Abstract
Objective:
To validate the 17-gene Oncotype DX Genomic Prostate Score (GPS) as a predictor of adverse pathology (AP) in African American (AA) men and to assess the distribution of GPS in AA and European American (EA) men with localized prostate cancer.
Methods:
The study populations were derived from two multi-institutional observational studies. Between February 2009 and September 2014, AA and EA men who elected immediate radical prostatectomy after a ≥10-core transrectal ultrasound biopsy were included in the study. Logistic regressions, area under the receiver operating characteristic curves (AUC), calibration curves, and predictive values were used to compare the accuracy of GPS. AP was defined as primary Gleason grade 4, presence of any Gleason pattern 5, and/or non-organ-confined disease (≥pT3aN0M0) at radical prostatectomy.
Results:
Overall, 96 AA and 76 EA men were selected and 46 (26.7%) had AP. GPS result was a significant predictor of AP (odds ratio per 20 GPS units [OR/20 units] in AA: 4.58; 95% confidence interval (CI) 1.8-11.5, p=0.001; and EA: 4.88; 95% CI 1.8-13.5, p=0.002). On multivariate analysis, there was no significant interaction between GPS and race (p >0.10). GPS remained significant in models adjusted for either National Comprehensive Cancer Network (NCCN) risk group or Cancer of the Prostate Risk Assessment (CAPRA) score. In race-stratified models, AUC for GPS/20 units was 0.69 for AAs vs. 0.74 for EAs (p=0.79). The GPS distributions were not statistically different by race (all p >0.05).
Conclusion:
In this clinical validation study, the Oncotype DX GPS is an independent predictor of AP at prostatectomy in AA and EA men with similar predictive accuracy and distributions.
Keywords: Oncotype DX, GPS score, prostate cancer biomarkers, African American, biomarker validation, active surveillance
Introduction
Only 16% of clinically localized prostate cancer (PCa) cases progress to cause mortality1. Active surveillance (AS) has been deployed as a way to manage very low to intermediate risk PCa and preserve quality of life by delaying treatment (i.e. radical prostatectomy (RP) or radiotherapy), while monitoring patients’ PCa progression by using serial PSA measurements, rectal examinations and prostate biopsies2. Overtreatment of biologically indolent disease results in substantial cost and unnecessary morbidity, which has led to some authorities doubting the value of routine screening3,4. Serum, urinary and tissue biomarkers are increasingly used to predict tumor aggressiveness and risk stratify patients who may elect AS5.
The Oncotype DX (ODX) Prostate Cancer Assay is a clinically validated assay based on the expression of 12 informative cancer-related genes relative to 5 housekeeping genes using tumor epithelium RNA macro-dissected from formalin fixed paraffin embedded needle biopsy specimens6. The expression values are weighted and added to provide a Genomic Prostate Score (GPS) for each patient’s tumor. The ODX report displays the patient’s GPS score on a normal GPS distribution for men within the same National Comprehensive Cancer Network (NCCN) risk group to see how an individual patient compares to the others and provides an estimated probability of adverse pathology (AP) at the time of RP. Validations of GPS have been limited to largely European ancestry populations7. Few studies have validated the GPS in African American (AA) men8. Among PCa patients, AA with low risk PCa have increased rates of AP and varied prostate tumor expression, especially in the androgen signaling pathway 9-14. In the publication by Cullen et al., 82 AA men were included in their analysis of AP and GPS. They found similar GPS distributions by race but a lower odds ratio for AP prediction in AAs relative to EAs (OR: 2.86 vs. 4.05). These data suggest that the ODX assay may have different predictive accuracy between AA and EA men eligible for AS8. In the present study, we aim to compare the accuracy of GPS as a predictor of AP in AA and EA men with clinically localized PCa who underwent RP. We will additionally compare the distributions of GPS score between AA and EA men.
