Abstract
Background
Mutations in the X-linked gene WDR45 cause neurodegeneration with brain iron accumulation type 5 (NBIA5). Global developmental delay is seen at an early age with a slow progression to dystonia, parkinsonism, and dementia due to progressive iron accumulation in the brain.
Methodology
We present 17 new cases and reviewed 106 reported cases of NBIA5. Detailed information related to developmental history and key time to event measures was collected.
Results
Within this cohort, there were 19 males. Most individuals were molecularly diagnosed by whole exome testing. Overall 10 novel variants were identified across 11 subjects. All individuals were affected by developmental delay, most prominently in verbal skills. Most individuals experienced a decline in motor and cognitive skills. While most individuals were affected by seizures, the spectrum ranged from provoked seizures to intractable epilepsy. The imaging findings varied as well, often evolving over time. The classic iron accumulation in the globus pallidus and substantia nigra was noted half of our cohort and was associated with an older age of image acquisition, while myelination abnormalities were associated with a younger age.
Conclusions
WDR45 is a progressive and evolving disorder, which is often delayed in diagnosis. Developmental delay and seizures predominate early childhood, followed by a progressive decline of neurologic function. There is variable expressivity in the clinical phenotypes of individuals with WDR45 mutations, suggesting that this gene should be considered in the diagnostic evaluation of children with myelination abnormalities, iron deposition, developmental delay, and epilepsy depending on the age at evaluation.
Keywords: Hypomyelination, Epileptic encephalopathy, WDR45, Developmental delay
Introduction
The X-chromosomal gene WDR45 is associated with dominantly inherited beta-propeller protein-associated neurodegeneration (BPAN) also known as neurodegeneration with brain iron accumulation, type 5 (NBIA5; MIM 300894).1 WDR45 encodes a beta-propeller protein involved in lysosomal autophagy and endoplasmic reticulum homeostasis.2,3 The clinical role of this gene was originally identified through exome sequencing of individuals within an NBIA cohort.4 Clinically, children affected by BPAN typically present with developmental delay, seizures, and disordered sleep.4–7 Later in the disease course, individuals typically develop dementia and Parkinsonian features.7 Affected males have been rarely reported in the literature, as this disorder was first considered to be embryonic lethal in males.4,8,9
With the growing availability of broader genetic platforms such as whole exome and whole genome sequencing (WES and WGS, respectively), there is a growing appreciation of a broader phenotypic range for previously characterized disorders.10 This is of key importance as targeted therapies are developed. This report describes 123 individuals with WDR45 variants, outlining a spectrum of clinical and radiographic findings found in this rare disorder.
Methodology
We identified 123 individuals with WDR45-related disease through natural history studies and a review of the medical literature (Table 1, Supplemental table). All case reports with individual-level developmental and/or radiographic information were included in this cohort. Informed consent was obtained for novel cases through one of three IRB-approved protocols: “Myelin Disorders Bioregistry Project (MDBP)”, “The Epilepsy Genetics Research Project”, or “Metabolic Consequences of Primary Mitochondrial Disease”. As available, the complete histories, imaging, and WDR45 sequencing were reviewed. Demographic information, including age at last evaluation, was collected. Date of acquisition of milestones was obtained from medical record and/or literature review. When age of acquisition was noted to be “normal”, the p50 data was used from the Denver Developmental Screening Test II (DDST-II).11 When the specific age at which a skill was acquired was unknown, but noted as delayed, two standard deviations beyond the DDST-II p90 was used. Microcephaly was identified in medical records and defined as more than 2 standard deviations from the mean for head circumference, or as noted “microcephalic”. Available images were reviewed by a neuroradiologist, and otherwise the radiographic reports were used for source data.
Table 1.
