulatory activities mediated by the CNS, including the regulation of heart rate, blood pressure, respiration, and blood glucose levels. Not surprisingly, all of these counter-regulatory relays are essential in performing exercise and most are influenced by the melanocortin system in both rodents and humans [69, 83, 87, 131, 150, 171, 172, 207]. Thus, the rapid changes occurring at the beginning of exercise might reflect compensatory counter-regulatory mechanisms to facilitate exercise performance. Moreover, there are distinct temporally restricted changes in POMC and NPY/ AgRP neurons that occur after exercise [97]. Specifically, the exercise-induced inhibition of NPY/AgRP neurons lasted for only a few hours while POMC neurons remained activated for at least 48 h after a single bout of exercise [97]. While speculative, the temporal regulation of melanocortin activity in response to exercise has very interesting implications on the regulation of metabolism and on behavior. For instance, ARH AgRP neurons have been shown to regulate acute phases of feeding behavior (from minutes to hours) whereas arcuate POMC neurons appear to regulate chronic feeding (over hours to days) [9, 211]. The effects of exercise on feeding behavior remain controversial, with studies suggesting compensatory food intake in response to increased activity [21, 121]. However, many of these studies have looked at activity dependent feeding over prolonged periods (i.e. days, weeks, or months). Human studies suggest a decrease in hunger/feeding may occur during high intensity exercise bouts and in the hours immediately post exercise [21, 31, 60]. Similarly, mice display acute hypophagia immediately after high intensity exercise [140]. This exercise-induced hypophagia may correlate with the suppression of NPY/AgRP neuronal activity observed after exercise. Additionally, exercise (single bouts or training) results in improved insulin sensitivity across multiple species up to 48 h after exercise [139, 143, 154, 191]. Importantly, the physiological role of leptin receptors in POMC neurons is to improve insulin sensitivity and glucose metabolism [12, 17, 42]. As the leptin receptor expressing POMC neurons remained activated for 48 h after a single exercise bout, this activity might be a cellular correlate contributing to exercise-induced improvements in insulin sensitivity observed in multiple species.
Fourth, how are counter-regulatory responses induced during and after exercise relayed to arcuate neurons? Counter-regulatory activities depend upon both neuronal circuits, which function as a “reflex” as well as humoral signals [26, 56, 74, 92, 125, 141, 142, 145, 193, 214]. As hormone-mediated regulatory activity is slower than direct neuronal activity, the changes observed at the beginning of exercise (within seconds to minutes) likely involve circuit-mediated changes in activity (possibly recruitment of various reflexes) while the long-term (lasting for hours to days) changes in neuronal activity is likely dependent upon humoral signals. These signals may cause differential changes to arcuate neuron activity, perhaps accounting for the activity differences observed during and after exercise. Leptin has been implicated in the rapid rewiring of POMC and NPY/AgRP neuronal circuitry within the arcuate as well as projection patterns to downstream target nuclei. In support of a role for leptin in the plasticity of the melanocortin circuit occurring after exercise, POMC neurons that express leptin receptors appeared more sensitive to the effects of exercise [97]. In contrast, NPY/AgRP neurons that express leptin receptors and those that do not express leptin receptors were both equally sensitive to the effects of exercise [97]. These data may support a role for leptin or leptin signaling in the rewiring of arcuate POMC neurons after exercise. However, it is important to note that leptin levels have generally been reported to fall after aerobic exercise training [88, 157, 158]. The changes in leptin levels may depend upon either exercise intensity/ duration or body mass changes [59, 62]. Decreased leptin levels corresponded to decreased adiposity after exercise training, however leptin levels have also been shown to fall in response to acute exercise independent of changes in adiposity [11, 151, 208]. In any case, a decrease in leptin would not be in agreement with the activity profile exhibited by either POMC or NPY/AgRP neurons after exercise [97]. However, exercise has also been shown to increase leptin sensitivity [63, 75, 175]. Thus, it might be possible that there is an increase in leptin signaling contributing to plasticity of the melanocortin circuit, independent of falling leptin levels. In addition to leptin levels, there are multiple factors, proteins, and signaling cascades that have been suggested to change in response to exercise [64, 81, 94, 184]. Thus, it is also possible that, similar to the overlap in the acute effects of various metabolic receptors in the ARH, exercise-induced plasticity in POMC and NPY/AgRP neurons may be influenced by several factors. [82, 99, 108, 109, 111, 116, 160, 173, 174, 180, 195, 197, 198]. Ultimately, identification of these signals and understanding how they influence the neural activity of discrete ARH neuron subpopulations will be important for understanding the neural basis for the beneficial effects of exercise.
A final salient aspect of exercise performance is the need for efficient utilization of energy substrates [54, 95]. In particular, there is a preference for carbohydrate utilization as exercise intensity is increased [18, 54, 95]. Recent work demonstrated that increasing AgRP neuron activity drives carbohydrate utilization [43]. In the current study, whole cell calcium dynamics of AgRP neurons were increased during exercise in ad libitum fed mice and decreased in food restricted mice (Fig 5). As carbohydrates are more readily available in ad libitum fed animals [144, 156], the increased activity of AgRP neurons during exercise of these mice might reflect a shift toward carbohydrate utilization. Interestingly, aerobic exercise performed in a fasted state has been associated with an increased reliance on fat, at least during exercise of low-to moderate-intensity [2, 95]. While currently unclear, the decreased activity of AgRP neurons during exercise in food restricted mice might reflect a shift toward fat utilization. Physiological correlates to the rapid changes in POMC activity observed during exercise might be less apparent. Based on pharmacological blockade or genetic deficiency of melanocortin signaling studies [6, 35, 112, 152], the decreased POMC activity during exercise of ad-lib fed mice predicts a similar shift toward carbohydrate utilization. However, if this activity represents substrate utilization, a shift towards carbohydrate utilization would also persist in food restricted mice as POMC calcium dynamics are still suppressed. Acute/rapid changes in NPY/AgRP and POMC activity during exercise may also have implications on energy expenditure, blood pressure, as well as glucose metabolism (Fig. 2), which warrants further investigation. Overall, there are varying energy demands that need to be accounted for during exercise and these likely differ from the long-term benefits of exercise. The melanocortin system is a key neural network integrating/relaying these demands and as such, it maybe less surprising that the activity patterns of melanocortin neurons vary with respect to various metabolic states which include before, during, and after exercise.
5.3. Conclusion and future directions
In summary, there is increasing evidence that the melanocortin system is highly sensitive to metabolic state. The activity of melanocortin neurons rapidly adapts to interoceptive (e.g. hormones and neurotransmitters) and exteroceptive (e.g. diet, fasting/fed state, over-/ under-nutrition, exercise) signals. Alterations in the activity of melanocortin neurons can be transient or sustained; lasting from hours to days. This is reminiscent of cellular properties that may underlie metabolic set points that contribute to homeostatic model systems including body weight and blood glucose regulation [47, 133, 176]. While these data highlight the plasticity of melanocortin neurons, these results also raise several questions for future investigation.