Materials and Methods
Study Population
The study populations were derived from 2 related observational studies investigating the associations between vitamin D status (serum and prostatic) and aggressive PCa called Biological and Environmental Mediators of Vitamin D and Prostate Cancer Risk (Vitamin D study, n = 95) and Vitamin D Effects on Apoptosis and Proliferation (Apoptosis study, n = 77). Both studies were institutional review board approved at Northwestern University and Jesse Brown VA Medical Center. The Vitamin D study accrued 954 men immediately before prostate biopsy from 2009-2014 and the second study accrued 191 men after they chose RP for primary treatment of their clinically localized PCa from 2013-2018. We compared the baseline characteristics of the Apoptosis and the Vitamin D study cohorts using nonparametric Mann-Whitney tests for continuous variables and Pearson-χ2 tests for categorical variables (see supplementary table 1). All men had ≥10 core transrectal ultrasound (TRUS) guided prostate needle biopsies and targeted the peripheral zone. Due to our recruitment period, MRI was not used before biopsy or before prostatectomy. Participants were the subset of 40-79y/o men who were NCCN very low to unfavorable intermediate risk, selfidentified as AA (non-Hispanic Black/African American) or EA (non-Hispanic White), had available biopsy tumor blocks, had undergone RP at our sites, and consented to the use of their data and specimen for future PCa research. There were 208 AAs and 152 EAs who fit eligibility criteria for this protocol and had their DNA extracted for ancestry estimation. Of these men, 96 AA and 76 EA provided consent and had available and adequate biopsy cores with ≥0.7 mm tumor in the highest Gleason grade core. Exclusion criteria included delay in RP >12 months and prior therapy for PCa such as pelvic- or prostate-focused external beam or interstitial seed radiotherapy, orchiectomy, androgen deprivation therapy, high-intensity focused ultrasound therapy, or cryotherapy. In addition, six AA men with PSA >20.00ng/ml as their high-risk feature had an ODX assay performed to assess AA’s GPS distribution in this subgroup. All patients provided written informed consent.
Pathology
Biopsies were selected where the dominant tumor nodule had at least 0.7mm of tumor length in the core containing the highest Gleason score. Both the prostate biopsy and RP tumor blocks were de-identified by adhering new labels on all slides with study ID number for all eligible subjects. Biopsy and prostatectomy specimen were read in separate sessions for centralized review by 3 experienced uropathologists at both sites in accordance with the 2014 International Society of Urological Pathology Consensus guidelines: Gleason Grade Group (GG)1 = Gleason score ≤6; GG2 = Gleason score 3+4 =7; GG3 = Gleason score 4+3 =7; GG4 = Gleason score 8; and GG5 = Gleason score 9–1015. Biopsy tumor blocks were submitted to the Genomic Health Inc. laboratory (Redwood City, CA) and were manually macrodissected as previously described6, 8.
Statistical Methods
Descriptive statistics were used to compare notable clinical, demographic, and cancer related covariates. Genetic West African ancestry was also compared to ensure accurate racial stratification. AP was defined as primary Gleason grade 4, presence of any Gleason pattern 5, and/or non-organ-confined disease (≥pT3aN0M0) at RP. To compare racial groups, nonparametric Mann-Whitney tests were performed for continuous variables, and Pearson-χ2 tests were used for categorical variables.
Our prespecified objectives were to compare the GPS distributions between EA and AA men and the associations of GPS testing with AP in the pathologic specimen with NCCN very low-, low-, favorable intermediate- and unfavorable intermediate-risk who elected RP16. The association of GPS and AP was evaluated using bivariate and multivariate logistic regression models to compare the odds ratios. GPS was explored as a continuous variable with GPS score divide by 2017. Multiplicative interactions tested for differential prediction of GPS accuracy by race (AA vs. EA). Receiver operating characteristic (ROC) curves were used to compare accuracy of models adjusted for GPS/20 and NCCN risk group or CAPRA score for prediction of AP. Additionally, in a post-hoc analysis, the accuracy of the ODX assay was compared to the Memorial Sloan Kettering Cancer Calculator (MSKCC) for the prediction of non-organ confined PCa since this is already available at no cost. We were powered to detect a 10% AUC difference between AA and EA with 80% power, assuming an AUC of 0.72 for GPS in EAs8. An alpha <0.05 was considered statistically significant. Statistical analyses were performed using SPSS 25 (IBM Corporation 2016, United States).
Results
Of 172 subjects, 96 (55.8%) identified as AA (NNMH = 21/NVA = 75) and 76 (44.2%) as EA (NNMH = 58/NVA = 18). Median age was 61.5 years and a total of 46 men (26.7%) had AP. NCCN risk groups at biopsy were as follows: 40% very low to low risk, 39% favorable intermediate risk, and 21% unfavorable intermediate risk. Compared to EA men, AAs had higher PSA (6.0ng/mL vs. 4.6ng/mL), PSA density (0.16ng/cm3 vs. 0.12ng/cm3), CAPRA scores (3.0 vs. 2.0), and greater frequencies of current smoking (25.0% vs. 2.7%) (all p<0.001). On univariate analysis, there were no statistical differences between racial groups in GPS, AP rates, pathological staging, age, family history of PCa, abnormal DRE, 5-α reductase inhibitor use, high school completion, and income less than $30,000/year (see Table 1). Although the study populations statistically differed in some clinical parameters, the rate of AP was similar in both groups: 24.7% for Vitamin D vs. 29.3% for Apoptosis study (supplementary table 1). The other parameters were clinically in line with AS cohorts and the distributions of NCCN risk strata were not statistically different.
