Abstract
High mobility group box 1 (HMGB1) is a key player in retinal inflammation. HMGB1 is a danger associated protein pattern receptor which can sense high glucose as a stressor. Increased HMGB1 levels have been found in patients with late stage diabetic retinopathy. HMGB1 can bind toll-like receptor 4 (TLR4) and the receptor for advanced glycation end-products (RAGE), leading to increased inflammation commonly through nuclear factor kappa beta (NFkB). Because diabetic patients have been found to have increased HMGB1 and RAGE levels, as well as polymorphisms of TLR4, a number of investigations have focused on inhibition of these pathways in the diabetic retina. Work in diabetic animal models and cell culture have demonstrated a number of factors that can inhibit HMGB1/TLR4/RAGE signaling. This regulation offers potential new avenues for therapeutic development. This review is focused on HMGB1 signaling and downstream pathways leading to inflammation in the diabetic retina.
Keywords: HMGB1, Diabetic retinopathy, TLR4, RAGE, inflammation
Introduction:
Diabetic retinopathy remains the leading cause of blindness in working age adults. With rates of obesity ever increasing, there remains a real need to better understand the retinal damage associated with diabetes. In the past decade, the role for retinal inflammation in the diabetic-induced retina damage has become increasingly important [1, 2]. In addition to specific cytokine actions in the retina, sterile inflammation has also recently been observed to cause retinal damage [3, 4]. Sterile inflammation is a form of pathogen-free inflammation that can be caused by trauma, ischemia, or other stressors. High glucose due to diabetes can activate a form of sterile inflammation[5]. High mobility group box 1 (HMGB1) is a key mediator of sterile inflammation in the retina [6, 7].
Role of HMGB1 in retinal inflammation.
HMGB1 is secreted by immune cells [8]. In response to high glucose or other stressors, HMGB1 will bind toll-like receptor 4 (TLR4) and the receptor for advanced glycation end-products (RAGE) (Figure 1) [9]. HMGB1 is a nuclear protein that leads to inflammation once translocated to the cytoplasm. Studies in diabetic patients with proliferative retinal disease showed a significant link of disease severity and increased HMGB1 levels [10, 11]. Based upon these clinical findings, researchers have investigated the role of HMGB1 in the diabetic retina in rodent models. HMGB1 appears to mediate diabetes-induced damage in retinal pericytes [12] and Müller cells [13]. Others reported a significant role for HMGB1 in retinal inflammation in retinal ganglion cells (RGC) [14] and retinal pigmented epithelial cells (RPE) [15]. Using diabetic rats, authors demonstrated that diabetes increased HMGB1 levels, which was reduced when a HMGB1 inhibitor, glycyrrhizin, was used [16]. Similarly, another groups showed that diabetes and high glucose culturing conditions in retinal cells significantly increased HMGB1 levels [17]. Silencing of HMGB1 in the cells reduced apoptosis and inflammatory mediators [17]. Another group reported that HMGB1 caused retinal neuropathy in response to diabetes which was mitigated by glycyrrhizin [18]. These findings agree with our recent data showing that glycyrrhizin reduced loss of retinal thickness and loss of RGC numbers due to streptozotocin [19].
Figure 1.