Demographics and key outcome measures within the WDR45 cohort
| 1A. Demographics | |
|---|---|
| Cases: N | 123 |
| Cases from literature review: N (%) | 106 (86%) |
| Sex (F): N (%) | 104 (8.5%) |
| Diagnosis | |
| Average age in years (from n=60) | 16.7 +/− 14.3 (SD) |
| Range age in years (from n=60) | 0.8 – 52.0 years |
| Molecular diagnostic method (from n=83) | |
| Targeted (Single-Gene) or Panel Test: N (%) | 24 (28.9%) |
| Whole exome testing: N (%) | 55 (66.3%) |
| Whole genome testing: N (%) | 4 (4.8%) |
| Clinical presentation | |
| Average age (from 91 individuals) | 0.9 +/− 0.8 |
| Range of ages in years (from 91 individuals) | 0.1–6.0 |
| Clinical features at presentation (from n=123) | |
| Developmental delay: N (%) | 74 (60.5) |
| Seizures: N (%) | 32 (26.0) |
| Movement disorder: N (%) | 3 (2.4) |
| Autistic features: N (%) | 1 (0.8) |
| Microcephaly noted (from 47 individuals): N (%) | 13 (27.7) |
| Clinical Trajectory | |
| Progressive loss of motor function (from 84 individuals) : N (%) | 41 (51.2) |
| Progressive loss of verbal function (from 71 individuals) : N (%) | 27 (38.0) |
| 1B. Imaging Findings | |
| MRIs: N | 159 |
| Individuals with MRIs available: N | 98 |
| Average age in years (from n=89) | |
| Range age in years (from n=60) | |
| MRI features | |
| Atrophy | |
| Supratentorial: N (%) | 104 (65.4) |
| Corpus Collosum: N (%) | 60 (37.7) |
| Brainstem: N (%) | 6 (3.8) |
| Cerebellum: N (%) | 37 (23.3) |
| Abnormal Myelination: N (%) | 45 (28.3) |
| Iron deposition: N (%) | 82 (51.6) |
Statistical Analysis
The total number of cases analyzed for each parameter varied based on the availability of the pertinent medical information. Developmental milestones were grouped by sex and compared to the normative data derived from the Denver Developmental Screening Test (DDST-II) 12. Skill acquisition was binned by percentage of the cohort who had attained a skill at a given age. In order to compare achievement of milestones by sex, we created Kaplan-Meier curves for acquisition of developmental milestones. Log-rank (Mantel-Cox) tests were used to compare acquisition of developmental milestones by sex. Age of acquisition of milestones was also plotted, including the age at which percentiles of the population (p10 for 10%, p25 for 25%, etc.) had achieved that specific milestone. Statistical analysis was performed in SAS and figures were prepared in Prism 8.0. CaseLog was used to generate the genetics figure (Yanshen Yang, Jörg Hakenberg, and Kyle Farh, Personal communication, January 2020). The Mann-Whitney test is used to compare differences between two independent groups, using a 2 tailed p value.
Results
We identified 123 individuals, including 19 males, with pathogenic or likely pathogenic WDR45 variants. Of these, 10 were novel variants found in 11 individuals. These variants had a range of consequences including splice site variants, nonsense variants, frameshift variants, missense variant, and deletions (Figure 1C). All affected individuals carried de novo variants except for five affected males who inherited variants from their reportedly asymptomatic mothers. Males on average presented at a younger age than females (Figure 1B). The median age at presentation 0.8 years for females vs 0.3 years for males (Mann Whitney test, 2-tailed p value <0.0001).
Figure 1.
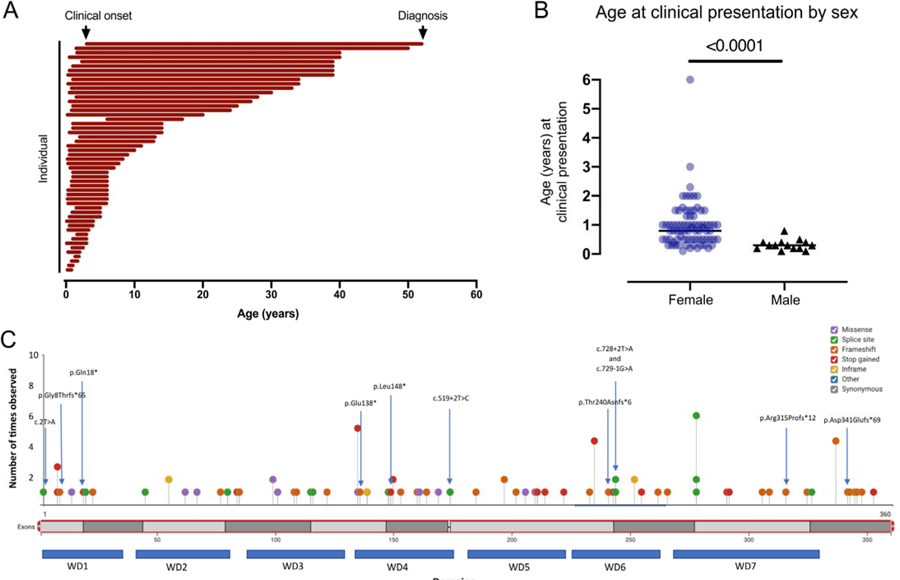
Presentation and diagnosis of WDR45. (A) Each line represents the interval in years between clinical onset and diagnosis (n=54, including n=6 males). (B) The age at clinical presentation was compared by sex (line= median value; n=77 females, n=14 males; Mann Whitney test, 2-tailed p value <0.001). (C) Distribution of variants found in the cohort of individuals with WDR45-related disease.
The age at diagnosis was available for 60 individuals (Figure 1A). From this subset, there was a mean age at diagnosis of 16.7 years (standard deviation [SD] of 14.3 years; range 0.8–52.0 years). The method of molecular diagnosis was identified for 83 individuals, of whom 55 were found by whole exome testing and an additional 4 were by whole genome testing (Table 1A). The remaining individuals were identified by targeted or panel testing. Both the age at diagnosis and the age at clinical presentation was available for 54 individuals, including 5 males (Figure 1A). For this cohort, there was an average delay of 15.7 years from presentation to diagnosis (SD 14.6 years, range 0.6 – 49.0 years).
