Abstract
Background and Purpose:
Spreading depolarizations (SDs) are recurrent and ostensibly spontaneous depolarization waves that may contribute to infarct progression after stroke. Somatosensory activation of the metastable peri-infarct tissue triggers peri-infarct SDs at a high rate.
Methods:
We directly measured the functional activation threshold to trigger SDs in peri-infarct hot zones using optogenetic stimulation after distal middle cerebral artery occlusion in Thy1-ChR2-YFP mice.
Results:
Optogenetic activation of peri-infarct tissue triggered SDs at a strikingly high rate (64%) compared with contralateral homotopic cortex (8%; p=0.004). Laser speckle perfusion imaging identified a residual blood flow of 31±2% of baseline marking the metastable tissue with a propensity to develop SDs.
Conclusions:
Our data reveal a spatially distinct increase in SD susceptibility in peri-infarct tissue where physiological levels of functional activation are capable of triggering SDs. Given the potentially deleterious effects of peri-infarct SDs, the effect of sensory overstimulation in hyperacute stroke should be examined more carefully.
Keywords: cerebral ischemia, migraine aura, middle cerebral artery occlusion, laser speckle imaging
Graphical Abstract

(A) Laser speckle flowmetry (LSF) and optical intrinsic signal (OIS) imaging was performed during distal middle cerebral artery occlusion (MCAO) or sham. Optogenetic ramp stimulation were delivered every 10 minutes. (B) Distal MCAO induced a focal perfusion defect in dorsolateral cortex. Three regions of interest were selected corresponding to core, peri-infarct and mildly ischemic cortex for stimulation. (C) The rate of SD occurrence during stimulation in MCAO and sham groups. ** and * vs. contralateral; †† and † vs. homotopic regions in MCAO (Fisher's exact test). The spontaneous peri-infarct SD occurrence in the absence of stimulation was 5.4%/5 minutes.
INTRODUCTION
Spreading depolarizations (SDs) are intense neuronal and glial depolarization waves associated with massive transmembrane ion and water shifts, elevated extracellular excitatory amino acid levels, and a tremendous metabolic burden to restore the homeostasis. In focal ischemic brain, recurrent waves of SDs originate from the peri-infarct tissue in an apparently spontaneous and random fashion and propagate throughout the vulnerable hemisphere. Peri-infarct SDs are believed to exacerbate ischemic injury by imposing additional metabolic burden coupled to a vasoconstrictive effect and by contributing to ischemic swelling.1-4 However, why recurrent SDs occur in the ischemic brain has been poorly understood.
We have recently identified oxygen supply-demand mismatch transients as a trigger for peri-infarct SDs.5 We showed that while the tissue surrounding the depolarized core has sufficient blood flow and oxygen delivery to maintain the resting membrane potentials (i.e. penumbra), it is not stable, such that any worsening in oxygen supply-demand mismatch precipitates anoxic depolarization, thereby triggering a peri-infarct SD. We also showed that in addition to hypoxic or hypotensive events diminishing oxygen supply, increased oxygen demand upon functional activation was also capable of triggering peri-infarct SDs in metastable ‘hot zones’ where residual cortical blood flow (CBF) is sufficient to maintain synaptic transmission but fails to match the increased demand when the tissue is activated. These data suggested that metastable peri-infarct hot zones are more susceptible and thus may have a lower threshold to develop SD, consistent with Hossmann’s definition of viability thresholds in ischemic penumbra.6
Somatosensory stimulation, however, cannot be easily titrated to achieve graded and targeted functional activation in order to quantify SD thresholds in different cortical regions. Optogenetic stimulation is a non-invasive alternative to achieve direct functional activation of neurons.7 The method allows precise titration of stimulus intensity, location and timing, without the confounding effects of anesthesia on the transmission of somatosensory stimuli through subcortical relay nuclei. Here, we examined the propensity of peri-infarct hot zones to develop SD upon physiological levels of functional activation by employing focal optogenetic stimulation to directly activate the cortex non-invasively through intact skull in transgenic mice expressing channelrhodopsin-2, ChR2(+), in neurons (Thy1-ChR2-YFP).8, 9 Our data indicate markedly elevated susceptibility to SD initiation upon functional activation in peri-infarct hot zones while the surrounding mildly ischemic cortex becomes relatively resistant compared with contralateral homotopic regions. The data also suggest that functional activation is capable of triggering an SD even in normal brain.
MATERIALS AND METHODS
Experiments were approved by the MGH Institutional Animal Care and Use Committee and carried out in accordance with the Guide for Care and Use of Laboratory Animals (NIH Publication No. 85-23, 1996). We used 33 ChR2(+) (10-week-old, male, B6.Cg-Tg(Thy1-COP4/EYFP)18Gfng/J, Jackson Laboratories, Bar Harbor, ME, USA)8, 9, and three wild type mice (ChR2(−), C57Bl/6, Charles River Laboratories, Wilmington, MA, USA).