First, arcuate POMC and NPY/AgRP neurons have defined projection patterns to multiple intra- and extra-hypothalamic nuclei (Fig. 1). Many of these downstream target nuclei/cell populations have also been implicated in direct regulation of energy and glucose homeostasis [84, 148, 185]. Additionally, these same nuclei send reciprocal projections back to the arcuate nucleus and in some cases directly back to arcuate POMC and NPY/AgRP neurons [8, 19, 192]. This is important as much of the plasticity observed in arcuate POMC and NPY/AgRP neurons involve both excitatory and inhibitory pre-synaptic activity [57, 97, 135, 159]. Thus, in addition to the plasticity occurring directly within the arcuate nucleus, there is a need to determine how various metabolic challenges alter the plasticity of these other metabolically relevant downstream/upstream brain regions in order to better understand the effects of various metabolic challenges in the brain and the ultimate contribution to metabolism.
While the plasticity of these metabolic circuits has been largely defined in males, detailed investigation into the cellular and systemic mechanisms involved in this process in females has been greatly understudied. This is a striking deficiency, given the widely recognized sexual dimorphism in metabolism including energy utilization/storage and activity/exercise capacity. Sex-based differences in the metabolic response to exercise in humans has also been reported (e.g. transient suppression of hunger after exercise in men but not women exposed to the same exercise regimen) [21, 120, 122]. However, less is known about the sex-dependent effects of exercise-induced synaptic rewiring of POMC, NPY/AgRP, and other neuronal populations, which warrants further investigation.
Finally, the adaptive capacity of metabolically relevant circuits has largely focused on the plasticity of these circuits in lean subjects. While these data may highlight a cellular mechanism possibly contributing to metabolic dysfunction, understanding if and how these circuits can adapt to metabolic improvements in disease states might prove more important. In particular, do these circuits retain their plasticity after chronic metabolic dysfunction? Similarly, do interventions known to improve metabolic dysfunction result in plasticity predictive of metabolic improvements? These critical questions will need to be answered in order to better understand metabolic disease and facilitate the development of targeted therapies.
6. Methods
6.1. Fiber photometry
6.1.1. Experimental model
Agrp-IRES-Cre (Jackson Labs 012,899, Agrptm1(cre)Lowl/J) [186] and Pomc1-Cre (Jackson Labs 005,965, POMCtg(Pomc1−Cre)16Lowl/J) [12] mice were used for experimentation. All mice were habituated to handling and experimental conditions prior to experiments. All procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
6.1.2. Exercise groups
All mice were familiarized to the treadmills for 2 days prior to the exercise bout [Habituation day 1: 5 min rest on the treadmill followed by 5 min at the speed of 8 m/min and then for 5 min at the speed of 10 m/min; Habituation day 2: 5 min rest on the treadmill followed by 5 min at the speed of 10 m/min and then for 5 min at the speed of 12 m/min [91, 97]
6.1.3. Exercise protocol
On the exercise test day, mice were subjected to a single ramp of high intensity interval exercise (HIIE) bout [91, 97, 140] to assess exercise-induced changes in calcium dynamics of NPY/AgRP and POMC neurons. Mice were rested on the treadmill for 5 min prior to performing the 20 min of exercise consisting of 1 × 20 min interval (5 min at the speed of 12 m/min, followed by 10 min at the speed of 17 m/min, and then 5 min at the speed of 22 m/min).
6.1.4. Viral injections and fiber optic implantation
Mice were anesthetized with isoflurane (1.5–3%, Clipper, 0,010,250) and pretreated with subcutaneous injections of ketoprofen (5 mg/kg, Santa Cruz Animal Health, sc-363115Rx) and bupivacaine (2 mg/kg, Moore Medical, 52,683). Mice were placed into a stereotaxic apparatus (Stoelting, 51725D) and viral injections were performed as previously described [1], [58]. Unilateral injections of the genetically encoded calcium indicator GCaMP6s [AAV1.Syn.Flex.GCaMP6s.WPRE.SV40 (titer: 4.216e13 GC/ml, Penn Vector Core) were performed in the arcuate hypothalamic nucleus (ARC, 400 μl) according to the following coordinates: 1.35 mm posterior to bregma, 0.25 mm lateral to midline, and 6.15–6.3 mm ventral to skull. A ferrule-capped optical fiber (400 μm core, NA 0.48, Doric, MF2.5, 400/430–0.48) was implanted 0.2 mm above the injection site and secured to the skull with Metabond cement (Parkell, S380) and dental cement (Lang Dental Manufacturing, Ortho-jet BCA Liquid, B1306 and Jet Tooth Shade Powder, 143,069). All mice were given at least 2 weeks for recovery and viral expression before experiments were performed.
6.1.5. Dual-wavelength fiber photometry
Dual-wavelength fiber photometry (FP) was performed as we and others have previously described [97, 134, 179]. Two excitation wavelengths were used: 470 and 405 nm. 470 nm excites calcium-dependent fluorescence from GCaMP6s protein, providing a measure of AgRP or POMC neuron activity. 405 nm excites calcium-independent fluorescence from GCaMP6s protein and serves as a control for movement and bleaching artifacts. Excitation light intensities were modulated at different frequencies (211 and 566 Hz for 470 and 405 nm, respectively) to avoid contamination from overhead lights (120 Hz and harmonics) and cross-talk between excitation lights. Excitation lights were generated through fiber-coupled LEDs (Thorlabs, M470F3 for 470 nm and M405F1 for 405 nm) and modulated by a real-time amplifier (Tucker–Davis Technology, RZ5P). GCaMP6s emission fluorescence signals were collected through a patch cord, collimated, passed through a GFP emission filter (Thorlabs, MF525–39), and focused onto a femtowatt photoreceiver (Newport, Model 2151, gain set to AC LOW). The emission lights were converted to electrical signals and demodulated by the RZ5P real-time processor. The FP experiments were controlled by Synapse software (Tucker–Davis Technology).
6.1.6. Food restriction
Mice were weighed at the same time each day and given chow once daily (1.5–3.0 g) after experimentation to maintain 85–90% of their starting body weight.
6.1.7. Fiber photometry hiie experiment
Ad libitum fed mice underwent the single ramp HIIE protocol as described above. On the second session, mice were tethered to a patch fiber to habituate to fiber photometry procedures. On test day, AgRP/POMC neuron activity was recorded during HIIE. Only mice that completed the running interval were included in the data analysis. AgRP/POMC neuron activity was analyzed during the 20 min of running and for 10 min pre-running.
6.1.8. Analysis and statistics
Results are reported as the mean ± SEM unless indicated other wise, as indicated in each figure legend. Fiber photometry data were exported from Synapse to MATLAB (MathWorks) using a script provided by Tucker–Davis Technology. The 470 and 405 nm signals were independently processed and normalized to baseline signals to determine ΔF/F, where ΔF/F = (F−Fpre-stimulus)/Fpre-stimulus and Fpre-stimulus is the median of pre-stimulus signal (before running). Data were down-sampled to 1 Hz in MATLAB, and mean ΔF/F was calculated by integrating ΔF/F over a period of time and then dividing by the integration time. All data were evaluated using a ANOVA with post hoc Holm-Sidak comparisons or two-tailed Student’s t-test, with *p<0.05 being considered statistically significant.
Acknowledgement
This work was supported by NIH grants to K.W.W. (R01 DK100699, R01 DK119169, and DK119130–5830), A.L.A. (R00 DK119574), and J.N.B. (R01 DK114014), and the American Diabetes Association (118IBS116 to J.N.B.).
Footnotes
Declaration of Competing Interest
The authors have nothing to disclose.
