Abstract
Objective:
To examine factors associated with PET scan use in the preoperative evaluation of patients diagnosed with bladder cancer.
Methods:
Using SEER Medicare data, we identified bladder cancer patients who underwent radical cystectomy from 2006–2011 (n=4138). The primary outcome was PET scan use within 6 months before surgery. To examine predictors of PET scan use, we fit a mixed logit model with health service area as a random effect to account for patients nested within health service areas. We also calculated the adjusted probability of use over time and examined variation among the highest volume surgeons.
Results:
Among the 4138 patients, 406 (10%) received a preoperative PET scan. The adjusted probability of a patient undergoing a PET scan increased from 0.04 in 2004 to 0.10 in 2011 (p < 0.001). Amongst the 78 highest volume surgeons, there was significant variation in PET scan use (p < 0.001). Patients with non-urothelial histology, measurement of alkaline phosphatase levels, and receipt of neoadjuvant chemotherapy were more likely to receive PET scan (all p <0.05).
Conclusion:
Use of PET prior to radical cystectomy doubled over a 5-year period, suggesting its increased use in patients with muscle-invasive bladder cancer, particularly those with high risk disease. Whether its use is warranted and improves patient outcomes is not clear and requires further studies.
Keywords: SEER, Medicare, Bladder cancer, Neoplasm Staging, Positron-Emission Tomography, Health Services Research
Introduction
The standard of care for patients diagnosed with non-metastatic, muscle-invasive bladder cancer is radical cystectomy. This surgery causes significant morbidity and is typically not performed in patients who have metastatic disease. As part of the preoperative staging evaluation, patients typically receive either a computed tomography scan or magnetic resonance imaging.
While computed tomography scan and magnetic resonance imaging are the most common imaging modalities used for preoperative staging, there are some concerns with their accuracy in detection of metastatic disease, leading some clinicians to advocate for positron emission tomography (PET) scans. PET scan relies on functional features (e.g., as radioactive tracer uptake) as opposed to morphological features for computed tomography scan or magnetic resonance imaging (e.g., lymph node size). PET tracer is a marker of increased glucose uptake and, since malignant neoplasms and metastasis have increased glucose utilization, PET scans may offer improved micro-metastatic disease detection.(Swinnen et al., 2010) The data for PET scan use are mixed. Some studies show improved detection of nodal disease with PET scan compared with computed tomography. (Brunocilla et al., 2014; Hitier‐Berthault et al., n.d.; Kibel et al., 2009; Soubra et al., 2016) Yet others show no difference in identifying occult metastasis between the two modalities and argue that the diagnostic yield is not worth the increased cost. (Aljabery et al., 2015; Ha, Koo, & Kim, 2018; Jensen et al., 2011; Maurer et al., 2012; Pichler et al., 2017) Currently, the rate of PET scan use as part of preoperative evaluation in cystectomy patients is unknown. Further, it is unclear what patient specific factors are associated with PET scan use.
For these reasons, we sought to assess utilization of abdominopelvic PET scan prior to radical cystectomy and evaluate factors associated with its use. Understanding the incidence and determinates of PET scan use may help refine preoperative imaging guidelines in this patient population.
Materials and Methods
Study Population
Using SEER-Medicare data, we identified patients who underwent radical cystectomy (International Classification of Diseases, Ninth Revision [ICD-9] codes 57.7, 57.71, 57.79, 68.8) for the treatment of bladder cancer between 2006 and 2011(Turner, Yabes, Davies, Heron, & Jacobs, 2017). We identified the date of surgery using the Medicare Provider Analysis and Review (MEDPAR) files.(Medicare, Baltimore, & Usa, 2017) We included patients between the ages of 66 and 99 who were continuously enrolled in Medicare Parts A and B during the 12 months before and one month after cystectomy.(Turner et al., 2017) We excluded patients participating in a health maintenance organization or Medicare Part C to guarantee all health care was captured. We also excluded patients diagnosed at autopsy or death and those with another diagnosis of a nonurothelial malignancy, including prostate cancer, prior to cystectomy. We included those with a concomitant diagnosis of prostate cancer at the time of cystectomy.(Turner et al., 2017)
Outcomes
The primary outcome was completion of a PET scan within 6 months prior to radical cystectomy. PET scan use was identified in the Medicare outpatient and carrier files by using Healthcare Common Procedure Coding System (HCPCS) codes 78811, 78812, 78813, 78814, 78815, 78816, and G0235.
We obtained patient demographic and pathological information using the SEER Patient Entitlement and Diagnosis Summary File (PEDSF).(“SEER-Medicare Linked Database,” n.d.) Demographics included age, sex, race, marital status, geographic region, and local census tract information (i.e., percentage of ZIP code population with at least a high school education, population of the county of residence, and median household income within the ZIP code). We categorized geographic region (northeast, south, central, west) based on the SEER region at the time of the bladder cancer diagnosis that most closely preceded surgery.(Turner et al., 2017) Pathologic information included histology type and tumor grade and stage. Histology type was categorized based on the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) histology codes and tumor stage was based on the reported SEER Collaborative Stage.