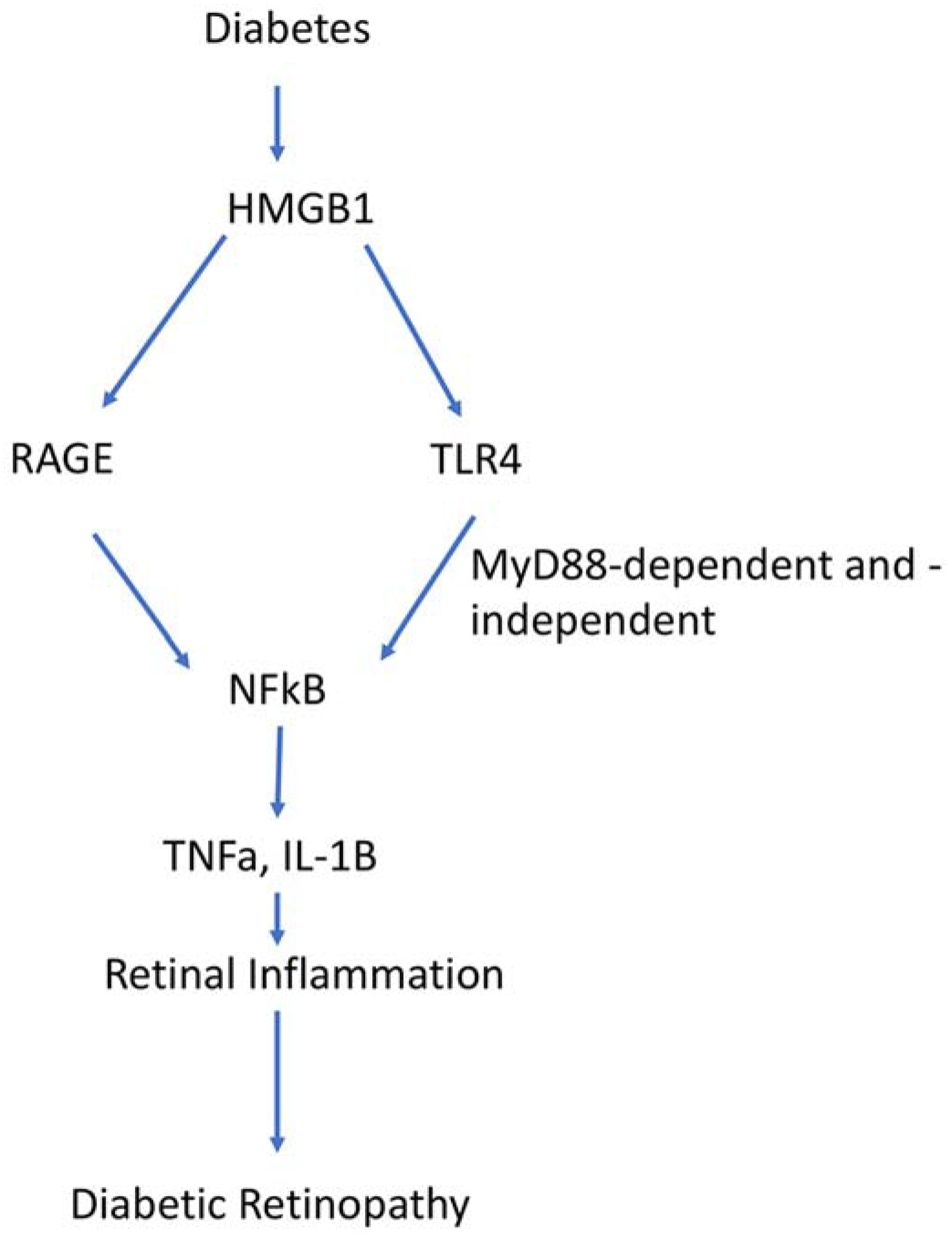
Schematic of the signaling of HMGB1 through RAGE/TLR4 to inflammation in diabetes.
In addition to inhibition of HMGB1 by siRNA or glycyrrhizin, increasing sirtuin 1 (SIRT1) levels appears key to blocking HMGB1 actions [20]. We found that both protein kinase A (PKA) and exchange protein for cAMP1 (Epac1) could increase SIRT1 to reduce HMGB1 actions in retinal cells [21, 22]. Another option to reduce HMGB1 actions in the diabetic retina is to block its interaction with TLR4 and RAGE [23]. Table 1 provides a list of HMGB1 inhibitors and their link to specific inflammatory diseases.
Table 1.
