Abstract
Background and Aims:
Women with bacterial vaginosis (BV) have increased risk of sexually transmitted infections and other adverse health outcomes. Based on the composition of their vaginal microbiota, women can broadly be classified into low-Lactobacillus (termed molecular-BV) and Lactobacillus-dominated profiles. Our objective was to determine the association between dietary macronutrient intake and molecular-BV.
Methods:
In a cross-sectional study of 104 reproductive-age women, dietary intake data were obtained using the Block Brief 2000 food frequency questionnaire. Vaginal microbiota composition was characterized by sequencing amplicons of the 16S rRNA gene V3-V4 regions and clustering into community state types (CST). Logistic regression was used to determine the association of macronutrient intake with molecular-BV (low-Lactobacillus vs. Lactobacillus-dominated CSTs combined).
Results:
Participants had a median age of 25.9 (interquartile range: 21.9-29.6), 58% were white (30% black), 51% overweight/obese and 52% on hormonal contraception. In multivariable models, diets richer in fiber were inversely associated with molecular-BV (adjusted odds ratio: 0.49 per standard deviation increase in energy-adjusted fiber intake, 95% confidence interval: 0.24-0.99; p=0.049).
Conclusions:
Our results indicate that diets richer in fiber were associated with lower odds of molecular-BV. Further studies are needed to confirm these findings and to test whether increasing fiber intake can modulate the microbiota towards a more optimal Lactobacillus-dominant profile.
Keywords: Diet, Nutrition, Fiber, vaginal microbiota, microbiome
Introduction:
Bacterial vaginosis (BV) is a common condition among reproductive-age women, characterized by low levels of Lactobacillus spp. and higher levels of a variety of primarily anaerobic bacteria, which can result in symptoms such as vaginal discharge or odor [1, 2]. Further, BV has also been associated with an increased risk of acquisition of sexually transmitted infections, including HIV [3–5], and with preterm delivery [4, 6, 7]. Prior studies have demonstrated a range of behavioral and demographic risk factors for BV, including race/ethnicity, hormonal contraception, sexual behaviors [2, 8], and intravaginal practices[9, 10]. Interestingly, some studies [11], but not others [12], have highlighted obesity as a potentially modifiable risk factor for BV [11]; however, the role of diet has not been well studied [13].
BV is clinically diagnosed based on Amsel’s criteria [14] and by Nugent score, a microscopy based-assay used in research settings [15]. Recent studies using 16S rRNA gene sequencing methods have allowed high resolution characterization of the vaginal microbiota composition. For example, using such compositional data, a study of 396 non-pregnant reproductive-age women showed that 27% of the women in the study had a low-Lactobacillus vaginal microbiota with a wide array of anaerobes such as Prevotella, Sneathia, Atopobium and Gardnerella [8]. In contrast, other types of microbiota were dominated by one of four species of Lactobacillus: L. crispatus, L. gasseri, L. iners and L. jensenii [8]. Although the specific proportion of women with either the low-Lactobacillus or Lactobacillus-dominated microbiota varies by setting, studies from diverse settings have also confirmed these two broad groupings [3, 16]. Importantly, a low-Lactobacillus vaginal microbiota is associated with increased vaginal pH and higher Nugent scores [8], and thus resembles BV. As a result, the term “molecular-BV” has been proposed to define a low-Lactobacillus vaginal microbiota to distinguish it from Nugent score-based diagnosis (i.e. “Nugent-BV”) which only relies on morphologic evaluation of the bacteria upon Gram-stain examination [17].
Prior studies [13, 18, 19], including from our group [20], have assessed associations of micronutrient intakes with BV but studies of macronutrient intake and BV are lacking. In one of these studies [13], a large longitudinal study of 1521 women from the United States (86% African-American), Neggers et al. demonstrated using a food frequency questionnaire that total fat intake was the only macronutrient associated with Nugent-BV. However, the relationship between dietary macronutrient intake with molecular-BV, a more accurate and in-depth characterization of the vaginal microbiota, has not been previously examined. In this study, we utilized a food frequency questionnaire and vaginal microbiota data from a study of reproductive-age non-pregnant women in order to evaluate the relationship between dietary macronutrient intake and molecular-BV.