Several preoperative factors were obtained from Medicare outpatient and carrier files, including assessment of alkaline phosphatase level, receipt of neoadjuvant chemotherapy, and type of urinary diversion (Supplemental table).(“SEER-Medicare Linked Database,” n.d.) We determined patient comorbidities by evaluating diagnoses from Medicare claims for the 12 months prior to cystectomy.(Klabunde, Potosky, Legler, & Warren, 2000) The surgeon and provider specialty were also identified using Medicare outpatient and carrier files.
Statistical Analysis
Demographic, socioeconomic, and pathologic characteristics of the study population were compared between groups (PET scan use: yes/no) using chi-square tests. Then we analyzed predictors of PET scan use using a mixed logit model with health service area as a random effect to account for patients nested within health service areas. Heath service area for each patient was identified in the PEDSF. Covariates included age, sex, race, comorbidity, marital status, education level and median income in ZIP code of residence, county of residence population, geographic region, grade, stage, measurement of an alkaline phosphatase level, and year of surgery. Variables used in the adjusted model included age, race, comorbidity, and those that had p < 0.1 in the univariable analyses. Next, a cohort of the highest volume surgeons, defined as those who performed 10 or more cystectomies over the study period, was identified. We assessed the proportion of each surgeon’s patients who underwent preoperative PET scan. We fit a multivariable mixed logit model, with surgeon as a random effect, to assess the variance in the proportion of patients undergoing PET scan explained at the surgeon level. Variables used in the adjusted model included those identified as independent predictors of PET scan use, namely receipt of neoadjuvant chemotherapy, year of cystectomy, and tumor histology.
Statistical analyses were performed using R (version 13.2)(“R,” n.d.) using the packages dpylr(Wickham, 2009) for data manipulation, compareGroups for descriptive tables, and ggplot2 for graphics.(Building Bivariate Tables, n.d.) Statistical significance was defined as p < 0.05. The University of Pittsburgh institutional review board reviewed the study and deemed it exempt from reviewer protocol.
Results
Among the 4138 patients who underwent radical cystectomy during the study period, 406 (10%) completed a preoperative PET scan. The demographic, clinical, and pathological information for the cohort is summarized in Table 1. Those who received PET scans more frequently received neoadjuvant chemotherapy, had a preoperative alkaline phosphatase level, and underwent a scan in the latter years of the study (p <0.001).
Table 1.
Demographic, socioeconomic, and pathological characteristics of the study population.
| Characteristics | No PET Scan (N = 3732) | PET Scan (N = 406) | p value* |
|---|---|---|---|
| Age at cystectomy (%) | 0.01 | ||
| 66–69 | 662 (18) | 87 (21) | |
| 70–74 | 1052 (28) | 114 (28) | |
| 75–79 | 983 (26) | 121 (30) | |
| 80 or more | 1033 (28) | 84 (21) | |
| Sex | 0.64 | ||
| Male | 2802 (75) | 300 (74) | |
| Female | 930 (25) | 106 (26) | |
| Race (%) | 0.89 | ||
| White | 3657 (88) | 357 (88) | |