Developmental information was available on 100 individuals. For the 91 individuals with detailed medical information, the average age at clinical onset was 0.9 years (SD 0.8, range 0.1–6.0) (Table 1A, Figure 1A–B). The clinical presentation features were divided into non-mutually exclusive categories: developmental delay, movement disorders, autistic features, and seizures. The most common presenting signs were developmental delay (74/123 individuals, 60.5%) and seizures (32/123, 26%). Head circumference data was available for 47 individuals. Of these, 13 (27.7%) were noted to be microcephalic.
All individuals within this cohort were noted to have developmental delay (Figure 2C–D). This was more profound in males (Figure 2C–D). The individuals included in this cohort reached an average age of 17.0 years (SD 14.4, range 0.8–52 years) at the time of last data collection or publication. Over the course of the available data, ultimately 43 of 84 individuals (51.2%) demonstrated a loss of motor skills, and 27 of 71 individuals (38.0%) demonstrated a loss of verbal skills, underscoring the progressive nature of this disorder. Approximately 40% of females (24 of 59 individuals) and the one male who was known to have gained independent ambulation (IA) lost this skill over the duration of record collection or up to the time of publication. The maximum available age for the individuals without decline was lower than the maximum age available for individuals with progressive disease (6.6 years versus 29.0 for motor progression; 10.9 years versus 24.1 for verbal progression). Furthermore, the ability to ambulate with assistance (such as with a walker or support) was lost in 22.6% (14/62) females and in 1 of 2 of the males with this skill (Figure 2A). Other key events, such as feeding tube placement, were not consistently found in the medical records or literature.
Figure 2.
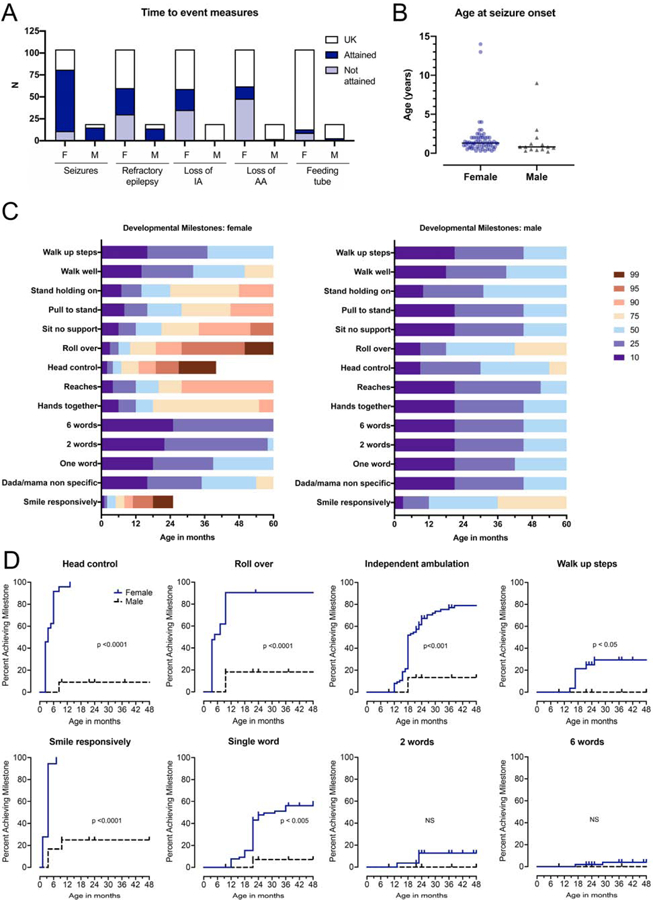
Key time to event measures and milestones in WDR45-related disease. (A) In the WDR45 population, important events included the presence of seizures, refractory epilepsy, loss of independent ambulation (IA) and loss of assisted ambulation (AA). Other events, such as feeding tube placement, were not consistently found in the medical records or literature. The dark blue bars represent event attained, light blue is not attained, while white bars represent unknown (UK). (B) Age at first seizure was compared across sex cohorts and was not significantly different (n=63 females; n=14 males; Mann Whitney test, two-tailed p value p=0.0590). (C) Developmental skill acquisition plots (“developmental heat maps”) were created to visualize the difference in development between females (left) and males (right). The designation of ‘10’ represents less than 10% of the population has acquired a milestone by the age indicated on the x-axis, while the designation of ‘90’ represents that up to 90% of the population has acquired the skill by the given age. The color-coded heat map indicates the percentage of the population who has attained the milestone at a given age, as shown on the x-axis. milestone acquisition by genotype. (D) Age at developmental skill acquisition was presented with Kaplan-Meier curves as compared across females (blue, solid line) and males (black, dotted line). P-values were calculated using the logrank test comparing sexes (p values included in figure).