References
- [1].Hetherington AW, R SW, Hypothalamic lesions and adiposity in the rat, The Anatomical Records 78 (1940) 149–172. [Google Scholar]
- [2].Achten J, Jeukendrup AE, Maximal fat oxidation during exercise in trained men, Int J Sports Med 24 (2003) 603–608. [DOI] [PubMed] [Google Scholar]
- [3].Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS, Role of leptin in the neuroendocrine response to fasting, Nature 382 (1996) 250–252. [DOI] [PubMed] [Google Scholar]
- [4].Aklan I, Sayar Atasoy N, Yavuz Y, Ates T, Coban I, Koksalar F, Filiz G, Topcu IC, Oncul M, Dilsiz P, et al. , NTS Catecholamine Neurons Mediate Hypoglycemic Hunger via Medial Hypothalamic Feeding Pathways, Cell Metab 31 (2020) 313–326 e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, Selman C, Piipari K, Clements M, Lingard S, et al. , Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons, Cell Metab 10 (2009) 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Albarado DC, McClaine J, Stephens JM, Mynatt RL, Ye J, Bannon AW, Richards WG, Butler AA, Impaired coordination of nutrient intake and substrate oxidation in melanocortin-4 receptor knockout mice, Endocrinology 145 (2004) 243–252. [DOI] [PubMed] [Google Scholar]
- [7].Alhadeff AL, Su Z, Hernandez E, Klima ML, Phillips SZ, Holland RA, Guo C, Hantman AW, De Jonghe BC, Betley JN, A Neural Circuit for the Suppression of Pain by a Competing Need State, Cell 173 (2018) 140–152 e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Anderson EJ, Cakir I, Carrington SJ, Cone RD, Ghamari-Langroudi M, Gillyard T, Gimenez LE, Litt MJ, 60 YEARS OF POMC: regulation of feeding and energy homeostasis by alpha-MSH, J. Mol. Endocrinol 56 (2016) T157–T174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Aponte Y, Atasoy D, Sternson SM, AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training, Nat. Neurosci 14 (2011) 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Babinski JFF, Tumeur du corps pituitaire sans acromégalie et avec arrét de développement des organes génitaux. Rev. Neurol. (Paris) 8 (1900) 531–533. [Google Scholar]
- [11].Baltaci AK, Vurucu N, Uzun A, Mogulkoc R, Kilic M, The effect of acute swimming exercise on plasma leptin in rats, Bratisl Lek Listy 113 (2012) 592–594. [DOI] [PubMed] [Google Scholar]
- [12].Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC Jr., Elmquist JK, et al. , Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis, Neuron 42 (2004) 983–991. [DOI] [PubMed] [Google Scholar]
- [13].Baver SB, Hope K, Guyot S, Bjorbaek C, Kaczorowski C, O’Connell KM, Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus, J Neurosci 34 (2014) 5486–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bear MF, Malenka RC, Synaptic plasticity: LTP and LTD, Curr. Opin. Neurobiol 4 (1994) 389–399. [DOI] [PubMed] [Google Scholar]
- [15].Bentivoglio M, Kuypers HG, Catsman-Berrevoets CE, Dann O, Fuorescent retrograde neuronal labeling in rat by means of substances binding specifically to adenine-thymine rich DNA, Neurosci Lett 12 (1979) 235–240. [DOI] [PubMed] [Google Scholar]
- [16].Berglund ED, Liu C, Sohn JW, Liu T, Kim MH, Lee CE, Vianna CR, Williams KW, Xu Y, Elmquist JK, Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis, J Clin Invest 123 (2013) 5061–5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Berglund ED, Vianna CR, Donato J Jr., Kim MH, Chuang JC, Lee CE, Lauzon DA, Lin P, Brule LJ, Scott MM, et al. , Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice, J Clin Invest 122 (2012) 1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bergman BC, Brooks GA, Respiratory gas-exchange ratios during graded exercise in fed and fasted trained and untrained men, J Appl Physiol (1985) 86 (1999) 479–487. [DOI] [PubMed] [Google Scholar]
- [19].Betley JN, Cao ZF, Ritola KD, Sternson SM, Parallel, redundant circuit organization for homeostatic control of feeding behavior, Cell 155 (2013) 1337–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bliss TV, Lomo T, Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path, J Physiol 232 (1973) 331–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Blundell JE, King NA, Physical activity and regulation of food intake: current evidence, Med Sci Sports Exerc 31 (1999) S573–S583. [DOI] [PubMed] [Google Scholar]
- [22].Bouret SG, Crossing the border: developmental regulation of leptin transport to the brain, Endocrinology 149 (2008) 875–876. [DOI] [PubMed] [Google Scholar]
- [23].Bouret SG, Draper SJ, Simerly RB, Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice, J Neurosci 24 (2004) 2797–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bouret SG, Draper SJ, Simerly RB, Trophic action of leptin on hypothalamic neurons that regulate feeding, Science 304 (2004) 108–110. [DOI] [PubMed] [Google Scholar]
- [25].Bouret SG, Simerly RB, Minireview: leptin and development of hypothalamic feeding circuits, Endocrinology 145 (2004) 2621–2626. [DOI] [PubMed] [Google Scholar]
- [26].Boychuk CR, Smith KC, Peterson LE, Boychuk JA, Butler CR, Derera ID, McCarthy JJ, Smith BN, A hindbrain inhibitory microcircuit mediates vagally-coordinated glucose regulation, Sci Rep 9 (2019) 2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Brandt C, Nolte H, Henschke S, Engstrom Ruud L, Awazawa M, Morgan DA, Gabel P, Sprenger HG, Hess ME, Gunther S, et al. , Food Perception Primes Hepatic ER Homeostasis via Melanocortin-Dependent Control of mTOR Activation, Cell 175 (2018) 1321–1335 e1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Brobeck JR, Mechanism of the development of obesity in animals with hypothalamic lesions, Physiol Rev 26 (1946) 541–559. [DOI] [PubMed] [Google Scholar]
- [29].Brobeck JR, Tepperman J, Long CN, The Effect of Experimental Obesity upon Carbohydrate Metabolism, Yale J Biol Med 15 (1943) 893–904. [PMC free article] [PubMed] [Google Scholar]
- [30].Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T, The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice, Proc Natl Acad Sci U S A 95 (1998) 15043–15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Broom DR, Stensel DJ, Bishop NC, Burns SF, Miyashita M, Exercise-induced suppression of acylated ghrelin in humans, J Appl Physiol (1985) 102 (2007) 2165–2171. [DOI] [PubMed] [Google Scholar]
- [32].Bumaschny VF, Yamashita M, Casas-Cordero R, Otero-Corchon V, de Souza FS, Rubinstein M, Low MJ, Obesity-programmed mice are rescued by early genetic intervention, J Clin Invest 122 (2012) 4203–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Burke LK, Doslikova B, D’Agostino G, Greenwald-Yarnell M, Georgescu T, Chianese R, Martinez de Morentin PB, Ogunnowo-Bada E, Cansell C, Valencia-Torres L, et al. , Sex difference in physical activity, energy expenditure and obesity driven by a subpopulation of hypothalamic POMC neurons, Mol Metab 5 (2016) 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Burmeister MA, Ayala JE, Smouse H, Landivar-Rocha A, Brown JD, Drucker DJ, Stoffers DA, Sandoval DA, Seeley RJ, Ayala JE, The Hypothalamic Glucagon-Like Peptide 1 Receptor Is Sufficient but Not Necessary for the Regulation of Energy Balance and Glucose Homeostasis in Mice, Diabetes 66 (2017) 372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD, A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse, Endocrinology 141 (2000) 3518–3521. [DOI] [PubMed] [Google Scholar]