HMGB1 inhibitors
| HMGB1 inhibitors | Inflammatory disease | Reference-PMID |
|---|---|---|
| Glycyrrhizin | ||
| Diabetic Kidney | 29241180 | |
| Box A | Retinal ischemia/reperfusion | 28542588 |
| Cilostazol | Rheumatoid Arthritis | 25126750 |
| Zeaxanthin | Diabetic retinopathy | 18385086 |
| Resveratrol | ||
| Hypoxic Brain injury | 31362164 | |
| MnTBAP | Diabetic Oxidative Stress | 19833897 |
| Astilbin | Diabetic CV disease | 24211745 |
| N-acetyl cysteine (NAC) | Diabetic Kidney disease | 25896065 |
| Empagliflozin | Diabetic Kidney disease | 31168342 |
| CaMKIV | Diabetic Neuropathic Pain | 27216039 |
| Peptide P5779 | Sepsis, pulmonary hypertension | Reviewed in Yang et al. 2020 DOI 10.3389 |
| M2G7 | Sepsis, arthritis | Reviewed in Yang et al. 2020 DOI 10.3389 |
| Dexmedetomide | Endotoxemia | 24803295 |
| Haptoglobin | Anti-inflammatory | 30568040 |
| Metformin | LPS-induced inflammation | 31772144 |
TLR4 actions in the diabetic retina.
TLR4 is a transmembrane protein that serves as a pattern recognition receptor, and its activation leads to inflammatory cytokine production [24]. TLR4 is typically activated by lipopolysaccharide (LPS) [25]. TLR4 can signal through a MyD88-dependent or -independent pathway leading to NFkB and activation of inflammatory cytokines [26]. TLR4 has a large number of agonists and antagonists, due to its role in opioid analgesia and sepsis [27, 28]. Focusing on diabetic retinopathy, studies have shown that TLR4 polymorphisms are linked to non-proliferative diabetic retinopathy in type 2 diabetic patients of Romanian descent [29]. Additionally, studies in monocytes freshly isolated from type 2 diabetic patients demonstrated increased TLR4 activation and downstream signaling [30]. Another group found that TLR4 polymorphisms were not linked to type 2 diabetes but that this polymorphism did contribute to progression of diabetic retinopathy [31]. Taken together, the exact role of TLR4 in diabetic retinopathy is not clear, but a link to diabetic retinopathy is established potentially through its activation by HMGB1 [7].
Based upon the work in humans, investigators initiated studies to better understand TLR4 in mouse and cell models of diabetic retinopathy. Work in human retinal endothelial cells showed that high glucose increased TLR2 and TLR4, leading to increased MyD88-dependent and independent signaling [32]. Similarly, others showed that increased TLR4 levels led to retinol-binding protein 4 (RBP4)-mediated inflammation [33]. In RGC grown in high glucose, inhibition of TLR4 reduced apoptosis and inflammatory markers [34]. Using diabetic TLR4 knockout mice, authors showed that bone marrow transplantation from wild-type mice to mutant mice led to increased vascular endothelial cell grown factor (VEGF), tumor necrosis factor alpha (TNFα) and interleukin-1β (IL-β) levels in the retina, suggesting that TLR4 is key to inflammation in diabetic retinopathy [35]. Additional studies on diabetic TLR4 knockout mice also showed that TLR4 drives inflammation in the retina [36].
Inhibition of TLR4 offers a novel avenue for therapeutic development. Using diabetic mice treated with miR-499 had increased progression of diabetic retinopathy markers through TLR4 actions [37]. In contrast, studies showed that miR-145 reduced TLR4/nuclear factor kappa beta (NFkB)-induced inflammation in REC [38]. Similarly, we found that miR-146 reduced TLR4mediated inflammation in the retina [39]. In addition to miRNA, we previously demonstrated that Compound 49b, a β-adrenergic receptor agonist, reduced TLR4 actions in REC, retinal Müller cells, and in the diabetic retina [40]. In a follow-up study, we showed that Epac1 inhibited TLR4 signaling in REC and in whole retina using Epac1 endothelial cell specific knockout mice [41]. Using whole retinal lysates from Müller cell specific TLR4 knockout mice, we demonstrated reduced MyD88-dependent and -independent signaling, leading to reduced inflammatory mediator levels [42]. The studies in cell-specific Epac1 KO and TLR4 KO mice were done in non-diabetic animals; however, the data suggest that TLR4 can be inhibited by a number of pathways that could lead to novel therapeutics for diabetic retinopathy.