Most individuals with WDR45-related disease had seizures (70 of 81 females and 14 of 15 males) (Figure 2B), although there was a wide range of severity from fever-provoked seizures to subclinical seizures to infantile spasms. Most males had refractory epilepsy (13/14; 92.9%) versus half of the female population (30 of 60 individuals). The spectrum of seizure semiologies included atonic, tonic-clonic, focal, myoclonic, and absence seizures. On electroencephalogram (EEG), a mix of generalized and focal discharges were common, as previously described 6. Age at first seizure was available for 65 of the 70 females with a history of seizure and all males (Figure 2B). There was no statistical difference in the onset of seizures by sex.
Classically, WDR45 mutations are associated with iron accumulation noted on cerebral imaging. Magnetic resonance imaging (MRI) results from 159 scans were available for 98 individuals within the cohort (Table 1B). The average age at imaging was 11.2 years (range 0.1 – 50 years). Iron deposition (82/159; 51.6%), atrophy (104/159; 65.4%), and abnormal myelination (45/159; 28.3%) were all commonly identified features (Figure 3). The age at image acquisition was available for 149 of the 159 scans. Abnormal myelination was significantly associated with a younger age, while the detection of iron was associated with an older age at image acquisition (Mann Whitney test with 2 tailed p values, both p <0.0001) (Figure 4).
Figure 3.
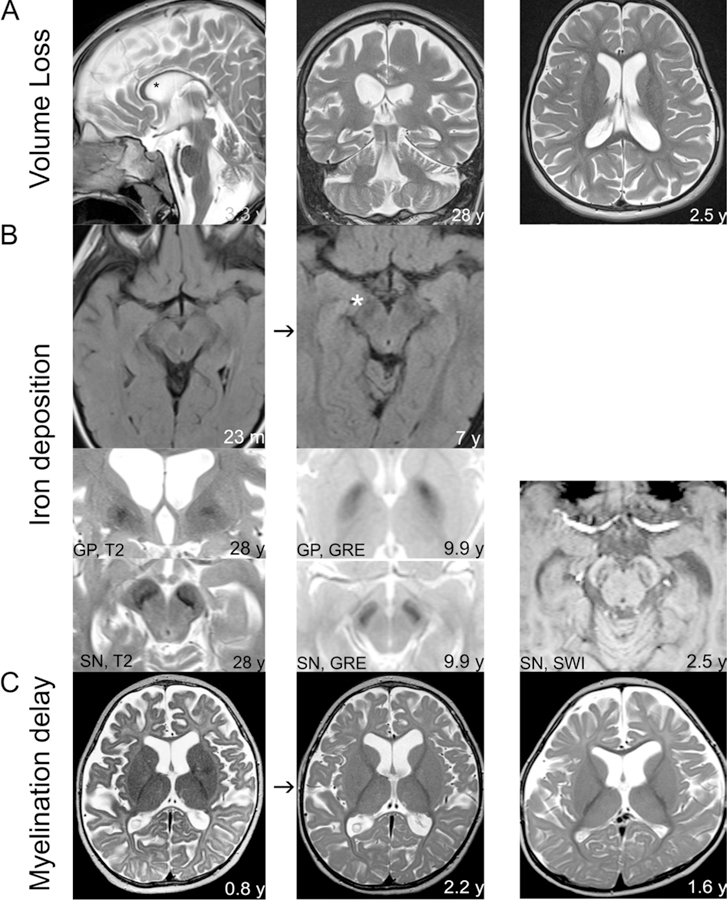
The spectrum of imaging findings in individuals affected by changes in WDR45. A. Imaging from three individuals demonstrating low volume, including of the corpus callosum (* left panel) and supra- and infra-tentorial regions middle panel). B. In some individuals, iron deposition was absent in the initial set of images, but later was evident (second row). Evidence of iron deposition was found in a subset of individuals in the Substantia Nigra (SN) and/or Globus Pallidus (GP) using T2, GRE, and SWI modalities. C. In a subset of individuals, myelination was delayed (left and middle panels) and low in volume (right panel).
Figure 4.