Mice were anesthetized with isoflurane (4% induction, 1.5% maintenance in 70%N2/30%O2). A femoral artery catheter was used to monitor arterial blood pressure, pH, pO2, and pCO2 (Supplementary Table I, please see https://www.ahajournals.org/journal/str). Rectal temperature was monitored and maintained at 37°C via a servo-controlled heating pad. Animals were placed in a stereotaxic frame. After a midline scalp incision and retraction, the skull surface was covered with a thin layer of mineral oil to prevent drying and enhance transparency for optical imaging.
Laser speckle and optical intrinsic signal imaging
A typical experimental timeline is shown in Fig. 1A. CBF imaging started before and continued during distal middle cerebral artery occlusion (MCAO) through intact skull by laser speckle flowmetry (LSF) using a near infrared laser diode (785 nm, 75mW) and a CCD camera (CoolSnap cf, 1392×1040 pixels; Photometrics, Tucson, AZ), as described previously.10 Raw speckle frames were acquired at 17 Hz and speckle contrast frames averaged, to yield a CBF image every 2.7 s. The experimental setup provided a full field view of regional CBF changes throughout the dorsal cortex and allowed precise placement of the optogenetic stimulus with respect to residual CBF. Importantly, LSF provides relative changes in CBF rather than the absolute CBF values. The optical intrinsic signal (OIS) was simultaneously imaged through intact skull to confirm SDs detected by LSF, using a UVC-compliant webcam (OT-HD, Opti-TekScope, Chandler, AZ, USA) as described in detail previously.11 Ambient room light served as the light source for OIS. Time-lapse images were taken every 2 s.
Figure 1. Experimental protocols.
(A) Timeline shows the stimulation protocol. Optogenetic stimulation of core, peri-infarct and mildly ischemic brain were delivered in random alternating order in different animals to control for time. Laser speckle flowmetry (LSF) and optical intrinsic signal (OIS) imaging started before and continued during distal middle cerebral artery occlusion (MCAO). Optogenetic stimulation was delivered every 10 minutes.
(B) Optogenetic stimulus started at 1 mW, 8 Hz, 6 ms and light power was increased by 1 mW every 30 seconds over a period of 5 min (1 to 10 mW), or until a spreading depolarization (SD) was identified on LSF.
(C) Distal MCAO induced a focal perfusion defect in dorsolateral cortex. Three regions of interest were selected corresponding to core, peri-infarct and mildly ischemic cortex, guided by real time LSF perfusion maps, for optogenetic stimulation, cerebral blood flow (CBF) measurements and evoked potentials.
(D) Peri-infarct SDs were easily detected by the propagating large-amplitude CBF transients on full-field images, as shown in the representative CBF tracing from ischemic penumbra, and subsequently confirmed using OIS (see Supplementary Movie I). Optogenetic stimulation (red bar) was terminated when a peri-infarct SD occurred (yellow triangle).
Transtemporal middle cerebral artery occlusion
The temporalis muscle was separated from the temporal bone and removed. A burr hole (2 mm diameter) was drilled in the temporal bone overlying the distal middle cerebral artery just above the zygomatic arch. Inadvertent mechanically triggered SDs during drilling were detected by LSF and OIS and served as an exclusion criterion. The middle cerebral artery was occluded just distal to the inferior cerebral vein using a microvascular clip as described previously.10, 12, 13
Optogenetic functional activation
Optogenetic light stimulus was applied through intact skull with a 400 μm diameter fiber (numerical aperture 0.39), a 470 nm LED light source (LED: MF470F3; LED driver: DC2100; Thorlabs, Newton, NJ, USA), and a data acquisition system (PowerLab, ADInstruments, Colorado Springs, CO, USA). Light power was calibrated before each experiment (PM16-130, Thorlabs). The stimulation paradigm is shown on Fig. 1B. In ChR2(+) mice, optogenetic stimulus intensity started at 1 mW and increased by 1 mW every 30 seconds over a period of 5 min (1 to 10 mW), or until an SD was identified on LSF. In wild type controls (ChR2(−); n= 3 C57Bl6), optogenetic stimulation intensity was set at 10 mW for the entire 5-minute period. Stimulus frequency (8 Hz) and pulse duration (6 ms) were chosen to evoke functional activation based on prior experience.7 Optogenetic functional activation was applied in ischemic core (whisker barrel field), peri-infarct cortex (primary somatosensory cortex), and mildly ischemic cortex (motor cortex, 1 mm lateral and 1 mm anterior to bregma), guided by the perfusion defect on LSF (Fig. 1C). Ipsilesional stimulation was alternated with contralesional homotopic regions of interest. SDs were detected by LSF in real time during ischemia (Fig. 1D) and subsequently confirmed off-line using OIS. The light power sufficient to trigger an SD was taken as the threshold in mW. At least 5 minutes were allowed after MCAO onset before the first stimulation, and then between each stimulation. All 3 regions were stimulated in each animal in random order.