Materials and Methods:
Study Population:
For these analyses, we analyzed the baseline visit of the Hormonal Contraception Longitudinal (HCL) Study [20], a longitudinal study of the impact of hormonal contraception (HC) on the vaginal microbiota. In the HCL study, 125 non-pregnant women of reproductive age were recruited from three Gynecology clinics at the Johns Hopkins Hospital system. Women were recruited who were using HC or reported an intention to initiate or cease HC, or were not using HC. Women with immunodeficiencies (including HIV), or medication conditions (such as polycystic ovary syndrome) which could impact endogenous sex hormone production were excluded from the study.
Ethics Statement:
The guidelines for United States Department of Health and Human Services on human studies were followed. Institutional review boards from Johns Hopkins University, University of Maryland and Columbia University approved this study and informed consent was obtained from all study participants.
Data and Sample Collection:
Clinical, behavioral and dietary intake data were collected from study participants at the baseline visit using detailed questionnaires. We used the Block Brief 2000 food frequency questionnaire (FFQ) to assess usual dietary intake. Participants are queried about the usual frequency of consumption and portion size of over 60 food items [21, 22]. Because of the reduced food list, estimates of nutrient intakes obtained from the Block Brief FFQ are lower than the estimates obtained from the full Block FFQ; the Brief FFQ is designed to rank individuals based on the distribution of usual intakes of each nutrient [21, 22]. The software supporting the FFQ also provides estimates of other dietary characteristics including intake by type of fat, fiber intake by source (grain, legumes), total sugar intake, and usual dietary glycemic index and glycemic load. At the baseline visit, clinicians collected mid-vaginal swabs from the study participants using the Eswabs system (Copan USA), followed by elution in liquid Amies media. The samples were then stored at −80°C until processing.
Laboratory assessments:
As previously described in detail [20], genomic DNA was isolated from 500 μL of vaginal swab eluate with the QS DSP Virus/Pathogen Midi Kit on the QiaSymphony Platform (Qiagen). After sequencing (see below), for samples with less than 15,000 sequence reads, we used a custom automated protocol to isolate DNA from 200 μL of vaginal swab eluate using the MagAttract Microbial DNA Kit (Qiagen) automated on a Microlab STAR system (Hamilton). Prior to nucleic acid extraction, cells were disrupted by shaking at 20 Hz for 20 minutes on a TissueLyser (Qiagen). The same procedures were followed for negative controls (water).
After DNA isolation, a 2-step polymerase chain reaction (PCR) targeting the V3-V4 region of the 16S rRNA gene was used for DNA library preparation as previously described [23]. Next-generation sequencing was performed on the Illumina HiSeq 2500 platform in Rapid Run mode and the sequence data were processed as previously described [23]. We used DADA2 to generate amplicon sequence variants (ASVs), and SILVA v128 16S rRNA gene database to train the RDP Naïve Bayesian classifier for taxonomic classification of ASVs [23–25]. Species-level annotations of ASVs were done using speciateIT (http://ravel-lab.org/speciateit). We excluded samples with <5,000 reads and taxa with study wide abundance <10−5.5
For community state type (CST) assignment, taxa relative abundances from a pool of 4,479 vaginal samples from the parent cohort were clustered into seven CSTs based on Jensen-Shannon distances and Ward linkage. Four CSTs were dominated by one species of Lactobacillus: CST I (L. crispatus), CST II (L. gasseri), CST III (L. iners) and CST V (L. jensenii) (Figure 1). Samples with low lactobacilli were assigned to CST IV (n= 23, diverse anaerobes including Gardnerella vaginalis, Atopobium vaginae and BVAB1), CST VI (n= 2, Streptococcus) and CST VII (n=1, Bifidobacterium), and thus were designated part of the molecular-BV group (Figure 1).
Figure 1: Heatmap of vaginal microbiota, stratified by community state type and fiber intake, n=104.