| Black | 156 (4) | 17 (4) | |
| Hispanic | 170 (4) | 15 (4) | |
| Other | 155 (4) | 17 (5) | |
| Comorbidity (%) | 0.98 | ||
| 0 | 1553 (42) | 168 (41) | |
| 1 | 1039 (28) | 110 (27) | |
| 2 | 590 (16) | 66 (16) | |
| 3 or more | 547 (15) | 62 (15) | |
| Marital status (%) | 0.64 | ||
| Married | 2454 (66) | 275 (68) | |
| Not married | 1121 (30) | 117 (29) | |
| Unknown | 157 (4) | 14 (3) | |
| Education level in ZIP code of residence (%) | 0.99 | ||
| Low (≤75% with high school education) | 398 (11) | 43 (11) | |
| High (>75% with high school education) | 3259 (89) | 352 (89) | |
| County of residence population (%) | 0.02 | ||
| 1,000,000 or more | 2051 (55) | 242 (60) | |
| 250,000–999,000 | 629 (17) | 77 (19) | |
| Less than 250,000 | 1052 (28) | 87 (21) | |
| Median household income in ZIP code of residence(%) | 0.002 | ||
| Less than $50,000 | 1310 (36) | 118 (30) | |
| $50,000–$70,000 | 1174 (32) | 116 (29) | |
| More than $70,000 | 1169 (32) | 161 (41) | |
| U.S. geographic region (%) | 0.01 | ||
| Northeast | 873 (23) | 92 (23) | |
| South | 872 (23) | 84 (21) | |
| Central | 561 (15) | 42 (10) | |
| West | 1426 (38) | 188 (46) | |
| Preoperative alkaline phosphatase level (%) | <0.001 | ||
| No | 638 (17) | 32 (8) | |
| Yes | 3094 (83) | 374 (92) | |
| Neoadjuvant chemotherapy (%) | <0.001 | ||
| No | 3235 (87) | 261 (64) | |
| Yes | 497 (13) | 145 (36) | |
| Type of Urinary Diversion (%) | 0.52 | ||
| Conduit | 2719 (73) | 285 (70) | |
| Continent diversion | 561 (15) | 67 (17) | |
| Other/Unknown | 452 (12) | 57 (13) | |
| Histology (%) | <0.001 | ||
| Urothelial carcinoma | 3471 (93) | 355 (87) | |
| Squamous cell carcinoma | 103 (3) | 16 (4) | |
| Other | 158 (4) | 35 (9) | |
| Grade (%) | 0.13 | ||
| Well/moderately differentiated | 358 (11) | 27 (7) | |
| Poorly/undifferentiated | 3125 (84) | 348 (86) | |
| Unknown | 249 (7) | 31 (8) | |
| T stage (%) | 0.02 | ||
| T1 or less | 1092 (31) | 96 (25) | |
| T2 | 1134 (33) | 152 (39) | |
| T3 | 815 (23) | 91 (24) | |
| T4 | 440 (13) | 48 (12) | |
| Year of cystectomy (%) | <0.001 | ||
| 2006 | 687 (18) | 34 (8) | |
| 2007 | 668 (18) | 47 (12) | |
| 2008 | 681 (18) | 79 (20) | |
| 2009 | 596 (16) | 65 (16) | |
| 2010 | 547 (15) | 94 (23) | |
| 2011 | 553 (15) | 87 (21) |
Abbreviations: PET, positron emission tomography; SD, standard deviation;. Percentages might not sum to 100 because of rounding.
P values determined using Chi-square tests.
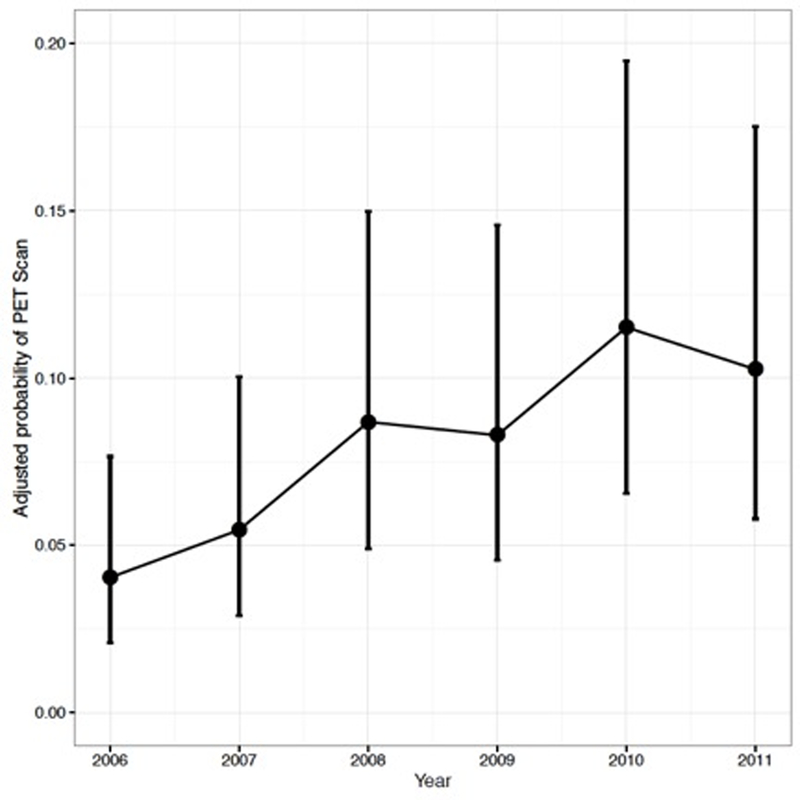
The univariable and multivariable analyses examining predictors of PET scan use are summarized in Table 2. After adjusting for covariates, preoperative measurement of an alkaline phosphatase level (adjusted odds ratio [aOR] 1.55, 95% confidence interval [CI] 1.05–2.30) and receipt of neoadjuvant chemotherapy (aOR 3.21; 95% [CI] 2.48–4.17) were independently associated with PET scan use. Compared to patients with urothelial carcinoma, those with squamous cell carcinoma (aOR 1.97; 95% [CI] 1.10–3.51) and other tumor histology (aOR 2.26; 95% [CI] 1.43–3.56) were more likely to have a PET scan. Additionally, the adjusted probability of undergoing a PET scan increased over time, from 0.04 in 2006 to 0.10 in 2011 (p<0.001, multivariable mixed logit model; Figure 1).
Table 2.