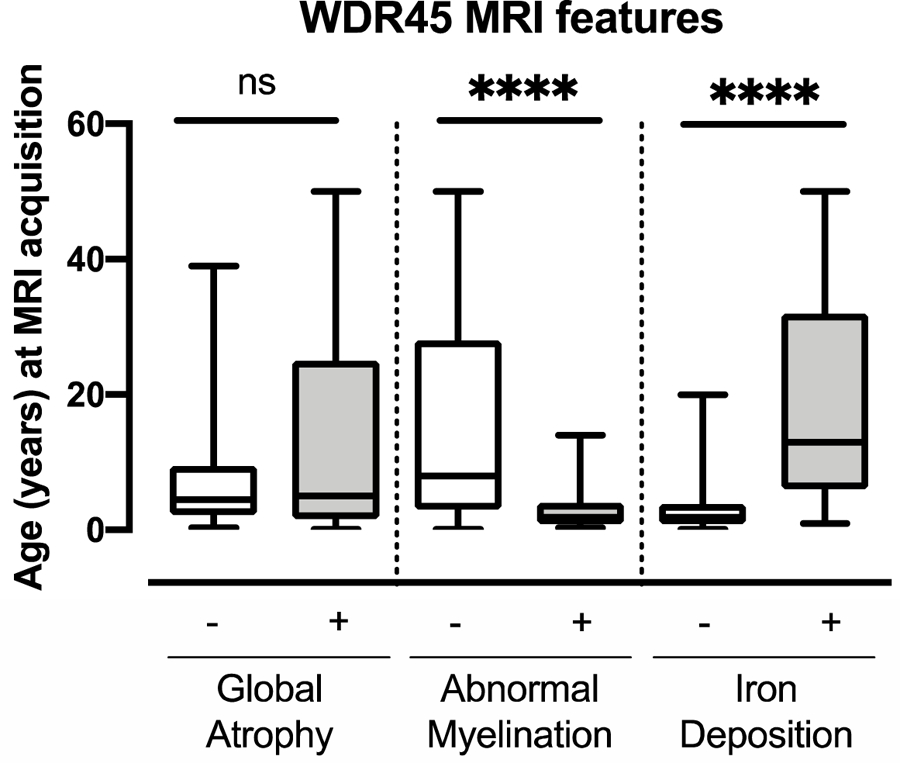
The characteristics identified by MRI correlate with age at imaging acquisition. Age at acquisition was available for 149 MRIs. The Mann Whitney test was used to compare the age of image acquisition by the presence of key radiographic features (2 tailed p <0.0001 for abnormal myelination and iron deposition).
Discussion
In this report, we present 123 individuals with WDR45-related disease, including 17 new cases. This analysis of both novel cases as well an in-depth review of cases from the literature emphasizes the evolution this rare disease. Depending on the sex and the age at evaluation, there are variable clinical features and imaging findings. Across the cohort, there was early developmental delay, frequently accompanied by seizures. Imaging revealed early myelination delay, followed by evidence of iron deposition.
Our cohort includes 19 males, and our results suggest that males develop a more severe phenotype, with earlier presentation. In females, the developmental delay was more variable. Unfortunately, the majority of individuals had a delay to diagnosis from the time of clinical presentation of 16 years on average. While this report represents the largest series of WDR45 cases to date, it is limited by the availability of medical reports and information from case reports. The findings however are consistent with previously published case reports and clinical experience. Additionally, as this was a cohort collected by enrollment and published cases, there is a bias towards the inclusion of individuals with a stronger phenotype. There are several reports of males inheriting the variant from an asymptomatic mother. Similar cases, of mild or normal phenotypes, would not have been captured in this study.
Leukoencephalopathy is not an uncommon finding in individuals with epileptic encephalopathy and has been previously identified in individuals affected by BPAN.13–19 In our cohort, we found earlier MRIs to reflect abnormal myelination, while later imaging was more likely to be notable for iron deposition. This underscores that WDR45-related imaging findings evolve over time.6,19 As found in other early onset epileptic encephalopathies, we hypothesize that delayed myelination will continue to improve over time and ultimately may normalize.
Our report underscores the importance of expanding the phenotypic spectrum of rare genetic disorders as children are often referred to different specialists at different ages. The majority of individuals in this cohort were identified through more broad-based diagnostic platforms, including whole exome and whole genome testing. The spectrum of variable clinical features observed in individuals affected by WDR45 variants as described here overlaps with other genetic conditions. This suggests that use of whole genome or whole exome sequencing as a first-tier diagnostic test may shorten the diagnostic odyssey and expedite appropriate clinical care and counseling.
Supplementary Material
Acknowledgements:
We would like to acknowledge the support and participation of the families affected by WDR45. We would also like to thank Erin Thorpe, Krista Bluske, Alison Coffey, Anjana Chandrasekhar, and Amirah Khouzam for scientific input on variants detected in this gene.
Funding disclosures:
LA: Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number KL2TR001879 and Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number K23NS114113. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
AV: Supported by the Kamens endowed chair for Translational Neurotherapeutics and the Myelin Disorders Bioregistry Project.