Optogenetic evoked potentials
To confirm cortical activation, we recorded epicranial evoked field potentials in a separate cohort following the same surgical preparation and distal MCAO as above, with the exception of arterial catheterization to minimize invasive surgery and allow for longer recordings. Single pulses (1, 5 or 10 mW, 6 msec) were applied every 5 seconds over the three ROIs as described above in ipsilesional and contralesional homotopic regions of interest in random order. Evoked potentials were recorded from the stimulated region as well as the contralesional homotopic region simultaneously using silver ball electrodes in contact with intact skull via conducting gel fed to an amplifier (Axoprobe 1A, Axon Instruments, Molecular Devices, San Jose, CA, USA) and digitized for offline analysis (Scope, ADInstruments, Colorado Springs, MO, USA). For each stimulus level in each region, 20 evoked potentials were averaged.
Cortical temperature measurement
Because optogenetic stimulation can raise tissue temperature,14, 15 in a separate group of ChR2(+) mice (n = 3), we measured brain temperature during light stimulation using a thermocouple microprobe thermometer (BAT-12/IT-23; Physitemp, Clifton, NJ, USA) inserted through a burr hole. The tip of the thermometer was placed below the skull surface directly under the light source. Optogenetic stimuli were delivered on whisker barrel cortex using stimulus parameters identical to functional activation as described above. Stimulations were delivered 3 times in each animal and temperature values were averaged. We did not detect a significant change during 1-10 mW, 6ms, 8Hz stimulation (Supplementary Fig. I), as previously reported.7
Data Analysis and Statistics
Study design and reporting followed ARRIVE guidelines. In the absence of an intervention, blinding was not applicable. Data are presented as fraction of total (%) or mean ± standard error. The statistical methods used to analyze each dataset is indicated in the results and figure legends. Statistical analyses were performed using Prism 8 (GraphPad Software, La Jolla, CA) or SPSS (IBM, Chicago, IL). P<0.05 was considered statistically significant.
Data availability
The raw data that support the findings of this manuscript are available upon request to the corresponding author.
RESULTS
Distal MCAO resulted in a discrete perfusion defect on LSF images (Fig. 1C) guiding the selective optogenetic activation of core, peri-infarct and mildly ischemic regions of interest based on CBF. Spontaneous and stimulus-induced SDs were readily identified on LSF and OIS by the characteristic vasoconstriction and hypoperfusion transients propagating across the cortex (Fig. 1D; Supplementary Movie I).
Peri-infarct SD occurrence
In non-stimulated ChR2(+) mice, peri-infarct SDs occurred spontaneously at a rate of 0.7±0.3/hr after MCAO without a particular temporal predilection (Supplementary Fig. II, white circles), as reported previously.5 Because of the apparently random background occurrence of spontaneous peri-infarct SDs, a probabilistic approach was required to statistically test whether optogenetic activation triggered SDs higher than the spontaneous background rate during any 5-minute stimulation period. The calculated spontaneous peri-infarct SD rate was 5.4% within any 5-minute period in non-stimulated mice (Fig. 2, horizontal dotted line).
Figure 2. Optogenetic stimulation-induced spreading depolarization rates and thresholds.
Upper panel and the diagrams show the rate of peri-infarct SD occurrence as percent of trials during 5-minute optogenetic stimulations of the three regions of interest in MCAO and sham groups. Statistical comparisons were made between ipsilesional and contralesional homotopic regions (left panel) and between homotopic regions in MCAO (left panel) and sham (right panel) groups using Fisher’s exact test. Horizontal dotted line indicates the 5-minute rate of spontaneous peri-infarct SD occurrence in the absence of optogenetic stimulation (5.4%) for comparisons with the stimulus-induced SD rates. Lower panel shows the light intensity threshold when SD was triggered. Trials that failed to trigger an event are plotted as threshold above 10 mW. Statistical comparisons were made between ipsilesional and contralesional homotopic regions (left panel, MCAO cohort) and between homotopic regions in MCAO (left panel) and sham (right panel) groups (Mann-Whitney U test; a and b: p=0.042 and 0.097, respectively, vs. ipsilesional core, c and d: p=0.004 and 0.003, respectively, vs. ipsilesional peri-infarct, e and f: p=0.015 and 0.036, respectively, vs. ipsilesional mild ischemic region of interest. The timelines for the entire dataset are shown in Supplementary Fig. II
Focal optogenetic stimulation with stepwise escalating light intensity (1-10 mW, 8 Hz, 6 ms) for up to 5 minutes indeed triggered SDs, the probability of which varied among the three regions of interest, between ipsilesional and contralesional hemispheres, and between sham and MCAO groups (n=5 and 10, respectively). The timelines for the entire dataset are shown in Supplementary Fig. II. An SD was considered as stimulus-induced only if it originated from within the stimulated region rather than remotely. When an SD originated outside of the stimulated region during the stimulus, it was considered spontaneous.