Unadjusted and adjusted estimated effects of each predictor on the use of preoperative positron emission tomography (PET).
| Predictor | Univariable analysis | Multivariable analysis* | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p value** | Adjusted Odds Ratio (95% CI) | p value | |
| Age at cystectomy | 0.01 | 0.09 | ||
| 66–69 | reference | reference | ||
| 70–74 | 0.82 (0.61–1.11) | 0.83 (0.60–1.16) | ||
| 75–79 | 0.94 (0.70–1.26) | 1.09 (0.78–1.52) | ||
| 80 or more | 0.62 (0.45–0.85) | 0.76 (0.53–1.08) | ||
| Race | 0.89 | 0.78 | ||
| White | reference | reference | ||
| Black | 1.14 (0.66–1.86) | 1.18 (0.65–2.14) | ||
| Hispanic | 0.90 (0.50–1.50) | 0.77 (0.41–1.45) | ||
| Other | 1.15 (0.66–1.87) | 1.09 (0.62–1.91) | ||
| Comorbidity | 0.98 | 0.97 | ||
| 0 | reference | reference | ||
| 1 | 0.98 (0.76–1.26) | 0.95 (0.72–1.26) | ||
| 2 | 1.04 (0.76–1.39) | 1.03 (0.74–1.43) | ||
| 3 or more | 1.05 (0.77–1.42) | 0.96 (0.67–1.36) | ||
| County of residence population | 0.02 | 0.65 | ||
| 1,000,000 or more | reference | reference | ||
| 250,000–999,000 | 1.04 (0.79–1.36) | 1.14 (0.73–1.79) | ||
| Less than 250,000 | 0.70 (0.54–0.90) | 0.93 (0.59–1.47) | ||
| Median household income in ZIP code of incidence | 0.002 | 0.12 | ||
| Less than $50,000 | reference | reference | ||
| $50,000–$40,000 | 1.10 (0.84–1.43) | 1.03 (0.74–1.44) | ||
| More than $70,000 | 1.53 (1.19–1.97) | 1.37 (0.96–1.95) | ||
| U.S. geographic region | 0.01 | 0.12 | ||
| Northeast | reference | reference | ||
| South | 0.91 (0.67–1.25) | 1.11 (0.60–2.04) | ||
| Central | 0.71 (0.48–1.03) | 0.89 (0.43–1.85) | ||
| West | 1.25 (0.96–1.63) | 1.64 (0.93–2.88) | ||
| Alkaline phosphatase | <0.001 | 0.03 | ||
| No | reference | reference | ||
| Yes | 3.62 (2.89–4.52) | 1.55 (1.05–2.30) | ||
| Neoadjuvant chemotherapy | <0.001 | <0.001 | ||
| No | reference | reference | ||
| Yes | 3.62 (2.89–4.52) | 3.21 (2.48–4.17) | ||
| Histology | <0.001 | <0.001 | ||
| Urothelial | reference | reference | ||
| Squamous cell | 1.53 (0.86–2.55) | 1.97 (1.10–3.51) | ||
| Other | 2.17 (1.46–3.15) | 2.26 (1.43–3.56) | ||
| T stage | 0.02 | 0.14 | ||
| T1 or less | reference | reference | ||
| T2 | 1.52 (1.17–2.00) | 1.40 (1.04–1.87) | ||
| T3 | 1.27 (0.94–1.72) | 1.27 (0.92–1.76) | ||
| T4 | 1.24 (0.86–1.78) | 1.13 (0.76–1.68) | ||
| Year of cystectomy | <0.001 | <0.001 | ||
| 2006 | reference | reference | ||
| 2007 | 1.42 (0.90–2.25) | 1.37 (0.83–2.26) | ||
| 2008 | 2.34 (1.55–3.59) | 2.26 (1.43–3.57) | ||
| 2009 | 2.20 (1.44–3.41) | 2.15 (1.34–3.44) | ||
| 2010 | 3.46 (2.32–5.27) | 3.09 (1.97–4.86) | ||
| 2011 | 3.17 (2.12–4.84) | 2.71 (1.72–4.28) | ||
Multivariable analysis incorporated a mixed logit model with health service area as a random effect to account for patients nested within health service areas. Estimates are adjusted for age, race, comorbidity, county of residence population, median income in ZIP code of residence, geographic region, measurement of an alkaline phosphatase level, year of cystectomy, receipt of neoadjuvant chemotherapy, histology, and stage.
Overall P values determined using partial likelihood-ratio tests.
Figure 1.
Adjusted probability of preoperative positron emission tomography (PET) use over time.
Abbreviations: PET, positron emission tomography
The adjusted probability of completing a PET scan increased from 0.04 in 2006 to 0.10 in 2011 (p <0.001, multivariable mixed logit model). Estimates are adjusted for age, race, comorbidity, county of residence population, median income in ZIP code of residence, geographic region, measurement of an alkaline phosphatase level, receipt of neoadjuvant chemotherapy, histology, and stage.