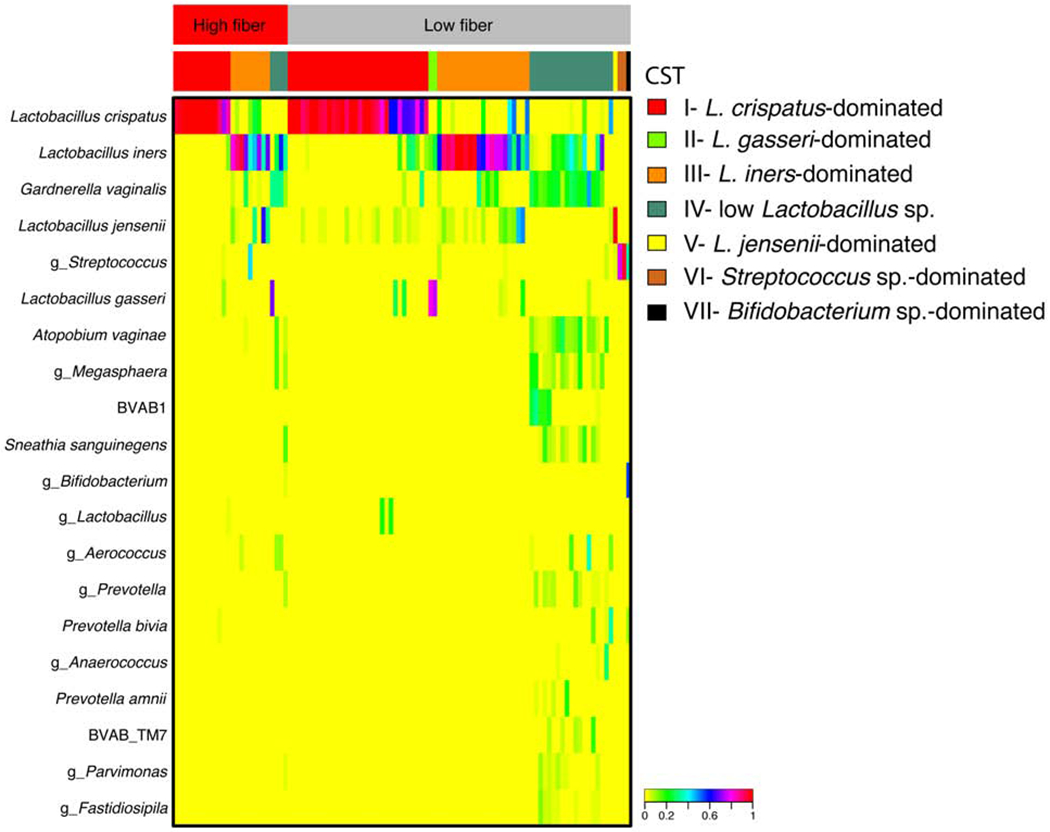
Heatmap shows the relative abundance for the top 20 most abundant taxa that were used to define each community state type (CST). The top bar displays the fiber quartile for the samples displayed below, and red is the highest quartile and gray represents all others. The second bar displays the CST stratifications. The heatmap shows the relative abundance of each taxa based on the coloring scheme in the legend.
Statistical analysis
We omitted individuals with no dietary intake data (n=13) and those (n=8) with improbable total energy intakes (<500 or >3500 kcal) from our analyses. The vaginal microbiota of women was categorized as a binary variable: Molecular-BV (CSTs IV, VI and VII) or not (i.e. Lactobacillus-dominant CSTs I, II, III and V). To assess differences in confounding and demographic variables by molecular-BV status, we used Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables.
Univariable (adjusted for total energy intake) and multivariable logistic regression models assessed the association between aspects of dietary intake and molecular-BV. Multivariable models were adjusted for known confounding factors (total energy, age, body mass index (BMI) and hormonal contraception use). Race was considered in multivariable models but was dropped as it was not significant in this analysis. As CST III (L. iners-dominated microbiota) has also been associated with BV and with increased risk of sexually transmitted infections [26], we performed a sensitivity analysis using multinomial logistic regression based on three categories for the outcome variable: a) L. iners-dominated (CST III) or b) other Lactobacillus-dominated CSTs (I, II and V) compared to c) molecular-BV (CSTs IV, VI and VII). We performed another sensitivity analysis in which CST VII (Bifidobacterium) was dropped from analysis because limited data are available on this CST to determine whether it is appropriate to define it as molecular-BV.
Separate analyses were performed for each dietary intake characteristic. For analysis purposes, all of the dietary intake variables were treated as continuous variables with regression results showing the odds of molecular-BV per unit increase of dietary intake variable. The unit for total energy was calculated per 100 Kcal. The unit for all other variables (except for glycemic index and daily servings of food groups) were calculated per one standard deviation (SD) increase.