Stimulation of the ipsilesional peri-infarct cortex triggered an SD in 64% of trials (9/14; Fig. 2). In contrast, stimulation of the contralesional homotopic region triggered an SD in only 8% of the trials (1/13; p=0.004 vs. ipsilesional peri-infarct, Fisher’s exact test). The duration of optogenetic stimulation required to trigger an SD in peri-infarct cortex was under 1 minute (median 56 seconds, interquartile range 25-197). In sham-operated non-ischemic mice, stimulation of the homotopic somatosensory cortex did not trigger an SD on either hemisphere (0/10 each, p=0.002 vs. ipsilesional peri-infarct in MCAO). Stimulation of the ipsilesional core triggered an SD in 33% of trials (5/15) vs. none in the contralesional homotopic region (0/14, p=0.042 vs. ipsilesional core). Once again in non-ischemic mice stimulation of the homotopic whisker barrel cortex did not trigger an SD on either hemisphere (0/10 each, p=0.061 vs. ipsilesional core in MCAO). These data showed that ipsilesional peri-infarct cortex, and to a lesser extent the core, are highly susceptible to functional activation-induced peri-infarct SD initiation compared with homotopic regions in the contralesional hemisphere and in sham-operated non-ischemic mice.
Contrary to peri-infarct and core regions, however, stimulation of the ipsilesional mild ischemic brain did not trigger any SD in 12 trials, whereas stimulation of contralesional homotopic region triggered an SD in 46% of trials (6/13, p=0.015 vs. ipsilesional mild ischemic), suggesting that ipsilesional mildly ischemic brain developed resistance to SD initiation upon functional activation. Moreover, in sham-operated non-ischemic mice, stimulation of the homotopic motor cortex triggered 3 SDs in one hemisphere and 4 SDs in the other out of 7 trials each (p=0.036 vs. ipsilesional mild ischemic), suggesting that intense neuronal activity is capable of triggering an SD even in normal brain.
Induction of peri-infarct SDs by optogenetic stimulation appeared to decrease the spontaneous peri-infarct SD occurrence, presumably because of the refractory period after induced SDs. Importantly, optogenetic stimulation of the contralesional cortex never triggered an SD remotely in the ipsilesional cortex, consistent with the inhibitory nature of transcallosal projections of optogenetically activated neurons in this transgenic strain.7 In ChR2(−) mice optogenetic light stimulation at the highest intensity (10 mW for 5 minutes) did not trigger an SD (n=3; Supplementary Fig. II).
The threshold light intensities at the time of SD onset were significantly lower in ipsilesional core and peri-infarct cortex compared with both the contralesional homotopic regions (p=0.042 and 0.004, a and c on Fig. 2 lower panel, respectively; Mann-Whitney U test) and the whisker barrel and somatosensory cortices in sham-operated non-ischemic mice (p=0.097 and p=0.003, b and d, respectively). In contrast, thresholds were significantly higher in ipsilesional mildly ischemic region compared with contralesional homotopic region (p=0.015, e), as well as the motor cortex in non-ischemic mice (p=0.036, f).
Optogenetic cortical evoked potentials
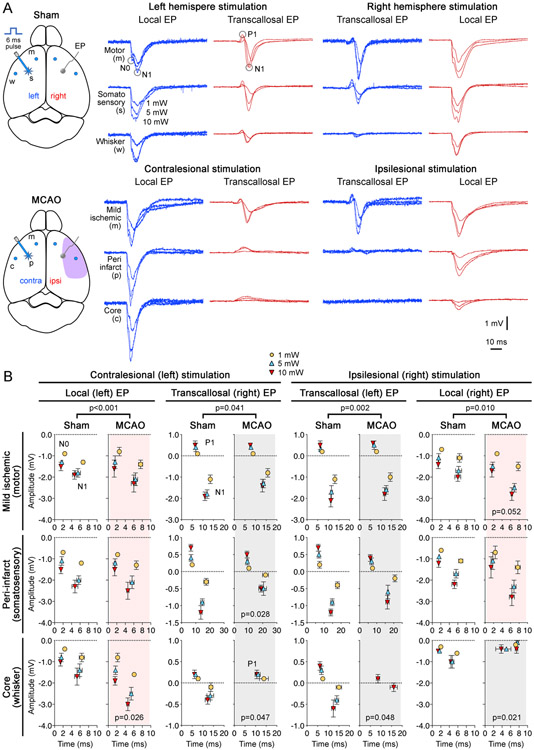
To confirm functional activation upon optogenetic stimulation, in a separate group of mice we recorded epicranial cortical evoked potentials from the stimulated area adjacent to the light source in core, peri-infarct and mildly ischemic regions of interest, as well as the contralateral homotopic regions (Fig. 3). In non-ischemic brain (Fig. 3A, sham; n=5), a single 6 ms light pulse evoked an immediate local negative potential shift (N0; Fig. 3A), likely reflecting the activation of ChR2 as previously described.7, 15 A larger negative field potential (N1) was superimposed on this early response. The transcallosal evoked response over the contralateral homotopic region was biphasic with an initial positive (P1) followed by a negative (N1) potential shift. Peak amplitudes and latencies of local N0/N1 and transcallosal P1/N1 are summarized in Fig. 3B.