AM, DLP, RJT, TH are Illumina employees
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hayflick SJ, Kurian MA, Hogarth P. Neurodegeneration with brain iron accumulation. Handbook of clinical neurology. 2018;147:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan H, Wang Q, Chen X, et al. WDR45 contributes to neurodegeneration through regulation of ER homeostasis and neuronal death. Autophagy. 2019:1–17. [DOI] [PMC free article] [PubMed]
- 3.Stanga D, Zhao Q, Milev MP, Saint-Dic D, Jimenez-Mallebrera C, Sacher M. TRAPPC11 functions in autophagy by recruiting ATG2B-WIPI4/WDR45 to preautophagosomal membranes. Traffic (Copenhagen, Denmark). 2019;20(5):325–345. [DOI] [PubMed] [Google Scholar]
- 4.Haack TB, Hogarth P, Kruer MC, et al. Exome sequencing reveals de novo WDR45 mutations causing a phenotypically distinct, X-linked dominant form of NBIA. American journal of human genetics. 2012;91(6):1144–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haack TB, Hogarth P, Gregory A, Prokisch H, Hayflick SJ. BPAN: the only X-linked dominant NBIA disorder. International review of neurobiology. 2013;110:85–90. [DOI] [PubMed] [Google Scholar]
- 6.Hayflick SJ, Kruer MC, Gregory A, et al. beta-Propeller protein-associated neurodegeneration: a new X-linked dominant disorder with brain iron accumulation. Brain : a journal of neurology. 2013;136(Pt 6):1708–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long M, Abdeen N, Geraghty MT, Hogarth P, Hayflick S, Venkateswaran S. Novel WDR45 Mutation and Pathognomonic BPAN Imaging in a Young Female With Mild Cognitive Delay. Pediatrics. 2015;136(3):e714–717. [DOI] [PubMed] [Google Scholar]
- 8.Saitsu H, Nishimura T, Muramatsu K, et al. De novo mutations in the autophagy gene WDR45 cause static encephalopathy of childhood with neurodegeneration in adulthood. Nature genetics. 2013;45(4):445–449, 449e441. [DOI] [PubMed] [Google Scholar]
- 9.Abidi A, Mignon-Ravix C, Cacciagli P, Girard N, Milh M, Villard L. Early-onset epileptic encephalopathy as the initial clinical presentation of WDR45 deletion in a male patient. European journal of human genetics : EJHG. 2016;24(4):615–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanderver A, Simons C, Helman G, et al. Whole exome sequencing in patients with white matter abnormalities. Annals of neurology. 2016;79(6):1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frankenburg WK. Denver II. In: Denver, CO: : Denver Developmental Materials, [1990] ©1990; 1990. [Google Scholar]
- 12.Jeong SU, Kim GC, Jeong HJ, et al. The Validity of the Bayley-III and DDST-II in Preterm Infants With Neurodevelopmental Impairment: A Pilot Study. Annals of rehabilitation medicine. 2017;41(5):851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvill GL, Liu A, Mandelstam S, et al. Severe infantile onset developmental and epileptic encephalopathy caused by mutations in autophagy gene WDR45. Epilepsia. 2018;59(1):e5–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burger BJ, Rose S, Bennuri SC, et al. Autistic Siblings with Novel Mutations in Two Different Genes: Insight for Genetic Workups of Autistic Siblings and Connection to Mitochondrial Dysfunction. Frontiers in pediatrics. 2017;5:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakashima M, Takano K, Tsuyusaki Y, et al. WDR45 mutations in three male patients with West syndrome. Journal of human genetics. 2016;61(7):653–661. [DOI] [PubMed] [Google Scholar]
- 16.Morikawa M, Takano K, Motobayashi M, et al. Clinical features of a female with WDR45 mutation complicated by infantile spasms: a case report and literature review. Brain & development. 2017. [DOI] [PubMed]
- 17.Takano K, Shiba N, Wakui K, et al. Elevation of neuron specific enolase and brain iron deposition on susceptibility-weighted imaging as diagnostic clues for beta-propeller protein-associated neurodegeneration in early childhood: Additional case report and review of the literature. American journal of medical genetics Part A. 2016;170a(2):322–328. [DOI] [PubMed] [Google Scholar]
- 18.Khalifa M, Naffaa L. Exome sequencing reveals a novel WDR45 frameshift mutation and inherited POLR3A heterozygous variants in a female with a complex phenotype and mixed brain MRI findings. European journal of medical genetics. 2015;58(8):381–386. [DOI] [PubMed] [Google Scholar]
- 19.Rathore GS, Schaaf CP, Stocco AJ. Novel mutation of the WDR45 gene causing beta-propeller protein-associated neurodegeneration. Movement disorders : official journal of the Movement Disorder Society. 2014;29(4):574–575. [DOI] [PubMed] [Google Scholar]
- 20.Kaleka G, McCormick ME, Krishnan A. Beta-Propeller Protein-Associated Neurodegeneration (BPAN) Detected in a Child with Epileptic Spasms. Cureus. 2019;11(8):e5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christoforou S, Christodoulou K, Anastasiadou V, Nicolaides P. Early-onset presentation of a new subtype of beta-Propeller protein-associated neurodegeneration (BPAN) caused by a de novo WDR45 deletion in a 6 year-old female patient. European journal of medical genetics. 2019:103765. [DOI] [PubMed]