Table 1.
Clinical & Socioeconomic Factors by Racial Groups.
| Continuous Variables | AA (n = 96) |
EA (n = 76) |
p-value1 |
|---|---|---|---|
| Median [IQR] | Median [IQR] | ||
| Age, years | 61.5 [57.5, 67.0] | 62.0 [59.0, 66.8] | 0.78 |
| BMI, kg/m2 | 28.1 [25.8, 30.8] | 27.4 [26.0, 29.9] | 0.34 |
| West African Ancestry, % | 78.6% [67.5%, 85.8%] | 4.0% [1.7%, 6.6%] | <0.001 |
| PSA, ng/ml | 6.0 [4.5, 8.9] | 4.6 [3.85, 7.14] | <0.001 |
| PSA density, ng/cm3 | 0.16 [0.10, 0.24] | 0.12 [0.08, 0.19] | <0.001 |
| CAPRA | 3.0 [2.0, 4.0] | 2.0 [1.0, 3.0] | <0.001 |
| GPS | 29.5 [23.3, 37.5] | 27.0 [23.0, 35.0] | 0.27 |
| Number of Positive Cores | 3.0 [2.0, 6.0] | 2/0 [ 1.0, 4.0] | 0.004 |
| Percent of Positive Cores | 27.9% [16.7%, 45.7%] | 17/4% [8.3%, 3.3%] | 0.001 |
| Categorical Variables | AA n (%) |
EA n (%) |
p-value2 |
| Pre-orostatectomy | |||
| Current Smoking | 24/96 (25.0) | 2/74 (2.7) | <0.001 |
| High school completion | 76/95 (80.0) | 59/74 (79.7) | 0.96 |
| Income < $30,000/year | 24/95 (25.2) | 11/71 (15.5) | 0.13 |
| FHx of PCa | 27/96 (28.1) | 16/76 (21.1) | 0.35 |
| Abnormal DRE | 17/96 (17.7) | 19/76 (25.0) | 0.24 |
| 5α-reductase inhibitor use | 1/96 (1.0) | 3 (3.9) | 0.19 |
| NCCN Risk Group | |||
| Very Low/Low | 31 (32.3) | 38 (50.0) | 0.02 |
| Favorable Intermediate | 35 (36.5) | 32 (42.1) | 0.45 |
| Unfavorable Intermediate | 30 (31.2) | 6 (7.9) | <0.001 |
| Post-prostatectomy | |||
| Adverse Pathology | 24/96 (25.0) | 22/76 (28.9) | 0.56 |
| Pathology Staging | |||
| T2a-T2b | 8 (8.3) | 6 (7.9) | 0.97 |
| T2c | 71 (74.0) | 54 (71.1) | 0.67 |
| T3a-T3b | 17 (17.7) | 16 (21.0) | 0.58 |
Using Mann-Whitney tests
Using χ2 tests; Bold type indicates p values <0.05
Abbreviations: AA: African American; EA: European American; BMI: Body Mass Index; PSA: Prostate Specific Antigen; CAPRA: Cancer of the Prostate Risk Assessment; GPS: Genomic Prostate Score; FHx of PCa: 1st degree Family History of Prostate cancer; DRE: digital rectal examination; NCCN: National Comprehensive Cancer Network.
The distribution of biopsy and pathological Gleason score by race is depicted in Table 2. Overall 85 (48.3%) subjects had a Gleason score of 3+3 PCa, out of which 39 (45.9%) were AA and 46 (54.1%) were EA men. AAs had a 43.6% rate of upgrading from Gleason 3+3 to Gleason 7-10. GPS scores across strata of NCCN risk groups by race is illustrated in Table 3 and Supplementary Figure 1. AA men with NCCN high-risk PCa were included for the purposes of assessing the distribution of GPS by risk group. Similar median GPS scores were observed for both racial groups, with GPS score increasing with higher NCCN risk groups. The rate of AP was substantially higher in EA men in the favorable intermediate (43.8% vs. 14.3%) and unfavorable intermediate (66.7% vs. 50.0%) risk groups relative to their AA counterparts. In line with this, the GPS scores were also higher or more varied for the EA men in the favorable and unfavorable intermediate risk groups, though differences were not statistically significant. To evaluate the GPS range in higher risk AAs, we also included 6 AA men with NCCN high risk PCa by PSA, out of which 5 (83.3%) demonstrated AP. No EA men were staged as high risk.