Results:
Study population characteristics
Information on dietary intake and vaginal microbiota was available from 104 women. Women in this study had a median age of 25.9 (interquartile range (IQR): 21.9-29.6) and a median BMI of 25.2 (IQR: 21.9-32.9) kg/m2 (Table 1). Based on BMI, 51% of the study participants were overweight or obese (BMI >25). Fifty-eight percent of the women were white, 30% were black and 12% were from other races. Nearly half of the women reported use of hormonal contraception, 82% cited one or more sexual partners in the last two months and 7% were currently engaged in vaginal douching practices (Table 1).
Table 1:
Demographic and behavioral factors associated with Lactobacillus-dominated vaginal microbiota and molecular-BV, N=104
| Overall N=104 | Lactobacillus-dominant (CST I, II, III, V) N=78 | Molecular-BV CST IV N=26 | p-value* | |
|---|---|---|---|---|
| Age median (IQR) | 25.9 (21.9-29.6) | 25.9 (22.0-29.1) | 24.4 (21.9-29.7) | 0.83 |
| Race | ||||
| White | 60 (57.7) | 49 (63.0) | 11 (42.3) | 0.10 |
| Black | 31 (29.8) | 19 (24.4) | 12 (46.2) | |
| Other | 13 (12.5) | 10 (12.8) | 3 (11.5) | |
| BMI (kg/m2) median (IQR) | 25.2 (21.9-32.9) | 24.3 (21.5-29.8) | 31.9 (23.8-36.6) | 0.002 |
| Current douching | 7 (6.7) | 1 (1.2) | 6 (23.1) | <0.01 |
| # Sex partners last 2 mo | ||||
| 0 | 18 (17.3) | 15 (19.2) | 3 (11.5) | 0.55 |
| ≥1 | 86 (82.7) | 63 (80.8) | 23 (88.5) | |
| Current hormonal contraception use | 50 (48.1) | 43 (55.1) | 7 (26.9) | 0.01 |
Data are presented as number (%) of subjects or median value (interquartile range)
Abbreviations: IQR, interquartile range; BMI, body mass index; mo, months
Low-Lactobacillus microbiota were women whose microbiota was not dominated by Lactobacillus (CST IV, VI and VII); Lactobacillus-dominant microbiota were women whose microbiota was dominated by Lactobacillus (CST I, II, III and V).
P-values were calculated used Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables to determine the difference between low and high Lactobacillus microbiota
In this cohort, 25% of the women had molecular-BV at study entry (Figure 1). Women with molecular-BV or Lactobacillus-dominant microbiota profile differed in terms of BMI (p=0.002), current engagement in douching (p<0.01) and current hormonal contraception use (p=0.01), but did not differ in terms of age (p=0.83), race (p=0.10) or number of male partners in last two months (p=0.39) (Table 1). When compared with women with a Lactobacillus-dominant profile, women with molecular-BV had a higher median BMI (31.9 vs. 24.3), and were more likely to be currently engaged in douching (23.1% vs. 1.2%) but were less likely to be using hormonal contraception (26.9% vs. 55.1%) (Table 1).
Association of dietary intake with molecular-BV
In logistic regression models only adjusting for total energy intake, usual intakes richer in total fiber (odds ratio (OR: 0.45, 95% confidence intervals (CI): 0.23-0.87) and fiber intake density from grains (OR: 0.51, 95% CI: 0.26-0.99) were inversely associated with molecular-BV, whereas trans fat (OR: 2.00, 95% CI: 1.02-3.95) was positively associated with molecular-BV (Table 2). Fiber intake density from beans (OR: 0.59, 95% CI: 0.32-1.08) were also negatively associated with molecular-BV but this association was of borderline statistical significance (Table 2). Among individuals in the highest quartile of fiber intake, 15% had molecular-BV; in contrast, 28% of individuals with diets in lower levels (i.e. lower three quartiles) of fiber intake had molecular-BV (data not shown).