- 22.Hornemann F, Le Duc D, Roth C, Pfaffle R, Huhle D, Merkenschlager A. Childhood Dystonia-Parkinsonism Following Infantile Spasms-Clinical Clue to Diagnosis in Early Beta-Propeller Protein-Associated Neurodegeneration. Neuropediatrics. 2019. [DOI] [PubMed]
- 23.Khoury J, Kotagal P, Moosa ANV. Epileptic encephalopathy and brain iron accumulation due to WDR45 mutation. Seizure. 2019;71:245–246. [DOI] [PubMed] [Google Scholar]
- 24.Xiao HXY, Liu Y, Li W, Zhao N, Xiong Q, Li P, Wu C, and Yang Y. Novel de novo Mutation in the Autophagy Gene WDR45 Causes BPAN
- 25.Xiong Q, Li W, Li P, Zhao Z, Wu C, Xiao H. Functional evidence for a de novo mutation in WDR45 leading to BPAN in a Chinese girl. Molecular genetics & genomic medicine. 2019;7(9):e858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chard M, Appendino JP, Bello-Espinosa LE, et al. Single-center experience with Beta-propeller protein-associated neurodegeneration (BPAN); expanding the phenotypic spectrum. Molecular genetics and metabolism reports. 2019;20:100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo C, Ardissone A, Freri E, et al. Substantia Nigra Swelling and Dentate Nucleus T2 Hyperintensity May Be Early Magnetic Resonance Imaging Signs of beta-Propeller Protein-Associated Neurodegeneration. Movement disorders clinical practice. 2019;6(1):51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akcakaya NH, Salman B, Gormez Z, et al. A Novel and Mosaic WDR45 Nonsense Variant Causes Beta-Propeller Protein-Associated Neurodegeneration Identified Through Whole Exome Sequencing and X chromosome Heterozygosity Analysis. Neuromolecular medicine. 2019;21(1):54–59. [DOI] [PubMed] [Google Scholar]
- 29.Rohani M, Fasano A, Akhoundi FH, et al. Beta-propeller protein associated neurodegeneration (BPAN); the first report of three patients from Iran with de novo novel mutations. Parkinsonism & related disorders. 2019;61:231–233. [DOI] [PubMed] [Google Scholar]
- 30.Sakamoto A, Arai R, Okamoto T, Yamada Y, Yamakado H, Matsuda S. Ischemic Fasciitis of the Left Buttock in a 40-Year-Old Woman with Beta-Propeller Protein-Associated Neurodegeneration (BPAN). The American journal of case reports. 2018;19:1249–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen H, Qian Y, Yu S, et al. Early onset developmental delay and epilepsy in pediatric patients with WDR45 variants. European journal of medical genetics. 2019;62(2):149–160. [DOI] [PubMed] [Google Scholar]
- 32.Hamdan FF, Srour M, Capo-Chichi JM, et al. De novo mutations in moderate or severe intellectual disability. PLoS genetics. 2014;10(10):e1004772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulikovskaja L, Sarajlija A, Savic-Pavicevic D, Dobricic V, Klein C, Westenberger A. WDR45 mutations may cause a MECP2 mutation-negative Rett syndrome phenotype. Neurology Genetics. 2018;4(2):e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fonderico M, Laudisi M, Andreasi NG, et al. Patient Affected by Beta-Propeller Protein-Associated Neurodegeneration: A Therapeutic Attempt with Iron Chelation Therapy. Frontiers in neurology. 2017;8:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zarate YA, Jones JR, Jones MA, et al. Lessons from a pair of siblings with BPAN. European journal of human genetics : EJHG. 2016;24(7):1080–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Percy AK, Lane J, Annese F, Warren H, Skinner SA, Neul JL. When Rett syndrome is due to genes other than MECP2. Translational science of rare diseases. 2018;3(1):49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willoughby J, Duff-Farrier C, Desurkar A, Kurian M, Raghavan A, Balasubramanian M. Functional mRNA analysis reveals aberrant splicing caused by novel intronic mutation in WDR45 in NBIA patient. American journal of medical genetics Part A. 2018;176(5):1049–1054. [DOI] [PubMed] [Google Scholar]
- 38.Nishioka K, Oyama G, Yoshino H, et al. High frequency of beta-propeller protein-associated neurodegeneration (BPAN) among patients with intellectual disability and young-onset parkinsonism. Neurobiology of aging. 2015;36(5):2004.e2009–2004.e2015. [DOI] [PubMed] [Google Scholar]
- 39.Ozawa T, Koide R, Nakata Y, et al. A novel WDR45 mutation in a patient with static encephalopathy of childhood with neurodegeneration in adulthood (SENDA). American journal of medical genetics Part A. 2014;164a(9):2388–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryu SW, Kim JS, Lee SH. Beta-Propeller-Protein-Associated Neurodegeneration: A Case of Mutation in WDR45. Journal of clinical neurology (Seoul, Korea). 2015;11(3):289–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoganathan S, Arunachal G, Sudhakar SV, Rajaraman V, Thomas M, Danda S. Beta Propellar Protein-Associated Neurodegeneration: A Rare Cause of Infantile Autistic Regression and Intracranial Calcification. Neuropediatrics. 2016;47(2):123–127. [DOI] [PubMed] [Google Scholar]