Table 2.
Distribution of Biopsy and Radical Prostatectomy Gleason Grade by Race.
| African American men | |||||||
|---|---|---|---|---|---|---|---|
| Radical Prostatectomy Gleason Grade | |||||||
| 3+3 | 3+4 | 4+3 | ≥4+4 | Total | Gleason Upgrade % |
||
| Biopsy Gleason Grade | 3+3 | 22 | 12 | 4 | 1 | 39 (40.6%) | 43.6% |
| 3+4 | 2 | 31 | 5 | 2 | 40 (41.7%) | 17.5% | |
| 4+3 | - | 6 | 9 | 2 | 17 (17.7%) | 11.8% | |
| European American men | |||||||
| Radical Prostatectomy Gleason Grade | |||||||
| 3+3 | 3+4 | 4+3 | ≥4+4 | Total | Gleason Upgrade % |
||
| Biopsy Gleason Grade | 3+3 | 35 | 4 | 5 | 2 | 46 (60.5%) | 23.9% |
| 3+4 | 5 | 15 | 9 | 1 | 30 (39.5%) | 33.3% | |
| 4+3 | - | - | - | - | - | - | |
Table 3.
Race-stratified distribution of GPS and Adverse Pathology at Prostatectomy by NCCN Risk Group.
| GPS Distribution Median [IQR] |
Adverse Pathology n (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| NCCN Risk Group |
All N=178 |
AA N=102 |
EA N=76 |
p- value1 |
All N=178 |
AA N=102 |
EA N=76 |
p- value2 |
| Very Low/Low N=69 |
27.0 [20.0,33.5] |
27.0 [20.0,34.0] |
27.0 [19.8,32.3] |
0.51 | 8/69 (11.6%) |
4/31 (12.9%) |
4/38 (10.5%) |
1.00 |
| Favorable Intermediate N=67 |
31.0 [26.0,40.0] |
30.0 [24.0,40.0] |
31.0 [27.0,39.5] |
0.47 | 19/67 (28.4%) |
5/35 (14.3%) |
14/32 (43.8%) |
0.01 |
| Unfavorable Intermediate N=36 |
35.0 [25.5,43.3] |
35.00 [26.5,41.8] |
35.0 [17.3,54.0] |
0.89 | 19/36 (52.8%) |
15/30 (50.0%) |
4/6 (66.7%) |
0.66 |
| High N=6 |
40.0 [36.8,46.5] |
40.0 [36.8,46.5] |
- | - | 5/6 (83.3%) |
5/6 (83.3%) |
0 (0%) |
- |
Using Mann-Whitney tests
Using Fisher’s exact test.
Abbreviations: AA: African American; EA: European American; GPS: Genomic Prostate Score; NCCN: National Comprehensive Cancer Network; AA: African American; EA: European American.
On the binary logistic regressions for AP at prostatectomy, GPS score was coded as a continuous variable divided by 20 [GPS/20] to assess the odds ratio per 20-point increase as in the publication by Cullen et al8. GPS/20 was a significant predictor of AP in both racial groups with an odds ratio in AA of 4.58 (95% CI 1.8-11.5, P =0.001) and in EA of 4.88 (95% CI 1.8-13.5, p=0.002). GPS/20 remained significant after adjustment for CAPRA score (2.55, p=0.17) and NCCN risk group (3.65, p<0.001). The interaction term of GPS/20 with race was not significant in both the CAPRA (p =0.38) and the NCCN (p =0.53) model.