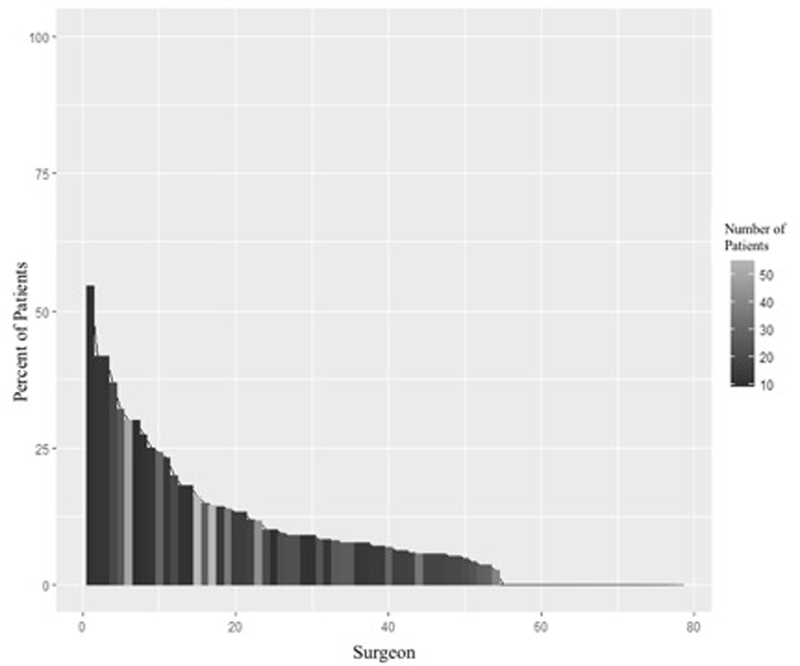
A total of 78 surgeons performed 10 or more radical cystectomies during the study period. The proportion of each provider’s patients who completed a preoperative PET scan is shown in Figure 2. After adjusting for alkaline phosphatase measurement, receipt of neoadjuvant chemotherapy, tumor histology, and year of surgery, there was significant variability in PET scan use at the provider level (p < 0.001).
Figure 2.
Variability of positron emission tomography (PET) use among highest volume surgeons.
Abbreviations: PET, positron emission tomography
Distribution of patients who underwent PET scan among the 78 high volume surgeons (>10 cystectomies). Surgeon IDs are sorted from maximum to minimum percentage of patients with a PET scan. The maximum percentage is 55%. There were 24 surgeons (31%) who did not have any patients with a PET scan.
Discussion
In this large population-based cohort, the adjusted probability of PET scan use prior to radical cystectomy increased from 0.04 in 2006 to 0.10 in 2011. Clinical factors independently associated with PET scan use include preoperative alkaline phosphatase, neoadjuvant chemotherapy, and tumor histology. After adjusting for these factors, there was also significant surgeon-level variation in the use of PET scans.
It makes sense that the factors associated with increased PET scan use are markers of high-risk disease. Patients with elevated alkaline phosphatase are at increased risk for bone metastases. (Chakraborty, Bhattacharya, Mete, & Mittal, 2013) Interestingly, a recent study showed PET scan had higher sensitivity and specificity compared to bone scan in identifying patients with bone metastasis(Chakraborty et al., 2013) Further, patients with more adverse tumor histologies were more likely to receive at PET scan. Given that non-transitional cell carcinoma histology is associated with rapid disease progression(Izard et al., 2015) and is an independent predictor of mortality(Rogers et al., 2006), one might expect these higher risk patients to undergo evaluation for metastatic disease with PET scan. In addition, patients who received neoadjuvant chemotherapy were more likely to undergo a preoperative PET scan. While neoadjuvant therapy has shown an overall survival benefit (Griffiths, Canc, & Grp, 2011; Grossman et al., 2003; Meeks et al., 2012), it tends to be used in higher risk patients(Nguyen & Thalmann, 2017), likely a reflection of the evidence showing that patients with cT3 disease or greater had the largest benefit from therapy.(Grossman et al., 2003) However, this observation may also reflect the involvement of medical oncologists who are more inclined to use PET scans in their practice.