Table 2:
Distributions of dietary intake characteristic and associations with molecular-BV
| Univariable Model* | Multivariable model** | ||||
|---|---|---|---|---|---|
| N=104 | Exposure, per 1000 kcal** Median (IQR) | Odds Ratio# (95% CI) Per 100 kcal or per SD | p-value | Adjusted OddsRatio# 95% CI Per 100 kcal or per SD | p-value |
| Total Energy, Kcal# | 1379 (1026-1690) | 1.01# (0.94-1.09) | 0.40 | 0.96# (0.88-1.04) | 0.28 |
| Macronutrients## | |||||
| Fat, g | 40.9 (36.7-44.7) | 0.93 (0.35-2.44) | 0.88 | 1.43 (0.46-4.49) | 0.54 |
| Protein, g | 38.1 (33.0-43.9) | 0.87 (0.36-2.10) | 0.75 | 1.62 (0.58-4.52) | 0.36 |
| Carbohydrate, g | 114.6 (102.2-126.6) | 1.24 (0.45-3.41) | 0.68 | 0.64 (0.18-2.23) | 0.48 |
| Fiber, g | 9.6 (7.1-12.5) | 0.45 (0.23-0.87) | 0.02 | 0.49 (0.24-0.99) | 0.049 |
| Type of Fat## | |||||
| Saturated Fat, g | 13.7 (12.0-15.8) | 1.05 (0.46-2.40) | 0.90 | 1.62 (0.61-4.32) | 0.34 |
| Monounsaturated Fat, g | 15.7 (13.4-17.4) | 0.94 (0.40-2.22) | 0.88 | 1.42 (0.51-3.97) | 0.51 |
| Polyunsaturated Fat, g | 7.6 (6.2-9.1) | 0.86 (0.39-1.89) | 0.71 | 0.80 (0.32-2.04) | 0.65 |
| Cholesterol, mg | 127.8 (97.8-169.1) | 1.20 (0.71-2.02) | 0.50 | 1.58 (0.85-2.95) | 0.15 |
| Trans Fat, g | 1.3 (1.0-1.6) | 2.00 (1.02-3.95) | 0.04 | 1.32 (0.64-2.72) | 0.46 |
| Total sugars, g## | 53.9 (44.2-69.1) | 1.36 (0.71-2.59) | 0.35 | 1.01 (0.49-2.11) | 0.97 |
| Type of Fiber## | |||||
| Fiber from beans, g | 0.99 (0.52-1.84) | 0.59 (0.32-1.08) | 0.09 | 0.61 (0.30-1.24) | 0.17 |
| Fiber from vegetables & fruit, g | 4.8 (3.4-7.2) | 0.81 (0.49-1.35) | 0.49 | 0.89 (0.52-1.55) | 0.69 |
| Fiber from grains, g | 2.9 (2.2-3.6) | 0.51 (0.26-0.99) | 0.049 | 0.57 (0.28-1.18) | 0.13 |
| Composite variables | |||||
| Glycemic Index, % reference | 37.2 (28.9-49.3) | 0.99 (0.88-1.12) | 0.93 | 0.99 (0.86-1.13) | 0.87 |
| Glycemic Load (GL), GL units## | 52.6 (46.5-59.2) | 1.63 (0.56-4.74) | 0.37 | 0.72 (0.21-2.55) | 0.62 |
Reference category for the regression model was Lactobacillus-dominant microbiota Lactobacillus (CST I, II, III and V).
Univariable models were adjusted for total energy intake
Median and interquartile range (IQR) per 1000 kcal are presented for all dietary variables, except for total energy. Note: these were not the units used in regression models
Multivariable models adjusted for: total energy, age, BMI and hormonal contraception use.
Odds ratio (OR) for total energy are expressed per 100 Kcal.
All other OR, except for glycemic index are expressed per 1 standard deviation (SD) increase
In multivariable models adjusting for total energy, age, BMI and hormonal contraception use, usual intakes richer in total fiber (adjusted odds ratio (aOR) for energy-adjusted fiber intake: 0.49, 95% CI: 0.24-0.99) were inversely associated with molecular-BV (Table 2). Consistent with this finding are non-statistically significant but inverse associations for fiber intake density from beans (aOR: 0.61, 95% CI: 0.30-1.24), fiber intake density from grains (aOR: 0.57, 95% CI: 0.28-1.18), and energy-adjusted daily servings of grains (aOR: 0.76, 95% CI: 0.52-1.12) (Table 2 and Supplementary Table 1). All other dietary characteristics - nutrients, food group servings, glycemic index and load (Table 2 and Supplementary Table 1) were not associated with molecular-BV in univariable or multivariable models.