RAGE actions in the diabetic retina.
RAGE is a transmembrane receptor in the immunoglobulin family, named for its primary agonist, advanced glycation products (AGEs) formed following chronic exposure to hyperglycemia [43, 44]. When RAGE interacts with its ligands, AGEs or HMGB1, activation of NFkB leads to a pro-inflammatory state [45]. AGE/RAGE have been associated with most of the complications of diabetic retinopathy, including reactive oxygen species (ROS), permeability changes, vascular damage, and angiogenic changes [46, 47]. RAGE has been localized in retinal glial cells, Müller cells, and REC [48]. Studies of patients with proliferative retinal disease found evidence of increased levels of RAGE and its ligands in the vitreous and epiretinal membranes [49]. The RAGE gene promoter is hypomethylated in patients with type 2 diabetes [50].
In support of the studies of RAGE actions in humans, a study using human samples, diabetic mice, and endothelial and Müller cells in culture demonstrated that both TLR4- and AGE-induced retinal inflammation through an upregulation of galectin-1 [51]. Studies using streptozotocin-induced diabetic mice and Müller glia showed that RAGE was significantly upregulated and primarily localized in Müller cells. When cells were cultured in high glucose, inhibition of RAGE abrogated the inflammatory response [52]. Use of a RAGE fusion protein in streptozotocin-induced diabetes showed that inhibition of RAGE prevented the vascular and permeability damage commonly associated with diabetes [53]. Studies using natural products demonstrated that an extract of Polygonum cuspidatum was able to block the HMGB1/RAGE pathway in diabetic rats [54]. An additional study showed that the class A scavenger receptor (SR-A) antagonized RAGE actions to reduce the inflammatory actions in the diabetic retina [55]. Somewhat similarly, studies also demonstrated that olmesartan, a drug altering the reninangiotensin pathway, blocked inflammatory actions and reactive oxygen species in REC mediated through RAGE [56]. Thus, the data suggest that HMGB1/RAGE/TLR4 activities lead to inflammatory-induced damage in the diabetic retina, and its inhibition may serve as a novel arena for therapeutic development (Figure 1).
NFkB is a key point for retinal inflammation in diabetes.
A key action of HMGB1/TLR4/RAGE is to phosphorylate NFkB, leading to activation of a number of inflammatory cytokines [57, 58]. NFkB is a protein complex controlling DNA transcription and production of a large number of cytokines [59]. Due to its role in regulating transcription and activation of cytokines, there are a plethora of agonists and antagonists for NFkB to treat a number of diseases, including cancers and inflammatory diseases [60, 61]. NFkB can also be regulated via acetylation/deacetylation, which can be regulated by epigenetic factors common to diabetic retinopathy [62, 63]. Because of its role in activation of a number of key cytokines observed in diabetic retinopathy, a large number of studies have investigated pathways that can inhibit/activate NFkB. For example, one study showed that resolvin D1 inhibited NFkB actions in STZ diabetic rats [64]. Similarly, additional studies have shown that selenium or coconut kernel protein can also block RAGE and NFkB in STZ-treated rats [58, 65]. These studies are representative of a larger number of studies showing that inhibition of NFkB can reduce retinal damage in response to diabetes.