Figure 3. Electrophysiological confirmation of cortical activation.
(A) Representative epicranial evoked field potentials (EP) in response to optogenetic stimulation (1, 5 or 10 mW light intensity, 6 ms pulse duration) are shown in sham-operated mice (upper panel) and after distal middle cerebral artery occlusion (MCAO; lower panel). We stimulated three regions on each hemisphere: ipsilesional peri-infarct, core and mildly ischemic regions, and their contralesional homotopic regions after MCAO, and corresponding right and left hemisphere somatosensory, whisker barrel and motor cortices in sham-operated mice. For each region, EPs were recorded from the stimulated area (local) adjacent to the light source as well as the contralateral homotopic region (transcallosal) simultaneously.
(B) Averaged response amplitudes of local evoked potentials (N0 and N1) and transcallosal evoked potentials (P1 and N1) for ipsilesional and contralesional stimulation after MCAO (n=5), and right and left hemisphere stimulation in the sham group (n=5). Standard errors of mean are also shown for both peak amplitude (mV) and latency (ms; time 0 indicates optogenetic stimulus onset). Grey and pink shades indicate diminished and augmented responses, respectively. P values comparing sham and MCAO groups indicate the result of multiple linear regression. P values shown on the graphs for individual regions indicate the result of two-way ANOVA for repeated measures (MCAO vs sham).
A multiple linear regression analysis (independent variables light intensity [1, 5 or 10 mW], region [core, peri-infarct or mildly ischemic], ischemia [sham or MCAO], dependent variable peak-to-peak amplitude) showed that MCAO significantly affected the evoked potentials within each stimulation-recording site pair when compared with sham (p=0.010 ipsilesional stimulation, local recording; p=0.002 ipsilesional stimulation, transcallosal recording; p<0.001 contralesional stimulation, local recording; p=0.041 contralesional stimulation, transcallosal recording; n=5). After MCAO, as expected, recordings in ischemic core showed severely diminished local and transcallosal evoked potentials (p=0.021 and p=0.048, respectively). Contralesional transcallosal evoked potentials upon stimulation of the ischemic core were also depressed (p=0.048). Transcallosal evoked potentials were also diminished when recorded in the peri-infarct region (p=0.028), consistent with depressed synaptic transmission. Interestingly, local evoked potentials in the mildly ischemic region and contralesional whisker barrel cortex were larger than non-ischemic (i.e. sham) controls (p=0.052 and p=0.026, respectively), suggesting disinhibition. Altogether these data confirmed successful optogenetic activation of the peri-infarct region and showed that local optogenetic evoked potentials are generally augmented after MCAO (red shades), with the exception of ischemic core, while transcallosal responses are diminished (grey shades).
Cerebral blood flow
We have recently shown that somatosensory stimulation triggers peri-infarct SDs by worsening oxygen supply-demand mismatch in the activated peri-infarct cortex.5 Therefore, we next turned our attention to CBF (Fig. 4). Using LSF, we determined the residual CBF in the stimulated regions of interest after MCAO, as well as the neurovascular coupling response (Fig. 4A). Residual CBF after MCAO was 43±3, 35±3 and 26±3% of pre-ischemic baseline in mildly ischemic, peri-infarct and core regions, respectively (Fig. 4B, at power 0 mW). In the mildly ischemic cortex, optogenetic stimulation evoked the anticipated hyperemic response in a power-dependent manner (Fig. 4B, left). In contrast, CBF in ischemic core failed to increase in response to optogenetic stimulation, presumably because maximal autoregulatory vasodilation has already been reached upon MCAO (Fig. 4B, right). In the peri-infarct region, CBF responses to optogenetic stimulation were mixed, ranging from no response to an attenuated response (Fig. 4B, middle).
Figure 4. Residual blood flow and functional hyperemic response predict SD occurrence during optogenetic cortical activation.
(A) Using laser speckle flowmetry (LSF), we measured the residual cerebral blood flow (CBF) and the superimposed neurovascular coupling response during optogenetic functional activation in each of the three regions of interest (core, peri-infarct and mildly ischemic brain) upon distal middle cerebral artery occlusion. Right panel shows a representative trace during optogenetic stimulation. A small CBF response to activation as well as a peri-infarct spreading depolarization (SD; yellow triangle) are seen. Stimulation (blue bar) was terminated when the peri-infarct SD occurred.