In sensitivity analysis, L. iners-dominated CST was considered a separate category (rather than part of the Lactobacillus-dominated profiles), the results for total fiber were more pronounced. Energy-adjusted total fiber intake was inversely associated with molecular-BV in univariable (OR: 0.40, 95% CI: 0.20-0.81) and in multivariable models (aOR: 0.42, 95% CI: 0.19-0.91), compared to Lactobacillus-dominated profiles, but not with L. iners-dominated CST III. Similarly, there were inverse associations for energy-adjusted fiber intake from beans (aOR: 0.52, 95% CI: 0.25-1.11), from grains (aOR: 0.46, 95% CI: 0.21-1.04) as well as for daily servings of grains (aOR: 0.66, 95% CI: 0.43-1.01) with molecular-BV in multivariable models, but the association were not statistically significant. No other dietary characteristic were associated with CST III. In another sensitivity analysis, dropping CST VII (Bifidobacterium-dominated CST; N=1) from analysis did not change the results.
Discussion:
In our study, women who reported consuming a diet richer in fiber (energy-adjusted fiber intake) were less likely to present with molecular-BV. Given the strength of the association, future interventional studies could evaluate if increasing dietary fiber intake, either alone or in combination with other treatment interventions, woud modulate the vaginal microbiota towards a more optimal Lactobacillus-dominant profile. Further, it will be important to test whether modulating fiber intake would improve other downstream cervicovaginal health outcomes such as reduced risk of premature birth or sexually transmitted infections.
To our knowledge, there are no prior studies that have assessed the association of macronutrients or fiber with molecular-BV. The only study with data directly relevant to our analysis is a prior study of dietary intake with Nugent-BV in 1,521 women (86% African-American) [13] in which BV was assessed with microscopic methods. The authors reported that diets richer in total fat, but not fiber, was associated with Nugent-BV [13]. Our data also revealed an increased odds for molecular-BV with diets richer in total fat, saturated fat and cholesterol, but the confidence intervals were relatively wide and not statistically significant, suggesting that our study may not have been powered adequately. It is also true that our dietary intake estimates are less precise because we utilized the brief questionnaire. Another potential reason for the difference in results could relate to differences in methodology. Neggers et. al. utilized the Nugent score to diagnose women with BV (Nugent-BV) whereas we used 16S rRNA gene amplicons sequencing to define molecular-BV, a more comprehensive assessment of the bacteria found in BV in studies without clinical examination. While those with Nugent-BV are more likely to have molecular-BV, the overlap is not 100% [8]. Other differences between the studies include distributions of race/ethnicity and geographic location. There may also be differences in fiber intake between the two populations, in terms of total fiber as well as by type of fiber consumed.
Previous observational and interventional studies have shown that dietary fiber can influence Lactobacillus species in the gut microbiota [27, 28], and our data suggests that this association may also be true for the vaginal microbiota. The potential for this relationship is further supported by data from an in vitro study showing that various prebiotic fibers stimulate the growth of monocultures of the major Lactobacillus species dominating the vaginal microbiota, while not affecting the BV-related microbes such as G. vaginalis [29]. Prior work has also demonstrated that there is some concordance in Lactobacillus species in the rectum and the vagina [30–32], although, in the gut microbiota, there are some major differences such as lack of L. iners and L. crispatus. In addition to gut microbiota, dietary fiber may also modulate other aspects of host physiology such as gut barrier integrity, microbial translocation and inflammation [33–36]; we hypothesize that the potential effect of dietary fiber on the vaginal microbiota might be more systemic rather than through direct colonization from the gut. Another link between diet and BV could be mediated through obesity, which has been associated with both BV [11] and lower fiber intake [37], although there is conflicting data on the relationship between obesity and BV [12]. In our study, women with molecular-BV had significantly higher BMI compared to Lactobacillus-dominant women, but we observed an association of diets richer in fiber and vaginal microbiota even after adjusting for BMI. Future studies will need to integrate assessment of the gut and vaginal microbiota along with host physiology to better understand the observed relationships.
This study has a few limitations. Our sample size for this study was relatively small (n=104) which may have limited power to detect potentially significant associations (e.g. fibers from beans, fiber from grains, cholesterol). Future larger studies will be needed to replicate these findings. We used a brief FFQ that allowed for ranking of participants with respect to their dietary exposures but not quantification of absolute intakes. The brief FFQ also did not query intake of fiber from dietary supplements, and this is a limitation that should be addressed in future studies. The cross-sectional nature of our study meant that we were only able to assess associations at one time point, and we could not assess the stability of the vaginal microbiota over time. Gajer and Brotman et al. have previously described community classes in which women with similar temporal microbiota can be clustered together [38] and we expect that women with an individual detection of a molecular BV observation will have a general pattern of fluctuating in that domain. Indeed, non-differential misclassification is known to suppress point estimates toward the null, making it less likely to detect an association, and therefore, suggests that the associations noted in this study between energy-adjusted fiber intake and Lactobacillus-dominated states may be underestimates. Despite these limitations, our study provides valuable new information by utilizing molecular approaches to characterize the vaginal microbiota to evaluate whether dietary characteristics related to macronutrient intake are related to molecular-BV presentation. Even with the limited sample size, we were able to observe an inverse association between diets richer in fiber with molecular-BV.