Conclusions:
Data demonstrate that HMGB1 levels are increased in patients with later stage diabetic retinopathy. Similarly, RAGE levels and polymorphisms of TLR4 are increased in diabetic patients. Studies in diabetic animal models and cells demonstrate that the HMGB1/TLR4/RAGE axis is linked to increased levels of retinal damage and inflammatory mediators in the retina. Taken together, HMGB1 signaling can activate both TLR4 and RAGE pathways, leading to increased NFkB actions and retinal inflammation in the diabetic retina.
Highlights.
HMGB1 mediates retinal inflammation in diabetic retinopathy
HMGB1 binds TLR4 and RAGE
Inhibition of HMGB1 offers new therapeutic development
Acknowledgements:
This study was supported by R01EY028442 and R01EY030284 (JJS), P30EY04068 (Hazlett), and an Unrestricted Grant to the Department of Ophthalmology from Research to Prevent Blindness (Kresge Eye Institute). The funders did not influence the design or execution of these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Joussen AM, Poulaki V, Le ML, et al. , A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 2004; 18 1450–2. [DOI] [PubMed] [Google Scholar]
- 2.Tang J and Kern TS, Inflammation in diabetic retinopathy. Progress in retinal and eye research, 2011; 30 343–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ulloa L and Messmer D, High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev, 2006; 17 189–201. [DOI] [PubMed] [Google Scholar]
- 4.Cox SS, Speaker KJ, Beninson LA, et al. , Adrenergic and glucocorticoid modulation of the sterile inflammatory response. Brain Behav Immun, 2014; 36 183–92. [DOI] [PubMed] [Google Scholar]
- 5.Donath MY and Shoelson SE, Type 2 diabetes as an inflammatory disease. Nat Rev Immunol, 2011; 11 98–107. [DOI] [PubMed] [Google Scholar]
- 6.Santos AR, Dvoriantchikova G, Li Y, et al. , Cellular mechanisms of high mobility group 1 (HMGB-1) protein action in the diabetic retinopathy. PloS one, 2014; 9 e87574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H, Wang H, and Andersson U, Targeting Inflammation Driven by HMGB1. Front Immunol, 2020; 11 Article 484. Page 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klune JR, Dhupar R, Cardinal J, Billiar TR, and Tsung A, HMGB1: endogenous danger signaling. Mol Med, 2008; 14 476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sims GP, Rowe DC, Rietdijk ST, Herbst R, and Coyle AJ, HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol, 2010; 28 367–88. [DOI] [PubMed] [Google Scholar]
- 10.Abu El-Asrar AM, Alam K, Garcia-Ramirez M, et al. , Association of HMGB1 with oxidative stress markers and regulators in PDR. Molecular vision, 2017; 23 853–871. [PMC free article] [PubMed] [Google Scholar]
- 11.El-Asrar AM, Nawaz MI, Kangave D, et al. , High-mobility group box-1 and biomarkers of inflammation in the vitreous from patients with proliferative diabetic retinopathy. Molecular vision, 2011; 17 1829–38. [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J, Kim CS, Sohn E, and Kim JS, Cytoplasmic translocation of high-mobility group box-1 protein is induced by diabetes and high glucose in retinal pericytes. Mol Med Rep, 2016; 14 3655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammad G, Jomar D, Siddiquei MM, Alam K, and Abu El-Asrar AM, High-Mobility Group Box-1 Protein Mediates the Regulation of Signal Transducer and Activator of Transcription-3 in the Diabetic Retina and in Human Retinal Muller Cells. Ophthalmic Res, 2017; 57 150–160. [DOI] [PubMed] [Google Scholar]
- 14.Zhao H, Zhang J, and Yu J, HMGB-1 as a Potential Target for the Treatment of Diabetic Retinopathy. Med Sci Monit, 2015; 21 3062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen XL, Zhang XD, Li YY, et al. , Involvement of HMGB1 mediated signalling pathway in diabetic retinopathy: evidence from type 2 diabetic rats and ARPE-19 cells under diabetic condition. Br J Ophthalmol, 2013; 97 1598–603. [DOI] [PubMed] [Google Scholar]