- [36].Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, Tenen D, Goldman M, Verstegen AM, Resch JM, McCarroll SA, et al. , A molecular census of arcuate hypothalamus and median eminence cell types, Nat Neurosci 20 (2017) 484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Campos CA, Bowen AJ, Roman CW, Palmiter RD, Encoding of danger by parabrachial CGRP neurons, Nature 555 (2018) 617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Camus J, Roussy G, Hypophysectomie et atrophie génitale. Contribution experimentale à ĺétude du syndrome adiposo-genital, Rev. Neurol. (Paris) 2 (1913). [Google Scholar]
- [39].Camus J, Roussy G, Hypophysectomie et polyurie expérimentales, Compt Rend Soc de Biol 75 (1913) 483–486. [Google Scholar]
- [40].Camus J, Roussy G, Polyurie expérimentale par lésions de la base du cerveau. La polyurie dite hypophysaire, Compt Rend Soc de Biol 75 (1913 c) 628–633. [Google Scholar]
- [41].Camus J, Roussy G, Experimental researches on the pituitary body. Diabetes Insipidus, Glycosuria and those dystrophies considered as hypophyseal in origin, Endinology 4 (1920) 507–522. [Google Scholar]
- [42].Caron A, Dungan Lemko HM, Castorena CM, Fujikawa T, Lee S, Lord CC, Ahmed N, Lee CE, Holland WL, Liu C, et al. , POMC neurons expressing leptin receptors coordinate metabolic responses to fasting via suppression of leptin levels, Elife 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cavalcanti-de-Albuquerque JP, Bober J, Zimmer MR, Dietrich MO, Regulation of substrate utilization and adiposity by Agrp neurons, Nat Commun 10 (2019) 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen SR, Chen H, Zhou JJ, Pradhan G, Sun Y, Pan HL, Li DP, Ghrelin receptors mediate ghrelin-induced excitation of agouti-related protein/neuropeptide Y but not pro-opiomelanocortin neurons, J Neurochem 142 (2017) 512–520. [DOI] [PubMed] [Google Scholar]
- [45].Chen Y, Essner RA, Kosar S, Miller OH, Lin YC, Mesgarzadeh S, Knight ZA, Sustained NPY signaling enables AgRP neurons to drive feeding, Elife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chen Y, Lin YC, Kuo TW, Knight ZA, Sensory detection of food rapidly modulates arcuate feeding circuits, Cell 160 (2015) 829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Chhabra KH, Adams JM, Jones GL, Yamashita M, Schlapschy M, Skerra A, Rubinstein M, Low MJ, Reprogramming the body weight set point by a reciprocal interaction of hypothalamic leptin sensitivity and Pomc gene expression reverts extreme obesity, Mol Metab 5 (2016) 869–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, et al. , AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons, J Clin Invest 117 (2007) 2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC, Differential sensitivity to central leptin and insulin in male and female rats, Diabetes 52 (2003) 682–687. [DOI] [PubMed] [Google Scholar]
- [50].Cone RD, Anatomy and regulation of the central melanocortin system, Nat Neurosci 8 (2005) 571–578. [DOI] [PubMed] [Google Scholar]
- [51].Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD, Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat, Neuron 24 (1999) 155–163. [DOI] [PubMed] [Google Scholar]
- [52].Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ, Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus, Nature 411 (2001) 480–484. [DOI] [PubMed] [Google Scholar]
- [53].Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, et al. , The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis, Neuron 37 (2003) 649–661. [DOI] [PubMed] [Google Scholar]
- [54].Coyle EF, Substrate utilization during exercise in active people, Am J Clin Nutr 61 (1995) 968S–979S. [DOI] [PubMed] [Google Scholar]
- [55].D’Agostino G, Lyons D, Cristiano C, Lettieri M, Olarte-Sanchez C, Burke LK, Greenwald-Yarnell M, Cansell C, Doslikova B, Georgescu T, et al. , Nucleus of the Solitary Tract Serotonin 5-HT2C Receptors Modulate Food Intake, Cell Metab 28 (2018) 619–630 e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dampney RAL, Resetting of the Baroreflex Control of Sympathetic Vasomotor Activity during Natural Behaviors: description and Conceptual Model of Central Mechanisms, Front Neurosci 11 (2017) 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dietrich MO, Horvath TL, Hypothalamic control of energy balance: insights into the role of synaptic plasticity, Trends Neurosci 36 (2013) 65–73. [DOI] [PubMed] [Google Scholar]
- [58].Dietrich MO, Zimmer MR, Bober J, Horvath TL, Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding, Cell 160 (2015) 1222–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Dirlewanger M, Di Vetta V, Giusti V, Schneiter P, Jequier E, Tappy L, Effect of moderate physical activity on plasma leptin concentration in humans, Eur J Appl Physiol Occup Physiol 79 (1999) 331–335. [DOI] [PubMed] [Google Scholar]
- [60].Douglas JA, King JA, Clayton DJ, Jackson AP, Sargeant JA, Thackray AE, Davies MJ, and Stensel DJ (2017). Acute effects of exercise on appetite, ad libitum energy intake and appetite-regulatory hormones in lean and overweight/ obese men and women. Int J Obes (Lond) 41, 1737–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Drucker DJ, Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1, Cell Metab 27 (2018) 740–756. [DOI] [PubMed] [Google Scholar]
- [62].Durstine JL, Thompson RW, Drowatzky KL, Bartoli WP, Leptin and exercise: new directions, Br J Sports Med 35 (2001) 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Dyck DJ, Leptin sensitivity in skeletal muscle is modulated by diet and exercise, Exerc Sport Sci Rev 33 (2005) 189–194. [DOI] [PubMed] [Google Scholar]
- [64].Egan B, Zierath JR, Exercise metabolism and the molecular regulation of skeletal muscle adaptation, Cell Metab 17 (2013) 162–184. [DOI] [PubMed] [Google Scholar]
- [65].Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK, Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area, Neuron 23 (1999) 775–786. [DOI] [PubMed] [Google Scholar]
- [66].Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK, Leptin activates hypothalamic CART neurons projecting to the spinal cord, Neuron 21 (1998) 1375–1385. [DOI] [PubMed] [Google Scholar]
- [67].Engstrom Ruud L, Pereira MMA, de Solis AJ, Fenselau H, Bruning JC, NPY mediates the rapid feeding and glucose metabolism regulatory functions of AgRP neurons, Nat Commun 11 (2020) 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Erdheim J, Über hypophysengangsgeschwulste und hirmcholesteatome, Sitzungsb Kais Akad Wissen Math Naturw Klin 113 (1904) 537. [Google Scholar]
- [69].Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD, Role of melanocortinergic neurons in feeding and the agouti obesity syndrome, Nature 385 (1997) 165–168. [DOI] [PubMed] [Google Scholar]
- [70].Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S, Effects of recombinant leptin therapy in a child with congenital leptin deficiency, N Engl J Med 341 (1999) 879–884. [DOI] [PubMed] [Google Scholar]