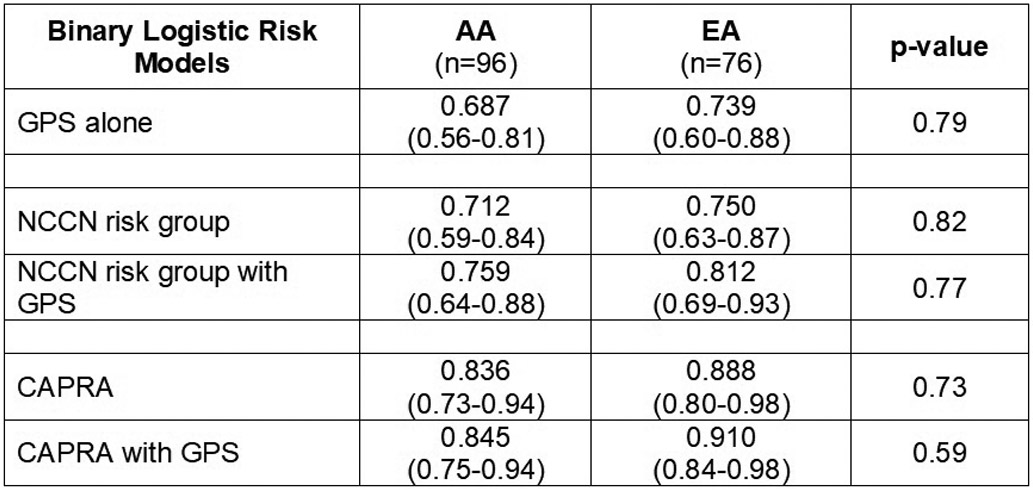
ROC curves assessed the accuracy of 5 binary logistic regression models: 1) GPS/20 alone; 2) NCCN risk group as a four-level ordinal variable (i.e. very low, low, favorable intermediate, and unfavorable intermediate) alone; 3) NCCN risk group with GPS/20; 4) CAPRA score (continuous from 0-1018) alone; and 5) CAPRA score with GPS/20. Using GPS/20 alone, the area under the receiver operating characteristics curve (AUCs) was 0.74 (SE: 0.60-0.88) in EA and 0.69 (SE: 0.56-0.81) in AA men (p = 0.79). The CAPRA model with GPS/20 had an AUC of 0.91 (0.84-0.98) in EA and 0.85 (SE: 0.75-0.94) in AA. The NCCN model, as used in the clinical assay, had an AUC of 0. 81 (SE: 0.69-0.93) in EA and 0.76 (SE: 0.64-0.88) in AA (p = 0.77) (see Figure 1).
Figure 1.
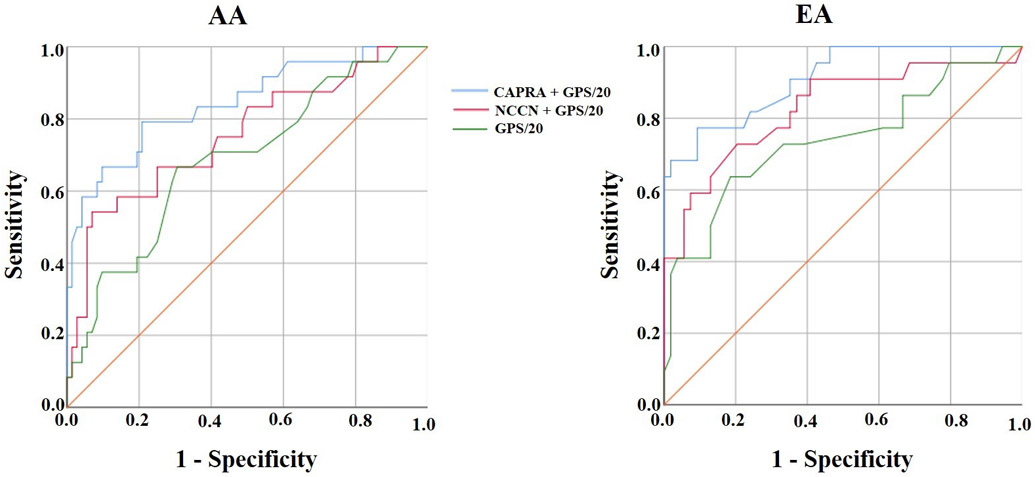
Prediction of adverse pathology at prostatectomy in univariate and multivariate analyses.
Legend 1a: Blue line = GPS with CAPRA model. Red Line = GPS with NCCN model. Green line = GPS alone.
Legend 1b: AUCs for all 5 models stratified by race.
The calibrations of the CAPRA risk model with GPS varied by race. In AAs, the model slightly underestimated the risk of AP in the 0-20% threshold, yet in EAs the model was well-calibrated through the 0-30% threshold. The NCCN risk model with GPS was fairly well calibrated for AA men. The differences between the CAPRA and NCCN models, however, were not significant in either racial group (AA: p =0.87, EA: p =0.29) (see Supplementary Figure 2).
In terms of its clinical application, at a 95% sensitivity the overall positive predictive value (PV) for the NCCN + GPS/20 model was 29% and the negative PV was 92%. No major differences were noted between racial groups. At a 90% sensitivity, the NCCN + GPS/20 model yielded a 29% positive and an 87% negative PV.