- 16.Mohammad G, Siddiquei MM, Othman A, Al-Shabrawey M, and Abu El-Asrar AM, High-mobility group box-1 protein activates inflammatory signaling pathway components and disrupts retinal vascular-barrier in the diabetic retina. Experimental eye research, 2013; 107 101–9. [DOI] [PubMed] [Google Scholar]
- 17.Jiang S and Chen X, HMGB1 siRNA can reduce damage to retinal cells induced by high glucose in vitro and in vivo. Drug Des Devel Ther, 2017; 11 783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu El-Asrar AM, Siddiquei MM, Nawaz MI, Geboes K, and Mohammad G, The proinflammatory cytokine high-mobility group box-1 mediates retinal neuropathy induced by diabetes. Mediators Inflamm, 2014; 2014 746415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L, Jiang Y, and Steinle JJ, Glycyrrhizin Protects the Diabetic Retina against Permeability, Neuronal, and Vascular Damage through Anti-Inflammatory Mechanisms. J Clin Med, 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammad GP, Abdelaziz GM, Siddiquei MMM, et al. , Cross-talk between Sirtuin 1 and the Proinflammatorty Mediator High-Mobility Group Box-1 in the Regulation of Blood-Retinal Barrier Breakdown in Diabetic Retinopathy. Current eye research, 201944, 1133–1143 [DOI] [PubMed] [Google Scholar]
- 21.Liu L, Patel P, and Steinle JJ, PKA regulates HMGB1 through activation of IGFBP-3 and SIRT1 in human retinal endothelial cells cultured in high glucose. Inflamm Res, 2018; 67 1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Jiang Y, and Steinle JJ, Epac1 and Glycyrrhizin Both Inhibit HMGB1 Levels to Reduce Diabetes-Induced Neuronal and Vascular Damage in the Mouse Retina. J Clin Med, 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Beijnum JR, Buurman WA, and Griffioen AW, Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis, 2008; 11 91–9. [DOI] [PubMed] [Google Scholar]
- 24.Vaure C and Liu Y, A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol, 2014; 5 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brubaker SW, Bonham KS, Zanoni I, and Kagan JC, Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol, 2015; 33 257–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palsson-McDermott EM and O’Neill LA, Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology, 2004; 113 153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shavit Y, Wolf G, Goshen I, Livshits D, and Yirmiya R, Interleukin-1 antagonizes morphine analgesia and underlies morphine tolerance. Pain, 2005; 115 50–9. [DOI] [PubMed] [Google Scholar]
- 28.Tidswell M, Tillis W, Larosa SP, et al. , Phase 2 trial of eritoran tetrasodium (E5564), a toll-like receptor 4 antagonist, in patients with severe sepsis. Crit Care Med, 2010; 38 72–83. [DOI] [PubMed] [Google Scholar]
- 29.Aioanei CS, Ilies RF, Bala C, et al. , The Role of Adiponectin and Toll-Like Receptor 4 Gene Polymorphisms on Non-Proliferative Retinopathy in Type 2 Diabetes Mellitus Patients. A Case-Control Study in Romanian Caucasians Patients. Acta Endocrinol (Buchar), 2019; −5 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dasu MR, Devaraj S, Park S, and Jialal I, Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care, 2010; 33 861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaharieva ET, Kamenov ZA, and Savov AS, TLR4 polymorphisms seem not to be associated with prediabetes and type 2 diabetes but predispose to diabetic retinopathy; TLR4 polymorphisms in glucose continuum. Endocr Regul, 2017; 51 137–144. [DOI] [PubMed] [Google Scholar]