- [71].Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S, Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene, N Engl J Med 348 (2003) 1085–1095. [DOI] [PubMed] [Google Scholar]
- [72].Farooqi IS, O’Rahilly S, Monogenic obesity in humans, Annu Rev Med 56 (2005) 443–458. [DOI] [PubMed] [Google Scholar]
- [73].Feldman DE, Synaptic mechanisms for plasticity in neocortex, Annu. Rev. Neurosci 32 (2009) 33–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Flak JN, Patterson CM, Garfield AS, D’Agostino G, Goforth PB, Sutton AK, Malec PA, Wong JT, Germani M, Jones JC, et al. , Leptin-inhibited PBN neurons enhance responses to hypoglycemia in negative energy balance, Nat Neurosci 17 (2014) 1744–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Flores MB, Fernandes MF, Ropelle ER, Faria MC, Ueno M, Velloso LA, Saad MJ, Carvalheira JB, Exercise improves insulin and leptin sensitivity in hypothalamus of Wistar rats, Diabetes 55 (2006) 2554–2561. [DOI] [PubMed] [Google Scholar]
- [76].Fröhlich A, Ein fall von tumor der hypophysis cerebri ohne akromegalie, Wiener klinische Rundschau 15 (1901) 833–836 906–908. [Google Scholar]
- [77].Fu S, Meng Y, Lin S, Zhang W, He Y, Huang L, Du H, Transcriptomic responses of hypothalamus to acute exercise in type 2 diabetic Goto-Kakizaki rats, PeerJ 7 (2019) e7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Fujikawa T, Castorena CM, Lee S, Elmquist JK, The hypothalamic regulation of metabolic adaptations to exercise, J Neuroendocrinol 29 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Fujikawa T, Castorena CM, Pearson M, Kusminski CM, Ahmed N, Battiprolu PK, Kim KW, Lee S, Hill JA, Scherer PE, et al. , SF-1 expression in the hypothalamus is required for beneficial metabolic effects of exercise, Elife 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gabery S, Salinas CG, Paulsen SJ, Ahnfelt-Ronne J, Alanentalo T, Baquero AF, Buckley ST, Farkas E, Fekete C, Frederiksen KS, et al. , Semaglutide lowers body weight in rodents via distributed neural pathways, JCI Insight 5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gabriel BM, Zierath JR, The Limits of Exercise Physiology: from Performance to Health, Cell Metab 25 (2017) 1000–1011. [DOI] [PubMed] [Google Scholar]
- [82].Gao Y, Yao T, Deng Z, Sohn JW, Sun J, Huang Y, Kong X, Yu KJ, Wang RT, Chen H, et al. , TrpC5 Mediates Acute Leptin and Serotonin Effects via Pomc Neurons, Cell Rep 18 (2017) 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Garfield AS, Lam DD, Marston OJ, Przydzial MJ, Heisler LK, Role of central melanocortin pathways in energy homeostasis, Trends Endocrinol Metab 20 (2009) 203–215. [DOI] [PubMed] [Google Scholar]
- [84].Gautron L, Elmquist JK, Williams KW, Neural control of energy balance: translating circuits to therapies, Cell 161 (2015) 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Goodin SZ, Kiechler AR, Smith M, Wendt D, Strader AD, Effect of gonadectomy on AgRP-induced weight gain in rats, Am J Physiol Regul Integr Comp Physiol 295 (2008) R1747–R1753. [DOI] [PubMed] [Google Scholar]
- [86].Goodpaster BH, Sparks LM, Metabolic Flexibility in Health and Disease, Cell Metab 25 (2017) 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, et al. , Modulation of blood pressure by central melanocortinergic pathways, N Engl J Med 360 (2009) 44–52. [DOI] [PubMed] [Google Scholar]
- [88].Guelfi KJ, Donges CE, Duffield R, Beneficial effects of 12 weeks of aerobic compared with resistance exercise training on perceived appetite in previously sedentary overweight and obese men, Metabolism 62 (2013) 235–243. [DOI] [PubMed] [Google Scholar]
- [89].Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM, Physiological response to long-term peripheral and central leptin infusion in lean and obese mice, Proc Natl Acad Sci U S A 94 (1997) 8878–8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM, Weight-reducing effects of the plasma protein encoded by the obese gene, Science 269 (1995) 543–546. [DOI] [PubMed] [Google Scholar]
- [91].Hamada T, Arias EB, Cartee GD, Increased submaximal insulin-stimulated glucose uptake in mouse skeletal muscle after treadmill exercise, J Appl Physiol (1985) 101 (2006) 1368–1376. [DOI] [PubMed] [Google Scholar]
- [92].Hammel HT, Regulation of internal body temperature, Annu Rev Physiol 30 (1968) 641–710. [DOI] [PubMed] [Google Scholar]
- [93].Hancock MB, Cells of origin of hypothalamo-spinal projections in the rat, Neurosci Lett 3 (1976) 179–184. [DOI] [PubMed] [Google Scholar]
- [94].Hawley JA, Hargreaves M, Joyner MJ, Zierath JR, Integrative biology of exercise, Cell 159 (2014) 738–749. [DOI] [PubMed] [Google Scholar]
- [95].Hawley JA, Leckey JJ, Carbohydrate Dependence During Prolonged, Intense Endurance Exercise, Sports Med 45 (Suppl 1) (2015) S5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].He Y, Shu G, Yang Y, Xu P, Xia Y, Wang C, Saito K, Hinton A Jr., Yan X, Liu C, et al. , A Small Potassium Current in AgRP/NPY Neurons Regulates Feeding Behavior and Energy Metabolism, Cell Rep 17 (2016) 1807–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].He Z, Gao Y, Alhadeff AL, Castorena CM, Huang Y, Lieu L, Afrin S, Sun J, Betley JN, Guo H, et al. , Cellular and synaptic reorganization of arcuate NPY/ AgRP and POMC neurons after exercise, Mol Metab 18 (2018) 107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].He Z, Gao Y, Lieu L, Afrin S, Cao J, Michael NJ, Dong Y, Sun J, Guo H, Williams KW, Direct and indirect effects of liraglutide on hypothalamic POMC and NPY/AgRP neurons - Implications for energy balance and glucose control, Mol Metab 28 (2019) 120–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].He Z, Gao Y, Lieu L, Afrin S, Guo H, Williams KW, Acute effects of zinc and insulin on arcuate anorexigenic proopiomelanocortin neurons, Br J Pharmacol 176 (2019) 725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Hebb DO (2002). The organization of behavior: a neuropsychological theory. (Mahwah, N.J.: L. Erlbaum Associates; ). [Google Scholar]
- [101].Heisler LK, Cowley MA, Kishi T, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro J, Zigman JM, et al. , Central serotonin and melanocortin pathways regulating energy homeostasis, Ann N Y Acad Sci 994 (2003) 169–174. [DOI] [PubMed] [Google Scholar]
- [102].Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H, et al. , Activation of central melanocortin pathways by fenfluramine, Science 297 (2002) 609–611. [DOI] [PubMed] [Google Scholar]
- [103].Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z, Liu HY, Zigman JM, Balthasar N, Kishi T, et al. , Serotonin reciprocally regulates melanocortin neurons to modulate food intake, Neuron 51 (2006) 239–249. [DOI] [PubMed] [Google Scholar]
- [104].Henry FE, Sugino K, Tozer A, Branco T, Sternson SM, Cell type-specific transcriptomics of hypothalamic energy-sensing neuron responses to weight-loss, Elife 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Hetherington AW, Ranson SW, Hypothalamic lesions and adiposity in the rat, Anat. Rec 78 (1940) 149–172. [Google Scholar]
- [106].Hetherington A.W.a.R., S.W. (1942). The relation of various hypothalamic lesions to adiposity in the rat. J. Comp. Neurol 76, 475–499. [Google Scholar]