Even after adjusting for these clinical factors, PET scan use increased over time. Reasons for this may include increased availability, better reimbursement, and cost-effectiveness. Since its introduction to clinical medicine in 2001, PET scan has had the largest growth worldwide of all the imaging modalities.(Buck et al., 2010) In the United States, the Centers for Medicare and Medicaid Services (CMS) constantly assesses additional oncologic areas of PET scan use and allows for reimbursement for most oncologic indications.(Buck et al., 2010; Tunis & Whicher, 2009) Additionally, recent evidence has highlighted the cost-effectiveness of PET scan in other malignancies, which may make its use more appealing.(Hollenbeak, Lowe, & Stack, n.d.; Kim et al., 2018)
Although PET scan use has been increasing, there is significant variation in its use at the surgeon level. Among the highest volume surgeons, as many as 55% to as low as 0% of patients underwent preoperative PET scan, even after controlling for possible influential factors such as alkaline phosphatase measurement, receipt of neoadjuvant chemotherapy, tumor histology, and year of surgery. This may suggest that some surgeons may have incorporated PET scan into routine preoperative staging, although there is no clear evidence to support this decision. It may also reflect the surgeons’ collaboration with medical oncologists more so than the surgeons themselves. NCCN guidelines do not provide definite recommendations, but state PET scan can be considered in patients with stage cT3 or greater, if metastatic disease is suspected, or to guide biopsy.(Spiess et al., 2017)
Given such variation in PET scan use, healthcare policy can help ensure appropriate use of this imaging modality, which will help our health system be more cost-effective. This can be done through initiatives that promote inquiry into the utility of PET scan in preoperative bladder cancer staging as well as cost-benefit analyses of PET scan use, such as those done for lung and head-and-neck cancer. (Hollenbeak et al., n.d.; Kim et al., 2018) CMS is already employing evidence based practice to guide reimbursement using a National Oncologic PET Registry (NOPR) that assesses how information from PET scans impacts the way physicians treat patients.(Tunis & Whicher, 2009) For example, recent studies have examined the impact of sodium-fluoride PET scans in the management of patients with osseous metastases, and found that results of sodium-fluoride PET led to an alteration in treatment plans in a substantial fraction of patients.(Hillner et al., 2015)
While PET scan use is increasing in the pre-operative setting for bladder cancer patients undergoing cystectomy, further studies are needed to elucidate if its growing use is warranted. Particularly since guidelines regarding the use of this expensive modality are lacking. A recent meta-analysis of 14 studies examining PET scan for regional lymph node staging showed a pooled sensitivity and specificity of 57% and 92% respectively (Ha et al., 2018). This is in comparison to a pooled sensitivity for CT scan of 35% (Soubra et al., 2016). In contrast, another study examined 51 patients who underwent preoperative PET scan and subsequent cystectomy with extended pelvic lymph node dissection regardless of node status and found PET and CT scans had similar sensitivities of 46% (Swinnen et al., 2010). The majority of studies are limited by statistical power due to limited sample sizes, however there may be a general trend towards showing a small advantage over CT in identifying regional nodal disease (Soubra et al., 2016). Cost-benefit analysis is still needed before widely adopting this expensive technology as it is unclear how often PET scan use will change clinical management. For instances, even a portion of patients with node positive disease may be cured with cystectomy and lymph node dissection (Herr & Donat, 2001). Interestingly, in a larger prospective study of 223 patients who underwent both PET and CT, PET scan was better at detecting distant metastasis and altered treatment course in 3% of patients (Goodfellow et al., 2014). Currently, there is a lack of data to promote routine use of PET scan in the preoperative setting, however it may have a role in patients at higher risk of distant metastasis (i.e. atypical histology or advanced stage) for whom management would be altered. Further large rigorous trials are needed to truly determine the utility of PET scan in the preoperative setting.
Our findings should be considered in the context of several limitations. First, the cohort is based on Medicare hospital claims which may limit the generalizability of our findings. However, the median age of bladder cancer patients is 73(Nielsen et al., 2014) and 82% of patients in this cohort are 70 or older. Second, this is a retrospective observational study and therefore confounders may exist including proximity/availability to a facility that contains PET scanners, use of alternative imaging modalities, and patient travel distance. However, we were able to adjust for several clinical and nonclinical factors to help minimize this bias. Third, the reported surgeon variation in PET scan use may be a reflection of other specialists who work together as part of a multidisciplinary team. Nonetheless, this variation exists and should be further investigated in future work.
Despite these limitations, our findings merit consideration for two reasons. Preoperative PET scan use is increasing in management of patients with muscle-invasive bladder cancer, and appears to be associated with higher risk disease. However, whether its increased use is merited and if its use improves outcomes requires further investigation. Future work should consider differences in treatment patterns and outcomes of high risk patients that received PET scans versus traditional imaging modalities. Second, we observed significant surgeon level variation in the use of PET scan. Additional studies examining the reasons for this variation are warranted.
Supplementary Material
Acknowledgments
Funding/Disclosures
Bruce Jacobs is supported in part by the University of Pittsburgh Physicians Academic Foundation, P30CA047904 from the National Cancer Institute, and the Henry L. Hillman Foundation.
Footnotes
The remaining authors declare no conflict of interest
The study was reviewed by the institutional review board and deemed exempt.