(B) CBF responses to optogenetic functional activation in ipsilesional core (n=12), peri-infarct (n=12) and mild ischemic cortex (n=10). Because no SD occurred during stimulation of the mildly ischemic cortex (left panel) CBF responses to optogenetic functional activation were averaged (mean ± standard error). In the peri-infarct and core regions (middle and right panels, respectively), CBF response to individual stimulations are shown along with the onset of SD in a subset (yellow triangles).
(C) Left panel shows residual CBF values within each region grouped based on SD occurrence (+ or -) and the region (core or peri-infarct). Peri-infarct SDs were triggered only when residual CBF in activated core and peri-infarct region were within a narrow range (p<0.001; one-way ANOVA). Right panel shows peak % CBF increase in SD (+) trials compared with the CBF increase at the same light intensity in SD (−) trials in peri-infarct region. Peak CBF increase was significantly larger in SD (−) trials than SD (+) trials at corresponding light intensities (n=8, 1% vs 4%, p=0.02, t-test). Mean ± standard errors are also shown.
A subset of stimulations in the peri-infarct and core regions culminated in SD (Fig. 4B, triangles). We found that the residual CBF within the stimulated cortex at the onset of optogenetic stimulation in trials that triggered an SD was approximately 31% (27-35%; 95% CI; n=8 and 4 in peri-infarct and core, respectively; Fig. 4C, left panel). Core stimulations failed to trigger an SD when residual CBF was lower than this narrow range (n=8, 23%, 21-25%; 95% CI), whereas peri-infarct stimulations failed to trigger an SD when residual CBF was higher (n=4, 39%, 27-50%; 95% CI; p<0.001, ANOVA followed by Holm-Sidak’s multiple comparisons). These data showed that a critical range of residual CBF in activated cortex was required to predispose tissue to functional activation induced peri-infarct SDs.
The magnitude of the hyperemic response to stimulation also predicted SD occurrence. We found significantly smaller CBF increases in trials that triggered an SD than those that did not at corresponding light intensities (1% vs 4%, p=0.02, t-test; Fig. 4C, right panel). Indeed, there was little net change in CBF during stimulations that eventually triggered an SD. These data showed that functional activation triggered an SD when CBF did not rise to match the increased demand. Importantly, functional activation of contralesional homotopic regions did not affect CBF in the ipsilesional cortex (see Supplementary Fig. III and Supplementary Fig. IV for all CBF responses in all regions of interest in MCAO as well as sham-operated mice). In ChR2(−) mice, optogenetic stimulation at 10 mW for 5 minutes did not change CBF (n=4, Supplementary Fig. V).
DISCUSSION
Our data show that acutely ischemic cortex is highly susceptible to develop SDs during optogenetic functional activation compared with contralesional (i.e. non-ischemic) homotopic cortex. High-resolution laser speckle imaging revealed a critical residual CBF of approximately one third of baseline in regions that developed a peri-infarct SD upon functional activation. This region was capable of synaptic transmission since transcallosal stimulation evoked local field potentials, and thus was outside ischemic penumbra, which, by definition, has preserved membrane potentials but synaptic silence. Regions with lower or higher CBF failed to develop an SD, presumably because the former was already persistently depolarized, and the latter had sufficient CBF to match the increased demand. Perfusion maps showed that regions that developed an SD upon functional activation did not mount a hyperemic response suggesting neurovascular uncoupling and worsening supply-demand mismatch during functional activation.
The lack of a hyperemic response to optogenetic activation in per-ischemic tissue was consistent with near-maximal autoregulatory dilation in penumbra, and conflicted the reports in a similar model of focal cerebral ischemia in rats.16-19 Our data suggest that focal functional activation of ischemic penumbra or core does not always recruit collateral CBF sufficient to match increased demand. As a result, supply-demand mismatch is worsened, culminating in the loss of membrane ion gradients and triggering an SD.
Peri-infarct stimulation triggered an SD more than 60% of the time and only after 1 minute of stimulation. In comparison, non-stimulated controls developed a spontaneous peri-infarct SD at a rate of 1% every minute, suggesting a strikingly elevated SD susceptibility during functional activation in peri-infarct cortex. In contrast, mildly ischemic ipsilesional motor cortex failed to develop an SD even at stimulation levels that triggered an SD in contralesional motor cortex, despite the trend for larger evoked potential amplitudes (Fig. 3B, upper right panel), suggesting reduced susceptibility to functional activation induced SDs. Mechanisms of this SD resistance in mildly ischemic cortex are unclear at this time. Since optogenetic SD susceptibility shows regional heterogeneity due to differences in ChR2 expression,15 we only compared ipsilesional and contralesional homotopic regions and did not attempt to compare different cortical regions within the same hemisphere. Unfortunately, non-invasive epicranial recordings precluded reliable electrophysiological analysis of SDs triggered by optogenetic stimulation.