We compared the ODX assay and the MSKCC19 for their prediction of non-organ confined prostate cancer at radical prostatectomy. Based on AUC curves, the MSKCC significantly outperformed the ODX assay for this adverse pathologic feature (AUC: 0.86 vs. 0.74, p = 0.015). Moreover, ODX GPS score did not add to the accuracy of the calculator (supplementary figure 3).
Discussion
This provides confirmatory evidence of the GPS score as an independent predictor of AP at RP in both AA and EA men with similar distributions and odds ratios by race. We believe this indicates that the Oncotype DX Assay is clinically valid for determining risk of AP independent of clinical risk factors with a high negative predictive value. Results of calibration analyses suggest that the GPS model with NCCN risk groups works well in predicting AP in the 0-30% range. In addition, we observe that GPS score distributions were similar for both AA and EA men across NCCN levels. This would suggest that tumor aggressiveness is similar stage for stage between EAs and AAs. In fact, similar GPS distributions were also seen with the ODX validation in 82 AAs and 305 EAs from Walter Reed and Madigan Army Medical Center8. Notably, both AA validations involved patient samples from ‘equal access’ sites where differences between EA and AA men in income and educational level are less pronounced. This is somewhat counter-intuitive given that AAs are known to harbor more aggressive PCa when eligible for AS9. However, our calibration plots demonstrate that the ODX assay with CAPRA under-estimated the rate of AP in AA men in the range from 0-20%. Despite similar distributions, ODX may benefit from re-calibration in this important prediction range to better match the actual rate of AP.
In the real world, AA men are less likely to be treated with AS20. Overall, ODX should allow AA men to confidently avoid active treatment when eligible for AS despite their increased risk of harboring AP9. Similar to MRI, the evidence provided in this study shows that the ODX assay may improve the safety of AS in AA men through improved patient selection21. The evidence provided in this study shows that GPS can provide orthogonal data to patients eligible for AS to facilitate their treatment decisions.
Several studies have described GPS as a strong independent predictor of AP in low to intermediate risk men8,22,23. The assay can increase urologists’ confidence when providing treatment recommendations for very low to low-intermediate risk patients, leading to increased uptake of AS24. In a prospective cohort, patients that received GPS scores were more likely to select AS and 96% reported finding the test useful25.
Few studies have analyzed the predictive accuracy of GPS in AAs. AA men eligible for AS progress more often and may harbor adverse pathologic features compared to EA men, which has led to an under-utilization of AS among AAs9,26-29. It was plausible that the GPS variables would not predict as well in AAs since there are known genetic and molecular differences between AA and EA PCas including: increased androgen signaling, differences in frequency of TMPRSS-ERG fusions and differences in tumor gene expression patterns12,30,31. On bivariate analysis, GPS prediction of AP proved to be similarly strong in both racial groups ([OR/20 units] in AA: 4.58, p =0.001; and EA: 4.88; p =0.002). Our results are consistent with Cullen et al.’s study which included 82 (20.4%) AA men in its cohort and demonstrated a statistically significant association between GPS and AP in both EAs and AAs: OR/20 4.05 and 2.86, respectively. Furthermore, similarly to Klein et al.’s large validation study, GPS/20 remained significant in multivariate analyses adjusting for CAPRA score and NCCN risk groups23. To our knowledge, this is the largest study to evaluate the accuracy of GPS in AA populations. Future studies with greater AA samples should externally validate our findings.
In our comparison of the ODX assay and the MSKCC calculator, it appears that the risk calculator outperforms the ODX assay for the prediction of non-organ confined PCa. The ODX GPS score did not provide orthogonal data to the calculator in our cohort. The benefit of the ODX assay over the MSKCC calculator is that it also predicts whether the patient harbors more aggressive Gleason patterns in the prostate, which is an important endpoint for AS patients. A future comparison between the ODX assay and prostate multi-parametric MRI could be assessed in men that have undergone both tests to predict the presence of a higher grade and higher stage tumor given their similar costs
Several limitations in the study should be noted. First, this is a retrospective analysis of multi-center studies where eligible AS men with available biopsy specimen were selected from a tertiary and VA medical center in a large metropolitan city, which might affect generalizability. Although we prospectively enrolled consecutive men in the parent cohorts we cannot rule out selection or spectrum bias. Our selection criteria most likely reduced any racial differences in risk factors. This is a common problem with retrospective biomarker validation in populations with diverse racial and socioeconomic characteristics. However, our demographic and clinical data suggests that these men are typical for their NCCN risk strata and AS cohorts, and while the selection criteria reduced racial differences in terms of risk, this would not necessarily introduce bias regarding the effect of race on GPS accuracy. Moreover, we conducted centralized pathologic review and blinded the pathologists to the race and clinical data of the participants. Second, 19 AS eligible men had insufficient tumor for the assay, which limited our AA men and possibly introduced a bias. Third, many of the biopsies were performed between 2009-2014 definitions of unfavorable risk PCa were not popular and centralized review upgraded several men, which led to our inclusion of unfavorable intermediate risk men in the sample. Our population was partially recruited prior to the widespread adoption of AS in EA men and increased concern regarding the safety of AS in AA men. Therefore, we recommend repeating our study design in a completely contemporary cohort. Lastly, the population size was relatively small, and limited our ability to include other ethnic/racial groups. In addition, GPS now reports risk of metastasis and death which was unable to be validated due to insufficient follow up time23.