- [107].Hetherington AW, R AW, Experimental Hypothalamico-Hypophyseal Obesity in the Rat, Exp. Biol. Med 41 (1939). [Google Scholar]
- [108].Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, et al. , Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility, Cell Metab 11 (2010) 286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK, Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice, J Clin Invest 118 (2008) 1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Hillman CH, Erickson KI, Kramer AF, Be smart, exercise your heart: exercise effects on brain and cognition, Nat Rev Neurosci 9 (2008) 58–65. [DOI] [PubMed] [Google Scholar]
- [111].Huang Y, He Z, Gao Y, Lieu L, Yao T, Sun J, Liu T, Javadi C, Box M, Afrin S, et al. , PI3K is integral for the acute activity of leptin and insulin in arcuate NPY/ AgRP neurons in males, Journal of the Endocrine Society (2018) js.2018–00061–js.02018–00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Hwa JJ, Ghibaudi L, Gao J, Parker EM, Central melanocortin system modulates energy intake and expenditure of obese and lean Zucker rats, Am J Physiol Regul Integr Comp Physiol 281 (2001) R444–R451. [DOI] [PubMed] [Google Scholar]
- [113].Ingalls AM, Dickie MM, Snell GD, Obese, a new mutation in the house mouse, J Hered 41 (1950) 317–318. [DOI] [PubMed] [Google Scholar]
- [114].Biggart JH, A GL, Experimental diabetes insipdus, Path. and Bact 48 (1939) 405–425. [Google Scholar]
- [115].Jacobowitz DM, O’Donohue TL, alpha-Melanocyte stimulating hormone: immunohistochemical identification and mapping in neurons of rat brain, Proc Natl Acad Sci U S A 75 (1978) 6300–6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Jeong JH, Lee DK, Liu SM, Chua SC Jr., Schwartz GJ, Jo YH, Activation of temperature-sensitive TRPV1-like receptors in ARC POMC neurons reduces food intake, PLoS Biol. 16 (2018) e2004399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kandel ER, The molecular biology of memory storage: a dialogue between genes and synapses, Science 294 (2001) 1030–1038. [DOI] [PubMed] [Google Scholar]
- [118].Kanter I, Kalisky T, Single cell transcriptomics: methods and applications, Front Oncol 5 (2015) 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Kessels HW, Malinow R, Synaptic AMPA receptor plasticity and behavior, Neuron 61 (2009) 340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].King NA, Blundell JE, High-fat foods overcome the energy expenditure induced by high-intensity cycling or running, Eur J Clin Nutr 49 (1995) 114–123. [PubMed] [Google Scholar]
- [121].King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE, Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss, Int J Obes (Lond) 32 (2008) 177–184. [DOI] [PubMed] [Google Scholar]
- [122].King NA, Snell L, Smith RD, Blundell JE, Effects of short-term exercise on appetite responses in unrestrained females, Eur J Clin Nutr 50 (1996) 663–667. [PubMed] [Google Scholar]
- [123].Kirchgessner AL, Sclafani A, PVN-hindbrain pathway involved in the hypothalamic hyperphagia-obesity syndrome, Physiol Behav 42 (1988) 517–528. [DOI] [PubMed] [Google Scholar]
- [124].Kirchgessner AL, Sclafani A, Nilaver G, Histochemical identification of a PVN-hindbrain feeding pathway, Physiol Behav 42 (1988) 529–543. [DOI] [PubMed] [Google Scholar]
- [125].Kirchheim HR, Systemic arterial baroreceptor reflexes, Physiol Rev 56 (1976) 100–177. [DOI] [PubMed] [Google Scholar]
- [126].Kohno D, Gao HZ, Muroya S, Kikuyama S, Yada T, Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin, Diabetes 52 (2003) 948–956. [DOI] [PubMed] [Google Scholar]
- [127].Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, et al. , Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production, Cell Metab. 5 (2007) 438–449. [DOI] [PubMed] [Google Scholar]
- [128].Kow LM, Montgomery MO, Pfaff DW, Effects of spinal cord transections on lordosis reflex in female rats, Brain Res 123 (1977) 75–88. [DOI] [PubMed] [Google Scholar]
- [129].Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB, Rapid, reversible activation of AgRP neurons drives feeding behavior in mice, J. Clin. Invest 121 (2011) 1424–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Krashes MJ, Shah BP, Koda S, Lowell BB, Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP, Cell Metab. 18 (2013) 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Kuo JJ, da Silva AA, Tallam LS, Hall JE, Role of adrenergic activity in pressor responses to chronic melanocortin receptor activation, Hypertension 43 (2004) 370–375. [DOI] [PubMed] [Google Scholar]
- [132].Lam BYH, Cimino I, Polex-Wolf J, Nicole Kohnke S, Rimmington D, Iyemere V, Heeley N, Cossetti C, Schulte R, Saraiva LR, et al. , Heterogeneity of hypothalamic pro-opiomelanocortin-expressing neurons revealed by single-cell RNA sequencing, Mol Metab 6 (2017) 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Leibel RL, Is obesity due to a heritable difference in ‘set point’ for adiposity? West J Med 153 (1990) 429–431. [PMC free article] [PubMed] [Google Scholar]
- [134].Lerner TN, Shilyansky C, Davidson TJ, Evans KE, Beier KT, Zalocusky KA, Crow AK, Malenka RC, Luo L, Tomer R, et al. , Intact-Brain Analyses Reveal Distinct Information Carried by SNc Dopamine Subcircuits, Cell 162 (2015) 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, Ding JB, Yang Z, Sabatini BL, Lowell BB, Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone, Neuron 73 (2012) 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Luquet S, Perez FA, Hnasko TS, Palmiter RD, NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates, Science 310 (2005) 683–685. [DOI] [PubMed] [Google Scholar]
- [137].Malenka RC, Synaptic plasticity in the hippocampus: LTP and LTD, Cell 78 (1994) 535–538. [DOI] [PubMed] [Google Scholar]
- [138].Mandelblat-Cerf Y, Ramesh RN, Burgess CR, Patella P, Yang Z, Lowell BB, Andermann ML, Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales, Elife 4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Manders RJ, Van Dijk JW, van Loon LJ, Low-intensity exercise reduces the prevalence of hyperglycemia in type 2 diabetes, Med Sci Sports Exerc 42 (2010) 219–225. [DOI] [PubMed] [Google Scholar]
- [140].Mani BK, Castorena CM, Osborne-Lawrence S, Vijayaraghavan P, Metzger NP, Elmquist JK, Zigman JM, Ghrelin mediates exercise endurance and the feeding response post-exercise, Mol Metab 9 (2018) 114–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Marty N, Dallaporta M, and Thorens B (2007). Brain glucose sensing, counterregulation, and energy homeostasis. Physiology (Bethesda) 22, 241–251. [DOI] [PubMed] [Google Scholar]
- [142].Meek TH, Nelson JT, Matsen ME, Dorfman MD, Guyenet SJ, Damian V, Allison MB, Scarlett JM, Nguyen HT, Thaler JP, et al. , Functional identification of a neurocircuit regulating blood glucose, Proc Natl Acad Sci U S A 113 (2016) E2073–E2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H, Effect of physical exercise on sensitivity and responsiveness to insulin in humans, Am J Physiol 254 (1988) E248–E259. [DOI] [PubMed] [Google Scholar]
- [144].Morgulis S, Fasting and Undernutrition, E. P. Dutton & company, New York, 1923. [Google Scholar]