- 42.Xixis KI, Mikati MA. Epileptic spasms: a previously unreported manifestation of WDR45 gene mutation. Epileptic disorders : international epilepsy journal with videotape. 2015;17(4):467–472. [DOI] [PubMed] [Google Scholar]
- 43.Kasai-Yoshida E, Kumada S, Yagishita A, et al. First video report of static encephalopathy of childhood with neurodegeneration in adulthood. Movement disorders : official journal of the Movement Disorder Society. 2013;28(3):397–399. [DOI] [PubMed] [Google Scholar]
- 44.Kimura Y, Sato N, Sugai K, et al. MRI, MR spectroscopy, and diffusion tensor imaging findings in patient with static encephalopathy of childhood with neurodegeneration in adulthood (SENDA). Brain & development. 2013;35(5):458–461. [DOI] [PubMed] [Google Scholar]
- 45.Hoffjan S, Ibisler A, Tschentscher A, Dekomien G, Bidinost C, Rosa AL. WDR45 mutations in Rett (-like) syndrome and developmental delay: Case report and an appraisal of the literature. Molecular and cellular probes. 2016;30(1):44–49. [DOI] [PubMed] [Google Scholar]
- 46.Araujo R, Garabal A, Baptista M, et al. Novel WDR45 mutation causing beta-propeller protein associated neurodegeneration (BPAN) in two monozygotic twins. Journal of neurology. 2017;264(5):1020–1022. [DOI] [PubMed] [Google Scholar]
- 47.Ichinose Y, Miwa M, Onohara A, et al. Characteristic MRI findings in beta-propeller protein-associated neurodegeneration (BPAN). Neurology Clinical practice. 2014;4(2):175–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srivastava S, Cohen JS, Vernon H, et al. Clinical whole exome sequencing in child neurology practice. Annals of neurology. 2014;76(4):473–483. [DOI] [PubMed] [Google Scholar]
- 49.Wynn DP, Pulst SM. A novel WDR45 mutation in a patient with beta-propeller protein-associated neurodegeneration. Neurology Genetics. 2017;3(1):e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tschentscher A, Dekomien G, Ross S, et al. Analysis of the C19orf12 and WDR45 genes in patients with neurodegeneration with brain iron accumulation. Journal of the neurological sciences. 2015;349(1–2):105–109. [DOI] [PubMed] [Google Scholar]
- 51.Verhoeven WM, Egger JI, Koolen DA, et al. Beta-propeller protein-associated neurodegeneration (BPAN), a rare form of NBIA: novel mutations and neuropsychiatric phenotype in three adult patients. Parkinsonism & related disorders. 2014;20(3):332–336. [DOI] [PubMed] [Google Scholar]
- 52.Endo H, Uenaka T, Satake W, et al. Japanese WDR45 de novo mutation diagnosed by exome analysis: A case report. Neurology and clinical neuroscience. 2017;5(4):131–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Redon S, Benech C, Schutz S, et al. Intragenic deletion of the WDR45 gene in a male with encephalopathy, severe psychomotor disability, and epilepsy. American journal of medical genetics Part A. 2017;173(5):1444–1446. [DOI] [PubMed] [Google Scholar]
- 54.Fieremans N, Van Esch H, Holvoet M, et al. Identification of Intellectual Disability Genes in Female Patients with a Skewed X-Inactivation Pattern. Human mutation. 2016;37(8):804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohba C, Nabatame S, Iijima Y, et al. De novo WDR45 mutation in a patient showing clinically Rett syndrome with childhood iron deposition in brain. Journal of human genetics. 2014;59(5):292–295. [DOI] [PubMed] [Google Scholar]
- 56.Takano K, Goto K, Motobayashi M, et al. Early manifestations of epileptic encephalopathy, brain atrophy, and elevation of serum neuron specific enolase in a boy with beta-propeller protein-associated neurodegeneration. European journal of medical genetics. 2017. [DOI] [PubMed]
- 57.Morisada N, Tsuneishi S, Taguchi K, et al. [A woman with beta-propeller protein-associated neurodegeneration identified by the WDR45 mutation presenting as Rett-like syndrome in childhood]. No to hattatsu Brain and development. 2016;48(3):209–212. [PubMed] [Google Scholar]
- 58.Okamoto N, Ikeda T, Hasegawa T, et al. Early manifestations of BPAN in a pediatric patient. American journal of medical genetics Part A. 2014;164a(12):3095–3099. [DOI] [PubMed] [Google Scholar]
- 59.Spiegel R, Shalev S, Bercovich D, et al. Severe infantile male encephalopathy is a result of early post-zygotic WDR45 somatic mutation. Clinical genetics. 2016;90(6):560–562. [DOI] [PubMed] [Google Scholar]
- 60.Stige KE, Gjerde IO, Houge G, Knappskog PM, Tzoulis C. Beta-propeller protein-associated neurodegeneration: a case report and review of the literature. Clinical case reports. 2018;6(2):353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uchino S, Saitsu H, Kumada S, Nakata Y, Matsumoto N. Stereotypic Hand Movements in beta-Propeller Protein-Associated Neurodegeneration: First Video Report. Movement disorders clinical practice. 2015;2(2):190–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hermann A, Kitzler HH, Pollack T, et al. A Case of Beta-propeller Protein-associated Neurodegeneration due to a Heterozygous Deletion of WDR45. Tremor and other hyperkinetic movements (New York, NY). 2017;7:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