References
- Aljabery F, Lindblom G, Skoog S, Shabo I, Olsson H, Rosell J, & Jahnson S. (2015). PET/CT versus conventional CT for detection of lymph node metastases in patients with locally advanced bladder cancer. BMC Urology, 15 10.1186/s12894-015-0080-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunocilla E, Ceci F, Schiavina R, Castellucci P, Maffione AM, Cevenini M, … Martorana G. (2014). Diagnostic Accuracy of 11C-Choline PET/CT in Preoperative Lymph Node Staging of Bladder Cancer: A Systematic Comparison With Contrast-Enhanced CT and Histologic Findings. Clinical Nuclear Medicine, 39(5), e308 10.1097/RLU.0000000000000342 [DOI] [PubMed] [Google Scholar]
- Buck AK, Herrmann K, Stargardt T, Dechow T, Krause BJ, & Schreyögg J. (2010). Economic Evaluation of PET and PET/CT in Oncology: Evidence and Methodologic Approaches. Journal of Nuclear Medicine Technology, 38(1), 6–17. 10.2967/jnmt.108.059584 [DOI] [PubMed] [Google Scholar]
- Building Bivariate Tables: The compareGroups Package for R | Subirana | Journal of Statistical Software. (n.d.). 10.18637/jss.v057.i12 [DOI] [Google Scholar]
- Chakraborty D, Bhattacharya A, Mete UK, & Mittal BR (2013). Comparison of 18F fluoride PET/CT and 99mTc-MDP bone scan in the detection of skeletal metastases in urinary bladder carcinoma. Clinical Nuclear Medicine, 38(8), 616–621. 10.1097/RLU.0b013e31828da5cc [DOI] [PubMed] [Google Scholar]
- Goodfellow H, Viney Z, Hughes P, Rankin S, Rottenberg G, Hughes S, … Khan MS (2014). Role of fluorodeoxyglucose positron emission tomography (FDG PET)-computed tomography (CT) in the staging of bladder cancer. BJU International, 114(3), 389–395. 10.1111/bju.12608 [DOI] [PubMed] [Google Scholar]
- Griffiths G, Canc M, & Grp N. (2011). International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: Long-term results of the BA06 30894 trial. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 29(16), 2171–2177. 10.1200/JCO.2010.32.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, … Crawford ED (2003). Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. The New England Journal of Medicine, 349(9), 859–866. 10.1056/NEJMoa022148 [DOI] [PubMed] [Google Scholar]
- Ha HK, Koo PJ, & Kim S-J (2018). Diagnostic Accuracy of F-18 FDG PET/CT for Preoperative Lymph Node Staging in Newly Diagnosed Bladder Cancer Patients: A Systematic Review and Meta-Analysis. Oncology, 95(1), 31–38. 10.1159/000488200 [DOI] [PubMed] [Google Scholar]
- Herr HW, & Donat SM (2001). Outcome of patients with grossly node positive bladder cancer after pelvic lymph node dissection and radical cystectomy. The Journal of Urology, 165(1), 62–64; discussion 64. 10.1097/00005392-200101000-00015 [DOI] [PubMed] [Google Scholar]
- Hillner BE, Siegel BA, Hanna L, Duan F, Quinn B, & Shields AF (2015). 18F-Fluoride PET Used for Treatment Monitoring of Systemic Cancer Therapy: Results from the National Oncologic PET Registry. Journal of Nuclear Medicine, 56(2), 222–228. 10.2967/jnumed.114.150391 [DOI] [PubMed] [Google Scholar]
- Hitier‐Berthault M, Ansquer C, Branchereau J, Renaudin K, Bodere F, Bouchot O, & Rigaud J. (n.d.). 18F-fluorodeoxyglucose positron emission tomography–computed tomography for preoperative lymph node staging in patients undergoing radical cystectomy for bladder cancer: A prospective study. International Journal of Urology, 20(8), 788–796. 10.1111/iju.12045 [DOI] [PubMed] [Google Scholar]
- Hollenbeak CS, Lowe VJ, & Stack BC (n.d.). The cost-effectiveness of fluorodeoxyglucose 18-F positron emission tomography in the N0 neck. Cancer, 92(9), 2341–2348. [DOI] [PubMed] [Google Scholar]
- Izard JP, Siemens DR, Mackillop WJ, Wei X, Leveridge MJ, Berman DM, … Booth CM (2015). Outcomes of squamous histology in bladder cancer: A population-based study. Urologic Oncology, 33(10), 425e7–13. 10.1016/j.urolonc.2015.06.011 [DOI] [PubMed] [Google Scholar]
- Jensen TK, Holt P, Gerke O, Riehmann M, Svolgaard B, Marcussen N, & Bouchelouche K. (2011). Preoperative lymph-node staging of invasive urothelial bladder cancer with 18F-fluorodeoxyglucose positron emission tomography/computed axial tomography and magnetic resonance imaging: Correlation with histopathology. Scandinavian Journal of Urology and Nephrology, 45(2), 122–128. 10.3109/00365599.2010.544672 [DOI] [PubMed] [Google Scholar]