Conclusion
In summary, the ODX Genomic Prostate Score has similar predictive accuracy for AP at RP in AA and EA men with very low-, low-, and intermediate-risk PCa. This establishes that Oncotype Dx assay can provide patients eligible for AS with orthogonal data to clinical factors to support treatment decisions. Future studies investigating the impact of these assays on patient treatment choice, especially in high-risk populations with potentially low health literacy is warranted.
Supplementary Material
Supplementary Figure 1. Box plots of GPS scores by race and NCCN risk group.
Legend: Mean indicated by “*”. Whiskers represent minimum and maximum value, or 1.5 * IQR (interquartile range) when outliers are present.
Supplementary Figure 2. Calibration plots of the CAPRA and NCCN Risk Model with GPS.
Legend: Black line = 45-degree line representing perfect calibration. Red line = African American men. Blue line = European American men. Green line = All men.
Supplementary Figure 3. Race-stratified Receiver Operating Characteristic Curves for Non-organ Confined Prostate Cancer.
Legend: Blue line = Memorial Sloan Kettering cancer calculator (MSKCC). Green line = ODX predictive score. Red Line = MSKCC + ODX model.
Acknowledgements
We would like to thank to the urologists, pathologists, the clinical and pathology staff, and the patients for their participation in our study. We also appreciate the cooperation of Genomic Health Inc. for defraying the costs of the assays and providing us the independence to analyze and publish our findings.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brawley OW. Trends in prostate cancer in the United States. Journal of the National Cancer Institute Monographs. 2012;2012(45):152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. Journal of Clinical Oncology. 2014;33(3):272–277. [DOI] [PubMed] [Google Scholar]
- 3.Bangma C, Roemeling S, Schröder F. Overdiagnosis and overtreatment of early detected prostate cancer. World Journal of Urology. 2007;25(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein EA, Maddala T, Millward C, et al. Development of a needle biopsy-based genomic test to improve discrimination of clinically aggressive from indolent prostate cancer. In: American Society of Clinical Oncology; 2012.
- 5.Loeb S, Tosoian JJ. Biomarkers in active surveillance. Translational Andrology Urology. 2018;7(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knezevic D, Goddard AD, Natraj N, et al. Analytical validation of the Onco type DX prostate cancer assay–a clinical RT-PCR assay optimized for prostate needle biopsies. BMC genomics. 2013;14(1):690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooperberg M, Simko J, Falzarano S, et al. Development and validation of the biopsy-based genomic prostate score (GPS) as a predictor of high grade or extracapsular prostate cancer to improve patient selection for active surveillance. The Journal of Urology. 2013. [Google Scholar]
- 8.Cullen J, Rosner IL, Brand TC, et al. A biopsy-based 17-gene genomic prostate score predicts recurrence after radical prostatectomy and adverse surgical pathology in a racially diverse population of men with clinically low-and intermediate-risk prostate cancer. European Urology. 2015;68(1):123–131. [DOI] [PubMed] [Google Scholar]
- 9.Sundi D, Ross AE, Humphreys EB, et al. African American men with very low–risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: should active surveillance still be an option for them? Journal of Clinical Oncology. 2013;31(24):2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chornokur G, Dalton K, Borysova ME, Kumar NB. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. The Prostate. 2011;71(9):985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vijayakumar S, Henegan JC, Zhang X, et al. Enriching gene expression profiles will help personalize prostate cancer management for African-Americans: a perspective. Paper presented at: Urologic Oncology: Seminars and Original Investigations2017. [DOI] [PubMed] [Google Scholar]
- 12.Powell IJ, Dyson G, Land S, et al. Genes associated with prostate cancer are differentially expressed in African American and European American men. Cancer Epidemiology Prevention Biomarkers. 2013;22(5):891–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kinseth MA, Jia Z, Rahmatpanah F, et al. Expression differences between African American and Caucasian prostate cancer tissue reveals that stroma is the site of aggressive changes. International Journal of Cancer. 2014;134(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faisal FA, Sundi D, Tosoian JJ, et al. Racial variations in prostate cancer molecular subtypes and androgen receptor signaling reflect anatomic tumor location. European Urology. 2016;70(1):14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. The American Journal of Surgical Pathology. 2016;40(2):244–252. [DOI] [PubMed] [Google Scholar]
- 16.Network NCCNJFWT. NCCN clinical practice guidelines in oncology: Prostate cancer version 1.2017. 2017.