- 32.Rajamani U and Jialal I, Hyperglycemia induces Toll-like receptor-2 and −4 expression and activity in human microvascular retinal endothelial cells: implications for diabetic retinopathy. Journal of diabetes research, 2014; 2014 790902. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Du M, Martin A, Hays F, et al. , Serum retinol-binding protein-induced endothelial inflammation is mediated through the activation of toll-like receptor 4. Molecular vision, 2017; 23 185–197. [PMC free article] [PubMed] [Google Scholar]
- 34.Hu L, Yang H, Ai M, and Jiang S, Inhibition of TLR4 alleviates the inflammation and apoptosis of retinal ganglion cells in high glucose. Graefes Arch Clin Exp Ophthalmol, 2017; 255 2199–2210. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Shi H, Zhang J, et al. , Toll-like receptor 4 in bone marrow-derived cells contributes to the progression of diabetic retinopathy. Mediators Inflamm, 2014; 2014 858763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devaraj S, Tobias P, and Jialal I, Knockout of toll-like receptor-4 attenuates the proinflammatory state of diabetes. Cytokine, 2011; 55 441–5. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Zhang Y, Liang H, Zhang Y, and Xu Y, microRNA-499–3p inhibits proliferation and promotes apoptosis of retinal cells in diabetic retinopathy through activation of the TLR4 signaling pathway by targeting IFNA2. Gene, 2020; 741 144539. [DOI] [PubMed] [Google Scholar]
- 38.Hui Y and Yin Y, MicroRNA-145 attenuates high glucose-induced oxidative stress and inflammation in retinal endothelial cells through regulating TLR4/NF-kappaB signaling. Life Sci, 2018; 207 212–218. [DOI] [PubMed] [Google Scholar]
- 39.Ye EA and Steinle JJ, miR-146a Attenuates Inflammatory Pathways Mediated by TLR4/NF-kappaB and TNFalpha to Protect Primary Human Retinal Microvascular Endothelial Cells Grown in High Glucose. Mediators Inflamm, 2016; 2016 3958453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berger EA, Carion TW, Jiang Y, et al. , beta-adrenergic receptor agonist, Compound 49b, inhibits TLR4 signaling pathway in diabetic retina. Immunol Cell Biol, 2016656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang Y, Liu L, Curtiss E, and Steinle JJ, Epac1 Blocks NLRP3 Inflammasome to Reduce IL-1beta in Retinal Endothelial Cells and Mouse Retinal Vasculature. Mediators Inflamm, 2017; 2017 2860956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu L and Steinle JJ, Loss of TLR4 in mouse Muller cells inhibits both MyD88-dependent and -independent signaling. PloS one, 2017; 12 e0190253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCance DR, Dyer DG, Dunn JA, et al. , Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J Clin Invest, 1993; 91 2470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beisswenger PJ, Makita Z, Curphey TJ, et al. , Formation of immunochemical advanced glycosylation end products precedes and correlates with early manifestations of renal and retinal disease in diabetes. Diabetes, 1995; 44 824–9. [DOI] [PubMed] [Google Scholar]
- 45.Bierhaus A, Schiekofer S, Schwaninger M, et al. , Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes, 2001; 50 2792808. [DOI] [PubMed] [Google Scholar]
- 46.Xu J, Chen LJ, Yu J, et al. , Involvement of Advanced Glycation End Products in the Pathogenesis of Diabetic Retinopathy. Cell Physiol Biochem, 2018; 48 705–717. [DOI] [PubMed] [Google Scholar]
- 47.Barile GR and Schmidt AM, RAGE and its ligands in retinal disease. Curr Mol Med, 2007; 7 758–65. [DOI] [PubMed] [Google Scholar]
- 48.Manigrasso MB, Juranek J, Ramasamy R, and Schmidt AM, Unlocking the biology of RAGE in diabetic microvascular complications. Trends Endocrinol Metab, 2014; 25 1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pachydaki SI, Tari SR, Lee SE, et al. , Upregulation of RAGE and its ligands in proliferative retinal disease. Experimental eye research, 2006; 82 807–15. [DOI] [PubMed] [Google Scholar]