In conclusion, we show that energy-adjusted fiber intake was inversely associated with molecular-BV, suggesting that consuming a diet higher in fiber could modulate the microbiota towards more Lactobacillus-dominant profiles and improve cervicovaginal health. Future interventional studies will be able to evaluate causal links.
Supplementary Material
Acknowledgements:
The authors thank the study participants for their time and contributions.
Funding This research was supported by NIH grant R01AI089878 (PI: KGG). RS is supported by R00HD089753. ST is supported by K23AI125715.
Abbreviations:
- BV
Bacterial Vaginosis
- CST
community state types
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None declared. ST is a consultant for Biofire Diagnostics and Roche Molecular Diagnostics. JR is co-founder of LUCA Biologics, a biotechnology company focusing on translating microbiome research into live biotherapeutics drugs for women’s health.
References:
- [1].Coleman JS, Gaydos CA. Molecular Diagnosis of Bacterial Vaginosis: an Update. Journal of clinical microbiology. 2018;56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Martin DH. The microbiota of the vagina and its influence on women’s health and disease. Am J Med Sci. 2012;343:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gosmann C, Anahtar MN, Handley SA, Farcasanu M, Abu-Ali G, Bowman BA, et al. Lactobacillus-Deficient Cervicovaginal Bacterial Communities Are Associated with Increased HIV Acquisition in Young South African Women. Immunity. 2017;46:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kroon SJ, Ravel J, Huston WM. Cervicovaginal microbiota, women’s health, and reproductive outcomes. Fertil Steril. 2018;110:327–36. [DOI] [PubMed] [Google Scholar]
- [5].Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12:1699–706. [DOI] [PubMed] [Google Scholar]
- [6].Elovitz MA, Gajer P, Riis V, Brown AG, Humphrys MS, Holm JB, et al. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nature communications. 2019;10:1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fettweis JM, Serrano MG, Brooks JP, Edwards DJ, Girerd PH, Parikh HI, et al. The vaginal microbiome and preterm birth. Nature medicine. 2019;25:1012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences of the United States of America. 2011;108 Suppl 1:4680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Brotman RM, Klebanoff MA, Nansel TR, Andrews WW, Schwebke JR, Zhang J, et al. A longitudinal study of vaginal douching and bacterial vaginosis--a marginal structural modeling analysis. AmJEpidemiol. 2008;168:188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hassan WM, Lavreys L, Chohan V, Richardson BA, Mandaliya K, Ndinya-Achola JO, et al. Associations between intravaginal practices and bacterial vaginosis in Kenyan female sex workers without symptoms of vaginal infections. Sex Transm Dis. 2007;34:384–8. [DOI] [PubMed] [Google Scholar]
- [11].Brookheart RT, Lewis WG, Peipert JF, Lewis AL, Allsworth JE. Association between obesity and bacterial vaginosis as assessed by Nugent score. Am J Obstet Gynecol. 2019;220:476 e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lokken EM, Richardson BA, Kinuthia J, Mwinyikai K, Abdalla A, Jaoko W, et al. A Prospective Cohort Study of the Association Between Body Mass Index and Incident Bacterial Vaginosis. Sexually transmitted diseases. 2019;46:31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Neggers YH, Nansel TR, Andrews WW, Schwebke JR, Yu KF, Goldenberg RL, et al. Dietary intake of selected nutrients affects bacterial vaginosis in women. The Journal of nutrition. 2007;137:2128–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. AmJMed. 1983;74:14–22. [DOI] [PubMed] [Google Scholar]
- [15].Nugent R, Krohn M, Hillier S. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991;29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Klatt NR, Cheu R, Birse K, Zevin AS, Perner M, Noel-Romas L, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science. 2017;356:938–45. [DOI] [PubMed] [Google Scholar]
- [17].McKinnon LR, Achilles SL, Bradshaw CS, Burgener A, Crucitti T, Fredricks DN, et al. The Evolving Facets of Bacterial Vaginosis: Implications for HIV Transmission. AIDS research and human retroviruses. 2019;35:219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Christian P, Labrique AB, Ali H, Richman MJ, Wu L, Rashid M, et al. Maternal vitamin A and beta-carotene supplementation and risk of bacterial vaginosis: a randomized controlled trial in rural Bangladesh. The American journal of clinical nutrition. 2011;94:1643–9. [DOI] [PubMed] [Google Scholar]