- [145].Morrison SF, Nakamura K, Central neural pathways for thermoregulation, Front Biosci (Landmark Ed) 16 (2011) 74–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD, Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain, Mol Endocrinol 8 (1994) 1298–1308. [DOI] [PubMed] [Google Scholar]
- [147].Mountjoy KG, Robbins LS, Mortrud MT, Cone RD, The cloning of a family of genes that encode the melanocortin receptors, Science 257 (1992) 1248–1251. [DOI] [PubMed] [Google Scholar]
- [148].Myers MG Jr., Olson DP, Central nervous system control of metabolism, Nature 491 (2012) 357–363. [DOI] [PubMed] [Google Scholar]
- [149].Newton AJ, Hess S, Paeger L, Vogt MC, Lascano Fleming, Nillni J, Bruning EA, Kloppenburg JC, Xu AW, AgRP innervation onto POMC neurons increases with age and is accelerated with chronic high-fat feeding in male mice, Endocrinology 154 (2013) 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Ni XP, Butler AA, Cone RD, Humphreys MH, Central receptors mediating the cardiovascular actions of melanocyte stimulating hormones, J Hypertens 24 (2006) 2239–2246. [DOI] [PubMed] [Google Scholar]
- [151].Nindl BC, Kraemer WJ, Arciero PJ, Samatallee N, Leone CD, Mayo MF, Hafeman DL, Leptin concentrations experience a delayed reduction after resistance exercise in men, Med Sci Sports Exerc 34 (2002) 608–613. [DOI] [PubMed] [Google Scholar]
- [152].Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, et al. , The central melanocortin system directly controls peripheral lipid metabolism, J Clin Invest 117 (2007) 3475–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Nohara K, Zhang Y, Waraich RS, Laque A, Tiano JP, Tong J, Munzberg H, Mauvais-Jarvis F, Early-life exposure to testosterone programs the hypothalamic melanocortin system, Endocrinology 152 (2011) 1661–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Oberlin DJ, Mikus CR, Kearney ML, Hinton PS, Manrique C, Leidy HJ, Kanaley JA, Rector RS, Thyfault JP, One bout of exercise alters free-living postprandial glycemia in type 2 diabetes, Med Sci Sports Exerc 46 (2014) 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Olofsson LE, Pierce AA, Xu AW, Functional requirement of AgRP and NPY neurons in ovarian cycle-dependent regulation of food intake, Proc Natl Acad Sci U S A 106 (2009) 15932–15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Owen OE, Smalley KJ, D’Alessio DA, Mozzoli MA, Dawson EK, Protein, fat, and carbohydrate requirements during starvation: anaplerosis and cataplerosis, Am J Clin Nutr 68 (1998) 12–34. [DOI] [PubMed] [Google Scholar]
- [157].Parr EB, Heilbronn LK, Hawley JA, A Time to Eat and a Time to Exercise, Exerc Sport Sci Rev 48 (2020) 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Pil-Byung C, Shin-Hwan Y, Il-Gyu K, Gwang-Suk H, Jae-Hyun Y, Han-Joon L, Sung-Eun K, Yong-Seok J, Effects of exercise program on appetite-regulating hormones, inflammatory mediators, lipid profiles, and body composition in healthy men, J Sports Med Phys Fitness 51 (2011) 654–663. [PubMed] [Google Scholar]
- [159].Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL, Rapid rewiring of arcuate nucleus feeding circuits by leptin, Science 304 (2004) 110–115. [DOI] [PubMed] [Google Scholar]
- [160].Ratner C, He Z, Grunddal KV, Skov LJ, Hartmann B, Zhang F, Feuchtinger A, Bjerregaard A, Christoffersen C, Tschop MH, et al. (2019). Long Acting Neurotensin Synergizes with Liraglutide to Reverse Obesity Through a Melanocortin-Dependent Pathway. Diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Ronnekleiv OK, Fang Y, Zhang C, Nestor CC, Mao P, Kelly MJ, Research resource: gene profiling of G protein-coupled receptors in the arcuate nucleus of the female, Mol Endocrinol 28 (2014) 1362–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Schwartz MW, Porte D Jr., Diabetes, obesity, and the brain, Science 307 (2005) 375–379. [DOI] [PubMed] [Google Scholar]
- [163].Secher A, Jelsing J, Baquero AF, Hecksher-Sorensen J, Cowley MA, Dalboge LS, Hansen G, Grove KL, Pyke C, Raun K, et al. , The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss, J Clin Invest 124 (2014) 4473–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Shi H, Seeley RJ, Clegg DJ, Sexual differences in the control of energy homeostasis, Front Neuroendocrinol 30 (2009) 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Shi H, Sorrell JE, Clegg DJ, Woods SC, Seeley RJ, The roles of leptin receptors on POMC neurons in the regulation of sex-specific energy homeostasis, Physiol Behav 100 (2010) 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Shi H, Strader AD, Sorrell JE, Chambers JB, Woods SC, Seeley RJ, Sexually different actions of leptin in proopiomelanocortin neurons to regulate glucose homeostasis, Am J Physiol Endocrinol Metab 294 (2008) E630–E639. [DOI] [PubMed] [Google Scholar]
- [167].Smith PE, The disabilities caused by hypophysectomy and their repair: the tuberal (hypothalamic) syndrome in the rat, JAMA 88 (1927) 158–161. [Google Scholar]
- [168].Smith RL, Soeters MR, Wust RCI, Houtkooper RH, Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease, Endocr Rev 39 (2018) 489–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR, Behavioral M, Lorcaserin for O, et al. , Multicenter, placebo-controlled trial of lorcaserin for weight management, N Engl J Med 363 (2010) 245–256. [DOI] [PubMed] [Google Scholar]
- [170].Sohn JW, Network of hypothalamic neurons that control appetite, BMB Rep 48 (2015) 229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Sohn JW, Elmquist JK, Williams KW, Neuronal circuits that regulate feeding behavior and metabolism, Trends Neurosci 36 (2013) 504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK, Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons, Cell 152 (2013) 612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Sohn JW, Williams KW, Functional heterogeneity of arcuate nucleus pro-opiomelanocortin neurons: implications for diverging melanocortin pathways, Mol Neurobiol 45 (2012) 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK, Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels, Neuron 71 (2011) 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Solomon TP, Sistrun SN, Krishnan RK, Del Aguila LF, Marchetti CM, O’Carroll SM, O’Leary VB, Kirwan JP, Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults, J Appl Physiol (1985) 104 (2008) 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Speakman JR, Levitsky DA, Allison DB, Bray MS, de Castro JM, Clegg DJ, Clapham JC, Dulloo AG, Gruer L, Haw S, et al. , Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity, Dis Model Mech 4 (2011) 733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Steculorum SM, Paeger L, Bremser S, Evers N, Hinze Y, Idzko M, Kloppenburg P, Bruning JC, Hypothalamic UDP Increases in Obesity and Promotes Feeding via P2Y6-Dependent Activation of AgRP Neurons, Cell 162 (2015) 1404–1417. [DOI] [PubMed] [Google Scholar]