- Kibel AS, Dehdashti F, Katz MD, Klim AP, Grubb RL, Humphrey PA, … Siegel BA (2009). Prospective Study of [18F]Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography for Staging of Muscle-Invasive Bladder Carcinoma. Journal of Clinical Oncology, 27(26), 4314–4320. 10.1200/JCO.2008.20.6722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CR, Kim B, Ning MS, Reddy JP, Liao Z, Tang C, … Gomez DR (2018). Cost Analysis of PET/CT Versus CT as Surveillance for Stage III Non–Small-Cell Lung Cancer After Definitive Radiation Therapy. Clinical Lung Cancer, 19(4), e517–e528. 10.1016/j.cllc.2018.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klabunde CN, Potosky AL, Legler JM, & Warren JL (2000). Development of a comorbidity index using physician claims data. Journal of Clinical Epidemiology, 53(12), 1258–1267. [DOI] [PubMed] [Google Scholar]
- Maurer T, Souvatzoglou M, Kübler H, Opercan K, Schmidt S, Herrmann K, … Treiber U. (2012). Diagnostic Efficacy of [11C]Choline Positron Emission Tomography/Computed Tomography Compared With Conventional Computed Tomography in Lymph Node Staging of Patients With Bladder Cancer Prior to Radical Cystectomy. European Urology, 61(5), 1031–1038. 10.1016/j.eururo.2011.12.009 [DOI] [PubMed] [Google Scholar]
- Medicare C for, Baltimore MS 7500 SB, & Usa M. (2017, January 9). Centers for Medicare and Medicaid Services. Medicare Provider Analysis and Review (MEDPAR). Retrieved July 16, 2018, from https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/IdentifiableDataFiles/index.html
- Meeks JJ, Bellmunt J, Bochner BH, Clarke NW, Daneshmand S, Galsky MD, … Sonpavde G. (2012). A systematic review of neoadjuvant and adjuvant chemotherapy for muscle-invasive bladder cancer. European Urology, 62(3), 523–533. 10.1016/j.eururo.2012.05.048 [DOI] [PubMed] [Google Scholar]
- Nguyen DP, & Thalmann GN (2017). Contemporary update on neoadjuvant therapy for bladder cancer. Nature Reviews.Urology, 14(6), 348–358. 10.1038/nrurol.2017.30 [DOI] [PubMed] [Google Scholar]
- Nielsen ME, Smith AB, Meyer A-M, Kuo T-M, Tyree S, Kim WY, … Millikan RC (2014). Trends in Stage-Specific Incidence Rates for Urothelial Carcinoma of the Bladder in the United States: 1988 to 2006. Cancer, 120(1), 86–95. 10.1002/cncr.28397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichler R, De Zordo T, Fritz J, Kroiss A, Aigner F, Heidegger I, … Uprimny C. (2017). Pelvic Lymph Node Staging by Combined 18F-FDG-PET/CT Imaging in Bladder Cancer Prior to Radical Cystectomy. Clinical Genitourinary Cancer, 15(3), e387–e395. 10.1016/j.clgc.2016.08.009 [DOI] [PubMed] [Google Scholar]
- R: A language and environment for statistical computing. (n.d.). Retrieved July 16, 2018, from https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing
- Rogers CG, Palapattu GS, Shariat SF, Karakiewicz PI, Bastian PJ, Lotan Y, … Schoenberg MP (2006). Clinical Outcomes Following Radical Cystectomy for Primary Nontransitional Cell Carcinoma of the Bladder Compared to Transitional Cell Carcinoma of the Bladder. The Journal of Urology, 175(6), 2048–2053. 10.1016/S0022-5347(06)00317-X [DOI] [PubMed] [Google Scholar]
- SEER-Medicare Linked Database. (n.d.). Retrieved July 16, 2018, from https://healthcaredelivery.cancer.gov/seermedicare/
- Soubra A, Hayward D, Dahm P, Goldfarb R, Froehlich J, Jha G, & Konety BR (2016). The diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography and computed tomography in staging bladder cancer: A single-institution study and a systematic review with meta-analysis. World Journal of Urology, 34(9), 1229–1237. 10.1007/s00345-016-1772-z [DOI] [PubMed] [Google Scholar]
- Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, Clark PE, … Gurski LA (2017). Bladder Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network, 15(10), 1240–1267. 10.6004/jnccn.2017.0156 [DOI] [PubMed] [Google Scholar]
- Swinnen G, Maes A, Pottel H, Vanneste A, Billiet I, Lesage K, & Werbrouck P. (2010). FDG-PET/CT for the Preoperative Lymph Node Staging of Invasive Bladder Cancer. European Urology, 57(4), 641–647. 10.1016/j.eururo.2009.05.014 [DOI] [PubMed] [Google Scholar]
- Tunis S, & Whicher D. (2009). The National Oncologic PET Registry: Lessons Learned for Coverage With Evidence Development. Journal of the American College of Radiology, 6(5), 360–365. 10.1016/j.jacr.2009.01.016 [DOI] [PubMed] [Google Scholar]
- Turner RM, Yabes JG, Davies BJ, Heron DE, & Jacobs BL (2017). Variations in Preoperative Use of Bone Scan among Medicare Beneficiaries Undergoing Radical Cystectomy. Urology, 103, 84–90. 10.1016/j.urology.2017.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. (2009). ggplot2: Elegant Graphics for Data Analysis. Retrieved from //www.springer.com/us/book/9780387981413
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.