- 50.Kan S, Wu J, Sun C, Hao J, and Wu Z, Correlation between RAGE gene promoter methylation and diabetic retinal inflammation. Exp Ther Med, 2018; 15 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanda A, Dong Y, Noda K, Saito W, and Ishida S, Advanced glycation endproducts link inflammatory cues to upregulation of galectin-1 in diabetic retinopathy. Sci Rep, 2017; 7 16168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zong H, Ward M, Madden A, et al. , Hyperglycaemia-induced pro-inflammatory responses by retinal Muller glia are regulated by the receptor for advanced glycation end-products (RAGE). Diabetologia, 2010; 53 2656–66. [DOI] [PubMed] [Google Scholar]
- 53.Li G, Tang J, Du Y, Lee CA, and Kern TS, Beneficial effects of a novel RAGE inhibitor on early diabetic retinopathy and tactile allodynia. Molecular vision, 2011; 17 3156–65. [PMC free article] [PubMed] [Google Scholar]
- 54.Sohn E, Kim J, Kim CS, Lee YM, and Kim JS, Extract of Polygonum cuspidatum Attenuates Diabetic Retinopathy by Inhibiting the High-Mobility Group Box-1 (HMGB1) Signaling Pathway in Streptozotocin-Induced Diabetic Rats. Nutrients, 2016; 8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma K, Xu Y, Wang C, et al. , A cross talk between class A scavenger receptor and receptor for advanced glycation end-products contributes to diabetic retinopathy. Am J Physiol Endocrinol Metab, 2014; 307 E1153–65. [DOI] [PubMed] [Google Scholar]
- 56.Yamagishi S, Matsui T, Nakamura K, et al. , Olmesartan blocks inflammatory reactions in endothelial cells evoked by advanced glycation end products by suppressing generation of reactive oxygen species. Ophthalmic Res, 2008; 40 10–5. [DOI] [PubMed] [Google Scholar]
- 57.Kern TS, Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res, 2007; 2007 95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pillai SS, Sugathan JK, and Indira M, Selenium downregulates RAGE and NFkappaB expression in diabetic rats. Biol Trace Elem Res, 2012; 149 71–7. [DOI] [PubMed] [Google Scholar]
- 59.Gilmore TD, Introduction to NF-kappaB: players, pathways, perspectives. Oncogene, 2006; 25 6680–4. [DOI] [PubMed] [Google Scholar]
- 60.Vlahopoulos SA, Aberrant control of NF-kappaB in cancer permits transcriptional and phenotypic plasticity, to curtail dependence on host tissue: molecular mode. Cancer Biol Med, 2017; 14 254–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monaco C, Andreakos E, Kiriakidis S, et al. , Canonical pathway of nuclear factor kappa B activation selectively regulates proinflammatory and prothrombotic responses in human atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America, 2004; 101 5634–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duraisamy AJ, Mishra M, Kowluru A, and Kowluru RA, Epigenetics and Regulation of Oxidative Stress in Diabetic Retinopathy. Investigative ophthalmology & visual science, 2018; 59 4831–4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kowluru RA, Mishra M, and Kumar B, Diabetic retinopathy and transcriptional regulation of a small molecular weight G-Protein, Rac1. Experimental eye research, 2016; 147 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yin Y, Chen F, Wang W, Wang H, and Zhang X, Resolvin D1 inhibits inflammatory response in STZ-induced diabetic retinopathy rats: Possible involvement of NLRP3 inflammasome and NF-kappaB signaling pathway. Molecular vision, 2017; 23 242–250. [PMC free article] [PubMed] [Google Scholar]
- 65.Salil G, Nithya R, Nevin KG, and Rajamohan T, Dietary coconut kernel protein beneficially modulates NFkappaB and RAGE expression in streptozotocin induced diabetes in rats. J Food Sci Technol, 2014; 51 2141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