- [178].Steculorum SM, Ruud J, Karakasilioti I, Backes H, Engstrom Ruud L, Timper K, Hess ME, Tsaousidou E, Mauer J, Vogt MC, et al. , AgRP Neurons Control Systemic Insulin Sensitivity via Myostatin Expression in Brown Adipose Tissue, Cell 165 (2016) 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Su Z, Alhadeff AL, Betley JN, Nutritive, Post-ingestive Signals Are the Primary Regulators of AgRP Neuron Activity, Cell Rep 21 (2017) 2724–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Sun J, Gao Y, Yao T, Huang Y, He Z, Kong X, Yu KJ, Wang RT, Guo H, Yan J, et al. , Adiponectin potentiates the acute effects of leptin in arcuate Pomc neurons, Mol Metab 5 (2016) 882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Suyama S, Maekawa F, Maejima Y, Kubota N, Kadowaki T, Yada T, Glucose level determines excitatory or inhibitory effects of adiponectin on arcuate POMC neuron activity and feeding, Sci Rep 6 (2016) 30796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Swanson LW, Kuypers HG, A direct projection from the ventromedial nucleus and retrochiasmatic area of the hypothalamus to the medulla and spinal cord of the rat, Neurosci Lett 17 (1980) 307–312. [DOI] [PubMed] [Google Scholar]
- [183].Takahashi KA, Cone RD, Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons, Endocrinology 146 (2005) 1043–1047. [DOI] [PubMed] [Google Scholar]
- [184].Thyfault JP, Wright DC, Weighing” the effects of exercise and intrinsic aerobic capacity: are there beneficial effects independent of changes in weight? Appl Physiol Nutr Metab 41 (2016) 911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Timper K, Bruning JC, Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity, Dis Model Mech 10 (2017) 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB, Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance, Nat Neurosci 11 (2008) 998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Uner AG, Kecik O, Quaresma PGF, De Araujo TM, Lee H, Li W, Kim HJ, Chung M, Bjorbaek C, Kim YB, Role of POMC and AgRP neuronal activities on glycaemia in mice, Sci Rep 9 (2019) 13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Urban JH, Bauer-Dantoin AC, Levine JE, Neuropeptide Y gene expression in the arcuate nucleus: sexual dimorphism and modulation by testosterone, Endocrinology 132 (1993) 139–145. [DOI] [PubMed] [Google Scholar]
- [189].van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, et al. , Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion, Endocrinology 149 (2008) 1773–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D, Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus, Nat Neurosci 7 (2004) 493–494. [DOI] [PubMed] [Google Scholar]
- [191].Van Dijk JW, Manders RJ, Canfora EE, Mechelen WV, Hartgens F, Stehouwer CD, Van Loon LJ, Exercise and 24-h glycemic control: equal effects for all type 2 diabetes patients? Med Sci Sports Exerc 45 (2013) 628–635. [DOI] [PubMed] [Google Scholar]
- [192].Wang D, He X, Zhao Z, Feng Q, Lin R, Sun Y, Ding T, Xu F, Luo M, Zhan C, Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons, Front Neuroanat 9 (2015) 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Watts AG, Donovan CM, Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation, Front Neuroendocrinol 31 (2010) 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Wei W, Pham K, Gammons JW, Sutherland D, Liu Y, Smith A, Kaczorowski CC, O’Connell KMS, Diet composition, not calorie intake, rapidly alters intrinsic excitability of hypothalamic AgRP/NPY neurons in mice, Sci Rep 5 (2015) 16810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Williams KW, Elmquist JK, From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior, Nat Neurosci 15 (2012) 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Williams KW, Liu T, Kong X, Fukuda M, Deng Y, Berglund ED, Deng Z, Gao Y, Liu T, Sohn JW, et al. , Xbp1s in Pomc neurons connects ER stress with energy balance and glucose homeostasis, Cell Metab 20 (2014) 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK, Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons, J Neurosci 30 (2010) 2472–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Williams KW, Scott MM, Elmquist JK, From observation to experimentation: leptin action in the mediobasal hypothalamus, Am J Clin Nutr 89 (2009) 985S–990S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Wu Q, Boyle MP, Palmiter RD, Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation, Cell 137 (2009) 1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Wu Q, Clark MS, Palmiter RD, Deciphering a neuronal circuit that mediates appetite, Nature 483 (2012) 594–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Wu Q, Howell MP, Cowley MA, Palmiter RD, Starvation after AgRP neuron ablation is independent of melanocortin signaling, Proc Natl Acad Sci U S A 105 (2008) 2687–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Wu Q, Palmiter RD, GABAergic signaling by AgRP neurons prevents anorexia via a melanocortin-independent mechanism, Eur J Pharmacol 660 (2011) 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Xu J, Bartolome CL, Low CS, Yi X, Chien CH, Wang P, Kong D, Genetic identification of leptin neural circuits in energy and glucose homeostases, Nature 556 (2018) 505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, et al. , 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis, Neuron 60 (2008) 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Xu Y, Jones JE, Lauzon DA, Anderson JG, Balthasar N, Heisler LK, Zinn AR, Lowell BB, Elmquist JK, A serotonin and melanocortin circuit mediates D-fen-fluramine anorexia, The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 30 (2010) 14630–14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Yang Y, Atasoy D, Su HH, Sternson SM, Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop, Cell 146 (2011) 992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O’Rahilly S, A frameshift mutation in MC4R associated with dominantly inherited human obesity, Nat Genet 20 (1998) 111–112. [DOI] [PubMed] [Google Scholar]
- [208].Zaccaria M, Ermolao A, Roi GS, Englaro P, Tegon G, Varnier M, Leptin reduction after endurance races differing in duration and energy expenditure, Eur J Appl Physiol 87 (2002) 108–111. [DOI] [PubMed] [Google Scholar]
- [209].Zeltser LM, Distinct Hypothalamic and Brainstem Contributions to Lorcaserin Action, Cell Metab 28 (2018) 533–534. [DOI] [PubMed] [Google Scholar]
- [210].Zeltser LM, Seeley RJ, Tschop M, Synaptic plasticity in circuits regulating energy balance, Nat Neurosci. (2012). [DOI] [PubMed] [Google Scholar]
- [211].Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, Luo M, Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively, The Journal of neuroscience: the official journal of the Society for Neuroscience 33 (2013) 3624–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM, Positional cloning of the mouse obese gene and its human homologue, Nature 372 (1994) 425–432. [DOI] [PubMed] [Google Scholar]
- [213].Zheng H, Patterson LM, Rhodes CJ, Louis GW, Skibicka KP, Grill HJ, Myers MG Jr., Berthoud HR, A potential role for hypothalamomedullary POMC projections in leptin-induced suppression of food intake, Am J Physiol Regul Integr Comp Physiol 298 (2010) R720–R728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Zsombok A, Smith BN, Plasticity of central autonomic neural circuits in diabetes, Biochim Biophys Acta 1792 (2009) 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]