Our data also show that functional activation is capable of triggering SDs even in non-ischemic brain tissue (Fig. 2, red bars, bilateral motor cortex in sham and contralesional motor cortex in MCAO). SD is widely recognized as the basis of migraine aura. For decades, the triggers for SD in apparently healthy brains of migraineurs has been a mystery. Our data support the notion that strong functional activation is capable of triggering an SD in otherwise normal cortex, providing one explanation for the origin of migraine aura. Historically, high-frequency tetanic stimulation reliably triggered SD in normal brain,20-22 but these models have been highly invasive and thus not physiological. Epileptic seizures have also been reported to culminate in SD.23-27 Moreover, when cortex is rendered hyperexcitable by genetic or pharmacological means, sensory stimulation has been shown to be capable of triggering SD.25, 28, 29 Indeed, electrophysiological signs of hyperexcitability have been reported in migraineurs.30, 31 Altogether, these data suggest that sufficiently intense neuronal activity within physiological range can trigger an SD, especially if the tissue is partially hypoperfused, supporting the stroke-migraine depolarization continuum.1
In summary, we show that peri-infarct ‘hot zones’ with a residual CBF ~30% of baseline are highly susceptible to developing SDs upon functional activation, although this CBF range should be taken with caution as different CBF measurement methods and different species might yield different CBF values for the hot-zones. Whether elevated SD susceptibility extends into the post-reperfusion period remains to be tested. Nevertheless, given the large body of evidence suggesting that peri-infarct SDs contribute to infarct growth,32 our data suggest that functional activation during focal cerebral ischemia may worsen the outcome of ischemic stroke, consistent with recent data against early mobilization after ischemic stroke due to a trend toward worse neurological outcomes.33
Supplementary Material
Acknowledgments
Funding: This work was funded by the National Institutes of Health (R25NS065743, KL2TR002542, and K08NS112601 to D.Y.C. and P01NS055104 and R01NS102969 to C.A.); the American Heart Association and American Stroke Association (18POST34030369 to D.Y.C.); the Andrew David Heitman Foundation (D.Y.C. and C.A.); the Aneurysm and AVM Foundation (D.Y.C.); the Brain Aneurysm Foundation’s Timothy P. Susco and Andrew David Heitman Foundation Chairs of Research (D.Y.C.); the Ellison Foundation (C.A.); the Boehringer Ingelheim Fonds (M.B.); the Turkish Society of Physical Medicine and Rehabilitation personal fees (S.A.); electroCore (A.H); and the Building Interdisciplinary Careers in Women’s Health (BIRCWH -K12 5 K12 HD051959 14 to A.H.).
Footnotes
Conflict-of-Interest: The authors report no conflict-of-interests. A.H. was a member of a scientific advisory committee for Bristol Myers Squibb. This role had no relationship to this work.
REFERENCES
- 1.Dreier JP, Reiffurth C. The stroke-migraine depolarization continuum. Neuron. 2015;86:902–922 [DOI] [PubMed] [Google Scholar]
- 2.Sadeghian H, Lacoste B, Qin T, Toussay X, Rosa R, Oka F, et al. Spreading depolarizations trigger caveolin-1-dependent endothelial transcytosis. Ann Neurol. 2018;84:409–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mestre H, Du T, Sweeney AM, Liu G, Samson AJ, Peng W, et al. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science. 2020;367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayata C, Lauritzen M. Spreading depression, spreading depolarizations, and the cerebral vasculature. Physiol Rev. 2015;95:953–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Bornstadt D, Houben T, Seidel JL, Zheng Y, Dilekoz E, Qin T, et al. Supply-demand mismatch transients in susceptible peri-infarct hot zones explain the origins of spreading injury depolarizations. Neuron. 2015;85:1117–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–565 [DOI] [PubMed] [Google Scholar]
- 7.Bohm M, Chung DY, Gomez CA, Qin T, Takizawa T, Sadeghian H, et al. Neurovascular coupling during optogenetic functional activation: Local and remote stimulus-response characteristics, and uncoupling by spreading depression. J Cereb Blood Flow Metab. 2020;40:808–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Peca J, Matsuzaki M, Matsuzaki K, Noguchi J, Qiu L, et al. High-speed mapping of synaptic connectivity using photostimulation in channelrhodopsin-2 transgenic mice. Proc Natl Acad Sci U S A. 2007;104:8143–8148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54:205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayata C, Dunn AK, Gursoy OY, Huang Z, Boas DA, Moskowitz MA. Laser speckle flowmetry for the study of cerebrovascular physiology in normal and ischemic mouse cortex. J Cereb Blood Flow Metab. 2004;24:744–755 [DOI] [PubMed] [Google Scholar]