- [19].Moore KR, Harmon QE, Baird DD. Serum 25-Hydroxyvitamin D and Risk of Self-Reported Bacterial Vaginosis in a Prospective Cohort Study of Young African American Women. J Womens Health (Larchmt). 2018;27:1278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tuddenham S, Ghanem KG, Caulfield LE, Rovner AJ, Robinson C, Shivakoti R, et al. Associations between dietary micronutrient intake and molecular-Bacterial Vaginosis. Reprod Health. 2019;16:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1:58–64. [DOI] [PubMed] [Google Scholar]
- [22].Delgado C, Ward P, Chertow GM, Storer L, Dalrymple L, Block T, et al. Calibration of the brief food frequency questionnaire among patients on dialysis. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2014;24:151–6 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Holm JB, Humphrys MS, Robinson CK, Settles ML, Ott S, Fu L, et al. Ultrahigh-Throughput Multiplexing and Sequencing of >500-Base-Pair Amplicon Regions on the Illumina HiSeq 2500 Platform. mSystems. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and environmental microbiology. 2007;73:5261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].van Houdt R, Ma B, Bruisten SM, Speksnijder A, Ravel J, de Vries HJC. Lactobacillus iners-dominated vaginal microbiota is associated with increased susceptibility to Chlamydia trachomatis infection in Dutch women: a case-control study. Sexually transmitted infections. 2018;94:117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Florowska A, Krygier K, Florowski T, Dluzewska E. Prebiotics as functional food ingredients preventing diet-related diseases. Food & function. 2016;7:2147–55. [DOI] [PubMed] [Google Scholar]
- [28].Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut microbes. 2017;8:172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Collins SL, McMillan A, Seney S, van der Veer C, Kort R, Sumarah MW, et al. Promising Prebiotic Candidate Established by Evaluation of Lactitol, Lactulose, Raffinose, and Oligofructose for Maintenance of a Lactobacillus-Dominated Vaginal Microbiota. Applied and environmental microbiology. 2018;84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Antonio MA, Rabe LK, Hillier SL. Colonization of the rectum by Lactobacillus species and decreased risk of bacterial vaginosis. The Journal of infectious diseases. 2005;192:394–8. [DOI] [PubMed] [Google Scholar]
- [31].El Aila NA, Tency I, Claeys G, Verstraelen H, Saerens B, Santiago GL, et al. Identification and genotyping of bacteria from paired vaginal and rectal samples from pregnant women indicates similarity between vaginal and rectal microflora. BMC infectious diseases. 2009;9:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Petricevic L, Domig KJ, Nierscher FJ, Krondorfer I, Janitschek C, Kneifel W, et al. Characterisation of the oral, vaginal and rectal Lactobacillus flora in healthy pregnant and postmenopausal women. European journal of obstetrics, gynecology, and reproductive biology. 2012;160:93–9. [DOI] [PubMed] [Google Scholar]
- [33].Jeffery IB, O’Toole PW. Diet-Microbiota Interactions and Their Implications for Healthy Living. Nutrients. 2013;5:234–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Claesson MJ, Jeffery IB, Conde S, Power SE, O’Connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–84. [DOI] [PubMed] [Google Scholar]
- [35].Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. Journal of clinical gastroenterology. 2006;40:235–43. [DOI] [PubMed] [Google Scholar]
- [36].Daien CI, Pinget GV, Tan JK, Macia L. Detrimental Impact of Microbiota-Accessible Carbohydrate-Deprived Diet on Gut and Immune Homeostasis: An Overview. Frontiers in immunology. 2017;8:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dahl WJ, Stewart ML. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. Journal of the Academy of Nutrition and Dietetics. 2015;115:1861–70. [DOI] [PubMed] [Google Scholar]
- [38].Gajer P, Brotman RM, Bai G, Sakamoto J, Schutte UM, Zhong X, et al. Temporal dynamics of the human vaginal microbiota. Science translational medicine. 2012;4:132ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