- 17.Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statistics in Medicine. 2008;27(2):157–172. [DOI] [PubMed] [Google Scholar]
- 18.Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. The Journal of Urology. 2005;173(6):1938–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Memorial Sloan Kettering Cancer Center: Prediction Tools/Prostate Cancer Nomograms: PreRadical Prostatectomy. Available at https://www.mskcc.org/nomograms/prostate/pre_op. Accessed November 22, 2019. .
- 20.Butler S, Muralidhar V, Chavez J, et al. Active Surveillance for Low-Risk Prostate Cancer in Black Patients. The New England Journal of Medicine. 2019;380(21):2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloom JB, Lebastchi AH, Gold SA, et al. Use of multiparmetric MRI and Fusion-Guided Biopsies to Properly Select and Follow African-American Men on Active Surveillance. BJU international. 2019. [DOI] [PMC free article] [PubMed]
- 22.Davis JW. Novel commercially available genomic tests for prostate cancer: a roadmap to understanding their clinical impact. BJU international. 2014;114(3):320–322. [DOI] [PubMed] [Google Scholar]
- 23.Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. European Urology. 2014;66(3):550–560. [DOI] [PubMed] [Google Scholar]
- 24.Badani KK, Kemeter MJ, Febbo PG, et al. The impact of a biopsy based 17-gene genomic prostate score on treatment recommendations in men with newly diagnosed clinically prostate cancer who are candidates for active surveillance. Urology Practice. 2015;2(4):181–189. [DOI] [PubMed] [Google Scholar]
- 25.Eure G, Germany R, Given R, et al. Use of a 17-gene prognostic assay in contemporary urologic practice: results of an interim analysis in an observational cohort. Urology. 2017;107:67–75. [DOI] [PubMed] [Google Scholar]
- 26.Iremashvili V, Soloway MS, Rosenberg DL, Manoharan M. Clinical and demographic characteristics associated with prostate cancer progression in patients on active surveillance. The Journal of Urology. 2012;187(5):1594–1600. [DOI] [PubMed] [Google Scholar]
- 27.Shao Y-H, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. Journal of the National Cancer Institute. 2009; 101 (18): 1280–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez-Ortiz RF, Troncoso P, Babaian RJ, Lloreta J, Johnston DA, Pettaway CA. African-American men with nonpalpable prostate cancer exhibit greater tumor volume than matched white men. Cancer. 2006;107(1):75–82. [DOI] [PubMed] [Google Scholar]
- 29.Powe J llI, Bock CH, Ruterbusch JJ, Sakr W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. The Journal of Urology. 2010;183(5):1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen P, Pfister D, Young D, et al. Differences in frequency of ERG oncoprotein expression between index tumors of Caucasian and African American patients with prostate cancer. Urology. 2012;80(4):749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farrell J, Petrovics G, McLeod D, Srivastava S. Genetic and molecular differences in prostate carcinogenesis between African American and Caucasian American men. International Journal of Molecular Sciences. 2013;14(8):15510–15531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Box plots of GPS scores by race and NCCN risk group.
Legend: Mean indicated by “*”. Whiskers represent minimum and maximum value, or 1.5 * IQR (interquartile range) when outliers are present.
Supplementary Figure 2. Calibration plots of the CAPRA and NCCN Risk Model with GPS.
Legend: Black line = 45-degree line representing perfect calibration. Red line = African American men. Blue line = European American men. Green line = All men.
Supplementary Figure 3. Race-stratified Receiver Operating Characteristic Curves for Non-organ Confined Prostate Cancer.
Legend: Blue line = Memorial Sloan Kettering cancer calculator (MSKCC). Green line = ODX predictive score. Red Line = MSKCC + ODX model.