- 11.Chung DY, Sugimoto K, Fischer P, Bohm M, Takizawa T, Sadeghian H, et al. Real-time non-invasive in vivo visible light detection of cortical spreading depolarizations in mice. J Neurosci Methods. 2018;309:143–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eikermann-Haerter K, Lee JH, Yuzawa I, Liu CH, Zhou Z, Shin HK, et al. Migraine mutations increase stroke vulnerability by facilitating ischemic depolarizations. Circulation. 2012;125:335–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata C. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab. 2006;26:1018–1030 [DOI] [PubMed] [Google Scholar]
- 14.Christie IN, Wells JA, Southern P, Marina N, Kasparov S, Gourine AV, et al. Fmri response to blue light delivery in the naive brain: Implications for combined optogenetic fmri studies. Neuroimage. 2013;66:634–641 [DOI] [PubMed] [Google Scholar]
- 15.Chung DY, Sadeghian H, Qin T, Lule S, Lee H, Karakaya F, et al. Determinants of optogenetic cortical spreading depolarizations. Cereb Cortex. 2019;29:1150–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hancock AM, Frostig RD. Testing the effects of sensory stimulation as a collateral-based therapeutic for ischemic stroke in c57bl/6j and cd1 mouse strains. PLoS One. 2017;12:e0183909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lay CC, Davis MF, Chen-Bee CH, Frostig RD. Mild sensory stimulation completely protects the adult rodent cortex from ischemic stroke. PLoS One. 2010;5:e11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lay CC, Frostig RD. Complete protection from impending stroke following permanent middle cerebral artery occlusion in awake, behaving rats. Eur J Neurosci. 2014;40:3413–3421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lay CC, Jacobs N, Hancock AM, Zhou Y, Frostig RD. Early stimulation treatment provides complete sensory-induced protection from ischemic stroke under isoflurane anesthesia. Eur J Neurosci. 2013;38:2445–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grafstein B. Mechanism of spreading cortical depression. Journal of neurophysiology. 1956;19:154–171 [DOI] [PubMed] [Google Scholar]
- 21.Ochs S, Hunt K, Booker H. Spreading depression using chronically implanted electrodes. The American journal of physiology. 1961;200:1211–1214 [DOI] [PubMed] [Google Scholar]
- 22.Leao AAP. Spreading depression of activity in cerebral cortex. Journal of neurophysiology. 1944:359–390 [DOI] [PubMed] [Google Scholar]
- 23.Fabricius M, Fuhr S, Bhatia R, Boutelle M, Hashemi P, Strong AJ, et al. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain. 2006;129:778–790 [DOI] [PubMed] [Google Scholar]
- 24.Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001;81:1065–1096 [DOI] [PubMed] [Google Scholar]
- 25.Vinogradova LV. Comparative potency of sensory-induced brainstem activation to trigger spreading depression and seizures in the cortex of awake rats: Implications for the pathophysiology of migraine aura. Cephalalgia. 2015;35:979–986 [DOI] [PubMed] [Google Scholar]
- 26.de Azeredo FA, Perret ML. Cortical slow potential changes during convulsions induced by maximal electroshock or penicillin focus. Metabolic brain disease. 1992;7:101–113 [DOI] [PubMed] [Google Scholar]
- 27.Koroleva VI, Vinogradova LV, Bures J. Reduced incidence of cortical spreading depression in the course of pentylenetetrazol kindling in rats. Brain Res. 1993;608:107–114 [DOI] [PubMed] [Google Scholar]
- 28.Van Harreveld A, Stamm JS. Cortical responses to metrazol and sensory stimulation in the rabbit. Electroencephalography and clinical neurophysiology. 1955;7:363–370 [DOI] [PubMed] [Google Scholar]
- 29.Vinogradova LV, Kuznetsova GD, Coenen AM. Unilateral cortical spreading depression induced by sound in rats. Brain Res. 2009;1286:201–207 [DOI] [PubMed] [Google Scholar]
- 30.Aurora SK, Wilkinson F. The brain is hyperexcitable in migraine. Cephalalgia. 2007;27:1442–1453 [DOI] [PubMed] [Google Scholar]
- 31.Welch KM, D'Andrea G, Tepley N, Barkley G, Ramadan NM. The concept of migraine as a state of central neuronal hyperexcitability. Neurologic clinics. 1990;8:817–828 [PubMed] [Google Scholar]
- 32.Hartings JA, Shuttleworth CW, Kirov SA, Ayata C, Hinzman JM, Foreman B, et al. The continuum of spreading depolarizations in acute cortical lesion development: Examining leao's legacy. J Cereb Blood Flow Metab. 2017;37:1571–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernhardt J, Langhorne P, Lindley RI, Thrift AG, Ellery F, Collier J, et al. Efficacy and safety of very early mobilisation within 24 h of stroke onset (avert): A randomised controlled trial. Lancet. 2015;386:46–55 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data that support the findings of this manuscript are available upon request to the corresponding author.